Abstract
Background
Procalcitonin (PCT)-guided antibiotic discontinuation appears to decrease antibiotic use in critically ill patients, but its impact on survival remains less certain.
Methods
We searched PubMed, Embase, Scopus, Web of Science, and CENTRAL for randomized controlled trials (RCTs) of PCT-guided antibiotic discontinuation in critically ill adults reporting survival or antibiotic duration. Searches were conducted without language restrictions from inception to July 23, 2018. Two reviewers independently conducted all review stages; another adjudicated differences. Data were pooled using random-effects meta-analysis. Study quality was assessed with the Cochrane risk of bias tool, and evidence was graded using GRADEpro.
Results
Among critically ill adults (5,158 randomized; 5,000 analyzed), PCT-guided antibiotic discontinuation was associated with decreased mortality (16 RCTs; risk ratio [RR], 0.89; 95% CI, 0.83-0.97; I2 = 0%; low certainty). Death was the primary outcome in only one study and a survival benefit was not observed in the subset specified as sepsis (10 RCTs; RR, 0.94; 95% CI, 0.85-1.03; I2 = 0%), those without industry sponsorship (nine RCTs; RR, 0.98; 95% CI, 0.87-1.10; I2 = 0%), high PCT-guided algorithm adherence (five RCTs; RR, 0.93; 95% CI, 0.71-1.22; I2 = 0%), and PCT-guided algorithms without C-reactive protein (eight RCTs; RR, 0.96; 95% CI, 0.87-1.06; I2 = 0%). PCT-guided antibiotic discontinuation decreased antibiotic duration (mean difference, 1.31 days; 95% CI, –2.27 to –0.35; I2 = 93%) (low certainty).
Conclusions
Our findings of increased survival and decreased antibiotic utilization associated with PCT-guided antibiotic discontinuation represent low-certainty evidence with a high risk of bias. This relationship was primarily observed in studies without high protocol adherence and in studies with algorithms combining PCT and C-reactive protein. Properly designed studies with mortality as the primary outcome are needed to address this question.
Trial Registry
International Prospective Register of Systematic Reviews (PROSPERO); No.: CRD42016049715; URL: http://www.crd.york.ac.uk/PROSPERO_REBRANDING/display_record.asp?ID=CRD42016049715
Key Words: antibiotic, critical illness, mortality, procalcitonin, sepsis
Abbreviations: MD, mean difference; PCT, procalcitonin; RCT, randomized controlled trial; RR, risk ratio
FOR EDITORIAL COMMENT, SEE PAGE 1085
Serial procalcitonin (PCT) levels are increasingly used by clinicians to guide antibiotic discontinuation after clinical stabilization, particularly in those with sepsis. Over 1.5 million people develop sepsis each year in the United States, and at least 5% of these patients undergo PCT testing.1, 2 While PCT-guided antibiotic discontinuation appears to reduce antibiotic duration in this population, nine meta-analyses of randomized clinical trials (RCTs) published before 2017 failed to show any statistically significant survival benefit in those with infection, sepsis, critical illness, or both sepsis and critical illness (e-Table 1).3, 4, 5, 6, 7, 8, 9, 10, 11 In 2017 and 2018, two meta-analyses reported improved survival with PCT-guided antibiotic discontinuation in critical illness, and two reported no survival benefit in those with sepsis.12, 13, 14, 15 Only one of these two latter sepsis meta-analyses graded the quality of evidence.12 One meta-analysis,13 which did not focus exclusively on sepsis, concluded their study provided robust evidence to support and expand the 2016 Surviving Sepsis Campaign (SSC) guidelines that PCT levels be used to shorten the duration of antimicrobial therapy in patients with sepsis.16
These conflicting findings compelled us to assess the certainty of evidence and plausibility of PCT-guided antibiotic discontinuation to improve survival in critically ill adults. We performed a systematic review and meta-analysis of PCT-guided antibiotic discontinuation RCTs. Using standardized tools, we assessed the risk of bias in individual RCTs and graded the overall certainty of evidence. We sought corroborating evidence within trials that might support the biologic plausibility of any survival advantage. Our main study aim was to estimate the effect of PCT-guided antibiotic discontinuation on survival in critically ill adults and the subset specified as having sepsis.
Methods
Data Sources and Searches
This systematic review was prepared according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement checklist17, 18 and registered in the PROSPERO (International Prospective Register of Systematic Reviews) database on October 31, 2016 (2016:CRD42016049715) (e-Appendixes 1, 2). We performed comprehensive literature searches (e-Appendix 3) for RCTs of procalcitonin-guided antibiotic discontinuation in critically ill adults in PubMed, Embase, Scopus, Web of Science, and CENTRAL (Cochrane Central Register of Controlled Trials). The searches were conducted without language restrictions from each database’s inception to July 23, 2018. Systematic reviews, guidelines, and review articles identified in our search3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 were searched for additional relevant references.
