Abstract
Eccentric exercise has been suggested to improve muscle atrophy, muscle function, and insulin sensitivity. The aim of this study was to examine the effect of acute eccentric exercise on appetite-related hormones, food preferences, and food intake. Fourteen moderately active men were recruited to participate in this study (age 24.2 ± 5.5 years; BMI 23.4 ± 3.3 kg/m2; VO2max 48.9 ± 3.1 ml/kg/min). Three different conditions were implemented; no exercise, flat running “inclination 0” and downhill running “inclination –12%.” Appetite-related hormones, subjective appetite sensations, food preference and reward, and ad libitum food intake were measured at pre-, immediately post-, and 24 h post exercise. There were no significant median changes in total ghrelin or pancreatic peptide concentrations between conditions. There were also no median differences in subjective appetite ratings or energy intake between conditions, but the median change in explicit liking of sweet versus savory foods differed significantly between pre-exercise and 24 h post exercise (p = .013). Post-hoc analysis observed a significant difference in the pre-exercise to 24 h post exercise change between front running and downhill running (p = .023), and indicated greater liking of savory foods over sweet foods in downhill running than front running. However, no further differences were seen between conditions for the remaining food preference parameters, suggesting there were no systematic trends in these data. In conclusion, there was no effect of front and downhill running on eating behavior as compared to a nonexercise control condition, but these data need to be replicated in a larger and more heterogeneous sample.
Keywords: eccentric exercise, eating behavior, appetite, food preferences
Eccentric exercise has distinct characteristics compared with isometric and concentric exercise, which have a number of physiological implications that affect acute responses to exercise (Douglas, Pearson, Ross, & McGuigan, 2017). Eccentric exercise has been suggested to improve muscle mass and total strength to a greater extent than concentric training (Roig et al., 2008). A recent review reported that fat and lean mass can be significantly improved with eccentric training protocols, especially in sedentary cohorts, with no worsening effect on insulin sensitivity (Gluchowski, Harris, Dulson, & Cronin, 2015). The high specificity of strength gains after eccentric training could be attributed to myogenic mechanisms (Franchi, Reeves, & Narici, 2017) and neuronal origin (Vikne et al., 2006) that characterizes eccentric in comparison with concentric exercise. The neuronal activation pattern during eccentric exercise serves to reduce metabolic demand and thus less muscle activity is needed for performing muscular actions (Julian et al., 2018). Eccentric exercise has greater effectiveness in term of enhancing isometric and isotonic strength at various velocities, particularly in elders (Raj, Bird, Westfold, & Shield, 2012).
Metabolic responses to eccentric exercise may differ to that seen following concentric exercise. For example, insulin concentrations were significantly increased for 4 days after a downhill running session. Adiponectin and visfatin remained unchanged, but resistin significantly increased only 2 days post exercise, relative to resting trial (Jamurtas et al., 2013). These hormones have been implicated in the control of appetite, which is also known to be transiently altered postexercise. However, the effect of eccentric aerobic exercise (relative to concentric based exercise) on appetite-regulated hormones is unclear and warrants further attention. A recent review reported that the responses of leptin to muscle action has not been examined, and ghrelin, an orexigenic hormone, is reduced with concentric exercise but may not change after eccentric exercise (Kraemer & Castracane, 2015). Weight-bearing (rope skipping) and nonweight-bearing (bicycle ergometer) exercise bouts have been reported to exert similar effects on appetite-regulating gut hormones (e.g., lowering of acylated ghrelin and increased total peptide YY with no effect on glucagon-like peptide-1), but weight-bearing bout suppressed subjective appetite sensations to a greater extent than nonweight-bearing exercise (Kawano et al., 2013). Thus, different modes and models of exercise may have different impacts on postexercise appetite-related hormones.
Several studies reported that eccentric exercise can prolong the elevation of resting energy expenditure in comparison with concentric exercise for at least 72 h (Hackney, Engels, & Gretebeck, 2008; Paschalis et al., 2011), which can be attributed to postexercise muscle protein synthesis. Protein turnover tends to raise after eccentric exercise (Tidball, 2011) to recondition muscular myofibrillar damage caused by eccentric exercise (Clarkson & Hubal, 2002). Likewise, fat oxidation was reported to increase after eccentric exercise, compared with concentric exercise (Paschalis et al., 2011). These two factors, elevation in resting energy expenditure and plasma lipid levels following eccentric exercise, can be proposed to indirectly alter appetite and eating behavior.
Food reward (e.g., “the momentary value of a food to the individual at the time of ingestion”; Rogers & Hardman, 2015) is an important component of eating behavior that might contribute to the amount and type of food intake (Finlayson, King, & Blundell, 2007). Research suggests that postexercise food reward may be influenced by the modality and intensity of exercise session. For example, chronic high intensity interval training tended to attenuate hedonic liking of high fat food (Alkahtani, Byrne, Hills, & King, 2014). The influence of exercise modality on food reward has also been examined, with McNeil, Cadieux, Finlayson, Blundell, and Doucet (2015) reporting that while the relative preference for high versus low fat foods was reduced following isoenergetic resistance and aerobic exercise, explicit liking for high versus low fat foods was found to be reduced following resistance exercise. This study examined the influence of acute eccentric aerobic exercise on appetite-related hormones, subjective appetite sensations, food reward, and ad libitum energy intake compared to concentric aerobic exercise and a resting control condition.
