Abstract
Adrenomedullin (ADM) is a peptide hormone first discovered in 1993 in pheochromocytoma. It is synthesized by endothelial and vascular smooth muscle cells and diffuses freely between blood and interstitium. Excretion of ADM is stimulated by volume overload to maintain endothelial barrier function. Disruption of the ADM system therefore results in vascular leakage and systemic and pulmonary oedema. In addition, ADM inhibits the renin–angiotensin–aldosterone system. ADM is strongly elevated in patients with sepsis and in patients with acute heart failure. Since hallmarks of both conditions are vascular leakage and tissue oedema, we hypothesize that ADM plays a compensatory role and may exert protective properties against fluid overload and tissue congestion. Recently, a new immunoassay that specifically measures the biologically active ADM (bio‐ADM) has been developed, and might become a biomarker for tissue congestion. As a consequence, measurement of bio‐ADM might potentially be used to guide diuretic therapy in patients with heart failure. In addition, ADM might be used to guide treatment of (pulmonary) oedema or even become a target for therapy. Adrecizumab is a humanized, monoclonal, non‐neutralizing ADM‐binding antibody with a half‐life of 15 days. Adrecizumab binds at the N‐terminal epitope of ADM, leaving the C‐terminal side intact to bind to its receptor. Due to its high molecular weight, the antibody adrecizumab cannot cross the endothelial barrier and consequently remains in the circulation. The observation that adrecizumab increases plasma concentrations of ADM indicates that ADM‐binding by adrecizumab is able to drain ADM from the interstitium into the circulation. We therefore hypothesize that administration of adrecizumab improves vascular integrity, leading to improvement of tissue congestion and thereby may improve clinical outcomes in patients with acute decompensated heart failure. A phase II study with adrecizumab in patients with sepsis is ongoing and a phase II study on the effects of adrecizumab in patients with acute decompensated heart failure with elevated ADM is currently in preparation.
Keywords: Heart failure, Adrenomedullin, Congestion, Vascular permeability, Decompensation
Introduction
Hospitalizations for heart failure are a major burden for patients, relatives, health care providers and society. Despite improvements in therapy for patients with heart failure with a reduced ejection fraction, we have not been able to reduce the risk of heart failure readmissions after a hospitalization for worsening heart failure. Approximately 35–50% of heart failure patients are rehospitalized within 6 months of discharge, making heart failure the most frequent diagnosis for 30‐day readmissions and incurring billions in costs.1, 2, 3 Therefore, preventing hospital (re‐)admissions is recognized as a major unmet need in the treatment of heart failure.
Risk prediction models have become reasonably accurate for predicting (cardiovascular) mortality, but models to predict hospital (re‐)admissions perform much worse.4 Given its poor predictive value, identifying patients for clinical trials who are at high risk of hospital (re‐)admission is difficult. Therefore, there is a need for markers to better identify patients who are at high risk for heart failure (re‐)hospitalization. Preferably, these markers should reflect a process that can be pathophysiologically linked to worsening heart failure.
Several studies have consistently shown that the main reason for (re‐)hospitalization for worsening heart failure is related to dyspnoea or breathlessness, mainly caused by pulmonary congestion.5, 6, 7, 8, 9 The great majority of patients are treated with loop diuretics to relieve congestion, but a large number of patients are discharged without losing body weight and with persistent signs of congestion.7, 9, 10, 11 This is particularly true when patients are discharged early and in patients without weight loss despite loop diuretic therapy. Consequently, studies have shown higher risks of hospital readmission in patients with a shorter duration of hospitalization, with a poor diuretic response, and with residual signs of congestion at discharge. Therefore, markers that reflect residual congestion might be useful to identify patients that are both epidemiologically and pathophysiologically at higher risk of hospital (re‐)admission. Currently, clinical signs at physical examination such as oedema, rales and jugular venous pressure are the mainstay for assessment of congestion. However, this examination is often not performed in clinical practice, is very dependent on the clinician's skills,12 has a low interrater reliability and specificity13, 14, 15 and is subjective due to the lack of standardized metrics or (de)congestion scores.16 In view of the high incidence of rehospitalization, clinical assessment of congestion is clearly insufficient. What clinicians therefore need is an easy‐to‐use surrogate for the assessment of a patient's (de)congestion status that may facilitate clinical decision making. The use of biomarkers for this purpose is attractive, since they are objective, easily available and have a known sensitivity and specificity. While by many heart failure clinicians natriuretic peptides are regarded as the main biomarker for congestion, recent studies show that there are several limitations in the use of natriuretic peptides as markers of congestion.17 Since natriuretic peptides reflect stretch and pressure of the heart, they mainly reflect intravascular volume overload, but do not reflect tissue and interstitial fluid. Therefore, better and more specific markers for tissue congestions are needed.
