Abstract
Purpose
Since thrombectomy has become a standard treatment technique for stroke, there is great demand for well-trained interventionalists. We offer practical courses on both silicone models and porcine models, and conducted a survey to evaluate whether ex vivo training models could replace in vivo models in the future.
Methods
In total, 110 neurointerventionalists participating in 30 training courses were included in our survey using a semi-structured questionnaire.
Results
The level of experience in thrombectomy maneuvers was almost balanced in our sample (52% experienced and 48% less-experienced participants). Silicone models were regarded as useful training tools regardless of the participants' experience (p = 1.000): 94% of less-experienced and 92% of experienced participants considered a silicone model to be a useful introduction for training with animal models. Of the participants, 95% indicated that training on animal models was helpful and necessary, even if they already had experience in performing interventions in humans (p = 1.000). After joining this course, 97% of all participants felt well prepared to perform thrombectomies in humans.
Conclusion
Even experienced participants benefit from silicone models. Silicone models are a good preparation for animal models but cannot replace them. Categorizing participants depending on their experience and their individual needs before practical training may allow for more efficient endovascular training.
Keywords: Animal model, endovascular neurointervention, silicone model, stroke, training
Introduction
Ischaemic stroke is the most frequent cause of disability in adulthood and one of the most frequent causes of death worldwide.1,2 Rapid and appropriate stroke treatment is crucial for therapeutic success.2,3 Since endovascular stroke therapy has been established as the standard treatment for large vessel occlusion stroke, there is great demand for neurointerventionalists. Neuroendovascular interventions represent one of the most complex and highest-risk procedures in medicine. Hence, effective and efficient endovascular training programmes are of major importance.4 In clinical practice, interventionalists are often trained on patients, a practice that is not only outdated but also dangerous: basic abilities and more sophisticated techniques should be trained in models.5,6 Today, sophisticated digital simulators, in vitro models and animal models allow for effective training before getting in contact with a patient.4,5,7–10
Our Department of Diagnostic and Interventional Neuroradiology, which has provided endovascular stroke treatment courses since 2011, is one of the leading national training centres with more than 30 interventional courses per year. Our courses include training on digital simulators, silicone models and animal models. The objectives of our courses are to acquire basic and advanced skills of material handling and endovascular techniques, depending on the course and the participants' experience.
According to the ‘3Rs principle’ (refinement, reduction and replacement) of laboratory animal science, we intend to reduce and replace our animal models by alternative models. This is why we evaluated our practical training on the issue regarding whether in vitro models could replace in vivo models. To this aim, we systematically assessed the subjective evaluations of novice and experienced participants with a postcourse questionnaire. We hypothesized that inexperienced attendees in particular profit from silicone models and feel better prepared for daily clinical practice after joining the courses, whereas experienced attendees would profit more from the in vivo model.
Materials and methods
In vitro silicone model
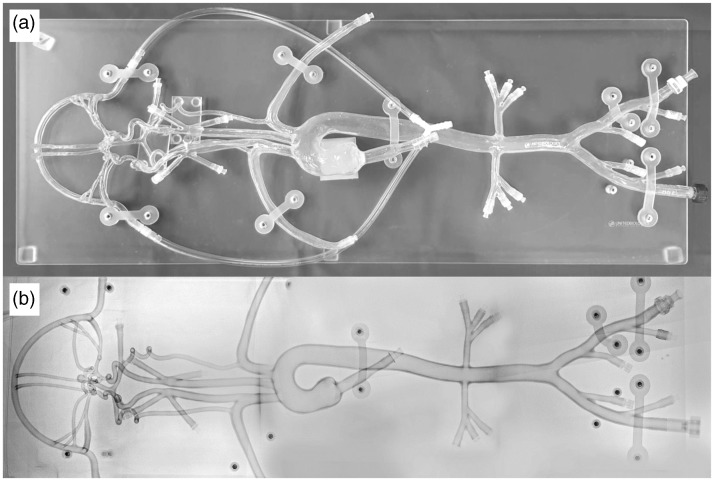
A silicone model (Neuro Testing Model Plus, United Biologics, Santa Ana, California) was incorporated into practical training for simulation of endovascular treatment procedures (Figure 1). This model mimics the human vasculature and is designed as a reconfigurable neuro-testing platform, with the Circle of Willis and aneurysms at the most common locations. The aortic branching consists of an anomalous common origin of the innominate and left common carotid artery. Another innominate artery allows training on difficult scenarios for carotid stenting. The model is made of soft and transparent silicone, which allows direct visualization without the need for fluoroscopic control. Nevertheless, endovascular techniques can be performed under fluoroscopic control as well. The model is filled with distilled water mixed with a small amount of shampoo to reduce friction. Systemic circulation is simulated by a pulsatile pump.