Materials and Methods
Participants
Fourteen moderately active men (mean age 24.2 ± 5.5 years, BMI 23.4 ± 3.3 kg/m2, VO2max 48.9 ± 3.1 ml/kg/min) who engaged in 2–5 h of structured aerobic exercise per week were recruited to participate in the study. Exclusion criteria included being sedentary and individuals who had been involved in resistance training in the past 2 months. All participants were informed of study requirements and signed written informed consent. The study was approved by the King Saud University (KSU) Institutional Review Board (IRB No. E-16-1831).
All participants were instructed to arrive at the laboratory between 8 am and 11 am. They were asked to have the same meals in the 24 h prior to test days. Participants were also asked to abstain from strenuous exercise and the consumption of caffeine in the 24 h prior to laboratory testing. All participants were asked to record their dietary intake before the first day exercise test, and between the first day and the second day (24 h post exercise), and their food records were given back to them in order to follow the same dietary intake in terms of amount and time. Blood samples and eating behavior tests were performed on the second day at the same time of day following an overnight fast (8–10 h).
Study Overview
The experiment was set at Exercise Physiology Laboratories at Exercise Physiology Department, College of Sport Sciences and Physical Activity, KSU. The laboratory is an air-conditioned laboratory with the temperature held constant at 21°C. Initially, a maximal incremental exercise test and a submaximal graded downhill running test were conducted to determine the speed that elicits 60% VO2max during front and downhill running. The Parvo Medics Analyser Module (TrueOne®2400, Metabolic Measurement System, Parvo Medics, Inc. USA) was used to monitor respiratory gas exchange. Further, one control session (no exercise [CON]) and two running sessions on a treadmill (flat running [FR], downhill running “inclination – 12%” [DHR]) were performed in a random order over a 12-week period, with each visit separated by 4 weeks to isolate any muscle damage which may happen after eccentric exercise. FR and DHR were performed at the speed that elicits 60% VO2max, and included five stages of 8 min interspersed by 2-min low-intensity exercise that elicits 30% VO2max. At pre-, immediately post- and 24 h post exercise (Pre-Ex, Post-Ex, and 24-h Post-Ex), appetite-related hormones, subjective appetite, food reward, and ad libitum energy intake were measured in all conditions (FR, DHR, and CON).
Appetite-Related Hormones
Blood samples were collected by a well-trained phlebotomist at the Biochemistry Laboratory at the College of Sport Sciences and Physical Activity at KSU, and were analyzed by experts in the field of human biomarkers at Prince Mutaib bin Abdullah Chair for Biomarkers Research on Osteoporosis, Faculty of Science, KSU. Total ghrelin and pancreatic polypeptide (PP) were measured using the commercially available specific enzyme-linked immunosorbent Luminex assay kits obtained from Millipore (Billerica, MA, Cat. # HMHEMAG-34K) and were performed as per manufacturer’s instructions to determine the serum levels of these proteins. Properly diluted serum samples were incubated with the antibody-coupled microspheres and then with biotinylated detection antibody before the addition of streptavidin-phycoerythrin. The captured bead complexes were measured with FLEXMAP 3D system (Luminex Corporation, Austin, TX) using the following instrument settings (events/bead, 35; sample size, 50 μl; discriminator gate, 8000–15,000). Fluid volume shifts were not accounted for in these values. Intra- and inter-assay CV were <10% and <20%, respectively. Due to missing values, total ghrelin concentrations are reported for 12 participants, and PP concentrations are reported for 11 participants.
Appetite and Energy Intake
Subjective appetite sensations including hunger, desire to eat, and fullness were measured at Pre-Ex, Post-Ex, and 24-h Post-Ex in all conditions, using a paper-based visual analogue scales (VAS) which is a 100 millimeter line with two extreme anchors of not at all at the left anchor and extremely agree at the right anchor. Three questions of appetite sensations are shown including “How hungry do you feel?” “How strong is your desire to eat?” and “How full do you feel?.” Scoring data followed the same procedure of the original instrument using the measure by millimeter (mm) to indicate participants’ responses. Within-subject appetite ratings are sensitive to experimental manipulation, display good test–retest reliability (Stubbs, Ferres, & Horgan, 2000), and are associated with subsequent food intake (Sadoul, Schuring, Mela, & Peters, 2014). Due to missing values, data are reported for eight participants.