Study Selection
We included RCTs that exclusively enrolled adults admitted to the ICU; and that also compared mortality rates or antibiotic duration in patients randomized to receive PCT-guided antibiotic discontinuation vs a group of control patients. Exclusion criteria included observational studies and studies that included adults not admitted to the ICU. Two authors (D. J. P., S. S. K.) reviewed search results to identify studies for inclusion. We performed dual review in a two-step process of first screening titles and abstracts followed by full-text review of selected articles. Author consensus resolved uncertainty regarding study inclusion. Details on data extraction, risk of bias assessment, and certainty assessment using GRADEpro (supplied by GRADEpro GDT) are provided in the online supplement (e-Appendix 4).35, 36 The primary outcome examined was mortality assessed as the relative risk of death and considered in the following hierarchy: 28-day, 30-day, 60-day, 90-day, hospital, or ICU. Other outcomes examined were hospital length of stay, ICU length of stay, and antibiotic duration or exposure.
Data Synthesis and Analysis
Mortality outcomes between intervention and comparator groups were analyzed as risk ratios (RRs) using random-effects models and the Knapp-Hartung adjustment for small study numbers.37, 38, 39 Because biologic plausibility would be strengthened if a survival benefit was observed in studies with high (> 80%) algorithm adherence, absence of industry funding, or those with only PCT in the intervention arm, we performed subgroup analyses according to these moderators. An 80% cutoff for high algorithm adherence was chosen as algorithm adherence was clustered in two groups (ie, low adherence: 41%-53% vs high adherence: 81%-97%). Mean differences (MDs) in the length of hospitalization, length of ICU stay, and duration of antibiotic use were analyzed using random-effects models, with conversion from medians (ranges/interquartile ranges) to means (standard deviations) when appropriate (e-Tables 2-4).40 Heterogeneity among studies was assessed using the Q statistic and I2 value.41 Two-sided P values < .05 were considered significant. Publication bias was assessed using funnel plots and Egger regression (P < .10 considered significant).42 All analyses were performed using R (version 3.4.4; R Foundation) with packages meta (version 4.9-1) and metafor (version 2.0-0).43, 44, 45
Results
Literature Search
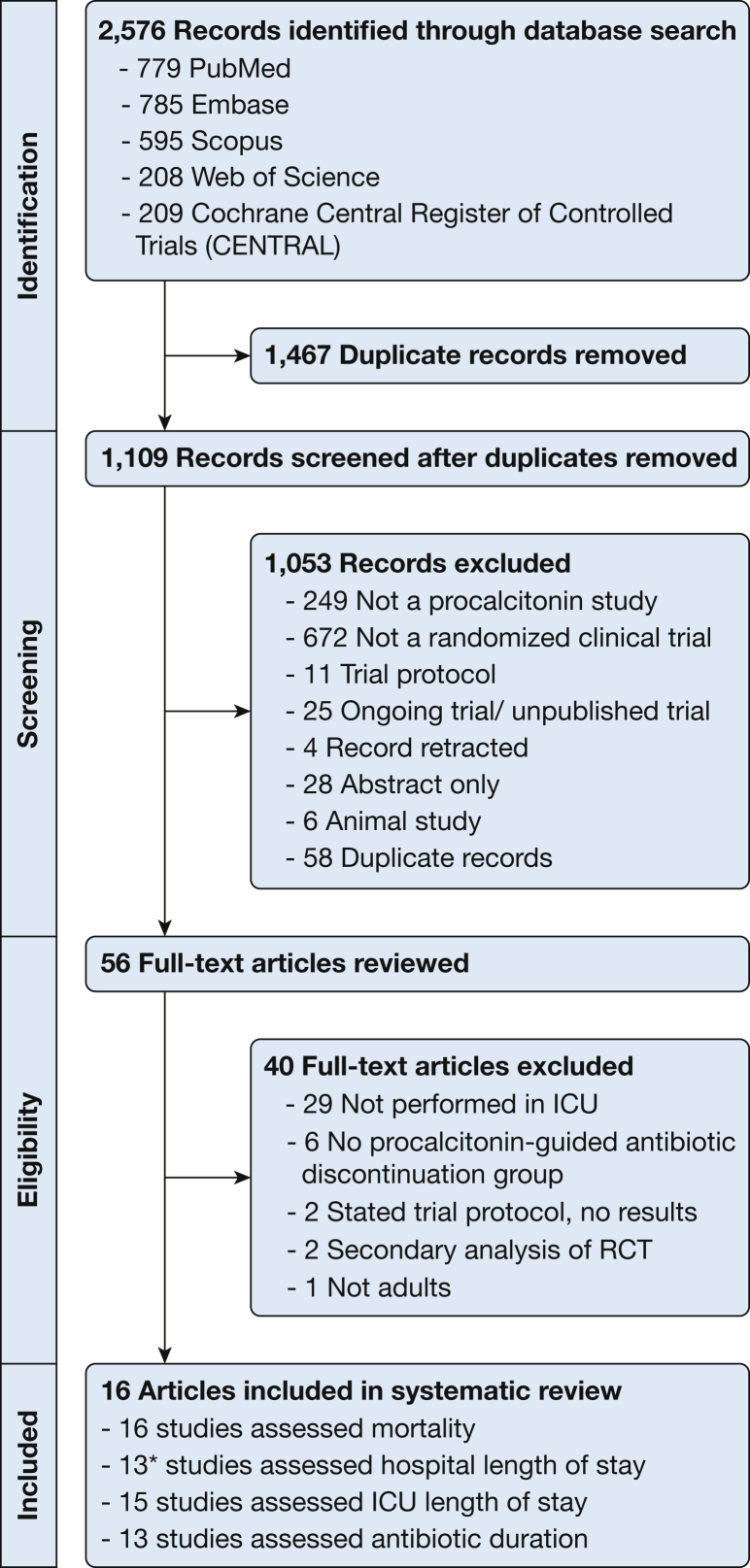
The literature search identified 2,576 references (Fig 1). Sixteen RCTs met inclusion criteria; 16 assessed mortality, 13 assessed hospital length of stay, 15 assessed ICU length of stay, and 13 assessed antibiotic duration or exposure.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34
Figure 1.
