Abstract
Background
Efficient incorporation of e‐health in patients with heart failure (HF) may enhance health care efficiency and patient empowerment. We aimed to assess the effect on self‐care of (i) the European Society of Cardiology/Heart Failure Association website ‘heartfailurematters.org’ on top of usual care, and (ii) an e‐health adjusted care pathway leaving out ‘in person’ routine HF nurse consultations in stable HF patients.
Methods and results
In a three‐group parallel‐randomized trial in stable HF patients from nine Dutch outpatient clinics, we compared two interventions ( heartfailurematters.org website and an e‐health adjusted care pathway) to usual care. The primary outcome was self‐care measured with the European Heart Failure Self‐care Behaviour Scale. Secondary outcomes were health status, mortality, and hospitalizations. In total, 450 patients were included. The mean age was 66.8 ± 11.0 years, 74.2% were male, and 78.8% classified themselves as New York Heart Association I or II at baseline. After 3 months of follow‐up, the mean score on the self‐care scale was significantly higher in the groups using the website and the adjusted care pathway compared to usual care (73.5 vs. 70.8, 95% confidence interval 0.6–6.2; and 78.2 vs. 70.8, 95% confidence interval 3.8– 9.4, respectively). The effect attenuated, until no differences after 1 year between the groups. Quality of life showed a similar pattern. Other secondary outcomes did not clearly differ between the groups.
Conclusions
Both the heartfailurematters.org website and an e‐health adjusted care pathway improved self‐care in HF patients on the short term, but not on the long term. Continuous updating of e‐health facilities could be helpful to sustain effects.
Clinical Trial registration: ClinicalTrials.gov ID NCT01755988.
Keywords: Telemedicine, Heart failure, Self‐care, Mortality, Hospitalization
Introduction
Heart failure (HF) is a chronic progressive disease, with an increasing prevalence with age. It has a major impact on health status, hospitalizations, and the health care budget.1, 2, 3
Due to aging of the population and substantial demand on health care resources, e‐health interventions to improve relevant patient outcomes, such as self‐care, are heavily promoted.4
An individual patient data meta‐analysis showed that self‐management interventions could have a beneficial effect on hospitalization, mortality, and HF‐related quality of life.5 An effect that may act through better adherence to evidence‐based treatment.6, 7 These programmes, however, demand considerable human resources, and are time‐consuming. Efficient incorporation of electronic health (e‐health) blended with existing care by replacing routine consultations could reduce the time investment of HF nurses per patient, creating time for the care of more patients. By monitoring vital signs such as blood pressure, heart rate, and body weight, imminent exacerbations might be timely identified and hospitalizations prevented.4 Incorporation of e‐health may be patient‐friendly as it enables self‐care activities at a time and place convenient for themselves, and reduces travel time to the hospital.
Earlier studies assessed the effect of e‐health tools (mostly telemonitoring) as part of disease management programmes. Some showed promising, but others neutral results in patients with HF.8, 9 These interventions were predominantly evaluated ‘on top of’ usual care (UC). In view of the anticipated shortage in health care facilities, evaluation of e‐health interventions with replacement of routine HF outpatient visits seems more relevant.
We created an interactive platform for HF disease management (e‐Vita platform) with telemonitoring facilities aimed at replacing routine consultations.
Another e‐health tool is the website ‘heartfailurematters.org’ (HFM website) with information targeted at patients and their family/carers to improve self‐care. Although the site is used intensively and translated in several languages, its effect on patient outcomes has never been evaluated.10
In our study we evaluated (i) an interactive platform for HF disease management (the e‐Vita platform) with telemonitoring facilities, replacing routine consultations, and (ii) the HFM website. Both were compared to UC. The primary outcome was self‐care and the secondary outcomes health status, hospitalizations and all‐cause mortality.
Methods
Study design
A three‐group parallel multicentre randomized pragmatic trial with 1:1:1 group allocation was performed. Patients in group I received UC, in group II UC plus the HFM website,10 and in group III an e‐health adjusted care pathway (EACP) with the e‐Vita platform including a link to the HFM website.
Overall, 450 HF outpatients were recruited from nine Dutch HF outpatient clinics between October 2013 and December 2014. Patients were followed up for 1 year. Patients were individually randomized by computerized block randomization (maximum of nine patients per block) to one of the three groups.
Details of the design of the e‐Vita HF study have been published elsewhere11 and a summary of the main design features is presented below.
The study was conducted according to the principles stated in the current Declaration of Helsinki12 and in accordance with the Dutch law on Medical Research Involving Human Subjects Act (WMO) and approved by the medical ethics committee of the University Medical Centre Utrecht, The Netherlands (number 12/456).
Study population
Heart failure patients were eligible to participate if (i) aged ≥ 18 years, and diagnosed with HF for at least 3 months; (ii) capable to fill out questionnaires, and perform blood pressure measurements and weighing (by standing on a weighing scale); (iii) they had access to internet and e‐mail, with basic user skills (or their spouses or carers); and (iv) able to read and understand Dutch.
Written informed consent was obtained during the first study visit at the HF outpatient clinic before any study procedure was undertaken.
Study groups
Usual care group
Allocated patients received UC from one of the nine HF outpatient clinic teams, at least comprising a cardiologist and a HF nurse. UC consisted of on average four routine consultations a year (typically three with the HF nurse, and one with the cardiologist).
