Abstract
Objective:
Epidemiologic data increasingly supports sleep as a determinant of cardiovascular disease risk. Fewer studies have investigated the mechanisms underlying this relationship using objective sleep assessment approaches. Therefore, the aim of this study was to examine associations between daily blood pressure and both objectively assessed sleep duration and efficiency.
Methods:
A diverse community sample of 300 men and women ages 21-70, enrolled in the North Texas Heart Study, participated in the study. Actigraphy assessed sleep was monitored over 2 consecutive nights with ambulatory blood pressure sampled randomly within 45-min blocks on the first and second day as well as the second night.
Results:
Overall, sleep duration results paralleled those of sleep efficiency. Individuals with lower sleep efficiency had higher daytime systolic (B=−0.35, SE=0.11, p=.0018, R2=0.26) but not diastolic BP (B=−0.043, SE=0.068, p=.52, R2=0.17) and higher nighttime BP (systolic: B=−0.37, SE=0.10, p<.001, R2=.15; diastolic: B=−0.20, SE=0.059, p<.001, R2=.14). Moreover, lower sleep efficiency on one night was associated with higher systolic (B=−0.51, SE=0.11, p<.001, R2=0.23) and diastolic BP (B=−0.17, SE=0.065, p=.012, R2 =.16) the following day. When both sleep duration and efficiency were assessed together, sleep efficiency was associated with daytime systolic BP, while sleep duration was associated with nighttime BP.
Conclusions:
Lower sleep duration and efficiency are associated with higher daytime systolic BP and higher nighttime BP when assessed separately. When assessed together, sleep duration and efficiency diverge in their associations with BP at different times of day. These results warrant further investigation of these possible pathways to disease.
Keywords: Sleep quality, Sleep duration, sleep efficiency, blood pressure, ambulatory, actigraphy, BP= Blood Pressure, CVD= Cardiovascular Disease, BMI = Body Mass Index, Polysomnography = PSG, Ecological Momentary Assessment = EMA
INTRODUCTION
Sleep and Cardiovascular Disease Risk
Sleep is increasingly recognized as a risk factor for cardiovascular disease (CVD). Numerous studies and reviews document relationships between sleep, including both individual variations and clinical sleep conditions, and a range of worse cardiovascular outcomes as well as mortality (1,2). For example, a recent meta-analysis of 512 studies involving 25,760 participants with obstructive sleep apnea (OSA) reported a 79% greater risk of CVD, a twofold increase in stroke, and a 92% increased risk of all-cause early mortality (3). Likewise, a recent prospective study of 23,447 men followed for 6 years documented associations between insomnia symptoms and greater CVD mortality (4).
In addition to clinical syndromes, everyday sleep inadequacy is also linked to CVD. Numerous reviews and meta-analyses have found an association between inadequate sleep duration and the prevalence and incidence of CVD (5,6). For example, in a 2016 meta-analytic review of literature regarding the association between sleep duration and incident CVD, the American Heart Association found short sleep duration is related to adverse cardiometabolic risk, including coronary heart disease and diabetes mellitus (7).Their findings underscore the importance of sleep as a behavior that contributes to cardiovascular health. Sleep quality, broadly defined, is also linked to CVD risk. A recent systematic review of 32 studies documented a significant association between poor objective and subjective insomnia and various measures of subclinical CVD, including endothelial dysfunction and carotid intima-media thickness (CIMT) (6). Taken together, these data provide strong evidence of the pathogenic effects of sleep inadequacy.
In addition to general quantity (i.e., sleep duration) issues, a significant portion of the population experiences difficulties with insomnia (8). Insomnia affects nearly 30% of adults in the U.S., and the best objective and continuous operational definition of insomnia severity is sleep efficiency. Sleep efficiency is the amount of total sleep time that occurs during time in bed (9), which accounts for the time spent awake lying in bed before sleep onset (i.e., difficulty initiating sleep), during the night (i.e., difficulty maintaining sleep), and in the morning (i.e., early morning awakenings). Despite emerging evidence linking sleep efficiency to CVD risk, the mechanisms underlying this relationship remain largely unexplored.
Emerging research has begun to examine the relationship between sleep efficiency and CVD risk broadly (6,10). For example, a study of 904 participants found a modest dose-response relationship between lower polysomnography-derived (PSG) sleep efficiency and higher Framingham cardiovascular risk scores, after controlling for BMI and age (11). Additionally, a large, prospective cohort study of 23,447 US men found a dose-dependent relationship between more self-reported sleep efficiency-related difficulties and increased risk of CVD mortality (4). Smaller studies have reported a relationship between decreased sleep efficiency and a diagnosis of hypertension vs. normotension (12).
In addition to studies of CVD risk factors and disease outcomes, a number of studies have linked sleep efficiency to CVD pathways and mechanisms. For example, a cross-sectional study of 340 women found an association between lower in-home PSG-assessed sleep efficiency and higher C-reactive protein and fibrogen levels -- markers of inflammation that contribute to atherosclerosis (13). In addition, a community-based observational study of 527 adults discovered that lower 7-day actigraphy-derived sleep efficiency was associated with lower baseline heart rate variability (14).
Sleep and Blood Pressure
One pathway by which chronic perturbations in daily sleep may affect CVD risk is through its effects on blood pressure (BP). Blood pressure is a well-established moderator of CVD (15,16). Psychological/behavioral factors have consistently demonstrated moderation of BP (17) with prospective data documenting their impact on underlying disease (18). Inadequate sleep, including issues of quantity and efficiency, may acutely affect BP with implications for disease depending on the chronicity of the sleep disturbance.
Specifically, sleep disturbance may affect BP by way of hypothalamic-pituitary-adrenal (HPA) axis activation. Studies have indicated perceived stress as a risk factor for sleep disturbance (19,20). A key component of the stress response involves HPA axis activation (21). ACTH and cortisol, products of the HPA axis, promote wakefulness (22) and increase blood pressure (23,24). Indeed, one 4-day PSG laboratory study comparing 11 insomniacs to 13 age- and BMI-matched healthy sleeping controls found that 24-hour ACTH and cortisol secretions are higher in sleep disturbed individuals (22). Other studies have shown that hypertension risk increases in a step-wise manner for individuals with insomnia and 24-hour hyperarousal (25). This finding is congruent with the reactivity hypothesis, which posits that higher psychophysiological reactivity to stress is a risk factor for CVD (26). Thus, it is plausible that sleep disturbance due to physiological hyperarousal may lead to increased BP via hormone secretion due to HPA axis activation.
To date, few studies have investigated the relationship between sleep efficiency and BP. The studies that have examined this relationship are methodologically mixed with most having various methodological shortcomings (27-29). Issues include reliance on self-report measures of sleep efficiency and single time point measures of BP (30,31). A few studies have used more preferred objective approaches but with various limitations. For example, a study of 87 Black and White college-aged men and women found that actigraphy-assessed sleep efficiency was associated with nighttime ambulatory diastolic BP, but not systolic BP (32). However, their sample was not community-based, and sleep efficiency and BP were only measured over 24 hours. A population-based single-night study of 2,040 ethnically diverse older adults found that in-home PSG-assessed sleep efficiency was associated with systolic BP, but not diastolic BP, before statistical adjustment (33). However, BP was only assessed with a single time point measure which raises questions about reliability. The few remaining studies using objective measures of both sleep efficiency and BP have been conducted with adolescent samples (34-36).
The aim of the current study was to examine the relationship between objectively assessed sleep duration and efficiency along with blood pressure using objective measures and a repeated measures design over two days in a large, diverse, community sample. The data also allows us to test whether sleep duration and efficiency are concurrently related to day and nighttime BP, their effects on BP the following day, and to disentangle the effects of duration and efficiency as either mirrored or unique determinants of BP in daily life.
Methods
Participants
Participants were a community sample of 300 adults (150 men, 150 women) ages 21 to 70 years (M=42.44, SD=12.76) from the North Texas Heart Study; a longitudinal investigation of stress and atherosclerotic risk (37). Inclusion criteria were 1) 21+ years of age, 2) residing within Denton County, Texas, 3) written and verbal fluency in English. Exclusionary criteria included, 1) cognitive impairment (i.e., dementia), 2) previous history of myocardial infarction or tertiary cardiac interventions (e.g., coronary artery bypass surgery, implanted cardiac defibrillator), 3) pregnancy within last year or anticipating pregnancy during study period, and 4) an occupation that requires shift work. The sample was stratified by age within sex and race/ethnicity in order to examine age-related effects. The diverse sample included 60% non-Hispanic Whites, 15% non-Hispanic Blacks, and 19% Hispanic/Latinos, of which 75% self-identified as being of Mexican descent.
Procedures
Overview.