Adrenomedullin: an introduction
Adrenomedullin (ADM) is a peptide hormone discovered in 1993 by Kitamura et al.18 ADM is a 52 amino acid peptide, containing a ring structure and a C‐terminal amide, both of which are essential for binding to ADM receptors. The ADM gene is located on chromosome 11 and consists of four exons.19 One of the key determinants for biological activity of ADM is the group of receptor activity‐modifying proteins (RAMP). The combination of RAMP 2 or 3 with the calcitonin receptor‐like receptor (CRLR) confers specificity of the receptor for ADM, and thus both RAMPs and CRLR are key in ADM expression.20, 21, 22, 23, 24, 25 While ADM was first discovered in pheochromocytoma originating from the adrenal medulla (hence the name ‘adrenomedullin’), further investigation showed that it was synthesized by many other tissues/cells, especially endothelial and vascular smooth muscle cells, and due to its small size (6 kDa) diffuses freely between blood and interstitium.26, 27 By proteolytic fragmentation of the pro‐hormone (pro‐ADM), a glycine‐extended, inactive ADM is formed, which subsequently is enzymatically converted from ADM‐glycine to the biologically active ADM‐amide. ADM receptors and binding sites are widespread in the body, but cardiovascular and lung tissues have highest density of these binding sites.28 The in vivo half‐life of ADM is approximately 22 min.29 ADM is assumed to mainly be metabolized by neutral endopeptidase, also known as neprilysin30 – a molecule that clinicians might recognize from the recent beneficial findings with sacubitril/valsartan as a novel treatment for patients with heart failure.31 Sacubitril is a neprilysin inhibitor, and thus is supposed to inhibit breakdown of ADM and several other peptide hormones. An additional mechanism by which ADM is cleared is through binding with its receptors and subsequent internalization and degradation.32, 33
Vascular effects of adrenomedullin
The most recognized function of ADM is vasodilatation in both vascular resistance and capacitance vessels. ADM lowers blood pressure, yet increases blood flow.18, 34 Even low doses induce vasodilatation, indicating that the plasma levels of ADM under conditions such as heart failure are in the range that directly affect vascular tone.34
Beside vasodilatation, ADM seems to play an important role in preservation of endothelial integrity. ADM expression can be induced by various stimuli, one of them being volume overload, and increased plasma ADM reflects excessive fluid volume.35 This is most likely the consequence of a counteracting response, as the ADM‐induced stabilization of endothelial barrier function is thought to limit tissue fluid overload. Indeed, disruption of the ADM system results in vascular leakage and systemic and pulmonary oedema.36, 37, 38 Also the role of the ADM–RAMP 2 system has been investigated. Mice lacking the gene encoding for RAMP 2 showed enhanced vascular permeability and systemic oedema.36 Similarly, mice with a conditional knock‐out of ADM in endothelial cells revealed increased vascular permeability in comparison with wild‐type littermates.39
Further support for the effects of ADM in maintaining vascular integrity comes from experimental studies showing that experimental overexpression of ADM inhibits systemic and pulmonary vascular leakage in animals.40, 41, 42 For example, in a rat model of Staphylococcus aureus‐toxin induced systemic inflammation, accompanied by extensive vascular leakage, ADM infusion protected endothelial barrier function via cyclic adenosine monophosphate (cAMP) elevation.40 Also, ADM dose‐dependently reduced experimentally induced endothelial hyperpermeability of cultured human umbilical vein endothelial cell and porcine pulmonary artery endothelial cell monolayers.41 Suppression of ADM contributes to vascular leakage and altered epithelial repair during asthma.42 In two animal models, intranasal ADM completely attenuated the acute‐induced airway hyper‐responsiveness and mucosal plasma leakage.42, 43 ADM acts on several pathways in order to stabilize the endothelial barrier, including the cAMP/protein kinase A (PKA) pathway that inhibits RhoA/ROCK and reduces subsequent myosin light chain kinase‐induced actomyosin contraction (the ‘pulling forces’ exerted on endothelial cell junctions), as well as the cAMP/PKA and possibly the PI3K/Akt pathway to promote production of (protective) cortical actin and stabilization of the VE‐cadherin/β‐catenin complex (part of adherens junctions).44 Finally, ADM inhibits the renin–angiotensin–aldosterone system.45 Although ADM increases plasma renin activity, it induces reductions in the aldosterone/plasma renin activity ratio and attenuates angiotensin II‐induced aldosterone secretion. In addition, ADM is upregulated by angiotensin II, and protects against cardiac hypertrophy and renal damage induced by angiotensin II. Altogether, it is suggested that ADM acts as a functional antagonist to angiotensin II, hereby inhibiting aldosterone secretion and thus compensating for renin–angiotensin–aldosterone system escalation.
In summary, vasodilatation and maintaining vascular integrity are the two most important functions of ADM. Importantly, the effects of ADM depend on its location. ADM is present both intravascular and in the interstitium. Its mode of action intravascular as opposed to the interstitium is depicted in Figure 1. Intravascular ADM is thought to improve vascular integrity and decrease vascular permeability through its effects on endothelial cells. Interstitial ADM however is thought to cause vasodilatation by acting on vascular smooth muscle cells, in an endothelium‐independent mechanism. Note that endothelial‐dependent pathways have also been described, although it remains unknown to what extent each pathway is involved in vivo in humans.46, 47, 48
Figure 1.
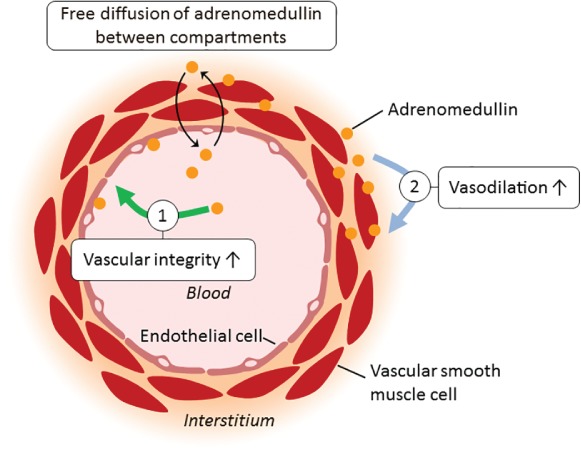
Simplistic representation of the mode of action of intravascular vs. interstitial adrenomedullin. (1) Adrenomedullin present within the blood vessels improved vascular integrity, thereby putatively reducing vascular permeability. (2) Adrenomedullin present in the interstitium acts on the vascular smooth muscle cells and causes dilatation of the vascular resistance and capacitance vessels.
Adrenomedullin is elevated in heart failure and related to congestion and clinical outcome in heart failure
In healthy humans, ADM circulates in the plasma in low concentrations. In 1995, it was first reported that ADM levels were elevated in heart failure.49 Plasma ADM concentration was 13 pg/mL in healthy subjects and 3–4 times higher in patients with chronic heart failure.49 The observation that ADM levels decreased after treatment with diuretics and digitalis led to the assumption that ‘volume expansion and an activated sympathetic nervous system may be associated with this increase and that plasma ADM levels change in response to the pathophysiologic changes of heart failure.’50 After this, many studies have shown elevated levels of ADM in patients with heart failure. In addition, several studies found a strong association between higher levels of ADM and adverse clinical outcome.51, 52 The majority of these studies used a stable part of the ADM precursor peptide, mid‐regional pro‐ADM (MR‐proADM).53 The drawback to this assay, however, is that it measures a stable fragment of a non‐functional ADM pro‐peptide, and therefore does not distinguish between the biologically active amidated ADM and the non‐functional ADM variant containing a glycine‐extended C‐terminal residue. Recently, a new immunoassay that specifically measures biologically active ADM (bio‐ADM) has been developed.53 Plasma bio‐ADM was measured in 246 patients admitted at the emergency department with suspicion of acute heart failure.54 Plasma bio‐ADM concentrations were higher among patients who experienced a cardiovascular event [median 80.5 pg/mL; interquartile range (IQR) 53.7–151.5 pg/Ml] compared with those who did not (median 54.4 pg/mL; IQR 43.4–78.4 pg/mL) (P < 0.01). After adjusting for the other biomarkers, plasma bio‐ADM remained a strong predictor of a cardiovascular event.54 Another study showed that bio‐ADM was a marker of impaired haemodynamics, organ dysfunction, and poor prognosis in patients with cardiogenic shock.55 We recently studied the clinical correlation and prognostic value of serial measurements of plasma bio‐ADM levels in 1562 patients admitted for acute decompensated heart failure.56 We showed that plasma bio‐ADM had the strongest association with clinically assessed congestion during hospital admission for acute decompensated heart failure. Moreover, bio‐ADM was a better predictor of residual congestion than any other individual baseline variable. In patients with clinical signs of residual congestion 7 days after hospital admission, bio‐ADM levels were high at baseline and remained high throughout the first week of hospitalization. This in contrast to brain natriuretic peptide (BNP) levels, which decreased in all patients irrespective of the presence and degree of residual congestion. Finally, plasma bio‐ADM concentrations, both at baseline and at day 7, provided significant added predictive value for 60‐day heart failure rehospitalization, even after adjustment for a pre‐defined 11‐item rehospitalization risk model, residual congestion by day 7 and BNP at day 7.
