Abstract
Aims
We examined the prognostic importance of N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) and troponin T (TnT) in heart failure patients with and without diabetes.
Methods and results
We measured NT‐proBNP and TnT in the biomarker substudy of the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial (PARADIGM‐HF). Of 1907 patients, 759 (40%) had diabetes. Median TnT in patients with diabetes was 18 (interquartile range 11–27) ng/L and 13 (9–21) ng/L in those without (P < 0.001). The TnT frequency‐distribution curve was shifted to the right in patients with diabetes, compared to those without diabetes. By contrast, NT‐proBNP did not differ between patients with and without diabetes. Diabetes and each biomarker were predictive of worse outcomes. Thus, patients with diabetes, an elevated TnT and a NT‐proBNP level in the highest tertile (9% of all patients) had an absolute risk of cardiovascular death or heart failure hospitalization of 265 per 1000 person‐years, compared to a rate of 42 per 1000 person‐years in those without diabetes, a TnT < 18 ng/L and a NT‐proBNP in the lowest tertile (16% of all patients). TnT remained an independent predictor of adverse outcomes in multivariable analyses including NT‐proBNP.
Conclusion
TnT is elevated to a greater extent in heart failure patients with diabetes compared to those without (whereas NT‐proBNP is not). TnT and NT‐proBNP are additive in predicting risk and when combined help identify diabetes patients at extremely high absolute risk.
Keywords: Troponin, NT‐proBNP, Diabetes, Heart failure with reduced ejection fraction
Introduction
Circulating B‐type natriuretic peptides are now measured routinely in patients with heart failure (HF) and it is well established that their levels are predictive of adverse clinical outcomes in these patients.1, 2, 3, 4 More recently, it has been demonstrated that B‐type natriuretic peptides are also predictive of cardiovascular outcomes and mortality in patients with type 2 diabetes mellitus.5, 6, 7
Although initially introduced to diagnose myocardial infarction in patients with suspected acute coronary syndromes, high‐sensitivity assays have demonstrated that elevated circulating troponin can be measured in most patients with HF and reduced ejection fraction (HFrEF). There is now a strong evidence base that troponin concentration is also predictive of outcomes in these patients.4, 8, 9 Latterly this has also been shown to be true in individuals with diabetes.10, 11, 12
However, troponin is not routinely measured in ambulatory patients with HF (or diabetes). We do not know how concentrations of these two peptides compare between HF patients with and without diabetes, with the potential confounding influences of renal impairment (which elevates both peptides), myocardial ischaemia (with coronary macro‐ and microvascular disease more common in individuals with diabetes) and obesity (which is associated with lower levels of natriuretic peptides and more common in individuals with diabetes).13 Likewise, we do not know their individual and combined predictive value in HF patients with and without diabetes. Specifically, does troponin add meaningful additional prognostic information to measurement of a B‐type natriuretic peptide (BNP) in HFrEF patients with and without diabetes, irrespective of HF aetiology (ischaemic or non‐ischaemic)?
We examined these questions in the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF), a large multicentre randomized controlled trial with long‐term follow‐up for fatal and non‐fatal outcomes in patients with HFrEF.
Methods
Study design and patients
The background, design and primary results of the PARADIGM‐HF trial are published.14, 15, 16 In brief, 8399 patients in New York Heart Association (NYHA) functional class II–IV with a left ventricular ejection fraction (LVEF) ≤ 40% receiving recommended treatment for HFrEF including an angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) and a beta‐blocker (unless contraindicated) and a mineralocorticoid receptor antagonist (MRA) (if indicated) were enrolled. Patients were required to have a plasma BNP ≥ 150 pg/mL [or N‐terminal pro‐BNP (NT‐proBNP) ≥ 600 pg/mL], or a BNP ≥ 100 pg/mL (or NT‐proBNP ≥ 400 pg/mL) and a HF hospitalization within the past 12 months. The key exclusion criteria included intolerance of ACE inhibitors or ARBs, a history of angioedema, symptomatic hypotension, a systolic blood pressure (SBP) < 100 mmHg at screening (< 95 mmHg at randomization), an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2, and a serum potassium level > 5.2 mmol/L at screening (> 5.4 mmol/L at randomization). Patients were randomized to sacubitril/valsartan (formerly known as LCZ696) or enalapril. Sacubitril/valsartan was superior to enalapril in reducing the risks of the primary composite endpoint of cardiovascular death or HF hospitalization, its components and all‐cause mortality. Of all patients randomized, 1907 (23%) were enrolled in a prospective biomarker substudy and had a measurement of high‐sensitivity troponin T (TnT) in addition to NT‐proBNP.
Definitions of diabetes mellitus and ischaemic aetiology
Diagnosis of diabetes at baseline and aetiology (ischaemic/non‐ischaemic) was based on investigator responses to the relevant questions in the case report form.
Outcomes
We investigated the association between TnT and NT‐proBNP, alone and in combination, and the primary composite outcome and all‐cause mortality.