Food reward was measured using The Leeds Food Preferences Questionnaire (LFPQ), which is a computer-based paradigm using the E-Prime experiment generator. The procedure uses 16 photographic food stimuli chosen to vary along two major dimensions: fat (high or low) and taste (sweet or nonsweet), such that there were four categories including high-fat sweet, high-fat nonsweet, low-fat sweet, and low-fat nonsweet. The Arab version of the LFPQ was previously examined and validated (Alkahtani, Dalton, Abuzaid, Obeid, & Finlayson, 2016). Data were presented using fat and taste appeal bias. For the fat appeal bias, a positive score indicated a preference for high fat foods over low fat foods, a negative score indicated a preference for low fat foods over high fat foods, and a score of zero indicated an equal preference for high and low fat foods. For the taste appeal bias, a positive score indicated a preference for sweet tasting foods over savory tasting foods, a negative score indicated a preference for savory tasting foods over sweet tasting foods, and a score of zero indicated an equal preference for sweet tasting and savory tasting foods. Due to missing values, data are reported for eight participants.
An ad libitum test meal was provided 30 min in the Post-Ex and 24-h Post-Ex. The meals included cheese and tuna pies and fruit juice. Fruit juice included 14.6 g of carbohydrate (CHO) in each 100 ml; cheese pie included 9.1 g protein, 36.2 g CHO, and 21.2 g fat in each 100 g; and tuna pie included 18.4 g protein, 36.2 g CHO, and 12.0 g fat in each 100 g. Participants were instructed to eat as much or as little as they wanted until comfortably full. Meals were weighed to the nearest 0.1 g on a digital scale before and after eating. The total amount of energy intake was calculated based on the manufacturers’ nutritional information and pies’ ingredients.
Statistical Analysis
Data were analyzed using SPSS version 21.0, IBM (Armonk, NY, USA). Due to the small sample size, nonparametric statistics were used and data for the main study outcomes are presented as the median (1st and 3rd) percentiles. All continuous variables were checked for normality using the Kolmogorov–Smirnov test. All the parameters was analyzed for tests for several related samples (time effect) and pairs samples by Friedman and Wilcoxon tests at baseline, Post-Ex, and 24-h Post-Ex for FR, DHR, and CON conditions. Furthermore, median change were analyzed by Kruskal–Wallis test and post hoc for several independent samples to check the median effect between FR, DHR, and CON conditions. Spearman’s correlation analysis was applied to check the association between change variables. A p value <.05 was considered statistically significant.
Results
Descriptive data demonstrated that oxygen consumption during FR and DHR were 29.89 ± 2.54 and 28.30 ± 2.36 ml/kg/min, which represents intensity at 61 ± 6% and 58 ± 5% VO2max for FR and DHR, respectively. Respiratory exchange ratio, CHO oxidation, fat oxidation, and energy expenditure for FR and DHR were respectively as follows: 0.85 ± 0.04 and 0.84 ± 0.04, 1.45 ± 0.51 and 1.29 ± 0.45 g/min, 0.54 ± 0.17 and 0.51 ± 0.14 g/min and 10.36 ± 1.72 and 9.51 ± 1.49 kcal/min.
The median (1st quartile–3rd quartile) percentiles for total ghrelin (pg/ml) and PP (pg/ml) are presented in Table 1. There were insignificant changes in both total ghrelin and PP concentrations for all conditions. There was no change or a decrease in total ghrelin for DHR and CON overtime, whereas there was a nonsignificant increase in Post-Ex value of total ghrelin concentration for FR (p = .063). Furthermore, condition differences according to median changes in concentrations were insignificant.
Table 1.
Median (Q1 and Q3) Percentile Responses of Appetite-Related Hormones at Pre-Ex, Post-Ex, and 24-h Post-Ex for All Conditions (FR, DHR, CON).
| Phase | Ghrelin
(pg/ml) (n = 12) |
PP (pg/ml) (n = 11) |
||||||
|---|---|---|---|---|---|---|---|---|
| FR | DHR | CON | p values | FR | DHR | CON | p values | |
| Pre-Ex | 39.4 (22.4–46.4) | 23.9 (9.4–38.2) | 34.9 (10.4–49.7) | 75.5 (27.1–129.5) | 58.2 (28.7–111.4) | 75.3 (25.3–105.7) | ||
| Post-Ex | 44.7 (24.8–52.2) | 22.5 (9.9–63.0) | 23.9 (9.5–45.9) | 39.6 (23.3–110.6) | 49.6 (13.9–88.6) | 66.4 (24.9–107.3) | ||
| 24-h Post-Ex | 21.0 (8.1–34.9) | 24.5 (6.7–42.8) | 10.1 (8.5–23.9) | 36.6 (22.2–58.8) | 56.5 (37.4–116.3) | 23.4 (9.1–43.3) | ||
| p values | .063 | .807 | .199 | .092 | .807 | .558 | ||
| Median change Pre-Ex with Post-Ex | 2.0 | −0.1 | −0.1 | .435 | −36.0 | 1.1 | −9.9 | .740 |
| Median change Pre-Ex with 24-h Post-Ex | −4.2 | 2.3 | −12.1 | .072 | −38.5 | 2.1 | −23.8 | .380 |
| Median change Post-Ex with 24-h Post-Ex | −11.6 | −3.2 | −1.5 | .739 | −2.6 | −7.8 | −15.1 | .808 |
Note. FR = front run; DHR = downhill run; CON = control rest; PP = pancreatic polypeptide.