‘Heartfailurematters.org’ website group
Participants received, on top of UC, information and 10 min instruction on the use of the HFM website from the HF nurse at the start of the study. During each routine consultation with the HF nurse, patients were encouraged to use the website, and experienced barriers were explored and solved. Additionally, participants received a leaflet with useful information, and every 3 months a reminder by e‐mail to use the website.
E‐health adjusted care pathway group
Participants in this group followed an EACP. They received identical initial information on the use of the HFM website as the participants of HFM group. In addition, the HF nurses instructed the patients and their caretakers on how to use the e‐Vita platform with telemonitoring facilities. Patients learned to record body weight, blood pressure and heart rate on a fixed time point everyday (or individually adjusted to a lower frequency if stable). All participants used a standardized weighing scale and blood pressure/heart rate device. The results of the vital parameters were automatically forwarded to the e‐Vita platform. At the start of the study, uniform pre‐specified alert limits for the values of body weight, blood pressure, and heart rate were set: body weight (+1 kg between two measurements, +2 kg in three consecutive measurements, −3 kg between two measurements, and +2 kg or −2 kg from baseline body weight), systolic blood pressure [average of 140 mmHg (upper limit) and average of 90 mmHg (lower limit) for three consecutive measurements], diastolic blood pressure [average of 100 mmHg (upper limit) and average of 50 mmHg (lower limit) for three consecutive measurements] and heart rate [100 b.p.m. (upper limit) and 50 b.p.m. (lower limit)]. To reduce unhelpful alerts, we encouraged the HF nurses to adjust these limits in shared decision with individual patients, and when necessary after consultation of the cardiologist or general practitioner (GP) of the patient.
If recordings of body weight, blood pressure, and/or heart rate were outside these limits or if measurements were not recorded, the HF nurse received an alert via the e‐Vita platform. If deemed necessary, the HF nurse contacted the patient by phone to explore symptoms, and possibly adjusted the individual management, asked the patient to visit the outpatient clinic, or visit the GP practice.
On the e‐Vita platform, co‐morbidities and medication were kept up to date by the patient, and checked by the HF nurse, who also encouraged the patients to keep it updated. Also, patients received monthly reminders by e‐mail. Finally, no routine face‐to‐face consultations with the HF nurse were scheduled, but if needed the patient could always contact the nurse.
Measurements and outcome parameters
Demographic and disease‐specific characteristics were collected at baseline.
Questionnaires were completed by patients at baseline, and after 3, 6 and 12 months, demanding ≈ 60 min per time period. Blood tests were performed at baseline, and after 6 and 12 months. Electronic medical files of the HF outpatient clinics and the GP were reviewed after 12 months of follow‐up.
The primary outcome was patient's self‐care. Self‐care was defined as the decision and strategies undertaken by the individual in order to maintain life, healthy functioning and well‐being.13 Self‐care was measured with the European Heart Failure Self‐care Behaviour (EHFScB) scale.14 The EHFScB scale includes both self‐reported consulting (i.e. ‘if shortness of breath increases, leg/feet are more swollen, I gain weight and/or experience fatigue I contact doctor or nurse’), and adherence to regimen behaviours (i.e. ‘I weigh myself every day’, ‘I limit the amount of fluids’, ‘I exercise regularly’, ‘I eat a low salt diet’, ‘I take my medication as prescribed’). It consists of nine items which are scored on a 5‐point Likert scale resulting in a standardized score from 0 to 100 with a higher score meaning better self‐care.14, 15
Secondary outcomes were (i) health‐related disease‐specific quality of life (hrQoL) measured with the Minnesota Living with HF Questionnaire, scoring between 0 and 105 with lower scores meaning better hrQoL,16 (ii) disease‐specific knowledge measured with the Dutch Heart Failure knowledge scale (DHFk), scoring between 0 and 15 with higher scores indicating more knowledge,17 (iii) patient satisfaction about the HF care measured with a visual analogue scale, scoring between 0 and 100 with higher scores meaning higher satisfaction, (iv) all‐cause mortality, (v) cardiovascular‐related mortality, (vi) HF‐related mortality, (vii) all‐cause hospitalizations, (viii) cardiovascular‐related hospitalizations, (ix) HF‐related hospitalizations, and (x) number of days of HF hospitalizations as captured by hospital and GP registries. The cause of death was assessed by an independent adjudication committee constituting a GP and two cardiologists who were unaware of the patient's allocation.
Statistical analysis
The sample size calculation was based on an overall comparison (with ANOVA) of the three study groups with an expected mean difference of the EHFScB scale score between HFM and UC, and between EAPC and UC of 0.5 and 2.0 points, respectively. These differences were based on previous studies.11 The estimated mean (standard deviation) EHFScB scale score in HF patients is 20 (5.54), based on unpublished data from a previous study.18 In addition, an alpha of 0.05 and a power of 80% were used. Based on the aforementioned assumptions, we required at least 414 patients (138 per group) for the study.
We performed an intention‐to‐treat analysis. Missing values were imputed by the multiple imputation method.19 The overall difference between the groups in self‐care at 3, 6 and 12 months was determined with an ANCOVA. Differences per comparison, between HFM vs. UC and EACP vs. UC were calculated with multiple linear regression models. Results of the crude regression model were presented. If residuals (i.e. an important assumption of a linear regression model) of the model were more sound with adjustment for the baseline values of self‐care, the results of the adjusted model were presented as well.
Differences in hrQoL, HF knowledge, and patient satisfaction about HF care, determined after 3, 6 and 12 months were also calculated with multiple linear regression models. Differences in mortality and hospitalizations were analysed with a Cox regression model, and the mean duration of HF hospitalizations with a multiple linear regression model. For all secondary outcomes, results of the crude model were presented.
