Abstract
Aims
To assess differences in diuretic dose requirements in patients treated with sacubitril/valsartan compared with enalapril in the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM‐HF) trial.
Methods and results
Overall, 8399 patients with New York Heart Association class II–IV heart failure and reduced LVEF were randomized to sacubitril/valsartan 200 mg bid or enalapril 10 mg twice daily. Loop diuretic doses were assessed at baseline, 6, 12, and 24 months, and furosemide dose equivalents were calculated via multiplication factors (2x for torsemide and 40x for bumetanide). Percentages of participants with reductions or increases in loop diuretic dose were determined. At baseline, 80.8% of participants were taking any diuretics (n = 6290 for loop diuretics, n = 496 for other diuretics); of those, recorded dosage data for loop diuretics were available on 5487 participants. Mean baseline furosemide equivalent doses were 48.2 mg for sacubitril/valsartan and 49.6 mg for enalapril (P = 0.25). Patients treated with sacubitril/valsartan were more likely to reduce diuretic dose and less likely to increase diuretic dose relative to those randomized to enalapril at 6, 12, 24 months post‐randomization, with an overall decreased diuretic use of 2.0% (P = 0.02), 4.1% (P < 0.001), and 6.1% (P < 0.001) at 6, 12, and 24 months, respectively, with similar findings in an on‐treatment analysis.
Conclusion
Treatment with sacubitril/valsartan was associated with more loop diuretic dose reductions and fewer dose increases compared with enalapril, suggesting that treatment with sacubitril/valsartan may reduce the requirement for loop diuretics relative to enalapril in patients with heart failure with reduced ejection fraction.
Keywords: Diuretics, Randomized clinical trial, Heart failure with reduced ejection fraction, Sacubitril/valsartan, Enalapril
Background
In the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM‐HF) trial, sacubitril/valsartan (formerly LCZ696) reduced the primary composite outcome of cardiovascular death or heart failure hospitalization compared to enalapril in patients with symptomatic heart failure.1 Sacubitril inhibits the enzyme neprilysin, which plays a role in the breakdown of natriuretic peptides, increases natriuretic peptide levels, which may result in a natriuretic effect.2 Loop diuretics, frequently used in patients with heart failure, can lead to neurohormonal activation,3, 4 electrolyte abnormalities, and worsening renal function,5 and higher doses of diuretics have been associated with worse outcomes.6, 7, 8
The goal of this analysis was to investigate post‐randomization differences in diuretic use among participants randomized to sacubitril/valsartan compared with enalapril in the PARADIGM‐HF trial. We hypothesized that sacubitril/valsartan would be associated with reduced diuretic dose requirements compared with enalapril.
Methods
Patients
The study design of PARADIGM‐HF has been previously reported. Briefly, patients with New York Heart Association (NYHA) class II–IV heart failure with reduced ejection fraction (≤ 40%) and mild elevation in natriuretic peptides entered sequential active run‐in phases in which they were up‐titrated to enalapril 10 mg bid followed by sacubitril/valsartan 200 mg bid. After run‐in, they were randomized to sacubitril/valsartan 200 mg bid, or enalapril 10 mg bid. Patients were followed for a median of 27 months. The trial complies with the Declaration of Helsinki; locally appointed ethics committees approved the research protocol and informed consent was obtained from all subjects.
Assessment of diuretic use
Use of diuretics (non‐loop and loop type) including name of drug and dose were collected at baseline, 6, 12, and 24 months post‐randomization on case report forms. For loop diuretics, furosemide dose equivalents were calculated, with bumetanide 1 mg or torsemide 20 mg considered equivalent to 40 mg of furosemide.9
Statistical analysis
Baseline characteristics were summarized by diuretic use (no diuretic, non‐loop diuretic, loop diuretic). For loop diuretic, categories were created based on dose: < 20 mg daily, 20–40 mg daily, > 40 mg daily). Data from participants with missing dose information for loop diuretics (n = 803) were excluded. Baseline characteristics between diuretic use groups were compared with chi‐square test for categorical variables and ANOVA for continuous variables. The percentage of patients with reductions or increases in loop diuretic dose was calculated at 6, 12, and 24 months post‐randomization and compared between enalapril and sacubitril/valsartan treatment arms via regression.
