Abstract
Over the past 13 years bone marrow‐derived mononuclear cells (BM‐MNCs) have been widely investigated for clinical efficacy in patients following acute myocardial infarction (AMI). These early phase II trials have used various surrogate markers to judge efficacy and, although promising, the results have been inconsistent. The phase III BAMI trial has therefore been designed to demonstrate that intracoronary infusion of BM‐MNCs is safe and will significantly reduce the time to first occurrence of all‐cause death in patients with reduced left ventricular ejection fraction after successful reperfusion for ST‐elevation AMI (powered with the aim of detecting a 25% reduction in all‐cause mortality). This is a multinational, multicentre, randomized, open‐label, controlled, parallel‐group phase III study aiming to enrol approximately 3000 patients in 11 European countries with at least 17 sites. Eligible patients who have impaired left ventricular ejection (≤45%) following successful reperfusion for AMI will be randomized to treatment or control group in a 1:1 ratio. The treatment group will receive intracoronary infusion of BM‐MNCs 2–8 days after successful reperfusion for AMI added on top of optimal standard of care. The control group will receive optimal standard of care. The primary endpoint is time from randomization to all‐cause death. The BAMI trial is pivotal and the largest trial to date of BM‐MNCs in patients with impaired left ventricular function following AMI. The aim of the trial is to provide a definitive answer as to whether BM‐MNCs reduce all‐cause mortality in this group of patients.
Keywords: Cell therapy, Cardiovascular disease, Bone marrow‐derived mononuclear cells, Myocardial infarction, Heart failure, Cardiac regeneration, BAMI
Introduction
Despite the widespread adoption of mechanical reperfusion for acute myocardial infarction (AMI) and the use of optimal medical therapy, the medium‐to‐long term mortality remains as high as 7–12%.1, 2, 3 Impaired left ventricular ejection fraction (LVEF) following AMI is a powerful predictor of mortality4 as well as morbidity and increased risk of hospitalization for heart failure.5 These realities have fuelled the search for novel therapies to improve cardiac function following AMI. Myocardial regeneration following bone marrow‐derived mononuclear cell (BM‐MNC) therapy was initially demonstrated in a murine model of AMI6 at the beginning of the 21st century. Ever since, there has been an enormous effort to try and translate this exciting pre‐clinical promise into clinically meaningful benefits for patients. The results of relatively small phase II clinical trials have been heterogeneous, some showing improvement in cardiac function and others no effect of cell therapy. This may be due to a number of reasons including the difference in cell isolation and preparation protocols (different number of cells and compositions), the timing of the infusion procedure, baseline ejection fraction, and study design. Importantly, all the studies have shown relative safety and feasibility of bone marrow aspiration and infusion of cells early after AMI. In fact, meta‐analysis studies have demonstrated a signal towards improved clinical outcome in patients treated with BM‐MNCs, including, importantly, overall mortality.7, 8 The European Society of Cardiology (ESC) task force on stem cells in cardiac disease comprises some of the key European trialists (17 members from 11 EU countries) that have contributed to clinical trials in this area and are leading experts in this field.9 The ESC Task Force has reached the consensus view that this therapy needs standardizing and testing in a definitive outcome endpoint clinical trial. This translated into the design of the BAMI study, the methods and rationale of which are described in this paper. The aim of the BAMI pivotal outcome trial is to demonstrate that intracoronary infusion of bone marrow‐derived progenitor cells is safe and will significantly reduce the time to first occurrence of all‐cause death in patients with reduced LVEF after successful reperfusion for acute ST‐elevation myocardial infarction (STEMI).
Methods
The full protocol can be found in Appendix S1 in the Supplementary material online.
Study objectives
The primary endpoint of the BAMI study is time from randomization to all‐cause death. Secondary efficacy endpoints include major adverse cardiac events—a full list of which is given in Table S1 in the Supplementary material online.
Overview of study flow
The BAMI study is a phase III study with a randomized controlled open‐label design. Approximately 3000 STEMI patients are planned to be enrolled in 11 European countries with at least 17 sites.