Search strategy. *In one study results were inconsistent and in another study the data were not normally distributed. Therefore two of these 13 studies were omitted and a total of 11 studies were included in the meta-analysis for hospital length of stay. RCT = randomized controlled trial.
Study Characteristics
Sixteen RCTs were conducted in nine countries from 2006 to 2016 (Table 1, e-Table 5). Of these RCTs, nine reported comorbid illnesses, nine reported baseline PCT levels, and three reported the interval from presentation to randomization (e-Table 6). In 10 RCTs, only patients with sepsis were enrolled. Fourteen RCTs specified the site of infection, predominantly pulmonary, abdominal, and urinary sites (e-Table 7).
Table 1.
Evidence Profile Table
| Study Characteristic | Data |
|---|---|
| No. of randomized clinical trials | 16 |
| Study years | Conducted, 2006-2016; published, 2008-2018 |
| Date of literature search | 1940-July 23, 2018 |
| No. of patients | 5,158 randomized; 5,000 analyzed |
| Race/ethnicity | Unavailable |
| Age | Adults |
| Setting | ICUs |
| Countries | Australia, Brazil, China, France, Germany, Netherlands, Serbia, Switzerland, USA |
| Comparison | Procalcitonin-guided antibiotic discontinuation vs control group |
| Primary outcome | Mortality (28-d mortality or hospital mortality) |
| Secondary outcomes | Antibiotic duration and length of stay |
Comparison of Interventions
All 16 RCTs described the intervention algorithm, and 15 described the control arm (e-Table 8). In the intervention algorithm, PCT levels were obtained daily in 12 RCTs, and at less frequent intervals in four RCTs. Criteria for antibiotic discontinuation were based on both absolute PCT values and percentage decrease from peak baseline PCT level (13 RCTs), or only on absolute PCT values (three RCTs). In two RCTs, the intervention duration was not specified; in the remaining 14 RCTs, the intervention duration ranged from 2 to 28 days or until ICU discharge or ICU transfer.
PCT algorithm adherence was reported in nine studies (eight in published manuscripts, one personal communication; e-Table 8). Algorithm adherence was clustered in two groups: five RCTs with reported algorithm adherence ≥ 80% (range, 81%-97%; considered high adherence) and four RCTs with reported adherence of 41%-53% (considered low adherence). Seven RCTs were industry funded and nine were not. Eight RCTs had only PCT in the intervention group and the other eight had both PCT and C-reactive protein in the intervention group (e-Tables 5, 8).
Outcomes
Only one RCT reported mortality as a properly powered and designated primary efficacy outcome (e-Table 9). This RCT, a two-by-two factorial trial studying sodium selenite and PCT-guided antimicrobial therapy, found no statistically significant decrease in mortality with PCT-guided antibiotic discontinuation.27 PCT guidance did not affect the frequency of diagnostic or therapeutic procedures, and resulted in a 4.5% reduction in antimicrobial exposure. One RCT, investigating 3-month mortality as the “primary non-inferiority endpoint,” found increased mortality with PCT-guided antibiotic discontinuation, and this increase was within the prespecified 12% noninferiority margin.40 Two trials examined mortality as a safety outcome in prospective noninferiority analyses with 80% and 90% power to exclude a 10% and > 8% mortality difference, respectively.32, 33 One of these trials showed a significant mortality benefit (19.6% vs 25.0%). Data were not provided to determine whether PCT monitoring resulted in patient management changes that explained this survival benefit.33 In the remaining 12 RCTs, 10 defined mortality a priori as a secondary outcome and two did not. None of these 12 RCTs demonstrated any statistically significant mortality benefit with PCT-guided antibiotic discontinuation. A total of 5,158 patients underwent randomization and 5,000 were included in the final analysis (e-Table 10). Ten RCTs reported an intention-to-treat analysis; six used a modified intention-to-treat analysis.
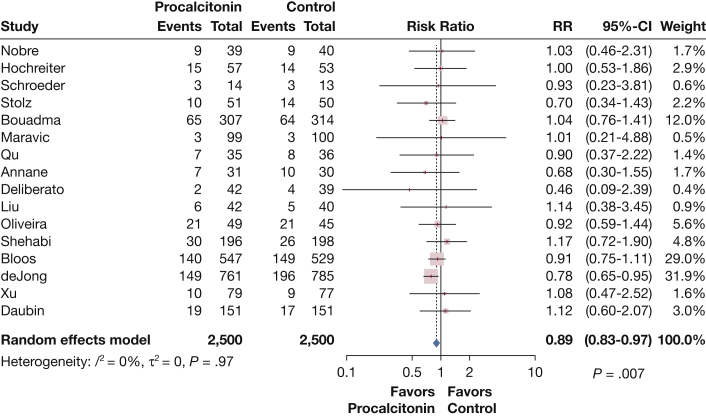
Meta-analysis of the 16 RCTs showed that PCT-guided antibiotic discontinuation had a statistically significant reduction in mortality compared with control subjects (RR, 0.89; 95% CI, 0.83-0.97; I2 = 0%) (Fig 2). Influence analysis showed that the omission of only one RCT39 caused these results to no longer be statistically significant (RR, 0.95; 95% CI, 0.88-1.02). This was the only RCT to report a statistically significant survival benefit with PCT-guided antibiotic discontinuation and had a low algorithm adherence of 44% to 53%. This RCT had a fragility index of nine, meaning that if nine cases in the intervention arm changed from “survived” to “died” the survival benefit would no longer be statistically significant. This RCT used a modified intention-to-treat analysis where the number of randomized participants differed from the number in the final analysis by 29 cases. In their discussion, the authors were unable to attribute the observed, unexpected survival benefit to a change in patient management.
