Abstract
Aims
The presence of central sleep apnoea (CSA) is associated with poor prognosis in patients with heart failure (HF). The aim of this analysis was to evaluate if using phrenic nerve stimulation to treat CSA in patients with CSA and HF was associated with changes in HF‐specific metrics.
Methods and results
All patients randomized in the remedē System Pivotal Trial and identified at baseline with HF were included (n = 96). Effectiveness data from treatment and former control groups were pooled based on months since therapy activation. Changes from baseline to 6 and 12 months in sleep metrics, Epworth Sleepiness Scale, patient global assessment health‐related quality of life, Minnesota Living with Heart Failure Questionnaire (MLHFQ), and echocardiographic parameters are reported. HF hospitalization, cardiovascular death, and the composite of HF hospitalization or cardiovascular death within 6 months are reported by the original randomized group assignment for safety assessment. Sleep metrics and quality of life improved from baseline to 6 and 12 months. At 12 months, MLHFQ scores changed by –6.8 ± 20.0 (P = 0.005). The 6‐month rate of HF hospitalization was 4.7% in treatment patients (standard error = 3.3) and 17.0% in control patients (standard error = 5.5) (P = 0.065). Reported adverse events were as expected for a transvenous implantable system.
Conclusions
Phrenic nerve stimulation reduces CSA severity in patients with HF. In parallel, this CSA treatment was associated with benefits on HF quality of life.
Keywords: Central sleep apnoea, Heart failure, Phrenic nerve stimulation
Introduction
Central sleep apnoea (CSA) is characterized by a temporary interruption of neural output from the respiratory control centre, resulting in cessation of respiratory muscle activity and airflow. This sleep disorder occurs in up to 40% of patients with heart failure (HF).1 The high prevalence of CSA in patients with HF is attributed to disease‐related processes that include augmented hypoxic and hypercapnic chemosensitivity, increased circulatory delay, altered cerebrovascular reactivity, and recurrent apnoeic events, each associated with hypoxia and a relative increase in blood carbon dioxide concentrations.1 These repeated episodes of apnoea, hypoxia, reoxygenation, and arousal lead to the pathophysiologic consequences of CSA, including sympathetic nervous system activation, oxidative stress, systemic inflammation, endothelial dysfunction, and an association with poor prognosis in patients with HF.2, 3, 4, 5
Positive airway pressure (PAP) for CSA is not widely employed because of scant effectiveness data, poor patient adherence, and potential safety risks.1, 6, 7, 8, 9 Transvenous unilateral phrenic nerve stimulation is a unique physiological approach to the treatment of CSA. The remedē® System (Respicardia, Inc., Minnetonka, MN, USA) stimulates the phrenic nerve to cause diaphragmatic movement similar to normal breathing and stabilizes carbon dioxide levels.10, 11 The recently published pivotal trial of the remedē System in patients with CSA from different aetiologies, including HF, showed that significantly more patients in the treatment than in the control group had an apnoea–hypopnoea index (AHI) reduction ≥ 50% from baseline to 6 months (51% vs. 11%; P < 0.0001) with an overall 12‐month freedom from implant‐, system‐, or therapy‐related adverse events of 91%.12
Preliminary observations from the randomized remedē System Pivotal Trial in the subset of patients with CSA and HF (with either reduced or preserved ejection fraction) demonstrated effectiveness on sleep and other CSA‐related measures similar to that observed in the full cohort of patients with CSA from various aetiologies.12 Therefore, the principal aims of these exploratory analyses were to determine if the improvements in CSA parameters (i.e. arousals, hypoxaemia, and other sleep metrics) induced by treatment with phrenic nerve stimulation were associated with changes in HF‐specific metrics such as cardiac performance by echocardiography and the Minnesota Living with Heart Failure Questionnaire (MLHFQ).
Methods
The design, methods, oversight, and primary results of the remedē System Pivotal Trial (NCT01816776) have been reported.11, 12 The protocol was approved by local ethics or institutional review boards; all patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice, and ISO‐14155:2011.
Briefly, the remedē System Pivotal Trial was a prospective, multicentre, randomized, open‐label, controlled trial of transvenous unilateral phrenic nerve stimulation vs. no stimulation in patients with CSA of different aetiologies. The system remained off in the control group until the primary effectiveness endpoint of the overall study was assessed at 6 months (as described in the endpoints section). After this time point, therapy was initiated in the control group and the treatment group remained on therapy (Figure 1). Full night polysomnograms were completed at baseline and at 6‐month intervals after therapy initiation through 24 months of follow‐up to assess the initial effectiveness of phrenic nerve stimulation and maintenance of the observed treatment effect. Patients and physicians were aware of treatment assignment, but the polysomnography core laboratory (Registered Sleepers, Leicester, NC, USA) remained masked throughout the study.
Figure 1.
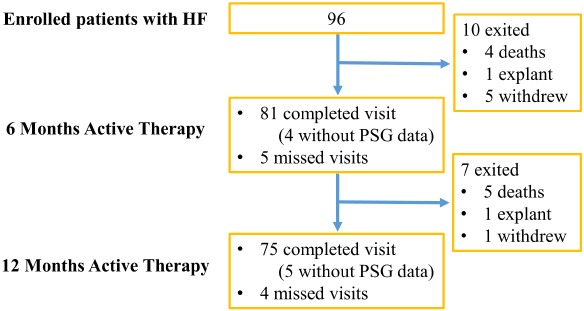
Composition of the pooled study population and follow‐up time points. As pre‐specified in the protocol, patients were implanted and randomized to treatment (therapy activated one month after implant) or control (therapy activated after the 6‐month assessments). This study design allowed for the pooling of 6‐ and 12‐month effectiveness data from the treatment and control groups based on months since therapy activation (baseline for these analyses). Patients in the treatment group accrued 6‐ and 12‐month data at the corresponding visits, whereas the control group accrued 6‐ and 12‐month data at the 12‐ and 18‐month visits due to the delay in initiating therapy. HF, heart failure; PSG, polysomnogram.
