Highlights
-
•
Recognition the etiologies of ATL is important due to its potentially reversible nature after treatment or removal of the toxin.
-
•
“CHOICES” is useful to memorize etiologies associated with PVWM injury in non-metabolic and non-infectious ATL patients.
-
•
Reduced diffusion is an early and important imaging finding to evaluate patients with non-metabolic and non-infectious ATL patients.
Abbreviations: ADEM, Acute disseminated encephalomyelitis; AEDs, Anti-epileptic drugs; AHE, Acute Hepatic/Hyperammonemic Encephalopathy; AHL, Acute hemorrhagic leukoencephalitis; ATL, Acute toxic leukoencephalopathy; CO, Carbon monoxide; EPM, Extrapontine myelinolysis; EtOH, Ethanol; HIE, Hypoxic-ischemic encephalopathy; LE, leukoencephalopathy; MBD, Marchiafava-Bignami Disease; MERS, Mild encephalitis/encephalopathy with reversible splenial lesion; NAWM, Normal-appearing white matter; ODS, Osmotic demyelination syndrome; PML, Progressive multifocal leukoencephalopathy; PRES, Posterior reversible encephalopathy syndrome; PVWM, Periventricular white matter; RIS, Radiology information system; RSL, Reversible splenial lesions
Keywords: Acute toxic leukoencephalopathy, Periventricular white matter, Diffusion-Weighted imaging
Abstract
Purpose
To describe non-metabolic, non-infectious etiologies of acute toxic leukoencephalopathy (ATL) on DWI MRI, and provide a useful acronym to remember them.
Material and Methods
Our PACS archive was reviewed, yielding 185 patients with suspected ATL per MRI reports and clinical follow up; infectious or metabolic causes were excluded.
Result/Discussion
The 87 included non-infectious, non-metabolic ATL patients' etiologies are represented by the acronym 'CHOICES': chemotherapy ('C',n = 34); heroin-induced ('H',n = 6), opioid analogues ('O',n = 14); immunosuppressant ('I',n = 11) or imidazole (n = 2); cocaine ('C',n = 1); environmental or ethanol abuse ('E',n = 5), splenial lesions ('S',n = 9), and 'other' (n = 5).
Conclusion
The "CHOICES" acronym delineates various toxic etiologies of ATL.
1. Introduction
The term “leukoencephalopathy” (LE) indicates an abnormality of cerebral white matter (WM) that predominantly damages myelin. Toxic LE predominately affects the cerebral WM and may occur from an exposure to a variety of agents, including environmental toxins, prescription medications, metabolic substances, and illicit drug usage [1]. Such leukotoxic agents can incite varying degrees of deterioration of higher cerebral function, which can clinically present acutely or chronically along a spectrum ranging from mild confusion, altered mental status, dementia, coma, or even death [1]. Acute toxic leukoencephalopathy (ATL) is a potentially reversible condition that may improve after treatment or following withdrawal of the offending toxin early in the course of disease; on MRI, ATL variably has abnormal signal on FLAIR/T2WI, but the abnormalities are typically visible as bright on DWI and dark on accompanying ADC maps relative to normal-appearing white matter (NAWM) [2,3]. ATL typically affects WM tracts extending from the periventricular white matter (PVWM) out to the subcortical WM, more commonly in a symmetric distribution. In a minority of cases, atypical areas of involvement include the basal ganglia, thalami, brainstem, internal capsules, and cerebellum [2,3]. While the exact pathophysiologic mechanism of ATL is unknown, preliminary histologic evidence suggests that the various forms of endothelial insults subsequently and generally result in intramyelinic edema [2,3]. Regarding potential contributing conditions to the onset of ATL, it is of note that uremia has recently been identified as a potential exacerbating factor in ATL [3].
The most common causes of ATL in adults include chemotherapeutic agents, immunosuppressant therapy, illicit drug use, and medication overuse, particularly from opioid overusage [2,3]. Environmental toxins such as carbon monoxide (CO) may also cause ATL [[1], [2], [3], [4], [5], [6], [7], [8]]. As reversible splenial lesions (RSL’s) may arise from similar potentially toxic substances and the callosal splenium is in fact periventricular, RSL may be considered a subtype of ATL [2,3].
In this study and accompanying review, a differential diagnosis of potential extrinsic causes of ATL (i.e. non-metabolic and non-infectious) in adults is proposed via the acronym “CHOICES”: ‘C’= chemotherapy; ‘H’= heroin-induced (illicit usage), ‘O’= opioid analogues (medication abuse or overdose); ‘I’= immunosuppressant or imidazole drugs; ‘C’= cocaine; ‘E’= environmental (such as CO) or ethanol abuse; ‘S’= splenial lesions of the corpus callosum (reversible, possibly due to multiple agents), as depicted in Table 1 [[1], [2], [3], [4], [5], [6], [7], [8]]. Hence, this review provides a novel acronym (“CHOICES”) to aid in the quick recall and subsequent treatment of the various toxic (i.e. non-metabolic and non-infectious) causes of ATL, as well as in describing the mimics of ATL.
Table 1.
Potential etiologies of ATL.
| C | Chemotherapy (n = 34) |
|---|---|
| H | Heroin-induced (n = 6), whether via intravenous (n = 3) or inhaled (n = 3) routes |
| O | Opioid analogue (n = 14), abuse via various routes of “non-heroin” medications |
| I | Immunosuppressant (n = 11) or Imidazole medications (n = 2) |
| C | “Crack” cocaine abuse (n = 1) |
| E | Environmental (CO, n = 3) and Ethanol-related(n = 2) |
| S | Splenial lesions (RSL), total n = 9): which include AEDs (n = 7), chemotherapy (n = 1), immunosuppressant medication (n = 1) |
Note: CO = Carbon monoxide, RSL = reversible splenial lesion, AEDs = anti-epileptic drugs.
2. Material and methods
This study was approved by our institutional review board. A search was performed of the radiology information system (RIS) at a multi-center institution for the terms “leukoencephalopathy” and “toxic” within the MRI reports, which includes both level 1 trauma and quaternary care centers. This search was conducted using 2 software programs: Vitrea Intelligence (Vital Images, Minnetonka, Minnesota) and Primordial (Nuance Communications, Burlington, Massachusetts), which yielded 185 patients over a 15 year period with potential non-metabolic and non-infectious ATL-related PVWM injury on DWI. The electronic records of these patients were then separately reviewed by a neuroradiology fellow, including their history, symptoms, laboratory values, and clinical followup. Pediatric patients, those with congenital disorders, metabolic conditions (such as hepatic encephalopathy, osmotic demyelination, hyperglycemia or uremia, to name a few), or patients with an active infection or sepsis were excluded. Also excluded were those with compromised imaging (e.g. MRI exams lacking ADC maps), or a lack of a clinical confirmation of ATL based on clinical followup. Thus, the final cohort consisted of only those with toxic causes of ATL. Notably, those with RSL were grouped and counted according to the etiology of ATL in the method described above.
3. Results
As above, the RIS search yielded 185 patients suspected of having ATL, based on MRI. However, 98 were ultimately excluded due to: 1) 73/98 as they either were confirmed not to have ATL (n = 53), or had inadequate imaging such as a lack of ADC maps (n = 14), or were children (n = 4), or were found to be a chronic form of toxic LE (n = 2); 2) the other 25/98 exclusions were due to being either metabolic/intrinsic or infectious causes, including acute hepatic encephalopathy (AHE, n = 14), sepsis-related ATL (n = 5, while 3/5 had an RSL-type appearance), hyperglycemia (n = 3), extrapontine myelinolysis (n = 1), uremia (n = 1), or hypertension (n = 1).
Hence, a total of 87 patients with non-metabolic (i.e. extrinsic) and non-infectious causes of ATL were included in the study, with the etiologies being ‘C’= chemotherapy (n = 34), ‘H’= heroin-induced (n = 6), ‘O’= opioid-related (n = 14), ‘I’= immunosuppressant (n = 11), and imidazole medication (n = 2), ‘C’= cocaine (n = 1), ‘E’= environmental: CO (n = 3), and EtOH-related (n = 2), reversible splenial lesion (n = 9), and, others such as: antibiotic (cefepime, n = 1), benzodiazepine (n = 1), antidepressant (sertraline, n = 1), and rituximab (n = 2) as shown in Table 1.
Among the 34 chemotherapeutic patients, being the largest ATL subcategory, the causative agents were: fludarabine (n = 16), methotrexate (n = 9), combined methotrexate + cytarabine (n = 3), 5-fluorouracil (n = 2), doxorubicin (n = 1), cisplatin (n = 1), erlotinib (n = 1), and cytarabine (n = 1).
Regarding the 20 heroin/opioid-related ATL patients, 6/20 were heroin-induced (inhaled n = 3, intravenous n = 3), while the other 14/20 were attributed to morphine (oral, n = 2), methadone (n = 1), transdermal fentanyl (n = 1), combined oxycodone + cocaine (n = 1), and an “unspecified opioid” (diagnosed via urine test, n = 9). Thus, in 9/20 patients the specific opioid agent was not discerned, demonstrating how causative opioid etiologies may not be determined.
Among the 11 immunosuppressant-related ATL patients, the causes were cyclosporine (n = 4), tacrolimus (n = 4), and cyclophosphamide (n = 1); the other 2/11 were an “unknown immunosuppressant”, where the records were too old to obtain (i.e. not electronic).
The etiologies of confirmed RSL-type lesions involving solely the callosal splenium were further sub-categorized by their cause of ATL, which included: anti-epileptic drugs (AEDs) (n = 7), chemotherapy (n = 1), and an immunosuppressant medication (n = 1), as in Table 1.