Rationale for bio‐adrenomedullin as a biomarker for tissue congestion
Adrenomedullin is a vasoactive peptide that is increased in patients that are volume overloaded. Main functions of ADM are vasodilatation and to maintain vascular integrity and decrease vascular leakage. Elevated levels are found in heart failure, but ADM is particularly elevated in patients with septic shock. A common factor between both diseases is vascular leakage and organ hypoperfusion. In heart failure, higher levels of ADM are associated with more severe heart failure, and are the strongest predictor of (residual) congestion in patients with acute decompensated heart failure. Increased ADM concentrations have been associated with impaired clinical outcome in several studies of patients with heart failure. In a recent study, higher levels of bio‐ADM were independently associated with a higher risk of hospital readmissions.56 We therefore propose the following concept, as depicted in Figure 2. We propose that higher bio‐ADM levels reflect residual tissue congestion, and residual tissue congestion is related to worse outcome after discharge, and a higher likelihood for frequent hospital readmissions in particular. Therefore, such a measurement might guide physicians to treat certain patients more intensively, and this might also facilitate discharge decisions.
Figure 2.
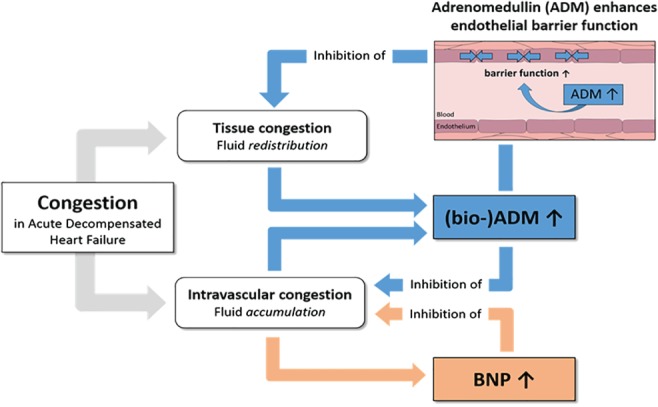
Bio‐adrenomedullin (ADM) as a marker and inhibitor of tissue congestion. Brain natriuretic peptide (BNP) as a marker and inhibitor of intravascular congestion.
Adrenomedullin as a target for therapy
Several pre‐clinical (Table 1)57, 58, 59, 60, 61, 62, 63, 64, 65, 66 and small clinical (Table 2)67, 68, 69, 70 studies have established the effects of exogenous administration of ADM in heart failure. Briefly, these effects included a reduction in myocardial infarct size, cardiac myocyte apoptosis, left ventricular remodelling (in animals) and aldosterone levels (animals and humans), while haemodynamics (in both humans and animals) and survival (in animals) were improved. In a rat coronary ligation model, ADM administration during the early period of a myocardial infarction improved survival and ameliorated progression of left ventricular remodelling and heart failure.62 Similar results were found in another study where chronic administration of ADM attenuated transition from left ventricular hypertrophy to heart failure in rats.60 In a case series of seven acute heart failure patients with dyspnoea and pulmonary congestion, the effects of long‐term intravenous administration of ADM in acute decompensated heart failure were studied. ADM infusion significantly reduced mean arterial pressure, pulmonary arterial pressure and systemic and pulmonary vascular resistance without changing heart rate, and increased cardiac output for most time‐points compared with those at baseline.71 In another small study of seven chronic heart failure patients and seven healthy subjects, ADM significantly decreased mean arterial pressure and increased heart rate in the healthy volunteers.68 In patients with heart failure, ADM also decreased mean arterial pressure and increased heart rate, but to a much lesser degree. ADM markedly increased cardiac index while decreasing pulmonary capillary wedge pressure.68
Table 1.
Overview of pre‐clinical studies investigating adrenomedullin in different models related to heart failure
| Author (year) | Intervention | Animal model | Effects |
|---|---|---|---|
| Nakamura57 (2002) | ADM infusion for 4 weeks via osmotic pump versus saline | Left coronary ligation‐induced myocardial infarction in rats |
↓ Heart weight/body weight ↓ Myocyte size ↓ Collagen volume fraction of non‐infarct LV area, without affecting infarct size ↓ LV end‐diastolic pressure |
| Okumura58 (2003) | ADM infusion for 60 min after coronary ligation | Ischaemia–reperfusion (30 min of left coronary artery ischaemia) in rats |
After 24 h: ↓ LV end‐diastolic pressure and ↓ cardiac myocyte apoptosis After 4 weeks: ↓ Myocardial fibrosis ↓ Myocardial infarct size |
| Niu59 (2003) | Heterozygous ADM(+/‐) knock‐out mice compared to wild‐type | Stress‐induced cardiac hypertrophy by angiotensin II infusion |
Knock‐out resulted in: ↑ Cardiac hypertrophy (heart weight/body weight and LV thickness) ↑ Renal dysfunction |
| Nishikimi60 (2003) | ADM infusion over 7 weeks using a micro‐osmotic pump, compared with placebo and diuretic treatment groups | Heart failure model of Dahl salt‐sensitive rats |
ADM infusion: ↓ LV end‐diastolic pressure, ↓ RV systolic pressure, ↓ RA pressure ↓ LV weight/body weight ↓ Renin–aldosterone and ANP ↑ Cardiac output and systemic vascular resistance ↑ LV end‐systolic elastance ↑ Survival compared to diuretic and placebo |
| Okumura61 (2004) | ADM infusion, or ADM + wortmannin, or placebo, for 60 min after coronary ligation | Ischaemia–reperfusion (30 min of left coronary artery ischaemia) in rats |
↓ Infarct size ↓ LV end‐diastolic pressure ↓ Myocardial apoptotic death Pre‐treatment with wortmannin abolished beneficial effects of ADM, indicating involvement of the PI3K/Akt dependent pathway |
| Nakamura62 (2004) | I.p. ADM or placebo over 7 days, immediately after induction of myocardial infarction | Left coronary ligation‐induced myocardial infarction in rats. Observed over 9 weeks |
At 9 weeks: ↑ Survival, ↓ heart/lung weight ↓ Oxidative stress and ACE transcription ↓ LV end‐diastolic pressure ↓ Collagen volume fraction of the non‐infarcted LV No effect on infarct size During 7 days of infusion: No effects on urinary output or haemodynamic parameters |