The complete North Texas Heart Study protocol is described elsewhere (37). Briefly, a representative community sample of men and women was recruited and participated in the study between November 2012 and September 2013. The study was approved by the University of North Texas’s Institutional Review Board. Following informed consent, participants attended a baseline session, which was conducted at a single-site, vascular medicine clinic located in the community that functioned as a general clinical research center. All laboratory sessions were conducted on Thursday mornings followed by a 2-day/1-night ambulatory/ecological momentary assessment (EMA) study. This study was comprised of two days of ABP collection (daytime of Thursday and Friday) and two nights of actigraphic data collection (nighttime of Thursday and Friday), and one night of ABP collection (Friday night). This ecological sampling design was developed through a collaboration between experts/leaders in sleep, EMA, and ABP assessment (38-40). Following arrival at the laboratory study and consent, all participants underwent a brief physical exam, personal and family medical history, current medications and physical conditions, health behaviors, and detailed cardiac disease history. Prior to leaving, all participants were fitted with an actigraph monitor, an ambulatory BP monitor (see details below), and given a cellular phone for the 2-day/1-night, ambulatory/EMA study and trained on their use. Participants were further instructed to complete two additional EMA-based surveys: 1) an end-of-day survey completed at bedtime, and 2) a morning survey upon awakening. On the third day, participants met the study coordinator at a designated site to return the EMA/ambulatory equipment and complete final measures.
Sleep and ambulatory protocol.
Sleep was assessed using three distinct methodologies: surveys, sleep diaries, and actigraphy. Sleep diaries and actigraphy (via Actiwatch) were used to assess subjective and objective aspects of sleep on each study night. The Actiwatch was used to objectively monitor sleep over the 48-hour measurement period. In addition, participants were asked to keep sleep diaries (41) to assess self-reported sleep and wake times as well as subjective experiences during the prior night’s sleep. Sleep diaries were completed upon waking each study day. Participants used an EMA-based sleep diary to report on the prior night’s sleep.
To assess the association between sleep and daily/nightly BP, participants were fitted with an ambulatory BP monitor (ABPM). The ABPM was programmed to assess BP at random times during 45-min intervals during the first and second study days and the second study night. Since repeated sampling may potentially alter participant behavior (42,43), the research team devised this methodology to randomize data capture while maintaining a measurement timing standard and burden. This random sampling procedure prevents participants from anticipating a reading and hence altering their activities. Participants were instructed to complete the EMA protocol in response to each BP sampling during hours awake. Participants were instructed to turn off the ABPM and remove it at bedtime on the first night, to attach and activate it upon awakening the next morning, and to contact the investigators immediately with questions. Participants were instructed to wear the ABPM on the second study night in order collect night-time BP data. This procedure allowed any disturbance in sleep due to the ABPM to be assessed and decreased participant burden. There was no indication of significant differences in sleep efficiency from one night to the next as indicated by t-test (p=0.21). However, night time awakenings significantly differed slightly (p=0.04).
Measures
Objective sleep quantity and efficiency.
AW Spectrum Actiwatches (Philips-Respironics, Inc., Bend, OR) utilize an accelerometer to monitor the occurrence and degree of motion (44). This information is analyzed with Actiware software using settings of medium activity threshold and ten immobile minutes to detect wakefulness. For the current study, derived variables included: bed time, wake time, time in bed, sleep onset latency, number of awakenings during the night, wake time after sleep onset, total sleep time, and sleep efficiency, using proprietary scoring algorithms in the software. To test the current aims, we focused on sleep duration (i.e., “total sleep time”) and sleep efficiency.
Sleep diaries and actigraphy data were combined to optimize assessment of sleep parameters. In the current study, there were moderately significant correlations between sleep diaries and actigraphy on total sleep time, sleep onset latency, wake time after sleep onset, and sleep efficiency (all p’s < .01).
Snoring, Tired, Observed Breathing, Blood Pressure (STOP).
Obstructive Sleep Apnea (OSA) risk was assessed using the STOP Sleep Apnea Screen, a 4-item measure to screen for sleep apnea. The STOP was developed and validated in 211 pre-operative surgical patients (45). Participants completed this assessment at each study visit.
Ambulatory BP Monitoring (ABPM).
Ambulatory BP was monitored using the Oscar 2 oscillometric ambulatory BP monitor (Suntech Medical Instruments, Inc., Raleigh, NC, USA). The Oscar 2 was designed specifically for ambulatory assessments and is the only ABPM clinically validated to all three international standards (46). The sensors and the cuff were unobtrusively worn under the participant’s clothing, and only a small control unit (approximately 4.7 × 2.8 × 1.2 inches; 284 grams) attached to the participant’s belt was partially exposed. An appropriately sized BP cuff was selected according to participant size and wrapped around the upper part of the non-dominant arm. Consistent with our extensive prior work (47-50), data was cleaned using the criteria set by Marler and colleagues (51). Specifically, outliers associated with artifactual readings were discarded if (a) SBP < 70 mmHg or > 250 mmHg, (b) DBP < 45 mmHg or > 150 mmHg, or (c) SBP / DBP < [1.065 + (.00125 X DBP)] or > 3.0. “Daytime” BP was defined as readings occurring between 7am and 10pm, while “Nighttime” BP was defined as readings occurring between 12am and 5am. Though it is possible individuals may have been asleep during “daytime” readings and awake during “nighttime” readings, these were the intervals during which it was most likely the highest percentage of participants would be awake and asleep, respectively.
Statistical Analyses
Ambulatory BP averages were created for each of the two study days and the one study night. Specific derived variables included: day one and two systolic and diastolic BP, and night two systolic and diastolic BP. In addition, average day systolic and diastolic BP were calculated by averaging all daytime measures between 7am and 10pm during the study period. In addition to nighttime BP, we sought to examine the more specific concept of “asleep blood pressure”. Similar to the International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcomes (IDACO) and methods employed in The Coronary Artery Risk Development in Young Adults (CARDIA) study (52) and in consultation with sleep and ambulatory BP experts, we operationalized asleep BP as BP readings occurring during a period of time that included at least 90% actigraphic sleep. For those whom less than 90% sleep occurred in the 12-5am window, but who had significant sleep period overlapping with some part of the 12-5am window, we identified the actigraphic parameters of their sleep window not to exceed the 5 hours total and averaged BP from within that window to represent “sleep BP.” The resulting BP average was one in which all “BP while sleeping” readings occurred during greater than 90% of sleep. The repeated measure BP sampling method afforded us the opportunity to examine mean person levels, which are a more important indicator of disease risk than individual BP readings. Consistent with established protocol (9), actigraphy-derived sleep duration was defined as the amount of time the participant was actually asleep during the night (i.e., TST). Actigraphy-derived sleep efficiency was calculated as the ratio between total sleep time (TST) and time in bed (TIB) (TST/TIB)*100. Specific derived variables included: night one sleep duration, night two sleep duration, night one sleep efficiency, night two sleep efficiency. In addition, average sleep duration and efficiency were calculated by averaging night one and two sleep duration and efficiency measured during the study period, respectively.
Linear regression was used to test hypotheses regarding the association between both actigraphic sleep duration and efficiency, and ambulatory BP. More specifically, we sought to examine whether both average sleep duration and sleep efficiency were associated with 1) average daytime BP, 2) average nighttime BP, and 3) whether average sleep duration and efficiency on one night were temporally associated with average daytime BP the next day.
First, simple correlations were performed between all sleep duration and efficiency variables, BP outcome variables, and continuous control variables. Next, linear regressions were conducted in steps. Separate unadjusted regressions were conducted analyzing the association between sleep duration and BP variables, and sleep efficiency and BP variables. Next, partially adjusted models were conducted, assessing the influence of demographic characteristics (i.e., age, sex, and race/ethnicity). Lastly, fully adjusted models were conducted to assess the added influence of BMI and sleep apnea risk—factors that are likely to explain variance in our models. Baseline BP was recorded, but was not included as a covariate in analyses as ambulatory BP (our outcome variable) predicts CVD outcome better relative to baseline BP (53). Each analysis was conducted for systolic and diastolic BP, respectively. Unadjusted, partially-, and fully-adjusted models that included both sleep duration and efficiency were then conducted to determine the differential impact of sleep duration and efficiency on BP.
In describing the analyses, we report the unstandardized (B) regression coefficients, standard error, 95% confidence intervals (CI), and p-values of the main outcomes and predictors. We also report the overall adjusted multiple correlation square (R2) for each model, as well as the change in R2 between unadjusted and partially-adjusted models, and between partially- and fully-adjusted models. F-statistics, p-values, and adjusted R2 of the overall models are reported. Model comparisons were made using the Akaike information criterion (AIC). Alpha level was set at p < .05 (two-tailed) and were performed using R version 3.3.2 and RStudio 1.0.136 (R Core Team, 2017).
Results
Sample Descriptives and Preliminary Analyses
Descriptive statistics for the total sample are shown in Table 1. Over the two study days, average daytime systolic BP was 146.15 mmHg while average daytime diastolic BP was 84.31 mmHg. Mean sleep efficiency was 82.52%, and mean sleep duration was 402.10 minutes, or approximately 6.7 hours
Table 1.
Total Sample Characteristics
| Variable | |
|---|---|
| Participants (n) | 300 |
| Sex | |
| Female (n) | 150 |
| Race/Ethnicity | |
| Non-Hispanic Black (n) | 46 |
| Non-Hispanic White (n) | 180 |
| Hispanic (n) | 57 |
| Non-Hispanic Other (n) | 17 |
| Mean age (years) (SD) | 42.44 (12.76) |
| Mean BMI (kg/m2) (SD) | 29.30 (6.48) |
| Mean STOP score (sleep apnea risk, 0 to 4) (SD) | 1.15 (0.96) |
| Mean Sleep Duration (minutes) (SD) | 402.10 (67.56) |
| Mean Sleep Efficiency (%) (SD) | 82.52 (9.63) |
| Mean daytime systolic BP (mmHg) (SD) | 146.15 (20.42) |
| Mean daytime diastolic BP (mmHg) (SD) | 84.31 (12.35) |
| Mean nighttime systolic BP (mmHg) (SD) | 128.97 (25.37) |
| Mean nighttime diastolic BP (mmHg) (SD) | 69.12 (15.43) |
Note. BMI= body mass index. SD=standard deviation. BP=Blood Pressure.
