Abstract
Aims
To analyse real‐world treatment patterns of sacubitril/valsartan (sac/val) using data from a pharmacy database in Germany.
Methods and results
A retrospective cohort study of 26 191 adult patients (aged ≥ 18 years) in the IMS® longitudinal prescriptions database in Germany who were dispensed sac/val from January 2016 to June 2017 was conducted. The analysis included sac/val dose titration assessed in the 6 months from first sac/val prescription; prescriptions of concomitant cardiovascular medications in the 6 months pre‐ and post‐index and compliance and persistence during 12 months post‐index. Two‐thirds of patients were prescribed the lowest sac/val dose of 50 mg twice daily (b.i.d.) at index and up‐titration during the first 6 months was attempted in 41% of these patients. Ten percent of patients prescribed 200 mg b.i.d. at index had to be stably down‐titrated; among patients prescribed 50 or 100 mg b.i.d. at index that were up‐titrated, > 80% remained on the higher dose. Overall, the mean daily diuretic dose decreased by 25% after initiation of sac/val. High compliance and persistence rates were observed across sac/val doses, increasing with higher sac/val dose at index. Prior dose of angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker had only minor impact on first sac/val dose, compliance and persistence.
Conclusions
Most patients prescribed sac/val are not initiated on the recommended dose nor up‐titrated as recommended by the EU Summary of Product Characteristics. Initiation of sac/val was associated with high persistence and compliance and a dose reduction of diuretics. Barriers to up‐titration must be explored.
Keywords: Heart failure, Angiotensin receptor–neprilysin inhibitor , Dose titration, Persistence, Compliance
Introduction
Sacubitril/valsartan (sac/val) is an angiotensin receptor–neprilysin inhibitor (ARNI) indicated for adults with symptomatic heart failure (HF) with reduced ejection fraction (HFrEF).1 Sac/val has been approved in the European Union (EU) since November 20152 based on the results of the Prospective comparison of ARNI with angiotensin‐converting enzyme inhibitor (ACEI) to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM‐HF) study3 and was launched in Germany in January 2016.4 The European Society of Cardiology included sac/val in the 2016 update of the guidelines for the diagnosis and treatment of acute and chronic HF with a class IB recommendation as replacement for ACEI treatment in ambulatory patients with HFrEF who remain symptomatic despite therapy with an ACEI, a beta‐blocker (BB) and a mineralocorticoid receptor antagonist (MRA).5
In a recent study of electronic medical records from Germany, we found that the majority of patients treated under real‐world conditions were not prescribed the recommended target sac/val dose of 200 mg twice daily (b.i.d.).6 This is in line with a previous report demonstrating under‐dosing of HF disease‐modifying medication.7
Factors related to treatment that influence patient outcomes in the real world include patient compliance (sometimes referred to as adherence), which describes the extent to which a patient takes a medicine in accordance with the prescribed interval and dosing regimen; and persistence, which refers to the duration of time from initiation to discontinuation of therapy.8 Many studies have reported poor compliance and persistence with chronic therapies, indicating a clear unmet need to improve compliance and persistence levels.9 Better compliance and persistence have been shown to improve outcomes in patients with HF,10, 11 and practitioners may be reassured when prescribing medications that are shown to be associated with high compliance and persistence.
We aimed to analyse the implementation, frequency and dynamics of up‐ and down‐titration, compliance and persistence of sac/val in real‐world clinical practice in Germany using a large pharmacy database. Additionally, concomitant use of other disease‐modifying therapies [e.g. renin–angiotensin–aldosterone system (RAAS) inhibitors, BBs and MRAs] and symptomatic therapy (diuretics) was analysed in the pre‐ and post‐index periods relative to initiation of sac/val.
Methods
Study design
This was a retrospective cohort study of adult patients (aged ≥ 18 years) identified in the IMS® longitudinal prescriptions (LRx) database in Germany who were dispensed sac/val from January 2016 to June 2017.
Database
The IMS® LRx captures anonymied patient‐level data on all dispensed prescriptions from retail pharmacies. The database covers approximately 60% of all reimbursed prescriptions from statutory health insured patients in Germany, and its suitability for research purposes has been demonstrated.12 While the database covers 90% of prescriptions in Southern Germany, the coverage in the North is only 10–20%, which is explained by the lack of some pharmacy data collection centres (e.g. the northern collection centre NARZ). Key information collected includes age, sex, date of dispensation, dispensed molecule/brand [cardiovascular (CV) and non‐CV medication], sac/val dose (50, 100 and 200 mg b.i.d.), pack size and prescriber specialty (general practice, cardiology and other). The collected data cover all Anatomical Therapeutic Chemical (ATC) codes (online supplementary Table S1) and prescriber specialties; therefore, the database provides a holistic view of outpatient treatments received by a patient; in‐hospital prescriptions are not captured.
Study population
The index date was defined as the date of the patient's first recorded sac/val dispensation. Subsets of patients were identified to perform the analyses (online supplementary Figure S1) and are described below.
Data analyses
Version 9.4 of the SAS statistical software (SAS Institute Inc., Cary, NC, USA) was used for all data extractions, manipulations and analyses. For each continuous variable, the number of patients or prescriptions, mean and standard deviation (SD) were summarised; categorical variables were described using frequencies and percentages. Differences between cohorts were deemed statistically significant if the P‐value was < 0.05.
Sacubitril/valsartan titration and treatment patterns
Sac/val dose and titration patterns were analysed during the 6 months after index in patients with an index date from January 2016 to December 2016, an observation time of at least 12 months before the index date, at least 12 months of pre‐index activity (defined as at least one prescription of any ATC code every 6 months) and a minimum of 6 months post‐index activity. Key outcomes included maximum individual dose reached, time to first dose up‐titration and time to target dose. Titration patterns were evaluated longitudinally at the patient level: ‘up‐titration’ corresponds to all patients who experienced an initial increase in sac/val dose post‐index, whereas ‘stable up‐titration’ corresponds to up‐titrated patients who experienced no subsequent decrease in sac/val dose. Likewise, ‘down‐titration’ corresponds to all patients who experienced an initial dose decrease in sac/val dose post‐index, whereas ‘stable down‐titration’ corresponds to the proportion of down‐titrated patients who experienced no subsequent up‐titration. All parameters were stratified by sac/val index dose and prescriber specialty. Titration patterns were also evaluated based on the 12‐month pre‐index use of ACEI/ARBs, for which patients were stratified as RAAS‐naïve patients (no ACEI or ARB use observed), low dose (< 50% ACEI target dose), medium dose (≥ 50% ACEI target dose < 100%) and ACEI 100% target dose.