As can be seen in Table 2, there were no significant changes in explicit liking appeal bias scores for high fat versus low fat foods, but the median change in explicit liking of sweet versus savory foods differed significantly between Pre-Ex with 24-h Post-Ex (p = .013). Post-hoc analysis indicated a significant difference in the Pre-Ex and 24-h Post-Ex change between FR and DHR (p = .023), suggesting greater liking of savory over sweet foods in DHR. Table 3 revealed that there were insignificant changes in both implicit wanting appeal bias scores for high fat versus low fat foods or sweet versus savory foods for all conditions (p > .05), and condition differences according to median changes were also insignificant (p > .05).
Table 2.
Median (Q1 and Q3) Percentile Responses at Pre-Ex, Post-Ex, and 24-h Post-Ex for All Conditions (FR, DHR, CON) for Explicit Liking Appeal Bias Scores of High Fat Versus Low Fat Foods and Sweet Versus Savory Foods.
| Phase | Explicit liking – Appeal fat bias (n = 8) | Explicit liking – Appeal taste bias (n = 8) | ||||||
|---|---|---|---|---|---|---|---|---|
| FR | DHR | CON | p values | FR | DHR | CON | p values | |
| Pre-Ex | −13.8 (−32.4–6.1) | −7.0 (−12.9–7.4) | 4.7 (−10.6–14.0) | −4.9 (−8.9–3.0) | 0.13 (−16.5–10.9) | −2.9 (−8.3–15.3) | ||
| Post-Ex | 1.5 (−19.5−7.4) | 1.0 (−19.5–9.0) | 2.9 (−6.0–18.6) | 1.2 (−24.3–17.5) | −5.5 (−14.0–13.8) | −5.2 (−16.3–10.0) | ||
| 24-h Post- Ex | −7.1 (−12.9–7.4) | 1.6 (−4.4–7.8) | 0.8 (−19.0–17.6) | −7.9 (13.2–10.6) | −10 (−26.8–10.8) | −2.3 (−18.3–12.9) | ||
| p values | .695 | .061 | .708 | .065 | .670 | .257 | ||
| Median change Pre-Ex with Post-Ex | −11.9 | 4.9 | 0.8 | .391 | 9.8 | −3.7 | −2.3 | .063 |
| Median change Pre-Ex with 24-h Post-Ex | 0.8 | 3.8 | 0.7 | .212 | 5.4 | −4.6* | −2.3 | .013 |
| Median change Post-Ex with 24-h Post-Ex | 12.4 | 1.8 | −2.3 | .314 | −3.7 | −1.7 | 1.4 | .553 |
Note. FR = front run; DHR = downhill run; CON = control rest. * indicates significant p value obtained from Friedman’s two-way ANOVA and Kruskal–Wallis test accordingly.
Table 3.
Median (Q1 and Q3) Percentile Responses at Pre-Ex, Post-Ex, and 24-h Post-Ex for All Conditions (FR, DHR, CON) for Implicit Wanting Appeal Bias Scores of High Fat Versus Low Fat Foods and Sweet Versus Savory Foods.
| Phase | Implicit wanting – Fat appeal
bias (n = 8) |
Implicit wanting – Taste appeal
bias (n = 8) |
||||||
|---|---|---|---|---|---|---|---|---|
| FR | DHR | CON | p values | FR | DHR | CON | p values | |
| Pre-Ex | −26.9 (−44.5–24.6) | 6.6 (−12.7–21.2) | 1.2 (−12.5–41.3) | −5.7 (−28.4–22.8) | −13.2 (−32.7–10.5) | −5.9 (−34.9–10.3) | ||
| Post-Ex | −18.3 (−28.1–62.6) | −10.2 (23.5–12.5) | 9.8 (−8.3–40.2) | −2.9 (−51.6–22.9) | −13.9 (−38.9–4.6) | −12.5 (−44–12.4) | ||
| 24-h Post-Ex | −3.03 (−32.5–12.9) | 1.9 (−14.8–35.3) | 11.2 (−19.4–41.1) | −7.5 (−38.7–23.6) | −14.1 (−35.8–15.7) | −5.8 (−38.1–14.5) | ||
| p values | .061 | .905 | .526 | .497 | .905 | .395 | ||
| Median change Pre-Ex with Post-Ex | 15.3 | −5.6 | 1.7 | .063 | 2.4 | −0.9 | 4.5 | .765 |
| Median change Pre-Ex with 24-h Post-Ex | 17.7 | 0.4 | −1.7 | .160 | 12.2 | 2.1 | 3.2 | .349 |
| Median change Post-Ex with 24-h Post-Ex | −11.6 | −4.8 | −2.3 | .618 | 2.4 | −7.5 | −0.2 | .519 |
Note. FR = front run; DHR = downhill run; CON = control rest.