4. Discussion
Acute toxic leukoencephalopathy (ATL) is a disorder that is caused by variety of toxic exposure including medications, illicit drug use, and environmental exposures [[2], [3], [4], [5], [6], [7], [8]]. Although the most common and recognizable MRI findings relate to reduced diffusivity as being bright on DWI, and dark on ADC maps, there may be WM hyperintensity on T2WI and FLAIR; these abnormalities are due to the toxins’ damage to myelin, where involved sites can change depending on the type of toxic substance and its severity [6]. It is important to point out that ATL is a less common effect of certain toxins relative to another entity, posterior reversible encephalopathy syndrome (PRES), which may occur from particular toxins that also cause ATL, most notably chemotherapy, immunosuppressants, and illicit drugs [10]. PRES is a more common acute and potentially reversible encephalopathic toxic and non-metabolic insult, also having an overlap in clinical symptom presentation (encephalopathic) as well as etiologies, but these two entities can be distinguished by their imaging appearances. For example, PRES has cortical and subcortical edema on routine MR imaging sequences such as FLAIR; in comparison, the extent of ATL is best visualized with reduced diffusion within the PVWM on DWI [2,3,9]. Also, the anatomic distribution and appearance on DWI help to differentiate these two potentially reversible entities, as ATL “starts” (i.e. in the mildest forms) within the PVWM (corona radiata and centrum semiovale), while the mildest cases of PRES are typically in the cortex/subcortical WM, most commonly being parietal and occipital in distribution [[10], [11], [12]]. Also, based on DWI, ATL has deep PVWM reduced diffusion, typically in a confluent and symmetric fashion, while PRES has DWI abnormalities in only a minority; when present in PRES, these DWI-positive foci are typically focal, asymmetric, cortical-based, and sometimes gyriform [[10], [11], [12], [13]]. Also, postcontrast imaging findings differ between the two entities, as ATL only rarely has contrast enhancement on T1WI MRI (described as punctate within the PVWM in <10%), while PRES can have varying degrees of avid enhancement in up to 50%, whether cortical, gyriform, or leptomeningeal [3,11]. Finally, on SWI, ATL has microhemorrhages in 15%, but rarely has macrohemorrhages (>1 cm); meanwhile PRES has microhemorrhages (< 1 cm in size) in nearly 60%, with macrohemorrhages in up to 10–20% [3,14]. Of note, several patients with combined ATL and PRES have recently been reported, corroborating the concept that these entities may have a certain degree of overlap in both etiology and pathophysiology, thought related to toxin-induced endothelial injury with subsequent loss of blood-brain barrier integrity [13,15]. Hence, the following discussion focuses on letters for the acronym “CHOICES” that represents toxic, non-metabolic, non-infectious causes of ATL, keeping in mind that many (but not all) of these agents may also cause PRES, particularly chemotherapeutic medications, immunosuppressant agents, and illicit drug usage.
4.1. ‘C’= chemotherapy (intravenous or intrathecal)
ATL is a clinical consideration in cancer patients exposed to anti-neoplastic agents who develop acute neurologic symptoms [4]. Chemotherapy is likely the most common cause of ATL (39% ATL patients in this study), based on this and prior studies, with fludarabine potentially being the most common agent in this study (47% of chemotherapy-related ATL), followed by methotrexate (26.5%) [3,4]. Notably, fludarabine-related ATL has relatively poorer outcomes as compared to other chemotherapeutic agents, as it can cause severe, late-onset symptoms even months after the exposure; hence, an exposure to fludarabine is important to recognize as it is likely the most common cause of chemotherapy-related death from ATL [2,16] (Fig. 1). With methotrexate, the second most common cause of chemotherapy-induced ATL in the current study, the neurotoxicity arises from disruption of CNS folate homeostasis or via direct toxic neuronal damage, typically involving the PVWM with focal or multifocal reduced diffusivity; notably, intrathecal methotrexate neurotoxicity is relatively well-described [2,6,10,17]. The cerebral and cerebellar cortices, subcortical WM, and thalami are other atypical sites of involvement by methotrexate, which, incidentally, can also cause PRES [2,6,10]. Various other agents, such as 5-fluorouracil and anti-metabolic agents such as cytarabine, seem to be more reversible and often have better outcomes if promptly discontinued. Of note, these agents can potentiate each other to cause ATL, as noted in this study’s 3 patients with combined methotrexate + cytarabine toxicity [2]. Also of interest is 5-fluorouracil neurotoxicity, where there is often reduced diffusion in the centrum semiovale, callosal splenium, and PVWM that usually reverses clinically and on imaging after removal of the offending agent; hence, 5-FU-related ATL seems less likely to result in permanent sequelae if recognized promptly (Fig. 2) [6,18].
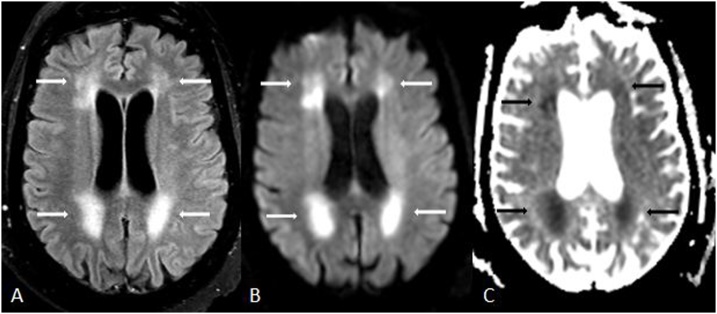
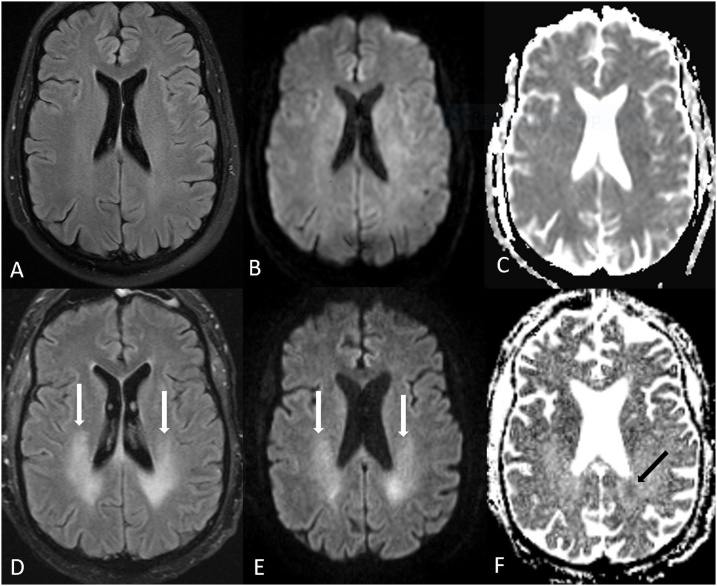
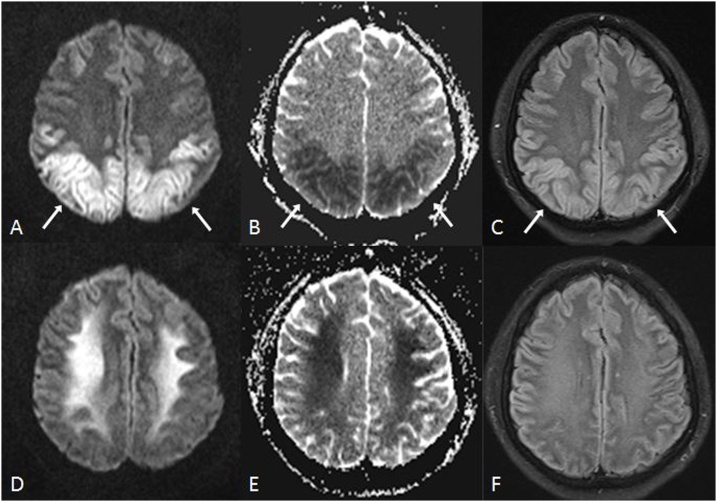
Fig. 1.
A 62 year old acutely encephalopathic female with acute toxic leukoencephalopathy (ATL), who had been administered fludarabine 3 weeks prior to imaging for treatment of multiple myeloma. She expired 19 days after MRI. 1A-C: there was bilateral, symmetric PVWM hyperintensity (arrows) on FLAIR (A), with reduced diffusion on DWI (B) and on ADC map (C).
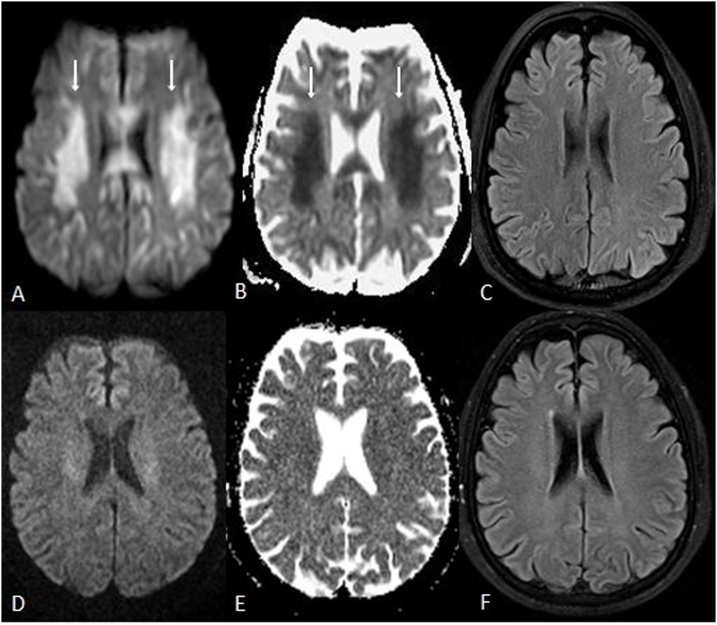
Fig. 2.
