Abstract
Iron deficiency is common in patients with chronic heart failure (CHF) and is associated with reduced exercise performance, impaired health‐related quality of life and an increased risk of mortality, irrespective of whether or not anaemia is present. Iron deficiency is a serious but treatable condition. Several randomized controlled clinical trials have demonstrated the ability of intravenous (IV) iron, primarily IV ferric carboxymaltose (FCM), to correct iron deficiency in patients with heart failure with reduced ejection fraction (HFrEF), resulting in improvements in exercise performance, CHF symptoms and health‐related quality of life. The importance of addressing the issue of iron deficiency in patients with CHF is reflected in the 2016 European Society of Cardiology (ESC) heart failure guidelines, which recognize iron deficiency as an important co‐morbidity, independent of anaemia. These guidelines recommend that all newly diagnosed heart failure patients are routinely tested for iron deficiency and that IV FCM should be considered as a treatment option in symptomatic patients with HFrEF and iron deficiency (serum ferritin < 100 µg/L, or ferritin 100–299 µg/L and transferrin saturation < 20%). Despite these specific recommendations, there is still a lack of practical, easy‐to‐follow advice on how to diagnose and treat iron deficiency in clinical practice. This article is intended to complement the current 2016 ESC heart failure guidelines by providing practical guidance to all health care professionals relating to the procedures for screening, diagnosis and treatment of iron deficiency in patients with CHF.
Keywords: Chronic heart failure, European Society of Cardiology, Iron deficiency, Guidelines, Ferric carboxymaltose
Iron deficiency in heart failure is common, important and treatable
Iron deficiency is a common co‐morbidity in patients with chronic heart failure (CHF) and can exist with or without anaemia.1 As many as ∼40–70% of patients with CHF are iron‐deficient.2, 3, 4, 5, 6, 7 Iron deficiency can be described as being either ‘absolute’ or ‘functional’.8 Absolute iron deficiency is the result of reduced iron stores [haemoglobin (Hb) and ferritin, present mainly in the liver, spleen and bone marrow].9 In patients with CHF, iron stores may be depleted by malabsorption, malnutrition, and gastrointestinal blood loss (may be caused or exacerbated by the use of anticoagulants, antithrombotics, or non‐steroidal anti‐inflammatory drugs).9 Functional iron deficiency is a result of impaired iron mobilization from storage sites. Patients with CHF usually have systemic chronic inflammation and elevated inflammatory cytokines, which increase hepcidin production by the liver. Hepcidin blocks iron absorption from the gastrointestinal tract and also iron mobilization from storage sites, including the reticuloendothelial system.9, 10, 11 In addition, existing co‐morbidities (e.g. renal dysfunction) and/or dietary restrictions (e.g. meat‐free diet) may contribute further to the development of iron deficiency in patients with CHF.12
The physiological impact of iron deficiency extends beyond impairment of erythropoiesis and the associated risk of anaemia. Non‐haematopoietic tissues, including skeletal and cardiac muscle, are dependent on iron as the key constituent of proteins involved in vital cellular processes, such as oxygen storage (as the component of myoglobin) and oxidative energy metabolism (as a component of oxidative enzymes and mitochondrial respiratory chain proteins).13, 14 Clinical studies in patients with CHF show that iron deficiency is associated with decreased exercise performance,4, 15, 16, 17 impaired health‐related quality of life (QoL)18, 19 and an increased risk of morbidity20 and mortality,2, 4, 21 irrespective of the presence of anaemia.
Iron deficiency is a treatable condition. Data from randomized clinical trials performed in patients with heart failure with reduced ejection fraction (HFrEF) and iron deficiency have demonstrated improvements in exercise capacity and QoL, and a reduction in hospitalizations following treatment with intravenous (IV) iron.22, 23, 24, 25, 26
The clinical benefit of treating iron deficiency in patients with CHF is reflected in the latest 2016 European Society of Cardiology (ESC) guidelines for heart failure (HF), in which iron deficiency is recognized as an important co‐morbidity, independent of anaemia, and specific recommendations relating to its diagnosis and treatment are provided.1
Despite these guideline recommendations, iron deficiency is still under‐diagnosed and under‐treated in clinical practice.27, 28 Therefore, many patients are being denied therapy that may potentially have a positive impact on both cardiovascular function and QoL. This may be partially due to the lack of practical advice on how to screen, diagnose and treat iron deficiency. In recognition of the lack of practical recommendations, a global working group of physicians with expertise in this field decided to address this clinical need. This article is intended to complement the 2016 ESC HF guidelines by providing practical guidance for all health care professionals (HCPs) worldwide who are involved in the management of patients with HF and are, therefore, in a position to identify and/or treat iron deficiency.