| Niu63 (2004) | Heterozygous ADM(+/‐) knock‐out mice compared to wild‐type | Stress‐induced cardiac hypertrophy by aortic constriction or angiotensin II infusion |
More pronounced in ADM knock‐out mice: ↑ Heart weight/body weight ratio ↑ LV wall thickness ↑ Perivascular fibrosis ↑ Expression of ACE, angiotensinogen, collagen type 1, BNP and c‐fos ↑ Renal damage with glomerular sclerosis Involvement of a PKA/PKC pathway |
| Looi64 (2006) | ADM bolus | Left coronary ligation‐induced myocardial infarction in anaesthetized rats |
↓ Ventricular arrhythmias Involvement of NO mechanism |
| Yoshizawa65 (2016) | Subcutaneous infusion of ADM using an osmotic minipump for 3 or 7 days | Doxorubicin‐induced cardiac damage in mice |
↑ 14 day survival ↓ LDH levels ↓ DOX‐induced cardiac tissue damage, mitochondrial abnormalities and cell death |
| Li66 (2018) | Myocardial transplantation of MSCs overexpressing ADM compared to GFP MSCs | Isoproterenol‐induced global heart failure |
↑ Cardiac function ↓ Cardiac fibrosis |
ACE, angiotensin‐converting enzyme; ADM, adrenomedullin; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; DOX, doxorubicin; GFP, green fluorescent protein; i.p., intraperitoneal; LDH, lactate dehydrogenase; LV, left ventricular; MSC, mesenchymal stem cell; NO, nitric oxide; PKA, protein kinase A; PKC, protein kinase C; RA, right atrial; RV, right ventricular.
Table 2.
Overview of studies investigating adrenomedullin in human patients with forms of heart failure
| Author (year) | Intervention | Condition | Effects |
|---|---|---|---|
| Nakamura67 (1997) | FBF and SBF test with intra‐arterial ADM |
Healthy subjects (n = 10) Patients with CHF (n = 18) |
↑ FBF & ↑ SBF Effects partially NO‐mediated Impaired FBF and SBF responses in CHF group |
| Nagaya68 (2000) | I.v. infusion of ADM (n = 7) or placebo (n = 6), 90 min total | Patients with precapillary pulmonary hypertension (n = 13 total) |
↑ Cardiac index (44%) ↓ Pulmonary arterial pressure (32%) ↓ MAP (9 mmHg), ↑ HR ↓ Plasma aldosterone (but not renin) No effect ANP/BNP |
| Nishikimi69 (2009) | I.v. infusion of ADM + hANP for 12 h, followed by 12 h of hANP | Acute heart failure patients with dyspnoea and pulmonary congestion (n = 7) |
ADM + hANP: ↓ MAP, PAP, systemic and pulmonary vascular resistance ↑ Cardiac output, urine volume and urinary sodium excretion ↓ Aldosterone, BNP, free‐radical metabolites |
| Kataoka70 (2010) |
I.v. infusion of ADM for 12 h No placebo arm |
Patients with acute myocardial infarction before undergoing PCI (n = 10) |
During infusion, two patients showed unstable haemodynamics MRI 3 vs. 1 month after PCI: ↑ Wall motion index, ↓ infarct size |
ADM, adrenomedullin; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; CHF, chronic heart failure; FBF, forearm blood flow; hANP, human atrial natriuretic peptide; HR, heart rate; MAP, mean arterial pressure; MRI, magnetic resonance imaging; NO, nitric oxide; PAP, pulmonary artery pressure; PCI, percutaneous coronary intervention; SBF, skin blood flow.
Taken together, these data consistently show that ADM induces beneficial haemodynamic, hormonal and myocardial changes in both experimental models and patients with heart failure. These effects are likely related to the vasodilatory properties of intravascular ADM, although other pathways may also be involved. Many vasodilators have been studied in acute and chronic heart failure with mixed results. Therefore, it would be more interesting to be able to stimulate the effects of ADM on endothelial permeability, and to prevent decreases in blood pressure, since this might be deleterious in patients with worsening heart failure.
Adrecizumab for the treatment of heart failure and sepsis
Adrecizumab is a humanized, monoclonal, non‐neutralizing antibody against the N‐terminus of ADM. It has a half‐life of 15 days when administered by a single intravenous infusion. The mode of action of adrecizumab is presented in Figure 3. Administration of adrecizumab leads to a dose‐dependent increase in plasma ADM bound to the administered antibody. The increase occurs within a few minutes and is not caused by induction of de‐novo synthesis, because concentrations of MR‐proADM are not increased (an inactive peptide fragment originating from the same precursor as ADM, which is synthesized in a 1:1 ratio). It is hypothesized that translocation of pre‐existing ADM accounts for the observed increase in circulating ADM.72 Briefly, circulating adrecizumab cannot leave the blood compartment due to its high molecular weight (160 kDa), whereas ADM (with a much lower molecular weight of 6 kDa) can freely cross the endothelial barrier between the interstitium and the circulation.55 Binding of ADM by adrecizumab (present in the circulation in a large excess over ADM) prevents ADM from leaving the blood vessel, effectively ‘trapping’ ADM in the circulation. In addition, adrecizumab may translocate ADM from the interstitium into the circulation. Because adrecizumab is a non‐neutralizing antibody, the net effect is a significant increase of functional plasma ADM which leads to – as we hypothesize – the restoration of vascular integrity (endothelial effect) and less vasodilatation (vascular smooth muscle cell effect due to decreased concentrations of ADM in the interstitium). Further, it is thought that adrecizumab prevents ADM from being degraded by proteases and prolongs half‐life of ADM.72
Figure 3.