A 57 year old male with ATL on 5-Fluorouracil (5-FU) for esophageal cancer, who presented with altered mental status 5 days prior to MRI. 2A-C: The initial MRI showed reduced diffusion within the PVWM (arrows) on DWI (A) and ADC map (B); however, there was no evident abnormal signal on FLAIR (C). 2D-F: The MRI findings of ATL and symptoms nearly entirely resolved 19 days later on MRI, as shown on follow up DWI (D), ADC (E) and FLAIR (F) performed at that time.
4.2. ‘H’= heroin-induced ATL
Myelin is vulnerable to lipophilic substances and lipid peroxidation, due to its high lipid content [19]. Thus, as opioids are lipophilic, they can traverse the blood-brain barrier and damage myelin. Notably, the word “opioid” is a broad term encompassing both natural (i.e. true opiates such as heroin, morphine and codeine), semi-synthetic (i.e. hydrocodone, oxycodone), and synthetic substances (i.e. methadone, fentanyl) [20]. Hence, for the purposes of the ‘CHOICES’ acronym, we separate heroin-induced LE (usually intravenous or inhaled) from the opioid analogues (typically oral or intravenous), as the original descriptions of toxic LE from illicit opioids are largely based on heroin usage, as opposed to medication abuse [[21], [22], [23]]. A confounding issue in identifying the etiology of ATL can be when heroin or other opiates occur in tandem with other illicit drugs (such as cocaine), making it difficult to discern which is the causative etiology; such instances with a positive urine sample having an ‘unspecified opioid’ occurred as the cause in 9/87 patients (10.3%) in this study [2,3].
Heroin is one of the most commonly abused opiates, seen in about 5% of ATL patients, in accord with the finding of occurring in 6/87 patients (6.9%) in this study [3]. Clinically, whether intravenous or inhaled, heroin-induced ATL may progress through stages, beginning with a cerebellar syndrome, while the other end of the spectrum is a final clinical stage of symptomatology that includes spasms, akinetic mutism, and possibly death [20]. Characteristic areas of inhaled heroin-induced ATL (so-called “chasing the dragon”) include the posterior cerebral WM, the internal capsule’s posterior limbs, and occasionally cerebellar WM, whereas the subcortical U fibers and the internal capsules’ genu are more frequently involved in intravenous heroin-induced neurotoxicity [2,3,5,22]. Depending on the severity, lesions progress symmetrically from the deep PVWM to the subcortical WM, often spreading posterior to anterior; this can be entirely reversible, particularly in intravenous exposures [2,24]. Inhaled exposures (‘chasing the dragon’) tend to have poorer outcomes relative to intravenous exposures, and such severe cases may be result in diffuse cerebral atrophy and neurologic sequelae [2,3,5] (Fig. 3). Recent research has suggested that, of the major subtypes of etiologies of ATL involving the PVWM, opiates typically have the worst outcomes, followed by chemotherapy [2,3].
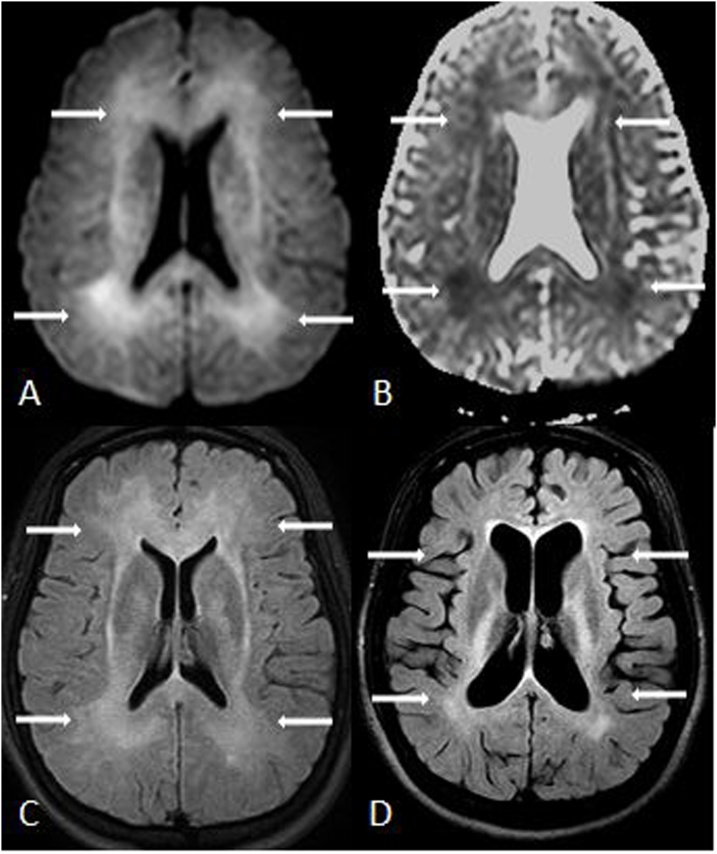
Fig. 3.
A 24 year old female was found unresponsive and quadriplegic from ATL after heroin inhalation (aka “chasing the dragon”). 3A-D: Reduced diffusion involves the PVWM relatively diffusely and symmetrically on DWI MRI (A) and ADC map (B), also with bilateral, symmetric periventricular hyperintensity on FLAIR (C), characteristic of ATL. Her paresis mostly resolved by 1 year, but there were residual PVWM abnormalities with severe cerebral atrophy on FLAIR (D).
4.3. ‘O’= opioid analogues (i.e. oxycodone, hydrocodone, methadone, fentanyl)
There are a number of opioid analogues which have been more recently described to cause ATL in contrast to that traditionally described of heroin-induced ATL [2,3]. For example, methadone hydrochloride is a synthetic opioid receptor agonist, where its primary symptoms of neurotoxicity are sedation, dizziness, abnormal sensation, respiratory depression, and coma [25]. On neuroimaging, a number of cases of methadone-induced LE have been described, having bilaterally high signal within the cerebral WM on FLAIR or T2WI, with sparing of the cortical U-fibers; in addition, symmetrical involvement of the cerebellar gray and WM, basal ganglia, hippocampi, central tegmental tracts, have also been reported [[25], [26], [27]].
Oxycodone- and fentanyl-induced ATL are less well described. As mentioned previously, in opioid-related ATL it can be difficult to discern the exact causative drug or medication since illicit usage may involve several drugs ingested together. In the current study, there were 9 patients categorized as an ‘unspecified opioid’, for which the exact causative opioid was unclear. Oxycodone is an orally ingested semi-synthetic opioid which has been reported to involve the PVWM, but also may involve the cerebellum and globi pallidi [3,28]. Fentanyl is a synthetic analogue, typically administered intravenously, but also can be transdermal, which can present on DWI and FLAIR MRI as PVWM and deep WM abnormalities in cerebral or cerebellar distributions [2,3]. It is important to note that in the setting of an opioid overdose such as fentanyl, it may be difficult to initially differentiate the MRI findings of ATL involving the PVWM from that of fentanyl-related (or other opioid-induced) delayed or subacute post-anoxic/hypoxic-ischemic encephalopathy (HIE), which can occur from respiratory depression or from the opioid’s toxic effects, or even via a combination of both ATL and HIE occurring simultaneously [2,29]. In pure ATL, the cerebral cortex overlying the PVWM injury remains normal signal intensity on FLAIR, while in patients with purely HIE the overlying cortex is mildly hyperintense [2] (Fig. 4).
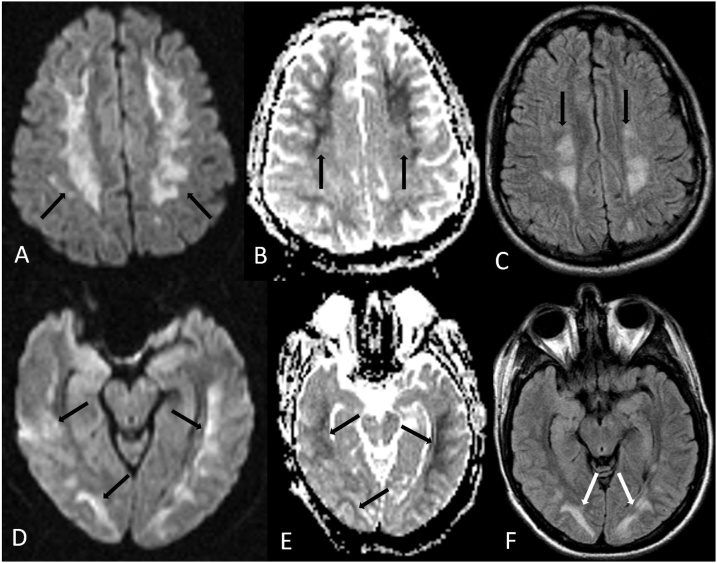
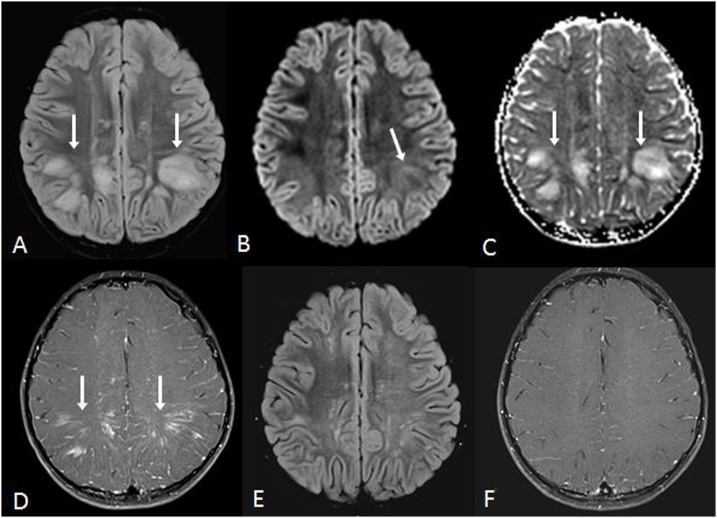
Fig. 4.