Sacubitril/valsartan persistence and compliance
Persistence up to 12 months post‐index was assessed in patients with an index date from January 2016 to June 2017 and at least 12 months of pre‐index activity. No post‐index activity was required. Persistence was assessed using the permissible gap8 and Kaplan–Meier methods,13 allowing for a maximum gap of 90 days between the end of the last day's supply and the next refill. In the analyses, treatment discontinuation was considered as a failure event, and censoring was applied at the end of the study period or after more than 90 days without any prescription activity for any ATC code. The number of days' supply was calculated as dispensed pack size divided by two (assuming that sac/val was prescribed to be taken b.i.d. in line with the approved posology). A multivariate Cox regression model was applied to assess the association between patient characteristics and non‐persistence using hazard ratios, adjusted for prescriber specialty, sex, age, sac/val dose at index and previous CV and non‐CV medication use, and associated 95% confidence intervals.
Compliance in the 12 months post‐index was analysed in a cohort with an index date from January 2016 to December 2016, with at least 12 months of pre‐index activity and evidence of 12 months of post‐index activity (defined as at least one sac/val prescription in the first 6 months post‐index and another during 7–12 months post‐index). Compliance was defined as the proportion of days covered (PDC), calculated as the total days' supply of medication divided by the length of the treatment period, with overlapping days' supply removed.
Concomitant medication
Concomitant medication was analysed in a cohort with an index date from January 2016 to December 2016, at least 12 months pre‐index activity and at least one sac/val prescription within 6 months post‐index. Oral diuretic usage was evaluated during the 6 months pre‐ and post‐index and calculated as daily diuretic dose using the dose of the four most frequently prescribed oral diuretics (furosemide, torasemide, hydrochlorothiazide, and xipamide). Usage was normalised per substance to the defined daily dose (DDD) according to the World Health Organisation definition and calculated as dispensed DDD divided by the number of days until the next prescription.14 Pre‐ and post‐index doses were compared in patients who had records of pre‐index diuretic prescriptions; the absence of a diuretic dose post‐index was defined as a dose of zero for calculation purposes. For ease of interpretation, DDD was transformed into the equivalent dose of furosemide.
Ethical standards
Only aggregated, anonymised patient data were used in these analyses. This study was performed in accordance with the guidelines for Good Practice of Secondary Data Analysis.15
Results
Baseline patient demographics and characteristics
A total of 26 191 patients received their first recorded prescription of sac/val between January 2016 and June 2017, equating to 127 803 sac/val prescriptions. General practitioners (GPs) and cardiologists accounted for approximately 78% and 17% of prescriptions, respectively. Among patients with a minimum of 12 months of pre‐index activity and 6 months of post‐index activity (n = 12 082), the first sac/val prescription in the database was issued by GPs for 68% of patients, compared with 25% by cardiologists and 6% by physicians in other specialties. Nearly one‐third (30%) of the 10 762 patients who received more than one prescription of sac/val received the prescriptions from different prescriber specialties over time. Of those who received their first prescription from a GP, 85% also received all subsequent sac/val prescriptions from a GP (online supplementary Table S2).
Patients receiving their first sac/val prescription from GPs tended to be older [mean age (SD) of 72.8 (11.8) years vs. 68.3 (11.4) years] and were more often female (28.4% vs. 20.9%) than those receiving their first sac/val prescription from cardiologists (Table 1). Overall, the use of HF medications prior to index (first recorded sac/val prescription) was high; 56.1% of patients had received an ACEI, 37.0% had received an angiotensin receptor blocker (ARB), 89.7% had received a BB and 64.1% had received an MRA.
Table 1.
Baseline patient demographics and use of cardiovascular and non‐cardiovascular drugs during 12 months pre‐index, stratified by prescriber specialty
| Characteristic | Total (n = 12 082) | GP (n = 8253) | Cardiology (n = 3063) | Other (n = 766) | P‐value GP vs. cardiology |
|---|---|---|---|---|---|
