Abstract
BACKGROUND:
Up to half of patients with oral cavity squamous cell carcinoma (OCSCC) have stage I to II disease. When adequate resection is attained, no further treatment is needed; however, re-resection or radiotherapy may be indicated for patients with positive or close margins. This multicenter study evaluated the outcomes and role of adjuvant treatment in patients with stage I to II OCSCC.
METHODS:
Overall survival (OS), disease-specific survival, local-free survival, and disease-free survival rates were calculated with Kaplan-Meier analysis.
RESULTS:
Of 1257 patients with T1–2N0M0 disease, 33 (2.6%) had positive margins, and 205 (16.3%) had close margins. The 5-year OS rate was 80% for patients with clear margins, 52% for patients with close margins, and 63% for patients with positive margins (P <.0001). In a multivariate analysis, age, depth of invasion, and margins were independent predictors of outcome. Close margins were associated with a >2-fold increase in the risk of recurrence (P <.0001). The multivariate analysis revealed that adjuvant treatment significantly improved the outcomes of patients with close/positive margins (P =.002 to .03).
CONCLUSIONS:
Patients with stage I to II OCSCC and positive/close margins have poor long-term outcomes. For this population, adjuvant treatment may be associated with improved survival.
Keywords: margins, oral cavity, squamous cell carcinoma, survival
INTRODUCTION
Oral cavity squamous cell carcinoma (OCSCC) is among the common malignant tumors worldwide, with an estimated 300,000 new cases per year.1 Surgery is the primary treatment modality for stage I to II OCSCC, whereas adjuvant treatment is indicated for advanced disease (stages III-IV).2 Although patients with T1–2N0M0 OCSCC can be treated with surgery alone, those with positive and close margins may benefit from adjunctive treatment.3,4 It has been suggested that adequate resection of OCSCC requires at least 5-mm margins,5 although smaller margins have also been suggested.6,7 Because of the complexity of these tumors, clear margins are achieved in only 50% to 80% of patients treated at cancer centers.8–12 Hence, many patients with early OCSCC undergo inadequate surgery.
Surprisingly, although the margin status is a key factor in determining outcomes, there is no consensus regarding the best treatment for patients with stage I to II OCSCC who have positive or close margins.13 Because adjuvant therapy may induce severe toxic effects, a considerable challenge is the determination of a reliable method for stratifying patients with early OCSCC for whom adjuvant treatment may be beneficial.
In this multicenter, international study, we evaluated patients with a T1–2 OCSCC classification and negative nodal metastases. We report the clinical significance of the margin status in this population and the effect of adjuvant treatment on outcomes.
MATERIALS AND METHODS
Patients
The cohort was composed of patients from several cancer centers worldwide who were treated for OCSCC with primary surgery, with or without adjuvant chemoradiotherapy, between 1970 and 2011. Oral cavity subsites included the following: lip, oral tongue, floor of mouth, alveolar ridge, hard palate, and buccal mucosa. Clinical data from the contributing centers are presented in Supporting Table 1. The study was approved by the local institutional review board committees. All patients underwent local tumor resection with a standardized neck dissection involving levels I to III, I to IV, or I to V, as described by the American Head and Neck Society.14 The type of neck dissection was specified in all patients before the operation. The data were double-checked at each center by 2 researchers independently. In addition, 2 different investigators (M.A. and E.F.) adjudicated the complete cohort from all centers. To determine the presence of between-center heterogeneity, we performed a 2-stage random effects model15,16 with the complete data set. We used univariate analyses because of the small number of patients and events at most institutions. In the second stage, the center-specific effect estimates were introduced into the random effects model of DerSimonian and Laird,17 which allows for unexplained sources of heterogeneity between centers; .1 was considered statistically significant because the test had limited power and was quantified with the I2 measure (the percentage of total variation across centers attributable to heterogeneity rather than chance).18 We did not find significant institutional heterogeneity for locoregional failure, disease-specific survival (DSS), or overall survival (OS; I2 = 0%; P > .7). Similarly, a 2-stage random effects model with the full database failed to show any evidence for heterogeneity between centers, and this suggested that the finding was robust and reliable.16
Histopathological Analysis
All specimens were evaluated by certified head and neck pathologists at each center independently. Specimen dissection and tissue sampling of the primary tumor were performed in accordance with the guidelines for the histopathological assessment of cancer. Staging was performed according to the seventh edition of the American Joint Committee on Cancer system. All margin assessments (clear, positive, or close) were done on the specimen itself (tumor and margins) and not on the patient’s margins (ie, the specimen’s bed after tumor resection). Positive margins were defined as the presence of tumor cells in the resected edge of the specimen, close margins were defined as the presence of tumor cells at a distance less than 5 mm, and clear margins were defined as free margins of at least 5 mm. When a re-excision was performed, the extension of the resection was analyzed in a similar manner and was calculated with the specimen.