Median responses in subjective appetite sensations for each condition can be seen in Table 4, and Figure 1 represents median responses of energy intake at Post-Ex and 24-h Post-Ex for all conditions. As can be seen, there were insignificant changes in median values of all conditions and time points. Furthermore, exercise-induced energy expenditure for FR and DHR (440 ± 70 and 398 ± 56 kcal) did not cause an increase in the Post-Ex energy intake, which means relative energy intake is lower than CON for both exercise conditions (FR and DHR).
Table 4.
Median (Q1 and Q3) Percentile Responses of Appetite Sensations at Pre-Ex, Post-Ex, and 24-h Post-Ex for All Conditions (FR, DHR, CON).
| Phase | Hunger
(mm) (n = 8) |
Fullness
(mm) (n = 8) |
Desire to eat
(mm) (n = 8) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FR | DHR | CON | p values | FR | DHR | CON | p values | FR | DHR | CON | p values | |
| Pre-Ex | 59.5 (30.0–89.0) | 70.5 (63.0–89.0) | 68.5 (54.0–81.0) | 48.0 (10.0–75.0) | 13.0 (4.0–35.0) | 10 (3.0–30.0) | 80.0 (42.0–100) | 75.5 (64.0–98.0) | 79.5 (50.0–89.5) | |||
| Post-Ex | 86.0 (57.6–98.5) | 89.0 (60.0–99.0) | 75.0 (48.0–95.0) | 29.0 (6.0–68.0) | 29.5 (6.5–40.0) | 18 (9.0–31.0) | 93.5 (63–99) | 84.5 (32.0–98.0) | 77.0 (74.0–95.0) | |||
| 24-h Post- Ex | 52.5 (22.0–94.5) | 72 (66.0–89.0) | 85.0 (73.0–89.0) | 40.5 (6.0−68) | 33.0 (11.0–37.0) | 13.0 (7.0–39) | 68.5 (38.5–98.5) | 82.5 (63.0–95.0) | 82.0 (74.0–89.0) | |||
| p values | .128 | .918 | .315 | .472 | .607 | .236 | .402 | .729 | .098 | |||
| Median change Pre-Ex with Post-Ex | 6.5 | 4.0 | −19.0 | .412 | −23.5 | −2.0 | −14.0 | .681 | 2.5 | 0.5 | −5.5 | .701 |
| Median change Pre-Ex with 24-h Post-Ex | 0.5 | −6.0 | −0.5 | .191 | −2.5 | −0.05 | −7.0 | .841 | 0.5 | −1.0 | 3.0 | .312 |
| Median change Post-Ex with 24-h Post-Ex | −7.0 | −6.0 | −2.0 | .081 | 14.0 | 1.0 | −3.0 | .413 | −7.5 | 4.5 | −3.0 | .072 |
Note. FR = front run; DHR = downhill run; CON = control rest.
Figure 1.
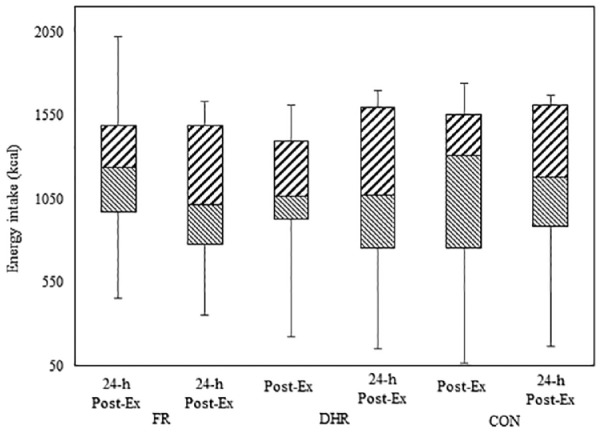
Median of energy intake (upper box is Q1 and lower box is Q3) of Post-Ex and 24-h Post-Ex at all conditions – front running (FR), downhill running (DHR), and control rest (CON) sessions. Error bar represents maximal and minimal values (n = 14).
Discussion
The principal finding from this study is that acute flat (concentric) and downhill (eccentric) running had no immediate or delayed effect (24-h) on total ghrelin or PP concentrations, subjective appetite sensations, food preference, or food intake. As such, the benefits of eccentric exercise can be utilized during exercise training program, and comparable impact with concentric exercise on eating behavior may be expected.
In contrast to the present study, it has previously been reported that total ghrelin can rise immediately after an exercise bout and decreases thereafter to reach a concentration lower than baseline levels, and this effect seems to be driven by the duration rather than intensity of exercise (Erdmann, Tahbaz, Lippl, Wagenpfeil, & Schusdziarra, 2007). Another study reported that acylated ghrelin was lower following concentric resistance exercise compared with both control (nonexercise) and aerobic exercise session (Balaguera-Cortes, Wallman, Fairchild, & Guelfi, 2011). However, a reduction in ghrelin and subjective hunger has also been reported after acute aerobic and resistance exercise compared with control session (Broom, Batterham, King, & Stensel, 2009). PP has been reported to increase significantly after resistance and aerobic exercise compared with control, but this did influence postexercise energy intake (Balaguera-Cortes et al., 2011).