Laboratory analyses
All samples were collected at the randomization visit. Sample handling and biomarker measurement using the Roche Elecsys NT‐proBNP (Roche Diagnostics, Indianapolis, IN, USA) and the fifth generation high‐sensitivity TnT assay (Roche Diagnostics GmbH, Mannheim, Germany) have been described previously.17, 18
Statistical analyses
Baseline characteristics are described by use of proportions for categorical variables and means with standard deviations or medians with quartiles for continuous variables. Differences in baseline characteristics between patients with and without diabetes were tested using a χ2 test for categorical variables and ANOVA or Kruskal–Wallis's test for continuous variables. Kaplan–Meier curves for all‐cause mortality and cumulative incidence curves for the primary endpoint were estimated and differences between groups were compared by use of log‐rank and Gray's test, respectively. Cox proportional hazard models were used to compare the risk of patients according to diabetes status and levels of TnT and NT‐proBNP for the primary endpoint and all‐cause mortality. The Cox regression models were adjusted for age, sex, randomized treatment, ejection fraction, NYHA class, body mass index, loge(NT‐proBNP), heart rate, systolic blood pressure, creatinine, low‐density lipoprotein (LDL), prior angina pectoris, atrial fibrillation, and pacemaker implantation. The assumption of linearity was tested for TnT, age, ejection fraction, and NT‐proBNP. Log [–log(survival)] curves were used to evaluate the proportional hazard assumption. Tests for interactions of diabetes status, age and sex with TnT and NT‐proBNP in relation to all outcomes were performed. Model discrimination was tested by use of Harrell's c‐statistic, the integrated discrimination improvement and the continuous net reclassification index adapted for survival models.19, 20 The associations between TnT and NT‐proBNP and outcomes were examined using the biomarkers in a categorical way and as continuous variables, according to history of diabetes and aetiology. TnT was dichotomized at or above/below the recently recommended threshold for prognostication in HF (18 ng/L) and NT‐proBNP divided by tertiles.21 The biomarkers were examined as continuous variables in an adjusted model using restricted cubic splines. P‐values of < 0.05 were considered significant. Analyses were performed using Stata version 15 and R version 3.3.2 (Stata Corp., College Station, TX, USA).
Results
Baseline characteristics
Among the patients (n = 1907) in the biomarker substudy, 759 (40%) had diabetes. Patients with diabetes had a higher body mass index, systolic blood pressure, and a lower LDL, high‐density lipoprotein and eGFR, compared to those without diabetes (Table 1). A larger proportion of patients with diabetes had a history of hypertension, myocardial infarction and coronary revascularization than those without diabetes. Diuretic use and prior implantable cardioverter defibrillator implantation were also more common in individuals with diabetes. Most patients were in NYHA functional class II and the distribution of NYHA class did not differ significantly between patients with and without diabetes.
Table 1.
Baseline characteristics according to diabetes status
| No diabetes | Diabetes | P‐value | |
|---|---|---|---|
| Patients, n (%) | 1148 (60) | 759 (40) | |
| Female sex, n (%) | 223 (19) | 136 (18) | 0.41 |
| Age, years, mean ± SD | 66 ± 10 | 67 ± 9 | 0.12 |
| Ischaemic aetiology, n (%) | 700 (61) | 533 (70) | <0.001 |
| White, n (%) | 1094 (95) | 717 (95) | 0.42 |
| Randomized to sacubitril/valsartan, n (%) | 573 (50) | 378 (50) | 0.96 |
| HbA1c, %, median [Q1–Q3] | 6.0 [5.7–6.3] | 7.0 [6.3–7.9] | <0.001 |
| Ejection fraction, %, mean ± SD | 30 ± 6 | 30 ± 6 | 0.94 |
| NYHA class, n (%) | 0.52 | ||
| I | 29 (3) | 16 (2) | |
| II | 845 (74) | 541 (71) | |
| III | 265 (23) | 197 (26) | |
| IV | 7 (0.6) | 5 (0.7) | |
| Body mass index, kg/m2, median [Q1–Q3] | 28 [25–31] | 30 [27–34] | <0.001 |
| Heart rate, b.p.m., median [IQR] | 70 [62–79] | 70 [64–80] | 0.04 |
| SBP, mmHg, median [IQR] | 120 [110–131] | 124 [113–135] | <0.001 |
| eGFR, mL/min/ 1.73 m2, median [IQR] | 65 [52–77] | 61 [48–73] | <0.001 |
| Creatinine, μmol/L, median [IQR] | 97 [83–116] | 102 [87–123] | <0.001 |
| Cholesterol, mmol/L, median [IQR] | |||
| Total | 4.6 [3.9–5.4] | 4.3 [3.6–5.0] | <0.001 |
| Low‐density lipoprotein | 2.5 [2.0–3.2] | 2.2 [1.7–2.8] | <0.001 |
| High‐density lipoprotein | 1.3 [1.1–1.5] | 1.1 [1.0–1.4] | <0.001 |
| Current smoker, n (%) | 156 (14) | 94 (12) | 0.45 |
| Oedema, n (%) | 251 (21) | 175 (23) | 0.54 |
| Rales, n (%) | 100 (9) | 66 (9) | 0.99 |
| Third heart sound, n (%) | 71 (6) | 57 (8) | 0.26 |
| Jugular vein distention, n (%) | 113 (10) | 60 (8) | 0.15 |
| Orthopnoea, n (%) | 68 (6) | 55 (7) | 0.25 |
| Dyspnoea on effort, n (%) | 1024 (89) | 694 (91) | 0.13 |
| Dyspnoea at rest, n (%) | 52 (5) | 33 (4) | 0.84 |
| Medical history, n (%) | |||
| Angina | 338 (29) | 244 (32) | 0.21 |
| CABG or PCI | 448 (39) | 413 (54) | <0.001 |
| Pacemaker | 221 (19) | 176 (23) | 0.04 |
| ICD | 291 (25) | 247 (33) | <0.001 |
| Atrial fibrillation | 551 (48) | 377 (50) | 0.47 |
| Hypertension | 837 (73) | 650 (86) | <0.001 |
| Myocardial infarction | 525 (47) | 411 (54) | <0.001 |
| Intermittent claudication | 54 (5) | 70 (9) | <0.001 |
| Stroke | 114 (10) | 79 (10) | 0.74 |
| Current medication, n (%) | |||
| Diuretics | 920 (80) | 648 (85) | 0.003 |
| ACEI or ARB | 1148 (100) | 759 (100) | – |
| Beta‐blockers | 1092 (95) | 723 (95) | 0.89 |
| MRA | 510 (44) | 349 (46) | 0.50 |
| Digoxin | 239 (21) | 184 (24) | 0.08 |
| Antiplatelets | 643 (56) | 450 (59) | 0.16 |
| Anticoagulants | 498 (43) | 331 (44) | 0.92 |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; ICD, implantable cardioverter defibrillator; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; Q, quartile; SBP, systolic blood pressure; SD, standard deviation.