Statistical Methods
Rates of 5- and 10-year OS, DSS, disease-free survival (DFS), and local recurrence–free survival (LFS) were calculated with the Kaplan-Meier method, and differences in survival were assessed with the log-rank test.19 OS was measured from the date of surgery to the date of death or the last follow-up. Local recurrence was considered only if there was recurrence at the original tumor site and did not include regional (or nodal) or distant recurrence. LFS and DFS were measured from the date of surgery to the date of local recurrence or the date of first recurrence (local, regional, or distant), respectively, or the last follow-up. For DSS, patients who died of causes other than OCSCC were censored at the time of death. The variables that had prognostic potential suggested by the univariate analysis were subjected to multivariate analysis with the Cox proportional hazards regression model.15 The analysis was performed with JMP software (SAS Institute, Inc, Cary, North Carolina). We additionally analyzed LFS with the cumulative incidence function (CIF) with OS and distant metastasis–free survival as competing factors.20 Similarly, DSS was analyzed with death from other causes as a competing factor. For the competing risk model, the analysis was performed with the R program21 and the cmprsk package.22 All statistical tests were 2-sided. A P value < .5 was considered to indicate statistical significance.
RESULTS
Patients with oral cavity cancer (n = 1257) were identified with T1–2N0 disease. Of those patients, 461 had the T1 classification (36.7%), and 796 had the T2 classification (63.3%). The median follow-up time from the date of surgery was 56 months. During the follow-up, 401 patients (32%) died; 214 of them (53%) died with disease recurrence. Table 1 shows the clinical and pathological characteristics of the patients. The overall rate of positive/close margins in patients with stage I to II OCSCC was 19.3%: 16.6% (n = 205) had close margins, and 2.7% (n = 33) had positive margins. We analyzed the effect of the nodal yield on outcomes with the Kaplan-Meier method. The nodal yield was analyzed as a categorical variable (<18 vs ≥ 18) as previously suggested.23 In this cohort (stages I and II), the number of negative nodes was not associated with the outcome (OS, DSS, DFS, or LFS).
TABLE 1.
Demographic and Clinical Characteristics of T1–2N0 Patients (n = 1257)
| Variable | No. | % |
|---|---|---|
| Age (n = 1257) | 100 | |
| Mean ± SD, y | 56.1 ± 13.11 | |
| Median (range), y | 56 (0.9–93.1) | |
| Sex (n = 1254) | ||
| Male | 931 | 74.2 |
| Female | 323 | 25.8 |
| pT (n = 1257) | ||
| T1 | 461 | 36.7 |
| T2 | 796 | 63.3 |
| Surgical margins (n = 1233) | ||
| Clear (≥5 mm) | 995 | 80.7 |
| Close (<5 mm) | 205 | 16.6 |
| Positive | 33 | 2.7 |
| Adjuvant treatment (n = 1255) | ||
| None | 900 | 71.7 |
| Radiotherapy | 284 | 22.6 |
| Chemoradiotherapy | 50 | 4.0 |
| Radiotherapy and Cetuximab | 21 | 1.7 |
| Follow-up, median (range), mo | 56 (0–302) |
Abbreviations: pT, pathological T stage; SD, standard deviation.