Bivariate correlations for all relevant continuous variables are displayed in Table 2. Replicating prior work, individuals with higher body mass index (BMI) had significantly higher daytime (r= 0.37, p<.01 for systolic; r=0.34, p<.01 for diastolic) and nighttime BP. Correlational analyses also revealed that there was a significant relationship between higher average sleep efficiency, and greater and average sleep duration, as well as lower average daytime systolic BP, and average nighttime systolic and diastolic BP. Night One and Night Two sleep duration variables were weakly correlated (r=0.059), whereas Night One and Night Two sleep efficiency variables were moderately correlated (r=0.25).
Table 2.
Correlations and Descriptive Statistics for Blood Pressure, Sleep, and Continuous Predictor Variables (N=300)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 1 | |||||||||||||||
| 2. BMI | −0.01 | 1 | ||||||||||||||
| 3. Average Actigraphic Sleep Duration - Night 1 and 2 | 0.19** | −0.03 | 1 | |||||||||||||
| 4. Actigraphic Sleep Duration - Night 1 | 0.07 | 0.04 | 0.69*** | 1 | ||||||||||||
| 5. Actigraphic Sleep Duration - Night 2 | 0.17** | −0.1 | 0.76*** | 0.06 | 1 | |||||||||||
| 6. Average Actigraphic Sleep Efficiency - Day 1 and 2 | 0.17** | −0.07 | 0.47*** | 0.1 | 0.56*** | 1 | ||||||||||
| 7. Actigraphic Sleep Efficiency - Night 1 | 0.15* | 0 | 0.4*** | 0.43*** | 0.18** | 0.71*** | 1 | |||||||||
| 8. Actigraphic Sleep Efficiency - Night 2 | 0.1 | −0.13* | 0.36*** | −0.16** | 0.66*** | 0.86*** | 0.25*** | 1 | ||||||||
| 9. Average Daytime SBP - Day 1 and 2 | 0.14* | 0.37*** | −0.15* | −0.1 | −0.16** | −0.18** | −0.17** | −0.18** | 1 | |||||||
| 10. Average Daytime DBP - Day 1 and 2 | 0.13* | 0.34*** | −0.08 | −0.09 | −0.05 | −0.1 | −0.11 | −0.11 | 0.85*** | 1 | ||||||
| 11. Average Daytime SBP - Day 1 | 0.13* | 0.38*** | −0.1 | −0.04 | −0.16** | −0.13* | −0.12 | −0.17** | 0.94*** | 0.78*** | 1 | |||||
| 12. Average Daytime DBP - Day 1 | 0.13* | 0.35*** | −0.05 | −0.06 | −0.06 | −0.07 | −0.08 | −0.1 | 0.79*** | 0.92*** | 0.82*** | 1 | ||||
| 13. Average Daytime SBP - Day 2 | 0.14* | 0.32*** | −0.19** | −0.15* | −0.14* | −0.2*** | −0.2*** | −0.17** | 0.95*** | 0.82*** | 0.78*** | 0.68*** | 1 | |||
| 14. Average Daytime DBP - Day 2 | 0.11 | 0.29*** | −0.11 | −0.11 | −0.04 | −0.12 | −0.12 | −0.1 | 0.79*** | 0.94*** | 0.65*** | 0.73*** | 0.84*** | 1 | ||
| 15. Average Nighttime SBP - Night 2 | 0.02 | 0.28*** | −0.26*** | −0.04 | −0.29*** | −0.31*** | −0.17** | −0.24*** | 0.66*** | 0.58*** | 0.57*** | 0.49*** | 0.67*** | 0.57*** | 1 | |
| 16. Average Nighttime DBP - Night 2 | 0.01 | 0.26*** | −0.28*** | −0.05 | −0.29*** | −0.29*** | −0.16* | −0.22*** | 0.53*** | 0.61*** | 0.46*** | 0.54*** | 0.54*** | 0.59*** | 0.84*** | 1 |
= p<.05;
= p<.01;
= p<.001
Data Quality
Of the 300 individuals enrolled in the study, 48 participants (16%) had missing data for average sleep efficiency and average sleep duration, 4 (1%) had missing data for both mean daytime systolic and diastolic BP, and 58 (19%) had missing data for mean nighttime systolic and diastolic BP. Specifically, the missing rate for ABP readings on the first day were 1% and 3% the following day. Such missing rates are relatively common, and values were not imputed (54). Participants were not excluded based on missing data. Rather, we relied on listwise deletion in order to maximize the n within specific models (55). We found that the only variable that predicted the missingness of nighttime BP, average sleep efficiency, and average sleep duration, was race/ethnicity. Race/ethnicity was included in all partially- and fully-adjusted models to account for this non-random missingness.
For all models, we assessed the normality of the residuals as well as any potential outliers and cases with significant leverage. We found no leverage points associated with our models. For models with significant associations, we followed up with a sensitivity analysis in which we removed outliers in the outcome variable. Most substantive effects remained after removal of outliers. All adjusted results shown are those conducted after outlier removal.
Associations between Sleep Duration and Blood Pressure
Association between average sleep duration and daytime blood pressure.
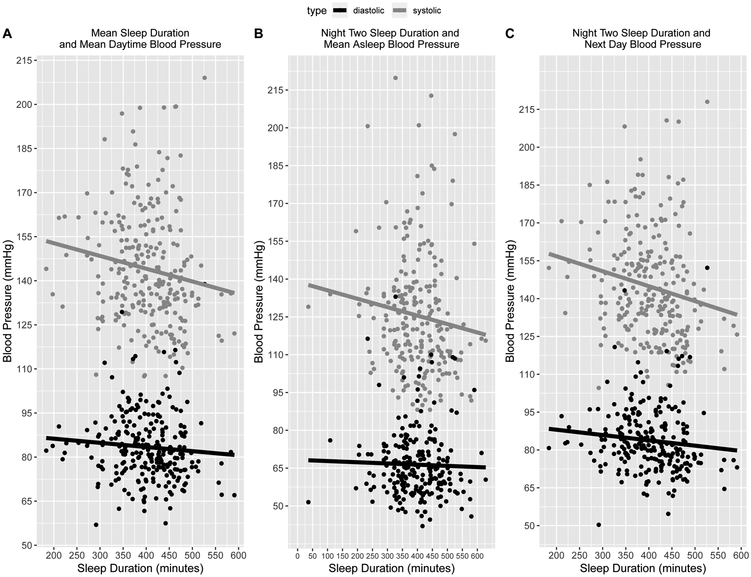
Multiple regression analyses were used to test whether actigraphic sleep duration, was associated with average daytime BP (Figure 1a). All main outcomes and predictors were averaged over the two study days. The results of all unadjusted, partially, and fully adjusted models are displayed in Table 3. Unadjusted analyses revealed that lower average sleep duration was associated with higher average daytime systolic (F(1,248)=5.77, p=.017, R2= 0.019) but not diastolic BP (F(1,248)=1.70, p=.19, R2= 0.0028). The association with systolic BP remained significant after partial (F(4,241)=8.16, p=.021, R2= 0.11) and full adjustment (F(6,229)=13.58, p=.046, R2=0.24) (Table 3) with all changes in R2 significant.
Figure 1.
Unadjusted Associations Between Sleep Duration and Blood Pressure
Table 3.