A 19 year old male with oral methadone overdose-related ATL presented with acute left sided weakness and upper motor neuron signs. 4A-C: The reduced diffusion bilaterally involves the PVWM (arrows) on DWI MRI (A) and ADC map (B), with bilateral symmetric periventricular hyperintensity on FLAIR (C). 4D-F: There is also reduced diffusion within the optic radiations on DWI MRI (D) and ADC map (E), also having abnormal signal on FLAIR (F).
4.4. ‘I’= immunosuppressant medications (e.g. cyclosporine, tacrolimus, etc.) and Imidazoles
Immunosuppressant‐related ATL is a less common subtype, typically due to medications such as cyclosporine, tacrolimus, and less commonly mycophenolate. In the current study, this occurred in 12.6% of patients, where cyclosporine and tacrolimus were the most common causes. As with chemotherapeutic agents, immunosuppressant-related neurotoxicity more commonly results in PRES, but a minority (about 20–28%) of patients with MRI findings of neurotoxicity may suffer purely ATL [[2], [3], [4],30]. The neurotoxicity is thought to result from endothelial injury that leads to blood–brain barrier dysfunction and subsequent PVWM injury, most often in the parietooccipital regions, usually with symmetric involvement of the deep and subcortical WM (Fig. 5) [2,3,31]. On DWI, there is often a milder degree of reduced diffusion relative to other causes of ATL, and the clinical severity and outcomes are also typically milder and more reversible in immunosuppressant‐related ATL, as compared to chemotherapy and opiate-related ATL [3,6].
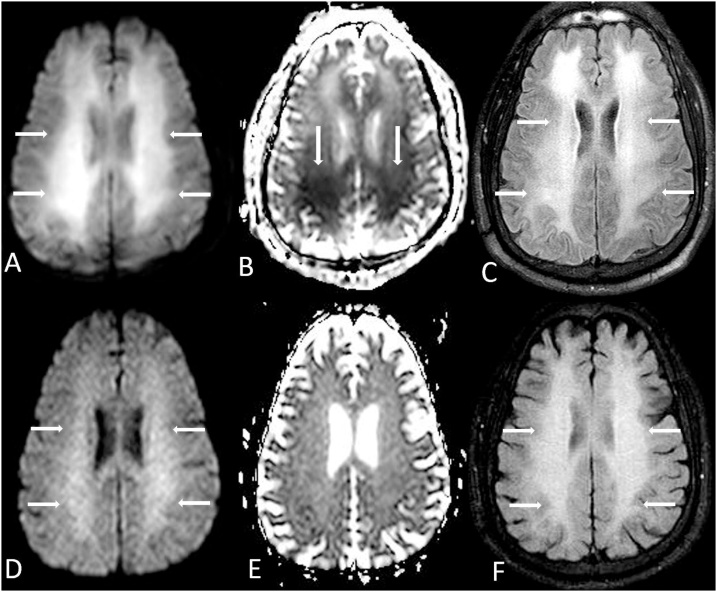
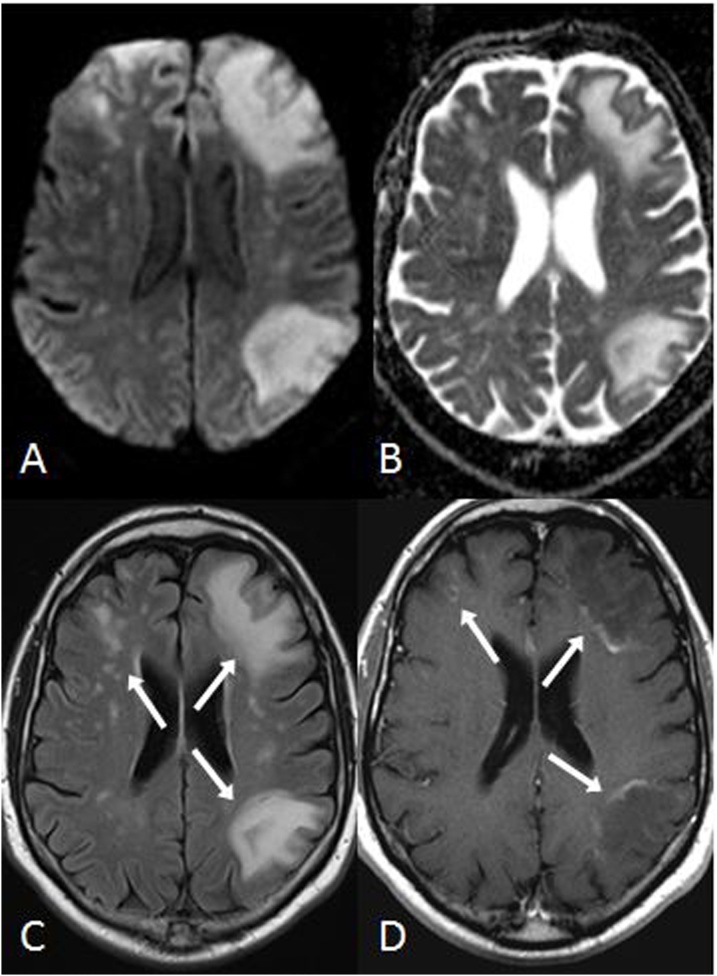
Fig. 5.
A 37 year old male with myelodysplastic syndrome underwent a normal-appearing baseline brain MRI prior to bone marrow transplantation (BMT), which was nearly normal on FLAIR (A), DWI (B) and ADC map (C). Nineteen days post-BMT, while the patient was on cyclosporine therapy (an immunosuppressant medication), regions of hyperintensity (arrows) from ATL developed within the bilateral posterior PVWM on FLAIR (D) and DWI (E); note mild, asymmetric reduced diffusion in the left posterior PVWM on the ADC map (F, arrow). The patient’s symptoms promptly resolved over several days after the cessation of cyclosporine.
Regarding imidazole medications, metronidazole (an antimicrobial) is a nitroimidazole derivative that can easily cross the blood-brain barrier. The characteristic pattern of involvement on MRI is the cerebellar dentate nuclei, but other affected areas may include the vestibular nuclei, cerebral PVWM, or callosal splenium, with or without dentate involvement (Fig. 6) [32]. In the current study, there were two such patients with ATL from metronidazole, one with characteristic dentate involvement, and the other having PVWM, callosal splenium, and bilateral corticospinal tract involvement. Hence, ATL should be considered in patients with PVWM DWI abnormalities receiving high doses of metronidazole, even if the dentate appears normal. This phenomenon can be entirely reversible both on imaging and clinically upon removal of the medication [32].
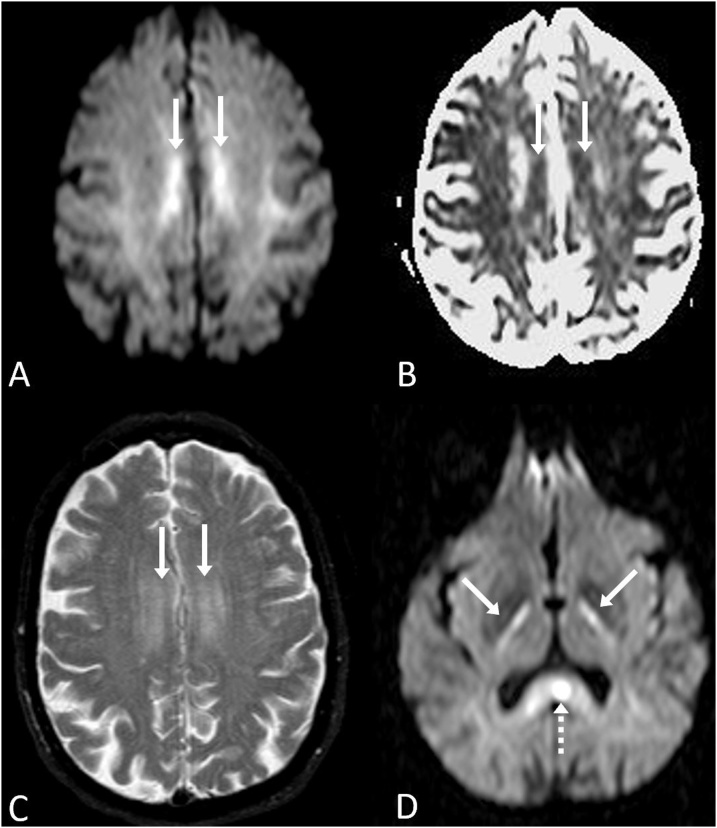
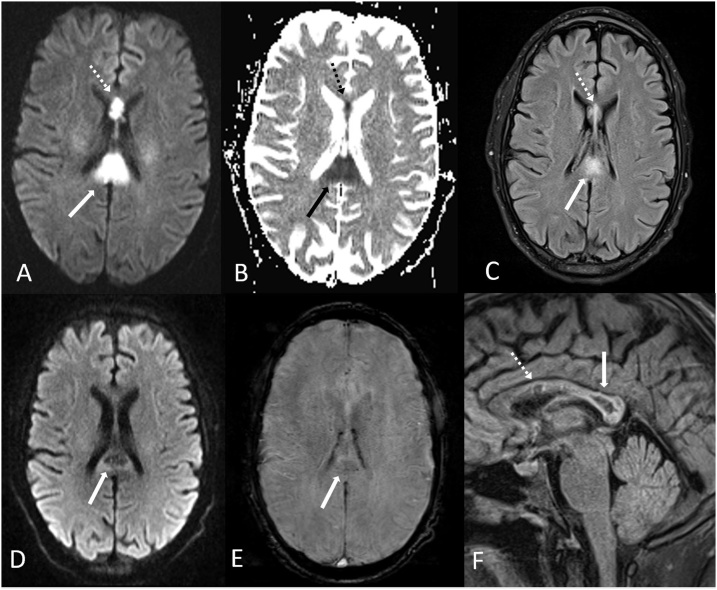
Fig. 6.