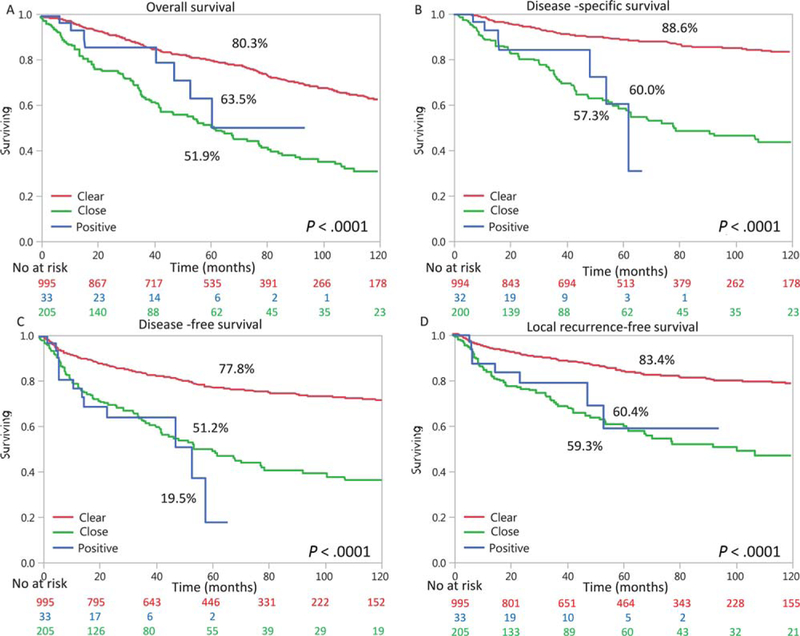
Adjuvant treatment was given to 355 patients (28%); 284 (22.6%) received postoperative radiation therapy, whereas only 71 patients (5.7%) received adjuvant chemotherapy or cetuximab in addition to radiation therapy. Radiation and chemotherapy, doses, and treatment regimens were chosen in accordance with each center’s decision and protocols accepted at that time. These protocols included radiation doses of 1.8 Gy 5 days a week for a total dose of 40 to 70 Gy according to the clinical setting. The concurrent chemotherapy used mostly was cisplatin at doses of 30 to 50 mg/m2/wk and 100 mg/m2/3 wk. In selected cases, carboplatin with or without 5-fluorouracil was used instead of cisplatin (secondary to hearing deficits and renal insufficiency). Survival outcomes of all patients with T1–2N0M0 disease are shown in Figure 1. The Kaplan-Meier estimates of 5-year OS, DSS, and DFS were 75%, 83% and 73%, respectively. At 5 years, the LFS rate was 79%. The 5-year DSS rates were 89%, 57%, and 60% for patients with clear margins, close margins, and positive margins, respectively (P < .0001). DSS was significantly better for clear margins than positive or close margins (P < .0001 for both); there was no significant difference between close and positive margins. The other outcome measures and associations with the margins status are shown in Figure 1.
Figure 1.
Outcomes of patients with T1–2N0M0 tumors according to the margin status (clear, close, or positive). The Kaplan-Meier analysis shows (A) overall survival, (B) disease-specific survival, (C) disease-free survival, and (D) local recurrence–free survival. Below the x-axis is the at-risk set, which shows attrition with time. Supporting table 2 specifies P values between groups.
The 10-year OS rates were 63% and 32% for clear and close/positive margins, respectively. Similarly, the 10-year DSS rate was 83% for patients with clear margins and 43% for those with positive or close margins. This represents an almost 2-fold difference in mortality according to the margin status.
We further analyzed data for patients with stage I OCSCC (n = 461) to examine whether inadequate tumor resection increases the risk of recurrence in this subgroup. For these patients, the variables that were associated with local recurrence in the univariate analysis were older age, depth of invasion, and margin status (Table 2). In the multivariate analysis, older age, depth of invasion, and margin status remained independent predictors of outcome. Close margins alone were associated with a more than 2-fold increase in the risk of local recurrence.
TABLE 2.
Univariate and Multivariate Analyses of Prognostic Factors for Local Recurrence in T1N0 Oral Cavity Squamous Cell Carcinoma (n = 461)
| Univariate Analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|
| Variable | No. | HR (95% CI) | P | HR (95% CI) | P |
| Sex | .134 | .124 | |||
| Male | 320 | 1 | 1 | ||
| Female | 140 | 1.39 (0.9–2.12) | 0.63 (0.33–1.13) | ||
| Missing | 1 | ||||
| Age | .0006 | .05 | |||
| <70 y | 376 | 1 | 1 | ||
| ≥70 y | 85 | 2.39 (1.48–3.76) | 1.93 (1.01–3.46) | ||
| Missing | 0 | ||||
| Treatment | .58 | .55 | |||
| Surgery | 343 | 1 | 1 | ||
| S+ADJ | 117 | 1.15 (0.69–1.87) | 0.83 (0.43–1.51) | ||
| Missing | 1 | ||||
| DOI | .0004 | .0007 | |||
| <4 mm | 133 | 1 | 1 | ||
| ≥4 mm | 245 | 2.69 (1.53–5.05) | 2.67 (1.49–5.11) | ||
| Missing | 83 | ||||
| Margins | <.0001 | .02 | |||
| Clear | 375 | 1 | 1 | ||
| Close | 68 | 3.42 (2.17–5.28) | 2.24 (1.22–3.90) | ||
| Positive | 14 | 5.6 (1.94–12.91) | 3.4 × 10−9 (0–3.24) | ||
| Missing | 4 | ||||
Abbreviations: CI, confidence interval; DOI, depth of invasion; HR, hazard ratio; S+ADJ, surgery and adjuvant treatment.