Participants
For inclusion in the overall study, eligible patients had to be medically stable for 30 days on guideline‐directed medical therapy prior to baseline assessments and have a qualifying polysomnogram.12 This post‐hoc analysis was performed in the subset of patients in the remedē System Pivotal Trial with HF as determined at baseline by the investigator.12 As pre‐specified in the protocol, patients were implanted and randomized to treatment (therapy activated one month after implant) or control (therapy activated after the 6‐month assessments). This study design allowed for the pooling of 6‐ and 12‐month effectiveness data from the treatment and control groups based on months since therapy activation. The time of initiation of treatment was the baseline for the analyses presented here. Patients in the treatment group accrued 6‐ and 12‐month data at the corresponding visits, whereas the control group accrued 6‐ and 12‐month data at the 12‐ and 18‐month visits due to the 6‐month delay in initiating therapy, as per study design (online supplementary Figure S1).
Intervention and follow‐up procedures
The remedē System has an implanted pulse generator and lead (placed in the left pericardiophrenic or right brachiocephalic vein) that stimulates a phrenic nerve to produce diaphragm contraction akin to normal breathing. The system automatically stimulates the phrenic nerve throughout the scheduled time at night when patients are at rest and in a sleeping posture, which is detected by position and motion sensors within the device. In addition to polysomnogram testing as previously described, echocardiograms were interpreted by a core laboratory (United Heart and Vascular Center, St. Paul, MN, USA) blinded to the time of the visit and duration of therapy to assess left ventricular ejection fraction (LVEF), left ventricular end‐systolic volume (LVESV), and left ventricular end‐diastolic volume (LVEDV).
Endpoints
The post‐hoc endpoints were the proportion of patients in the pooled study groups who achieved a reduction in AHI of ≥ 50% from baseline to 6 and 12 months. In addition, changes in central apnoea index, AHI, arousal index, oxygen desaturation index of ≥ 4%, percent of sleep time with oxygen saturation < 90%, and percent of sleep spent in rapid eye movement were assessed within the pooled group. Quality of life was assessed by the Epworth Sleepiness Scale, the proportion of patients with moderate or marked improvement in the patient global assessment instrument, and MLHFQ. Changes from baseline in echocardiographic parameters (LVEF, LVESV, and LVEDV) were analysed in the CSA patients who had HF, baseline LVEF ≤ 45%, and did not have permanent atrial fibrillation. Patients with this rhythm disorder (n = 19) were excluded because of the high variability in estimated cardiac volumes in these patients.13, 14 The exclusion of patients with permanent atrial fibrillation is consistent with the choice made in other studies evaluating serial changes in cardiac volumes, and the echocardiographic protocol was not designed with procedures to address the variability of cardiac volumes in the presence of atrial fibrillation (i.e. to average data over 10 cardiac cycles).15, 16
Freedom from serious adverse events associated with the implantation procedure, the remedē System, or delivered therapy through 12 months post‐implant was summarized for the pooled population. Three additional safety analyses were conducted by randomization assignment: HF hospitalization, cardiovascular death, and the composite of HF hospitalization or cardiovascular death through 6 months.
Statistical analysis
Due to the exploratory nature of this analysis, all statistical tests in the HF subgroup are post‐hoc, completed after unblinding of the pivotal trial data, and are unadjusted for multiple testing, with all reported P‐values considered nominal. Imputation was not performed for missing data.
Respiratory, sleep and quality of life changes from baseline to 6 and 12 months within the pooled group were assessed using a paired t‐test and, due to distributional characteristics, echocardiographic data were analysed using non‐parametric Wilcoxon signed‐rank tests. All reported P‐values are two‐sided. The safety endpoint of freedom from related serious adverse events at 12 months was summarized as a binomial proportion.
The 6‐month HF hospitalization, cardiovascular death, and composite of HF hospitalization or cardiovascular death rates (time‐to‐first event) and P‐values comparing survival curves between the treatment and control groups were analysed using the Kaplan–Meier method to estimate and visualize survival functions. The log‐rank test was used for association testing. Control subjects were censored when therapy was activated. SAS software version 9.4 (SAS Institute, Inc., Cary, NC, USA) was used for all analyses.
Results
A total of 96 patients with CSA and HF (64% of the total pivotal trial population) were included in the pooled analyses, with 81 patients completing a 6‐month and 75 a 12‐month post‐activation visit. The flow of patients with HF in the trial is shown in Figure 1. These patients with HF had multiple co‐morbidities as shown in Table 1. Concomitant cardiac devices were present in 63% of patients. Baseline mean ± standard deviation (SD) AHI was 47.1 ± 18.5 events/hour. Average LVEF was 34.5 ± 12.1% with 78% of patients having a LVEF ≤ 45%. Of the 16 patients who were categorized in New York Heart Association (NYHA) class I, 12 (75%) had LVEF < 45%; two subjects with NYHA class I symptoms did not have an LVEF assessment at baseline.
Table 1.