Analyses were performed with SPSS version 21.0 (IBM Corp., Armonk, NY, USA).
Results
Demographics
From the 1988 invited patients, 450 (23%) consented to participate and were randomized (150 patients per group) (Figure 1). Mean age of the participants was 66.8 ± 11.0 years and 74.2% were male. At baseline, 78.8% was classified as New York Heart Association (NYHA) I or II, and the mean left ventricular ejection fraction (LVEF) was 35.7 ± 10.8, and 70.4% had a LVEF ≤ 40% (Table 1). Most baseline characteristics did not differ between the three study groups after randomization, although there were more smokers in the UC (19%) than in the HFM and EACP groups (12%, and 14%, respectively). The proportion of patients in NYHA class I was higher in the EACP (49%) than in the UC and HFM groups (40%).
Figure 1.
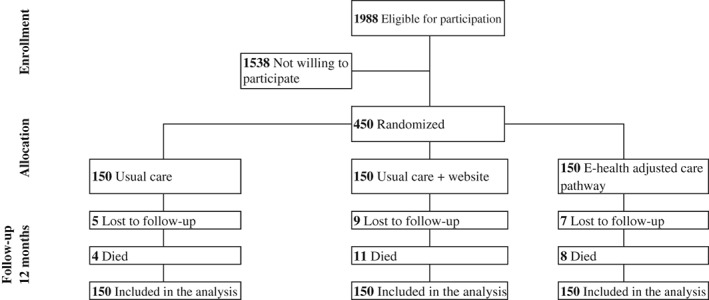
Flow chart of the study patients.
Table 1.
Baseline characteristics of the 450 participants in the e‐Vita heart failure study
| n | Usual care (n = 150) | Website (n = 150) | E‐health adjusted care pathway (n = 150) | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 66.9 ± 11.6 | 66.7 ± 10.4 | 66.6 ± 11.0 | |
| Male sex | 109 (72.7) | 112 (74.7) | 113 (75.3) | |
| BMI, kg/m2 | 28 ± 4.1 | 28.1 ± 5.1 | 27.9 ± 5.6 | |
| Education level | 449 | 149 | ||
| Low | 34 (22.8) | 31 (20.7) | 34 (22.7) | |
| Middle | 66 (44.3) | 67 (44.7) | 59 (39.3) | |
| High | 49 (32.7) | 52 (34.7) | 57 (38.0) | |
| Married or living with a partner | 110 (73.3) | 123 (82.0) | 107 (71.3) | |
| 449 | 149 | |||
| Living with others | 111 (74.5) | 124 (82.7) | 114 (76.0) | |
| Current smoking | 29 (19.3) | 18 (12.0) | 21 (14.0) | |
| 432 | 145 | 143 | 144 | |
| Self‐care score on EHFSBcB scale | 70.6 ± 14.6 | 69.3 ± 16.4 | 72.0 ± 16.0 | |
| 432 | 145 | 143 | 144 | |
| Median HF‐related QoL | 23.0 ± 32.5 | 24.0 ± 31.0 | 23.0 ± 27.8 | |
| HF‐related characteristics | ||||
| Duration of HF in months | 40.6 ± 36.0 | 45.3 ± 42.4 | 38.5 ± 35.7 | |
| 432 | 142 | 145 | 145 | |
| LVEF, % | 36.2 ± 10.0 | 35.2 ± 11.1 | 35.6 ± 11.2 | |
| LVEF ≤ 40% | 66.7% | 73.3% | 71.3% | |
| NYHA classa | 428 | 143 | 144 | 141 |
| I | 57 (39.9) | 57 (39.6) | 69 (48.9) | |
| II | 55 (38.5) | 53 (36.8) | 46 (32.6) | |
| III | 24 (16.8) | 17 (11.8) | 17 (12.1) | |
| IV | 7 (4.9) | 17 (11.8) | 9 (6.4) | |
| Hypertension | 70 (46.7) | 62 (41.3) | 65 (43.3) | |
| Acute coronary syndrome | 71 (47.3) | 69 (39.3) | 72 (48.0) | |
| Stable angina pectoris | 28 (18.7) | 26 (17.3) | 20 (13.3) | |
| Atrial fibrillation | 54 (36.0) | 68 (45.3) | 66 (44.0) | |
| Other heart rhythm disorders | 44 (29.3) | 44 (29.3) | 42 (28.0) | |
| Valvular heart disease | 58 (38.7) | 66 (44.0) | 57 (38.0) | |
| Other co‐morbidities | ||||
| CVA | 20 (13.3) | 9 (6.0) | 25 (16.7) | |
| Hypercholesterolaemia | 43 (28.7) | 52 (34.7) | 51 (34.0) | |
| Diabetes mellitus | 39 (26.0) | 36 (24.0) | 40 (26.7) | |
| Renal failure | 22 (14.7) | 23 (15.3) | 24 (16.0) | |
| COPD | 30 (20.0) | 44 (29.3) | 36 (24.0) | |
| Medication | ||||
| Diureticsb | 121 (80.7) | 115 (76.7) | 100 (66.7) | |
| MRA | 61 (40.7) | 66 (44.0) | 59 (39.3) | |
| ACEI/ARBs | 122 (81.3) | 115 (76.7) | 115 (76.7) | |
| Beta‐blockers | 128 (85.3) | 123 (82.0) | 121 (80.7) | |
| Oral anticoagulants | 71 (47.3) | 72 (48.0) | 69 (46.0) | |
| Antiplatelet agents | 50 (33.3) | 49 (32.7) | 52 (34.7) | |
| Lipid‐lowering drugs | 79 (52.7) | 81 (54.0) | 72 (48.0) |
Values are presented as n (%) or mean ± standard deviation, if not specified.