For patients with the T2N0M0 classification, the margin status was the only independent predictor of local recurrence (Table 3). Similarly to stage I cancer, the risk of local recurrence was more than 2-fold higher in patients with close margins versus clear margins (P < .0001).
TABLE 3.
Univariate and Multivariate Analyses of Prognostic Factors for Local Recurrence in T2N0 Oral Cavity Squamous Cell Carcinoma (n = 796)
| Univariate Analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|
| Variable | No. | HR (95% CI) | P | HR (95% CI) | P |
| Sex | .5157 | .41 | |||
| Male | 611 | 1 | 1 | ||
| Female | 183 | 1.13 (0.77–1.61) | 0.81 (0.49–1.3) | ||
| Missing | 2 | ||||
| Age | .243 | .66 | |||
| <70 y | 673 | 1 | 1 | ||
| ≥70 y | 123 | 1.29 (0.83–1.94) | 1.13 (0.64–1.86) | ||
| Missing | 0 | ||||
| Treatment | .63 | .23 | |||
| Surgery | 557 | 1 | 1 | ||
| S+ADJ | 238 | 0.92 (0.63–1.3) | 0.76 (0.48–1.18) | ||
| Missing | 1 | ||||
| DOI | .23 | .17 | |||
| <4 mm | 123 | 1 | 1 | ||
| ≥4 mm | 547 | 1.32 (0.85–2.17) | 1.37 (0.87–2.26) | ||
| Missing | 126 | ||||
| Margins | <.0001 | <.0001 | |||
| Clear | 620 | 1 | 1 | ||
| Close | 137 | 2.88 (2.03–4.04) | 2.91 (1.88–4.38) | ||
| Positive | 19 | 1.33 (0.33–3.54) | 1.53 (0.25–5.05) | ||
| Missing | 20 | ||||
Abbreviations: CI, confidence interval; DOI, depth of invasion; HR, hazard ratio; S+ADJ, surgery and adjuvant treatment.
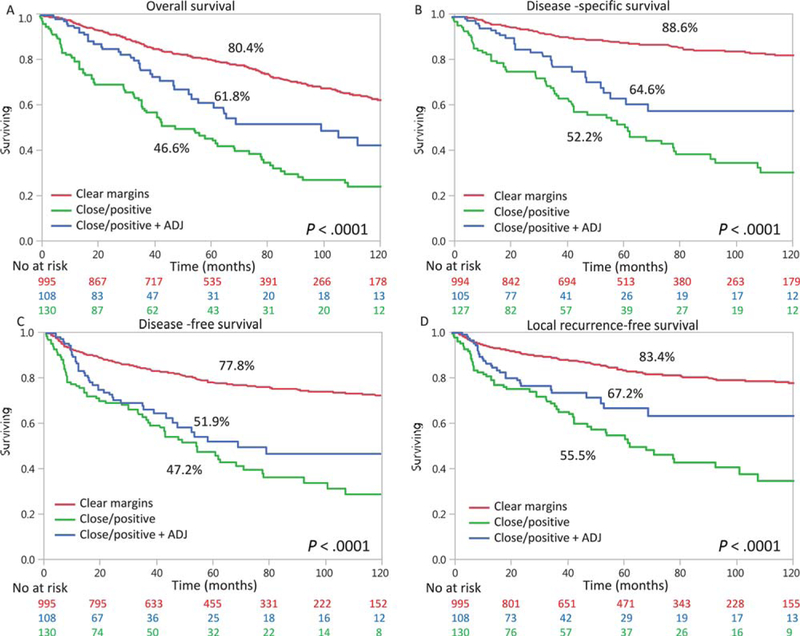
Finally, we investigated whether adjuvant treatment could improve the outcomes of patients with stage I to II OCSCC who had inadequate resection and positive/close margins. To examine whether adjuvant treatment was independently associated with improved outcomes in patients with T1–2N0M0 OCSCC and positive or close margins, we performed a subgroup analysis in a multivariate model. We adjusted for prognostic factors that might be known to be related to the prescription of adjuvant treatment. The variables that were analyzed were sex, age, adjuvant treatment, depth of invasion, and T classification (Table 4). In this model, adjuvant treatment was associated with a significantly lower hazard ratio for all outcome measures (P = .002 to .03). For example, the risk of local recurrence was reduced to a hazard ratio of 0.6 after adjuvant treatment (P = .02). Figure 2 shows Kaplan-Meier analyses according received adjuvant treatment. Kaplan-Meier analyses of patients with a T1–2 classification and a positive neck lymph node showed similar results, with improved outcomes