Figure 2.
Survival in 16 randomized clinical trials assessing procalcitonin-guided antibiotic discontinuation in critically ill adults. RR = risk ratio.
PCT-guided antibiotic discontinuation did not significantly improve survival in 10 RCTs that included only critically ill patients with sepsis (RR, 0.94; 95% CI, 0.85-1.03), in nine RCTs that were not industry sponsored (RR, 0.98; 95% CI, 0.87-1.10), in eight RCTs where only PCT was used in the intervention algorithm (RR, 0.96; 95% CI, 0.87-1.06), or in five RCTs where adherence to the PCT-guided algorithm exceeded 80% (RR, 0.93; 95% CI, 0.71-1.22) (e-Figs 1-3, 10). A meta-regression using exact compliance rates for each RCT showed that adherence did not affect the relationship between procalcitonin and survival (P = .30) (e-Fig 35).
In critically ill adults, PCT-guided antibiotic discontinuation did not decrease hospital length of stay (mean difference [MD], –0.59 days; 95% CI, –3.70 to 2.51; I2 = 83%) or ICU length of stay (MD, –0.48 days; 95% CI, –2.90 to 1.95; I2 = 86%) but decreased antibiotic exposure (MD, –1.31 days; 95% CI, –2.27 to –0.35; I2 = 93%). Similar results were found in critically ill adults with sepsis. All sensitivity analyses for each study outcome (survival, hospital length of stay, ICU length of stay, and antibiotic duration) are provided in e-Figures 1-34.
Study Limitations and Risk of Bias
No studies reported whether antibiotic stewardship programs were used in the control group. Ten RCTs reported adverse events (e-Table 9). Five RCTs documented trial registration before study initiation (e-Table 11). Because of the lack of blinding and the limited data reported for all RCTs, the potential for selection, performance, detection, or attrition bias was present in each RCT. Funnel plots and Egger regression analysis showed no publication bias (e-Figs 10, 11).
Certainty Assessment Using GRADE (Grading of Recommendations, Assessment, Development and Evaluation) Criteria
PCT-guided antibiotic discontinuation in critically ill adults has low certainty to improve survival or decrease antibiotic exposure (Table 2). Evidence does not support decreased hospital or ICU length of stay (low certainty). In critically ill adults with sepsis, evidence does not support improved survival or decreased hospital or ICU length of stay, but supports decreased antibiotic exposure (low certainty for all).
Table 2.
GRADE Assessment of Randomized Clinical Trials
| Certainty Assessment |
Summary of Findings |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients |
Effect |
Certainty | Importance | |||||||||
| Outcome Examined (No. of Studies) | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Procalcitonin Arm | Control Arm | Estimate (95% CI) | Absolute (95% CI) | ||
| Critical Illness | ||||||||||||
| Mortality (16) | Randomized trials | Seriousa | Not serious | Seriousb | Not serious | None | 496/2,500 (19.8%) | 552/2,500 (22.1%) | RR, 0.89 (0.83-0.97) | 24 fewer per 1,000 (from 10 to 40 fewer) | ⊕⊕◯◯ (low) | Critical |
| Hospital length of stay (11) | Randomized trials | Seriousc | Seriousd | Not serious | Not serious | None | MD, –0.59 d (–3.70 to 2.51) | … | ⊕⊕◯◯ (low) | Important | ||
| ICU length of stay (15) | Randomized trials | Seriouse | Seriousd | Not serious | Not serious | None | MD, –0.48 d (–2.90 to 1.95) | … | ⊕⊕◯◯ (low) | Important | ||
| Antibiotic duration or exposure (13) | Randomized trials | Seriousf | Seriousd | Not serious | Not serious | None | MD, –1.31 d (–2.27 to –0.35) | … | ⊕⊕◯◯ (low) | Important | ||
| Sepsis and Critical Illness | ||||||||||||
| Mortality (10) | Randomized trials | Seriousg | Not serious | Seriousb | Not serious | None | 243/1,096 (22.2%) | 250/1,064 (23.5%) | RR, 0.94 (0.85-1.03) | Not estimable | ⊕⊕◯◯ (low) | Critical |
| Hospital length of stay (7) | Randomized trials | Serioush | Seriousd | Not serious | Not serious | None | MD, –0.27 d (–5.00 to 4.46) | … | ⊕⊕◯◯ (low) | Important | ||
| ICU length of stay (10) | Randomized trials | Seriousg | Seriousd | Not serious | Not serious | None | MD, –0.69 d (–4.72 to 3.34) | … | ⊕⊕◯◯ (low) | Important | ||
| Antibiotic duration or exposure (9) | Randomized trials | Seriousi | Seriousd | Not serious | Not serious | None | MD, –0.96 d (–1.82 to –0.10) | … | ⊕⊕◯◯ (low) | Important | ||
GRADE = Grading of Recommendations, Assessment, Development and Evaluation; MD = mean difference; RR = risk ratio.