Patients with HF of ischaemic origin (65%) were older, had a lower eGFR and less use of diuretics and digoxin than patients with non‐ischaemic aetiology. Fewer ischaemic patients had atrial fibrillation compared to those with non‐ischaemic HF, irrespective of diabetes status (online supplementary Table S1). Patients in the biomarker substudy differed from those not included in a number of ways. They were older, more were men and they had a different racial composition, compared with patients not included in the biomarker substudy. A higher proportion had an ischaemic aetiology and they had a greater burden of co‐morbidities, including more obesity and renal impairment (Table 2).
Table 2.
Baseline characteristics of patients with and without a troponin T measurement
| Measurement of TnT | No measurement of TnT | P‐value | |
|---|---|---|---|
| Patients, n (%) | 1907 (23) | 6492 (77) | |
| Female sex, n (%) | 359 (19) | 1473 (23) | <0.001 |
| Age, years, mean ± SD | 67 ± 10 | 62 ± 11 | <0.001 |
| Ischaemic aetiology, n (%) | 1233 (65) | 3803 (59) | <0.001 |
| White, n (%) | 1811 (95) | 3733 (58) | <0.001 |
| Randomized to sacubitril/valsartan, n (%) | 951 (50) | 3236 (50) | 0.99 |
| NT‐proBNP, pg/mL, median [Q1–Q3] | 1481 [855–2812] | 1671 [903–3400] | <0.001 |
| Ejection fraction, %, mean ± SD | 30 ± 6 | 29 ± 6 | <0.001 |
| NYHA class, n (%) | <0.001 | ||
| I | 45 (2) | 344 (5) | |
| II | 1386 (73) | 4533 (70) | |
| III | 462 (24) | 1556 (24) | |
| IV | 12 (0.6) | 48 (0.7) | |
| Body mass index, kg/m2, median [Q1–Q3] | 29 [26–32] | 27 [24–31] | <0.001 |
| Heart rate, b.p.m., median [IQR] | 70 [63–79] | 72 [64–80] | <0.001 |
| SBP, mmHg, median [IQR] | 121 [110–132] | 120 [110–130] | <0.001 |
| eGFR, mL/min/ 1.73 m2, median [IQR] | 63 [51–75] | 67 [55–81] | <0.001 |
| Creatinine, μmol/L, median [IQR] | 99 [85–119] | 94 [80–112] | <0.001 |
| Cholesterol, mmol/L, median [IQR] | |||
| Total | 4.4 [3.8–5.3] | 4.4 [3.8–5.3] | 0.8358 |
| Low‐density lipoprotein | 2.4 [1.8–3.1] | 2.4 [1.9–3.1] | 0.1377 |
| High‐density lipoprotein | 1.2 [1.0–1.5] | 1.2 [1.0–1.4] | <0.001 |
| Current smoker, n (%) | 250 (13) | 958 (15) | 0.0715 |
| Medical history, n (%) | |||
| Diabetes | 759 (40) | 2148 (33) | <0.001 |
| Angina | 582 (31) | 1722 (27) | <0.001 |
| CABG or PCI | 861 (45) | 1779 (27) | <0.001 |
| Pacemaker | 538 (28) | 705 (11) | <0.001 |
| ICD | 397 (21) | 691 (11) | <0.001 |
| Atrial fibrillation | 928 (49) | 2163 (33) | <0.001 |
| Hypertension | 1487 (78) | 4453 (69) | <0.001 |
| Myocardial infarction | 936 (49) | 2698 (42) | <0.001 |
| Intermittent claudication | 124 (7) | 268 (4) | <0.001 |
| Stroke | 193 (10) | 532 (8) | 0.009 |
| Current medication, n (%) | |||
| Diuretics | 1568 (82) | 5170 (80) | 0.01 |
| ACEI or ARB | 1907 (100) | 6472 (100) | 0.02 |
| Beta‐blockers | 1815 (95) | 5996 (92) | <0.001 |
| MRA | 859 (45) | 3812 (59) | <0.001 |
| Digoxin | 423 (22) | 2116 (33) | <0.001 |
| Antiplatelets | 1093 (57) | 3643 (56) | 0.35 |
| Anticoagulants | 829 (44) | 1856 (29) | <0.001 |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter defibrillator; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; Q, quartile; SBP, systolic blood pressure; SD, standard deviation; TnT, troponin T.
Concentrations of troponin T
The median concentration of TnT at baseline was significantly higher in patients with diabetes [18 ng/L, interquartile range (IQR) 11–27 ng/L] compared to patients without diabetes (13 ng/L, IQR 9–21 ng/L) (P < 0.001). Half of patients with diabetes had a TnT value ≥ 18 ng/L compared to a third of those without diabetes (Table 3). These findings were consistent when patients were compared according to aetiology of HF (ischaemic or non‐ischaemic) (Table 3).
Table 3.
Troponin T and N‐terminal pro B‐type natriuretic peptide levels according to diabetes status
| No diabetes | Diabetes | P‐value | |
|---|---|---|---|
| Troponin T, ng/L, median [IQR] | |||
| Overall | 13 [9–21] | 18 [11–27] | <0.001 |
| Ischaemic | 13 [8–20] | 18 [12–27] | |
| Non‐ischaemic | 13 [9–20] | 16 [11–24] | |
| Troponin T ≥ 18 ng/L, n (%) | |||
| Overall | 394 (34) | 382 (50) | <0.001 |
| Ischaemic | 241 (34) | 279 (52) | |
| Non‐ischaemic | 153 (34) | 103 (46) | |
| NT‐proBNP, pg/mL, median [IQR] | |||
| Overall | 906 [516–1725] | 890 [538–1585] | 0.51 |
| Ischaemic | 988 [538–1782] | 883 [538–1654] | |
| Non‐ischaemic | 830 [455–1591] | 916 [542–1465] | |
| NT‐proBNP ‐ tertile 3, n (%) | |||
| Overall | 395 (34) | 240 (32) | 0.38 |
| Ischaemic | 253 (36) | 173 (32) | |
| Non‐ischaemic | 142 (32) | 67 (30) |
IQR, interquartile range; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide.