after adjuvant treatment. We also compared different treatments (surgery alone, surgery plus radiation, surgery plus radiochemotherapy, and surgery plus radiation and cetuximab). We found significant differences in outcomes between surgery alone and surgery with radiotherapy (OS, DSS, and LFS) among patients with clear margins versus close/positive margins. Among those with positive/close margins, there was a significant survival benefit from adding radiation. When we compared surgery with surgery plus chemoradiation or surgery plus radiation and Cetuximab we could not detect a significant difference between groups, probably because of the small number of patients in the chemoradiation/Cetuximab groups (50 and 21, respectively). We further conducted a subset multivariate analysis with only patients with close/positive margins, with adjuvant RT used as a covariate. We found that adjuvant radiotherapy remained a significant predictor of OS and DSS (Table 5).
TABLE 4.
Multivariate Analysis of Overall, Disease-Specific, and Local Recurrence–Free Survival in Patients With T1–2N0 Disease and ClosenPositive Margins According to Adjuvant Therapy (n = 238)
| Overall Survival |
Disease-Specific Survival |
Local Recurrence–Free Survival |
|||||
|---|---|---|---|---|---|---|---|
| Variable | No. | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Sex | .045 | .24 | |||||
| Male | 154 | 1 | 1 | ||||
| Female | 82 | 1.57 (1.03–2.47) | 1.3 (0.82–2.14) | ||||
| Missing | 2 | ||||||
| Age | .064 | ||||||
| <70 y | 177 | 1 | |||||
| ≥70 y | 61 | 1.65 (0.97–2.74) | |||||
| Missing | 0 | ||||||
| Treatment | .002 | .01 | .03 | ||||
| Surgery | 130 | 1 | 1 | 1 | |||
| S+ADJ | 108 | 0.55 (0.37–0.81) | 0.55 (0.35–0.87) | 0.6 (0.37–0.94) | |||
| Missing | 0 | ||||||
| DOI | |||||||
| <4 mm | 35 | ||||||
| ≥4 mm | 146 | ||||||
| Missing | 57 | ||||||
| pT | |||||||
| 1 | 82 | ||||||
| 2 | 156 | ||||||
| Missing | 0 | ||||||
Abbreviations: CI, confidence interval; DOI, depth of invasion; HR, hazard ratio; pT, pathological T stage; S+ADJ, surgery and adjuvant therapy.
Figure 2.
Outcomes of patients with T1–2N0M0 disease according to the margin status (clear or close/positive) and ADJ. The Kaplan-Meier analysis shows (A) overall survival, (B) disease-specific survival, (C) disease-free survival, and (D) local recurrence–free survival. Below the x-axis is the at-risk set, which shows attrition with time. ADJ indicates adjuvant therapy. Supporting table 2 specifies P values between groups.
TABLE 5.