All studies unblinded to participants and personnel; incomplete outcome data (7/15 studies); no data on random sequence generation (4/15 studies); no data on allocation concealment (8/15 studies).
A sensitivity analysis showed that studies with higher adherence (> 80%) to procalcitonin-guided algorithm showed no significant benefit whereas studies with lower adherence (< 80%) showed benefit.
All studies unblinded to participants and personnel; incomplete outcome data (7/10 studies), no data on random sequence generation (1/10 studies); no data on allocation concealment (5/10 studies).
I2 values exceed 75%.
All studies unblinded to participants and personnel; incomplete outcome data (7/14 studies); no data on random sequence generation (5/14 studies); no data on allocation concealment (9/14 studies).
All studies unblinded to participants and personnel; incomplete outcome data (5/12 studies); no data on random sequence generation (4/12 studies); no data on allocation concealment (7/12 studies).
All studies unblinded to participants and personnel; incomplete outcome data (5/10 studies); no data on random sequence generation (3/10 studies); no data on allocation concealment (7/10 studies).
All studies unblinded to participants and personnel; incomplete outcome data (5/7 studies); no data on allocation concealment (4/7 studies).
All studies unblinded to participants and personnel; incomplete outcome data (4/9 studies); no data on random sequence generation (2/9 studies); no data on allocation concealment (6/9 studies).
Discussion
This systematic review examined > 2,500 references and identified 16 RCTs of PCT-guided antibiotic discontinuation in critically ill adults. PCT-guided antibiotic discontinuation appears to decrease antibiotic utilization by 1 day and improve mortality. However, these findings are tempered by low-certainty evidence given the substantial risk of bias, indirectness of effect, and the unknown application of antibiotic stewardship programs in control arms. PCT use has no statistically significant mortality reduction in those with sepsis, in those with > 80% protocol adherence, in those without industry sponsorship, or in those where only PCT is used in the intervention algorithm.
Only one trial showed a mortality benefit, which the authors acknowledge was unexpected as death was examined as a safety outcome using a noninferiority analysis.33 Influence analysis shows that this one RCT drives any attributed survival benefit to PCT testing. Several features of this RCT raise concern: low algorithm compliance; lack of reporting of differences across baseline comorbidities; and the modified intention-to-treat analysis with a fragility index of nine, substantially less than the number excluded from the final analysis (29 adults). Finally, the speculated reason for the observed decrease in mortality was not tested—the knowledge of PCT concentrations leads to earlier and more adequate diagnoses and treatments.33 Therefore, how PCT guidance might produce the observed survival benefit remains unexplained. The only trial designed and powered to examine survival as a primary efficacy outcome found no differences in frequency of diagnostic procedures, interventions for source control, readjustment of empirical antimicrobial therapy, or relapses of hospitalization.27
Previous meta-analyses have sought to address whether PCT-guided antibiotic discontinuation improves survival in sepsis, critical illness, and both critical illness and sepsis (e-Table 1). Our search involved a greater number of data sources and articles. We identified and translated additional articles from the Chinese literature24, 28 and assessed algorithm adherence, industry sponsorship, and the use of cointerventions in the intervention and control arms. Our findings are strengthened by our certainty assessment using GRADEpro (Table 2). We found substantial risk of bias, inconsistency, and indirectness. Therefore, there is low certainty for the use of PCT-guided antibiotic discontinuation to improve survival. The recent patient-level meta-analysis of adults with infection and sepsis by Wirz et al46 found improved survival in those with sepsis. Several important differences exist between their meta-analysis and ours. Ours analyzed only those studies with patients (n = 2,160) who met a definition of sepsis preceding the 2016 Sepsis-3 definition. Theirs included patients meeting the Sepsis-3 definition (n = 3,235). They performed multivariable hierarchical regression analysis and adjusted for treatment arm, age, sex, and type of infection but not adherence to study protocol or whether the treatment algorithm specified procalcitonin only in the intervention arm. Also, in the article text, their reported survival benefit of PCT-guided therapy in the subgroup meeting the Sepsis-3 definition (95% CI, 0.76-0.98) differs from the forest plot of 30-day mortality (95% CI crosses 1). Their analysis was supported by industry and used a writing service to draft the manuscript, and they acknowledge that “pathophysiologic mechanisms [for the survival advantage] are incompletely understood.” The validity of their observed survival benefit is not questioned and future studies to investigate this unexpected result are not proposed. In another recent patient-level meta-analysis of 523 bacteremic patients from 13 trials (including six trials of patients with sepsis), PCT-guided antibiotic discontinuation resulted in fewer antibiotic days, and no survival benefit overall.47
Although its impact on survival is debatable, many clinicians may conclude that PCT is useful if it can decrease antibiotic exposure without harming patients. Investigators concluded that PCT-based algorithms could potentially save government health systems millions of euros annually,48 based on a decision tree analysis of six RCTs.19, 20, 21, 22, 23, 30 However, these six RCTs all had high or unknown risk of bias and do not mention whether an antibiotic stewardship program was used in the control arm. Future studies need to minimize bias and determine the true benefit of PCT-based algorithms on antibiotic exposure using an appropriately rigorous control arm. Among patients with lower respiratory tract infection, a well-designed large multicenter RCT showed that the provision of PCT results to ED- and hospital-based clinicians did not result in less use of antibiotics than did usual care.49
We acknowledge several limitations. Baseline characteristics such as comorbidities, site of infection, baseline PCT levels, and the interval from presentation to randomization were not uniformly reported across both study arms for all RCTs. Baseline comparability of illness severity is important as substantial clinical heterogeneity occurs in those admitted to the ICU. Procalcitonin algorithms differed considerably across RCTs and algorithm adherence was either not reported or low. Low algorithm compliance suggests some clinicians disagreed with algorithm-directed changes in care, raising concerns about reproducibility in general practice settings. Our sepsis subgroup only included patients from RCTs that explicitly studied septic populations. No RCT compared PCT-guided antibiotic discontinuation to control arms compliant with sound principles of antibiotic stewardship. In RCTs that assessed adverse events, data were missing on the development of antibiotic resistance, allergic reactions and Clostridium difficile infections. Only 10 RCTs used an intention-to-treat analysis. We excluded observational studies to minimize residual confounding and heterogeneity.