| Age, years, mean (SD) | 71.3 (12.0) | 72.8 (11.8) | 68.3 (11.4) | 67.1 (13.0) | <0.001 |
| Sex (%) | |||||
| Male | 54.0 | 51.3 | 62.0 | 52.0 | <0.0001 |
| Female | 25.8 | 28.4 | 20.9 | 17.6 | <0.0001 |
| Unknown | 20.2 | 20.4 | 17.2 | 30.2 | <0.0001 |
| CV medication use pre‐index (%) | |||||
| ACEI | 56.1 | 54.8 | 59.7 | 55.1 | <0.0001 |
| ARB | 37.0 | 36.6 | 37.0 | 41.8 | 0.6458 |
| BB | 89.7 | 88.7 | 91.7 | 91.5 | <0.0001 |
| MRA | 64.1 | 60.5 | 71.7 | 72.8 | <0.0001 |
| Diuretics (excluding MRAs) | 86.4 | 87.1 | 84.6 | 85.9 | <0.0001 |
| Oral diuretics (including MRAs) | 93.3 | 92.7 | 94.6 | 94.4 | <0.0001 |
| Potassium sparing | 0.0 | 0.0 | 0.0 | 0.0 | 1.000 |
| Loop diuretics | 73.5 | 73.7 | 73.2 | 72.3 | 0.5297 |
| Thiazides | 14.4 | 15.4 | 11.7 | 15.1 | <0.0001 |
| Selective nephron blockadea | 9.6 | 10.3 | 7.9 | 9.0 | <0.0001 |
| Vitamin K antagonists | 31.3 | 31.2 | 31.5 | 30.7 | 0.7201 |
| Antiplatelet medications | 33.7 | 34.2 | 32.4 | 33.7 | 0.0357 |
| Lipid‐lowering drugs | 61.8 | 60.2 | 65.2 | 64.2 | <0.0001 |
| Non‐CV medication use pre‐index (%) | |||||
| Glucose‐lowering drugs | 34.8 | 35.4 | 33.5 | 32.6 | 0.0279 |
| Insulin | 19.2 | 19.3 | 18.4 | 20.8 | 0.2069 |
| Dipeptidyl peptidase‐4 inhibitors | 14.0 | 14.7 | 12.3 | 12.8 | 0.0011 |
| SGLT2 | 2.0 | 2.0 | 1.8 | 2.9 | 0.4924 |
| Other glucose‐lowering drugs | 17.3 | 17.2 | 18.2 | 14.9 | 0.2230 |
| Antidepressants | 15.7 | 17.2 | 12.0 | 13.7 | <0.0001 |
| NSAIDs | 28.5 | 29.8 | 25.9 | 24.8 | <0.0001 |
| Gout treatments | 35.5 | 35.5 | 34.7 | 39.7 | 0.3548 |
| COPD treatment | 29.6 | 31.2 | 25.4 | 28.7 | <0.0001 |
| CV medication naïve (%) | |||||
| ACEI/ARB | 6.3 | 7.3 | 4.0 | 4.4 | <0.0001 |
| BB | 6.8 | 7.8 | 4.5 | 5.1 | <0.0001 |
| MRA | 28.9 | 32.5 | 21.0 | 21.8 | <0.0001 |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta‐blocker; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; GP, general practice; MRA, mineralocorticoid receptor antagonist; NSAID, non‐steroidal anti‐inflammatory drug; SD, standard deviation; SGLT2, sodium–glucose co‐transporter‐2.
Selective nephron blockade defined as a prescription of both a loop diuretic and a thiazide on the same day.
A high proportion of patients were prescribed other CV drugs during the 12 months pre‐index, including lipid‐lowering drugs, antiplatelet medications and vitamin K antagonists (61.8%, 33.7% and 31.3% respectively; Table 1). The most frequently prescribed non‐CV drugs were gout treatments, glucose‐lowering drugs and treatments for chronic obstructive pulmonary disease (35.5%, 34.8% and 29.6%, respectively; Table 1).
A total of 73.5% of patients were prescribed a loop diuretic during the 12 months pre‐index; 14.4% of all patients were prescribed a thiazide, and 9.6% were prescribed both a loop diuretic and a thiazide on the same day (Table 1).
Sacubitril/valsartan treatment patterns during the first 6 months post‐index
Of the 12 082 patients with 12 months of pre‐index activity and a minimum of 6 months of follow‐up, 64% were prescribed a first observed sac/val dose of 50 mg b.i.d., 32% were prescribed 100 mg b.i.d. and 4% were prescribed 200 mg b.i.d. (Figure 1).
Figure 1.
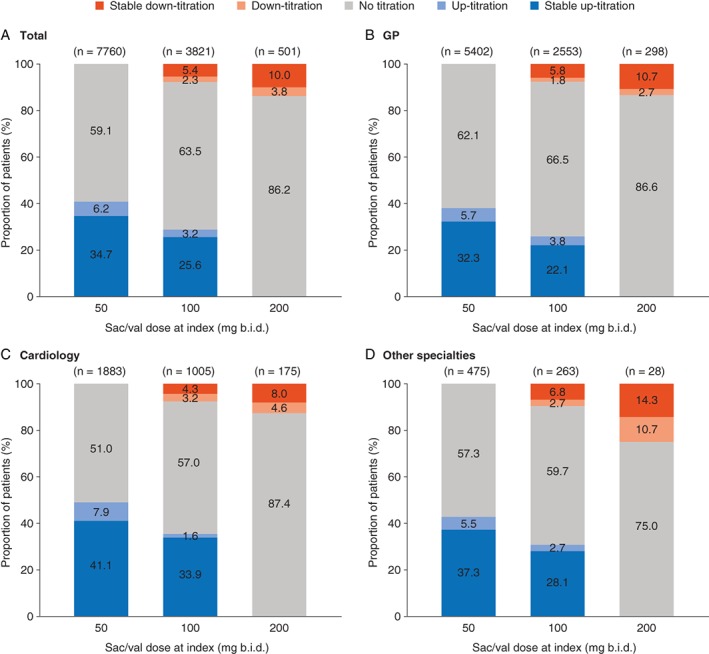
Titration patterns during the 6 months post‐index stratified by sacubitril/valsartan (sac/val) dose at index and prescriber specialty (A–D). ‘Up‐titration’ corresponds to all patients who experience an initial increase in sac/val dose; ‘stable up‐titration’ corresponds to up‐titrated patients who experienced no subsequent decrease in sac/val dose; ‘down‐titration’ corresponds to all patients who experienced an initial decrease in sac/val dose; ‘stable down‐titration’ corresponds to down‐titrated patients who experienced no subsequent increase in sac/val dose. Percentages may not sum to 100 owing to rounding. b.i.d., twice daily; GP, general practice.
Of the patients prescribed 50 mg b.i.d. or 100 mg b.i.d. at index, more than 80% of those who were up‐titrated were able to maintain a stable higher dose in the first 6 months post‐index (Figure 1). Only 10% of patients prescribed a dose of 200 mg b.i.d. at index were down‐titrated to a stable lower dose. Overall, 62% of patients had no change in their sac/val dose during the 6 months of follow‐up.
The mean (SD) time to first up‐titration was 54 (44) days, while the mean time to reach the target dose varied from 79 (44) days to 57 (47) days for patients on 50 mg b.i.d. and 100 mg b.i.d. at index, respectively (P < 0.001; data not shown). No notable differences in the time to first titration were observed across specialties at index.