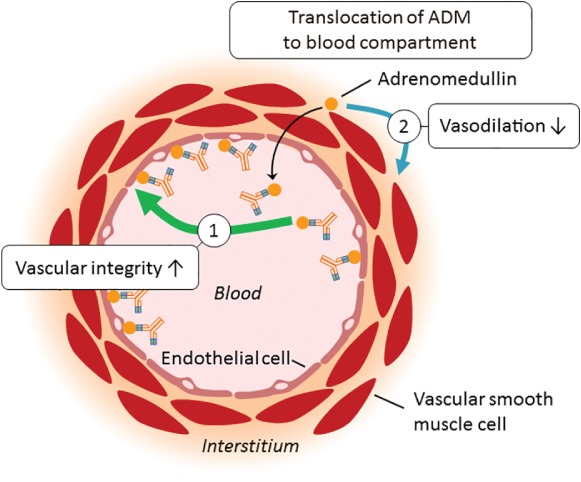
Mode of action of adrecizumab. Administration of adrecizumab leads to a dose‐dependent increase of plasma adrenomedullin (ADM) bound to the administered antibody. Circulating adrecizumab cannot leave the blood compartment due to its high molecular weight (160 kDa), whereas ADM (with a much lower molecular weight of 6 kDa) can freely cross the endothelial barrier between the interstitium and the circulation. Binding of ADM by adrecizumab (present in the circulation in a large excess over ADM) prevents ADM from leaving the blood vessel, effectively ‘trapping’ ADM in the circulation.
In animal models of systemic inflammation and septic shock, adrecizumab improved haemodynamics, renal function, systemic inflammation and reduced inducible nitric oxide synthase expression.72 Currently, adrecizumab is being evaluated in a phase II trial in human septic shock (http://clinicaltrials.gov identifier: NCT03085758). There were no safety concerns observed in pre‐clinical studies, as well as in two phase I studies in which 0.5, 2 and 8 mg/kg were administered to healthy volunteers.72 Importantly, even though adrecizumab induced great increases in circulating levels of ADM, this did not cause hypotension. In a phase Ib study, where healthy subjects received an infusion of bacterial lipopolysaccharide to induce a systemic inflammatory response, administration of adrecizumab significantly reduced the perception of illness/sickness, as assessed by clinical scores.73
A phase II proof of concept study in patients with worsening heart failure and elevated bio‐ADM levels after initial stabilization is currently being considered. Patients with elevated bio‐ADM have (residual) congestion and are at high risk for clinical events, and a heart failure readmission in particular. Adrecizumab is expected to increase intravascular ADM and decrease interstitial ADM, since adrecizumab may translocate ADM from the interstitium into the circulation. The hypothesis of this study is that by improving vascular integrity, it is expected that adrecizumab will decrease tissue congestion and thereby improve dyspnoea, potentially reducing heart failure readmissions. However, it should be noted that long‐term effects of ADM on development and/or progression of heart failure have never been clinically investigated. In addition, adrecizumab itself has not been investigated in pre‐clinical models of heart failure, but only in septic shock and systemic inflammation. Tissue congestion was never an outcome parameter in pre‐clinical studies with ADM.
Conclusions
Adrenomedullin is an endogenous hormone that is released as a counteracting response to volume overload. Levels of ADM are clearly increased in patients with heart failure, and higher levels are related to more advanced heart failure and worse outcomes. Since elevation of ADM is a feedback response to volume overload to maintain vascular integrity and decrease vascular leakage of fluid from the the vasculature to the tissues, measurement of ADM might reflect tissue and pulmonary oedema. Such a measurement might guide physicians to more intensively treat patients with heart failure hospitalization and facilitate discharge decisions. In addition, ADM might become a target of therapy in heart failure. The mode of action of adrecizumab, a humanized monoclonal antibody that binds but does not significantly inhibit ADM, might be of particular interest, since it is assumed to translocate ADM from the interstitium into the vasculature to improve vascular integrity and prevent vascular leakage. A clinical study with adrecizumab is currently being conducted in patients with sepsis and a study in hospitalized heart failure patients is currently being prepared.
Conflict of interest: A.A.V. has received research support from Sphingotec GmbH. C.G. has received travel reimbursements from Adrenomed AG. J.S. is employed by Adrenomed AG and Sphingotec GmbH and holds shares in Adrenomed AG. A.B. is employed by Adrenomed AG and Sphingotec GmbH and holds shares in both. P.P. received travel reimbursements and consultancy fees from Sphingotec GmbH. The other authors report no conflicts of interest.
References
- 1. Fida N, Piña IL. Trends in heart failure hospitalizations. Curr Heart Fail Rep 2012;9:346–353. [DOI] [PubMed] [Google Scholar]
- 2. Harjola VP, Parissis J, Brunner‐La Rocca HP, Celutkiene J, Chioncel O, Collins SP, De Backer D, Filippatos GS, Gayat E, Hill L, Lainscak M, Lassus J, Masip J, Mebazaa A, Miro O, Mortara A, Mueller C, Mullens W, Nieminen MS, Rudiger A, Ruschitzka F, Seferovic PM, Sionis A, Vieillard‐Baron A, Weinstein JM, de Boer RA, Crespo‐Leiro MG, Piepoli M, Riley JP. Comprehensive in‐hospital monitoring in acute heart failure: applications for clinical practice and future directions for research. A statement from the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2018;20:1081–1099. [DOI] [PubMed] [Google Scholar]
- 3. Gupta A, Fonarow GC. The Hospital Readmissions Reduction Program – learning from failure of a healthcare policy. Eur J Heart Fail 2018;20:1169–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ouwerkerk W, Voors AA, Zwinderman AH. Factors influencing the predictive power of models for predicting mortality and/or heart failure hospitalization in patients with heart failure. JACC Heart Fail 2014;2:429–436. [DOI] [PubMed] [Google Scholar]
- 5. Cleland JG, Swedberg K, Follath F, Komajda M, Cohen‐Solal A, Aguilar JC, Dietz R, Gavazzi A, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, van Gilst WH, Widimsky J, Freemantle N, Eastaugh J, Mason J. The EuroHeart Failure survey programme — a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J 2003;24:442–463. [DOI] [PubMed] [Google Scholar]