Whether changes in eating behavior or appetite-related hormones are influenced by the differing metabolic responses or degree of muscle damage following concentric or eccentric muscular contraction has not been specifically studied. It has previously been reported that the acute effects of muscle-damaging eccentric exercise (downhill running) and concentric exercise (flat running) did not differ in terms of their effect glucose tolerance or insulin during a 2-hr 75-g oral glucose tolerance test (Cook, Myers, Kelly, & Willems, 2015). However, despite again reporting no differences in glucose tolerance following acute uphill (concentric) and downhill (eccentric) walking, an increase in interleukin-6 and a decrease in tumor necrosis factor alpha concentrations were seen immediately and 24 h following downhill but not uphill walking in healthy but sedentary females (Philippe, Junker, Gatterer, Melmer, & Burtscher, 2016). Furthermore, a recent review reported lower responses of growth hormone and accumulated lactate after eccentric compared to concentric exercise, but comparable response of testosterone, insulin, and cortisol. It was concluded that hormonal responses after eccentric exercise are largely influenced by a combination of load and time under tension rather than contraction type (Douglas et al., 2017). In the present study, no changes in total ghrelin or PP concentrations were seen between conditions, but given the sample size and the fact that fluid volume shifts were not accounted for in these values, further research is needed to examine these responses.
In the present study, there were no differences in subjective appetite between conditions and no consistent changes in food reward. There was a difference between FR and DHR in the median change score (Pre-Ex to 24-h Post-Ex) for the explicit liking for sweet versus savory foods, but given this was an isolated finding in a small sample it should be approached with caution. It is still unclear how exercise mode (e.g., aerobic vs. resistance) and contraction type (e.g., concentric vs. eccentric) influences appetite and food intake. For example, it has been reported that acute resistance exercise did not affect postexercise energy intake (Cadieux, McNeil, Lapierre, Riou, & Doucet, 2014), but the preference for high fat foods has been shown to decrease after acute resistance exercise to a greater extent than aerobic exercise (McNeil et al., 2015). In the longer term, subjective fullness has been shown to increase following 12 weeks of aerobic training but not resistance training in sedentary men who are overweight and obese (Guelfi, Donges, & Duffield, 2013). This highlights the need to not only examine the effect of exercise mode on appetite and food intake, but to do so under chronic rather than acute exercise conditions.
Absolute energy intake immediately or 24 h after FR, DHR, or CON did not differ between conditions. As such, when relative energy intake was calculated, there was no evidence of compensation in energy intake for the exercise-induced energy expenditure of FR or DHR as compared to the control condition. While the energy expenditure of FR and DHR differed (10.36 ± 1.72 and 9.51 ± 1.49 kcal/min), these findings are in line with a recent meta-analysis which reported that individuals tended not to compensate for energy expenditure in a few hours after exercise by altering food intake (Schubert, Desbrow, Sabapathy, & Leveritt, 2013), particularly among active individuals (Rocha, Paxman, Dalton, Winter, & Broom, 2015). The short duration of exercise in the current study may also contribute to the current outcome. For example, energy intake increased after long duration at 120 min compared with lower durations at 30 and 60 min (Erdmann et al., 2007). Thus, DHR can be utilized in exercise training, with no expected increase in energy intake after exercise.
The strengths of this study included the use of different instruments of eating behavior to better understand the responses of homeostasis and nonhomeostasis appetite sensations and food preferences. Control resting day condition was also important to compare the outcome of eccentric condition with rest and concentric condition. However, the small sample size, which was compounded by missing data, should clearly be acknowledged when interpreting findings. Furthermore, food consumption was not controlled during the 24 h period after FR, DHR, or CON. This is important because it was found that specific nutrition after eccentric exercise can help reducing muscle damage and soreness (Gavin, Myers, & Willems, 2015; Machin et al., 2014).
Conclusion
Appetite sensations, appetite-related hormones, and food preference and reward are not effected by acute eccentric exercise among moderately active young men. Thus, the benefits of eccentric exercise can be utilized with no expected impact on postexercise eating behavior. Future studies should reexamine the current hypothesis using a larger and heterogeneous sample and chronic exercise training rather than acute exercise.
Acknowledgments
The authors thank all participants, the Cardiovascular Laboratory, and all research assistants, particularly Mr. Abdullah Al-Qawati and Mr. Thabit Al-Aizari. Blood samples were collected by Mr. Abdul Aziz Alsahali from the Department of Clinical Laboratory Sciences at College of Applied Medical Sciences at KSU, and were analyzed by experts in the field of human biomarkers at Prince Mutaib bin Abdullah Chair for Biomarkers Research on Osteoporosis, Faculty of Science, KSU. The authors also thank Prof. Mark Willems from the University of Chichester for providing a consultation of downhill running.
Footnotes
Authors’ Contributions: SA, AD, and MH designed the study; SA supervised the data collection process, and all authors contributed to data management, and SA and MH performed statistical analysis. SA wrote the first draft of manuscript and AD and MH revised some sections. All authors approved the final draft for publication submission.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No (RG-1439-82).