Development of practical recommendations
Practical recommendations complementing ESC guidelines have previously been developed for the management of other treatments in CHF (e.g. angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers).29, 30 The practical recommendations outlined in this manuscript were developed, discussed and agreed by the authors during a series of working group meetings that took place in 2016 and 2017. This practical guidance addresses the management of patients with iron deficiency in a stepwise manner, from initial screening and diagnosis through to treatment and follow‐up.
Practical recommendations on the screening and diagnosis of iron deficiency in patients with heart failure
The current 2016 ESC HF guidelines1 recommend that evaluation of iron status should be considered in the diagnostic work‐up of all newly diagnosed HF patients (class of recommendation I, level of evidence C). A practical recommendation of the working group is that iron status should also be evaluated in patients with existing CHF, particularly if they are symptomatic despite receiving optimal background HF medications. In addition, as part of routine follow‐up, consideration should be given to the re‐evaluation of iron status 1–2 times per year, as well as after hospitalization for HF.
Ferritin and transferrin saturation (TSAT) are widely available blood markers for evaluating iron status. Their use for the diagnosis of iron deficiency is recommended by the 2016 ESC HF guidelines.1 Ferritin is an intracellular iron‐storage protein secreted by iron‐storing tissues (e.g. liver and reticuloendothelial system). Serum ferritin concentrations are a surrogate marker of stored iron quantity.9 TSAT (defined as % of transferrin that has iron bound to it) is used as a marker of the availability of circulating iron to supply metabolizing cells.9 TSAT value is calculated by dividing the serum iron concentration by the total iron‐binding capacity (TIBC):
The 2016 ESC HF guidelines recommend treating iron deficiency based on a serum ferritin level < 100 µg/L, or 100–299 µg/L when TSAT < 20%. Two different cut‐offs are used because ferritin levels may become elevated in the presence of inflammation and may, therefore, appear to be within the normal range (100–300 µg/L). As ferritin, an acute‐phase protein, is elevated as a result of inflammation, TSAT levels < 20% indicate that insufficient circulating iron is available to supply metabolizing cells (functional iron deficiency).9 Therefore, in accordance with the 2016 ESC HF guidelines, it is important to ensure that both ferritin and TSAT testing is performed simultaneously and evaluated together when assessing iron status (Figure 1). Lower ferritin cut‐offs (e.g. < 30 µg/L) may be used to confirm the presence of iron deficiency in other disease settings and are often displayed on laboratory test results as the lower limit of normal. However, the thresholds defined above are recommended to diagnose iron deficiency in patients with CHF.
Figure 1.
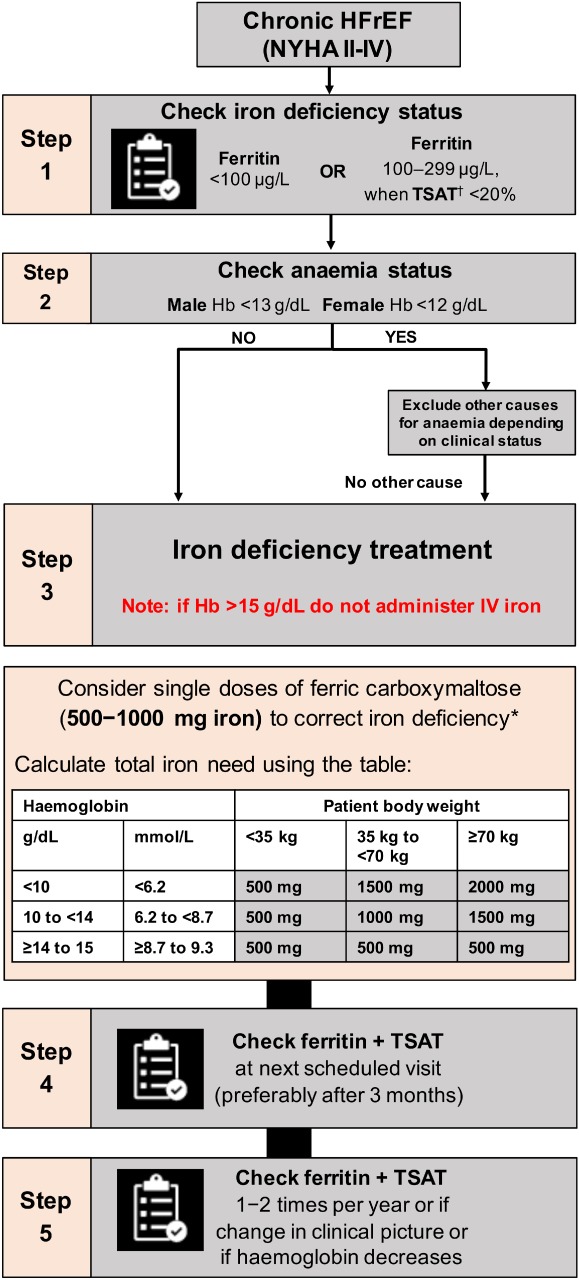
Algorithm for screening/diagnosis and treatment/follow‐up of iron deficiency in patients with chronic heart failure. Hb, haemoglobin; HFrEF, heart failure with reduced ejection fraction; IV, intravenous; NYHA, New York Heart Association; TSAT, transferrin saturation†. *Note: The use of ferric carboxymaltose has not been studied in children, and therefore is not recommended in children under 14 years. For full prescribing information, please refer to the Summary of Product Characteristics.39 †TSAT = (serum iron concentration/total iron‐binding capacity) x 100. Algorithm adapted from McDonagh and Macdougall.12
Note that mean corpuscular volume, mean corpuscular Hb (MCH) and MCH concentration have been found to be unreliable markers of iron deficiency status.31 Measuring their levels is not recommended for assessment of iron deficiency in patients with HF. In addition, serum iron concentrations can vary substantially between HF patients, and also exhibit large diurnal variations and, therefore, serum iron alone should not be used as a marker of iron status.32 Ferritin and TSAT should be used instead.