Multiple Regression Results: Fully Adjusted Associations Between Sleep Duration and Blood Pressure
| B(se) | 95% CI | p | B(se) | 95% CI | p | ||
|---|---|---|---|---|---|---|---|
|
Avg Daytime Systolic BP a |
Avg Daytime
Diastolic BP b |
||||||
| Avg SDur | −0.03(0.02) | (−0.07, −0.001) | .046* | Avg SDur | −0.01(0.01) | (−0.03, 0.01) | .43 |
| Gender | −8.43(2.24) | (−12.84, −4.02) | <.001*** | Gender | −3.88(1.33) | (−6.50, −1.26) | .004** |
| Age | 0.25(0.09) | (0.08, 0.42) | .004** | Age | 0.10(0.051) | (0.002, 0.20) | .045* |
| R/E | −1.42(1.46) | (−4.29, 1.46) | .33 | R/E | −1.25(0.87) | (−2.96, 0.47) | .15 |
| BMI | 1.07(0.17) | (0.73, 1.41) | <.001*** | BMI | 0.56(0.10) | (0.36, 0.76) | <.001*** |
| STOP | 0.71(1.15) | (−1.56, 2.98) | .54 | STOP | 0.25(0.68) | (−1.10, 1.60) | .72 |
|
Night 2 Avg Systolic BP c |
Night 2 Avg Diastolic BP d |
||||||
| Night 2 SDur | −0.06(0.02) | (−0.09, −0.45) | <.001*** | Night 2 SDur | −0.04(0.01) | (−0.06, −0.02) | <.001*** |
| Gender | −5.76(3.01) | (−11.68, 0.16) | .057 | Gender | −0.55(1.79) | (−4.07, 2.98) | .76 |
| Age | 0.10(0.12) | (−0.13, 0.32) | .42 | Age | 0.07(0.07) | (−0.07, 0.20) | .32 |
| R/E | −0.44(2.04) | (−4.47, 3.58) | .83 | R/E | −0.72(1.20) | (−3.08, 1.64) | .55 |
| BMI | 0.89(0.23) | (0.43, 1.34) | <.001*** | BMI | 0.57(0.14) | (0.30, 0.84) | <.001*** |
| STOP | 2.21(1.52) | (−0.79, 5.21) | .15 | STOP | 1.77(0.90) | (−0.01, 3.54) | .051 |
|
Avg Day 2 Systolic BP e |
Avg Day 2 Diastolic BP f |
||||||
| Night 1 SDur | −0.04(0.01) | (−0.07, −0.01) | .007** | Night 1 SDur | −0.02(0.01) | (−0.03, −0.002) | .028* |
| Gender | −7.84(2.47) | (−12.70, −2.97) | .002** | Gender | −3.78(1.46) | (−6.66, −0.90) | .010* |
| Age | 0.31(0.10) | (0.12, 0.50) | .002** | Age | 0.12(0.06) | (0.01, 0.24) | .032* |
| R/E | −0.15(1.65) | (−3.40, 3.09) | .93 | R/E | −0.70(0.98) | (−2.63, 1.23) | .48 |
| BMI | 0.98(0.19) | (0.61, 1.36) | <.001*** | BMI | 0.53(0.11) | (0.31, 0.75) | <.001*** |
| STOP | 0.21(1.30) | (−2.35, 2.78) | .87 | STOP | 0.04(0.77) | (−1.48, 1.55) | .96 |
Note.
: N=236, Adjusted R2= 0.24, Δ R2=0.13;
: N=235, Adjusted R2= 0.17, Δ R2=0.11;
: N=218, Adjusted R2=0.18, Δ R2=0.08;
: N=219, Adjusted R2= 0.18, Δ R2=0.084;
: N=235, Adjusted R2=0.19, Δ R2=0.096;
: N=237, Adjusted R2=0.14, Δ R2=0.074; The reference is a 55 year-old White woman with a low BMI (17.6 kg/m), no sleep apnea risk, and an average SD of 7.1 hours.
=p<.05;
=p<.01;
=p<.001. Avg.= Average. SDur= Sleep Duration. R/E= Combined Race and Ethnicity. BMI= Body Mass Index. STOP= sleep apnea risk score. Partially adjusted model adjusted for gender age, and race/ethnicity; Fully adjusted model additionally adjusted for body mass index, and sleep apnea risk score. All adjusted values are those after removal of outliers.
Association between sleep duration and same night blood pressure.
Multiple regression analyses were used to determine whether sleep duration was associated with average nighttime systolic and diastolic BP on the same night (Figure 1b). These analyses focused on night two of the study when sleep and BP were measured concurrently. Unadjusted analyses revealed that lower sleep duration was significantly associated with higher average systolic F(1,230)=21.81, p<.001, R2=0.083) and diastolic BP (F(1,230)=20.73, p<.001, R2=0.079). These associations remained significant after both partial (systolic: F(4,223)=7.56, p<.001, R2=0.10; diastolic: F(4,223)=7.02, p<.001, R2=0.096) and full adjustment (systolic: F(6,211)=8.92, p<.001, R2=0.18; diastolic: F(6,212)=8.98, p<.001, R2=0.18), with full adjustment accounting for up to 18% of the variance in both systolic and diastolic BP, respectively (Table 3). Only changes in R2 between the partially- and fully-adjusted models were significant.
Next, we investigated the association between objectively measured sleep duration and asleep BP. Under these more specific conditions, the preceding effects largely disappear for both systolic and diastolic BP at the unadjusted (systolic: F(1,219)=3.24, p=0.073, R2=0.01; diastolic: F(1,219)=0.20, p=0.66, R2=−0.003), partial (systolic: F(4,214)=2.91, p=0.36, R2=0.03; diastolic: F(4,212)=2.59, p=0.99, R2=0.02), and fully adjusted levels (systolic: F(6,200)=6.03, p=0.23, R2=0.13; diastolic: F(6,200)=7.23, p=0.86, R2=0.15).
Association between sleep duration and next day blood pressure.
Additional multiple regression analyses were used to determine whether a temporal trend existed between sleep duration on one night and BP the next day (Figure 1c). Unadjusted analyses revealed that lower sleep duration on one night was associated with higher systolic (F(1,251)=5.46, p=.020, R2=0.017), but not diastolic BP (F(1,251)=3.10, p=.08, R2=0.0083), during the following day. The association between lower Night One sleep duration remained associated with higher average Day Two systolic BP after partial (F(4,245)=7.46, p=.019, R2= 0.094) and full adjustment (F(6,228)=10.07, p=.0065, R2= 0.19) (Table 3). Adjustment rendered associations with diastolic BP significant; partial (F(4,245)=5.37, p=.033, R2=0.066), full adjustment (F(6,230)=7.29, p=.028, R2=0.14) (Table 3). All changes in R2 were significant.
Associations between Sleep Efficiency and Blood Pressure
Association between average sleep efficiency and daytime blood pressure.
Multiple regression analyses were used to test whether actigraphic sleep efficiency, averaged across the two study days, was associated with average daytime BP, also averaged across the two study days. The results of all unadjusted-, partially-, and fully-adjusted models are displayed in Table 4. Unadjusted analyses revealed that lower average sleep efficiency was associated with higher average daytime systolic (F(1,248)=7.96, p=0.005, R2= 0.027), but not diastolic BP (F(1,248)=2.66, p=.10, R2=.0066). The association for systolic BP remained significant after partial (F(4,242)=9.18, p=.002, R2=0.12) and full adjustment (F(6, 229)=14.91; p=.002, R2=0.26) (Table 4) with all changes in R2 significant.
Table 4.
Multiple Regression Results: Fully Adjusted Associations Between Sleep Efficiency and Blood Pressure
| B(se) | 95% CI | p | B(se) | 95% CI | p | |||
|---|---|---|---|---|---|---|---|---|
|
Avg Daytime Systolic BP a |
Avg Daytime Diastolic BP b |
|||||||
| Avg SEff | −0.35(0.11) | (−0.56, −0.13) | .002** | Avg SEff | −0.04(0.07) | (−0.18, 0.09) | .52 | |
| Gender | −9.07(2.13) | (−13.26, −4.88) | <.001*** | Gender | −4.28(1.28) | (−6.80, −1.77) | <.001*** | |
| Age | 0.27(0.08) | (0.10, 0.43) | .002** | Age | 0.12(0.050) | (0.02, 0.22) | .015** | |
| R/E | −1.35(1.44) | (−4.19, 1.49) | .35 | R/E | −1.23(0.86) | (−2.93, 0.47) | .16 | |
| BMI | 1.05(0.17) | (0.72, 1.38) | <.001*** | BMI | 0.54(0.10) | (0.34, 0.74) | <.001*** | |
| STOP | 0.62(1.14) | (−1.62, 2.86) | .59 | STOP | −0.019(0.68) | (−1.36, 1.33) | .98 | |
|
Night 2 Avg Systolic
BP c |
Night 2 Avg
Diastolic BP d |
|||||||
| Night 2 SEff | −0.37(0.10) | (−0.56, −0.17) | <.001*** | Night 2 SEff | −0.20(0.06) | (−0.32, −0.09) | <.001*** | |
| Gender | −6.60(2.95) | −12.42, −0.78) | .027* | Gender | −2.13(1.74) | −5.56, 1.29) | .22 | |
| Age | 0.06(0.12) | (−0.17, 0.29) | .61 | Age | 0.04(0.07) | (−0.09, 0.17) | .55 | |
| R/E | −0.19(2.03) | (−4.19, 3.82) | .93 | R/E | −0.97(1.20) | (−3.33, 1.40) | .42 | |
| BMI | 0.80(0.23) | (0.35, 1.26) | <.001*** | BMI | 0.51(0.14) | (0.24, 0.77) | <.001*** | |
| STOP | 2.06(1.51) | (−0.92, 5.04) | .18 | STOP | 1.05(0.89) | (−0.71, 2.81) | .24 | |
|
Day 2 Avg Systolic BP e |
Day 2 Avg Diastolic f | |||||||
| Night 1 SEff | −0.51(0.11) | (−0.73, −0.28) | <.001*** | Night 1 SEff | −0.17(0.07) | (−0.29, −0.04) | .012* | |
| Gender | −8.66(2.41) | (−13.40, −3.93) | <.001*** | Gender | −4.88(1.39) | (−7.62, −2.14) | <.001*** | |
| Age | 0.32(0.10) | (0.14, 0.51) | <.001*** | Age | 0.14(0.06) | (0.031, 0.25) | .012* | |
| R/E | −0.69(1.63) | (−3.90, 2.53) | .67 | R/E | −0.55(0.95) | (−2.41, 1.32) | .56 | |
| BMI | 1.02(0.19) | (0.66, 1.39) | <.001*** | BMI | 0.53(0.11) | (0.32, 0.74) | <.001*** | |
| STOP | 0.75(1.29) | (−1.79, 3.29) | .56 | STOP | −0.20(0.75) | (−1.67, 1.27) | .79 |
Note.