During the 6‐month post‐index period, 26% of patients receiving their first prescription from a cardiologist were prescribed the target dose of 200 mg b.i.d., compared with 19% of patients with GP prescribers and 20% with prescribers in other specialties at index (P < 0.001; Table 2). From the 7609 eligible patients who were using ACEI in the pre‐index period or who were RAAS‐naïve, only 21% were prescribed the ACEI target dose. Among pre‐index RAAS‐naïve and low‐dose ACEI patients, around 72% to 74% were initiated on sac/val 50 mg b.i.d. compared to 65% of patients on pre‐index ACEI target dose. The proportion of patients with sac/val 50 mg b.i.d. dose at index decreased with the increasing ACEI dose strata at baseline, whereas the proportion of patients prescribed the sac/val target dose (200 mg b.i.d.) during the first 6 months increased from 15% to 27% (P < 0.001) from low to target pre‐index ACEI dose patients.
Table 2.
Maximum dose of sacubitril/valsartan reached within 6 months post‐index, stratified by sacubitril/valsartan dose at index and prescriber specialty
| Index dose | Prescriber specialty at index | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | GP | Cardiology | Other | |||||||||||||
| Total (n = 12 082) | 50 mg b.i.d. (n = 7760) | 100 mg b.i.d. (n = 3821) | 200 mg b.i.d. (n = 501) | Total (n = 8253) | 50 mg b.i.d. (n = 5402) | 100 mg b.i.d. (n = 2553) | 200 mg b.i.d. (n = 298) | Total (n = 3063) | 50 mg b.i.d. (n = 1883) | 100 mg b.i.d. (n = 1005) | 200 mg b.i.d. (n = 175) | Total (n = 766) | 50 mg b.i.d. (n = 475) | 100 mg b.i.d. (n = 263) | 200 mg b.i.d. (n = 28) | |
| 50 mg b.i.d. | 38 | 59 | N/A | N/A | 41 | 62 | N/A | N/A | 31 | 51 | N/A | N/A | 36 | 57 | N/A | N/A |
| 100 mg b.i.d. | 41 | 30 | 71 | N/A | 41 | 27 | 74 | N/A | 43 | 35 | 64 | N/A | 44 | 34 | 68 | N/A |
| 200 mg b.i.d. | 21 | 11 | 29 | 100 | 19 | 11 | 26 | 100 | 26 | 14 | 36 | 100 | 20 | 9 | 32 | 100 |
Values are given as %.
b.i.d., twice daily; GP, general practice; N/A, not available.
Patients on the pre‐index target ACEI dose presented slightly higher rates of down‐titration following up‐titration to target dose compared to patients with low pre‐index ACEI doses (90% vs. 84%) and similar to that observed in RAAS‐naïve patients (89%). Patients who were on the pre‐index ACEI target dose had a marginally shorter time to the first sac/val dose titration, and time to individual maximum dose compared to those with low ACEI doses (< 50% target dose) [mean (SD): 51.9 (43.8) days vs. 55.3 (44.2) days and 60.7 (46.6) days vs. 64.4 (47.1) days, respectively]. Times to first sac/val up‐titration and to the individual maximum dose observed in pre‐index ACEI target dose patients are very similar to those observed in RAAS‐naïve patients [mean (SD): 53.6 (40.5) days and 60.8 (42.8) days, respectively] (online supplementary Table S3).
Persistence and compliance analyses
Among the 22 275 patients with 12 months of pre‐index activity, sac/val persistence at 12 months was estimated to be 71% (Figure 2). In the majority of cases where discontinuation of sac/val therapy was observed, this happened within the first 90 days after index. Persistence at 12 months was similar across the different specialties prescribing sac/val at index, while time to discontinuation was shorter for patients prescribed sac/val 50 mg b.i.d. at index (77 days) than those prescribed 100 mg b.i.d. and 200 mg b.i.d. at index (110 days and 111 days, respectively).
Figure 2.
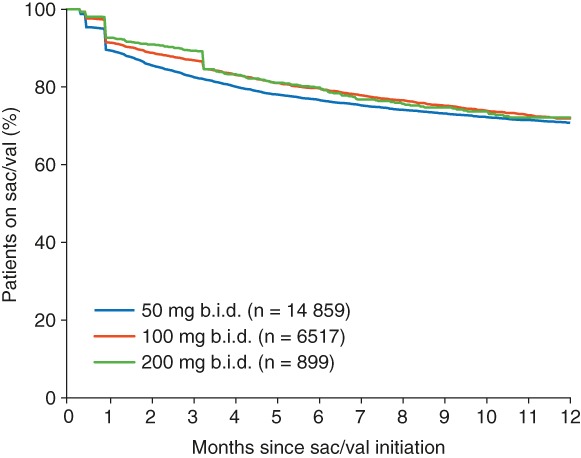
Kaplan–Meier plots for persistence with sacubitril/valsartan (sac/val) over 12 months post‐index, by sac/val dose at index. b.i.d., twice daily.
A multivariate analysis revealed that patients who were younger than 75 years, male, received an initial sac/val dose of 100 mg b.i.d., and used ACEI, ARB, BB, MRA, lipid‐lowering drugs, oral diuretics or novel oral anticoagulants in the 12 months pre‐index, had a significantly lower risk of therapy discontinuation (online supplementary Table S4).
Among the 8226 patients with a record of 12 months of pre‐ and post‐index activity, high compliance with sac/val (PDC > 80%) was observed during 12 months post‐index (Figure 3); this increased with sac/val dose at index (80% PDC for 50 mg b.i.d., 83% for 100 mg b.i.d., and 85% for 200 mg b.i.d.). The same trend was observed for compliance stratified by maximum dose reached by 6 months post‐index (77% PDC for 50 mg b.i.d., 82% for 100 mg b.i.d., and 85% for 200 mg b.i.d.; data not shown).
Figure 3.