In patients who are both iron‐deficient and anaemic, it is important to investigate the underlying causes of reduced Hb levels and to exclude other pathologies, including occult blood loss. Measurement of Hb levels is important as this has implications for IV iron dosing (discussed in the next section).
Practical recommendations on the treatment of iron deficiency in patients with chronic heart failure
The current treatment options for the correction of iron deficiency in the general population consist of IV or oral iron. The 2016 ESC HF guidelines specifically recommend that, in patients with symptomatic HF (HFrEF), iron deficiency is treated with IV ferric carboxymaltose (FCM).1 This recommendation is based on clinical trial evidence demonstrating the benefit of IV FCM therapy in this patient population. In turn, oral iron therapy has been found to be ineffective for replenishment of iron stores and improvement of clinical status in patients with CHF (further details below).33
Intravenous iron therapy
Of the IV iron preparations licensed for therapeutic use, IV FCM has been most extensively studied for the treatment of iron deficiency in patients with HFrEF. The efficacy of FCM has been evaluated in several randomized clinical trials, including FAIR‐HF,22 CONFIRM‐HF24 and EFFECT‐HF,34 which enrolled symptomatic patients with stable CHF [left ventricular ejection fraction (LVEF) ≤45%] and iron deficiency (Table 1).
Table 1.
Design and dosing regimens used in the FAIR‐HF, CONFIRM‐HF and EFFECT‐HF studies
| Study | Study design and duration | No. of patients treated | Key inclusion criteria | Dosing regimen | Single dose of iron used | Administration method | Mean total iron dose | Total no. of injections in FCM group |
|---|---|---|---|---|---|---|---|---|
|
FAIR‐HF (Anker et al.)22 |
Double‐blind, placebo‐controlled, randomized; 24 weeks |
FCM: 304 Placebo: 155 |
NYHA class II (LVEF ≤40%) or III (LVEF ≤45%) Hb 9.5–13.5 g/dL Ferritin <100 µg/L or 100–299 µg/L + TSAT <20% |
Dose calculated according to Ganzoni formula FCM 200 mg iron/week until iron repletion (correction phase) then every 4 weeks during maintenance phase |
100 mg or 200 mg | Bolus injection | 1850 mg | Median 6 (3–7) in the correction phase |
|
CONFIRM‐HF (Ponikowski et al.)24 |
Double‐blind, placebo‐controlled, randomized; 52 weeks |
FCM: 150 Placebo: 151 |
NYHA class II/III (LVEF ≤45%) Hb <15 g/dL Ferritin <100 µg/L or 100–300 µg/L + TSAT <20% |
FCM 500–2000 mg iron in therapy phase (baseline and week 6); 500 mg iron as maintenance (weeks 12, 24, 36) if iron deficiency still present | 500 mg or 1000 mg | Bolus injection | 1500 mg | >75% of patients receiving FCM required a maximum of 2 injections to achieve iron repletion during the study |
|
EFFECT‐HF (van Veldhuisen et al.)34 |
Open‐label, standard of care‐controlled, randomized; 24 weeks |
FCM: 86 Standard of care: 86 |
NYHA class II/III (LVEF ≤45%) Hb <15 g/dL Ferritin <100 µg/L or 100–300 µg/L + TSAT <20% Peak VO2 10–20 mL/kg/min (reproducible) |
FCM 500–2000 mg iron in therapy phase (baseline and week 6); 500 mg iron as maintenance (week 12) if iron deficiency still present | 500 mg or 1000 mg | Bolus injection or infusion | 1204 mg | 96% of patients received a maximum of 2 injections |
FCM, ferric carboxymaltose; Hb, haemoglobin; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; TSAT, transferrin saturation†; VO2, oxygen uptake.
TSAT = (serum iron concentration/total iron‐binding capacity) x 100.