: N=236, Adjusted R2= 0.26, Δ R2=0.14;
: N=235; Adjusted R2= 0.17, Δ R2=0.11;
: N=217, Adjusted R2=0.15, Δ R2=0.073;
: N=217, Adjusted R2= 0.14, ΔR2=0.11;
: N=237; Adjusted R2=0.23, Δ R2=0.11;
: N=235, Adjusted R2=0.16, Δ R2=0.092. The reference is a 55 year-old White woman with a low BMI (17.6 kg/m), no sleep apnea risk, and an average SEff of 87.5%.
=p<.05;
=p<.01;
=p<.001. Avg.= Average. SEff= Sleep Efficiency. R/E= Combined Race and Ethnicity. BMI= Body Mass Index. STOP= sleep apnea risk score. Partially adjusted model adjusted for gender age, and race/ethnicity; Fully adjusted model additionally adjusted for body mass index, and sleep apnea risk score. All adjusted values are those after removal of outliers.
Association between sleep efficiency and same night blood pressure.
Unadjusted analyses revealed that lower sleep efficiency on night two was significantly associated with higher average systolic (F(1,230)=14.64, p<.001, R2=0.056) and diastolic BP (F(1,230)=12.08, p<.001, R2=0.046) the same night. These associations remained significant after both partial (systolic: F(4,224)=5.77, p<.001, R2=0.077; diastolic: F(4,223)=2.89, p=.006, R2=0.032) and full adjustment (systolic: F(6,210)=7.39, p<.001, R2=0.15; diastolic: F(6,210)=6.69, p<.001, R2=0.14), with full adjustment accounting for up to 15% and 14% of the variance in systolic and diastolic BP, respectively (Table 4). Only the change in R2 from the partially to fully adjusted model was significant.
Next, as we did for sleep duration, we investigated the association between objectively measured sleep efficiency and asleep BP. Under these more specific conditions, the preceding effects largely disappear for both systolic and diastolic BP at the unadjusted (systolic: F(1,219)=4.34, p=0.038, R2=0.015; diastolic: F(1,219)=1.54, p=0.22, R2=0.002), partial (systolic: F(4,213)=3.28, p=0.09, R2=0.040; diastolic: F(4,213)=3.12, p=0.39, R2=0.038), and fully adjusted levels (systolic: F(6,200)=6.17, p=0.15, R2=0.13; diastolic: F(6,199)=7.57, p=0.83, R2=0.16) except for unadjusted systolic BP.
Association between sleep efficiency and next day blood pressure.
Temporal trend analyses for sleep efficiency mirrored those of sleep duration. Unadjusted analyses revealed that lower sleep efficiency on one night was associated with higher systolic (F(1,251)=10.50, p=.0014, R2=0.036), but not diastolic (F(1,251)=3.63, p=0.058, R2=0.01), BP during the following day. The association between lower sleep efficiency one night and higher systolic BP the following day remained after partial (F(4,245)=9.05, p<.001, R2=0.12) and full adjustment (F(6,230)=12.73, p<.001, R2=0.23) (Table 4). Additionally, the association between lower sleep efficiency on one night and higher diastolic BP the next day was significant after partial (F(4,245)=5.56, p=0.022, R2=0.068) and full adjustment (F(6,228)=8.36, p=0.012, R2=0.16) (Table 4). All changes in R2 for systolic BP models were significant.
Associations between both Sleep Duration and Sleep Efficiency and Blood Pressure: Combined Model
Given the conceptual overlap between sleep duration and sleep efficiency, we tested whether sleep duration and efficiency confer unique influence in a single competing model. The results of all unadjusted-, partially-, and fully-adjusted models are displayed in Table 5.
Table 5.
Multiple Regression Results: Fully Adjusted Associations Between both Sleep Duration and Sleep Efficiency with Blood Pressure
| B(se) | 95% CI | p | B(se) | 95% CI | p | ||
|---|---|---|---|---|---|---|---|
|
Avg Daytime Systolic BP a |
Avg Daytime Diastolic BP b |
||||||
| Avg SDur | −0.013(0.02) | (−0.05, 0.02) | .47 | Avg SDur | −0.004(0.01) | (−0.03, 0.02) | .70 |
| Avg SEff | −0.31(0.12) | (−0.55, −0.07) | .012* | Avg SEff | −0.07(0.07) | (−0.21, 0.08) | .37 |
| Gender | −8.63(2.21) | (−12.99, −4.27) | <.001*** | Gender | −3.82(1.33) | (−6.44, −1.20) | .005** |
| Age | 0.27(0.09) | (0.11, 0.44) | .002** | Age | 0.12(0.05) | (0.02, 0.22) | .024* |
| R/E | −1.32(1.44) | (−4.16, 1.53) | .36 | R/E | −1.26(0.87) | (−2.97, 0.46) | .15 |
| BMI | 1.05(0.17) | (0.72, 1.38) | <.001*** | BMI | 0.56(0.10) | (0.36, 0.76) | <.001*** |
| STOP | 0.65(1.14) | (−1.59, 2.90) | .57 | STOP | 0.17(0.68) | (−1.18, 1.52) | .81 |
|
Night 2 Systolic BP c |
Night 2 Diastolic BP d |
||||||
| Night 2 SDur | −0.06(0.02) | (−0.09, −0.02) | .005** | Night 2 SDur | −0.03(0.01) | (−0.05, −0.01) | .015* |
| Night 2 SEff | −0.10(0.13) | (−0.36, 0.16) | .46 | Night 2 SEff | −0.08(0.08) | (−0.23, 0.08) | .31 |
| Gender | −5.82(3.01) | (−11.76, 0.11) | .054 | Gender | −1.16(1.79) | (−4.69, 2.36) | .51 |
| Age | 0.10(0.12) | (−0.13, 0.32) | .41 | Age | 0.08(0.07) | (−0.06, 0.21) | .27 |
| R/E | −0.37(2.05) | (−4.40, 3.66) | .86 | R/E | −0.35(1.20) | (−2.70, 2.01) | .77 |
| BMI | 0.87(0.23) | (0.41, 1.32) | <.001*** | BMI | 0.52(0.14) | (0.25, 0.79) | <.001*** |
| STOP | 2.20(1.52) | (−0.81, 5.20) | .15 | STOP | 1.38(0.90) | (−0.40, 3.17) | .13 |
|
Day 2 Avg Systolic BP e |
Day 2 Avg
Diastolic BP f |
||||||
| Night 1 SDur | −0.01(0.02) | (−0.04, 0.02) | .34 | Night 1 SDur | −0.012(0.009) | (−0.03, 0.006) | .19 |
| Night 1 SEff | −0.47(0.12) | (−0.71, −0.23) | <.001*** | Night 1 SEff | −0.13(0.07) | (−0.28, 0.02) | .082 |
| Gender | −8.06(2.41) | (−12.81, −3.31) | <.001*** | Gender | −3.92(1.46) | (−6.79, −1.04) | .008** |
| Age | 0.34(0.09) | (0.15, 0.53) | <.001*** | Age | 0.14(0.06) | (0.03, 0.25) | .016* |
| R/E | −0.13(1.61) | (−3.29, 3.04) | .94 | R/E | −0.69(0.98) | (−2.61, 1.24) | .48 |
| BMI | 0.98(0.18) | (0.62, 1.35) | <.001*** | BMI | 0.53(0.11) | (0.31, 0.75) | <.001*** |
| STOP | 1.02(1.26) | (−1.47, 3.51) | .42 | STOP | 0.04(0.77) | (−1.47, 1.55) | .96 |
Note.
: N=237, Adjusted R2=0.26, Δ R2=0.14;
: N=237, Adjusted R2=0.17, Δ R2=0.11;
: N=218, Adjusted R2=0.18, Δ R2=0.07;
: N=218, Adjusted R2=0.15, Δ R2=0.076;
: N=235, Adjusted R2=0.24, Δ R2=0.11;
: N=237, Adjusted R2=0.15, Δ R2=0.079. The reference is a 55 year-old non-Hispanic White woman with a low BMI (17.6 kg/m), no sleep apnea risk, an average Sleep Duration of 7.1 hours and an average Sleep Efficiency of 87.5%.
=p<.05;
=p<.01;
=p<.001. Avg.= Average. SDur= Sleep Duration. SEff= Sleep Efficiency. R/E= Combined Race and Ethnicity. BMI= Body Mass Index. STOP= sleep apnea risk score. Partially adjusted model adjusted for gender age, and race/ethnicity; Fully adjusted model additionally adjusted for body mass index and sleep apnea risk score. All adjusted values are those after removal of outliers.
Association between average sleep duration and efficiency and daytime blood pressure.
First, we tested the relationships between sleep duration and efficiency, each averaged across the two study days, and daytime BP, also averaged across the two study days. Unadjusted analyses revealed that when entered together, neither average sleep duration nor efficiency was associated with average daytime systolic or diastolic BP, all Fs (2,247)<4.74; p>.05. However, the association between lower sleep efficiency and higher systolic BP was significant after partial (F(5,240)=7.81, p=.008, R2=0.12) and full adjustment (F(7,228)=12.83, p=.012, R2=0.26) (Table 5) with all changes in R2 significant. Sleep duration remained unassociated with BP after partial and full adjustment.
Association between sleep duration and efficiency and same night blood pressure.