A 69 year old female with acutely altered mental status developed ATL after receiving high doses of metranidazole for over 2 months, prescribed for a recto-vaginal fistula. Her symptoms resolved after cessation of metronidazole. 6A-D: The reduced diffusion was symmetric and bilateral within the centrum semiovale (arrows) on DWI MRI (A) and ADC map (B), with hyperintensity on T2WI (C). Also, there was bilateral abnormal signal within the posterior limbs of the internal capsules (D, arrows) and midline callosal splenium (D, dotted arrow) on DWI. Notably, the dentate nuclei were normal in this patient (not shown); hence, this represents an atypical case of metronidazole toxicity causing ATL.
4.5. ‘C’= “crack” or cocaine
The neuroimaging findings of cocaine toxicity can have either of two appearances related to differing underlying mechanisms: 1) ischemic events due to vasospasm, or 2) direct neurotoxicity causing diffuse PVWM injury [33]. Regarding direct WM toxicity, the abnormalities are best visualized on DWI or FLAIR MRI, with or without reduced diffusion [33]. While heroin-induced LE usually involves the posterior cerebral and cerebellar WM, cocaine-related LE usually involves the frontal lobes, often sparing the brainstem and cerebellum [34,35]. In the current study, only one such patient was diagnosed with cocaine-related ATL (Fig. 7).
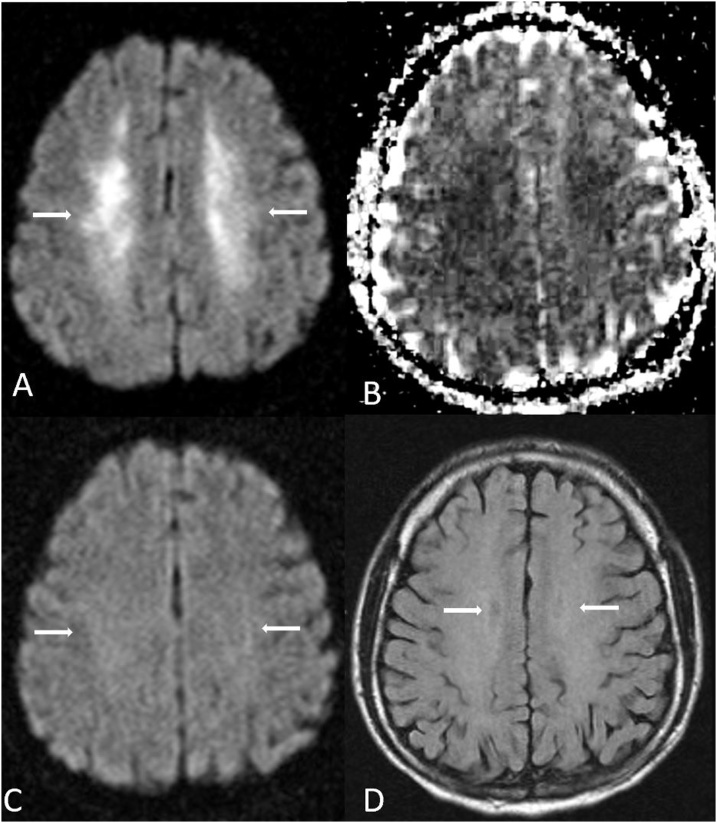
Fig. 7.
A 44 year old male presented with an altered and decreased level of consciousness for 2–3 days following a reported large amount of “crack” cocaine inhalation, which caused ATL. An initial MRI demostrated bilateral, symmetric reduced diffusion throughout the PVWM (arrows) on DWI MRI (A) and ADC map (B), also with PVWM hyperintensity on FLAIR (C). Four months later, the areas of reduced diffusion within the PVWM evolved into predominantly “T2 shine-through” (i.e. bright on ADC), as shown on DWI MRI (D), ADC map (E), and FLAIR (F).
4.6. ‘E’= environmental (carbon monoxide) or ethanol abuse
Carbon monoxide (CO) is a colorless and odorless gas, the toxic levels of which may be present in several scenarios, such as smoke inhalation, suicide attempts, or abandoned buildings. The symptoms of acute CO intoxication vary, and may include chest or abdominal pain, dizziness, or headaches, or even coma and death [6]. CO toxicity can result from induced hypoxia, respiratory chain blockage, or direct cellular toxicity; such direct toxic effects on WM can result when CO activates polymorphonuclear leukocytes, causing lipid peroxidation and resultant acute demyelination [7,36]. In CO-related encephalopathy, there are three general patterns of involvement: PVWM (most common), basal ganglial, and hippocampal (least common), usually with ADC reduction in the acute phase (Fig. 8) [6,7,37,38]. The WM abnormalities are usually reversible, however basal ganglial insults are more prone to permanent damage due to the higher metabolic activity of those nuclei [6,38]. Accordingly, in this study, three such patients with CO-related ATL were noted, each having PVWM involvement. Interestingly, both CO and inhaled opiate neurotoxicity may appear similar, due to either toxic demyelination or spongiform degeneration, as both syndromes can involve the PVWM, basal ganglia, or rarely the hippocampi [2]. Thus, in patients presenting as acutely obtunded with PVWM involvement on MRI, toxicity screening for both CO and opiate toxicity may be warranted if the clinical history is limited.
Fig. 8.
A 33 year old male with ATL from carbon monoxide (CO) poisoning, in which the symptoms and imaging findings later improved, having severely elevated serum CO levels. 8A-B: an MRI demonstrates reduced diffusion in bilateral PVWM (arrows) on DWI (A) and ADC map (B), these abnormalities had mostly resolved 9 months later (arrows), as shown on DWI (C) and with mild resultant diffuse cerebral atrophy on FLAIR (D).
Ethanol (EtOH) neurotoxicity uncommonly causes ATL, albeit rare, as hippocampal edema (from withdrawal seizures) is the most common MRI finding of EtOH neurotoxicity; less common findings are Wernicke encephalopathy from thiamine deficiency, and rarely ATL [39]. The rare form of ATL that is EtOH-related is Marchiafava-Bignami Disease (MBD), which is an uncommon acute presentation of chronic EtOH abuse, with a rapid onset of altered mental status, gait abnormalities, loss of consciousness, and speech impairment [40]. In the acute form on MRI, the genu and splenium of the corpus callosum are more commonly involved on FLAIR and DWI with reduced diffusion, and lesions may extend from the corpus callosum into adjacent PVWM, occasionally with involvement of other regions such as the cerebral cortex, middle cerebellar peduncles, and internal capsules [41]. In the late phase, there is hyperintensity on ADC map and FLAIR, with loss of the DWI-bright signal. In this regard, in the current study there were two EtOH-related ATL patients, one of which had the typical ATL-type of PVWM affectation; the other appeared in the distribution of MBD with callosal and PVWM involvement (Fig. 9). Hence, MBD should be kept in mind in any acutely encephalopathic patients with a history of alcohol abuse and ATL [40,41].
Fig. 9.
A 37 year old male with alcohol abuse presented with visual changes, who was ultimately diagnosed with ATL from Marchiafava-Bignami disease. 9A-C: On the initial MRI, reduced diffusion is noted in the body (dotted arrows) and the splenium (arrows) of the corpus callosum on DWI MRI (A) and ADC map (B), while these same areas are hyperintense on FLAIR (C). On a follow up MRI 2 months later, subsequent focal atrophy was present within the callosal splenium (arrows), having elevated diffusion that appears dark on DWI (D) that is less visible on SWI (E); focal atrophy is also noted within the callosal body (dotted arrow) on sagittal FLAIR (F).
4.7. ‘S’= splenial lesions
Focal splenial lesions that spare, or only minimally involve, the PVWM are relatively uncommon abnormalities on DWI MRI in acutely encephalopathic patients, usually being related to anti-epileptic drugs (AEDs) [3,42,43]. While AEDs are likely the most commonly reported etiology of a reversible splenial lesion (RSL), RSL’s may also arise from chemotherapy, immunosuppressive medications, sepsis, metabolic disorders, or even antimicrobials such as metronidazole [3,[42], [43], [44]]. In this regard, the term RSL has also been alternated with the term “mild encephalitis with reversible splenial lesions” (MERS), a reversible phenomenon thought related solely to infectious etiologies (i.e. sepsis); however, the term RSL is used here to exclude the “infectious” subtypes, as AEDs have now been found to be the most common likely cause of RSLs, although infectious causes lacking exposure to an AED also occur [[43], [44], [45]]. In RSL, the abnormal signal on DWI MRI (often invisible on FLAIR) is either present solely within the splenium of the corpus callosum near the midline, or occasionally has additional, symmetric involvement of the posterior WM as well; the abnormalities typically do not enhance, and resolve within 2–3 weeks following the insult if the offending medication is withdrawn [2,3,[43], [44], [45]]. In the current study, an AED was the causative etiology in 7/9 patients, while the other two patients having RSL were from chemotherapy and immunosuppressant therapy (Fig. 10). Interestingly, as RSL variably involves the PVWM, it may be considered a focal subtype of ATL; another reason to consider it as a subtype (or relative) of ATL is that there is overlap with the various etiologies that may cause ATL [3,42,44,45]. Again, as sepsis may also cause RSL-type lesions, septic RSL (termed MERS) is discussed under the following section on ‘Mimics of ATL and ATL-like appearances’, as this is a non-toxic disorder.