Results
Out of 8399 validly randomized patients in PARADIGM‐HF, 80.8% of participants were taking any diuretics [n = 6290 for loop diuretics, n = 496 for other diuretics (e.g. thiazide)] at baseline. Of those, recorded, dosage data for loop diuretics were available on 5487 participants out of 6290 (furosemide equivalent < 20 mg, n = 438, 7.3%; furosemide equivalent 20–40 mg, n = 3625, 60.6%; furosemide > 40 mg, n = 1424, 23.8%). A total of 7259 participants had diuretic doses available at baseline and at least at one additional time point. Use of any diuretics was associated with higher NYHA class, lower ejection fraction, higher body mass index, greater likelihood of prior heart failure hospitalization, hypertension, diabetes, atrial fibrillation, digoxin use, mineralocorticoid receptor antagonist (MRA) use, higher creatinine, higher N‐terminal pro brain natriuretic peptide (NT‐proBNP), and lower incidence of ischaemic aetiology (Table 1). Higher diuretic dose was associated with worse NYHA class, higher likelihood of prior heart failure hospitalization, hypertension, diabetes, atrial fibrillation, use of digoxin, higher creatinine, and higher NT‐proBNP. In a multivariable model, changes in diuretic dose from baseline to 6 months were positively associated with baseline body mass index and NT‐proBNP, and inversely associated with baseline use and dose of MRA, digoxin, baseline weight, and randomization to sacubitril/valsartan (all P < 0.01). Doses of spironolactone and eplerenone at 6 and 12 months were also associated with 6 and 12‐month diuretic changes, but the effect of sacubitril/valsartan on diuretic dose reductions remained significant after adjustment for MRA dose changes and post‐baseline changes in systolic blood pressure and weight. Systolic blood pressure, weight, and MRA use and dose did not modify the effect of sacubitril/valsartan on diuretic dose changes at 6 and 12 months (all P > 0.1).
Table 1.
Baseline characteristics by diuretic use groups
| Characteristic | No diuretics (n = 1620) | Non‐loop diuretics (n = 496) | Furosemide equivalent dose | P‐value | ||
|---|---|---|---|---|---|---|
| < 20 mg (n = 438) | 20–40 mg (n = 3625) | > 40 mg (n = 1424) | ||||
| Randomized to sacubitril/valsartan, n (%) | 811 (50.1) | 223 (45.0) | 206 (47.0) | 1847 (51.0) | 688 (48.3) | 0.06 |
| Age, years, mean (SD) | 64 (11) | 65 (11) | 64 (11) | 64 (11) | 63 (12) | 0.03 |
| Female sex, n (%) | 346 (21.4) | 131 (26.4) | 101 (23.1) | 788 (21.7) | 277 (19.5) | 0.02 |
| Caucasian, n (%) | 985 (60.8) | 311 (62.7) | 326 (74.4) | 2340 (64.6) | 974 (68.4) | <0.001 |
| NYHA class III/IV, n (%) | 266 (16.5) | 163 (32.9) | 147 (33.6) | 796 (22.0) | 435 (30.6) | <0.001 |
| Ejection fraction, mean (SD) | 30.3 (5.9) | 31.2 (5.7) | 30.3 (5.9) | 29.3 (6.2) | 28.0 (6.7) | <0.001 |