The distribution of plasma TnT concentrations in patients with and without diabetes, stratified by aetiology (ischaemic/non‐ischaemic) is shown in Figure 1. The frequency distribution curve was shifted to the right in patients with diabetes, compared to those without diabetes, in both ischaemic and non‐ischaemic HFrEF patients; this pattern was quite distinct from that seen for NT‐proBNP (see below).
Figure 1.
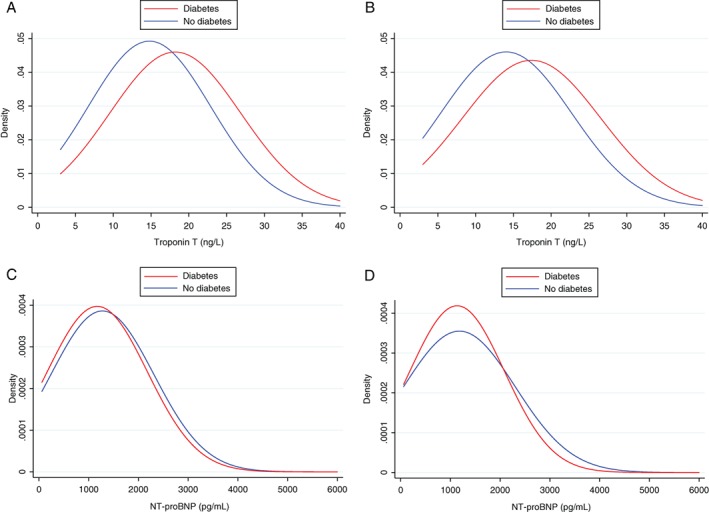
Distribution of troponin T and N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) according to diabetes status and heart failure aetiology in PARADIGM‐HF. (A) Troponin T in patients with ischaemic heart failure. (B) Troponin T in patients with non‐ischaemic heart failure. (C) NT‐proBNP in patients with ischaemic heart failure. (D) NT‐proBNP in patients with non‐ischaemic heart failure.
Concentrations of NT‐proBNP
In contrast to TnT, patients with diabetes and without diabetes had similar median levels of NT‐proBNP at baseline (890 pg/mL, IQR 538–1585 pg/mL vs. 906 pg/mL, IQR 516–1725 pg/mL; P = 0.51). Around a third of patients with and without diabetes had a NT‐proBNP value in the top tertile (i.e. > 1342 pg/mL; P = 0.38) (Table 3). Median NT‐proBNP levels were also similar in patients with and without diabetes, irrespective of aetiology (ischaemic or non‐ischaemic). In contrast to TnT, the frequency distribution curves for concentration of NT‐proBNP were similar in patients with and without diabetes, in both ischaemic and non‐ischaemic HFrEF patients (Figure 1).
Baseline troponin T and clinical outcomes
During follow‐up, patients with TnT ≥ 18 ng/L had a significantly higher risk of the composite outcome of cardiovascular death or HF hospitalization, irrespective of diabetes status and ischaemic or non‐ischaemic aetiology (Figure 2). Patients with diabetes were at higher risk than those without and patients with an ischaemic aetiology were at a higher risk than those with a non‐ischaemic aetiology. Consequently, patients with diabetes and ischaemic HF and an elevated TnT were at a very high absolute risk (203 per 1000 person‐years) compared to those without diabetes, a non‐ischaemic aetiology who had a TnT < 18 ng/L (46 per 1000 person‐years).
Figure 2.
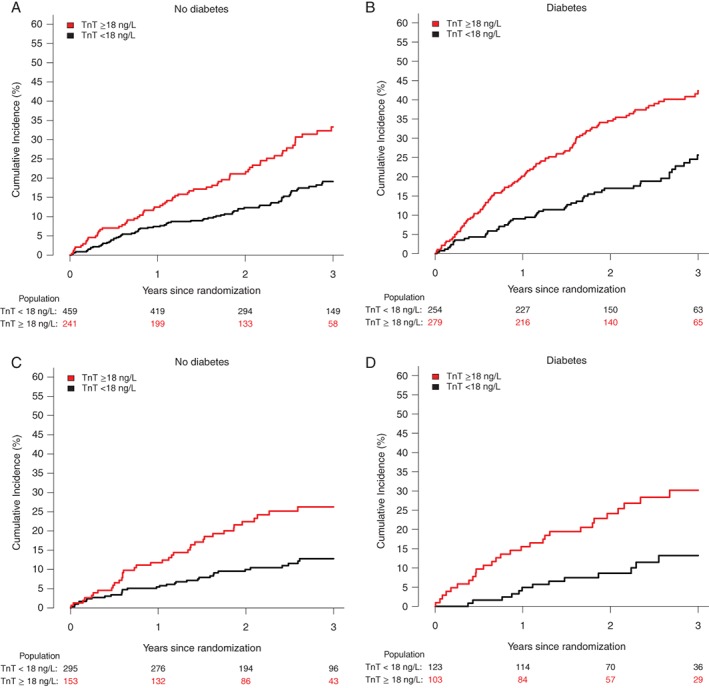
Cumulative incidence of heart failure hospitalization or cardiovascular death with death as competing risk according to troponin T (TnT) ≥ 18 or < 18 ng/L among patients with and without heart failure of ischaemic origin and diabetes in PARADIGM‐HF. (A) Patients with no diabetes and heart failure of ischaemic origin. (B) Patients with diabetes and heart failure of ischaemic origin. (C) Patients with no diabetes and heart failure of non‐ischaemic origin. (D) Patients with diabetes and heart failure of non‐ischaemic origin.
The elevated risk related to TnT persisted in adjusted Cox regression models incorporating TnT in a categorical way (≥ 18 vs. < 18 ng/L) or as a continuous variable (per loge increase in TnT) (Table 4). The relationship between TnT and the composite outcome of cardiovascular death or HF hospitalization was similar in patients with and without diabetes, across the spectrum of TnT concentrations, with a linear increase in risk of 91% and 73% in patients with and without diabetes, respectively, per log unit increase in TnT (Table 4 and online supplementary Figure S1).