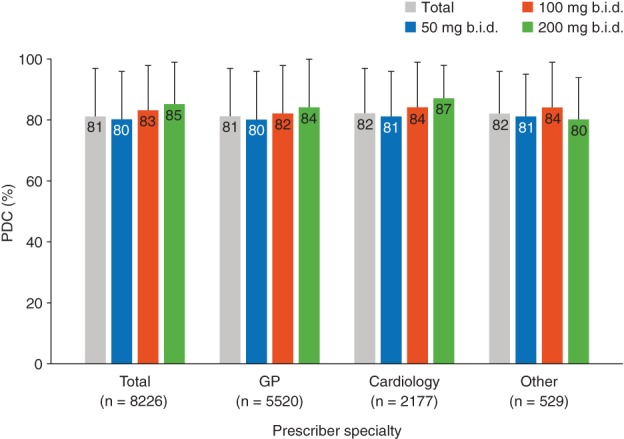
Compliance with sacubitril/valsartan during 12 months post‐index stratified by sacubitril/valsartan dose at index. Proportion of days covered (PDC) was calculated as the total days' supply of sacubitril/valsartan divided by the length of treatment period, with overlapping days' supply removed. b.i.d., twice daily; GP, general practice.
Among RAAS‐naïve patients the mean compliance was estimated to be 77.2%, which is significantly lower than compliance in non‐naïve patients (81.4%) (P < 0.001).
Concomitant medication
Among the cohort of 10 566 patients eligible for analyses of concomitant medication, use of BBs in patients first prescribed sac/val by GPs and cardiologists was 84% and 86%, respectively (P = 0.027), in the 6 months post‐index, whereas MRA use was lower in patients prescribed sac/val by GPs than cardiologists (54% vs. 63%; P < 0.001; data not shown). Similarly, the use of these drug classes in the 12 month pre‐index period was comparable between patients prescribed sac/val by GPs and cardiologists (BBs, 89% vs. 92%, P < 0.001; MRAs, 61% vs. 72%, P < 0.001; Table 1).
The majority of patients (77%) were prescribed an oral diuretic during the 6‐month pre‐index period compared with 73% in the 6‐month post‐index period (P < 0.001; data not shown). A diuretic was prescribed in both the pre‐ and post‐index periods for 64% of all patients, while 12% discontinued and 9% started diuretics post‐index. The post‐index daily diuretic dose was 50% higher in patients prescribed sac/val by GPs than those prescribed sac/val by cardiologists (P < 0.001).
In the entire sac/val cohort, the mean furosemide equivalent dose was reduced by 25% from 218 mg during the 6 months pre‐index to 163 mg in the 6 months post‐index (P < 0.001). The daily diuretic dose decreased from pre‐ to post‐index by 48 mg (−26%) in patients stably up‐titrated on sac/val (P < 0.001), decreased by 35 mg (−14%) in those who were stably down‐titrated on sac/val (P = 0.003) and decreased by 67 mg (−28%) in patients who were neither up‐ nor down‐titrated (P < 0.001) (Figure 4).
Figure 4.
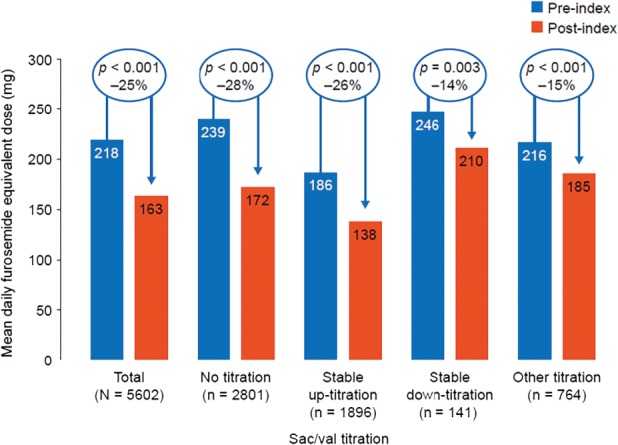
Oral diuretic furosemide equivalent doses during the 6 months pre‐ vs. the 6 months post‐index in the full sacubitril/valsartan (sac/val) cohort, stratified by sac/val titration patterns. ‘Stable up‐titration’ corresponds to patients who initially up‐titrated and experienced no subsequent decrease in sac/val dose; ‘stable down‐titration’ corresponds to patients who initially down‐titrated and experienced no subsequent increase in sac/val dose. Mean daily furosemide equivalent doses have been rounded using values to zero decimal places.
Discussion
Our study has five main findings: (i) two‐thirds of patients prescribed sac/val in Germany are initially prescribed the lowest dose of sac/val; (ii) almost two‐thirds (62%) of all patients stay on their initial dose in the 6 months post‐index, while over 80% of patients who are up‐titrated stay on the higher dose; (iii) on average, across all doses the estimated persistence and compliance with sac/val at 12 months was high (71% and > 80%, respectively); (iv) while other HF drug classes are used stably pre‐ and post‐initiation of sac/val, symptomatic diuretic treatment is reduced irrespective of prescribed sac/val dose; and (v) patients receiving their first sac/val prescription from a cardiologist are more likely to receive the target dose of 200 mg b.i.d. within 6 months after index than patients receiving a first prescription from a GP or other prescriber specialty.
Baseline patient demographics and characteristics
Our findings support those recently observed in a study of another German database, which showed that patients prescribed sac/val in the real world are often older and more likely to be female than patients enrolled in the pivotal PARADIGM‐HF trial [in which the mean age (SD) of patients was 64 (12) years; 21% were female].3, 6
Prior use of disease‐modifying HF medication in patients prescribed sac/val was high, suggesting that sac/val may be preferentially used in patients who have received optimal therapy but remain symptomatic, as recommended by current guidelines. Only 6% of patients with a sac/val prescription were RAAS‐naïve, suggesting that these patients may have been newly diagnosed (which is likely considering the population definition for this analysis).