Figure 1.
Silicone model.
In vitro silicone model for the simulation of endovascular treatment procedures with direct visualization (a) and under fluoroscopic control (b).
In vivo porcine stroke model
Animal training was performed on female Landrace swine in accordance with the (blinded) legislation governing animal studies following the ‘Guide for the Care and Use of Laboratory Animals’ (National Research Council, 8th edition, 2011) and the ‘Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes’ (European Union Official Journal, 2010). Official permission was granted from the governmental animal care and use office (blinded).
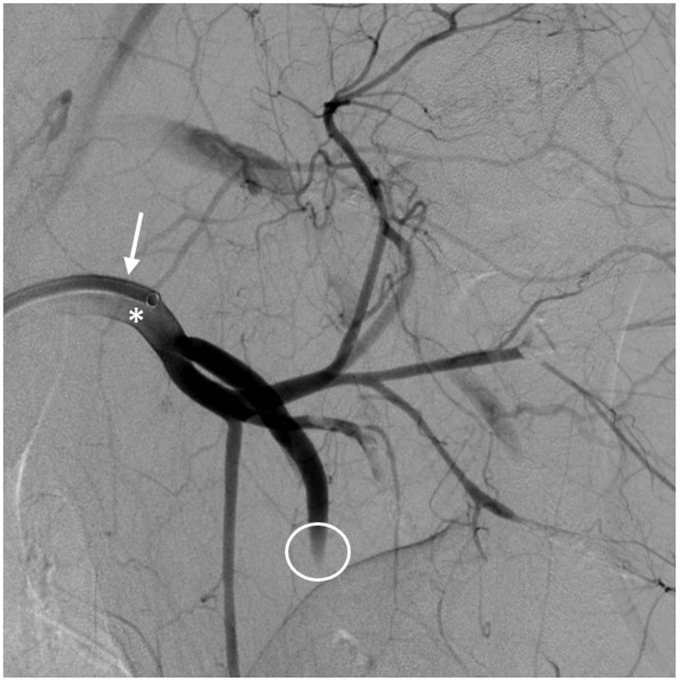
The animals receive premedication by intramuscular injection of atropine (Atropinsulfat, B. Braun Melsungen AG, Melsungen, Germany), azaperone (Stresnil ad usum veterinarium (ad. us. vet.); Sanochemia Pharmazeutika AG, Neufeld, Austria) and ketamine (10% Ketavet ad usum veterinarium (ad. us. vet.), Zoetis Deutschland GmbH, Berlin, Germany). The animals are intubated and mechanically ventilated with an oxygen–air mixture. Anaesthesia is maintained using either an oxygen–isoflurane mixture (isofluran 1.5 vol% and oxygen 1L/min) or continuous intravenous infusion with propofol (propofol 2% MCT Fresenius; Fresenius Kabi Deutschland GmbH, Neuss, Germany). To prevent blood coagulation during interventions, the animals are treated with aspirin (500 mg) and heparin (3000 IU, heparin-natrium-5000; Ratiopharm GmbH, Ulm, Germany) intravenously. For analgesia, fentanyl (Fentanyl-Janssen 0,5mg, Janssen-Cilag GmbH, Neuss, Germany) is continuously administered. The animals are positioned on the table in a supine position and are punctured at the femoral artery. Afterwards, we insert an 8F sheath. Due to the rete mirabile, it is not possible to induce stroke in intracerebral vessels of the swine.11 Therefore, endovascular techniques are performed in the carotid arteries and in the arteries of the forelimb (Figure 2). We synthesize barium sulphate-marked clots consisting of the pig's blood using a chandler loop and inject them for selective thromboembolization. Training conditions are made to be as realistic as possible: training takes place in our angiography suites or operating rooms, with the same set-up as in a clinical environment.
Figure 2.