ACEI, angiotensin‐converting‐enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident (including transient ischaemic attack); HF, heart failure; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; QoL, quality of life.
LVEF was < 40% in patients first diagnosed at admission to hospital, on average 3 years before participation in the trial.
Patient‐reported NYHA classes.
Includes loop diuretics and thiazides.
Primary endpoint (patient's self‐care)
At baseline, the mean self‐care on the EHFScB scale was 70.6 ± 14.6 in the UC, 69.3 ± 16.4 in the HFM, and 72.0 ± 16.0 in the EACP group. After 3 months, there was a significant (overall P < 0.001) difference in self‐care between the study groups; HFM vs. UC and EACP vs. UC; mean 73.5 vs. 70.8 [95% confidence interval (CI) 0.6–6.2], and 78.2 vs. 70.8 (95% CI 3.8–9.4), respectively. The significant effect attenuated during the following 9 months, with at 6 months HFM vs. UC mean 74.7 vs. 74.2, and EACP vs. UC 78.6 vs. 74.2, respectively (overall P = 0.070), and at 12 months HFM vs. UC mean 72.1 vs. 72.7, and EACP vs. UC 76.1 vs. 72.7, respectively (overall P‐value = 0.184) (Table 2).
Table 2.
Overall effect and effect per comparison of a website and an e‐health adjusted care pathway on patient self‐care after 3, 6, and 12 months unadjusted and adjusted for self‐care at baseline
| Mean | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Overall effect between the groups | Adjusted for self‐care at baseline | Overall effect between the groups | |||
| 95% CI | P‐value | 95% CI | P‐value | |||
| 3 months | <0.001 | <0.001 | ||||
| Usual care | 70.8 | ref | ref | |||
| Website | 73.5 | (−0.61 to 6.14) | (0.60 to 6.22) | |||
| E‐healtha | 78.2 | (4.05 to 10.80) | (3.80 to 9.43) | |||
| 6 months | 0.034 | 0.070 | ||||
| Usual care | 74.2 | ref | ref | |||
| Website | 74.7 | (−3.08 to 4.21) | (−2.08 to 4.38) | |||
| E‐healtha | 78.6 | (0.81 to 8.10) | (0.48 to 6.94) | |||
| 12 months | 0.082 | 0.184 | ||||
| Usual care | 72.7 | ref | ref | |||
| Website | 72.1 | (−4.45 to 3.21) | (−3.71 to 3.44) | |||
| E‐healtha | 76.1 | (−0.39 to 7.27) | (−0.74 to 6.41) | |||
CI, confidence interval.
E‐health adjusted care pathway.
Secondary outcomes
After 3 and 6 months, significant differences were observed in hrQoL between EACP and UC (median EACP 19.0 vs. UC 22.8, P = 0.029 and EACP 21.0 vs. UC 24.0, P‐value = 0.003), and at 3 months in HF knowledge (median EACP 13 vs. UC 13, P = 0.014). This effect attenuated during follow‐up, with at 12 months no clear differences between the groups in HrQoL and HF knowledge (Table 3).
Table 3.
Effect of a website and an e‐health adjusted care pathway on secondary outcomes after 3, 6, and 12 months
| Outcomes | 3 months | 6 months | 12 months | |||
|---|---|---|---|---|---|---|
| Median (n = 150) | 95% CI of the difference between the groups | Median n = 150) | 95% CI of the difference between the groups | Median (n = 150) | 95% CI of the difference between the groups | |
| Patient satisfaction about their HF care (0 = no satisfaction, 100 = maximal satisfaction) | ||||||
| Usual care | 75.7 | ref | 75.5 | ref | 75.3 | ref |
| Website | 76.1 | −6.33 to 7.39 | 75.2 | −7.03 to 6.48 | 71.5 | −12.32 to 1.79 |
| E‐healtha | 77.8 | −1.32 to 12.39 | 80.5 | −0.19 to 13.32 | 71.7 | −10.65 to 3.46 |
| HF‐related QoLb (0 = best QoL, 105 = worst QoL) | ||||||
| Usual care | 22.8 | ref | 24.0 | ref | 26.5 | ref |
| Website | 26.5 | −4.42 to 4.81 | 26.0 | −5.70 to 3.80 | 28.3 | −3.63 to 6.08 |
| E‐healtha | 19.0 | −9.76 to −0.53* | 21.0 | −11.90 to −2.40* | 25.5 | −7.90 to 1.81 |
| Disease‐specific knowledgec (0 = most insufficient knowledge, 15 = most sufficient knowledge) | ||||||
| Usual care | 13.0 | ref | 13.0 | ref | 13.0 | ref |
| Website | 13.0 | −0.18 to 10.49 | 13.0 | −0.19 to 0.50 | 13.0 | −0.28 to 0.39 |
| E‐healtha | 13.0 | 0.09 to 0.75* | 13.0 | −0.06 to 0.63 | 13.0 | −0.14 to 0.53 |
CI, confidence interval; HF, heart failure; QoL, quality of life.
E‐health adjusted care pathway.
Measured with the Minnesota Living with Heart Failure Questionnaire.
Measured with Dutch Heart Failure knowledge (DHFk) scale.
Significant.
There was no difference between groups in patient's satisfaction about the received HF care.