Sacubitril/valsartan treatment patterns during the first 6 months post‐index
Good adherence to treatment and dosing guidelines by physicians when prescribing HF medications has been associated with better clinical outcomes.10 However, almost two‐thirds (64%) of the patients analysed in the current study were initially prescribed a sac/val dose of 50 mg b.i.d.; this is in line with several other recent real‐world studies of sac/val16, 17, 18 but conflicts with the recommendations outlined in the EU Summary of Product Characteristics that suggest starting most patients on a sac/val dose of 100 mg b.i.d.1 The mean (SD) time to first titration was 54 (44) days, which is considerably longer than the 2–4 weeks recommended in the EU Summary of Product Characteristics.1 Moreover, only 21% of patients received the target dose of 200 mg b.i.d. and 62% of all patients had no change in their sac/val dose within 6 months post‐index. Only 10% of patients receiving 200 mg b.i.d. sac/val at index were stably down‐titrated and more than 80% of all attempted up‐titration efforts in patients receiving 50 mg b.i.d. or 100 mg b.i.d. were successful (i.e. 43% and 31% were stably up‐titrated, respectively). In comparison, in the recent TITRATION randomised controlled trial, 76% of patients were able to be up‐titrated to 200 mg b.i.d. within 12 weeks.19 This gap in percentage of patients reaching target dose of sac/val under study conditions as compared to real‐world data requires further study. However, the main explanation is that up‐titration is just not attempted rather than higher dosages are not tolerated.
Additionally, only marginal differences in sac/val titration patterns were observed across patients on prior ACEI vs RAAS‐naïve patients; overall, approximately half of the patients were prescribed sac/val doses of 100 or 200 mg b.i.d. during the first 6 months. Similar findings have been encountered in the TITRATION randomised controlled trial where no notable differences between up‐titration regimens in the proportion achieving and maintaining the target dose over the entire study period were observed among RAAS‐naïve patients.19
The reasons why patients in the real world are initiated on lower doses and are less often up‐titrated are unknown. Co‐morbidities (e.g. renal impairment) and side effects of sac/val (e.g. hypotension, hypercalcemia and dizziness) may occur more often in older patients than younger patients, which may discourage physicians from up‐titrating. This theory is supported by the predictors of persistence we identified (e.g. younger age and male sex), but cannot fully explain the difference between the recommended and real‐world doses of sac/val. The fact that most patients in this study were able to tolerate up‐titration may be an indication that sac/val can be well tolerated at higher doses and that the use of 50 mg b.i.d. as a first dose may be an unnecessary precautionary measure. This is a common observation for the early adoption period of other HF drugs.20, 21 A study of patients with chronic HF receiving ACEIs and ARBs has similarly shown that only 29% and 24% of patients were receiving the target dose of ACEIs and ARBs, respectively.7 These findings imply that the lack of up‐titration of sac/val is not a drug‐specific phenomenon, and rather may be inherent to prescription of any RAAS inhibitor therapy. A multivariable regression model performed on data from the PARADIGM‐HF study identified significant predictors of dose reduction, including (but not limited to) increased serum creatinine, N‐terminal pro B‐type natriuretic peptide levels and heart rate, older age and lower systolic blood pressure.22
Overall, sac/val initiation and titration patterns identified in the German IMS® LRx database are very similar to those observed by other real‐world studies of German patients with HF.6, 18 These findings support the idea that clinician inertia in up‐titration of HF medication still exists, which has also been observed in studies of other HF medications.7 Identifying reasons for clinician inertia is necessary, and educational efforts regarding the benefit of up‐titrating need to be reinforced. Encouragingly, recent observational studies conducted in Spain, Portugal and Canada have observed that high proportions of patients are achieving the sac/val target dose of 200 mg b.i.d. in real‐world clinical practice (35%, 52% and 70% of patients, respectively).23, 24, 25
Persistence and compliance analysis
Sac/val persistence (71%) and compliance (PDC > 80%) at 12 months post‐index were found to be high and in line with those observed in other real‐world studies.26, 27 Persistence and compliance were shown to be higher in patients who were prescribed higher initial doses of sac/val than those patients who were prescribed an initial sac/val dose of 50 mg b.i.d. Additionally, compliance was slightly lower in RAAS‐naïve patients (77.2%), which could be potentially explained by the fact that these patients may be de novo patients. The absence of clinical data meant we were unable to further investigate reasons for the association between sac/val dosing and compliance and persistence. However, it is encouraging that lower compliance and higher rates of treatment dropout were not observed in this real‐world population, which may have been the case had many patients that experienced tolerability issues with the target dose. These data support the initiation of patients on to higher doses of sac/val if possible, particularly because higher persistence and compliance are associated with improved patient outcomes.11
Concomitant medication
Patients who were up‐titrated on sac/val tended to be prescribed lower daily diuretic doses post‐index than those who were down‐titrated on sac/val. This has been reported in other observational cohorts,28 and may suggest a small diuretic effect of sac/val or reflect the reduction in HF symptoms by sac/val, which allows a lowering of diuretic dose. Additionally, an analysis of the PARADIGM‐HF study cohort also indicated that patients receiving sac/val were more likely to experience a diuretic dose reduction than patients receiving enalapril.29 Although diuretics may resolve the signs and symptoms of HF, they have no prognostic benefit in HF,5 but increasing doses of diuretics can promote kidney injury and renal impairment, which are associated with increased mortality. In the PARADIGM‐HF study, renal impairment was less frequent with sac/val than with enalapril, and the reduction in diuretic dose may be one potential explanation for this finding.
Study strengths and limitations
The large sample size of patient‐level, pharmacy data in the IMS® LRx database allows for unbiased and representative characterization of the real‐world use of sac/val in Germany, capturing 60% of statutory health‐insured dispensed prescriptions (approximately 90% of all patients in Germany are statutory health insured). Additionally, the fact that the database collects pharmacy dispensing data ensures that unclaimed prescriptions are eliminated from the data set, which is not true for studies using electronic medical record data (which may not account for therapies that do not reach the patient). The limitations associated with this study include those inherent to secondary use of data.30 Further limitations include a lack of diagnosis recording and clinical parameters, co‐morbidities, ejection fraction, laboratory data, symptoms (e.g. New York Heart Association class), lack of hospitalisation data (including drugs administered or prescribed in a hospital setting), the incomplete coverage of all pharmacies in Germany and lack of continuous enrolment. Consecutively, we were not able to identify the total number of patients potentially eligible for sac/val in Germany neither to stratify patients by clinical subgroups. A small proportion of patients (4%) were prescribed the target dose of sac/val of 200 mg b.i.d. at index, which is not recommended as the starting dose in the product label.1 As dispensations of sac/val from hospital pharmacies and from pharmacies beyond the scope of the German IMS® LRx database are not captured, there is a possibility that these patients may have received an initial, lower sac/val dose (e.g. 100 mg b.i.d.) in a hospital setting and were then up‐titrated after hospital discharge by a primary care physician. The implementation of an activity requirement (i.e. a minimum number of any ATC code and/or sac/val prescriptions) during a pre‐specified time frame prior to index was implemented to reduce ‘misclassified first prescriptions’ and overcome the lack of continuous enrolment. The label for sac/val also confines use of sac/val to patients with HFrEF; however, owing to the lack of echocardiographic data, this could not be confirmed. Additionally, information on the prescribed dose frequency is not captured within the database. These analyses therefore assumed that sac/val was prescribed to be taken twice daily according to the approved posology.