The FAIR‐HF study found that, compared with placebo, FCM therapy was associated with significant improvements in patient self‐reported QoL (measured using patient self‐reported global assessment) and HF symptoms [measured by New York Heart Association (NYHA) class] over a 6‐month period, irrespective of anaemia status.
The CONFIRM‐HF study followed patients for 52 weeks. The primary endpoint — demonstrating that FCM therapy was associated with significant improvements in exercise capacity (assessed using the 6‐minute walk test) after 24 weeks — was met. A secondary endpoint analysis found that FCM also reduced the risk of hospitalization in patients with CHF, compared with placebo.
The open‐label, randomized EFFECT‐HF study evaluated the impact of FCM vs. standard of care on exercise capacity [assessed by change in peak oxygen uptake (VO2) to week 24] in 174 patients with stable CHF and iron deficiency. After 24 weeks, FCM treatment was associated with a significantly beneficial effect on peak VO2, vs. standard of care, in the primary analysis. This was observed in both anaemic and non‐anaemic patients.34
Based on the data from FAIR‐HF,22 CONFIRM‐HF24 and a meta‐analysis of randomized clinical trials26 that evaluated the impact of IV iron therapy in patients with HFrEF, the current 2016 ESC HF guidelines recommend the use of IV FCM for the treatment of iron deficiency in CHF (class of recommendation IIa, level of evidence A). Furthermore, a recent meta‐analysis of individual patient data has demonstrated a reduction in recurrent cardiovascular hospitalizations and cardiovascular mortality with IV FCM in systolic HF patients with iron deficiency.35 The subsequent sections will provide some practical guidance relating to the use of IV FCM for the treatment of iron deficiency in patients with CHF.
Oral iron
Oral iron therapy is frequently used as a first‐line treatment in the correction of iron deficiency in patients with CHF. However, there is a lack of clinical data supporting its efficacy for use in this setting.
A recent randomized, placebo‐controlled clinical trial (IRONOUT HF) evaluated the impact of high‐dose oral iron polysaccharide on exercise capacity (assessed by change in peak VO2 from baseline to week 16) and HF symptom improvement in patients with HFrEF and iron deficiency.33 The study found that oral iron minimally replenished iron stores in this patient population, compared with placebo, and did not improve exercise capacity or HF symptoms. The results of the IRONOUT HF study do not support the use of oral iron therapy for the correction of iron deficiency in patients with HFrEF. There have been no randomized controlled trials where IV iron has been compared directly with oral iron in patients with CHF.
Furthermore, oral iron is poorly tolerated in patients with CHF, with gastrointestinal side effects occurring in up to 60% of patients.12 The low rate of iron absorption from oral iron preparations means that they are less rapidly effective than IV iron, and therefore a relatively long duration of oral iron therapy (in some cases > 6 months) may be required to achieve iron repletion.12
Which patients are suitable candidates for intravenous iron?
The FAIR‐HF, CONFIRM‐HF and EFFECT‐HF studies enrolled CHF patients with LVEF ≤ 45%, and demonstrated the efficacy of IV FCM with respect to improving functional capacity, HF symptoms and health‐related QoL,22, 24, 34 while potentially reducing risk of HF‐related hospitalizations in this patient population.24 These clinical trials were not designed for, or powered to, evaluate the effect of treatment on survival. Therefore, the potential efficacy of IV iron therapy in reducing mortality in CHF patients has not been demonstrated to date. The efficacy and safety of IV iron therapy has not yet been established in acute HF, or in HF with preserved ejection fraction (HFpEF, defined as LVEF ≥ 50%). These gaps in knowledge are being addressed in ongoing clinical trials.36, 37, 38
Contraindications for the use of FCM in patients with CHF and iron deficiency are few and include: hypersensitivity to the active substance, to FCM, or any of its excipients; known serious hypersensitivity to other parenteral iron products; the presence of anaemia not attributed to iron deficiency (e.g. other microcytic anaemia); evidence of iron overload; or disturbances in the utilization of iron39 (Table 2).
Table 2.
Practical guidance on the treatment of iron deficiency in patients with chronic heart failure
| Why screen for iron deficiency? |
|---|
|
| In whom and when to give IV iron therapy? |
|
| Which iron preparation/route? |
|
| How should IV iron be administered? |
|
| Where to perform the treatment? |
|
| Monitoring of iron status |
|
CHF, chronic heart failure; ESC, European Society of Cardiology; FCM, ferric carboxymaltose; Hb, haemoglobin; HF, heart failure; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IV, intravenous; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; TSAT, transferrin saturation†.
TSAT = (serum iron concentration/total iron‐binding capacity) x 100.