Table 4.
Adjusted hazard ratios for cardiovascular death/heart failure hospitalization and all‐cause mortality according to troponin T and N‐terminal pro B‐type natriuretic peptide levels
| HR (95% CI) | ||
|---|---|---|
| No diabetes | Diabetes | |
| CV death/HF hospitalization | ||
| Troponin T | ||
| < 18 ng/L | 1.00 (ref) | 1.00 (ref) |
| ≥ 18 ng/L | 1.65 (1.20–2.28) | 1.49 (1.05–2.12) |
| Per loge increase | 1.73 (1.34–2.23) | 1.91 (1.45–2.51) |
| NT‐proBNP | ||
| T1 | 1.00 (ref) | 1.00 (ref) |
| T2 | 1.33 (0.88–2.01) | 1.75 (1.15–2.67) |
| T3 | 2.77 (1.88–4.07) | 3.35 (2.19–5.11) |
| Per loge increase | 1.64 (1.40–1.93) | 1.85 (1.54–2.23) |
| All‐cause mortality | ||
| Troponin T | ||
| < 18 ng/L | 1.00 (ref) | 1.00 (ref) |
| ≥ 18 ng/L | 1.51 (1.05–2.17) | 1.49 (1.01–2.26) |
| Per loge increase | 1.66 (1.24–2.22) | 1.71 (1.23–2.37) |
| NT‐proBNP | ||
| T1 | 1.00 (ref) | 1.00 (ref) |
| T2 | 1.41 (0.87–2.27) | 2.00 (1.19–3.37) |
| T3 | 2.45 (1.55–3.86) | 3.34 (1.98–5.65) |
| Per loge increase | 1.51 (1.26–1.82) | 1.60 (1.30–1.97) |
CI, confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide.
All models were adjusted for age, sex, treatment effect, ejection fraction, New York Heart Association class, body mass index, heart rate, systolic blood pressure, creatinine, low‐density lipoprotein, prior angina pectoris, atrial fibrillation and pacemaker implantation. Models with troponin T levels as exposure were also adjusted for loge(NT‐proBNP).
Baseline NT‐proBNP and clinical outcomes
Patients with NT‐proBNP in the highest tertile had a significantly higher risk of the composite outcome of cardiovascular death or HF hospitalization, irrespective of diabetes status and ischaemic or non‐ischaemic aetiology. Patients with diabetes and an ischaemic aetiology were at the highest risk of all outcomes. Thus, patients with diabetes and ischaemic HF and NT‐proBNP in the highest tertile were at a very high absolute risk of the composite outcome of cardiovascular death or HF hospitalization (267 per 1000 person‐years) compared to those without diabetes, a non‐ischaemic aetiology and NT‐proBNP in the lowest tertile (53 per 100 person‐years).
In adjusted Cox regression models incorporating NT‐proBNP as tertiles or as a continuous variable (per loge increase in NT‐proBNP), the elevated risk associated with increased levels of NT‐proBNP persisted (Table 4). The relationship between NT‐proBNP and the composite outcome of cardiovascular death or HF hospitalization was similar in patients with and without diabetes, across the spectrum of NT‐proBNP concentrations (online supplementary Figure S2).
Combination of troponin T and NT‐proBNP in predicting clinical outcomes
The risks associated with TnT and NT‐proBNP were additive in predicting outcomes, both in patients with and without diabetes.
Thus, patients with diabetes, an elevated TnT and a NT‐proBNP level in the highest tertile (n = 178, 9% of all patients) had an absolute risk of the primary composite outcome of 265 per 1000 person‐years, compared to a rate of 42 per 1000 person‐years in those without diabetes, a TnT < 18 ng/L and a NT‐proBNP in the lowest tertile (n = 305, 16% of all patients). This contrast in absolute rates was even more striking when ischaemic aetiology was also considered. Patients with an ischaemic aetiology, diabetes, an elevated TnT and a NT‐proBNP level in the highest tertile (n = 130, 7% of all patients) had an absolute risk of the primary composite outcome of 295 per 1000 person‐years, compared to a rate of 40 per 1000 person‐years in those with non‐ischaemic HF, without diabetes, a TnT < 18 ng/L and a NT‐proBNP in the lowest tertile (n = 133, 7% of all patients).
In multivariable predictive models, among patients with diabetes, those having a TnT level ≥ 18 ng/L and a NT‐proBNP in the highest tertile had a 4.5‐fold higher risk (4.1 for ischaemic and 15.6 for non‐ischaemic patients) of the primary outcome, compared to those with a TnT < 18 ng/L and a NT‐proBNP in the lowest tertile. The risk was 4.2‐fold higher (3.6 for ischaemic and 5.0 for non‐ischaemic patients) in individuals without diabetes (Figure 3). Among patients with diabetes, TnT and NT‐proBNP were associated with increased risk of cardiovascular death or HF hospitalization, irrespective of glycated haemoglobin levels (data not shown).
Figure 3.
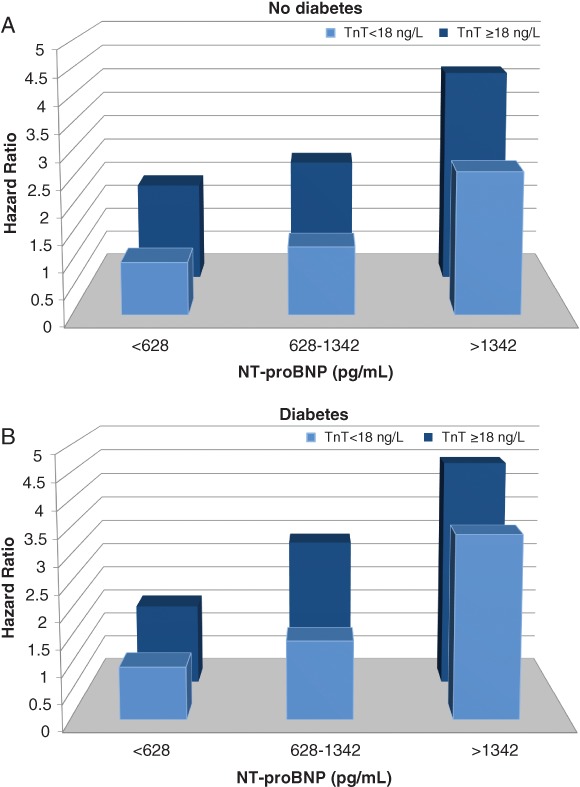
Risk of cardiovascular death or heart failure hospitalization according to troponin T (TnT) ≥ 18 or < 18 ng/L and tertiles of N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) levels among patients with and without diabetes in PARADIGM‐HF (A: no diabetes; B: diabetes). Adjusted for age, sex, treatment effect, ejection fraction, New York Heart Association class, body mass index, heart rate, systolic blood pressure, creatinine, low‐density lipoprotein, prior angina pectoris, atrial fibrillation, and pacemaker implantation.