Baseline characteristics of the heart failure subgroup
| Pooled (n) | 96 |
| Age (years) | 67 ± 12 |
| Male sex | 87 (91) |
| White race | 89 (93) |
| BMI (kg/m2) | 30.7 ± 5.8 |
| Neck circumference (cm) | 43 ± 4 (n = 95) |
| Heart rate (b.p.m.) | 72.4 ± 11.7 |
| SBP (mmHg) | 120.3 ± 18.2 |
| DBP (mmHg) | 71.9 ± 11.3 |
| RR (breaths/min) | 17.4 ± 2.8 |
| LVEF (%) | 34.5 ± 12.1 (n = 91) |
| LVEF ≤45% | 71/91 (78) |
| NYHA class | |
| I | 18 (19) |
| II | 41 (43) |
| III | 37 (39) |
| IV | 0 (0) |
| Previous history of atrial fibrillation | 50 (52) |
| Coronary artery disease | 69 (72) |
| Hypertension | 77 (80) |
| Diabetes | 35 (36) |
| Previous stroke | 7 (7) |
| Renal impairment | 31 (32) |
| Concomitant cardiac devices | 60 (63) |
| ICD | 33 (34) |
| CRT‐D | 20 (21) |
| Non‐CRT‐P | 6 (6) |
| CRT‐P | 1 (1) |
| Medications | |
| ACE inhibitor or ARB | 79 (82) |
| Statin | 67 (70) |
| Beta‐blocker | 85 (89) |
| Antiplatelet | 63 (66) |
| Mineralocorticoid receptor antagonist | 46 (48) |
| Loop diuretic | 62 (65) |
| Thiazide diuretic | 22 (23) |
| Digoxin | 23 (24) |
| Calcium channel blocker | 16 (17) |
Values are mean ± standard deviation, or number (%), unless otherwise noted.
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CRT‐D, cardiac resynchronization therapy‐defibrillator; CRT‐P, cardiac resynchronization therapy‐pacemaker; DBP, diastolic blood pressure; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; RR, respiratory rate; SBP, systolic blood pressure.
In patients with HF, 53% (41/77) had a ≥ 50% reduction in AHI from baseline to 6 months and 57% (40/70) from baseline to 12 months (Table 2). A reduction in AHI occurred in 61 of 70 (87%) patients at 12 months. The percentage change in AHI for each patient with HF in the pooled group following 12 months of active therapy is shown in Figure 2.
Table 2.
Changes in sleep metrics in the pooled heart failure population
| Baseline observed | 6–month active therapy | 12–month active therapy | |||
|---|---|---|---|---|---|
| Observed | Paired change from baseline | Observed | Paired change from baseline | ||
| Proportion of patients with ≥50% reduction in AHI (%) | 53 (41/77) (42%, 64%) | 57 (40/70) (45%, 68%) | |||
| CAI (events/h)* | 26.2 ± 17.7 (93) 22.1 [13.4–37.2] | 4.1 ± 6.0 (77) 1.4 [0.2–5.4] | –21.8 ± 18.1 (77) –20.0 [–35.0 to –8.3] P < 0.001 | 3.5 ± 6.5 (70) 0.9 [0.0–3.5] | –23.2 ± 16.9 (70) –19.9 [–34.6 to –11.8] P < 0.001 |
| AHI (events/h)* | 47.1 ± 18.5 (93) 45.8 [32.0–58.3] | 25.2 ± 18.9 (77) 20.9 [10.0–34.5] | –21.2 ± 18.2 (77) –20.7 [–37.8 to –7.5] P < 0.001 | 24.9 ± 18.6 (70) 19.5 [10.7–34.4] | –22.6 ± 18.1 (70) –22.0 [–35.6 to –5.9] P < 0.001 |
| Arousal index (events/h)* | 43.0 ± 18.7 (93) 40.6 [30.0–57.3] | 25.2 ± 14.2 (77) 21.0 [16.7–31.2] | –16.8 ± 19.1 (77) –14.3 [–27.7 to –3.6] P < 0.001 | 24.5 ± 13.8 (70) 19.4 [15.0–32.8] | –18.4 ± 20.9 (70) –16.2 [–35.1 to –4.7] P < 0.001 |
| Percent sleep in REM* | 10.4 ± 7.2 (93) 9.6 [5.1–15.8] | 13.8 ± 8.2 (77) 13.4 [8.5–17.7] | 2.9 ± 8.2 (77) 1.1 [–3.3 to 7.7] P = 0.003 | 14.6 ± 8.8 (70) 13.9 [7.2–20.9] | 3.6 ± 9.0 (70) 2.7 [–2.6 to 8.0] P = 0.001 |
| ODI4 (events/h)* | 43.2 ± 20.2 (93) 41.0 [29.5–54.6] | 24.2 ± 19.8 (77) 20.1 [8.2–33.4] | –18.3 ± 16.9 (77) –17.1 [–30.4 to –5.3] P < 0.001 | 24.2 ± 19.4 (70) 18.9 [8.8–32.4] | –19.9 ± 19.9 (70) –20.4 [–32.3 to –4.7] P < 0.001 |
| Percent of sleep with O2 saturation < 90%* | 15.7 ± 16.6 (92) 9.8 [3.4–23.9] | 10.7 ± 15.1 (77) 4.8 [1.1–16.0] | –3.9 ± 13.7 (76) –4.1 [–9.1 to 1.5] P = 0.014 | 9.4 ± 13.2 (70) 4.4 [0.9–14.4] | –6.6 ± 15.8 (69) –4.1 [–9.8 to 0.1]P < 0.001 |
Values are mean ± standard deviation (n), or median [interquartile range].