It is also important to note that the efficacy and safety of IV FCM have not been evaluated in patients with Hb levels >15 g/dL, and therefore the drug should not be used in this patient population. Furthermore, IV iron should be used with caution in patients with acute or chronic infection according to the opinion of the treating physician, and treatment with IV FCM should be stopped in patients with ongoing bacteraemia.39
Who can administer intravenous ferric carboxymaltose therapy?
A range of HCPs, including the general practitioner, nurse and hospital physician, can administer IV FCM therapy. In clinical practice, this varies between treatment centre and country based on local prescribing regulations or laws. FCM can be administered in any health care setting where staff are trained and equipment is available to evaluate and treat a potential hypersensitivity reaction. The risk of hypersensitivity reactions with IV FCM is low, with a frequency (events/patients treated) of ≥ 0.1% to < 1.0% observed during clinical trials and post‐marketing surveillance.39 This is in contrast to the historical risk of older IV iron preparations (e.g. iron dextran), for which an increased risk of anaphylaxis has been observed.40
How to administer and monitor intravenous ferric carboxymaltose therapy?
Ferric carboxymaltose contains 50 mg iron/mL. A 2 mL ampoule contains 100 mg of iron and a 10 mL ampoule contains 500 mg of iron. The determination of the initial iron need is calculated based on body weight and Hb levels, rather than ferritin or TSAT levels (used to diagnose the presence of iron deficiency) (Figure 1). The maximum recommended cumulative dose of FCM is 1000 mg iron (20 mL FCM)/week.
Intravenous FCM can be given as an injection or infusion. It is easily administered as an undiluted slow bolus injection (100 mg/min, or 15 min for a 1000 mg dose). Note that if administered as an IV infusion, FCM should not be over‐diluted as this affects the stability of the drug. A dilution plan of FCM for IV infusion is displayed in Table 3. Patients should be observed for adverse effects for at least 30 min following each IV injection.
Table 3.
Dilution plan for ferric carboxymaltose for intravenous infusion
| Equivalent iron dose to be repleted | Volume of FCM required | Maximum amount of sterile 0.9% m/V sodium chloride solution | Minimum administration time |
|---|---|---|---|
| 500 mg | 10 mL | 100 mL | 6 min |
| 1000 mg | 20 mL | 250 mL | 15 min |
FCM, ferric carboxymaltose; m/V, mass/volume %.
After IV administration of the correction dose, we recommend that iron status be re‐evaluated at 3 months. Early re‐evaluation of iron status (i.e. within 4 weeks of IV iron administration) should be avoided as serum levels of ferritin can increase markedly following administration of IV iron and cannot be utilized as a marker of iron status during this time.
Further iron repletion should be provided as needed. If there is no response or Hb levels decrease, further investigation for other underlying causes should be considered as clinically indicated, particularly occult blood loss. After correction of iron deficiency, as part of routine follow‐up consider re‐evaluation of iron parameters (ferritin and TSAT) 1–2 times per year. Iron status should be re‐evaluated if patients remain symptomatic despite receiving optimal background HF medications, or in the event that Hb levels decrease (Figure 1).
Clinical studies have demonstrated that IV FCM is well tolerated with an acceptable safety profile in patients with CHF.22, 24 The most common side effects (occurring at a frequency of ≥ 1.0% to < 10.0%) reported during clinical trials and post‐marketing surveillance include dizziness, headache, hypertension, hypophosphataemia, injection‐site reactions, and nausea.39 In patients who are iron‐deficient and anaemic, appropriate investigation to determine the underlying cause of anaemia and to exclude other pathologies based on local clinical practice guidelines should be implemented as a safety measure. The risk of hypersensitivity reactions with IV FCM is low, with a frequency (events/patients treated) of ≥ 0.1% to < 1.0% observed during clinical trials and post‐marketing surveillance. Skin staining (or discolouration) can occur at the infusion site if there is extravasation of IV iron.39 This may be avoided by ensuring best practice in IV line placement and care. In case of paravenous leakage, the administration of FCM must be stopped immediately.39
Conclusions
There is evidence from randomized clinical trials to show that correction of iron deficiency with IV iron therapy in patients with CHF (LVEF ≤ 45%) provides improvements in functional capacity, HF symptoms and health‐related QoL, and may reduce the risk of HF‐related hospitalizations.22, 24, 34
The 2016 ESC HF guidelines recommend that iron status should be evaluated as part of the initial work‐up of all newly diagnosed HF patients.1 The working group also recommends that iron status is checked among patients with existing CHF independently of Hb level or when symptoms persist despite receiving optimal background HF medications.
Intravenous iron therapy with FCM is recommended by the 2016 ESC HF guidelines for the correction of iron deficiency, and should be considered in symptomatic patients with HFrEF and iron deficiency.1
Knowledge gaps and areas for further research
It is important to note that, in the absence of certain clinical data, some of the practical recommendations made in this article are based on the collective expert opinion of the working group.