- 6. Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, She L, Stough WG, Yancy CW, Young JB, Fonarow GC. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA 2006;296:2217–2226. [DOI] [PubMed] [Google Scholar]
- 7. O'Connor CM, Stough WG, Gallup DS, Hasselblad V, Gheorghiade M. Demographics, clinical characteristics, and outcomes of patients hospitalized for decompensated heart failure: observations from the IMPACT‐HF registry. J Card Fail 2005;11:200–205. [DOI] [PubMed] [Google Scholar]
- 8. Van Aelst LNL, Arrigo M, Placido R, Akiyama E, Girerd N, Zannad F, Manivet P, Rossignol P, Badoz M, Sadoune M, Launay JM, Gayat E, Lam CS, Cohen‐Solal A, Mebazaa A, Seronde MF. Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur J Heart Fail 2018;20:738–747. [DOI] [PubMed] [Google Scholar]
- 9. Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med 2006;119:S3–S10. [DOI] [PubMed] [Google Scholar]
- 10. Lucas C, Johnson W, Hamilton MA, Fonarow GC, Woo MA, Flavell CM, Creaser JA, Stevenson LW. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am Heart J 2000;140:840–847. [DOI] [PubMed] [Google Scholar]
- 11. Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, Maggioni AP, Cook T, Swedberg K, Burnett JC Jr, Grinfeld L, Udelson JE, Zannad F, Gheorghiade M, EVEREST Trial Investigators . Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J 2013;34:835–843. [DOI] [PubMed] [Google Scholar]
- 12. McGee SR. Physical examination of venous pressure: a critical review. Am Heart J 1998;136:10–18. [DOI] [PubMed] [Google Scholar]
- 13. Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, JJ MM, Filippatos G, European Society of Cardiology; European Society of Intensive Care Medicine . Assessing and grading congestion in acute heart failure: a scientific statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail 2010;12:423–433. [DOI] [PubMed] [Google Scholar]
- 14. Chaudhry A, Singer AJ, Chohan J, Russo V, Lee C. Interrater reliability of hemodynamic profiling of patients with heart failure in the ED. Am J Emerg Med 2008;26:196–201. [DOI] [PubMed] [Google Scholar]
- 15. Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA 1989;261:884–888. [PubMed] [Google Scholar]
- 16. Voors AA, Ter Maaten JM. Tackling early heart failure deaths and readmissions by estimating congestion. JACC Heart Fail 2015;3:894–895. [DOI] [PubMed] [Google Scholar]
- 17. Omar HR, Guglin M. A single BNP measurement in acute heart failure does not reflect the degree of congestion. J Crit Care 2016;33:262–265. [DOI] [PubMed] [Google Scholar]
- 18. Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun 1993;192:553–560. [DOI] [PubMed] [Google Scholar]
- 19. Ishimitsu T, Kojima M, Kangawa K, Hino J, Matsuoka H, Kitamura K, Eto T, Matsuo H. Genomic structure of human adrenomedullin gene. Biochem Biophys Res Commun 1994;203:631–639. [DOI] [PubMed] [Google Scholar]
- 20. McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin‐receptor‐like receptor. Nature 1998;393:333–339. [DOI] [PubMed] [Google Scholar]
- 21. Hay DL, Pioszak AA. Receptor activity‐modifying proteins (RAMPs): new insights and roles. Annu Rev Pharmacol Toxicol 2016;56:469–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brain SD, Grant AD. Vascular actions of calcitonin gene‐related peptide and adrenomedullin. Physiol Rev 2004;84:903–934. [DOI] [PubMed] [Google Scholar]
- 23. Tam CW, Husmann K, Clark NC, Clark JE, Lazar Z, Ittner LM, Götz J, Douglas G, Grant AD, Sugden D, Poston L, Poston R, McFadzean I, Marber MS, Fischer JA, Born W, Brain SD. Enhanced vascular responses to adrenomedullin in mice overexpressing receptor‐activity‐modifying protein 2. Circ Res 2006;98:262–270. [DOI] [PubMed] [Google Scholar]
- 24. Autelitano DJ, Ridings R. Adrenomedullin signalling in cardiomyocytes is dependent upon CRLR and RAMP2 expression. Peptides 2001;22:1851–1857. [DOI] [PubMed] [Google Scholar]
- 25. Kamitani S, Asakawa M, Shimekake Y, Kuwasako K, Nakahara K, Sakata T. The RAMP2/CRLR complex is a functional adrenomedullin receptor in human endothelial and vascular smooth muscle cells. FEBS Lett 1999;448:111–114. [DOI] [PubMed] [Google Scholar]
- 26. Sugo S, Minamino N, Kangawa K, Miyamoto K, Kitamura K, Sakata J, Eto T, Matsuo H. Endothelial cells actively synthesize and secrete adrenomedullin. Biochem Biophys Res Commun 1994;201:1160–1166. [DOI] [PubMed] [Google Scholar]
- 27. Schönauer R, Els‐Heindl S, Beck‐Sickinger AG. Adrenomedullin – new perspectives of a potent peptide hormone. J Pept Sci 2017;23:472–485. [DOI] [PubMed] [Google Scholar]
- 28. Owji AA, Smith DM, Coppock HA, Morgan DG, Bhogal R, Ghatei MA, Bloom SR. An abundant and specific binding site for the novel vasodilator adrenomedullin in the rat. Endocrinology 1995;136:2127–2134. [DOI] [PubMed] [Google Scholar]
- 29. Dschietzig T, Azad HA, Asswad L, Böhme C, Bartsch C, Baumann G, Stangl K. The adrenomedullin receptor acts as clearance receptor in pulmonary circulation. Biochem Biophys Res Commun 2002;294:315–318. [DOI] [PubMed] [Google Scholar]
- 30. Lisy O, Jougasaki M, Schirger JA, Chen HH, Barclay PT, Burnett JC Jr. Neutral endopeptidase inhibition potentiates the natriuretic actions of adrenomedullin. Am J Physiol 1998;275:F410–F414. [DOI] [PubMed] [Google Scholar]
- 31. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 32. Schönauer R, Kaiser A, Holze C, Babilon S, Köbberling J, Riedl B, Beck‐Sickinger AG. Fluorescently labeled adrenomedullin allows real‐time monitoring of adrenomedullin receptor trafficking in living cells. J Pept Sci 2015;21:905–912. [DOI] [PubMed] [Google Scholar]