For number of patients at baseline, three control subjects exited prior to the 6‐month therapy activation visit (their baseline for on‐therapy assessments); thus, a total of 93 subjects with heart failure were available for the pooled analyses of active therapy.
AHI, apnoea–hypopnoea index; CAI, central apnoea index; ODI4, oxygen desaturation index of ≥4%; REM, rapid eye movement.
Nominal two‐sided P‐value from paired t‐test for change from baseline.
Figure 2.
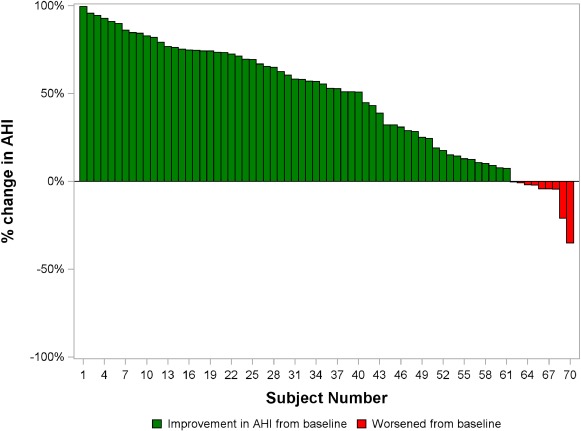
Percentage change in apnoea–hypopnoea index (AHI) from baseline to 12 months of therapy for each patient in the pooled population of patients with heart failure and polysomnogram data. The change from baseline following 12 months of active therapy for all subjects is shown. Patients with any decrease in AHI from baseline are shown in green bars and patients with any increase in AHI from baseline are shown in red bars.
All observed respiratory and sleep metrics improved from baseline to 6 and 12 months post‐therapy initiation (all P < 0.05) (Table 2). These include central apnoea index, AHI, arousal index, oxygen desaturation index of ≥ 4%, percent of sleep time with oxygen desaturation < 90%, and percent of sleep spent in rapid eye movement.
Quality of life as assessed by the Epworth Sleepiness Scale score showed a reduction of –2.8 ± 4.5 points at 6 months (P < 0.001) and –3.1 ± 4.7 at 12 months (P < 0.001). Moderate to marked improvement in patient global assessment was demonstrated by 47/81 (58%) patients at 6 months and 41/75 (55%) at 12 months. At 12 months, MLHFQ scores changed by –6.8 ± 20.0 (P = 0.005) (Table 3).
Table 3.
Changes in quality of life in the pooled heart failure population
| Baseline observed | 6‐month active therapy | 12‐month active therapy | |||
|---|---|---|---|---|---|
| Observed | Paired changefrom baseline | Observed | Paired changefrom baseline | ||
| Moderate or marked improvement in PGA | N/A | N/A | 58 (47/81) | N/A | 55 (41/75) |
| Epworth Sleepiness Scale* | 8.9 ± 5.1 (93) 8.0 [5.0–13.0] | 6.2 ± 4.1 (81) 6.0 [3.0–9.0] | –2.8 ± 4.5 (81) –2.0 [–6.0 to 0.0] P < 0.001 | 6.1 ± 3.7 (75) 5.0 [3.0–9.0] | –3.1 ± 4.7 (75) –2.0 [–5.0 to 0.0] P < 0.001 |
| Minnesota Living with Heart Failure score* | 39.2 ± 22.8 (91) 40.0 [21.0–55.0] | 35.3 ± 24.3 (81) 32.0 [16.0–52.0] | –2.6 ± 19.2 (79) –1.0 [–12.0 to 7.0] P = 0.227 | 31.0 ± 22.8 (75) 27.0 [13.0–46.0] | –6.8 ± 20.0 (73) –4.0 [–18.0 to 8.0] P = 0.005 |
Values are mean ± standard deviation (n), or median [interquartile range] for continuous data, or % (n/N) for categorical data.
N/A, not applicable; PGA, patient global assessment.
Nominal two‐sided P‐value from paired t‐test for change from baseline.
In patients with HF, LVEF ≤ 45% and no permanent atrial fibrillation (n = 50), the median change in LVESV at 12 months was –6.0 mL (interquartile range –21.0 to 5.0 mL; P = 0.078) and was accompanied by a change in the median LVEF at 12 months of 4.0% (interquartile range –1.0 to 8.0%; P = 0.004). The median change in LVEDV at 12 months was –7.0 mL (interquartile range –27.0 to 9.0 mL; P = 0.288) (Table 4).
Table 4.