Patients with an acute STEMI as defined by the universal definition of AMI undergoing acute revascularization [i.e. either acute percutaneous coronary intervention (PCI) within 24 h of symptom onset or thrombolysis within 12 h followed by acute PCI within 24 h of symptom onset] will be screened at investigational sites. Further details on screening is given in the ‘Patient screening and randomization’ section.
For patients assigned to the BM‐MNC group, 50 ml bone marrow will be aspirated under local anaesthesia from the iliac crest. Bone marrow will be sent to one of the central cell processing centres without delay allowing that cell processing can start within 24 h of bone marrow aspiration. The final investigational medicinal product with BM‐MNCs will be produced and sent back to the investigational site. Intracoronary infusion will be performed no later than 24 h after formulation of the final product and between 2 and 8 days after successfully reperfused ST elevation AMI (summarized in Figure 1). Intracoronary infusion of BM‐MNCs will be performed via conventional percutaneous intracoronary intervention techniques using an over‐the‐wire balloon and stop‐flow method with low pressure balloon inflation.10 Importantly, peri‐procedural anticoagulation must be performed with bivalirudin and not heparin at all recruiting institutions. Patients in the control group will not undergo any of the described interventions and will not receive the BM‐MNC therapy. All patients will be treated with optimal post‐myocardial infarction pharmacological treatment. Between 9 and 12 months post‐primary PCI, dual anti‐platelet therapy as per local practice should be applied.
Figure 1.
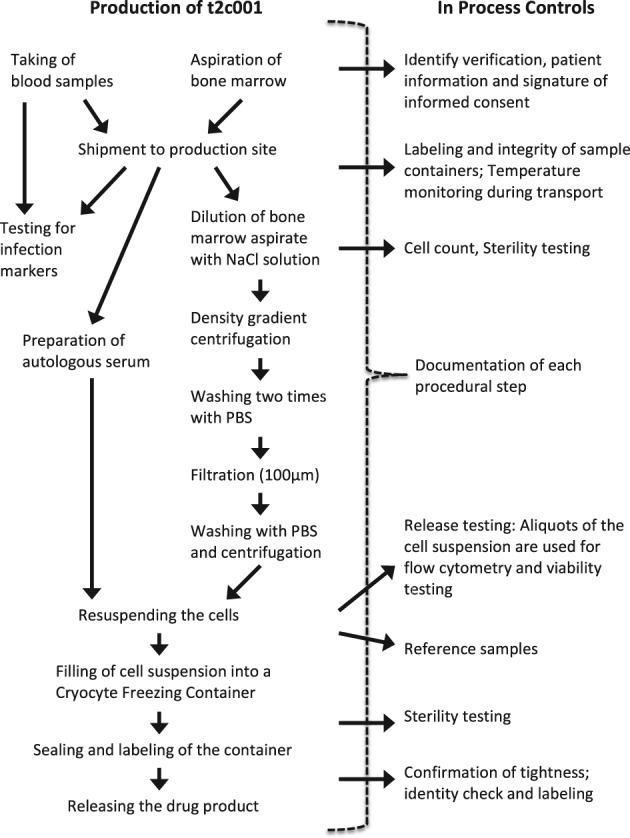
Flow‐chart of t2c001 drug substance production. PBS, phosphate‐buffered saline.
After hospital discharge, all study patients will return to the clinical centre for an onsite visit at 30 days. They will then be followed up by telephone at 3 months after randomization and every 3 months thereafter until the required number of events has been observed. At this point, all patients will attend a final site visit at the clinical centre. However, minimum follow‐up is 2 years for each patient, so the final study visit may be performed later for patients who are in the study for less than 2 years when the full number of events is observed. Endpoints will be reported as occurring throughout the follow‐up. The defined cardiovascular efficacy endpoints will be adjudicated by an independent Clinical Event Committee blinded to the patient treatment allocation. This ensures a consistent and unbiased adjudication of events across all investigational sites.