Porcine animal stroke model.
Digital subtraction angiography via an access catheter (arrow) that is placed in the left subclavian artery (asterisk). There is thromboembolic occlusion of one of the main branches (circle).
Practical training
The participants came from various European and non-European countries, and were assistant or senior physicians with various degrees of proficiency in endovascular techniques. The training programme took place on either one or two consecutive days, and includes a theoretical and a practical part. Before training on the animal model, trainees usually applied their (newly acquired) knowledge on the silicone model.
On 1-day courses, the participants could alternately spend time on the silicone or the animal model depending on their experience and knowledge. On 2-day courses, the theoretical part and training on silicone models took place on the first day, followed by a full day working on the animal model under realistic conditions. For both the silicone and the animal model, an experienced interventionalist from our department served as a supervisor, giving individualized instructions regarding tool manipulation in angiography and thrombectomy manoeuvres.
Survey
A standardized questionnaire was given to the participants after the course. We designed a semi-structured questionnaire in three languages: German, English and Russian. The questionnaire was blinded for personal data and contained open- and close-ended questions. The first part contained demographic questions referring to gender, professional position and associated department. Next, information about the number of performed or assisted endovascular procedures, and previous experience in training with both silicone and animal models, was evaluated. Participants were further asked if they found it helpful to train on animal models, if silicone models are a good introduction to animal models and which model they would have wanted to spend more time on. Finally, we asked how well the course prepares individuals for performing endovascular interventions on patients. After an interim analysis, we modified the questionnaire and added the following items: the participants are also asked (a) if they felt well prepared to perform thrombectomies in patients before joining the course, (b) if animal models are replaceable by silicone models, and (c) whether the training on silicone and animal models is useful before performing thrombectomies in humans.
Data and statistical analysis
We used Chi-square and Fisher's exact tests for our statistical analysis. Participants were divided into two groups according to their previous experience: the novice group had assisted in less than 10 interventions and had performed no endovascular procedures on their own. The advanced group consisted of those who had assisted in more than 10 interventions or had already performed endovascular procedures on their own.
All statistical analyses were calculated in SPSS V.23 software (IBM, Armonk, New York, USA) and p-values of an α level ≤ 0.05 were considered statistically significant.
Results
A total of 110 participants returned the questionnaire, of which 62 (56%) were fully filled out. The questionnaire was filled out by 23 female and 85 male participants; two attendees did not specify their gender. We received 1 response from a student, 33 responses from assistants and 65 responses from senior physicians. The remaining 11 participants provided no information on their position. In total, 39 of the participants were from neurosurgery, 43 from neuroradiology and 24 from radiology departments. Four participants did not specify their department.
We defined 57 (52%) and 53 (48%) of the participants as advanced and novice interventionalists, respectively. Table 1 provides the results of all participants in total, with a comparison between novice and advanced learners.
Table 1.
Comparison items between novice and advanced interventionalists. Agreement with item in the left column in relative and absolute numbers. The p-value refers to comparison between novice and advanced participants.
| Participant indicated that | Total | Novice | Advanced | p |
|---|---|---|---|---|
| He/she was well prepared for patient care before the course | 74% (23/31) | 56% (10/18) | 100% (13/13) | 0.010 |
| He/she had previous experience with silicone models | 66% (72/109) | 47% (25/53) | 84% (47/56) | <0.001 |
| He/she had previous experience with animal models | 62% (47/75) | 30% (10/33) | 89% (34/42) | <0.001 |
| Silicone models are a good preparation for patient care | 80% (82/103) | 84% (43/51) | 75% (39/52) | 0.241 |
| Silicone models are a good preparation for animal models | 93% (28/30) | 94% (17/18) | 92% (11/12) | <0.001 |
| Animal models are a good preparation for patient care | 97% (30/31) | 94% (17/18) | 100% (13/13) | <0.001 |
| Silicone models can replace animal models | 3% (1/30) | 0% (0/17) | 8% (1/13) | 0.433 |
| It was helpful to train on animal models even if they had already performed thrombectomies in humans | 95% (92/97) | 95% (38/40) | 95% (54/57) | 0.664 |
| He/she wanted to spend more time on the animal model | 77% (84/109) | 79% (42/53) | 75% (42/56) | 0.381 |
| He/she wanted to spend more time on the silicone model | 7% (8/109) | 9% (5/53) | 5% (3/56) | 0.381 |
| He/she felt well prepared for patient care after the course | 97% (103/106) | 94% (49/52) | 100% (54/54) | 0.115 |
In total, 84% of the novice and 75% of the advanced interventionalists indicated that they considered silicone models to be a good introduction to patient care. In addition, 65% of the novices and 46% of the experienced participants substantiated their answer regarding the possibility of using silicone models to train basic skills. In total, 14% of novices and 10% of the experienced interventionalists stated that silicone models allow realistic training; however, 75% of novices and 85% of the experienced participants indicated that the use of silicone models was not good preparation for patient care since silicone models do not allow for realistic training conditions.