Finally, there were no clear differences in all‐cause mortality [HFM vs. UC 11 vs. 4, hazard ratio (HR) 2.82 (95% CI 0.90–8.87) and EACP vs. UC 8 vs. 4, HR 2.06 (95% CI 0.62–6.84)], and hospitalizations [HFM vs. UC 66 vs. 66, HR 0.98 (95% CI 0.70–1.38) and EACP vs. UC 57 vs. 66, HR 0.85 (95% CI 0.59–1.21)] between the groups (Table 4). Neither was this the case for disease specific hospitalizations and the duration of HF‐related hospitalizations.
Table 4.
Effect of a website and an e‐health adjusted care pathway on mortality and hospitalization
| Outcomes | n | HR | 95% CI of the difference between the groups |
|---|---|---|---|
| All‐cause mortality | |||
| Usual care | 4 | ref | ref |
| Website | 11 | 2.82 | 0.90 to 8.87 |
| E‐healtha | 8 | 2.06 | 0.62 to 6.84 |
| HF‐related mortality | |||
| Usual care | 3 | ref | ref |
| Website | 7 | 2.39 | 0.62 to 9.24 |
| E‐healtha | 3 | 1.03 | 0.21 to 5.11 |
| All‐cause hospitalizations | |||
| Usual care | 66 | ref | ref |
| Website | 66 | 0.98 | 0.70 to 1.38 |
| E‐healtha | 57 | 0.85 | 0.59 to 1.21 |
| HF‐related hospitalizations | |||
| Usual care | 12 | ref | ref |
| Website | 8 | 0.65 | 0.27 to 1.60 |
| E‐healtha | 7 | 0.57 | 0.23 to 1.45 |
CI, confidence interval; HF, heart failure; HR, hazard ratio.
E‐health adjusted care pathway.
Discussion
We observed an improvement in self‐care at 3 months when stable HF patients received the HFM website in addition to UC compared to UC alone (mean score on EHFScBs 73.5 vs. 70.8; difference 2.7, 95% CI 0.6–6.2). An EACP resulted in an even higher improvement when compared to UC (mean 78.2 vs. 70.8; difference 7.4, 95% CI 3.8–9.4). These effects attenuated during the following 9 months, and no clear differences were seen at 12 months between the groups. Secondary outcomes such as hrQoL and HF knowledge showed a similar trend, but mortality and hospitalizations did not clearly differ between the groups.
We are the first to evaluate the health effects of the HFM website. Previously, only the very short‐term (2 weeks) effect of a website with HF information was evaluated showing a significant effect on self‐care knowledge.20 In addition, just a few studies evaluated the effect of e‐health/telemonitoring interventions on self‐care. One of these is the recently published Dutch TEHAF study, also with UC provided by Dutch HF outpatient clinics. The effect of our EACP is in line with this study, reporting significant improvement in self‐care on the EHFScB scale for the telemonitoring group vs. UC at 12 months (17.4 ± 4.5) vs. 20.8 ± 5.8), P < 0.001).21 These unstandardized scores correspond with mean standardized scores on the EHFScB scale of 76.7 and 67.3, respectively, which is similar to our EHFScB scale scores at 3 months. The TEHAF study monitored HF symptoms, knowledge, and related behaviour, with the HF nurse intervening when patients gave high‐risk (inadequate) responses.21 Our study monitored just body weight, blood pressure and heart rate, and HF nurses intervened with lifestyle advices or drug treatment adjustments when reported values fell outside the pre‐determined limits.
The fact that the adjusted care pathway in our study also increased HF knowledge after 3 months is not surprising as HF knowledge is strongly related to self‐care.11 The positive effect on hrQoL after 3 and 6 months of follow‐up we found was also observed in previous telemonitoring studies.22, 23
Our study was designed and powered for the primary outcome self‐care. We also recorded mortality and hospitalizations, but our sample size was insufficient (underpowered) to formally compare these outcomes between the groups. There were no significant differences between the study groups in all‐cause mortality or HF‐related mortality, neither in all‐cause hospitalizations or HF‐related hospitalizations (Table 4).
A large previous study also executed in the Netherlands evaluated a disease management programme in patients from the HF outpatient clinics, powered on death and HF hospitalization, and showed a non‐significant beneficial effect on mortality and HF hospitalizations.24 A systematic review of 41 studies on e‐health in HF could show a clear significant beneficial effect on both mortality and HF hospitalizations of e‐health on top of UC. 23 Compared to other studies, we included a high percentage of NYHA class I patients in the e‐Vita HF study. This might partly be due to the fact that NYHA class was self‐reported, not clinician‐reported. Self‐reporting may lead to an over‐optimistic assessment of the NYHA class as patients adapt to the their clinical situation. In contrast, clinicians tend to assign a less favourable NYHA class, because they not only use patient‐reported symptoms, but also information from medical history, and results from clinical tests.25 Regarding generalization of our results, the mild severity of HF (79% NHYA class I or II) should be acknowledged. We assume that effects of the studied interventions are larger in patients with more severe HF, and in settings with a lower level of care as usual for HF as in the Netherlands.