The overall study flow is depicted in Figure 2.
Figure 2.
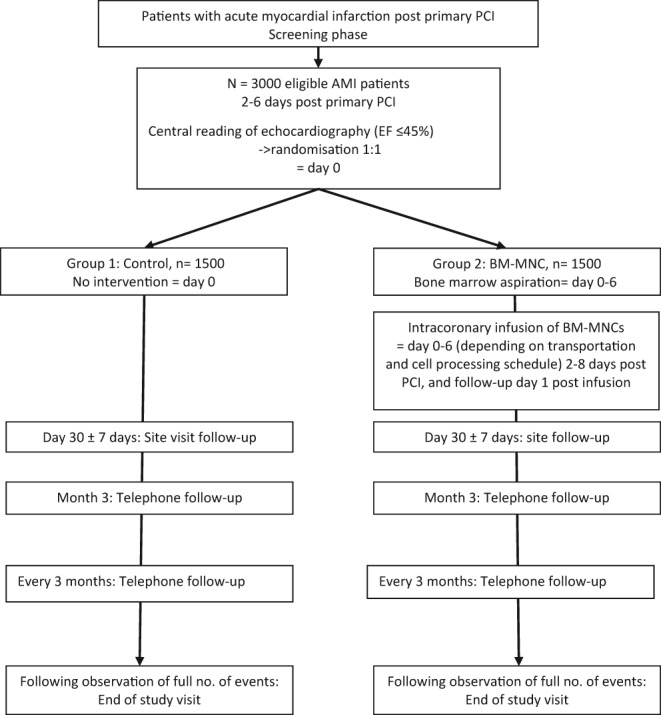
BAMI study flowchart. AMI, acute myocardial infarction; BM‐MNC, bone marrow‐derived mononuclear cell; EF, ejection fraction.
Study population
A total of approximately 3000 male and female patients are planned to be enrolled in the BAMI study. Enrolment will involve at least 17 sites in 11 countries. Investigational sites will keep a logbook of all patients proposed to be included.
Table S2 in the Supplementary material online provides a full list of the inclusion and exclusion criteria for enrolment in the BAMI trial.
Patient screening and randomization
Patients will be recruited based on the existing medical information from the pool of AMI patients at the clinical centres. Patients who had primary PCI performed at clinical centres that are different from the investigational sites can also be included: interested patients may be referred for screening to any of the participating study sites within 2–4 days. Informed consent and assessment of eligibility of patients with respect to inclusion and exclusion criteria will be done at the investigational site. Written informed consent must be obtained from patients prior to any study‐specific procedure. After consenting, patients undergo the procedures listed for the screening visit.
After ensuring that a patient meets all other eligibility criteria, the investigator will perform the echocardiographic examination. The electronic record of echocardiography will be transferred to the central Echocardiography Core Laboratory for quantitative analysis. Patients with LVEF ≤45% will be randomized to control arm (Group 1) or treatment arm (Group 2) in a 1:1 ratio, stratified by country. Patients with LVEF >45% will not be randomized, but will be classified as screening failures.
Patients in the BM‐MNC treatment group that were randomized but not treated with BM‐MNCs will be replaced. After treatment with investigational medicinal product, patients who discontinue the study will not be replaced. Patients in the control group will not be replaced after randomization.
Study duration for study patients
Each enrolled patient will remain in the study throughout the entire study duration, with a minimum follow‐up of 2 years for each patient. A study patient's participation may be terminated early for reasonable cause, such as the investigator's medical decision. At any time, the patient has the right to withdraw consent without a negative impact on her/his medical treatment. However, the investigators are encouraged to ask for the patient's permission for further follow‐up telephone contacts from those whose decision for discontinuation was based on the need for further outpatient visits.
Duration of the whole study
Patients will be enrolled during a recruitment phase of approximately 3 years. Since minimum study duration for one patient is 2 years, the overall study duration is approximately 5 years. The Data Monitoring Committee may terminate the study earlier based on safety concerns at any time. The sponsor may also terminate the study early for reasonable cause. Competent authorities/ethics committees retain the right for premature termination of the study according to applicable regulations. At an individual study centre, the study may be terminated early if the work performed is not compliant with Good Clinical Practice.