| Body mass index, kg/m2, mean (SD) | 27.0 (4.8) | 27.9 (5.6) | 27.6 (5.2) | 28.1 (5.4) | 29.9 (6.3) | <0.001 |
| Prior HF hospitalization, n (%) | 869 (53.6) | 235 (47.4) | 278 (63.5) | 2337 (64.5) | 1019 (71.6) | <0.001 |
| Hypertension, n (%) | 1091 (67.3) | 367 (74.0) | 320 (73.1) | 2571 (70.9) | 988 (69.4) | 0.01 |
| Diabetes mellitus, n (%) | 453 (28.0) | 150 (30.2) | 116 (26.5) | 1268 (35.0) | 638 (44.8) | <0.001 |
| Atrial fibrillation, n (%) | 472 (29.1) | 181 (36.5) | 177 (40.4) | 1305 (36.0) | 617 (43.3) | <0.001 |
| Systolic BP, mmHg, mean (SD) | 122 (15) | 124 (15) | 123 (14) | 121 (15) | 120 (16) | <0.001 |
| ICD, n (%) | 207 (12.8) | 36 (7.3) | 44 (10.0) | 527 (14.5) | 332 (23.3) | <0.001 |
| CRT, n (%) | 84 (5.2) | 16 (3.2) | 17 (3.9) | 221 (6.1) | 179 (12.6) | <0.001 |
| ACEi use, n (%) | 1213 (74.9) | 364 (73.4) | 354 (80.8) | 2817 (77.7) | 1135 (79.7) | 0.001 |
| ARB use, n (%) | 409 (25.2) | 133 (26.8) | 83 (18.9) | 818 (22.6) | 298 (20.9) | 0.002 |
| Beta‐blocker use, n (%) | 1496 (92.3) | 463 (93.3) | 405 (92.5) | 3357 (92.6) | 1336 (93.8) | 0.52 |
| Digoxin use, n (%) | 318 (19.6) | 171 (34.5) | 133 (30.4) | 1109 (30.6) | 536 (37.6) | <0.001 |
| MRA use, n (%) | 772 (47.7) | 227 (45.8) | 293 (66.9) | 2091 (57.7) | 856 (60.1) | <0.001 |
| Serum creatinine, mg/dL, mean (SD) | 1.07 (0.25) | 1.07 (0.26) | 1.11 (0.30) | 1.12 (0.30) | 1.21 (0.33) | <0.001 |
| NT‐proBNP, pg/mL, median (IQR) |
1304 (768–2566) |
1392 (806–2501) |
1599 (905–3134) |
1684 (921–3396) |
2026 (1067–4207) |
<0.001 |
| Ischaemic aetiology, n (%) | 1082 (66.8) | 324 (65.3) | 282 (64.4) | 2062 (56.9) | 783 (55.0) | <0.001 |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CRT, cardiac resynchronization therapy; HF, heart failure; ICD, implantable cardioverter defibrillator; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro brain natriuretic peptide; NYHA, New York Heart Association; SD, standard deviation.
Mean baseline furosemide equivalent doses were 48.2 mg for sacubitril/valsartan and 49.6 mg for enalapril (P = 0.25). Participants assigned to sacubitril/valsartan had more frequent diuretic dose reductions and less frequent dose increases compared to those taking enalapril at 6, 12, and 24 months (Figure 1). As a result, patients randomized to sacubitril/valsartan had lower use of diuretics at 6 months (net reduction 2.0%, P = 0.02), 12 months (net reduction 4.1%, P < 0.001) and 24 months (net reduction 6.1%, P < 0.001) relative to enalapril with similar differences seen in an on‐treatment analysis.
Figure 1.
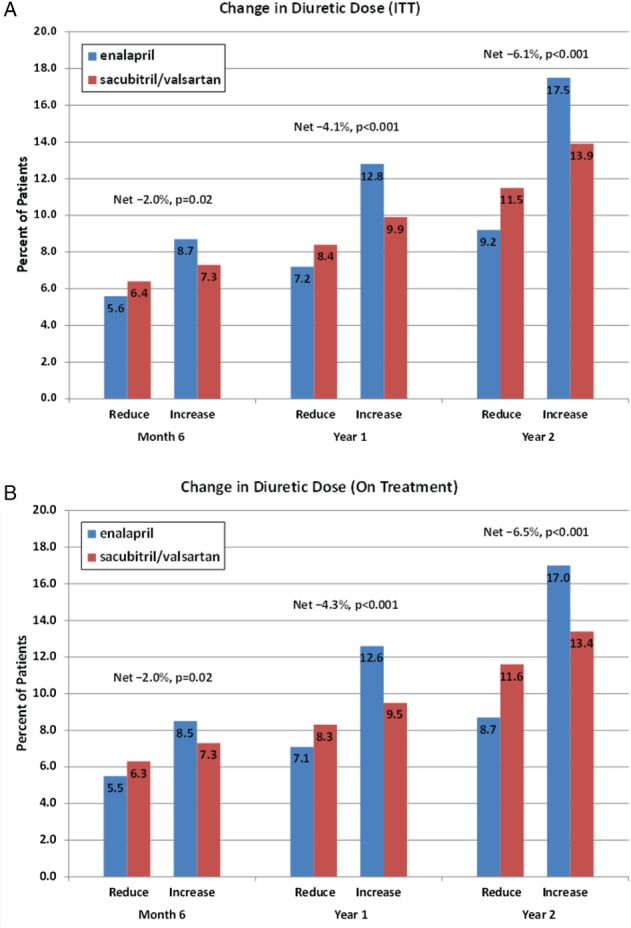
Changes in diuretic use during PARADIGM‐HF at 6 months, 1 year, and 2 years by treatment arm based on (A) intention‐to‐treat (ITT) and (B) per protocol treatment.