Changes in echocardiographic parameters in the pooled heart failure population
| Baseline observed (n = 50) | 6‐month active therapy | 12‐month active therapy | |||
|---|---|---|---|---|---|
| Observed (n = 43) | Paired change from baseline (n = 43) | Observed (n = 41) | Paired change from baseline (n = 41) | ||
| Left ventricular ejection fraction (%)* | 31.6 ± 8.5 (50) 31.0 [26.0–38.0] | 31.2 ± 9.9 (43) 30.0 [23.0–40.0] | 0.0 ± 5.9 (43) 1.0 [–4.0 to 4.0] P = 0.834 | 34.8 ± 12.4 (41) 32.0 [24.0–44.0] | 3.3 ± 7.6 (41) 4.0 [–1.0 to 8.0] P = 0.004 |
| Left ventricular end‐systolic volume (mL)* | 119.7 ± 63.6 (50) 109.0 [70.0–150.0] | 123.4 ± 69.4 (43) 122.0 [65.0–157.0] | 3.9 ± 32.3 (43) –5.0 [–14.0 to 17.0] P = 0.943 | 111.3 ± 68.4 (41) 100.0 [60.0–140.0] | –6.0 ± 27.5 (41) –6.0 [–21.0 to 5.0] P = 0.078 |
| Left ventricular end‐diastolic volume (mL)* |
169.3 ± 71.7 (50) 161.5 [110.0–219.0] |
172.5 ± 81.0 (43) 169.0 [105.0–212.0] |
4.6 ± 38.5 (43) –3.0 [−19.0 to 20.0] P = 0.966 |
161.4 ± 75.9 (41) 146.0 [103.0–200.0] | –4.3 ± 31.1 (41) –7.0 [–27.0 to 9.0] P = 0.288 |
Values are mean ± standard deviation (n), or median [interquartile range].
Nominal two‐sided P‐value from Wilcoxon signed‐rank test for change from baseline.
The pooled 12‐month freedom from serious adverse events related to implant procedure, device or therapy was 88/96 (92%; 95% confidence interval 84–96%). The related serious adverse events are shown in the online supplementary Table S1. Of 96 patients with HF, 32 (33%) reported non‐serious therapy‐related discomfort through 12 months, which resolved with remedē System reprogramming in all but one patient. Among patients with implantable cardiac devices, no ventricular arrhythmias were adjudicated as attributable to phrenic nerve stimulation. One case of oversensing resulted in inappropriate defibrillation, which was corrected by remedē System reprogramming without reoccurrences.
A Kaplan–Meier analysis of the time from therapy initiation visit (one month post‐implant) to first HF‐related hospitalization during the randomized portion of the trial (through the 6‐month visit) produced HF‐related hospitalization rates of 4.7% (standard error = 3.3) in the treatment group and 17.0% (standard error = 5.5) in the control group (P = 0.065) (online supplementary Figure S2 A).
There was no detectable evidence of a difference in cardiovascular mortality between groups (online supplementary Figure S2 B). Three deaths occurred through 6 months (during the randomized portion), one of which was in the treatment group (sudden cardiac death) and two were in the control group (two cardiac pump failure) with a 6‐month cardiovascular death rate of 2% (standard error = 2.3%) in the treatment group and 4% (standard error = 2.9%) in the control group (P = 0.617). One additional death occurred in the control group during the 6 months of active therapy (as noted on Figure 1). The rate of the composite endpoint of time‐to‐first HF hospitalization or cardiovascular death through 6 months was 7.0% (standard error = 3.9) and 17.0% (standard error = 5.5) for patients in the treatment and control groups, respectively (P = 0.148) (online supplementary Figure S2 C).
Discussion
These analyses characterize the effects of 6 and 12 months of active phrenic nerve stimulation on sleep, respiratory, cardiac, and quality of life outcomes in patients with CSA and HF enrolled in the remedē System Pivotal Trial. After 6 months of active therapy, these patients with HF experienced improvement in sleep metrics from baseline with a reduction in the severity of CSA, fewer arousals, less hypoxaemia, and improvement in rapid eye movement sleep. These effects were sustained at 12 months. Quality of life as assessed by the Epworth Sleepiness Scale and patient global assessment also improved from baseline after 6 and 12 months of therapy. The MLHFQ score improved at 6 and 12 months of active phrenic nerve stimulation. In the post‐hoc subgroup of patients in the remedē System Pivotal Trial with HF, a baseline LVEF ≤ 45% and no permanent atrial fibrillation, an increase in LVEF was observed at 12 months. The consistency of improvement in both sleep and HF‐specific quality of life measures together with the modest amelioration of cardiac volumes and systolic function suggest that effective treatment of CSA with phrenic nerve stimulation may have a parallel association with clinically relevant benefits in CSA patients with HF.
While the original trial did not compare phrenic nerve stimulation to PAP therapy, it is important to consider the known effects of PAP in patients with HF as reported in two large randomized trials. The Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure (CANPAP) trial was unable to demonstrate a reduction in arousals or a significant improvement in the Chronic Heart Failure Questionnaire in the continuous PAP group compared to the control group.6 Left ventricular volumes were not measured.6 In addition, the results of the Treatment of Sleep‐Disordered Breathing with Predominant Central Sleep Apnea by Adaptive Servo‐Ventilation in Patients with Heart Failure (SERVE‐HF) trial showed no significant differences between the adaptive servo‐ventilation (ASV) and control groups in MLHFQ scores.7 Furthermore, the SERVE‐HF trial showed an unexpected significant increase in the risk of cardiovascular mortality (P = 0.006) despite a substantial reduction in AHI from baseline to 12 months.7 Although the present study was not powered to detect a difference in mortality, this exploratory analysis does not unveil a signal toward an increase in mortality in HF patients with CSA treated with phrenic nerve stimulation.12 The authors of the SERVE‐HF trial considered two hypotheses to explain the increased mortality risk associated with ASV. First, it is possible that PAP itself had detrimental haemodynamic effects. The mechanism of action of phrenic nerve stimulation is different and opposite to that of ASV. Specifically, while ASV delivers PAP, normal breathing via diaphragmatic contraction triggered by neurostimulation generates negative intrathoracic pressure, and therefore favours venous return to the heart.1, 7, 17, 18, 19 The alternative hypothesis considered by the SERVE‐HF investigators is that CSA could be a beneficial compensatory mechanism in patients with advanced HF.7 The intermittent hypoxaemia and norepinephrine release associated with CSA events make it unlikely that this sleep disorder confers any long‐term benefits to patients with HF.1, 2, 7, 17, 18 Indeed, the recent multistate modelling analysis of the individual components of the SERVE‐HF primary endpoint showed the increased risk of cardiovascular death was primarily observed in patients with LVEF ≤ 30% and in those who died suddenly without a prior hospitalization for worsening HF.20
Reducing arousals may indirectly mitigate the surges of sympathetic activation that accompany these events. Reduction of hypoxaemia may be another important benefit.21 Indeed, decreases in both oxygen desaturation index of ≥ 4% and time spent with oxygen saturation < 90% occurred during therapy. These effects of phrenic nerve stimulation are potentially key mechanisms underlying the improvements in quality of life that were not observed in randomized trials of mask‐based therapies in this patient population.