Unadjusted analyses revealed that lower sleep duration (systolic: F(2,229)=11.46, p=.006, R2=0.083; diastolic: F(2,229)=10.58, p=.004, R2=0.077), but not sleep efficiency (systolic: F(2,229)=11.46, p=.30, R2=0.083; diastolic: F(2,229)=10.58, p=.49, R2=0.077), was significantly associated with higher average systolic and diastolic BP on the same night. The association between lower sleep duration and nighttime BP remained significant after both partial (systolic: F(5,222)=6.30, p=.006, R2=0.11; diastolic: F(5,221)=4.613, p=.004, R2=0.074) and full adjustment (systolic: F(7,210)=7.71, p=.0049, R2=0.18); diastolic: F(7,210)=6.60, p=.015, R2=0.15), with full adjustment accounting for up to 18% and 15% of the variance in systolic and diastolic BP, respectively (Table 5). Only the change in R2 from the partially to fully adjusted model was significant. 1
Next, we examined the associations between both objectively measured sleep duration and efficiency, and asleep BP. Under these more specific conditions, the preceding effects again largely disappear for both sleep duration and efficiency, all Fs (7,199)>2.65, p=ns.
Association between sleep duration and efficiency and next day blood pressure.
Unadjusted analyses revealed that lower sleep efficiency but not sleep duration was associated with higher systolic BP during the following day, F(2,250)=5.86, p=.014, R2=0.037. This relationship remained significant after partial (F(5,242)=8.42, p=.001, R2=0.13) and full adjustment (F(7,227)=11.35, p<.001, R2=0.24) (Table 5) with all changes in R2 significant. No other significant relationships were found.
Discussion
This study examined the association of sleep duration and sleep efficiency, respectively, with day and nighttime BP in a diverse community sample of adults. The key findings were that sleep duration and efficiency, when assessed separately, mirrored each other in their associations with day and nighttime BP. Namely, sleep inadequacy, in the form of lower sleep duration or lower sleep efficiency, was associated with higher daytime systolic BP, higher nighttime systolic and diastolic BP, and temporally associated with higher BP the next day. When sleep duration and efficiency were assessed together in the same model, unique patterns emerged; lower sleep duration was associated with higher nighttime systolic and diastolic BP, whereas lower sleep efficiency was associated on average and temporally with higher daytime systolic BP. However, these associations did not hold when we examined the more specific asleep BP. Together, these findings advance the science of sleep and blood pressure with implications for the epidemiology of sleep and CVD risk.
Increasingly robust data supports sleep as an important behavioral moderator of CVD risk. The current work helps to identify one potential psychophysiological pathway that may help to explain the epidemiological observations. In fact, blood pressure and blunted dipping status are broadly accepted risk determinants (56,57). Here we show that a good or bad night’s sleep may predict BP on the following day. In addition, the current work suggests that the level of specificity in how “nighttime” BP is measured must be considered, as such a shift in characterization has a strong impact on results. Here we show evidence that sleep is associated with nighttime BP but not asleep BP. This difference may have implications for how self-report sleep and nighttime BP is viewed. In addition, some caution may be warranted in discarding nighttime BP as it may still reflect a valid ecological experience of nighttime activity which may include periods of sleep and wakefulness for a significant minority of the population.
Despite these qualifications, the current work posits that the effects of sleep inadequacy on BP operate on a continuum and are not exclusively in the context of clinical syndromes such as insomnia or apnea. Here we show that natural variations in actigraphy-assessed sleep are associated with BP. Finally, the current study speaks to the ecological validity of these relationships in daily life. The focus here is on the pathway and not on individual differences in the frequency of the activation. However, we could hypothesize that chronic perturbations in sleep inadequacy (duration, efficiency, or both) would likely account, in part, for such differences.
Methodologically, this study is amongst the first to report on objectively assessed sleep characteristics and concurrent ambulatory blood pressure in a diverse community sample which may have important implications for the associated literature. Previous reviews and meta-analyses on the relationship between sleep duration and BP have yielded mixed results (58), with some positing a relationship between short sleep duration and high BP or hypertension (59,60) and others refuting this claim (61). The results of the present study may help to explain this heterogeneity in the literature. In the present study, the association between daytime BP and sleep duration was dependent upon whether sleep efficiency was considered in the same model. Thus, when sleep efficiency was not considered, sleep duration was associated with daytime and nighttime BP. This finding is relatively consistent with the current literature that often posits a relationship between short sleep duration and high BP (62) in the absence of an index of sleep continuity (i.e., sleep efficiency or insomnia).
The current study may also help to clarify seemingly mixed evidence in the literature. Specifically, several recent studies report mixed results regarding associations between sleep duration and BP or hypertension; some reporting associations between lower duration and higher daytime BP and some not (58,63). A key methodological difference between these studies appears to be treatment of sleep continuity variables. The majority of studies that control for either insomnia or sleep efficiency do not find a significant relationship between sleep duration and hypertension or BP. Studies that do not account for some index of sleep continuity such as insomnia or sleep efficiency generally report a significant relationship between sleep duration and BP whereas those that do account for continuity largely do not find such a relationship. The current findings support this possibility by demonstrating that when examined separately, duration is associated with BP but when sleep efficiency is added in a competing model (vs. simply covarying) it becomes the dominant factor in daytime BP and duration results are nullified. Hence, the literature may not be discrepant but rather in need of a more contextualized explanation. More broadly, the current findings suggest that these sleep parameters exert unique influence despite their high collinearity.
We would note a few key limitations of the current study. First, the data collection time was limited to 2 days/1 night and thus, the data may not prove representative of broader sleep patterns or be as stable as a longer data sampling window might yield. Future work should attempt to replicate these findings over a longer period of time and perhaps at different time periods. Second, despite our efforts, the ambulatory BP cuff may have perturbed sleep quality for some participants resulting in artificially inflated readings that may not be a true ecologically representation of normal sleep/BP patterns. In order to get a sense of this, we assessed whether sleep one night (without ABPM) was different from the next night (with ABPM). Sleep efficiency did not significantly differ one night to the next (p=0.21), but night time awakenings did (p=0.04). It is difficult to tell whether awakenings differed because of the ABPM. Third, the association between lower sleep duration and higher nighttime BP may be an artifact of higher arousal/wakefulness during the time at which nighttime BP was collected (12am-5am). To confirm this, future studies should map ABP onto patient-specific actigraphic data to confirm each individual’s unique “asleep” vs. “waking” BP readings. Fourth, we used an abbreviated form of the STOPBANG questionnaire to capture sleep apnea risk. This measure was included in fully adjusted models, but was not associated with BP. This may indicate that the abbreviated STOP questionnaire is not worth using as a meaningful sleep apnea risk indicator. Fifth, although we have a racially/ethnically diverse sample, we examined the relationships across the whole sample which may omit group differences. There is evidence to suggest there are racial/ethnic differences in sleep disturbance (64) and in the relationship between sleep and cardiovascular disease (65). Studies powered to look at those relationships should be conducted to elucidate those trends. Hence, this study should be viewed as conceptually consistent with expectations but in need of replication and extension. Finally, as is common in ecological sampling research, unknown/daily life factors occurring at random across participants may have influenced the individual data.
Despite these limitations, the present findings help to clarify the relationship between both sleep duration, sleep efficiency, and BP and support the sleep-BP relationship as a plausible risk pathway in CVD. Future work should examine 1) this relationship with subjective indices of sleep, and 2) how stable these relationships are over time in both representative samples and in clinical populations particularly as it may influence CVD morbidity and outcomes.
Acknowledgments
Source of Funding: National Heart, Lung, and Blood Institute
Footnotes
Conflicts of Interest: None
We also examined whether end-of-day survey questions about stress predicted BP. These items were not significantly associated with BP (p’s>0.2) at full adjustment, and were not included in final models.