Multivariate Analysis of Overall, Disease-Specific, and Local Recurrence–Free Survival in Patients With T1–2N0 Disease and Close\Positive Margins According to Adjuvant Radiotherapy (n = 208)
| Overall Survival |
Disease-Specific Survival |
Local Recurrence–Free Survival |
|||||
|---|---|---|---|---|---|---|---|
| Variable | No. | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Sex | .051 | .33 | |||||
| Male | 136 | 1 | 1 | ||||
| Female | 72 | 1.56 (0.99–2.43) | 0.7 (0.34–1.4) | ||||
| Missing | 0 | ||||||
| Age | .11 | ||||||
| <70 y | 157 | 1 | |||||
| ≥70 y | 51 | 1.88 (0.87–3.79) | |||||
| Missing | 0 | ||||||
| Treatment | .0094 | .02 | .24 | ||||
| Surgery | 130 | 1 | 1 | 1 | |||
| S+PORT | 78 | 0.58 (0.38–0.88) | 0.56 (0.33–0.92) | 0.71 (0.38–1.26) | |||
| Missing | 0 | ||||||
| DOI | .02 | ||||||
| <4 mm | 32 | 1 | |||||
| ≥4 mm | 125 | 2.54 (1.16–6.7) | |||||
| Missing | 51 | ||||||
| pT | |||||||
| 1 | 76 | ||||||
| 2 | 132 | ||||||
| Missing | 0 | ||||||
Abbreviations: CI, confidence interval; DOI, depth of invasion; HR, hazard ratio; pT, pathological T stage; S+PORT, surgery and postoperative radiation therapy.
Patients who received adjuvant treatment other than radiation were excluded from the analysis.
Using the CIF, we re-analyzed local recurrence with OS and distant metastasis–free survival as competing factors. Using CIF analysis, we found that margins and treatment remained significant predictors of local recurrence. Similarly, we re-analyzed DSS with death from other causes as a competing factor. Using the CIF analysis, we showed that margins and treatment remained significant predictors of DSS.
DISCUSSION
Oncologically sound treatment of early-stage OCSCC can be achieved with complete tumor resection with wide free margins, which is the single most important prognostic factor for these patients.13 Although prospective controlled data are lacking, current guidelines indicate that an adequate margin is more than 5 mm from the tumor front.5 If the surgeon has achieved adequate resection in the early stage and the neck dissection shows negative nodes, there is no need for further treatment. Factors that may influence the attainment of free margins include the tumor subsite, tumor size, depth of invasion, pattern of invasion, and prior treatment.5 In a prospective study, we recently demonstrated that a true intraoperative assessment of margins can be performed by a frozen section analysis of the specimen itself (specimen-driven margin).5 The accuracy of this technique is greater than 90%, and it is significantly more reliable than the traditional method of patient-driven margins.10 At an advanced tumor stage, when clear margins are not attained, adjuvant chemoradiation has been shown to reduce the risk of recurrence.4,24 In early tumors, however, both resurgery and adjuvant radiotherapy are recommended.13 Nevertheless, these modalities carry considerable morbidity, and their advantage in improving outcomes requires further evaluation.
In this international study, we focused on patients with T1–2 OCSCC with no nodal metastases. This population composes up to 50% of OCSCC patients in Western countries.25 In this study, we found that the rate of true positive margins in patients with T1–2 OCSCC was 3%. This finding is in agreement with previous reports of a similar population.26–28 We show that patients with clear margins had better outcomes than those with positive/close margins, with a 31% difference in 5-year DSS (from 58% to 89%). The 10-year DSS was 83% for patients with clear margins and 43% for those with positive/close margins. This represents an almost 2-fold difference in mortality according to the margin status.
An important finding of this study is that patients with close or positive margins who received adjuvant treatment had significantly better outcomes than did patients who did not receive adjuvant treatment. Yet, the outcomes remained worse than those for patients who had clear margins.
Our study indicates that clear margins should be pursued by the surgeon. We suggest that the use of intraoperative margin assessment should be promoted to improve the achievement of adequate margins. This method is now recommended by the Society of Surgical Pathologists.10,29 The data also suggest the consideration of adjuvant treatment for patients with early OCSCC who have positive/close margins; however, prospective studies are needed for the establishment of such a recommendation.
A number of small, single-institute, retrospective studies have shown that the margin status in early-stage OCSCC, with no other adverse features, does not affect local control or DSS.30,31 The margin status has been shown to be strongly associated with other known prognostic factors that could not be accounted for in this study, such as perineurial invasion and lymphovascular spread. However, because these studies included small numbers of patients, statistical significance is hard to achieve with a large number of variables.