The results of the current analysis may indicate a potential association between treatment of CSA with phrenic nerve stimulation and parallel changes in HF‐specific clinical parameters.22 The principal features of cardiac remodelling are left ventricular cavity enlargement and biochemical myocyte alterations, which lead to impaired cardiac contractility and relaxation.23 A reduction in cardiac volumes and improvement in left ventricular systolic function generally predict improvements in morbidity and mortality.24 It is not surprising that the improvement in systolic function in HF patients observed after effective treatment of CSA with phrenic nerve stimulation was not detected until 12 months of active therapy, as the time course of this process is highly variable among individual patients and treatments.22 One study of 207 patients with HF showed that 40% demonstrated left ventricular reverse remodelling in < 24 months after initiation of pharmacotherapy, 12% in ≥ 24 months, and 48% had no change.25 Patients with reverse remodelling had improved clinical outcomes regardless of whether reverse remodelling occurred early or late, compared to those without changes in left ventricular size.25 Among 127 cardiac resynchronization therapy recipients, patients exhibiting reverse remodelling in < 6 months had the best outcomes, but reverse remodelling ≥ 6 months still had significantly better clinical and echocardiographic outcomes than those who did not.26 These data from Viveiros Monteiro et al.26 support the hypothesis that in patients with HF, LVEF ≤ 45%, and without permanent atrial fibrillation, the observed signals of improved echocardiographic measures after 12 months of phrenic nerve stimulation have clinical relevance, because it suggests that effective treatment of CSA, as demonstrated by improvements in sleep and quality of life, may be associated with beneficial changes in measures of cardiac structure and function.
It is of interest that in patients with HF undergoing phrenic nerve stimulation for CSA, the increase in LVEF was due to a numerical reduction in LVESV. Changes in LVESV have been shown to be superior to other echocardiographic measurements in predicting outcomes after a myocardial infarction, identifying the optimal time for valvular surgical interventions, and assessing response to cardiac resynchronization therapy.27, 28, 29
Therefore, the signal for a decrease in LVESV after 12 months of active phrenic nerve stimulation provides support for the hypothesis that in patients with HF, LVEF ≤ 45%, and CSA, effective treatment of this sleep disorder may be associated with beneficial changes in cardiac structure and function that could influence clinical outcomes.24 Observations from the Kaplan–Meier analysis suggest a potentially longer time to first HF hospitalization within 6 months for the treatment compared to control group that merits additional study. In a recent observational study of 784 hospitalized patients with systolic HF who underwent inpatient polysomnography and were followed for 6 months, 165 (21%) had CSA. The rate ratio for cardiac readmission within 6 months in patients with CSA compared to patients without sleep disordered breathing was 1.53 (95% confidence interval 1.1–2.2; P = 0.03) after adjustment for demographics, clinical characteristics and co‐morbidities.4 The same study not only showed that CSA is an independent predictor of morbidity in patients hospitalized with HF, but it also identifies a novel and potentially modifiable risk factor for HF readmissions.
The analyses presented in this manuscript have limitations related to their exploratory nature and small sample size. All analyses are post‐hoc and non‐randomized except the hospitalization analyses which used the randomized portion of the trial; thus, causality cannot be ascribed to treatment. Phrenic nerve stimulation is designed to treat patients with predominantly CSA and it is not expected to treat obstructive apnoea. Because a period of approximately 3 months is needed to optimally titrate stimulation, we would not expect remodelling to be evident until after 6 months of maximum active therapy, which in these patients would occur between 9–12 months. By design, the control group patients only had their device programmed off for 6 months. Thus, randomized control group data are not available for the period of time in which remodelling would be reasonably expected to occur. For this reason, the control group was not included in this analysis. Future studies assessing echocardiographic measures of reverse myocardial remodelling will require a longer randomized period to permit firmer conclusions regarding between‐group differences. P‐values were unadjusted for multiple testing. Additionally, the definition used to identify patients with HF reflected the investigator's designation of HF diagnosis and NYHA class as recorded on the case report form. Other limitations include those already acknowledged for the overall remedē System Pivotal Trial.12 Although the present study was not powered to detect a difference in mortality, this exploratory analysis does not unveil a signal toward an increase in mortality in the population that was studied. Regardless, our analysis provides important new information specifically in patients with CSA and HF. Improvement in AHI was similar to that observed with continuous PAP in other studies, but there was a greater reduction in the central episodes with phrenic nerve stimulation (from 26.2 ± 17.7 to 4.1 ± 6.0) and, in addition, phrenic nerve stimulation was associated with improvements in arousals, sleep quality, and patient‐assessed quality of life scores from baseline to 6 and 12 months. However, despite these encouraging results, the nature of the residual AHI requires further investigation. These effects were associated with improved quality of life at 12 months specific to patients with HF as measured by the MLHFQ score.