Both HFM and EACP point to a short‐term effect on self‐care. Both e‐health tools, although, very different in nature, seem to address but not sustain the components necessary to maintain self‐care as measured with the EHFScB scale consisting of two components: self‐care maintenance (e.g. ‘I exercise regularly’) and self‐care management (e.g. ‘if I gain weight I contact a doctor or nurse’). Adding monitoring of shortness of breath and providing interactive learning functionalities like the pre‐set questions and dialogues in the TEHAF study (about symptoms, knowledge, and behaviour) enhance the sustainability of the effect given that the effect in the TEHAF study lasted over 12 months.21 Also, pro‐active e‐signals, for example ‘triggers’ and ‘push messages’ (i.e. any notification from an app while not actively in use) could be helpful to improve sustainability.26 In addition, incorporation of behavioural models in e‐health could be helpful for sustaining the effect on self‐care.27
The purpose of our study was to report on the effect of a website and the platform on several clinically relevant outcomes, not on actual use or uptake of the tools. How the adjusted care pathway affects usage of care, workload and costs in comparison to UC and the HFM website group will be reported in the cost‐effectiveness analysis of the e‐Vita HF study. We were able to include the number of patients as needed based on our sample size calculations, which allows us to draw robust conclusions regarding our primary outcome of self‐care.
Limitations
A participation rate of 23% is low, but rather comparable to previous telemonitoring studies that reported these rates.23 Low participation rates may impede generalizability, but are not a methodological problem, i.e. a flaw in design or bias. The low participation in our study is a realistic representation of the proportion actually willing to use e‐health, when in stable HF, which is valuable information for future use. We did not register the reasons for not participating, and therefore do not know how many patients refused because of a lack of reliable internet access.
We explicitly aimed to include stable outpatients, and the use of e‐health as a replacement of routine face‐to‐face contacts. Then, non‐invasive e‐health is most feasible and may safely (and cost‐effective) reduce routine control visits. This aim resulted, as could be expected, in a relatively young and healthy study population (on average 3.4 years known with HF, mean age 67 ± 11) years).28 Straightforward applicability to all outpatients with HF is not possible, and also not justified. In addition, participants had relatively low percentages of co‐morbidities. The aforementioned implies that cardiologists and HF nurses should realize that our results constrain to stable HF outpatients with a low NYHA class.
The low number of events, e.g. mortality and HF hospitalizations, in our study was due to the fact that we only included stable HF patients from the HF outpatient clinic, and the majority of these patients had NYHA class I–II. We therefore had (on purpose) no participants who were just discharged from hospital (including those who die within a few months), or patients who were unstable/in NYHA class III and IV.
Missing data (20% of the participants did not complete all questionnaires) was imputed by multiple imputation, allowing us to analyse the entire dataset. A method that results in more credible outcomes as was shown by simulation studies.29 We also imputed values on self‐care for the patients who died during the follow‐up. To exclude accompanying bias, we performed a sensitivity analysis excluding those patients (5.1%), which revealed similar results.
In our study we registered the medication use at the start and end of the study. We do not have longitudinal data and cannot assess if changes in drug use affected the results. Importantly, however, an item of the EHFScB scale is ‘intake of prescribed medication’, and we showed a significant improvement on this self‐care scale in the first 3 months, with a persisting but non‐significant trend over the following 9 months in the EACP. Because the scale consists of multiple items, it is however impossible to assess which items were most important.
Of notice, the care delivered by HF outpatient clinics, the Netherlands is intensive compared to many other European countries with on average three to four routine consultations a year.24, 30 Therefore, it is a challenge to surpass the effect of UC on self‐care and other outcomes with the interventions and effects of the studied interventions may be (much) larger in more deprived areas.31
Although the interpretability of the EHFScB scale was recently evaluated,32 it remains difficult to assess a clinically relevant difference in scores.
In addition, our results might suggest that replacement of routine care as performed in our study was safe. However, we can only conclude that we identified no clear safety concerns. To formally prove safety of an intervention in a study would require a non‐inferiority design instead of a superiority design. The former would imply a non‐inferiority trial, which in general requires a much larger sample size if events related to safety do not occur frequently.33
Finally, the HFM website is freely accessible since 2012 and even though we explicitly instructed the HF nurses not to encourage this, patients in the UC group may have visited the site themselves, which could have resulted in a smaller effect (i.e. difference) between the intervention groups and UC. Indeed, according to a patient reported questionnaire filled out at the end of the study, 21% of the patients in the UC group had visited the HFM website once or more often. In the HFM and EACP, where patients were stimulated to use the HFM website, 57%, and 66% respectively visited the website once or more often.
In conclusion, we showed that both the HFM website and e‐health platform improved self‐care in HF patient on the short term, but this effect attenuated during the following 9 months. Continuous updating of e‐health facilities to help sustain effects over longer time should be considered for evaluation.
Implications for clinical practice
Our primary outcome was a patient relevant outcome measure. The shortcoming of applying the EHFScB scale is that the clinical relevance is unclear of the significance difference on the scale we found in the first 3 months for both HFM and EAPC. There is yet no consensus on which change in this score is clinically meaningful. Our study may provide key information that is helpful to define such clinical meaningfulness with the help of future studies evaluating the EHFScB scale. Our results on the secondary outcomes of all‐cause and HF‐related mortality and hospitalizations are useful for updating the individual patient data systematic review on e‐health in HF. Nevertheless, based on our study results, the use of the HFM website may be recommended to educate HF patients. The website may positively effects self‐care, is freely accessible, and the use by patients or their relatives followed by discussion with the HF nurse does not require serious changes in the infrastructure of the health care system. To decide on implementation of an EACP, further research on sustainability of the effect and cost‐effectiveness would be needed.