The primary limitation of the current study is its retrospective design. Because no randomization was undertaken, there may have been multiple important confounders that were not adequately adjusted. Another limitation is the fact that it represents techniques of surgeons from several cancer centers worldwide. Also, the number of patients with positive margins was relatively small, and thus, a statistically significant distinction for this group was difficult to attain. The prolonged period of the study and the heterogeneity among institutions are also limitations of this work. Nevertheless, because of the latter, the conclusions of this international study can be generalized to medical centers across the globe. Other possible limitations are the lack of data on comorbidities that may affect survival outcomes and the lack of other known prognostic factors such as lymphovascular invasion and perineural invasion. These risk factors are known for their prognostic impact on head and neck cancers.32 However, their role in early OCSCC is undetermined,33,34 and further study is required to bring these factors into consideration.
The oral cavity includes several subsites for which we did not have information in this study. It is possible that variations in our ability to reach clear margins at different tumor subsites are inherent to the data. Recent data suggest that a 5-mm margin is redundant, and a redefinition of close and clear margins6,7 is needed. Nevertheless, because the current acceptable margin is 5 mm, our findings remain relevant for the current classification. A detailed assessment of the length of margins is required in future studies.
The 10-year DSS rate of only 43% highlights dramatically the unfavorable prognosis of patients who have early-stage OCSCC with close/positive margins. These results highlight the need for further prospective studies that will focus on the outcomes of early OCSCC. A lack of consensus regarding the significance of disease margins in this population poses potential impediments to the current policy of selecting adjuvant treatment.
In conclusion, our data suggest that positive and close margins are associated with poor outcomes for patients with early OCSCC. These patients may benefit from adjuvant radiotherapy. Further prospective studies are needed to show the utility of adjuvant treatment in patients with close margins.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
This work was supported by the Israel Science Foundation and the Israel Cancer Association.
We thank Cindy Cohen for her editorial assistance and Anat Reiner Benaim, PhD, for her statistical analysis and support.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Ziv Gil is the owner of a patent on a cold plasma–generating system (patent WO2016079742A1).
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLO-BOCAN 2012. Int J Cancer 2015;136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Shah JP, Gil Z. Current concepts in management of oral cancer—surgery. Oral Oncol 2009;45:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amit M, Yen TC, Liao CT, et al. The origin of regional failure in oral cavity squamous cell carcinoma with pathologically negative neck metastases. JAMA Otolaryngol Head Neck Surg 2014;140:1130–1137. [DOI] [PubMed] [Google Scholar]
- 4.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (#9501). Head Neck 2005;27:843–850. [DOI] [PubMed] [Google Scholar]
- 5.Amit M, Na’ara S, Leider-Trejo L, et al. Improving the rate of negative margins after surgery for oral cavity squamous cell carcinoma: a prospective randomized controlled study. Head Neck 2016;38(suppl 1):E1803–E1809. [DOI] [PubMed] [Google Scholar]
- 6.Zanoni DK, Migliacci JC, Xu B, et al. A proposal to redefine close surgical margins in squamous cell carcinoma of the oral tongue. JAMA Otolaryngol Head Neck Surg 2017;143:555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tasche KK, Buchakjian MR, Pagedar NA, Sperry SM. Definition of “close margin” in oral cancer surgery and association of margin distance with local recurrence rate. JAMA Otolaryngol Head Neck Surg 2017;143:1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutton D, Brown J, Rogers S, Vaughan E, Woolgar J. The prognostic implications of the surgical margin in oral squamous cell carcinoma. Int J Oral Maxillofac Surg 2003;32:30–34. [DOI] [PubMed] [Google Scholar]
- 9.Amit M, Yen TC, Liao CT, et al. Improvement in survival of patients with oral cavity squamous cell carcinoma: an international collaborative study. Cancer 2013;119:4242–4248. [DOI] [PubMed] [Google Scholar]