ORCID iD: Shaea Alkahtani  https://orcid.org/0000-0002-1443-6249
https://orcid.org/0000-0002-1443-6249
Availability of data and materials: All raw data presented in the manuscript are freely available to any scientist wishing to use them for non-commercial purposes, without breaching participants’ confidentiality, and with a condition that IRB office at King Saud University does not have any objections.
References
- Alkahtani S. A., Byrne N. M., Hills A. P., King N. A. (2014). Interval training intensity affects energy intake compensation in obese men. International Journal of Sport Nutrition and Exercise Metabolism, 24(6), 595–604. [DOI] [PubMed] [Google Scholar]
- Alkahtani S. A., Dalton M., Abuzaid O., Obeid O., Finlayson G. (2016). Validation of the Leeds food preference questionnaire in Arabs. Asia Pacific Journal of Clinical Nutrition, 25(2), 257–264. [DOI] [PubMed] [Google Scholar]
- Balaguera-Cortes L., Wallman K. E., Fairchild T. J., Guelfi K. J. (2011). Energy intake and appetite-related hormones following acute aerobic and resistance exercise. Applied Physiology, Nutrition, and Metabolism, 36(6), 958–966. [DOI] [PubMed] [Google Scholar]
- Broom D. R., Batterham R. L., King J. A., Stensel D. J. (2009). Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. American Journal of Physiology-Regulatory, Integrative, and Comparative Physiology, 296(1), R29–R35. doi: 10.1152/ajpregu.90706.2008 [DOI] [PubMed] [Google Scholar]
- Cadieux S., McNeil J., Lapierre M. P., Riou M.-È., Doucet É. (2014). Resistance and aerobic exercises do not affect post-exercise energy compensation in normal weight men and women. Physiology & Behavior, 130, 113–119. [DOI] [PubMed] [Google Scholar]
- Clarkson P. M., Hubal M. J. (2002). Exercise-induced muscle damage in humans. American Journal of Physical Medicine & Rehabilitation, 81(11 Suppl), S52–S69. doi: 10.1097/00002060-200211001-00007 [DOI] [PubMed] [Google Scholar]
- Cook M. D., Myers S. D., Kelly J. S. M., Willems M. E. T. (2015). Acute postexercise effects of concentric and eccentric exercise on glucose tolerance. International Journal of Sport Nutrition and Exercise Metabolism, 25(1), 14–19. doi: 10.1123/ijsnem.2013-0261 [DOI] [PubMed] [Google Scholar]
- Douglas J., Pearson S., Ross A., McGuigan M. (2017). Eccentric exercise: Physiological characteristics and acute responses. Sports Medicine, 47(4), 663–675. [DOI] [PubMed] [Google Scholar]
- Erdmann J., Tahbaz R., Lippl F., Wagenpfeil S., Schusdziarra V. (2007). Plasma ghrelin levels during exercise—Effects of intensity and duration. Regulatory Peptides, 143(1–3), 127–135. doi: 10.1016/j.regpep.2007.05.002 [DOI] [PubMed] [Google Scholar]
- Finlayson G., King N., Blundell J. E. (2007). Liking vs. wanting food: Importance for human appetite control and weight regulation. Neuroscience & Biobehavioral Reviews, 31(7), 987–1002. doi: 10.1016/j.neubiorev.2007.03.004 [DOI] [PubMed] [Google Scholar]
- Franchi M. V., Reeves N. D., Narici M. V. (2017). Skeletal muscle remodeling in response to eccentric vs. concentric loading: Morphological, molecular, and metabolic adaptations. Frontiers in Physiology, 8, 447. doi: 10.3389/fphys.2017.00447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin J. P., Myers S. D., Willems M. E. T. (2015). Neuromuscular responses to mild-muscle damaging eccentric exercise in a low glycogen state. Journal of Electromyography and Kinesiology, 25(1), 53–60. [DOI] [PubMed] [Google Scholar]
- Gluchowski A., Harris N., Dulson D., Cronin J. (2015). Chronic eccentric exercise and the older adult. Sports Medicine, 45, 1413–1430. doi: 10.1007/s40279-015-0373-0 [DOI] [PubMed] [Google Scholar]
- Guelfi K. J., Donges C. E., Duffield R. (2013). Beneficial effects of 12 weeks of aerobic compared with resistance exercise training on perceived appetite in previously sedentary overweight and obese men. Metabolism, 62(2), 235–243. [DOI] [PubMed] [Google Scholar]
- Hackney K. J., Engels H.-J., Gretebeck R. J. (2008). Resting energy expenditure and delayed-onset muscle soreness after full-body resistance training with an eccentric concentration. Journal of Strength and Conditioning Research, 22(5), 1602–1609. doi: 10.1519/jsc.0b013e31818222c5 [DOI] [PubMed] [Google Scholar]