Regarding the open-ended question regarding whether animal models are a good preparation for patient care, 94% of novices and 100% of the advanced participants agreed, with 88% of novices and 69% of the experienced participants reasoning that training on animal models best represents reality.
Silicone models were considered to be good preparation for animal models by 94% of the novices and 92% of the advanced participants. The majority (81% of novices and 55% of experienced participants) explained their answer with the fact that training on animal models is more effective if basic skills are trained on silicone models first.
The statement that silicone models can replace animal models was agreed with by one (8%) of the advanced interventionalists but none (0%) of the novice interventionalists agreed. As a justification of their views, 76% of the novices and 62% of the advanced participants indicated that silicone models cannot replace animal models because they do not enable training under realistic conditions.
Discussion
We intend to optimize our training programme and to reduce the number of animals needed by replacing them with silicone models. With this aim, we assessed to what degree silicone models can replace animal models in our training programme by evaluating our participants' subjective point of view. The vast majority of participants indicated that they felt better prepared for daily clinical practice after joining our training programme using both in vitro and in vivo models. This is most likely due to the combination of silicone models and animal models. The specific advantages and disadvantages of the respective models complement each other:
Silicone models are suitable for basic training purposes. They allow for safe and reproducible test conditions.12 Because of their transparency, silicone models allow direct observation without the need for fluoroscopy, and allow basic skills and new techniques to be learned with conscious orientation of hand-eye coordination without the application of contrast agents (and radiation).4,9 As a one-time purchase they are very cost-efficient.13 An additional benefit is the reduction of animal experiments.
One of the major disadvantages of silicone models is the absence of biological conditions, such as friction, haemodynamics, haemostasis and thrombosis. There is also a lack of fragility and spastic reaction of the arteries, and complication management is not possible (e.g. dissection or rupture of the vessel wall).14
Pigs are suitable in vivo models for endovascular stroke training, due to there being similar haemodynamic and haemostatic conditions as in humans.7,8,11,15,16 Vessel sizes are comparable to the intracranial setting in humans, which allows for the use of standard-sized devices. The autologous blood thrombus, enriched by a contrast agent, enables visualization of the interaction with devices, and possible dislocation or fragmentation during angiography.14 In contrast to silicone models, complication management is possible and the training setting is more realistic, allowing for ‘fine-tuning’ of skills and competences in realistic settings. Drawbacks are ethical considerations, the high purchase costs and low reproducibility.
There are also digital simulation systems (in silico models) that imitate human anatomy and physiology.17 Virtual simulation systems allow for the standardized assessment of skills by recording all procedures during the standardized scenarios.18,19 However, comparably high acquisition costs and the need for optimization of the degree of realism (for example force feedback, or implemented materials and techniques) are disadvantages that need to be addressed.20,21
In summary, simulations provide a risk-free environment, which is beneficial for both the patient and the learner.20,21)
Only a few studies to date have evaluated the efficiency of virtual simulators in endovascular training and provided evidence of a significant improvement in simulator performance.20–23 In a study by Berry et al.,24 the cumulative benefits of endovascular training on simulators and pigs were compared in endovascular novices. Both training methods turned out to improve endovascular skills equivalently, but the total scores were significantly higher at the simulator sessions, which attests that training on pigs is more challenging. As in our study, the participants favoured porcine experience over simulation experience because of experiencing the real intervention in a more realistic environment. In their comparison of computer-based simulators and animal models, Dayal et al.7 showed that it was mainly novice neurointerventionalists that benefitted from training with simulators. Remarkably, in our study, not only novices but also the majority of advanced interventionalists indicated that they had benefitted from silicone models and that they were a good introduction for training on animal models. The majority of participants indicated that this was due to the possibility of practicing new techniques in a simplified setting (silicone model) before applying them in a more realistic animal model.