- 10.Maxwell JH, Thompson LDR, Brandwein-Gensler MS, et al. Early oral tongue squamous cell carcinoma. JAMA Otolaryngol Neck Surg 2015;141:1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohiyuddin SMA, Padiyar BV, Suresh TN, et al. Clinicopathological study of surgical margins in squamous cell carcinoma of buccal mucosa. World J Otorhinolaryngol Neck Surg 2016;2:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loree TR, Strong EW. Significance of positive margins in oral cavity squamous carcinoma. Am J Surg 1990;160:410–414. [DOI] [PubMed] [Google Scholar]
- 13.Adelstein D, Gillison ML, Pfister DG, et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 2.2017. J Natl Compr Canc Netw 2017;15:761–770. [DOI] [PubMed] [Google Scholar]
- 14.Robbins KT, Shaha AR, Medina JE, et al. Consensus statement on the classification and terminology of neck dissection. Arch Otolaryngol Neck Surg 2008;134:536. [DOI] [PubMed] [Google Scholar]
- 15.Patel SG, Amit M, Yen TC, et al. Lymph node density in oral cavity cancer: results of the International Consortium for Outcomes Research. Br J Cancer 2013;109:2087–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebrahimi A, Gil Z, Amit M, et al. Comparison of the American Joint Committee on Cancer N1 versus N2a nodal categories for predicting survival and recurrence in patients with oral cancer: time to acknowledge an arbitrary distinction and modify the system. Head Neck 2016;38:135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 19.Gil Z, Carlson DL, Boyle JO, et al. Lymph node density is a significant predictor of outcome in patients with oral cancer. Cancer 2009;115:5700–5710. [DOI] [PubMed] [Google Scholar]
- 20.Dignam JJ, Kocherginsky MN. Choice and interpretation of statistical tests used when competing risks are present. J Clin Oncol 2008; 26:4027–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Core Team. R: A Language and Environment for Statistic Computing Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 22.Gray B cmprsk: Subdistribution Analysis of Competing Risks. Package Version 2.2–7 https://cran.r-project.org/web/packages/cmprsk/cmprsk.pdf. Accessed January 15, 2017.
- 23.Ebrahimi A, Clark JR, Amit M, et al. Minimum nodal yield in oral squamous cell carcinoma: defining the standard of care in a multicenter international pooled validation study. Ann Surg Oncol 2014; 21:3049–3055. [DOI] [PubMed] [Google Scholar]
- 24.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004;350:1937–1944. [DOI] [PubMed] [Google Scholar]
- 25.Montero PH, Yu C, Palmer FL, et al. Nomograms for preoperative prediction of prognosis in patients with oral cavity squamous cell carcinoma. Cancer 2014;120:214–221. [DOI] [PubMed] [Google Scholar]
- 26.Dik EA, Willems SM, Ipenburg NA, Adriaansens SO, Rosenberg AJWP, van Es RJJ Resection of early oral squamous cell carcinoma with positive or close margins: relevance of adjuvant treatment in relation to local recurrence. Oral Oncol 2014;50:611–615. [DOI] [PubMed] [Google Scholar]
- 27.Barry CP, Ahmed F, Rogers SN, et al. Influence of surgical margins on local recurrence in T1/T2 oral squamous cell carcinoma. Head Neck 2015;37:1176–1180. [DOI] [PubMed] [Google Scholar]
- 28.Jayasooriya PR, Pitakotuwage TN, Mendis BRRN, Lombardi T Descriptive study of 896 oral squamous cell carcinomas from the only university based oral pathology diagnostic service in Sri Lanka. BMC Oral Health 2016;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiosea SI. Intraoperative margin assessment in early oral squamous cell carcinoma. Surg Pathol Clin 2017;10:1–14. [DOI] [PubMed] [Google Scholar]
- 30.Low THH, Gao K, Gupta R, et al. Factors predicting poor outcomes in T1N0 oral squamous cell carcinoma: indicators for treatment intensification. ANZ J Surg 2016;86:366–371. [DOI] [PubMed] [Google Scholar]
- 31.Ch’ng S, Corbett-Burns S, Stanton N, et al. Close margin alone does not warrant postoperative adjuvant radiotherapy in oral squamous cell carcinoma. Cancer 2013;119:2427–2437. [DOI] [PubMed] [Google Scholar]
- 32.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004;350:1945–1952. [DOI] [PubMed] [Google Scholar]
- 33.Chen TC, Wang CP, Ko JY, et al. The impact of perineural invasion and/or lymphovascular invasion on the survival of early-stage oral squamous cell carcinoma patients. Ann Surg Oncol 2013;20:2388–2395. [DOI] [PubMed] [Google Scholar]
- 34.Michikawa C, Uzawa N, Kayamori K, et al. Clinical significance of lymphatic and blood vessel invasion in oral tongue squamous cell carcinomas. Oral Oncol 2012;48:320–324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.