- Jamurtas A. Z., Garyfallopoulou A., Theodorou A. A., Zalavras A., Paschalis V., Deli C. K., , . . . Koutedakis Y. (2013). A single bout of downhill running transiently increases HOMA-IR without altering adipokine response in healthy adult women. European Journal of Applied Physiology, 113(12), 2925–2932. doi: 10.1007/s00421-013-2717-5 [DOI] [PubMed] [Google Scholar]
- Julian V., Thivel D., Costes F., Touron J., Boirie Y., Pereira B., , . . . Richard R. (2018). Eccentric training improves body composition by inducing mechanical and metabolic adaptations: A promising approach for overweight and obese individuals. Frontiers in Physiology, 9, 1013. doi: 10.3389/fphys.2018.01013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano H., Mineta M., Asaka M., Miyashita M., Numao S., Gando Y., , . . . Higuchi M. (2013). Effects of different modes of exercise on appetite and appetite-regulating hormones. Appetite, 66, 26–33. doi: 10.1016/j.appet.2013.01.017 [DOI] [PubMed] [Google Scholar]
- Kraemer R. R., Castracane V. D. (2015). Endocrine alterations from concentric vs. eccentric muscle actions: A brief review. Metabolism, 64(2), 190–201. [DOI] [PubMed] [Google Scholar]
- Machin D. R., Christmas K. M., Chou T.-H., Hill S. C., Van Pelt D. W., Trombold J. R., Coyle E. F. (2014). Effects of differing dosages of pomegranate juice supplementation after eccentric exercise. Physiology Journal, 2014, 1–7. [Google Scholar]
- McNeil J., Cadieux S., Finlayson G., Blundell J. E., Doucet É. (2015). The effects of a single bout of aerobic or resistance exercise on food reward. Appetite, 84, 264–270. doi: 10.1016/j.appet.2014.10.018 [DOI] [PubMed] [Google Scholar]
- Paschalis V., Nikolaidis M. G., Theodorou A. A., Panayiotou G., Fatouros I. G., Koutedakis Y., Jamurtas A. Z. (2011). A weekly bout of eccentric exercise is sufficient to induce health-promoting effects. Medicine & Science in Sports & Exercise, 43(1), 64–73. doi: 10.1249/mss.0b013e3181e91d90 [DOI] [PubMed] [Google Scholar]
- Philippe M., Junker G., Gatterer H., Melmer A., Burtscher M. (2016). Acute effects of concentric and eccentric exercise matched for energy expenditure on glucose metabolism in healthy females: A randomized crossover trial. SpringerPlus, 5(1), 1455. doi: 10.1186/s40064-016-3062-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj I. S., Bird S. R., Westfold B. A., Shield A. J. (2012). Effects of eccentrically biased versus conventional weight training in older adults. Medicine & Science in Sports & Exercise, 44(6), 1167–1176. doi: 10.1249/mss.0b013e3182442ecd [DOI] [PubMed] [Google Scholar]
- Rocha J., Paxman J., Dalton C., Winter E., Broom D. (2015). Effects of an acute bout of aerobic exercise on immediate and subsequent three-day food intake and energy expenditure in active and inactive pre-menopausal women taking oral contraceptives. Appetite, 89, 183–191. doi: 10.1016/j.appet.2015.02.005 [DOI] [PubMed] [Google Scholar]
- Rogers P. J., Hardman C. A. (2015). Food reward. What it is and how to measure it. Appetite, 90, 1–15. doi: 10.1016/j.appet.2015.02.032 [DOI] [PubMed] [Google Scholar]
- Roig M., O’Brien K., Kirk G., Murray R., McKinnon P., Shadgan B., Reid W. D. (2008). The effects of eccentric versus concentric resistance training on muscle strength and mass in healthy adults: A systematic review with meta-analyses. British Journal of Sports Medicine, 43(8), 556–568. [DOI] [PubMed] [Google Scholar]
- Sadoul B. C., Schuring E. A. H., Mela D. J., Peters H. P. F. (2014). The relationship between appetite scores and subsequent energy intake: An analysis based on 23 randomized controlled studies. Appetite, 83, 153–159. [DOI] [PubMed] [Google Scholar]
- Schubert M. M., Desbrow B., Sabapathy S., Leveritt M. (2013). Acute exercise and subsequent energy intake. A meta-analysis. Appetite, 63, 92–104. doi: 10.1016/j.appet.2012.12.010 [DOI] [PubMed] [Google Scholar]
- Stubbs J., Ferres S., Horgan G. (2000). Energy density of foods: Effects on energy intake. Critical Reviews in Food Science and Nutrition, 40(6), 481–515. [DOI] [PubMed] [Google Scholar]
- Tidball J. G. (2011). Mechanisms of muscle injury, repair, and regeneration. Comprehensive Physiology, 1(4), 2029–2062. doi: 10.1002/cphy.c100092 [DOI] [PubMed] [Google Scholar]
- Vikne H., Refsnes P. E., Ekmark M., Medbø J. I., Gundersen V., Gundersen K. (2006). Muscular performance after concentric and eccentric exercise in trained men. Medicine and Science in Sports and Exercise, 38(10), 1770–1781. doi: 10.1249/01.mss.0000229568.17284.ab [DOI] [PubMed] [Google Scholar]