Sample size and statistical analysis
This trial is designed as an event‐driven trial. The estimated required number of events is based on the assumptions that there will be a 12% event (death) rate at 2 years in the control group, which will be reduced to 9% (25% relative reduction, 1.355 hazard ratio) in the BM‐MNC group. In addition, the study will be designed as a group‐sequential study with one interim analysis performed after 150 deaths have been observed. Early termination will be possible at this stage if the experimental group shows superiority in comparison to the control group at a statistical significance level of 1%. However, all patients recruited must complete a 2 year follow‐up, even if the trial is stopped prematurely. Using an alpha‐spending function according to Lan–DeMets,11 an initial total of 450 events will be required to have 90% statistical power to detect the above treatment effect.
With an accrual period of 3 years, total study duration of 5 years and an expected drop‐out rate of 10% at 2 years, it is estimated that a total of 3000 patients will be needed to observe the required number of events during the study period.
The efficacy analyses of this study will be performed on the full analysis set (FAS), including all randomized patients according to their randomized treatment. In case of many protocol violations, a per protocol set (PPS) will be defined excluding patients with serious protocol violations. Finally, a safety set will be defined, including all patients who were treated according to their actual treatment, for the assessment of safety parameters. The primary endpoint of all‐cause mortality will be presented using Kaplan–Meier curves by treatment group. The curves will be compared using a log‐rank test. Statistical significance will be claimed when the resulting P‐value is lower than 0.043. The secondary and safety endpoints will be summarized using cumulative incidence functions (CIFs), allowing for the competing risk of non‐cardiac death in the case of cardiac mortality and for the competing risk of all‐cause death without cardiac hospitalization in the case of cardiac rehospitalization or non‐cardiac hospitalization in the case of cardiac rehospitalization. Comparison of the CIF between treatment groups will be done by means of the Pepe–Mori test. Statistical significance for the secondary endpoints will be assessed at a significance level of 5%. Primary and secondary endpoints will be assessed for the FAS and the PPS, if defined.
Discussion
The design of the pivotal BAMI study described above has several important considerations. This is the first phase III study of BM‐MNCs in AMI, which is powered with the aim of detecting a 25% reduction in all‐cause mortality. Previous trials have mainly relied on surrogate markers such as LVEF to judge efficacy. The results of these trials, listed in Table S3 in the Supplementary material online, have been inconsistent with variable or no improvement in left ventricular function. Interestingly, improved clinical outcomes including mortality and rehospitalization for heart failure have been seen in long‐term follow‐up of the REPAIR‐AMI study.12 Furthermore, meta‐analysis studies have also demonstrated a signal towards reduced mortality and morbidity following BM‐MNC therapy.7, 8 It is also important to remember that dramatic changes in LVEF were not seen in clinical trials of therapies in patients with AMI that have subsequently been shown to have significant reductions in mortality.13 The purpose of the BAMI trial is therefore to investigate this potential mortality‐lowering effect of BM‐MNC therapy in an appropriately powered study.
A randomized design with a control group not undergoing any planned interventional procedure (i.e. bone marrow aspiration or PCI) was chosen to enable evaluation of the addition of the study treatment on top of the current best standard of care for three reasons. First, inclusion of a true placebo group (‘sham‐intervention’) is generally ethically controversial. Second, the previous double‐blind, placebo‐controlled, REPAIR‐AMI trial has already documented a beneficial effect of intracoronary administration of bone marrow‐derived progenitor cells on functional left ventricular contractile recovery post‐AMI as well as on long‐term mortality and morbidity.10, 12, 14 Third, there are small risks associated with the interventions required for BM‐MNC therapy, though these have been evaluated as acceptable in the risk/benefit assessment for the cell therapy group.