- 33. Lewis LK, Smith MW, Brennan SO, Yandle TG, Richards AM, Nicholls MG. Degradation of human adrenomedullin(1‐52) by plasma membrane enzymes and identification of metabolites. Peptides 1997;18:733–739. [DOI] [PubMed] [Google Scholar]
- 34. Cockcroft JR, Noon JP, Gardner‐Medwin J, Bennett T. Haemodynamic effects of adrenomedullin in human resistance and capacitance vessels. Br J Clin Pharmacol 1997;44:57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hirano S, Imamura T, Matsuo T, Ishiyama Y, Kato J, Kitamura K, Koiwaya Y, Eto T. Differential responses of circulating and tissue adrenomedullin and gene expression to volume overload. J Card Fail 2000;6:120–129. [DOI] [PubMed] [Google Scholar]
- 36. Tanaka M, Koyama T, Sakurai T, Kamiyoshi A, Ichikawa‐Shindo Y, Kawate H, Liu T, Xian X, Imai A, Zhai L, Hirabayashi K, Owa S, Yamauchi A, Igarashi K, Taniguchi S, Shindo T. The endothelial adrenomedullin‐RAMP2 system regulates vascular integrity and suppresses tumor metastasis. Cardiovasc Res 2016;111:398–409. [DOI] [PubMed] [Google Scholar]
- 37. Koyama T, Sakurai T, Kamiyoshi A, Ichikawa‐Shindo Y, Kawate H, Shindo T. Adrenomedullin–RAMP2 system in vascular endothelial cells. J Atheroscler Thromb 2015;22:647–653. [DOI] [PubMed] [Google Scholar]
- 38. Koyama T, Ochoa‐Callejero L, Sakurai T, Kamiyoshi A, Ichikawa‐Shindo Y, Iinuma N, Arai T, Yoshizawa T, Iesato Y, Lei Y, Uetake R, Okimura A, Yamauchi A, Tanaka M, Igarashi K, Toriyama Y, Kawate H, Adams RH, Kawakami H, Mochizuki N, Martínez A, Shindo T. Vascular endothelial adrenomedullin–RAMP2 system is essential for vascular integrity and organ homeostasis. Circulation 2013;127:842–853. [DOI] [PubMed] [Google Scholar]
- 39. Ochoa‐Callejero L, Pozo‐Rodrigálvarez A, Martínez‐Murillo R, Martínez A. Lack of adrenomedullin in mouse endothelial cells results in defective angiogenesis, enhanced vascular permeability, less metastasis, and more brain damage. Sci Rep 2016;6:33495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Temmesfeld‐Wollbrück B, Hocke AC, Suttorp N, Hippenstiel S. Adrenomedullin and endothelial barrier function. Thromb Haemost 2007;98:944–951. [DOI] [PubMed] [Google Scholar]
- 41. Hippenstiel S, Witzenrath M, Schmeck B, Hocke A, Krisp M, Krüll M, Seybold J, Seeger W, Rascher W, Schütte H, Suttorp N. Adrenomedullin reduces endothelial hyperpermeability. Circ Res 2002;91:618–625. [DOI] [PubMed] [Google Scholar]
- 42. Hagner S, Welz H, Kicic A, Alrifai M, Marsh LM, Sutanto EN, Ling KM, Stick SM, Müller B, Weissmann N, Renz H. Suppression of adrenomedullin contributes to vascular leakage and altered epithelial repair during asthma. Allergy 2012;67:998–1006. [DOI] [PubMed] [Google Scholar]
- 43. Ohbayashi H, Suito H, Yoshida N, Ilto Y, Kume H, Yamaki K. Adrenomedullin inhibits ovalbumin‐induced bronchoconstriction and airway microvascular leakage in guinea‐pigs. Eur Respir J 1999;14:1076–1081. [DOI] [PubMed] [Google Scholar]
- 44. Geven C, Kox M, Pickkers P. Adrenomedullin and adrenomedullin‐targeted therapy as treatment strategies relevant for sepsis. Front Immunol 2018;9:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Charles CJ, Lainchbury JG, Nicholls MG, Rademaker MT, Richards AM, Troughton RW. Adrenomedullin and the renin–angiotensin–aldosterone system. Regul Pept 2003;112:41–49. [DOI] [PubMed] [Google Scholar]
- 46. Dettmann ES, Vysniauskiene I, Wu R, Flammer J, Haefliger IO. Adrenomedullin‐induced endothelium‐dependent relaxation in porcine ciliary arteries. Invest Ophthalmol Vis Sci 2003;44:3961–3966. [DOI] [PubMed] [Google Scholar]
- 47. Nishimatsu H, Suzuki E, Nagata D, Moriyama N, Satonaka H, Walsh K, Sata M, Kangawa K, Matsuo H, Goto A, Kitamura T, Hirata Y. Adrenomedullin induces endothelium‐dependent vasorelaxation via the phosphatidylinositol 3‐kinase/Akt‐dependent pathway in rat aorta. Circ Res 2001;89:63–70. [DOI] [PubMed] [Google Scholar]
- 48. Yang BC, Lippton H, Gumusel B, Hyman A, Mehta JL. Adrenomedullin dilates rat pulmonary artery rings during hypoxia: role of nitric oxide and vasodilator prostaglandins. J Cardiovasc Pharmacol 1996;28:458–462. [DOI] [PubMed] [Google Scholar]
- 49. Jougasaki M, Wei CM, McKinley LJ, Burnett JC Jr. Elevation of circulating and ventricular adrenomedullin in human congestive heart failure. Circulation 1995;92:286–289. [DOI] [PubMed] [Google Scholar]
- 50. Nishikimi T, Saito Y, Kitamura K, Ishimitsu T, Eto T, Kangawa K, Matsuo H, Omae T, Matsuoka H. Increased plasma levels of adrenomedullin in patients with heart failure. J Am Coll Cardiol 1995;26:1424–1431. [DOI] [PubMed] [Google Scholar]
- 51. Gegenhuber A, Struck J, Dieplinger B, Poelz W, Pacher R, Morgenthaler NG, Bergmann A, Haltmayer M, Mueller T. Comparative evaluation of B‐type natriuretic peptide, mid‐regional pro‐A‐type natriuretic peptide, mid‐regional pro‐adrenomedullin, and copeptin to predict 1‐year mortality in patients with acute destabilized heart failure. J Card Fail 2007;13:42–49. [DOI] [PubMed] [Google Scholar]
- 52. Maisel A, Mueller C, Nowak R, Peacock WF, Landsberg JW, Ponikowski P, Mockel M, Hogan C, Wu AH, Richards M, Clopton P, Filippatos GS, Di Somma S, Anand I, Ng L, Daniels LB, Neath SX, Christenson R, Potocki M, McCord J, Terracciano G, Kremastinos D, Hartmann O, von Haehling S, Bergmann A, Morgenthaler NG, Anker SD. Mid‐region pro‐hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. J Am Coll Cardiol 2010;55:2062–2076. [DOI] [PubMed] [Google Scholar]
- 53. Weber J, Sachse J, Bergmann S, Sparwaßer A, Struck J, Bergmann A. Sandwich immunoassay for bioactive plasma adrenomedullin. J Appl Lab Med 2017. 10.1373/jalm.2017.023655. [DOI] [PubMed] [Google Scholar]