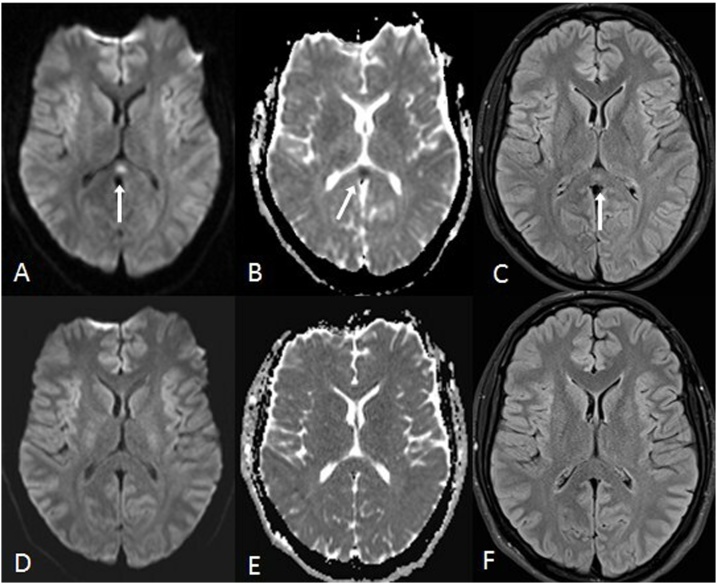
Fig. 10.
A 20 year old male with ganglioglioma on anti-epileptic therapy for 5 years presented with confusion, eventually being diagnosed with RSL-ATL. 10A-C: There is reduced diffusion within the callosal splenium (arrows) on DWI (A) and ADC map (B), with hyperintensity on FLAIR (C). 10C-D: The lesions resolved after 3 months, as on DWI (D), ADC (E) and FLAIR (F) sequences.
4.8. Mimics of ATL and ATL-like appearances
The terms ‘mimics’ or ‘ATL-like’ implies that the abnormalities are confined to PVWM with reduced diffusion in a symmetric and confluent fashion, usually lacking contrast enhancement. However, their characteristic imaging findings usually differ from that on sequences other than DWI, and may involve other anatomic locations. In addition, their clinical presentation is often discordant with ATL. These entities may appear similar to ATL, with corresponding PVWM involvement on DWI that causes their appearance to simulate ATL; such entities with PVWM DWI findings include metabolic entities such as osmotic demyelination syndrome (ODS, or EPM “extrapontine myelinolysis”), acute hepatic (or “hyperammonemic”) encephalopathy (AHE), as well as infectious processes (such as sepsis). However, additional areas of involvement within these disorders will usually hint that these are not true ATL.
As above, the ATL-like entities on DWI of PVWM include ODS/EPM, AHE, and MERS. Regarding ODS, while in “central” pontine myelinolysis, the pons is the predominant site of abnormality on MRI (especially DWI and FLAIR acutely), in “extrapontine myelinolysis”, the basal ganglia and PVWM may be involved, even without the pons (Fig. 11) [46]. Regarding AHE, it is a clinicoradiologic syndrome (usually with elevated plasma ammonia levels) which characteristically involves the insular cortex symmetrically on DWI or FLAIR MRI (both being >80%), while the thalami (70%, 85%), posterior limb of internal capsule (80%, 75%), and PVWM (85%, 80%) can be involved on DWI and FLAIR, respectively; less commonly, more severe insults of the cortex diffusely or basal ganglia may occur, and the clinical and imaging findings can be reversible with therapy (Fig. 12) [[47], [48], [49]]. Finally, as mentioned above, sepsis-related MERS can mimic AED-related RSL/ATL; as the callosal splenium is part of the PVWM, we include ‘splenial lesions’ (most commonly due to AEDs) in the ‘CHOICES’ acronym, recognizing that MERS-type lesions related to sepsis can resemble RSL [2,3].
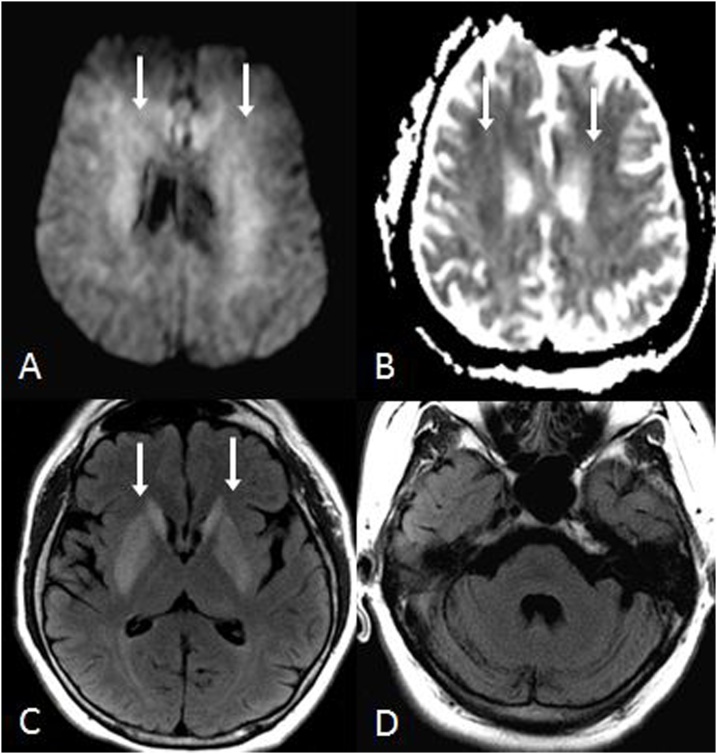
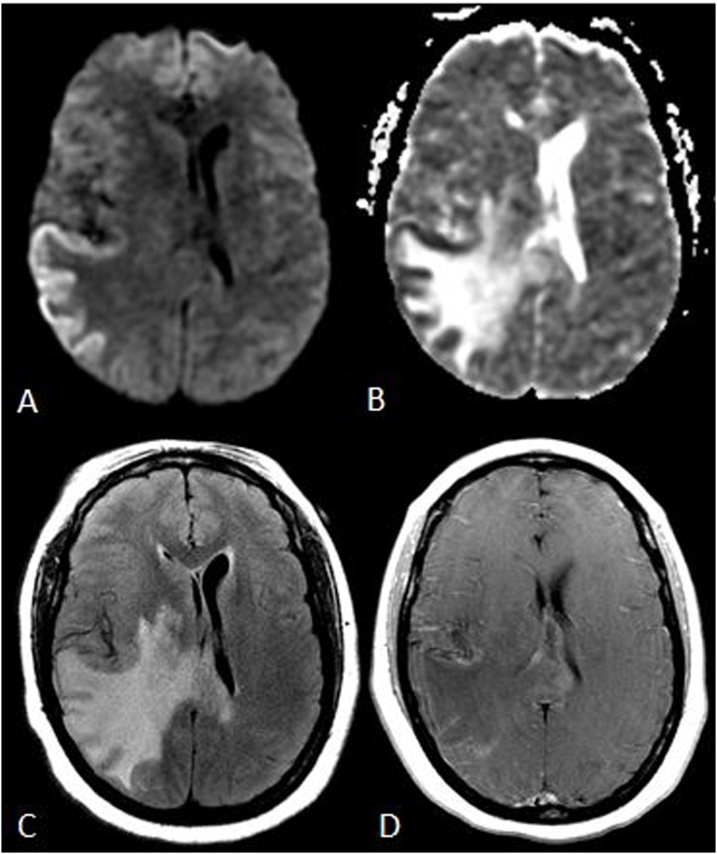
Fig. 11.
A 57 year old male with a Na+ level of 115mEq/L, corrected over 1-2days, with osmotic demyelination syndrome affecting the PVWM and basal ganglia. Mildly reduced diffusion is noted within the PVWM bilaterally (arrows) on DWI (A) and the ADC map (B), with basal ganglial hyperintensities also present on FLAIR (arrows, C), but sparing the pons on FLAIR (D). Hence, as the basal ganglia are additionally involved, this is not characteristic of ATL.
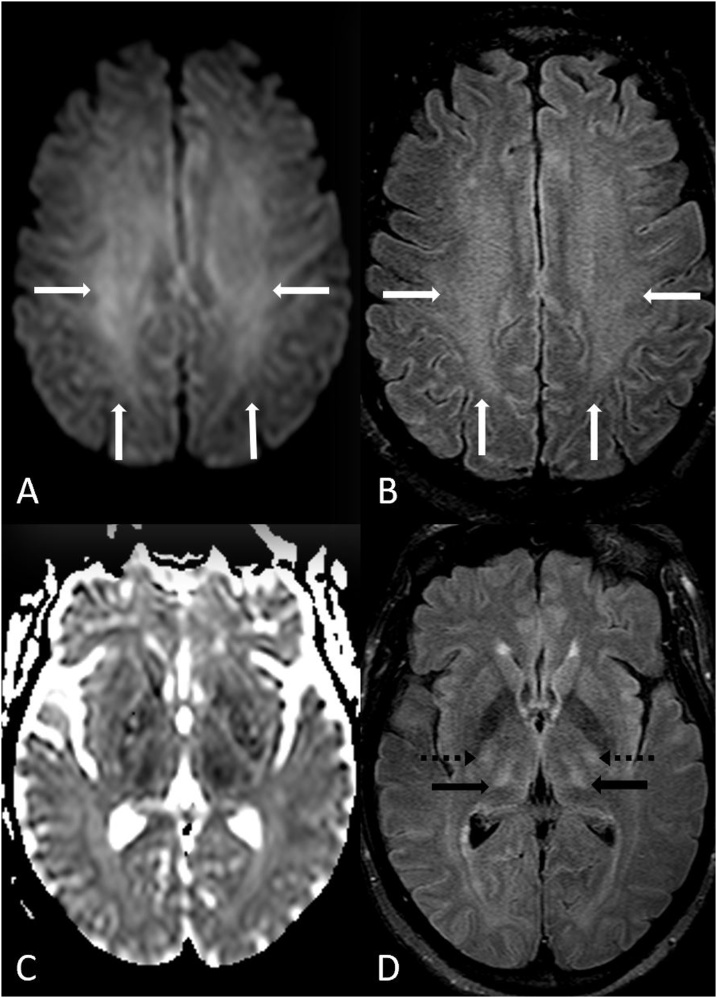
Fig. 12.