Concerning animal models, both novices and advanced interventionalists indicated equally that they profited from animal models and that an in vivo model cannot be completely replaced. This is consistent with data from Berry et al., who indicated that their participants did not think that training on a porcine model can be replaced.24 However, our results demonstrate that even if animal models cannot be replaced completely, initial appropriate and intensive training on silicone models can help to reduce animal experiments and make training more efficient. This implies that basic skills and new techniques should be learned first using silicone models and computer simulations, and that training should then be intensified in in vivo models.
Limitations
The transferability of simulation-learnt skills to actual clinical skills is a major limitation of simulation-based programmes and has not been addressed comprehensively in the literature.20 Only one study has indicated that endovascular skills trained on simulators can improve clinical skills.25 However, there is evidence from simulator training in other medical fields that shows that simulator training translates into clinical skills.26–29
Conclusion
Interventionalists benefit from practical training with silicone and animal models regardless of their experience. Silicone models can reduce but not replace the use of animal models. Before training, participants should be categorized depending on their experience and their individual needs, so that new techniques can be trained in a simplified in vitro model before being applied in a more realistic animal model.
Contributorship statement
Conception and design: all authors. Acquisition of data: all authors. Analysis and interpretation of data: all authors. Drafting of the original article: Johanna Sandmann. Critically revising the article: all authors.
Ethical standards
The animal studies were approved by the appropriate ethics committee and were therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MW: grants, Stryker Neurovascular and Siemens Healthcare; personal fees, Stryker Neurovascular, Silkroad Medical, Siemens Healthcare and Bracco; and non-financial support, Codman Neurovascular, Covidien, Abbott, St. Jude Medical, Phenox, Penumbra, Microvention/Terumo, B. Braun, Bayer, Acandis and ab medica. The other authors declare they have no conflict of interest.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Meyers PM, Schumacher HC, Connolly ES, et al. Current status of endovascular stroke treatment. Circulation 2011; 123: 2591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moussaddy A, Demchuk AM, Hill MD. Thrombolytic therapies for ischemic stroke: Triumphs and future challenges. Neuropharmacology 2018; 134: 272–279. [DOI] [PubMed] [Google Scholar]
- 3.Khatri P, Abruzzo T, Yeatts SD, et al. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology 2009; 73: 1066–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paramasivam S, Baltsavias G, Psatha E, et al. Silicone models as basic training and research aid in endovascular neurointervention–a single-center experience and review of the literature. Neurosurg Rev 2014; 37: 331–337. [DOI] [PubMed] [Google Scholar]
- 5.Liebig T, Holtmannspotter M, Crossley R, et al. Metric-based virtual reality simulation: A paradigm shift in training for mechanical thrombectomy in acute stroke. Stroke 2018; 49: e239–e242. [DOI] [PubMed] [Google Scholar]
- 6.Bridges M, Diamond DL. The financial impact of teaching surgical residents in the operating room. Am J Surg 1999; 177: 28–32. [DOI] [PubMed] [Google Scholar]
- 7.Dayal R, Faries PL, Lin SC, et al. Computer simulation as a component of catheter-based training. J Vasc Surg 2004; 40: 1112–1117. [DOI] [PubMed] [Google Scholar]
- 8.Hsu JH, Younan D, Pandalai S, et al. Use of computer simulation for determining endovascular skill levels in a carotid stenting model. J Vasc Surg 2004; 40: 1118–1125. [DOI] [PubMed] [Google Scholar]
- 9.Namba K, Mashio K, Kawamura Y, et al. Swine hybrid aneurysm model for endovascular surgery training. Interv Neuroradiol 2013; 19: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki Y, Fujitsuka M, Chaloupka JC. Simulation of endovascular neurointervention using silicone models: Imaging and manipulation. Neurol Med Chir (Tokyo) 2005; 45: 567–572. discussion 72–73. [DOI] [PubMed] [Google Scholar]