Acknowledgements
First of all, we thank all the participants, and patients that helped us with the development and testing of the e‐Vita platform and heartfailurematters.org website. Second, we want to thank the heart failire nurses of the participating outpatient clinics for the pleasant collaboration and their hard work: Patricia Ninaber, Will van Zimmeren, Wim Janssen, Joke Froon, Jeanine Zimmerman, Hester Vermeulen, Germa Tuin, Agneta Markusse, Netty Koot, Jacqueline van Santvoort, Vicky Kneijber, Marjan Aertsen, Judith Grooters, Inge Walstra, Maureen Roes, Lydia Molenaar, Els van Scherpenzeel, Mireille Kragting, Marlies Niesing‐Lut, Anneke van Haarlem, Jolanda Flaman‐van der Hoogt, Marijke Martherus, Elly Rodijk, Joke Pluimers, Bernarda Beverdam, Huub van Amerongen, Astrid Uitzetter, Danielle Doeschot, Diana Veldhuis, Carrolien Schouten, Petra op den Kelder, and Riet Schouten. Third, we thank the staff from the hospital laboratories. Fourth, we are grateful for the work of the e‐Vita platform helpdesks: Mireille Donkervoort, Leony van Kooten, Wietse Veenstra, Jan Willem Brakel. Fifth, we like to thank Mireille Kragting, Marjon van der Meer and Curt Brugman for helping us to carry out the study and their contribution to the data collection. Finally, we thank the MSc students for all their work in the data collection as well and Peter Zuithoff for helping us with the statistical analysis.
Funding
The study was supported by the Foundation ‘Care Within Reach’ (In Dutch: Stichting Zorg Binnen Bereik).
Conflict of interest: none declared.
References
- 1. Dunlay SM, Roger VL. Understanding the epidemic of heart failure: past, present, and future. Curr Heart Fail Rep 2014;11 (4):404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007;93:1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 4. Cowie MR, Bax J, Bruining N, Cleland JG, Koehler F, Malik M, Pinto F, van der Velde E, Vardas P. e‐Health: a position statement of the European Society of Cardiology. Eur Heart J 2016;37:63–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jonkman NH, Westland H, Groenwold RH, Agren S, Atienza F, Blue L, Bruggink‐Andre de la Porte PW, DA DW, Hebert PL, Heisler M, Jaarsma T, Kempen GI, Leventhal ME, Lok DJ, Martensson J, Muniz J, Otsu H, Peters‐Klimm F, Rich MW, Riegel B, Stromberg A, Tsuyuki RT, van Veldhuisen DJ, Trappenburg JC, Schuurmans MJ, Hoes AW. Do self‐management interventions work in patients with heart failure? An individual patient data meta‐analysis. Circulation 2016;133:1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Spall HG, Rahman T, Mytton O, Ramasundarahettige C, Ibrahim Q, Kabali C, Coppens M, Brian Haynes R, Connolly S. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta‐analysis. Eur J Heart Fail 2017;19:1427–1443. [DOI] [PubMed] [Google Scholar]
- 7. Adamson PB, Ginn G, Anker SD, Bourge RC, Abraham WT. Remote haemodynamic‐guided care for patients with chronic heart failure: a meta‐analysis of completed trials. Eur J Heart Fail 2017;19:426–433. [DOI] [PubMed] [Google Scholar]
- 8. Inglis SC, Clark RA, McAlister FA, Stewart S, Cleland JG. Telemedicine and remote management of heart failure. Lancet 2011;378:e9 author reply e10. [DOI] [PubMed] [Google Scholar]
- 9. Takahashi PY, Pecina JL, Upatising B, Chaudhry R, Shah ND, Van Houten H, Cha S, Croghan I, Naessens JM, Hanson GJ. A randomized controlled trial of telemonitoring in older adults with multiple health issues to prevent hospitalizations and emergency department visits. Arch Intern Med 2012;172:773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wagenaar KP, Rutten FH, Klompstra L, Bhana Y, Sieverink F, Ruschitzka F, Seferovic PM, Lainscak M, Piepoli MF, Broekhuizen BDL, Stromberg A, Jaarsma T, Hoes AW, Dickstein K. ‘heartfailurematters.org’, an educational website for patients and carers from the Heart Failure Association of the European Society of Cardiology: objectives, use and future directions. Eur J Heart Fail 2017;19:1447–1454. [DOI] [PubMed] [Google Scholar]
- 11. Wagenaar KP, Broekhuizen BD, Dickstein K, Jaarsma T, Hoes AW, Rutten FH. Effectiveness of an interactive platform, and the ESC/HFA heartfailurematters.org website in patients with heart failure: design of the multicentre randomized e‐Vita heart failure trial. Eur J Heart Fail 2015;17:1310–1316. [DOI] [PubMed] [Google Scholar]
- 12. World Medical A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 13. Jaarsma T, Stromberg A, Martensson J, Dracup K. Development and testing of the European Heart Failure Self‐Care Behaviour Scale. Eur J Heart Fail 2003;5:363–370. [DOI] [PubMed] [Google Scholar]
- 14. Jaarsma T, Arestedt KF, Martensson J, Dracup K, Stromberg A. The European Heart Failure Self‐care Behaviour scale revised into a nine‐item scale (EHFScB‐9): a reliable and valid international instrument. Eur J Heart Fail 2009;11:99–105. [DOI] [PubMed] [Google Scholar]