A 53 year old male with chronic hepatic failure presented with acute confusion from acute hepatic/hyperammonemic encephalopathy (AHE). The patient had an elevated serum level of NH4+ 101 ug/dl (normal: <20-40 ug/dl). 12A-D: bilateral symmetric PVWM involvement of the centrum semiovale is present on DWI (A) and FLAIR (B), also with involvement of the thalami (D arrows); involvement of the internal capsules’ posterior limbs (D dotted arrows) is noted on the ADC map (C) and FLAIR (D), being characteristic of AHE.
Mimics of ATL include the subacute phase (7–21 days) of hypoxic-ischemic encephalopathy (HIE, typically with cortical reduced diffusion and mild FLAIR hyperintensity in the acute phase, Fig. 13), acute disseminated encephalomyelitis (ADEM, typically asymmetric, multifocal, contrast-enhancing and possibly involving the basal ganglia, with only mild DWI abnormalities, Fig. 14), progressive multifocal leukoencephalopathy (PML, typically asymmetric, with elevated diffusivity on ADC within the PVWM, while having contrast enhancement in >90%). Also, less common ATL mimics are PML-IRIS (an inflammatory response occurring after treatment of PML due to recovery of the immune system in the immunocompromised) (Fig. 15), acute necrotizing encephalopathy (usually T1-bright foci with hemorrhage involving the basal ganglia and cerebellum), and acute hemorrhagic leukoencephalitis (AHL, typically having cystic necrosis with only peripheral reduced diffusion and enhancement extending beyond the PVWM (Fig. 16) [2,3,[50], [51], [52], [53], [54], [55]]. The latter three entities are rather rare, even relative to ATL, and while involving the PVWM, these syndromes have imaging appearances quite different from ATL on other MR sequences, and usually enhance avidly after the use of intravenous contrast. In practice, of these mimics, ADEM may be the one entity that might truly mimic ATL on neuroimaging, as a small percentage of ADEM patients resemble ATL by having confluent and symmetric abnormalities on DWI, and being limited to the PVWM, without contrast enhancement [51]. Another discriminating point regarding the subacute phase of HIE is that it could mimic ATL on DWI and ADC maps, but the clinical history and imaging in the acute phase (1–6 days’ post-insult) usually clearly discerns this entity from ATL [2,3,50]. Hence, these six entities were not included in the CHOICES acronym, as their typical MRI appearances are usually readily identifiable on imaging sequences other than DWI, they can occur in locations other than the PVWM, and the clinical history is usually discordant with a toxic insult.
Fig. 13.
A 38 year old female with acute pulmonary and cardiac failure and arrest causing hypoxic-ischemic encpehalopathy (HIE). 13A-C: an MRI the day after the insult shows gyriform reduced diffusion (arrows) on DWI (A), ADC map (B) and hyperintensities on FLAIR (C) within the parietal cortices bilaterally. 13D-F: Six days later, in the subacute phase, the reduced diffusion is symmetric and bilateral within the centrum semiovale of the PVWM on DWI (D) and ADC map (E), with slight hyperintensities on FLAIR (F), consistent with delayed PVWM anoxic injury from HIE, which could mimic ATL if the initial MRI was not obtained. However, ATL usually lacks the mild cortical hyperintensity as noted here (arrows, 13C).
Fig. 14.
A 5 year old male with ADEM. Multifocal and asymmetric hyperintensities on FLAIR (A) have only very minimal reduced (and mostly elevated) diffusion (arrows) on DWI (B) and ADC map (C), and multifocal enhancement (arrows) on post-contrast T1WI (D). After 6 weeks, the hyperintensities have mostly regressed on FLAIR (E), and there is resolution of enhancement on postcontrast T1-WI (F). The multifocal contrast enhancement is not typical for ATL.
Fig. 15.
A 62 year old immunocompromised male, with PML, has “T2-shine through” (i.e. highly elevated diffusion) on DWI (A) and elevated diffusivity on ADC map (B); typically asymmetric lesions of PML are also seen (arrows) on FLAIR (C). Such multifocal lesions usually have an incomplete rim of enhancement (arrows) on postcontrast T1WI, suggestive for PML-IRIS (D).
Fig. 16.
A 37 year old female with acute confusion was diagnosed with AHL, having histopathologic confirmation. Reduced diffusion is noted within the cortex unilaterally on DWI (A) and ADC map (B), with accompanying hyperintensity on FLAIR (C) and mild cortical enhancement on postcontrast T1WI (D). The unilaterality, asymmetry, mild enhancement, and mass effect are not typical of ATL, as well as the lack of PVWM reduced diffusion. Although no hemorrhage was noted on T2WI, diffuse microhemorrhages were confirmed on histopathology.
This study is limited by its retrospective nature, descriptive/observational design, and the small number of patients; for example, only one patient had cocaine-related ATL. However, as this observational study is for the purposes of developing an acronym and a review about PVWM involvement of ATL, it should be sufficient to have a small number of patients for each letter in the acronym to aid in memorization of this potentially difficult differential diagnosis. Addtionally, another potential limitation is that in many patients, there were overlapping causes that gave rise to uncertainty of an exact etiology, such as with the ‘unspecified’ category for opioids.
5. Conclusion
In summary, we describe an acronym (“CHOICES”) for non-metabolic and non-infectious etiologies of ATL-related PVWM injury on DWI, and provide relevant examples. The intent is to increase awareness of possible non-metabolic and non-infectious etiologies of ATL due to its potentially reversible nature after treatment or removal of the toxin. Mimics of ATL and ATL-like disorders are described. Hopefully this improves recall of associated etiologies, which may result in prompt diagnosis and therapy.
References
- 1.Filley C.M., Kleinschmidt-DeMasters B.K. Toxic leukoencephalopathy. N. Engl. J. Med. 2001;345(6):425–432. doi: 10.1056/NEJM200108093450606. [DOI] [PubMed] [Google Scholar]
- 2.McKinney A.M. Acute toxic leukoencephalopathy: potential for reversibility clinically and on MRI with diffusion-weighted and FLAIR imaging. AJR Am. J. Roentgenol. 2009;193(1):192–206. doi: 10.2214/AJR.08.1176. [DOI] [PubMed] [Google Scholar]
- 3.Ozutemiz C. Acute toxic leukoencephalopathy: etiologies, imaging findings, and outcomes in 101 patients. AJNR Am. J. Neuroradiol. 2019;40(2):267–275. doi: 10.3174/ajnr.A5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beitinjaneh A. Toxic leukoencephalopathy following fludarabine-associated hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2011;17(3):300–308. doi: 10.1016/j.bbmt.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Keogh C.F. Neuroimaging features of heroin inhalation toxicity: "chasing the dragon". AJR Am. J. Roentgenol. 2003;180(3):847–850. doi: 10.2214/ajr.180.3.1800847. [DOI] [PubMed] [Google Scholar]
- 6.Rimkus Cde M. Toxic leukoencephalopathies, including drug, medication, environmental, and radiation-induced encephalopathic syndromes. Semin. Ultrasound CT MR. 2014;35(2):97–117. doi: 10.1053/j.sult.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Lo C.P. Brain injury after acute carbon monoxide poisoning: early and late complications. AJR Am. J. Roentgenol. 2007;189(4):W205–11. doi: 10.2214/AJR.07.2425. [DOI] [PubMed] [Google Scholar]
- 8.Takanashi J. Widening spectrum of a reversible splenial lesion with transiently reduced diffusion. AJNR Am. J. Neuroradiol. 2006;27(4):836–838. [PMC free article] [PubMed] [Google Scholar]
- 9.Rykken J.B., McKinney A.M. Posterior reversible encephalopathy syndrome. Semin. Ultrasound CT MR. 2014;35(2):118–135. doi: 10.1053/j.sult.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 10.McKinney A.M. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am. J. Roentgenol. 2007;189(4):904–912. doi: 10.2214/AJR.07.2024. [DOI] [PubMed] [Google Scholar]
- 11.Karia S.J. Utility and significance of gadolinium-based contrast enhancement in posterior reversible encephalopathy syndrome. AJNR Am. J. Neuroradiol. 2016;37(3):415–422. doi: 10.3174/ajnr.A4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao B. Controversy of posterior reversible encephalopathy syndrome: what have we learnt in the last 20 years? J. Neurol. Neuro. Psych. 2018;89(1):14–20. doi: 10.1136/jnnp-2017-316225. [DOI] [PubMed] [Google Scholar]
- 13.Ozutemiz C. Concomitant acute toxic leukoencephalopathy and posterior reversible encephalopathy syndrome. J. Neuroimaging. 2018;28(5):535–541. doi: 10.1111/jon.12526. [DOI] [PubMed] [Google Scholar]