Troponin T added significant discriminatory power to the models predicting cardiovascular death or HF hospitalization, although the effect sizes were modest (online supplementary Table S2). In models with all‐cause mortality as outcome, only the integrated discrimination improvement was improved when TnT was added to the models.
Discussion
This study has three key findings. First, HFrEF patients with diabetes had higher concentrations of TnT, compared to HFrEF patients without diabetes, and this was true for patients with non‐ischaemic HF, as well as those with an ischaemic aetiology. This contrasted to the lack of difference in NT‐proBNP between patients with and without diabetes. Second, higher levels of TnT and NT‐proBNP were each associated with worse outcomes, irrespective of diabetes status and HF aetiology, i.e. diabetes did not alter the prognostic performance of either biomarker. TnT was an independent predictor of outcomes when added to a multivariable model including NT‐proBNP. The risks associated with increasing levels of the two biomarkers were additive, and this additivity was similar in individuals with and without diabetes. Thirdly, as a result of the foregoing, and perhaps most importantly clinically, these biomarkers used in combination with patient phenotype identified individuals at remarkably high absolute risk. For example, those with the triad of diabetes, an ischaemic aetiology and an elevated TnT had a primary endpoint rate of 203 per 1000 person‐years (compared with 46 per 1000 person‐years in those with non‐ischaemic HF, no diabetes and a TnT < 18 ng/L).
A few prior studies have reported that patients with diabetes have elevated levels of TnT and, in HF patients, diabetes seems to be more prevalent among those with high levels of TnT.8, 10, 11, 22 In accordance with this, we found higher average levels of TnT in HFrEF patients with diabetes compared to those without diabetes. Using the newly recommended TnT prognostication threshold for HF of ≥ 18 ng/L, we found that the proportion of diabetes patients with an elevated TnT was 50% compared to 34% of patients without diabetes. The most obvious explanation for more frequent elevation of TnT in patients with diabetes is the higher prevalence of coronary artery disease in those individuals. However, we found TnT was similarly elevated in patients with non‐ischaemic and ischaemic HFrEF. Diabetes also causes microvascular disease and this may lead to myocardial ischaemia and cardiomyocyte injury in the absence of or in addition to macrovascular coronary disease.23 Unfortunately, information on microvascular complications was not collected in PARADIGM‐HF. An alternative explanation might be the greater likelihood of renal dysfunction in patients with diabetes, as renal dysfunction is also associated with raised troponin concentrations.24, 25, 26 However, there was only a small difference in eGFR and creatinine between those with and without diabetes, and NT‐proBNP, levels of which are also influenced by renal function, did not differ between those with and without diabetes. Additional pathophysiologic processes operating in patients with diabetes might cause cardiomyocyte injury. For example, inflammation appears to be more common in individuals with diabetes compared to those without.27
Elevation of TnT has previously been associated with worse prognosis in each of HFrEF and diabetes.8, 12 Our study adds to these prior findings by showing that TnT is an independent predictor of adverse outcomes in patients with these two conditions combined, even when added to a multivariable model containing NT‐proBNP, which is in itself the single most powerful predictor of prognosis in HFrEF.28, 29, 30 That the prognostic value of TnT is similar in HFrEF patients with and without diabetes is important, given the greater prevalence of elevated concentrations of TnT in individuals with diabetes. This highlights the considerable contribution of TnT to population‐attributable risk in HFrEF patients with diabetes. Moreover, this could be important and relevant for clinical practice as by use of both routinely‐available and relatively inexpensive biomarkers combined with a diabetic phenotype (and especially a diabetic, ischaemic phenotype) we were able to identify an extremely high‐risk subset of patients in PARADIGM‐HF. Such patients may merit close surveillance, appropriate counselling, intensive efforts to optimize pharmacological, device and surgical therapies (including coronary revascularization). This sizeable minority of patients might also be the target for new strategies to lower risk.
Limitations
Both biomarkers were only available in the pre‐specified biomarker substudy of approximately 2000 patients, and the characteristics of these patients were significantly different from the patients not included in the biomarker substudy. Diabetes status and ischaemic aetiology were investigator reported and a degree of misclassification will have occurred as, for example, it is known that some HFrEF patients have undiagnosed diabetes and some patients with a presumed non‐ischaemic HFrEF aetiology are found to have coronary artery disease on angiography or at autopsy. However, the clinical characteristics and difference in event rates between patients with the different phenotypes of interest suggest at least reasonably accurate categorization.
Conclusions
In HFrEF, patients with diabetes had higher levels of TnT than patients without diabetes, contrasting with NT‐proBNP, which did not differ between patients with and without diabetes. TnT was similarly elevated in ischaemic and non‐ischaemic patients. TnT was independently predictive of outcomes, when added to a multivariable model including NT‐proBNP, irrespective of diabetes status. TnT and NT‐proBNP are therefore additive in predicting risk and, when combined with clinical phenotype (diabetes, ischaemic aetiology), help identify patients at extremely high absolute risk.
Conflict of interest: none declared.