Discussion
We found that in PARADIGM‐HF diuretic use at baseline was associated with a more severe burden of illness. Patients randomized to sacubitril/valsartan had lower subsequent use of diuretics, with fewer loop diuretic dose increases and more frequent dose reductions compared with those taking enalapril.
Loop diuretic use has been associated in prior studies with worse outcomes in heart failure, although the extent to which diuretic use represents a marker for sicker patients and thus increased risk, or plays a causal role remains unclear. Several studies, including those with adjustment for the propensity to be treated with loop diuretics, have documented an association between loop diuretics and a greater risk for hospitalization or death due to worsening heart failure, all‐cause mortality, cardiovascular death, and sudden cardiac death compared to non‐use.5, 6, 7, 8, 10 There appears to be a dose‐related association, such that higher doses of diuretics are associated with greater risk compared with lower doses.5
Several mechanisms have been proposed by which loop diuretics may increase risk in heart failure. Loop diuretics, through their actions on the sodium/potassium/2–chloride co‐transporter, lead to secretion of renin, with resulting neurohormonal activation.3, 11, 12 Increased levels of plasma renin activity, suggestive of neurohormonal activation, have been independently associated with increased risk of mortality in patients with heart failure.13 Diuretic use in the presence of neurohormonal activation has also been shown to portend worse outcomes.14
The reduced relative need for diuretics in patients randomized to sacubitril/valsartan may potentially be secondary to the natriuretic effects of sacubitril or the presumed improvement in haemodynamics that may occur with sacubitril/valsartan. Sacubitril/valsartan is not associated with weight loss, however, suggesting that any diuretic effect is weak. While there are not any demonstrated haemodynamic data in heart failure patients treated with sacubitril/valsartan, sacubitril/valsartan is known to decrease NT‐proBNP substantially within 4 weeks of therapy, consistent with a relatively rapid improvement in haemodynamics with administration.15 Since investigators were blinded to therapy, diuretic dose reductions were prompted by changes in patient symptoms, which improved in patients randomized to sacubitril/valsartan.16 Conversely, it is also possible that sacubitril/valsartan lowered blood pressure more than enalapril, necessitating reduction of diuretic doses.
Diuretic use has been implicated in greater risk for hypotension in patients receiving renin–angiotensin system inhibitors; if diuretic doses were not down‐titrated in patients taking sacubitril/valsartan in response to reduced clinical need, this may have resulted in over‐diuresis that could contribute to hypotension. This possibility underscores the importance of assessment and potential adjustment of diuretic doses prior to and following initiation of an angiotensin receptor neprilysin inhibitor.
Several limitations of this analysis should be noted. The differential use of diuretics among participants randomized to sacubitril/valsartan compared to enalapril was not a pre‐specified analysis. Doses of diuretics were not available for all participants, which could lower the precision of dose change comparisons between groups. We compared diuretic use at discrete time points and may have missed interim changes in diuretic use that may not have been captured at study visits. Diuretic dose changes were not available at earlier time points after randomization, which could have been of interest given the quick onset of NT‐proBNP reduction by sacubitril/valsartan. Additionally, there was limited information on medication dose changes during the run‐in period of the PARADIGM‐HF trial; these data could have further informed trends on diuretic dose adjustments after initiation of sacubitril/valsartan. Reasons for diuretic dose changes were not captured in the study. Lastly, there could be incomplete capture of non‐loop diuretic changes or addition of MRAs.
In summary, treatment with sacubitril/valsartan was associated with more loop diuretic dose reductions and fewer dose increases compared with enalapril in the PARADIGM‐HF study, suggesting that treatment with sacubitril/valsartan may reduce the relative requirement for loop diuretics in patients with heart failure with reduced ejection fraction. These findings may be relevant to clinicians treating patients concomitantly with sacubitril/valsartan and diuretics.
Funding
This manuscript is the result of work supported with resources and use of facilities of the Minneapolis VA Health Care System. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Conflict of interest: O.V., A.S.D., J.R., M.R.Z., K.S., J.J.V.Mc.M., and S.D.S. have consulted for or received research support from Novartis, sponsor of the PARADIGM‐HF trial. S.D.S. has received research grants from Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Sanofi Pasteur, Theracos, and has consulted for Akros, Alnylam, Amgen, AstraZeneca, Bayer, BMS, Cardior, Corvia, Cytokinetics, Gilead, GSK, Ironwood, Merck, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions outside the scope of this work. M.P. has consulted for Novartis, Actelion, Sanofi, Cardiokinetix, BioControl, Janssen, Amgen, AMAG, Daiichi, CardioMEMS, and Cardiorentis. J.J.V.Mc.M.'s employer, University of Glasgow, was paid by Novartis for his time spent as co‐chairman of the PARADIGM‐HF trial. J.K., M.L., and V.S. are employees of Novartis Pharmaceuticals Corporation. B.C. does not have disclosures.