Phrenic nerve stimulation reduces CSA severity in patients with HF, and reported adverse events were as expected for a transvenous implantable system. This CSA treatment was associated with favourable changes in HF quality of life and disease progression as suggested by MLHFQ scores and echocardiographic findings, respectively. Larger studies in HF populations should further explore the effects of treatment of CSA by phrenic nerve stimulation on outcomes of patients with both reduced and preserved LVEF.
Funding
Respicardia, Inc., Minnetonka, MN, USA.
Conflict of interest: M.R.C.: personal fees from Respicardia (consulting and study principal investigator for remedē® System Pivotal Trial). P.P.: research grants from Respicardia and Coridea; personal fees from Respicardia, Coridea, Philips Respironics GK. A.C.: personal fees from Respicardia (consulting fees). S.J.: personal fees from Respicardia (Advisory Board and Steering Committee member). R.A.: personal fees from Respicardia (training of implanting physicians) and Medtronic (Advisory Board); research grants from Medtronic. L.R.G.: research grants from Respicardia; personal fees from Medtronic, St. Jude, Boston Scientific. R.H.: personal fees from Respicardia for statistical consulting and review of study results. A.K.: research grant to institution from Respicardia to conduct the study. O.O.: personal fees for lectures from Novartis, Sorin/LivaNova, ResMed, and Bayer; research grants from ResMed, Novartis, Bayer, Sorin/LivaNova. C.S.: research grants from Respicardia, St. Jude Medical, Biotronik, Medtronic, and Sorin/LivaNova; advisory board for Sorin/LivaNova. S.M.: employee at Respicardia. W.T.A.: research grants to institution from Respicardia; personal fees from Respicardia (consulting, Advisory Board). The other author (R.N.K.) has no conflicts of interest to disclose.
References
- 1. Costanzo MR, Khayat R, Ponikowski P, Augostini R, Stellbrink C, Mianulli M, Abraham WT. Mechanisms and clinical consequences of untreated central sleep apnea in heart failure. J Am Coll Cardiol 2015;65:72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Javaheri S, Parker TJ, Liming JD, Corbett WS, Nishiyama H, Wexler L, Roselle GA. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation 1998;97:2154–2159. [DOI] [PubMed] [Google Scholar]
- 3. Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol 2007;49:2028–2034. [DOI] [PubMed] [Google Scholar]
- 4. Khayat R, Abraham W, Patt B, Brinkman V, Wannemacher J, Porter K, Jarjoura D. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail 2012;18:534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khayat R, Jarjoura D, Porter K, Sow A, Wannemacher J, Dohar R, Pleister A, Abraham WT. Sleep disordered breathing and post‐discharge mortality in patients with acute heart failure. Eur Heart J 2015;36:1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bradley TD, Logan AG, Kimoff RJ, Series F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Tomlinson G, Floras JS; CANPAP Investigators . Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005;353:2025–2033. [DOI] [PubMed] [Google Scholar]
- 7. Cowie MR, Woehrle H, Wegscheider K, Angermann C, d'Ortho MP, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, Teschler H. Adaptive servo‐ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015;373:1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Javaheri S, Brown LK, Randerath W, Khayat R. SERVE‐HF: more questions than answers. Chest 2016;149:900–904. [DOI] [PubMed] [Google Scholar]
- 9. Woehrle H, Cowie MR, Eulenburg C, Suling A, Angermann C, d'Ortho MP, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, Teschler H, Wegscheider K. Adaptive servo ventilation for central sleep apnoea in heart failure: SERVE‐HF on‐treatment analysis. Eur Respir J 2017;50:1601692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang XL, Ding N, Wang H, Augostini R, Yang B, Xu D, Ju W, Hou X, Li X, Ni B, Cao K, George I, Wang J, Zhang SJ. Transvenous phrenic nerve stimulation in patients with Cheyne–Stokes respiration and congestive heart failure: a safety and proof‐of‐concept study. Chest 2012;142:927–934. [DOI] [PubMed] [Google Scholar]
- 11. Costanzo MR, Augostini R, Goldberg LR, Ponikowski P, Stellbrink C, Javaheri S. Design of the remede System Pivotal Trial: a prospective, randomized study in the use of respiratory rhythm management to treat central sleep apnea. J Card Fail 2015;21:892–902. [DOI] [PubMed] [Google Scholar]
- 12. Costanzo MR, Ponikowski P, Javaheri S, Augostini R, Goldberg L, Holcomb R, Kao A, Khayat RN, Oldenburg O, Stellbrink C, Abraham WT; remede System Pivotal Trial Study Group . Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet 2016;388:974–982. [DOI] [PubMed] [Google Scholar]
- 13. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 14. Donal E, Lip GY, Galderisi M, Goette A, Shah D, Marwan M, Lederlin M, Mondillo S, Edvardsen T, Sitges M, Grapsa J, Garbi M, Senior R, Gimelli A, Potpara TS, Van Gelder IC, Gorenek B, Mabo P, Lancellotti P, Kuck KH, Popescu BA, Hindricks G, Habib G, Cardim NM, Cosyns B, Delgado V, Haugaa KH, Muraru D, Nieman K, Boriani G, Cohen A. EACVI/EHRA Expert Consensus Document on the role of multi‐modality imaging for the evaluation of patients with atrial fibrillation. Eur Heart J Cardiovasc Imaging 2016;17:355–383. [DOI] [PubMed] [Google Scholar]