Supporting information
Figure S1. Risk of cardiovascular death or heart failure hospitalization according to troponin T levels among patients with and without diabetes in PARADIGM‐HF (A: no diabetes; B: diabetes). Adjusted for loge(NT‐proBNP), age, sex, treatment effect, ejection fraction, New York Heart Association class, body mass index, heart rate, systolic blood pressure, creatinine, low‐density lipoprotein, prior angina pectoris, atrial fibrillation and pacemaker implantation.
Figure S2. Risk of cardiovascular death or heart failure hospitalization according to N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) levels among patients with and without diabetes in PARADIGM‐HF (A: no diabetes; B: diabetes). Adjusted for age, sex, treatment effect, ejection fraction, New York Heart Association class, body mass index, heart rate, systolic blood pressure, creatinine, low‐density lipoprotein, prior angina pectoris, atrial fibrillation and pacemaker implantation.
Table S1. Baseline characteristics according to diabetes status and heart failure aetiology.
Table S2. C‐index, net reclassification index and integrated discrimination improvement for the added effect of troponin T to the baseline model.
References
- 1. Logeart D, Thabut G, Jourdain P, Chavelas C, Beyne P, Beauvais F, Bouvier E, Solal AC. Predischarge B‐type natriuretic peptide assay for identifying patients at high risk of re‐admission after decompensated heart failure. J Am Coll Cardiol 2004;43:635–641. [DOI] [PubMed] [Google Scholar]
- 2. Sugiura T, Takase H, Toriyama T, Goto T, Ueda R, Dohi Y. Circulating levels of myocardial proteins predict future deterioration of congestive heart failure. J Card Fail 2005;11:504–509. [DOI] [PubMed] [Google Scholar]
- 3. Jourdain P, Jondeau G, Funck F, Gueffet P, Le Helloco A, Donal E, Aupetit JF, Aumont MC, Galinier M, Eicher JC, Cohen‐Solal A, Juilliere Y. Plasma brain natriuretic peptide‐guided therapy to improve outcome in heart failure: the STARS‐BNP Multicenter Study. J Am Coll Cardiol 2007;49:1733–1739. [DOI] [PubMed] [Google Scholar]
- 4. Fonarow GC, Peacock WF, Horwich TB, Phillips CO, Givertz MM, Lopatin M, Wynne J. Usefulness of B‐type natriuretic peptide and cardiac troponin levels to predict in‐hospital mortality from ADHERE. Am J Cardiol 2008;101:231–237. [DOI] [PubMed] [Google Scholar]
- 5. Hillis GS, Welsh P, Chalmers J, Perkovic V, Chow CK, Li Q, Jun M, Neal B, Zoungas S, Poulter N, Mancia G, Williams B, Sattar N, Woodward M. The relative and combined ability of high‐sensitivity cardiac troponin T and N‐terminal pro‐B‐type natriuretic peptide to predict cardiovascular events and death in patients with type 2 diabetes. Diabetes Care 2014;37:295–303. [DOI] [PubMed] [Google Scholar]
- 6. Bhalla MA, Chiang A, Epshteyn VA, Kazanegra R, Bhalla V, Clopton P, Krishnaswamy P, Morrison LK, Chiu A, Gardetto N, Mudaliar S, Edelman SV, Henry RR, Maisel AS. Prognostic role of B‐type natriuretic peptide levels in patients with type 2 diabetes mellitus. J Am Coll Cardiol 2004;44:1047–1052. [DOI] [PubMed] [Google Scholar]
- 7. Wolsk E, Claggett B, Pfeffer MA, Diaz R, Dickstein K, Gerstein HC, Lawson FC, Lewis EF, Maggioni AP, McMurray JJ, Probstfield JL, Riddle MC, Solomon SD, Tardif JC, Kober L. Role of B‐type natriuretic peptide and N‐terminal prohormone BNP as predictors of cardiovascular morbidity and mortality in patients with a recent coronary event and type 2 diabetes mellitus. J Am Heart Assoc 2017;6:e004743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Latini R, Masson S, Anand IS, Missov E, Carlson M, Vago T, Angelici L, Barlera S, Parrinello G, Maggioni AP, Tognoni G, Cohn JN, Val‐HeFT Investigators . Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 2007;116:1242–1249. [DOI] [PubMed] [Google Scholar]
- 9. Nagarajan V, Hernandez AV, Tang WH. Prognostic value of cardiac troponin in chronic stable heart failure: a systematic review. Heart 2012;98:1778–1786. [DOI] [PubMed] [Google Scholar]
- 10. Wallace TW, Abdullah SM, Drazner MH, Das SR, Khera A, McGuire DK, Wians F, Sabatine MS, Morrow DA, de Lemos JA. Prevalence and determinants of troponin T elevation in the general population. Circulation 2006;113:1958–1965. [DOI] [PubMed] [Google Scholar]
- 11. Hallen J, Johansen OE, Birkeland KI, Gullestad L, Aakhus S, Endresen K, Tjora S, Jaffe AS, Atar D. Determinants and prognostic implications of cardiac troponin T measured by a sensitive assay in type 2 diabetes mellitus. Cardiovasc Diabetol 2010;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Everett BM, Cook NR, Magnone MC, Bobadilla M, Kim E, Rifai N, Ridker PM, Pradhan AD. Sensitive cardiac troponin T assay and the risk of incident cardiovascular disease in women with and without diabetes mellitus: the Women's Health Study. Circulation 2011;123:2811–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nadruz W, Claggett BL, McMurray JJ, Packer M, Zile MR, Rouleau JL, Desai AS, Swedberg K, Lefkowitz M, Shi VC, MF P, Solomon SD. Impact of body mass index on the accuracy of N‐terminal pro‐brain natriuretic peptide and brain natriuretic peptide for predicting outcomes in patients with chronic heart failure and reduced ejection fraction. Insights from the PARADIGM‐HF Study (Prospective Comparison of ARNI With ACEI toDetermine Impact on Global Mortality and Morbidity in Heart Failure Trial). Circulation 2016;134:1785–1787. [DOI] [PubMed] [Google Scholar]