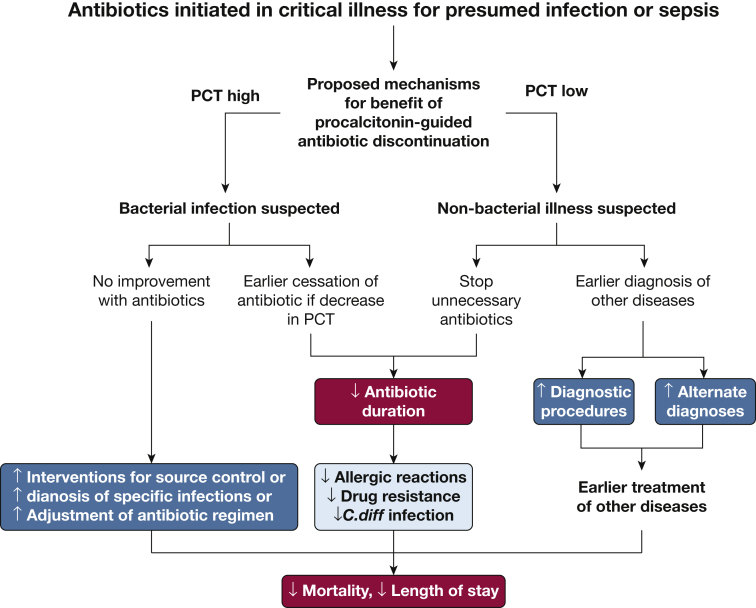
Future studies need to designate survival as the primary outcome and collect data on other potential impactful secondary end points. Knowledge of PCT results might affect other important aspects of care such as antibiotic adequacy, source control, use of diagnostic testing, and diagnosing alternative illnesses that influence survival. Figure 3 summarizes outcomes previously assessed in RCTs (red boxes), as well as putative mechanisms (blue boxes) of potential benefit. In addition to reporting all these outcomes, future RCTs should (1) exclusively use PCT and no other biomarkers in the intervention arm, (2) rigorously report rates and rationales for PCT algorithm nonadherence or overruling, (3) report side effects and complications of antibiotic administration and antibiotic discontinuation, and (4) test whether these purported mechanisms for improved survival actually decrease mortality. If future RCTs establish proof-of-concept and elucidate mechanisms of benefit,50 comparative effectiveness trial designs could assess whether PCT-guided antibiotic discontinuation has generalizable benefits beyond those provided by well-managed antibiotic stewardship programs, which are increasingly the standard of care in hospitals.51, 52
Figure 3.
Testable hypotheses for the potential mechanism of a survival benefit from procalcitonin-guided antibiotic discontinuation. C.diff = Clostridium difficile; PCT = procalcitonin.
We found low-certainty evidence with a high risk of bias to support PCT-guided antibiotic discontinuation to increase survival among critically ill adults. The plausibility of this survival benefit is weakened as this occurred primarily in studies with low protocol adherence (ie, providers frequently overruled PCT guidance) and studies with algorithms combining PCT with other biomarkers (C-reactive protein). Antibiotic discontinuation in recovering critically ill adults remains a challenge for intensivists and administrators, and the routine use of PCT requires ongoing evaluation for biologic plausibility and efficacy.
Acknowledgments
Author contributions: D. J. P. and S. S. K. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. D. J. P., J. S., C. R., J. W., J. H. P., R. L. D., and S. S. K. contributed substantially to the study design, data analysis, and interpretation. D. J. P. and S. S. K. drafted the manuscript, and J. S., C. R., J. W., J. H. P., and R. L. D. revised it critically for important intellectual content. D. J. P., J. S., C. R., J. W., J. H. P., R. L. D., and S. S. K. approve the final version to be published.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendixes, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: Funded in part by the Intramural Research Program of the National Institutes of Health Clinical Center, in part by the National Cancer Institute [Contract No. HHSN261200800001E], and in part by the Agency for Healthcare Research and Quality [Grant No. K08HS025008].