As outlined in the article, several knowledge gaps relating to the role of IV iron therapy in HF patients remain, many of which are being addressed by ongoing randomized clinical trials. These include the need for robust clinical data evaluating the impact of IV iron therapy on morbidity and mortality in systolic CHF patients with iron deficiency; these will be provided by the ongoing FAIR‐HF2 (NCT03036462) and IRONMAN (NCT02642562) studies. Furthermore, the efficacy and safety of IV iron has not yet been established in patients with HFpEF or acute HF and iron deficiency — the ongoing FAIR‐HFpEF (NCT03074591) and AFFIRM‐AHF (NCT02937454) studies should provide a better insight into the role of IV iron therapy in each of these patient populations.
Funding
Development of this article and funding to pay the Open Access publication charges was supported by Vifor Pharma, Glattbrugg, Switzerland.
Conflict of interest: T.M. reports honoraria for speaking for Vifor Pharma in satellite symposia. T.D. reports research support/consultation fees and travel support from Vifor Pharma, RESMED, Daiichi Sankyo, Pfizer, GSK, Alnylam, Prothena, Boston Scientific, Bayer, Medtronic, Novartis, Takeda, Merck, Astra Zeneca, Janssen Research & Development, LLC, Servier, and Abbott Diagnostics. W.D. reports research support/consultation fees and travel support from Vifor Pharma, Bayer, Boehringer Ingelheim, Sanofi‐Aventis, and Sphingotec. C.L. reports research support/consultation fees from Vifor Pharma, Boston Scientific, Bayer, Thermofisher, Medtronic, Novartis, Takeda, Merck, AstraZeneca, Janssen Research & Development, LLC, Menarini, Boehringer Ingelheim, and Abbott Diagnostics. A.S. reports honoraria, travel support or has appeared on expert panels for Amgen, Aspen, AstraZeneca, Bayer, Biotronik, Boehringer Ingelheim, Bristol‐Myers Squibb, Menarini, Merck Sharp & Dohme, Mylan, Novartis, Pfizer, Servier, and Vifor Pharma. P.v.d.M. reports grant support and consultancy fees from Vifor Pharma. A.C.S. reports research support/consultation fees from Vifor Pharma, CVRX, Novartis, Merck, Pfizer, Servier, Amgen, Menarini, Boehringer Ingelheim, and Roche Diagnostics. I.K. reports honoraria, travel support and consultation fees from Vifor Pharma, Novartis, Boehringer Ingelheim, Bayer, Pfizer, and Servier. N.M. reports fees for speaking for Vifor Pharma. O.P. reports consulting fees from Novartis, MSD, and Vifor Pharma. H.P.M. reports fees for speaking for Vifor Pharma, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Cardiome, Pfizer, and being member of CONFIRM‐HF and FAIR‐HF Steering Committees. J.T. reports consulting fees, travel support, speaker fees from Vifor Pharma, Servier and Novartis. J.C.C. reports fees for speaking for Vifor Pharma and being member of CONFIRM‐HF and FAIR‐HF Steering Committees.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) . Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 2. Klip IT, Comin‐Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013;165:575–582.e3. [DOI] [PubMed] [Google Scholar]
- 3. Nanas JN, Matsouka C, Karageorgopoulos D, Leonti A, Tsolakis E, Drakos SG, Tsagalou EP, Maroulidis GD, Alexopoulos GP, Kanakakis JE, Anastasiou‐Nana MI. Etiology of anemia in patients with advanced heart failure. J Am Coll Cardiol 2006;48:2485–2489. [DOI] [PubMed] [Google Scholar]
- 4. Okonko DO, Mandal AK, Missouris CG, Poole‐Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol 2011;58:1241–1251. [DOI] [PubMed] [Google Scholar]
- 5. Parikh A, Natarajan S, Lipsitz SR, Katz SD. Iron deficiency in community‐dwelling US adults with self‐reported heart failure in the National Health and Nutrition Examination Survey III: prevalence and associations with anemia and inflammation. Circ Heart Fail 2011;4:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yeo TJ, Yeo PS, Ching‐Chiew Wong R, Ong HY, Leong KT, Jaufeerally F, Sim D, Santhanakrishnan R, Lim SL, Chan MM, Chai P, Low AF, Ling LH, Ng TP, Richards AM, Lam CS. Iron deficiency in a multi‐ethnic Asian population with and without heart failure: prevalence, clinical correlates, functional significance and prognosis. Eur J Heart Fail 2014;16:1125–1132. [DOI] [PubMed] [Google Scholar]