- 15. Kutyifa V, Goldenberg I, Moss AJ. Lessons learned from the Multicenter Automatic Defibrillator Implantation Trial‐Cardiac Resynchronization Therapy (MADIT‐CRT). Trends Cardiovasc Med 2016;26:137–146. [DOI] [PubMed] [Google Scholar]
- 16. St John Sutton M, Cerkvenik J, Borlaug BA, Daubert C, Gold MR, Ghio S, Chirinos JA, Linde C, Ky B. Effects of cardiac resynchronization therapy on cardiac remodeling and contractile function: results from Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE). J Am Heart Assoc 2015;4:e002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cowie MR, Wegscheider K, Teschler H. Adaptive servo‐ventilation for central sleep apnea in heart failure. N Engl J Med 2016;374:690–691. [DOI] [PubMed] [Google Scholar]
- 18. Naughton MT. Cheyne–Stokes respiration: friend or foe? Thorax 2012;67:357–360. [DOI] [PubMed] [Google Scholar]
- 19. Le Pimpec‐Barthes F, Gonzalez‐Bermejo J, Hubsch J, Duguet A, Morelot‐Panzini C, Riquet M, Similowski T. Intrathoracic phrenic pacing: a 10‐year experience in France. J Thorac Cardiovasc Surg 2011;142:378–383. [DOI] [PubMed] [Google Scholar]
- 20. Eulenburg C, Wegscheider K, Woehrle H, Angermann C, d'Ortho MP, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, Teschler H, Cowie MR. Mechanisms underlying increased mortality risk in patients with heart failure and reduced ejection fraction randomly assigned to adaptive servoventilation in the SERVE‐HF study: results of a secondary multistate modelling analysis. Lancet Respir Med 2016;4:873–881. [DOI] [PubMed] [Google Scholar]
- 21. Oldenburg O, Wellmann B, Buchholz A, Bitter T, Fox H, Thiem U, Horstkotte D, Wegscheider K. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur Heart J 2016;37:1695–1703. [DOI] [PubMed] [Google Scholar]
- 22. Waring AA, Litwin SE. Redefining reverse remodeling: can echocardiography refine our ability to assess response to heart failure treatments? J Am Coll Cardiol 2016;68:1277–1280. [DOI] [PubMed] [Google Scholar]
- 23. Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling – concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 2000;35:569–582. [DOI] [PubMed] [Google Scholar]
- 24. Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta‐analytic approach. J Am Coll Cardiol 2010;56:392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ikeda Y, Inomata T, Iida Y, Iwamoto‐Ishida M, Nabeta T, Ishii S, Sato T, Yanagisawa T, Mizutani T, Naruke T, Koitabashi T, Takeuchi I, Nishii M, Ako J. Time course of left ventricular reverse remodeling in response to pharmacotherapy: clinical implication for heart failure prognosis in patients with idiopathic dilated cardiomyopathy. Heart Vessels 2016;31:545–554. [DOI] [PubMed] [Google Scholar]
- 26. Viveiros Monteiro A, Martins Oliveira M, Silva Cunha P, Nogueira da Silva M, Feliciano J, Branco L, Rio P, Pimenta R, Delgado AS, Cruz Ferreira R. Time to left ventricular reverse remodeling after cardiac resynchronization therapy: better late than never. Rev Port Cardiol 2016;35:161‐167. [DOI] [PubMed] [Google Scholar]
- 27. Yu CM, Bleeker GB, Fung JW, Schalij MJ, Zhang Q, van der Wall EE, Chan YS, Kong SL, Bax JJ. Left ventricular reverse remodeling but not clinical improvement predicts long‐term survival after cardiac resynchronization therapy. Circulation 2005;112:1580–1586. [DOI] [PubMed] [Google Scholar]
- 28. White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end‐systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation 1987;76:44–51. [DOI] [PubMed] [Google Scholar]
- 29. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57–185. [DOI] [PubMed] [Google Scholar]
Supporting information
Figure S1. Patient flow. Flow of the heart failure population through 6 and 12 months of active therapy.
Figure S2. (A) Heart failure (HF) hospitalization. Kaplan–Meier curve of months to first HF hospitalization through 6 months. (B) Cardiovascular (CV) death. Kaplan–Meier curve of months to CV death through 6 months. (C) Composite of cardiovascular (CV) death and heart failure (HF) hospitalization. Kaplan–Meier curve of months to first HF hospitalization or CV death through 6 months.
Table S1. Related serious adverse events in the heart failure subgroup through 12 months.
Acknowledgements
The study investigators acknowledge United Heart and Vascular Clinic's Echocardiography Core Lab, supervised by Dr. Alan Bank. The authors acknowledge the contribution of Wendy Gattis Stough, PharmD for editorial assistance with manuscript preparation. Dr. Stough worked under the supervision of Dr. Costanzo and was supported by Respicardia, Inc.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Patient flow. Flow of the heart failure population through 6 and 12 months of active therapy.
Figure S2. (A) Heart failure (HF) hospitalization. Kaplan–Meier curve of months to first HF hospitalization through 6 months. (B) Cardiovascular (CV) death. Kaplan–Meier curve of months to CV death through 6 months. (C) Composite of cardiovascular (CV) death and heart failure (HF) hospitalization. Kaplan–Meier curve of months to first HF hospitalization or CV death through 6 months.
Table S1. Related serious adverse events in the heart failure subgroup through 12 months.