Supplementary Data
References
- 1.Rhee C., Dantes R., Epstein L. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA. 2017;318(13):1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balk R.A., Kadri S.S., Cao Z., Robinson S.B., Lipkin C., Bozzette S.A. Effect of procalcitonin testing on health-care utilization and costs in critically ill patients in the United States. Chest. 2017;151(1):23–33. doi: 10.1016/j.chest.2016.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang H., Huang T., Jing J., Shen H., Cui W. Effect of procalcitonin-guided treatment in patients with infections: a systematic review and meta-analysis. Infection. 2009;37(6):497–507. doi: 10.1007/s15010-009-9034-2. [DOI] [PubMed] [Google Scholar]
- 4.Kopterides P., Siempos, Tsangaris I., Tsantes A., Armaganidis A. Procalcitonin-guided algorithms of antibiotic therapy in the intensive care unit: a systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2010;38(11):2229–2241. doi: 10.1097/CCM.0b013e3181f17bf9. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal R., Schwartz D.N. Procalcitonin to guide duration of antimicrobial therapy in intensive care units: a systematic review. Clin Infect Dis. 2011;53(4):379–387. doi: 10.1093/cid/cir408. [DOI] [PubMed] [Google Scholar]
- 6.Heyland D.K., Johnson A.P., Reynolds S.C., Muscedere J. Procalcitonin for reduced antibiotic exposure in the critical care setting: a systematic review and an economic evaluation. Crit Care Med. 2011;39(7):1792–1799. doi: 10.1097/CCM.0b013e31821201a5. [DOI] [PubMed] [Google Scholar]
- 7.Schuetz P., Chiappa V., Briel M., Greenwald J.L. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322–1331. doi: 10.1001/archinternmed.2011.318. [DOI] [PubMed] [Google Scholar]
- 8.Schuetz P., Briel M., Christ-Crain M. Procalcitonin to guide initiation and duration of antibiotic treatment in acute respiratory infections: an individual patient data meta-analysis. Clin Infect Dis. 2012;55(5):651–662. doi: 10.1093/cid/cis464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthaiou D.K., Ntani G., Kontogiorgi M., Poulakou G., Armaganidis A., Dimopoulos G. An ESICM systematic review and meta-analysis of procalcitonin-guided antibiotic therapy algorithms in adult critically ill patients. Intensive Care Med. 2012;38(6):940–949. doi: 10.1007/s00134-012-2563-7. [DOI] [PubMed] [Google Scholar]
- 10.Soni N.J., Samson D.J., Galaydick J.L. Procalcitonin-guided antibiotic therapy: a systematic review and meta-analysis. J Hosp Med. 2013;8(9):530–540. doi: 10.1002/jhm.2067. [DOI] [PubMed] [Google Scholar]
- 11.Prkno A., Wacker C., Brunkhorst F.M., Schlattmann P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock: a systematic review and meta-analysis. Crit Care. 2013;17(6):R291. doi: 10.1186/cc13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andriolo B.N., Andriolo R.B., Salomão R., Atallah Á.N. Effectiveness and safety of procalcitonin evaluation for reducing mortality in adults with sepsis, severe sepsis or septic shock. Cochrane Database Syst Rev. 2017;1:CD010959. doi: 10.1002/14651858.CD010959.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang H.B., Peng J.M., Weng L., Wang C.Y., Jiang W., Du B. Procalcitonin-guided antibiotic therapy in intensive care unit patients: a systematic review and meta-analysis. Ann Intensive Care. 2017;7(1):114. doi: 10.1186/s13613-017-0338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iankova I., Thompson-Leduc P., Kirson N.Y. Efficacy and safety of procalcitonin guidance in patients with suspected or confirmed sepsis: a systematic review and meta-analysis. Crit Care Med. 2018;46(5):691–698. doi: 10.1097/CCM.0000000000002928. [DOI] [PubMed] [Google Scholar]
- 15.Lam S.W., Bauer S.R., Fowler R., Duggal A. Systematic review and meta-analysis of procalcitonin-guidance versus usual care for antimicrobial management in critically ill patients: focus on subgroups based on antibiotic initiation, cessation, or mixed strategies. Crit Care Med. 2018;46(5):684–690. doi: 10.1097/CCM.0000000000002953. [DOI] [PubMed] [Google Scholar]
- 16.Rhodes A., Evans L.E., Alhazzani W. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 17.Moher D., Shamseer L., Clarke M., PRISMA-P Group Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shamseer L., Moher D., Clarke M., PRISMA-P Group Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 19.Nobre V., Harbarth S., Graf J.D., Rohner P., Pugin J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177(5):498–505. doi: 10.1164/rccm.200708-1238OC. [DOI] [PubMed] [Google Scholar]
- 20.Hochreiter M., Köhler T., Schweiger A.M. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care. 2009;13(3):R83. doi: 10.1186/cc7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroeder S., Hochreiter M., Koehler T. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg. 2009;394(2):221–226. doi: 10.1007/s00423-008-0432-1. [DOI] [PubMed] [Google Scholar]