- 7. von Haehling S, Gremmler U, Krumm M, Mibach F, Schon N, Taggeselle J, Dahm JB, Angermann CE. Prevalence and clinical impact of iron deficiency and anaemia among outpatients with chronic heart failure: The PrEP Registry. Clin Res Cardiol 2017;106:436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manito N, Cerqueiro JM, Comin‐Colet J, Garcia‐Pinilla JM, Gonzalez‐Franco A, Grau‐Amoros J, Peraira JR, Manzano L. Consensus Document of the Spanish Society of Cardiology and the Spanish Society of Internal Medicine on the diagnosis and treatment of iron deficiency in heart failure. Rev Clin Esp 2017;217:35–45. [DOI] [PubMed] [Google Scholar]
- 9. Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013;34:816–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jankowska EA, Malyszko J, Ardehali H, Koc‐Zorawska E, Banasiak W, von Haehling S, Macdougall IC, Weiss G, McMurray JJ, Anker SD, Gheorghiade M, Ponikowski P. Iron status in patients with chronic heart failure. Eur Heart J 2013;34:827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol 2009;122:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McDonagh T, Macdougall IC. Iron therapy for the treatment of iron deficiency in chronic heart failure: intravenous or oral? Eur J Heart Fail 2015;17:248–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr 2001;131:568S–580S. [DOI] [PubMed] [Google Scholar]
- 14. Anker SD, Colet JC, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Mori C, von Eisenhart Rothe B, Pocock S, Poole‐Wilson PA, Ponikowski P; FAIR‐HF Committees and Investigators . Rationale and design of Ferinject assessment in patients with IRon deficiency and chronic Heart Failure (FAIR‐HF) study: a randomized, placebo‐controlled study of intravenous iron supplementation in patients with and without anaemia. Eur J Heart Fail 2009;11:1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin‐Nadzieja L, von Haehling S, Doehner W, Banasiak W, Polonski L, Filippatos G, Anker SD, Ponikowski P. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011;17:899–906. [DOI] [PubMed] [Google Scholar]
- 16. Haas JD, Brownlie T. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr 2001;131:676S–690S. [DOI] [PubMed] [Google Scholar]
- 17. Enjuanes C, Bruguera J, Grau M, Cladellas M, Gonzalez G, Merono O, Moliner‐Borja P, Verdu JM, Farre N, Comin‐Colet J. Iron status in chronic heart failure: impact on symptoms, functional class and submaximal exercise capacity. Rev Esp Cardiol (Engl Ed) 2016;69:247–255. [DOI] [PubMed] [Google Scholar]
- 18. Comin‐Colet J, Enjuanes C, Gonzalez G, Torrens A, Cladellas M, Merono O, Ribas N, Ruiz S, Gomez M, Verdu JM, Bruguera J. Iron deficiency is a key determinant of health‐related quality of life in patients with chronic heart failure regardless of anaemia status. Eur J Heart Fail 2013;15:1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Enjuanes C, Klip IT, Bruguera J, Cladellas M, Ponikowski P, Banasiak W, van Veldhuisen DJ, van der Meer P, Jankowska EA, Comin‐Colet J. Iron deficiency and health‐related quality of life in chronic heart failure: results from a multicenter European study. Int J Cardiol 2014;174:268–275. [DOI] [PubMed] [Google Scholar]
- 20. Nunez J, Comin‐Colet J, Minana G, Nunez E, Santas E, Mollar A, Valero E, Garcia‐Blas S, Cardells I, Bodi V, Chorro FJ, Sanchis J. Iron deficiency and risk of early readmission following a hospitalization for acute heart failure. Eur J Heart Fail 2016;18:798–802. [DOI] [PubMed] [Google Scholar]
- 21. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin‐Nadzieja L, Banasiak W, Polonski L, Filippatos G, McMurray JJ, Anker SD, Ponikowski P. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J 2010;31:1872–1880. [DOI] [PubMed] [Google Scholar]
- 22. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole‐Wilson PA, Ponikowski P; FAIR‐HF Trial Investigators . Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009;361:2436–2448. [DOI] [PubMed] [Google Scholar]
- 23. Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole‐Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency. FERRIC‐HF: a randomized, controlled, observer‐blinded trial. J Am Coll Cardiol 2008;51:103–112. [DOI] [PubMed] [Google Scholar]
- 24. Ponikowski P, van Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD; CONFIRM‐HF Investigators . Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015;36:657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toblli JE, Lombrana A, Duarte P, Di Gennaro F. Intravenous iron reduces NT‐pro‐brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol 2007;50:1657–1665. [DOI] [PubMed] [Google Scholar]