- 15. Vellone E, Jaarsma T, Stromberg A, Fida R, Arestedt K, Rocco G, Cocchieri A, Alvaro R. The European Heart Failure Self‐care Behaviour Scale: new insights into factorial structure, reliability, precision and scoring procedure. Patient Educ Couns 2014;94:97–102. [DOI] [PubMed] [Google Scholar]
- 16. Garin O, Ferrer M, Pont A, Rue M, Kotzeva A, Wiklund I, Van Ganse E, Alonso J. Disease‐specific health‐related quality of life questionnaires for heart failure: a systematic review with meta‐analyses. Qual Life Res 2009;18:71–85. [DOI] [PubMed] [Google Scholar]
- 17. van der Wal MH, Jaarsma T, Moser DK, van Veldhuisen DJ. Development and testing of the Dutch Heart Failure Knowledge Scale. Eur J Cardiovasc Nurs 2005;4:273–277. [DOI] [PubMed] [Google Scholar]
- 18. Jaarsma T, Van Der Wal MH, Hogenhuis J, Lesman I, Luttik ML, Veeger NJ, Van Veldhuisen DJ. Design and methodology of the COACH study: a multicenter randomised Coordinating study evaluating Outcomes of Advising and Counselling in Heart failure. Eur J Heart Fail 2004;6:227–233. [DOI] [PubMed] [Google Scholar]
- 19. Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol 2006;59:1087–1091. [DOI] [PubMed] [Google Scholar]
- 20. Jovicic A, Chignell M, Wu R, Straus SEI. web‐only self‐care education sufficient for heart failure patients? AMIA Annu Symp Proc 2009;2009:296–300. [PMC free article] [PubMed] [Google Scholar]
- 21. Boyne JJ, Vrijhoef HJ, Spreeuwenberg M, De Weerd G, Kragten J, Gorgels AP; TEHAF Investigators . Effects of tailored telemonitoring on heart failure patients’ knowledge, self‐care, self‐efficacy and adherence: a randomized controlled trial. Eur J Cardiovasc Nurs 2014;13:243–252. [DOI] [PubMed] [Google Scholar]
- 22. Seto E, Leonard KJ, Cafazzo JA, Barnsley J, Masino C, Ross HJ. Mobile phone‐based telemonitoring for heart failure management: a randomized controlled trial. J Med Internet Res 2012;14:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inglis SC, Clark RA, Dierckx R, Prieto‐Merino D, Cleland JG. Structured telephone support or non‐invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev 2015;10:CD007228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jaarsma T, van der Wal MH, Lesman‐Leegte I, Luttik ML, Hogenhuis J, Veeger NJ, Sanderman R, Hoes AW, van Gilst WH, Lok DJ, Dunselman PH, Tijssen JG, Hillege HL, van Veldhuisen DJ; Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH) Investigators. Counseling in Heart Failure I . Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH). Arch Intern Med 2008;168:316–324. [DOI] [PubMed] [Google Scholar]
- 25. Holland R, Rechel B, Stepien K, Harvey I, Brooksby I. Patients’ self‐assessed functional status in heart failure by New York Heart Association class: a prognostic predictor of hospitalizations, quality of life and death. J Card Fail 2010;16:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu S, Dunford SD, Leung YW, Brooks D, Thomas SG, Eysenbach G, Nolan RP. Reducing blood pressure with Internet‐based interventions: a meta‐analysis. Can J Cardiol 2013;29:613–621. [DOI] [PubMed] [Google Scholar]
- 27. Nolan RP, Payne AY, Ross H, White M, D'Antono B, Chan S, Barr SI, Gwadry‐Sridhar F, Nigam A, Perreault S, Farkouh M, McDonald M, Goodman J, Thomas S, Zieroth S, Isaac D, Oh P, Rajda M, Chen M, Eysenbach G, Liu S, Zbib A. An Internet‐based counseling intervention with email reminders that promotes self‐care in adults with chronic heart failure: randomized controlled trial protocol. JMIR Res Protoc 2014;3:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wagenaar KP, Hakim N, Broekhuizen BD, Jaarsma T, Rutten FH, Hoes AW. Representativeness of participants in heart failure e‐health trials: a report from the e‐vita HF study. J Card Fail 2017;23:88–89. [DOI] [PubMed] [Google Scholar]
- 29. Groenwold RH, Donders AR, Roes KC, Harrell FE Jr, Moons KG. Dealing with missing outcome data in randomized trials and observational studies. Am J Epidemiol 2012;175:210–217. [DOI] [PubMed] [Google Scholar]
- 30. Boyne JJ, Vrijhoef HJ, Crijns HJ, De Weerd G, Kragten J, Gorgels AP, TEHAF Investigators . Tailored telemonitoring in patients with heart failure: results of a multicentre randomized controlled trial. Eur J Heart Fail 2012;14:791–801. [DOI] [PubMed] [Google Scholar]
- 31. Dendale P, De Keulenaer G, Troisfontaines P, Weytjens C, Mullens W, Elegeert I, Ector B, Houbrechts M, Willekens K, Hansen D. Effect of a telemonitoring‐facilitated collaboration between general practitioner and heart failure clinic on mortality and rehospitalization rates in severe heart failure: the TEMA‐HF 1 (TElemonitoring in the MAnagement of Heart Failure) study. Eur J Heart Fail 2012;14:333–340. [DOI] [PubMed] [Google Scholar]
- 32. Wagenaar KP, Broekhuizen BD, Rutten FH, Stromberg A, van Stel HF, Hoes AW, Jaarsma T. Interpretability of the European Heart Failure Self‐care Behaviour scale. Patient Prefer Adherence 2017;11:1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schumi J, Wittes JT. Through the looking glass: understanding non‐inferiority. Trials 2011;12:106. [DOI] [PMC free article] [PubMed] [Google Scholar]