References
- 1.Badran M, Yassin BA, Fox N, Laher I, Ayas N. Epidemiology of Sleep Disturbances and Cardiovascular Consequences. Canadian Journal of Cardiology [Internet]. 2015. [cited 2017 July 26];31:873–79. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26037823 [DOI] [PubMed] [Google Scholar]
- 2.Javaheri S, Redline S. Insomnia and Risk of Cardiovascular Disease. Chest [Internet]. 2017. [cited 2017 August 16];152:435–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28153671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Ouyang Y, Wang Z, Zhao G, Liu L, Bi Y. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. International journal of cardiology [Internet]. 2013. [cited 2017 August 16];169:207–14. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0167527313016719 [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Zhang X, Winkelman JW, Redline S, Hu FB, Stampfer M, Ma J, Gao X. Association between insomnia symptoms and mortality: a prospective study of U.S. men. Circulation [Internet]. 2014. [cited 2017 August 16];129:737–46. Available from: http://circ.ahajournals.org/cgi/doi/10.1161/CIRCULATIONAHA.113.004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Covassin N, Singh P. Sleep Duration and Cardiovascular Disease Risk. Sleep Medicine Clinics [Internet]. 2016. [cited 2016 December 7];11:81–89. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26972035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aziz M, Ali SS, Das S, Younus A, Malik R, Latif MA, Humayun C, Anugula D, Abbas G, Salami J, Elizondo JV, Veledar E, Nasir K. Association of Subjective and Objective Sleep Duration as well as Sleep Quality with Non-Invasive Markers of Sub-Clinical Cardiovascular Disease (CVD): A Systematic Review. Journal of atherosclerosis and thrombosis [Internet]. 2017. [cited 2017 August 3];24:208–26. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27840384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St-Onge M-P, Michael Grandner CA, Brown D, Conroy MB, Jean-Louis G, Coons M, Bhatt DL. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement From the American Heart Association. [cited 2017. September 26]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5567876/pdf/nihms891245.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth T Insomnia: definition, prevalence, etiology, and consequences. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine [Internet]. 2007. [cited 2016 December 8];3:S7–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17824495 [PMC free article] [PubMed] [Google Scholar]
- 9.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine [Internet]. 2008. [cited 2017 November 1];4:487–504. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18853708 [PMC free article] [PubMed] [Google Scholar]
- 10.Ekstedt M, Åkerstedt T, Söderström M. Microarousals During Sleep Are Associated With Increased Levels of Lipids, Cortisol, and Blood Pressure. Psychosomatic Medicine [Internet]. 2004. [cited 2017 September 28];66:925–31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15564359 [DOI] [PubMed] [Google Scholar]
- 11.Cintra F, Bittencourt LRA, Santos-Silva R, Andersen M, de Paola A, Poyares D, Tufik S. The association between the Framingham risk score and sleep: A São Paulo epidemiological sleep study. Sleep Medicine [Internet]. 2012. [cited 2016 December 7];13:577–82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22516609 [DOI] [PubMed] [Google Scholar]
- 12.Friedman O, Bradley TD, Ruttanaumpawan P, Logan AG. Independent association of drug-resistant hypertension to reduced sleep duration and efficiency. American journal of hypertension [Internet]. 2010. [cited 2017 September 26];23:174–79. Available from: https://academic.oup.com/ajh/article-lookup/doi/10.1038/ajh.2009.220 [DOI] [PubMed] [Google Scholar]
- 13.Hale L, Parente V, Dowd JB, Sands M, Berger JS, Song Y, Martin LW, Allison MA. Fibrinogen may mediate the association between long sleep duration and coronary heart disease. Journal of Sleep Research [Internet]. 2013. [cited 2017 July 24];22:305–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23217092 [DOI] [PubMed] [Google Scholar]
- 14.Castro-Diehl C, Diez Roux AV, Redline S, Seeman T, McKinley P, Sloan R, Shea S. Sleep Duration and Quality in Relation to Autonomic Nervous System Measures: The Multi-Ethnic Study of Atherosclerosis (MESA). SLEEP [Internet]. 2016. [cited 2016 December 7];39:1927–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27568797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lichtenstein MJ, Shipley MJ, Rose G. Systolic and diastolic blood pressures as predictors of coronary heart disease mortality in the Whitehall study. British medical journal (Clinical research ed.) [Internet]. 1985. [cited 2017 June 14];291:243–45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3926137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, McManus RJ. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ (Clinical research ed.) [Internet]. 2016. [cited 2017 June 14];354:i4098 Available from: http://www.ncbi.nlm.nih.gov/pubmed/27511067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pointer MA, Yancey S, Abou-Chacra R, Petrusi P, Waters SJ, McClelland MK. State Anxiety Is Associated with Cardiovascular Reactivity in Young, Healthy African Americans. International Journal of Hypertension [Internet]. 2012. [cited 2017 September 28];2012:1–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22263105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jennings JR, Kamarck TW, Everson-Rose SA, Kaplan GA, Manuck SB, Salonen JT. Exaggerated Blood Pressure Responses During Mental Stress Are Prospectively Related to Enhanced Carotid Atherosclerosis in Middle-Aged Finnish Men. Circulation [Internet]. 2004. [cited 2017 September 28];110:2198–2203. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15451789 [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Liu C, Tian X, Zou G, Li G, Kong L, Li P. Associations of Perceived Stress, Resilience and Social Support with Sleep Disturbance Among Community-dwelling Adults. Stress and Health [Internet]. 2016. [cited 2019 February 10];32:578–86. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26669814 [DOI] [PubMed] [Google Scholar]
- 20.Palagini L, Bruno RM, Cheng P, Mauri M, Taddei S, Ghiadoni L, Drake CL, Morin CM. Relationship between insomnia symptoms, perceived stress and coping strategies in subjects with arterial hypertension: psychological factors may play a modulating role. Sleep Medicine [Internet]. 2016. [cited 2019 February 10];19:108–15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27198955 [DOI] [PubMed] [Google Scholar]
- 21.McEwen BS. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiological Reviews [Internet]. 2007. [cited 2019 February 10];87:873–904. Available from: http://www.physiology.org/doi/10.1152/physrev.00041.2006 [DOI] [PubMed] [Google Scholar]
- 22.Vgontzas AN, Bixler EO, Lin H-M, Prolo P, Mastorakos G, Vela-Bueno A, Kales A, Chrousos GP. Chronic Insomnia Is Associated with Nyctohemeral Activation of the Hypothalamic-Pituitary-Adrenal Axis: Clinical Implications. The Journal of Clinical Endocrinology & Metabolism [Internet]. 2001. [cited 2019 February 10];86:3787–94. Available from: https://academic.oup.com/jcem/article-lookup/doi/10.1210/jcem.86.8.7778 [DOI] [PubMed] [Google Scholar]
- 23.Kelly JJ, Mangos G, Williamson PM, Whitworth JA. Cortisol and hypertension. Clinical and experimental pharmacology & physiology. Supplement [Internet]. 1998. [cited 2019 February 10];25:S51–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9809193 [DOI] [PubMed] [Google Scholar]
- 24.Whitworth JA, Williamson PM, Mangos G, Kelly JJ. Cardiovascular consequences of cortisol excess. Vascular health and risk management [Internet]. 2005. [cited 2019 February 10];1:291–99. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17315601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Vgontzas AN, Fernandez-Mendoza J, Bixler EO, Sun Y, Zhou J, Ren R, Li T, Tang X. Insomnia With Physiological Hyperarousal Is Associated With Hypertension. Hypertension [Internet]. 2015. [cited 2019 February 10];65:644–50. Available from: https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.114.04604 [DOI] [PubMed] [Google Scholar]
- 26.Krantz DS, Manuck SB. Acute psychophysiologic reactivity and risk of cardiovascular disease: A review and methodologic critique. Psychological Bulletin [Internet]. 1984. [cited 2019 February 10];96:435–64. Available from: http://doi.apa.org/getdoi.cfm?doi=10.1037/0033-2909.96.3.435 [PubMed] [Google Scholar]
- 27.MorrellL MJ, Finn L, Kim H, Peppard PE, Safwan Badr M, Young T. Sleep Fragmentation, Awake Blood Pressure, and Sleep-Disordered Breathing in a Population-based Study. American Journal of Respiratory and Critical Care Medicine [Internet]. 2000. [cited 2017 September 28];162:2091–96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11112120 [DOI] [PubMed] [Google Scholar]
- 28.Fung MM, Peters K, Ancoli-Israel S, Redline S, Stone KL, Barrett-Connor E, Osteoporotic Fractures in Men (MrOS) Research Group. Total Sleep Time and Other Sleep Characteristics Measured by Actigraphy Do Not Predict Incident Hypertension in a Cohort of Community-Dwelling Older Men. Journal of Clinical Sleep Medicine [Internet]. 2013. [cited 2017 September 28];9:585–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23772192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chouchou F, Pichot V, Pépin JL, Tamisier R, Celle S, Maudoux D, Garcin A, Lévy P, Barthélémy JC, Roche F, PROOF Study Group. Sympathetic overactivity due to sleep fragmentation is associated with elevated diurnal systolic blood pressure in healthy elderly subjects: the PROOF-SYNAPSE study. European Heart Journal [Internet]. 2013. [cited 2017 October 5];34:2122–31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23756334 [DOI] [PubMed] [Google Scholar]
- 30.Bruno RM, Palagini L, Gemignani A, Virdis A, Di Giulio A, Ghiadoni L, Riemann D, Taddei S. Poor sleep quality and resistant hypertension. Sleep Medicine. 2013; 14:1157–63. [DOI] [PubMed] [Google Scholar]
- 31.Corbalán-Tutau MD, Madrid JA, Garaulet M. Timing and duration of sleep and meals in obese and normal weight women. Association with increase blood pressure. Appetite [Internet]. 2012. [cited 2016 December 7];59:9–16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22450522 [DOI] [PubMed] [Google Scholar]
- 32.Hughes J, Kobayashi I, Deichert N. Ethnic Differences in Sleep Quality Accompany Ethnic Differences in Night-time Blood Pressure Dipping. American Journal of Hypertension [Internet]. 2007. [cited 2016 December 7];20:1104–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17903695 [DOI] [PubMed] [Google Scholar]