References
- 1. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM‐HF Investigators and Committees. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 2. Vardeny O, Miller R, Solomon SD. Combined neprilysin and renin‐angiotensin system inhibition for the treatment of heart failure. JACC Heart Fail 2014;2:663‐670. [DOI] [PubMed] [Google Scholar]
- 3. Francis GS, Siegel RM, Goldsmith SR, Olivari MT, Levine TB, Cohn JN. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure. Activation of the neurohumoral axis. Ann Intern Med 1985;103:1‐6. [DOI] [PubMed] [Google Scholar]
- 4. Ellison DH, Felker GM. Diuretic treatment in heart failure. N Engl J Med 2017;377:1964‐1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Damman K, Kjekshus J, Wikstrand J, Cleland JG, Komajda M, Wedel H, Waagstein F, McMurray JJ. Loop diuretics, renal function and clinical outcome in patients with heart failure and reduced ejection fraction. Eur J Heart Fail 2016;18:328‐336. [DOI] [PubMed] [Google Scholar]
- 6. Cooper HA, Dries DL, Davis CE, Shen YL, Domanski MJ. Diuretics and risk of arrhythmic death in patients with left ventricular dysfunction. Circulation 1999;100:1311‐1315. [DOI] [PubMed] [Google Scholar]
- 7. Domanski M, Norman J, Pitt B, Haigney M, Hanlon S, Peyster E; Studies of Left Ventricular Dysfunction. Diuretic use, progressive heart failure, and death in patients in the Studies Of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol 2003;42:705‐708. [DOI] [PubMed] [Google Scholar]
- 8. Domanski M, Tian X, Haigney M, Pitt B. Diuretic use, progressive heart failure, and death in patients in the DIG study. J Card Fail 2006;12:327‐332. [DOI] [PubMed] [Google Scholar]
- 9. DeVore AD, Hasselblad V, Mentz RJ, O'Connor CM, Armstrong PW, McMurray JJ, Ezekowitz JA, Tang WH, Starling RC, Voors AA, Califf RM, Hernandez AF. Loop diuretic dose adjustments after a hospitalization for heart failure: insights from ASCEND‐HF. Eur J Heart Fail 2015;17:340‐346. [DOI] [PubMed] [Google Scholar]
- 10. Ahmed A, Husain A, Love TE, Gambassi G, Dell'Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J 2006;27:1431‐1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sarraf M, Masoumi A, Schrier RW. Cardiorenal syndrome in acute decompensated heart failure. Clin J Am Soc Nephrol 2009;4:2013‐2026. [DOI] [PubMed] [Google Scholar]
- 12. Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure: physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 2009;54:1747‐1762. [DOI] [PubMed] [Google Scholar]
- 13. Masson S, Solomon S, Angelici L, Latini R, Anand IS, Prescott M, Maggioni AP, Tognoni G, Cohn JN; Val‐HeFT Investigators. Elevated plasma renin activity predicts adverse outcome in chronic heart failure, independently of pharmacologic therapy: data from the Valsartan Heart Failure Trial (Val‐HeFT). J Card Fail 2010;16:964‐970. [DOI] [PubMed] [Google Scholar]
- 14. Testani JM, Cappola TP, Brensinger CM, Shannon RP, Kimmel SE. Interaction between loop diuretic‐associated mortality and blood urea nitrogen concentration in chronic heart failure. J Am Coll Cardiol 2011;58:375‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zile MR, Claggett BL, Prescott MF, McMurray JJ, Packer M, Rouleau JL, Swedberg K, Desai AS, Gong J, Shi VC, Solomon SD. Prognostic implications of changes in N‐terminal pro‐B‐type natriuretic peptide in patients with heart failure. J Am Coll Cardiol 2016;68:2425‐2436. [DOI] [PubMed] [Google Scholar]
- 16. Lewis EF, Claggett BL, McMurray JJ, Packer M, Lefkowitz MP, Rouleau JL, Liu J, Shi VC, Zile MR, Desai AS, Solomon SD, Swedberg K. Health‐related quality of life outcomes in PARADIGM‐HF. Circ Heart Fail 2017;10:e003430. [DOI] [PubMed] [Google Scholar]