As mentioned previously, the results of previous phase II trials have been inconsistent with regard to efficacy and potential reasons for this include heterogeneity in study design and cell isolation protocols. An important aspect of the BAMI trial has been standardization of the cell processing (using multiple core lab facilities) and intracoronary infusion techniques. For example, only bivalirudin is recommended for peri‐procedural anticoagulation during infusion, in view of recent data demonstrating impaired migration and homing function of BM‐MNCs exposed to heparin.15 Also, the infusion procedure will be performed within 2–8 days after AMI in all patients as this appears to be the most efficacious time‐point from meta‐analysis.8 The trial has been designed using the intracoronary rather than intramyocardial infusion method based on existing data (Supplementary material online, Table S3) and the concerns of ventricular perforation using the currently available percutaneous intramyocardial injection systems at the time of AMI.
Finally, objective unbiased measurement of LVEF in the Echocardiography Core Lab after web‐based transmission will ensure that only patients with LVEF ≤45% will be included in order to select for high‐risk survivors of AMI.
The BAMI trial is therefore an ambitious venture but with a robust study design which addresses the limitations of the previous smaller phase II trials. Hard clinical outcome data have been lacking in this field and this trial will for the first time provide an answer as to whether BM‐MNC therapy lowers mortality following AMI.
Conclusions
The pivotal BAMI study will be the largest study of BM‐MNCs in patients with impaired left ventricular systolic function following successful reperfusion for acute STEMI. The objective of the trial is simple with a single primary endpoint aiming to detect a 25% reduction in all‐cause mortality with BM‐MNC therapy. The results of the BAMI trial will be highly anticipated, as the field of regenerative therapy will finally have an appropriately sized trial to provide an answer for the most important clinical outcome of all.
Funding
The BAMI Project is partially funded by the European Commission under the 7th Framework Programme (Grant Agreement number 278967). The trial is registered with ClinicalTrials.gov Identifier: NCT01569178.
Conflict of interest: A.H.‐N. is an employee of t2cure, which licensed the cell product used in the trial. B.A. reports grants from LOEWE (Landes‐Offensive zur Entwicklung Wissenschaftlich‐ökonomischer Exzellenz) Center for Cell and Gene Therapy (State of Hessen), during the conduct of the study; grants and personal fees from St Jude Medical, personal fees from Novartis, Servier, Boehringer‐Ingelheim, Pfitzer, outside the submitted work. J.H. reports grants from State (Finland) Research Fund (to institution) during the conduct of the study. S.D. reports grants from null during the conduct of the study; grants, personal fees, non‐financial support and other from null, outside the submitted work. In addition, S.D. has a patent null licensed and is a Founder and shareholder of t2cure. All other authors have nothing to disclose.
Supporting information
Appendix S1. BAMI protocol.
Appendix S2. BAMI trial logistics ‐ current trial and recruitment status.
Table S1. BAMI trial endpoints.
Table S2. BAMI trial inclusion and exclusion criteria.
Table S3. Clinical trials of stem cell therapy in acute myocardial infarction.