- 33.Dean DA, Wang R, Jacobs DR, Duprez D, Punjabi NM, Zee PC, Shea S, Watson K, Redline S. A Systematic Assessment of the Association of Polysomnographic Indices with Blood Pressure: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep [Internet]. 2015. [cited 2017 September 28];38:587–96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25348124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep Quality and Elevated Blood Pressure in Adolescents. Circulation [Internet]. 2008. [cited 2016 December 7];118:1034–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18711015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Au CT, Ho CKW, Wing YK, Lam HS, Li AM. Acute and Chronic Effects of Sleep Duration on Blood Pressure. Pediatrics. 2014;133. [DOI] [PubMed] [Google Scholar]
- 36.Mezick EJ, Hall M, Matthews KA. Sleep Duration and Ambulatory Blood Pressure in Black and White Adolescents. Hypertension [Internet]. 2012. [cited 2018 August 10];59:747–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22275538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz JM, Taylor DJ, Uchino BN, Smith TW, Allison M, Ahn C, Johnson JJ, Smyth JM. Evaluating the longitudinal risk of social vigilance on atherosclerosis: study protocol for the North Texas Heart Study. BMJ Open [Internet]. 2017. [cited 2017 October 14];7:e017345 Available from: http://www.ncbi.nlm.nih.gov/pubmed/28808040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ancoli-Israel S, Martin JL, Blackwell T, Buenaver L, Liu L, Meltzer LJ, Sadeh A, Spira AP, Taylor DJ. The SBSM Guide to Actigraphy Monitoring: Clinical and Research Applications. Behavioral Sleep Medicine [Internet]. 2015. [cited 2019 March 12];13:S4–38. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26273913 [DOI] [PubMed] [Google Scholar]
- 39.Scott SB, Sliwinski MJ, Zawadzki M, Stawski RS, Kim J, Marcusson-Clavertz D, Lanza ST, Conroy DE, Buxton O, Almeida DM, Smyth JM. A Coordinated Analysis of Variance in Affect in Daily Life. Assessment [Internet]. 2018. [cited 2019 March 12];107319111879946 Available from: http://www.ncbi.nlm.nih.gov/pubmed/30198310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchino BN, Smith TW, Carlisle M, Birmingham WC, Light KC. The Quality of Spouses’ Social Networks Contributes to Each Other’s Cardiovascular Risk Preis T, editor. PLoS ONE [Internet]. 2013. [cited 2019 March 12];8:e71881 Available from: http://www.ncbi.nlm.nih.gov/pubmed/23990999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, Morin CM. The Consensus Sleep Diary: Standardizing Prospective Sleep Self-Monitoring. Sleep [Internet]. 2012. [cited 2018 February 4];35:287–302. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22294820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meier E, Miller MB, Lombardi N, Leffingwell T. Assessment reactivity: A randomized controlled trial of alcohol-specific measures on alcohol-related behaviors. Addictive Behaviors [Internet]. 2017. [cited 2019 March 12];67:44–48. Available from: https://www.sciencedirect.com/science/article/pii/S0306460316304154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song M-K, Ward SE. Assessment Effects in Educational and Psychosocial Intervention Trials: An Important but Often-Overlooked Problem. Research in Nursing & Health [Internet]. 2015. [cited 2019 March 12];38:241–47. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25728502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Philips Respironics ∣ Clinical monitoring devices. [cited 2018. September 6]. Available from: http://www.actigraphy.com/solutions/ [Google Scholar]
- 45.Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, Khajehdehi A, Shapiro CM. STOP Questionnaire. Anesthesiology [Internet]. 2008. [cited 2018 February 4];108:812–21. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18431116 [DOI] [PubMed] [Google Scholar]
- 46.Oscar 2 Ambulatory Blood Pressure Monitor - SunTech Medical. [cited 2018. September 6]. Available from: https://www.suntechmed.com/bp-products/ambulatory-blood-pressure-monitoring/oscar-2-ambulatory-blood-pressure-monitor [Google Scholar]
- 47.Bowen KS, Uchino BN, Birmingham W, Carlisle M, Smith TW, Light KC. The stress-buffering effects of functional social support on ambulatory blood pressure. Health Psychology [Internet]. 2014. [cited 2019 March 22];33:1440–43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24245843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanbonmatsu DM, Uchino BN, Birmingham W. On the importance of knowing your partner’s views: attitude familiarity is associated with better interpersonal functioning and lower ambulatory blood pressure in daily life. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine [Internet]. 2011. [cited 2019 March 22];41:131–37. Available from: https://academic.oup.com/abm/article/41/1/131-137/4569550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cundiff JM, Birmingham WC, Uchino BN, Smith TW. Marital Quality Buffers the Association Between Socioeconomic Status and Ambulatory Blood Pressure. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine [Internet]. 2016. [cited 2019 March 22];50:330–35. Available from: https://academic.oup.com/abm/article/50/2/330-335/4296054 [DOI] [PubMed] [Google Scholar]
- 50.Uchino BN, Smith TW, Carlisle M, Birmingham WC, Light KC. The quality of spouses’ social networks contributes to each other’s cardiovascular risk Preis T, editor. PloS one [Internet]. 2013. [cited 2019 March 22];8:e71881 Available from: https://dx.plos.org/10.1371/journal.pone.0071881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marler MR, Jacob RG, Lehoczky JP, Shapiro AP. The statistical analysis of treatment effects in 24-hour ambulatory blood pressure recordings. Statistics in medicine [Internet]. 1988. [cited 2018 February 4];7:697–716. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3406600 [DOI] [PubMed] [Google Scholar]
- 52.Booth JN, Anstey DE, Bello NA, Jaeger BC, Pugliese DN, Thomas SJ, Deng L, Shikany JM, Lloyd‐Jones D, Schwartz JE, Lewis CE, Shimbo D, Muntner P. Race and sex differences in asleep blood pressure: The Coronary Artery Risk Development in Young Adults (CARDIA) study. The Journal of Clinical Hypertension [Internet]. 2019. [cited 2019 March 22];21:jch.13474. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30719843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory Blood Pressure Monitoring and Risk of Cardiovascular Disease: A Population Based Study. 2006. [cited 2018 September 21]; Available from: https://academic.oup.com/ajh/article-abstract/19/3/243/251714 [DOI] [PubMed] [Google Scholar]
- 54.Ustinov Y, Lichstein KL. Actigraphy Reliability with Normal Sleepers. Behavioral Sleep Medicine [Internet]. 2013. [cited 2019 February 7];11:313–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23268697 [DOI] [PubMed] [Google Scholar]
- 55.Little RJA. Regression With Missing X’s: A Review. Journal of the American Statistical Association [Internet]. 1992. [cited 2019 March 22];87:1227 Available from: https://www.jstor.org/stable/2290664?origin=crossref [Google Scholar]
- 56.Sherwood A, Bower JK, Routledge FS, Blumenthal JA, McFetridge-Durdle JA, Newby LK, Hinderliter AL. Nighttime blood pressure dipping in postmenopausal women with coronary heart disease. American journal of hypertension [Internet]. 2012. [cited 2017 June 12];25:1077–82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22785406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, Williams B, Hingorani A, Hemingway H. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. The Lancet [Internet]. 2014. [cited 2019 February 8];383:1899–1911. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24881994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bathgate CJ, Fernandez-Mendoza J. Insomnia, Short Sleep Duration, and High Blood Pressure: Recent Evidence and Future Directions for the Prevention and Management of Hypertension. Current Hypertension Reports [Internet]. 2018. [cited 2018 August 20];20:52 Available from: http://link.springer.com/10.1007/s11906-018-0850-6 [DOI] [PubMed] [Google Scholar]
- 59.Guo X, Zheng L, Wang J, Zhang X, Zhang X, Li J, Sun Y. Epidemiological evidence for the link between sleep duration and high blood pressure: A systematic review and meta-analysis. Sleep Medicine [Internet]. 2013. [cited 2018 August 20];14:324–32. Available from: https://www.sciencedirect.com/science/article/pii/S1389945712004443 [DOI] [PubMed] [Google Scholar]
- 60.Gangwisch JE. A Review of Evidence for the Link Between Sleep Duration and Hypertension. American Journal of Hypertension [Internet]. 2014. [cited 2018 February 6];27:1235–42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24778107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Q, Xi B, Liu M, Zhang Y, Fu M. Short sleep duration is associated with hypertension risk among adults: a systematic review and meta-analysis. Hypertension Research [Internet]. 2012. [cited 2018 March 8];35:1012–18. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22763475 [DOI] [PubMed] [Google Scholar]
- 62.Grandner M, Mullington JM, Hashmi SD, Redeker NS, Watson NF, Morgenthaler TI. Sleep Duration and Hypertension: Analysis of > 700,000 Adults by Age and Sex. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine [Internet]. 2018. [cited 2018 August 21];14:1031–39. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29852916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo J, Fei Y, Li J, Zhang L, Luo Q, Chen G. Gender- and age-specific associations between sleep duration and prevalent hypertension in middle-aged and elderly Chinese: a cross-sectional study from CHARLS 2011–2012. BMJ Open [Internet]. 2016. [cited 2018 August 24];6:e011770 Available from: http://bmjopen.bmj.com/lookup/doi/10.1136/bmjopen-2016-011770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hicken MT, Lee H, Ailshire J, Burgard SA, Williams DR. “Every shut eye, ain’t sleep”: The role of racism-related vigilance in racial/ethnic disparities in sleep difficulty. Race and social problems [Internet]. 2013. [cited 2017 July 22];5:100–112. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23894254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalmbach DA, Pillai V, Arnedt JT, Drake CL. DSM-5 Insomnia and Short Sleep: Comorbidity Landscape and Racial Disparities. SLEEP [Internet]. 2016. [cited 2018 March 16];39:2101–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27634805 [DOI] [PMC free article] [PubMed] [Google Scholar]