Conclusion
To conclude, in this large real‐world cohort study, two‐thirds of patients on sac/val received the lowest dose of 50 mg b.i.d. at index and attempts to up‐titrate the dose were made in only 41% of these patients during the following 6 months. Persistence and compliance at 12 months post‐index were high, and symptomatic diuretic treatment was reduced following sac/val index, irrespective of sac/val dose prescribed. Barriers to up‐titration must be further explored, and educational efforts to promote up‐titration must be intensified.
Supporting information
Figure S1. Study population selection.
Table S1. List of Anatomical Therapeutic Chemical (ATC) codes.
Table S2. Patients receiving sacubitril/valsartan prescriptions from multiple prescriber specialties during follow‐up.
Table S3. Sacubitril/valsartan titration patterns by angiotensin‐converting enzyme inhibitor baseline dosage strata.
Table S4. Multivariate Cox regression model for predictors of discontinuing sacubitril/valsartan.
Acknowledgements
Medical writing support was provided by Abigail Morris of PharmaGenesis London, London, UK, and was funded by Novartis Pharma AG, Basel, Switzerland.
Funding
This research was funded by Novartis Pharma AG, Basel, Switzerland.
Conflict of interest: R.W. has been a consultant for, or received speaker's bureau honoraria from Bayer, Berlin‐Chemie, Boehringer Ingelheim, Bristol‐Myers Squibb, CVRx, Daiichi, Johnson & Johnson, Medtronic, Novartis, Pfizer, Sanofi and Servier. His institution has received research funding from Boehringer Ingelheim, the European Union and The Federal Ministry of Education and Research. A.F.F. and B.B. and R.S. were employees of Novartis Pharma AG, Basel, Switzerland at the time of the study. B.B. is now an employee of F. Hoffman‐La Roche Ltd, Basel, Switzerland. S.K. is an employee of Novartis Pharma GmbH, Nuremberg, Germany. S.B.W. was an employee of Novartis Sweden AB, Stockholm, Sweden at the time of this study and is now an employee of Janssen‐Cilag AB, Solna, Sweden. E.K., J.E. and K.K. were employees of IQVIA, Frankfurt, Germany at the time of this study. IQVIA was commissioned to conduct the study on behalf of Novartis Pharma AG, and has ongoing consulting and research relationships with Novartis Pharma AG.
References
- 1. European Medicines Agency . Summary of Product Characteristics, Entresto. 2015. https://www.ema.europa.eu/documents/product‐information/entresto‐epar‐product‐information_en.pdf (11 March 2019).
- 2. European Medicines Agency . EPAR Summary for the public, Entresto. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Summary_for_the_public/human/004062/WC500197539.pdf (11 March 2019).
- 3. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 4.Pharmazeutische Zeitung. Sacubitril/valsartan|Entresto®|53|2016. https://www.pharmazeutische‐zeitung.de/arzneistoffe/daten/2016/sacubitrilvalsartanentrestor532016 (11 March 2019).
- 5. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 6. Wachter R, Viriato D, Klebs S, Grunow SS, Schindler M, Engelhard J, Proenca CC, Calado F, Schlienger R, Dworak M, Balas B, Bruce Wirta S. Early insights into the characteristics and evolution of clinical parameters in a cohort of patients prescribed sacubitril/valsartan in Germany. Postgrad Med 2018;130:308–316. [DOI] [PubMed] [Google Scholar]
- 7. Maggioni AP, Anker SD, Dahlstrom U, Filippatos G, Ponikowski P, Zannad F, Amir O, Chioncel O, Leiro MC, Drozdz J, Erglis A, Fazlibegovic E, Fonseca C, Fruhwald F, Gatzov P, Goncalvesova E, Hassanein M, Hradec J, Kavoliuniene A, Lainscak M, Logeart D, Merkely B, Metra M, Persson H, Seferovic P, Temizhan A, Tousoulis D, Tavazzi L; Heart Failure Association of the ESC . Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2013;15:1173–1184. [DOI] [PubMed] [Google Scholar]
- 8. Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health 2008;11:44–47. [DOI] [PubMed] [Google Scholar]
- 9. Malo S, Aguilar‐Palacio I, Feja C, Lallana MJ, Rabanaque MJ, Armesto J, Menditto E. Different approaches to the assessment of adherence and persistence with cardiovascular‐disease preventive medications. Curr Med Res Opin 2017;33:1329–1336. [DOI] [PubMed] [Google Scholar]
- 10. Komajda M, Cowie MR, Tavazzi L, Ponikowski P, Anker SD, Filippatos GS. Physicians' guideline adherence is associated with better prognosis in outpatients with heart failure with reduced ejection fraction: the QUALIFY international registry. Eur J Heart Fail 2017;19:1414–1423. [DOI] [PubMed] [Google Scholar]
- 11. Gislason GH, Rasmussen JN, Abildstrom SZ, Schramm TK, Hansen ML, Buch P, Sorensen R, Folke F, Gadsboll N, Rasmussen S, Kober L, Madsen M, Torp‐Pedersen C. Persistent use of evidence‐based pharmacotherapy in heart failure is associated with improved outcomes. Circulation 2007;116:737–744. [DOI] [PubMed] [Google Scholar]
- 12. Richter H, Dombrowski S, Hamer H, Hadji P, Kostev K. Use of a German longitudinal prescription database (LRx) in pharmacoepidemiology. Ger Med Sci 2015;13:Doc14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zwiener I, Blettner M, Hommel G. Survival analysis: part 15 of a series on evaluation of scientific publications. Dtsch Arztebl Int 2011;108:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO Collaborating Center for Drug Statistics Methodology. Definition and general considerations. https://www.whocc.no/ddd/definition_and_general_considera (11 March 2019).