ClinicalTrials.gov Identifier: NCT01569178
References
- 1. Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Fahy M, Parise H, Mehran R; HORIZONS‐AMI Trial Investigators . Heparin plus a glycoprotein IIb/IIIa inhibitor versus bivalirudin monotherapy and paclitaxel‐eluting stents versus bare‐metal stents in acute myocardial infarction (HORIZONS‐AMI): final 3‐year results from a multicentre, randomised controlled trial. Lancet 2011;377:2193–2204. [DOI] [PubMed] [Google Scholar]
- 2. Setoguchi S, Glynn RJ, Avorn J, Mittleman MA, Levin R, Winkelmayer WC. Improvements in long‐term mortality after myocardial infarction and increased use of cardiovascular drugs after discharge: a 10‐year trend analysis. J Am Coll Cardiol 2008;51:1247–1254. [DOI] [PubMed] [Google Scholar]
- 3. Keeley EC, Boura JA, Grines CL. Comparison of primary and facilitated percutaneous coronary interventions for ST‐elevation myocardial infarction: quantitative review of randomised trials. Lancet 2006;367:579–588. [DOI] [PubMed] [Google Scholar]
- 4. Ng VG, Lansky AJ, Meller S, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie B, Shah R, Mehran R, Stone GW. The prognostic importance of left ventricular function in patients with ST‐segment elevation myocardial infarction: the HORIZONS‐AMI trial. Eur Heart J Acute Cardiovasc Care 2014;3:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelly DJ, Gershlick T, Witzenbichler B, Guagliumi G, Fahy M, Dangas G, Mehran R, Stone GW. Incidence and predictors of heart failure following percutaneous coronary intervention in ST‐segment elevation myocardial infarction: the HORIZONS‐AMI trial. Am Heart J 2011;162:663–670. [DOI] [PubMed] [Google Scholar]
- 6. Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal‐Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature 2001;410:701–705. [DOI] [PubMed] [Google Scholar]
- 7. Jeevanantham V, Butler M, Saad A, Abdel‐Latif A, Zuba‐Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long‐term improvement in cardiac parameters: a systematic review and meta‐analysis. Circulation 2012;126:551–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin‐Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J 2008;29:1807–1818. [DOI] [PubMed] [Google Scholar]
- 9. Bartunek J, Dimmeler S, Drexler H, Fernandez‐Aviles F, Galinanes M, Janssens S, Martin J, Mathur A, Menasche P, Priori S, Strauer B, Tendera M, Wijns W, Zeiher A. The consensus of the task force of the European Society of Cardiology concerning the clinical investigation of the use of autologous adult stem cells for repair of the heart. Eur Heart J 2006;27:1338–1340. [DOI] [PubMed] [Google Scholar]
- 10. Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Süselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM; REPAIR‐AMI Investigators . Intracoronary bone marrow‐derived progenitor cells in acute myocardial infarction. N Engl J Med 2006;355:1210–1221. [DOI] [PubMed] [Google Scholar]
- 11. Lan KK, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659–663. [Google Scholar]
- 12. Assmus B, Rolf A, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Tillmanns H, Yu J, Corti R, Mathey DG, Hamm CW, Süselbeck T, Tonn T, Dimmeler S, Dill T, Zeiher AM, Schächinger V; REPAIR‐AMI Investigators . Clinical outcome 2 years after intracoronary administration of bone marrow‐derived progenitor cells in acute myocardial infarction. Circ Heart Fail 2010;3:89–96. [DOI] [PubMed] [Google Scholar]
- 13. Reffelmann T, Konemann S, Kloner RA. Promise of blood‐ and bone marrow‐derived stem cell transplantation for functional cardiac repair: putting it in perspective with existing therapy. J Am Coll Cardiol 2009;53:305–308. [DOI] [PubMed] [Google Scholar]
- 14. Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Süselbeck T, Werner N, Haase J, Neuzner J, Germing A, Mark B, Assmus B, Tonn T, Dimmeler S, Zeiher AM; REPAIR‐AMI Investigators . Improved clinical outcome after intracoronary administration of bone‐marrow‐derived progenitor cells in acute myocardial infarction: final 1‐year results of the REPAIR‐AMI trial. Eur Heart J 2006;27:2775–2783. [DOI] [PubMed] [Google Scholar]
- 15. Seeger FH, Rasper T, Fischer A, Muhly‐Reinholz M, Hergenreider E, Leistner DM, Sommer K, Manavski Y, Henschler R, Chavakis E, Assmus B, Zeiher AM, Dimmeler S. Heparin disrupts the CXCR4/SDF‐1 axis and impairs the functional capacity of bone marrow‐derived mononuclear cells used for cardiovascular repair. Circ Res 2012;111:854–862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. BAMI protocol.
Appendix S2. BAMI trial logistics ‐ current trial and recruitment status.
Table S1. BAMI trial endpoints.
Table S2. BAMI trial inclusion and exclusion criteria.
Table S3. Clinical trials of stem cell therapy in acute myocardial infarction.