- 54. Self WH, Storrow AB, Hartmann O, Barrett TW, Fermann GJ, Maisel AS, Struck J, Bergmann A, Collins SP. Plasma bioactive adrenomedullin as a prognostic biomarker in acute heart failure. Am J Emerg Med 2016;34:257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tolppanen H, Rivas‐Lasarte M, Lassus J, Sans‐Roselló J, Hartmann O, Lindholm M, Arrigo M, Tarvasmäki T, Köber L, Thiele H, Pulkki K, Spinar J, Parissis J, Banaszewski M, Silva‐Cardoso J, Carubelli V, Sionis A, Harjola VP, Mebazaa A. Adrenomedullin: a marker of impaired hemodynamics, organ dysfunction, and poor prognosis in cardiogenic shock. Ann Intensive Care 2017;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kremer D, ter Maaten JM, Voors AA. Bio‐adrenomedullin as a potential quick, reliable and objective marker of congestion in heart failure. Eur J Heart Fail 2018;20:1363–1365. [DOI] [PubMed] [Google Scholar]
- 57. Nakamura R, Kato J, Kitamura K, Onitsuka H, Imamura T, Marutsuka K, Asada Y, Kangawa K, Eto T. Beneficial effects of adremedullin on left ventricular remodeling after myocardial infarction in rats. Cardiovasc Res 2002;56:373–380. [DOI] [PubMed] [Google Scholar]
- 58. Okumura H, Nagaya N, Kangawa K. Adrenomedullin infusion during ischemia/reperfusion attenuates left ventricular remodeling and myocardial fibrosis in rats. Hypertens Res 2003;26:S99–S104. [DOI] [PubMed] [Google Scholar]
- 59. Niu P, Shindo T, Iwata H, Ebihara A, Suematsu Y, Zhang Y, Takeda N, Iimuro S, Hirata Y, Nagai R. Accelerated cardiac hypertrophy and renal damage induced by angiotensin II in adrenomedullin knockout mice. Hypertens Res 2003;26:731–736. [DOI] [PubMed] [Google Scholar]
- 60. Nishikimi T, Yoshihara F, Horinaka S, Kobayashi N, Mori Y, Tadokoro K, Akimoto K, Minamino N, Kangawa K, Matsuoka H. Chronic administration of adrenomedullin attenuates transition from left ventricular hypertrophy to heart failure in rats. Hypertension 2003;42:1034–1041. [DOI] [PubMed] [Google Scholar]
- 61. Okumura H, Nagaya N, Itoh T, Okano I, Hino J, Mori K, Tsukamoto Y, Ishibashi‐Ueda H, Miwa S, Tambara K, Toyokuni S, Yutani C, Kangawa K. Adrenomedullin infusion attenuates myocardial ischemia/reperfusion injury through the phosphatidylinositol 3‐kinase/Akt‐dependent pathway. Circulation 2004;109:242–248. [DOI] [PubMed] [Google Scholar]
- 62. Nakamura R, Kato J, Kitamura K, Onitsuka H, Imamura T, Cao Y, Marutsuka K, Asada Y, Kangawa K, Eto T. Adrenomedullin administration immediately after myocardial infarction ameliorates progression of heart failure in rats. Circulation 2004;110:426–431. [DOI] [PubMed] [Google Scholar]
- 63. Niu P, Shindo T, Iwata H, Iimuro S, Takeda N, Zhang Y, Ebihara A, Suematsu Y, Kangawa K, Hirata Y, Nagai R. Protective effects of endogenous adrenomedullin on cardiac hypertrophy, fibrosis, and renal damage. Circulation 2004;109:1789–1794. [DOI] [PubMed] [Google Scholar]
- 64. Looi YH, Kane KA, McPhaden AR, Wainwright CL. Adrenomedullin acts via nitric oxide and peroxynitrite to protect against myocardial ischaemia‐induced arrhythmias in anaesthetized rats. Br J Pharmacol 2006;148:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yoshizawa T, Takizawa S, Shimada S, Tokudome T, Shindo T, Matsumoto K. effects of adrenomedullin on doxorubicin‐induced cardiac damage in mice. Biol Pharm Bull 2016;39:737–746. [DOI] [PubMed] [Google Scholar]
- 66. Li LL, Peng C, Zhang M, Liu Y, Li H, Chen H, Sun Y, Zhu C, Zhang Y. Mesenchymal stem cells overexpressing adrenomedullin improve heart function through antifibrotic action in rats experiencing heart failure. Mol Med Rep 2018;17:1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nakamura M, Yoshida H, Makita S, Arakawa N, Niinuma H, Hiramori K. Potent and long‐lasting vasodilatory effects of adrenomedullin in humans. Comparisons between normal subjects and patients with chronic heart failure. Circulation 1997;95:1214–1221. [DOI] [PubMed] [Google Scholar]
- 68. Nagaya N, Satoh T, Nishikimi T, Uematsu M, Furuichi S, Sakamaki F, Oya H, Kyotani S, Nakanishi N, Goto Y, Masuda Y, Miyatake K, Kangawa K. Hemodynamic, renal, and hormonal effects of adrenomedullin infusion in patients with congestive heart failure. Circulation 2000;101:498–503. [DOI] [PubMed] [Google Scholar]
- 69. Nishikimi T, Karasawa T, Inaba C, Ishimura K, Tadokoro K, Koshikawa S, Yoshihara F, Nagaya N, Sakio H, Kangawa K, Matsuoka H. Effects of long‐term intravenous administration of adrenomedullin (AM) plus hANP therapy in acute decompensated heart failure: a pilot study. Circ J 2009;73:892–898. [DOI] [PubMed] [Google Scholar]
- 70. Kataoka Y, Miyazaki S, Yasuda S, Nagaya N, Noguchi T, Yamada N, Morii I, Kawamura A, Doi K, Miyatake K, Tomoike H, Kangawa K. The first clinical pilot study of intravenous adrenomedullin administration in patients with acute myocardial infarction. J Cardiovasc Pharmacol 2010;56:413–419. [DOI] [PubMed] [Google Scholar]
- 71. Nishikimi T, Karasawa T, Inaba C, Ishimura K, Tadokoro K, Koshikawa S, Yoshihara F, Nagaya N, Sakio H, Kangawa K, Matsuoka H. Effects of long‐term intravenous administration of adrenomedullin (AM) plus hANP therapy in acute decompensated heart failure: a pilot study. Clin Sci (Lond) 2012;122:429–437. [DOI] [PubMed] [Google Scholar]
- 72. Geven C, Bergmann A, Kox M, Pickkers P. Vascular effects of adrenomedullin and the anti‐adrenomedullin antibody adrecizumab in sepsis. Shock 2018;50:132–140. [DOI] [PubMed] [Google Scholar]
- 73. Geven C, van Lier D, Blet A, Peelen R, ten Elzen B, Mebazaa A, Kox M, Pickkers P. Safety, tolerability and pharmacokinetics/‐dynamics of the adrenomedullin antibody adrecizumab in a first‐in‐human study and during experimental human endotoxemia in healthy subjects. Br J Clin Pharmacol 2018;84:2129–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]