- 15. Swart E, Gothe H, Geyer S, Jaunzeme J, Maier B, Grobe TG, Ihle P. Good practice of secondary data analysis (GPS): guidelines and recommendations. Gesundheitswesen 2015;77:120–126. [DOI] [PubMed] [Google Scholar]
- 16. Barrett MJ, Earls S, Zhou S, McDonald K. Use of sacubitril/valsartan in a dedicated heart failure centre – real world experience from the first 110 patients. Eur J Heart Fail 2017;19:408 (abstr). [Google Scholar]
- 17. Martens P, Belien H, Dupont M, Mullens W. Insights into implementation of sacubitril/valsartan into clinical practice. ESC Heart Fail 2018;5:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wachter R, Viriato D, Klebs S, Grunow SS, Schindler M, Engelhard J, Proenca CC, Calado F, Schlienger R, Dworak M, Balas B, Bruce Wirta S. Dosing patterns and evolution of clinical parameters in patients prescribed sacubitril/valsartan in Germany. Circulation 2017;136:A15751 (abstr). [Google Scholar]
- 19. Senni M, McMurray JJ, Wachter R, McIntyre HF, Reyes A, Majercak I, Andreka P, Shehova‐Yankova N, Anand I, Yilmaz MB, Gogia H, Martinez‐Selles M, Fischer S, Zilahi Z, Cosmi F, Gelev V, Galve E, Gómez‐Doblas JJ, Nociar J, Radomska M, Sokolova B, Volterrani M, Sarkar A, Reimund B, Chen F, Charney A. Initiating sacubitril/valsartan (LCZ696) in heart failure: results of TITRATION, a double‐blind, randomized comparison of two uptitration regimens. Eur J Heart Fail 2016;18:1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Remme WJ, McMurray JJ, Hobbs FD, Cohen‐Solal A, Lopez‐Sendon J, Boccanelli A, Zannad F, Rauch B, Keukelaar K, Macarie C, Ruzyllo W, Cline C. Awareness and perception of heart failure among European cardiologists, internists, geriatricians, and primary care physicians. Eur Heart J 2008;29:1739–1752. [DOI] [PubMed] [Google Scholar]
- 21. Lenzen MJ, Boersma E, Reimer WJ, Balk AH, Komajda M, Swedberg K, Follath F, Jimenez‐Navarro M, Simoons ML, Cleland JG. Under‐utilization of evidence‐based drug treatment in patients with heart failure is only partially explained by dissimilarity to patients enrolled in landmark trials: a report from the Euro Heart Survey on Heart Failure. Eur Heart J 2005;26:2706–2713. [DOI] [PubMed] [Google Scholar]
- 22. Vardeny O, Claggett B, Packer M, Zile MR, Rouleau J, Swedberg K, Teerlink JR, Desai AS, Lefkowitz M, Shi V, McMurray JJ, Solomon SD; Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF) Investigators. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM‐HF trial. Eur J Heart Fail 2016;18:1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pandey AK, Reinhart J, Pandey AS, Verma S. The differential effects of valsartan/sacubirtril on cardiac function in ischemic versus dilated cardiomyopathy: a 1 year real world study. Circulation 2018;136:A19506 (abstr). [Google Scholar]
- 24. Goena Vives A, Gomez Ramirez C, Quintas Ovejero L, Garcia Martin R, Luis Serret I, Natividad Andres R, Sagasti Aboitiz I, Campana Lazaro M. Tolerability of sacubitril/valsartan when initiating/uptitrating in real‐life patients. Initial experience in two centres. Eur J Heart Fail 2018;20 (Suppl. 1):273 (abstr).
- 25. Neiva JN, Gomez‐Otero IG, Varela‐Roman AV, Moure Gonzalez MM, Seoane Blanco AS. Tolerability and safety of sacubitril/valsartan in real‐life practice. Eur J Heart Fail 2018;20 (Suppl. 1):73 (abstr).
- 26. Poluzzi E, Strahinja P, Vaccheri A, Vargiu A, Silvani MC, Motola D, Marchesini G, De Ponti F, Montanaro N. Adherence to chronic cardiovascular therapies: persistence over the years and dose coverage. Br J Clin Pharmacol 2007;63:346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mohanty A, Levitan EB, Dodson JA, He T, Russo PA, Bress AP. Adherence and healthcare utilization following initiation of sacubitril/valsartan among veterans with heart failure and reduced ejection fraction. Circulation 2017;136:A13540 (abstr). [Google Scholar]
- 28. Canu A, Maurin V, Dos Santos P, Picard F. Results of a single center experience on 200 consecutive patients treated with Entresto (sacubitril/valsartan). Eur J Heart Fail 2017;19 (Suppl. 1):413 (abstr). [Google Scholar]
- 29. Vardeny O, Claggett B, Kachadourian J, Packer M, Zile M, Rouleau J, Swedberg K, Shi V, Lefkowitz M, McMurray J, Solomon SD. Reduced loop diuretic use in patients taking Sacubitril/valsartan compared with enalapril. Circulation 2016;134:A17948 (abstr). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheng HG, Phillips MR. Secondary data analysis of existing data: opportunities and implementation. Shanghai Arch Psychiatry 2014;26:371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study population selection.
Table S1. List of Anatomical Therapeutic Chemical (ATC) codes.
Table S2. Patients receiving sacubitril/valsartan prescriptions from multiple prescriber specialties during follow‐up.
Table S3. Sacubitril/valsartan titration patterns by angiotensin‐converting enzyme inhibitor baseline dosage strata.
Table S4. Multivariate Cox regression model for predictors of discontinuing sacubitril/valsartan.