- 14.McKinney A.M. Detection of microhemorrhage in posterior reversible encephalopathy syndrome using susceptibility-weighted imaging. AJNR Am. J. Neuroradiol. 2012;33(5):896–903. doi: 10.3174/ajnr.A2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luckman J. Difficulty in distinguishing posterior reversible encephalopathy syndrome, hypoxic-ischemic insult, and acute toxic leukoencephalopathy in children. Neuropediatrics. 2016;47(1):33–38. doi: 10.1055/s-0035-1569154. [DOI] [PubMed] [Google Scholar]
- 16.Lee M.S. Clinical and imaging features of fludarabine neurotoxicity. J. Neuroophthalmol. 2010;30(1):37–41. doi: 10.1097/WNO.0b013e3181ce8087. [DOI] [PubMed] [Google Scholar]
- 17.Rollins N. Acute methotrexate neurotoxicity: findings on diffusion-weighted imaging and correlation with clinical outcome. AJNR Am. J. Neuroradiol. 2004;25(10):1688–1695. [PMC free article] [PubMed] [Google Scholar]
- 18.Lucato L.T. Reversible findings of restricted diffusion in 5-fluorouracil neurotoxicity. Australas. Radiol. 2006;50(4):364–368. doi: 10.1111/j.1440-1673.2006.01602.x. [DOI] [PubMed] [Google Scholar]
- 19.Tan T.P. Toxic leukoencephalopathy after inhalation of poisoned heroin: MR findings. AJNR Am. J. Neuroradiol. 1994;15(1):175–178. [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong S.C., Wynn G.H., Sandson N.B. Pharmacokinetic drug interactions of synthetic opiate analgesics. Psychosomatics. 2009;50(2):169–176. doi: 10.1176/appi.psy.50.2.169. [DOI] [PubMed] [Google Scholar]
- 21.Wolters E.C. Leucoencephalopathy after inhaling "heroin" pyrolysate. Lancet. 1982;2(8310):1233–1237. doi: 10.1016/s0140-6736(82)90101-5. [DOI] [PubMed] [Google Scholar]
- 22.Cheng M.Y. Different routes of heroin intake cause various heroin-induced leukoencephalopathies. J. Neurol. 2019;266(2):316–329. doi: 10.1007/s00415-018-9131-1. [DOI] [PubMed] [Google Scholar]
- 23.Richter R.W. Neurological complications of addiction to heroin. Bull. N. Y. Acad. Med. 1973;49(1):3–21. [PMC free article] [PubMed] [Google Scholar]
- 24.Offiah C., Hall E. Heroin-induced leukoencephalopathy: characterization using MRI, diffusion-weighted imaging, and MR spectroscopy. Clin. Radiol. 2008;63(2):146–152. doi: 10.1016/j.crad.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Zanin A. A delayed methadone encephalopathy: clinical and neuroradiological findings. J. Child Neurol. 2010;25(6):748–751. doi: 10.1177/0883073809343318. [DOI] [PubMed] [Google Scholar]
- 26.Mills F. Severe cerebellitis following methadone poisoning. Pediatr. Radiol. 2008;38(2):227–229. doi: 10.1007/s00247-007-0635-6. [DOI] [PubMed] [Google Scholar]
- 27.Anselmo M. Methadone intoxication in a child: toxic encephalopathy? J. Child Neurol. 2006;21(7):618–620. doi: 10.1177/08830738060210071101. [DOI] [PubMed] [Google Scholar]
- 28.Morales Odia Y., Jinka M., Ziai W.C. Severe leukoencephalopathy following acute oxycodone intoxication. Neurocrit. Care. 2010;13(1):93–97. doi: 10.1007/s12028-010-9373-y. [DOI] [PubMed] [Google Scholar]
- 29.Foy L., Seeyave D.M., Bradin S.A. Toxic leukoencephalopathy due to transdermal fentanyl overdose. Pediatr. Emerg. Care. 2011;27(9):854–856. doi: 10.1097/PEC.0b013e31822c281f. [DOI] [PubMed] [Google Scholar]
- 30.Trullemans F. Clinical findings and magnetic resonance imaging in severe cyclosporine-related neurotoxicity after allogeneic bone marrow transplantation. Eur. J. Haematol. 2001;67(2):94–99. doi: 10.1034/j.1600-0609.2001.t01-1-00440.x. [DOI] [PubMed] [Google Scholar]
- 31.Small S.L. Immunosuppression-induced leukoencephalopathy from tacrolimus (FK506) Ann. Neurol. 1996;40(4):575–580. doi: 10.1002/ana.410400406. [DOI] [PubMed] [Google Scholar]
- 32.Kim E. MR imaging of metronidazole-induced encephalopathy: lesion distribution and diffusion-weighted imaging findings. AJNR Am. J. Neuroradiol. 2007;28(9):1652–1658. doi: 10.3174/ajnr.A0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bianco F. Recurrent leukoencephalopathy in a cocaine abuser. Neurotoxicology. 2011;32(4):410–412. doi: 10.1016/j.neuro.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Duarte A., Williams R. Cocaine-induced recurrent leukoencephalopathy. Neuroradiol. J. 2013;26(5):511–513. doi: 10.1177/197140091302600503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartzokis G. The incidence of T2-weighted MR imaging signal abnormalities in the brain of cocaine-dependent patients is age-related and region-specific. AJNR Am. J. Neuroradiol. 1999;20(9):1628–1635. [PMC free article] [PubMed] [Google Scholar]
- 36.Thom S.R., Bhopale V.M., Fisher D. Hyperbaric oxygen reduces delayed immune-mediated neuropathology in experimental carbon monoxide toxicity. Toxicol. Appl. Pharmacol. 2006;213(2):152–159. doi: 10.1016/j.taap.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Teksam M. Diffusion-weighted MR imaging findings in carbon monoxide poisoning. Neuroradiology. 2002;44(2):109–113. doi: 10.1007/s002340100639. [DOI] [PubMed] [Google Scholar]
- 38.O’Donnell P. The magnetic resonance imaging appearances of the brain in acute carbon monoxide poisoning. Clin. Radiol. 2000;55(4):273–280. doi: 10.1053/crad.1999.0369. [DOI] [PubMed] [Google Scholar]
- 39.Kim T.E. Wernicke encephalopathy and ethanol-related syndromes. Semin. Ultrasound CT MR. 2014;35(2):85–96. doi: 10.1053/j.sult.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Tung C.S. Marchiafava-Bignami disease with widespread lesions and complete recovery. AJNR Am. J. Neuroradiol. 2010;31(8):1506–1507. doi: 10.3174/ajnr.A1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menegon P. Marchiafava-Bignami disease: diffusion-weighted MRI in corpus callosum and cortical lesions. Neurology. 2005;65(3):475–477. doi: 10.1212/01.wnl.0000171348.55820.89. [DOI] [PubMed] [Google Scholar]
- 42.Kim S.S., Chang K.-H., Kim S.T. Focal lesion in the splenium of the corpus callosum in epileptic patients: antiepileptic drug toxicity? AJNR Am. J. Neuroradiol. 1999;20:125–129. [PubMed] [Google Scholar]
- 43.Polster T., Hoppe M., Ebner A. Transient lesion in the splenium of the corpus callosum: three further cases in epileptic patients and a pathophysiological hypothesis. J Neurol Neuro. Psychiatry. 2001;70:459–463. doi: 10.1136/jnnp.70.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Monco J.C. Reversible splenial lesion syndrome (RESLES): what's in a name? J. Neuroimaging. 2011;21(2):e1–14. doi: 10.1111/j.1552-6569.2008.00279.x. [DOI] [PubMed] [Google Scholar]
- 45.Tada H., Takanashi J., Barkovich A.J. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology. 2004;63:1854–1858. doi: 10.1212/01.wnl.0000144274.12174.cb. [DOI] [PubMed] [Google Scholar]
- 46.Howard S.A. Best cases from the AFIP: osmotic demyelination syndrome. Radiographics. 2009;29(3):933–938. doi: 10.1148/rg.293085151. [DOI] [PubMed] [Google Scholar]
- 47.McKinney A.M. Acute hepatic encephalopathy: diffusion-weighted and fluid-attenuated inversion recovery findings, and correlation with plasma ammonia level and clinical outcome. AJNR Am. J. Neuroradiol. 2010;31(8):1471–1479. doi: 10.3174/ajnr.A2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benson J.C. Delineation of microhemorrhage in acute hepatic encephalopathy using susceptibility-weighted imaging. Eur. J. Radiol. 2016;85(3):629–634. doi: 10.1016/j.ejrad.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 49.JM, U.K.-I Acute hyperammonemic encephalopathy in adults: imaging findings. AJNR Am. J. Neuroradiol. 2011;32(2):413–418. doi: 10.3174/ajnr.A2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arbelaez A., Castillo M., Mukherji S.K. Diffusion-weighted MR imaging of global cerebral anoxia. AJNR Am. J. Neuroradiol. 1999;20(6):999–1007. [PMC free article] [PubMed] [Google Scholar]
- 51.Balasubramanya K.S. Diffusion-weighted imaging and proton MR spectroscopy in the characterization of acute disseminated encephalomyelitis. Neuroradiology. 2007;49(2):177–183. doi: 10.1007/s00234-006-0164-2. [DOI] [PubMed] [Google Scholar]
- 52.Wattjes M.P. MRI characteristics of early PML-IRIS after natalizumab treatment in patients with MS. J Neurol Neurosurg Psychiatry. 2016;87(8):879–884. doi: 10.1136/jnnp-2015-311411. [DOI] [PubMed] [Google Scholar]
- 53.Cosottini M. Diffusion-weighted imaging in patients with progressive multifocal leukoencephalopathy. Eur. Radiol. 2008;18(5):1024–1030. doi: 10.1007/s00330-007-0845-1. [DOI] [PubMed] [Google Scholar]
- 54.Albayram S. Diffusion-weighted MR imaging findings of acute necrotizing encephalopathy. AJNR Am. J. Neuroradiol. 2004;25(5):792–797. [PMC free article] [PubMed] [Google Scholar]
- 55.Lee H.Y. Serial MR imaging findings of acute hemorrhagic leukoencephalitis: a case report. AJNR Am. J. Neuroradiol. 2005;26(8):1996–1999. [PMC free article] [PubMed] [Google Scholar]