- 14. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 15. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau J, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Committees and Investigators . Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin‐converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM‐HF). Eur J Heart Fail 2013;15:1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz M, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Committees and Investigators . Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial (PARADIGM‐HF). Eur J Heart Fail 2014;16:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile M, Andersen K, Arango JL, Arnold JM, Belohlavek J, Bohm M, Boytsov S, Burgess LJ, Cabrera W, Calvo C, Chen CH, Dukat A, Duarte YC, Erglis A, Fu M, Gomez E, Gonzalez‐Medina A, Hagege AA, Huang J, Katova T, Kiatchoosakun S, Kim KS, Kozan O, Llamas EB, Martinez F, Merkely B, Mendoza I, Mosterd A, Negrusz‐Kawecka M, Peuhkurinen K, Ramires FJ, Refsgaard J, Rosenthal A, Senni M, Sibulo AS Jr, Silva‐Cardoso J, Squire IB, Starling RC, Teerlink JR, Vanhaecke J, Vinereanu D, Wong RC, PARADIGM‐HF Investigators and Coordinators . Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015;131:54–61. [DOI] [PubMed] [Google Scholar]
- 18. Zile MR, Claggett BL, Prescott MF, McMurray JJ, Packer M, Rouleau JL, Swedberg K, Desai AS, Gong J, Shi VC, Solomon SD. Prognostic implications of changes in N‐terminal pro‐B‐type natriuretic peptide in patients with heart failure. J Am Coll Cardiol 2016;68:2425–2436. [DOI] [PubMed] [Google Scholar]
- 19. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 20. Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aimo A, Januzzi JL Jr, Vergaro G, Ripoli A, Latini R, Masson S, Magnoli M, Anand IS, Cohn JN, Tavazzi L, Tognoni G, Gravning J, Ueland T, Nymo SH, Brunner‐La Rocca HP, Bayes‐Genis A, Lupon J, de Boer RA, Yoshihisa A, Takeishi Y, Egstrup M, Gustafsson I, Gaggin HK, Eggers KM, Huber K, Tentzeris I, Tang WH, Grodin J, Passino C, Emdin M. Prognostic value of high‐sensitivity troponin T in chronic heart failure: an individual patient data meta‐analysis. Circulation 2018;137:286–297. [DOI] [PubMed] [Google Scholar]
- 22. Masson S, Anand I, Favero C, Barlera S, Vago T, Bertocchi F, Maggioni AP, Tavazzi L, Tognoni G, Cohn JN, Latini R, Valsartan Heart Failure Trial (Val‐HeFT) and Gruppo Italiano per lo Studio della Sopravvivenza nell'Insufficienza Cardiaca‐Heart Failure (GISSI‐HF) Investigators . Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation 2012;125:280–288. [DOI] [PubMed] [Google Scholar]
- 23. Laakso M. Heart in diabetes: a microvascular disease. Diabetes Care 2011;34 Suppl 2:S145–S149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Freda BJ, Tang WH, Van Lente F, Peacock WF, Francis GS. Cardiac troponins in renal insufficiency: review and clinical implications. J Am Coll Cardiol 2002;40:2065–2071. [DOI] [PubMed] [Google Scholar]
- 25. Aviles RJ, Askari AT, Lindahl B, Wallentin L, Jia G, Ohman EM, Mahaffey KW, Newby LK, Califf RM, Simoons ML, Topol EJ, Berger P, Lauer MS. Troponin T levels in patients with acute coronary syndromes, with or without renal dysfunction. N Engl J Med 2002;346:2047–2052. [DOI] [PubMed] [Google Scholar]
- 26. Diris JH, Hackeng CM, Kooman JP, Pinto YM, Hermens WT, van Dieijen‐Visser MP. Impaired renal clearance explains elevated troponin T fragments in hemodialysis patients. Circulation 2004;109:23–25. [DOI] [PubMed] [Google Scholar]
- 27. Calle MC, Fernandez ML. Inflammation and type 2 diabetes. Diabetes Metab 2012;38:183–191. [DOI] [PubMed] [Google Scholar]
- 28. Aspromonte N, Feola M, Milli M, Scardovi AB, Coletta C, Carbonieri E, Giovinazzo P, Di Giacomo T, Barro S, Rosso GL, Ceci V, Milani L, Valle R. Prognostic role of B‐type natriuretic peptide in patients with diabetes and acute decompensated heart failure. Diabet Med 2007;24:124–130. [DOI] [PubMed] [Google Scholar]
- 29. Hartmann F, Packer M, Coats AJ, Fowler MB, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Anker SD, Amann‐Zalan I, Hoersch S, Katus HA. Prognostic impact of plasma N‐terminal pro‐brain natriuretic peptide in severe chronic congestive heart failure. A substudy of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial. Circulation 2004;110:1780–1786. [DOI] [PubMed] [Google Scholar]
- 30. Bettencourt P, Azevedo A, Pimenta J, Friões F, Ferreira S, Ferreira A. N‐terminal–pro‐brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation 2004;110:2168–2174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Risk of cardiovascular death or heart failure hospitalization according to troponin T levels among patients with and without diabetes in PARADIGM‐HF (A: no diabetes; B: diabetes). Adjusted for loge(NT‐proBNP), age, sex, treatment effect, ejection fraction, New York Heart Association class, body mass index, heart rate, systolic blood pressure, creatinine, low‐density lipoprotein, prior angina pectoris, atrial fibrillation and pacemaker implantation.
Figure S2. Risk of cardiovascular death or heart failure hospitalization according to N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) levels among patients with and without diabetes in PARADIGM‐HF (A: no diabetes; B: diabetes). Adjusted for age, sex, treatment effect, ejection fraction, New York Heart Association class, body mass index, heart rate, systolic blood pressure, creatinine, low‐density lipoprotein, prior angina pectoris, atrial fibrillation and pacemaker implantation.
Table S1. Baseline characteristics according to diabetes status and heart failure aetiology.
Table S2. C‐index, net reclassification index and integrated discrimination improvement for the added effect of troponin T to the baseline model.