- 26. Jankowska EA, Tkaczyszyn M, Suchocki T, Drozd M, von Haehling S, Doehner W, Banasiak W, Filippatos G, Anker SD, Ponikowski P. Effects of intravenous iron therapy in iron‐deficient patients with systolic heart failure: a meta‐analysis of randomized controlled trials. Eur J Heart Fail 2016;18:786–795. [DOI] [PubMed] [Google Scholar]
- 27. Wienbergen H, Pfister O, Hochadel M, Michel S, Bruder O, Remppis BA, Maeder MT, Strasser R, von Scheidt W, Pauschinger M, Senges J, Hambrecht R; RAID‐HF (Registry Analysis of Iron Deficiency‐Heart Failure) Registry Study Group . Usefulness of iron deficiency correction in management of patients with heart failure [from the Registry Analysis of Iron Deficiency‐Heart Failure (RAID‐HF) Registry]. Am J Cardiol 2016;118:1875–1880. [DOI] [PubMed] [Google Scholar]
- 28. Cohen‐Solal A, Damy T, Terbah M, Kerebel S, Baguet JP, Hanon O, Zannad F, Laperche T, Leclercq C, Concas V, Duvillie L, Darne B, Anker S, Mebazaa A. High prevalence of iron deficiency in patients with acute decompensated heart failure. Eur J Heart Fail 2014;16:984–991. [DOI] [PubMed] [Google Scholar]
- 29. McMurray J, Cohen‐Solal A, Dietz R, Eichhorn E, Erhardt L, Hobbs R, Maggioni A, Pina I, Soler‐Soler J, Swedberg K; Clinical Research Initiative in Heart Failure . Practical recommendations for the use of ACE inhibitors, beta‐blockers and spironolactone in heart failure: putting guidelines into practice. Eur J Heart Fail 2001;3:495–502. [DOI] [PubMed] [Google Scholar]
- 30. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) . Developed with the special contribution of the Heart Failure Association (HFA) of the ESC – Web Addenda. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 31. Tkaczyszyn M, Comin‐Colet J, Voors AA, van Veldhuisen DJ, Enjuanes C, Moliner‐Borja P, Rozentryt P, Polonski L, Banasiak W, Ponikowski P, van der Meer P, Jankowska EA. Iron deficiency and red cell indices in patients with heart failure. Eur J Heart Fail 2018;20:114–122. [DOI] [PubMed] [Google Scholar]
- 32. Cohen‐Solal A, Leclercq C, Mebazaa A, De Groote P, Damy T, Isnard R, Galinier M. Diagnosis and treatment of iron deficiency in patients with heart failure: expert position paper from French cardiologists. Arch Cardiovasc Dis 2014;107:563–571. [DOI] [PubMed] [Google Scholar]
- 33. Lewis GD, Malhotra R, Hernandez AF, McNulty SE, Smith A, Felker GM, Tang WH, LaRue SJ, Redfield MM, Semigran MJ, Givertz MM, Van Buren P, Whellan D, Anstrom KJ, Shah MR, Desvigne‐Nickens P, Butler J, Braunwald E. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: the IRONOUT HF randomized clinical trial. JAMA 2017;317:1958–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Veldhuisen DJ, Ponikowski P, van der Meer P, Metra M, Böhm M, Doletsky A, Voors AA, Macdougall IC, Anker SD, Roubert B, Zakin L, Cohen‐Solal A; EFFECT‐HF Investigators . Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation 2017;136:1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin‐Colet J, Ruschitzka F, Luscher TF, Arutyunov GP, Motro M, Mori C, Roubert B, Pocock SJ, Ponikowski P. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron‐deficient heart failure patients: an individual patient data meta‐analysis. Eur J Heart Fail 2018;20:125–133. [DOI] [PubMed] [Google Scholar]
- 36. ClinicalTrials.gov . Study to Compare Ferric Carboxymaltose With Placebo in Patients With Acute Heart Failure and Iron Deficiency (AFFIRM‐AHF) https://clinicaltrials.gov/ct2/show/NCT02937454. (3 August 2018)
- 37. EU Clinical Trials Register . FAIR‐HF2: Intravenous iron in patients with systolic heart failure and iron deficiency to improve morbidity & mortality https://www.clinicaltrialsregister.eu/ctr‐search/trial/2016‐000068‐40/PT. (3 August 2018)
- 38. ClinicalTrials.gov . Randomized Placebo‐controlled Trial of FCM as Treatment for Heart Failure With Iron Deficiency (HEART‐FID) https://clinicaltrials.gov/ct2/show/NCT03037931. (3 August 2018)
- 39. Vifor Pharma Ltd . Ferinject (ferric carboxymaltose). Summary of Product Characteristics https://www.medicines.org.uk/emc/medicine/24167/SPC/Ferinject. (3 August 2018)
- 40. Wang C, Graham DJ, Kane RC, Xie D, Wernecke M, Levenson M, MaCurdy TE, Houstoun M, Ryan Q, Wong S, Mott K, Sheu TC, Limb S, Worrall C, Kelman JA, Reichman ME. Comparative risk of anaphylactic reactions associated with intravenous iron products. JAMA 2015;314:2062–2068. [DOI] [PubMed] [Google Scholar]
Acknowledgements
Editorial assistance was provided by AXON Communications, London, UK.
The copyright line for this article was changed on 15 May 2019 after original online publication.