- 22.Annane D., Maxime V., Faller J.P. Procalcitonin levels to guide antibiotic therapy in adults with non-microbiologically proven apparent severe sepsis: a randomised controlled trial. BMJ Open. 2013;3(2):e002186. doi: 10.1136/bmjopen-2012-002186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deliberato R.O., Marra A.R., Sanches P.R. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn Microbiol Infect Dis. 2013;76(3):266–271. doi: 10.1016/j.diagmicrobio.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Liu B.H., Li H.F., Lei Y., Zhao S.X., Sun M.L. [Clinical significance of dynamic monitoring of procalcitonin in guiding the use of antibiotics in patients with sepsis in ICU] [article in Chinese] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2013;25(11):690–693. doi: 10.3760/cma.j.issn.2095-4352.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira C.F., Botoni F.A., Oliveira C.R. Procalcitonin versus C-reactive protein for guiding antibiotic therapy in sepsis: a randomized trial. Crit Care Med. 2013;41(10):2336–2343. doi: 10.1097/CCM.0b013e31828e969f. [DOI] [PubMed] [Google Scholar]
- 26.Shehabi Y., Sterba M., Garrett P.M. ProGUARD Study Investigators; ANZICS Clinical Trials Group. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis: a randomized controlled trial. Am J Respir Crit Care Med. 2014;190(10):1102–1110. doi: 10.1164/rccm.201408-1483OC. [DOI] [PubMed] [Google Scholar]
- 27.Bloos F., Trips E., Nierhaus A. Effect of sodium selenite administration and procalcitonin-guided therapy on mortality in patients with severe sepsis or septic shock: a randomized clinical trial. JAMA Intern Med. 2016;176(9):1266–1276. doi: 10.1001/jamainternmed.2016.2514. [DOI] [PubMed] [Google Scholar]
- 28.Xu X.L., Yan F.D., Yu J.Q., Chen Q.H., Lin H., Zheng R.Q. [Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment of sepsis patients] [article in Chinese] Zhonghua Yi Xue Za Zhi. 2017;97(5):343–346. doi: 10.3760/cma.j.issn.0376-2491.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Stolz D., Smyrnios N., Eggimann P. Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: a randomised study. Eur Respir J. 2009;34(6):1364–1375. doi: 10.1183/09031936.00053209. [DOI] [PubMed] [Google Scholar]
- 30.Bouadma L., Luyt C.E., Tubach F., PRORATA Trial Group Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375(9713):463–474. doi: 10.1016/S0140-6736(09)61879-1. [DOI] [PubMed] [Google Scholar]
- 31.Maravić-Stojković V., Lausević-Vuk L., Jović M., Ranković A., Borzanović M., Marinković J. Procalcitonin-based therapeutic strategy to reduce antibiotic use in patients after cardiac surgery: a randomized controlled trial. Srp Arh Celok Lek. 2011;139(11-12):736–742. [PubMed] [Google Scholar]
- 32.Qu R., Ji Y., Ling Y. Procalcitonin is a good tool to guide duration of antibiotic therapy in patients with severe acute pancreatitis: a randomized prospective single-center controlled trial. Saudi Med J. 2012;33(4):382–387. [PubMed] [Google Scholar]
- 33.de Jong E., van Oers J.A., Beishuizen A. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819–827. doi: 10.1016/S1473-3099(16)00053-0. [DOI] [PubMed] [Google Scholar]
- 34.Daubin C., Valette X., Thiollière F. Procalcitonin algorithm to guide initial antibiotic therapy in acute exacerbations of COPD admitted to the ICU: a randomized multicenter study. Intensive Care Med. 2018;44(4):428–437. doi: 10.1007/s00134-018-5141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkins D., Best D., Briss P.A., GRADE Working Group Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.GRADEpro. https://gradepro.org/
- 37.Dersimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 38.Cornell J.E., Mulrow C.D., Localio R. Random-effects meta-analysis of inconsistent effects: a time for change. Ann Intern Med. 2014;160(4):267–270. doi: 10.7326/M13-2886. [DOI] [PubMed] [Google Scholar]
- 39.Knapp G., Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22(17):2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 40.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 42.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.R Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. http://www.R-project.org/
- 44.Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7(3):40–45. [Google Scholar]
- 45.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 46.Wirz Y., Meier M.A., Bouadma L. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: a patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meier MA, Branche A, Neeser OL, et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: a patient-level meta-analysis of randomized trials. Clin Infect Dis. In press. [DOI] [PubMed]
- 48.Kip M.M., Kusters R., IJzerman M.J., Steuten L.M. A PCT algorithm for discontinuation of antibiotic therapy is a cost-effective way to reduce antibiotic exposure in adult intensive care patients with sepsis. J Med Econ. 2015;18(11):944–953. doi: 10.3111/13696998.2015.1064934. [DOI] [PubMed] [Google Scholar]
- 49.Huang D.T., Yealy D.M., Filbin M.R. ProACT Investigators. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379(3):236–249. doi: 10.1056/NEJMoa1802670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fiore L.D., Lavori P.W. Integrating randomized comparative effectiveness research with patient care. N Engl J Med. 2016;374(22):2152–2158. doi: 10.1056/NEJMra1510057. [DOI] [PubMed] [Google Scholar]
- 51.Fridkin S., Baggs J., Fagan R. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194–200. [PMC free article] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention Antibiotic Prescribing and Use in Hospitals and Long-Term Care: Core Elements of Hospital Antibiotic Stewardship Programs. https://www.cdc.gov/antibiotic-use/healthcare/implementation/core-elements.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.