- 11.Burbridge B, Matte G, Remedios A. Complex intracranial arterial anatomy in swine is unsuitable for cerebral infarction projects. Can Assoc Radiol J 2004; 55: 326–329. [PubMed] [Google Scholar]
- 12.Sugiu K, Tokunaga K, Sasahara W, et al. Training in neurovascular intervention usefulness of in-vitro model and clinical practice. Interv Neuroradiol 2004; 10: 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneko N, Mashiko T, Ohnishi T, et al. Manufacture of patient-specific vascular replicas for endovascular simulation using fast, low-cost method. Sci Rep 2016; 6: 39168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gralla J, Schroth G, Remonda L, et al. A dedicated animal model for mechanical thrombectomy in acute stroke. AJNR Am J Neuroradiol 2006; 27: 1357–1361. [PMC free article] [PubMed] [Google Scholar]
- 15.Crisóstomo V, Sun F, Maynar M, et al. Common swine models of cardiovascular disease for research and training. Lab Anim (NY) 2016; 45: 67–74. [DOI] [PubMed] [Google Scholar]
- 16.Arikan F, Martinez-Valverde T, Sanchez-Guerrero A, et al. Malignant infarction of the middle cerebral artery in a porcine model. A pilot study. PLoS One 2017; 12: e0172637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson DL, Meyer J, Lee ES, et al. Training with simulation improves residents' endovascular procedure skills. J Vasc Surg 2007; 45: 149–154. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal R, Black SA, Hance JR, et al. Virtual reality simulation training can improve inexperienced surgeons' endovascular skills. Eur J Vasc Endovasc Surg 2006; 31: 588–593. [DOI] [PubMed] [Google Scholar]
- 19.Aggarwal R, Moorthy K, Darzi A. Laparoscopic skills training and assessment. Br J Surg 2004; 91: 1549–1558. [DOI] [PubMed] [Google Scholar]
- 20.Fargen KM, Arthur AS, Bendok BR, et al. Experience with a simulator-based angiography course for neurosurgical residents: Beyond a pilot program. Neurosurgery 2013; 73: 46–50. [DOI] [PubMed] [Google Scholar]
- 21.Spiotta AM, Rasmussen PA, Masaryk TJ, et al. Simulated diagnostic cerebral angiography in neurosurgical training: A pilot program. J Neurointerv Surg 2013; 5: 376–381. [DOI] [PubMed] [Google Scholar]
- 22.Fargen KM, Siddiqui AH, Veznedaroglu E, et al. Simulator based angiography education in neurosurgery: Results of a pilot educational program. J Neurointerv Surg 2012; 4: 438–441. [DOI] [PubMed] [Google Scholar]
- 23.Pannell JS, Santiago-Dieppa DR, Wali AR, et al. Simulator-based angiography and endovascular neurosurgery curriculum: A longitudinal evaluation of performance following simulator-based angiography training. Cureus 2016; 8: e756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berry M, Lystig T, Beard J, et al. Porcine transfer study: Virtual reality simulator training compared with porcine training in endovascular novices. Cardiovasc Intervent Radiol 2007; 30: 455–461. [DOI] [PubMed] [Google Scholar]
- 25.Chaer RA, Derubertis BG, Lin SC, et al. Simulation improves resident performance in catheter-based intervention: Results of a randomized, controlled study. Ann Surg 2006; 244: 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burden C, Preshaw J, White P, et al. Usability of virtual-reality simulation training in obstetric ultrasonography: A prospective cohort study. Ultrasound Obstet Gynecol 2013; 42: 213–217. [DOI] [PubMed] [Google Scholar]
- 27.Roy MJ, Sticha DL, Kraus PL, et al. Simulation and virtual reality in medical education and therapy: A protocol. Cyberpsychol Behav 2006; 9: 245–247. [DOI] [PubMed] [Google Scholar]
- 28.Dawe SR, Pena GN, Windsor JA, et al. Systematic review of skills transfer after surgical simulation-based training. Br J Surg 2014; 101: 1063–1076. [DOI] [PubMed] [Google Scholar]
- 29.Koch A, Pfandler M, Stefan P, et al. Say, what is on your mind? Surgeons' evaluations of realism and usability of a virtual reality vertebroplasty simulator. Surg Innov. Epub ahead of print 15 January 2019. DOI: 10.1177/1553350618822869. [DOI] [PubMed] [Google Scholar]