Abstract
Background
The Scepter XC is a dual-lumen balloon catheter that accommodates a 0.014-inch microwire and can be used for balloon-assisted coiling of cerebral aneurysms. We describe our experience with the use of this device.
Methods
Two high-volume institution neurointerventional databases were retrospectively reviewed for cerebral aneurysms treated with balloon-assisted coiling using the Scepter XC balloon catheter. Patient demographics, aneurysm characteristics, and procedural details were recorded. Major procedure-related neurologic complications were defined as events that caused an increase in modified Rankin Scale that persisted for more than 1 week after the procedure. Follow-up aneurysm occlusion was assessed using the Raymond-Roy classification.
Results
During the study period, 231 aneurysms were treated in 219 patients (152 women, 67 men) with a mean age of 58.4 ± 12.2 years. Mean aneurysm size was 6.1 ± 3.1 mm, with a mean neck diameter of 3.1 ± 1.3 mm. In total, 77.5% of aneurysms were wide necked, and 39.8% were treated in the setting of subarachnoid hemorrhage. The major complication rate was 0.9% (2/231) per treated aneurysm, including one stroke and one death related to intraoperative aneurysm rupture. Excluding patients who died, angiographic follow up was available for 85.3% (191/224) of aneurysms. During a mean follow up of 17.4 ± 13.0 months (range, 1.7–66.5 months), Raymond-Roy 1 and 2 occlusion rates were 56.5% (108/191) and 35.6% (68/191), respectively. The retreatment rate was 12.6% (24/191).
Conclusion
Our experience using the coaxial dual-lumen Scepter XC for balloon-assisted coiling demonstrates acceptable aneurysm occlusion and complication rates.
Keywords: Aneurysm, angiography, balloon, coiling, subarachnoid hemorrhage
Introduction
Balloon-assisted coiling (BAC) of cerebral aneurysms was first described by Moret et al. in 1997,1 and has since become a well-established technique for the endovascular treatment of wide-neck aneurysms.2–5 The balloon is inflated across the neck of the aneurysm until enough coils have been deployed to create a stable coil mass within the aneurysm. The balloon is then deflated and removed. The balloon can also be used to stabilize the coiling microcatheter within the aneurysm and protect the origins of branch vessels arising from the aneurysm neck. Over the decades, there have been several advances in balloon catheter technology, including the Scepter XC (MicroVention, Aliso Viejo, CA), a dual-lumen catheter attached to a compliant balloon that accommodates a 0.014 inch microwire. This technical report describes our experience using the Scepter XC for BAC.
Methods
Retrospective review of the neurointerventional databases of two high-volume neurovascular centers was approved by respective institutional review boards to identify patients with cerebral aneurysms treated with BAC using the Scepter XC between January 2012 and January 2018. Both participating institutions perform > 100 endovascular aneurysm treatments per year. Patient information recorded included age, sex, smoking status, medical comorbidities, and clinical presentation. Active tobacco use was defined as smoking within 6 months prior to treatment. Recorded aneurysm characteristics included three-dimensional dome and neck measurements, location, and morphology. Wide-neck aneurysms were defined as those with a neck width ≥ 4 mm or a dome-to-neck ratio < 2 measured on digital subtraction angiography (DSA). Procedural details, including peri-procedural complications, were collected from operative reports. Major procedure-related neurologic complications were defined as events that caused an increase in modified Rankin Scale (mRS) that persisted for more than 1 week after the procedure. Aneurysm occlusion at the conclusion of each procedure was assessed on DSA images by the attending neurointerventionalist using the Raymond-Roy classification.6 Follow-up occlusion status was assessed with time-of-flight magnetic resonance angiography (MRA) for aneurysms < 10 mm, MRA with and without gadolinium contrast for aneurysms > 10 mm, or DSA, at the discretion of the attending neurointerventionalist.
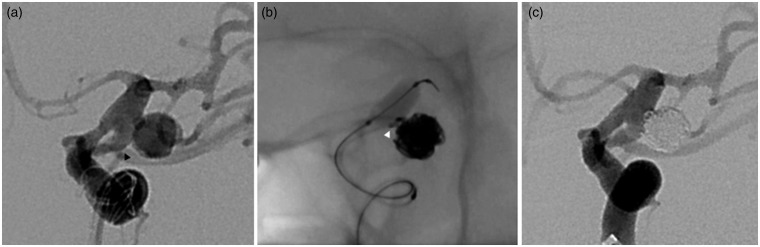
Written informed consent was obtained prior to all procedures. All elective patients received peri-procedural antiplatelet therapy and platelet function testing according to individual institution protocol. Intraprocedural heparin was administered to all patients. Goal-activated clotting times were two to three times baseline for elective cases and 1.5 to two times baseline for ruptured aneurysms. The choice to use BAC versus another adjunctive technique was made by the attending neurointerventionalist. In general, BAC was used whenever feasible, particularly in the setting of subarachnoid hemorrhage (SAH) or an elective case in which it was preferable to avoid postoperative dual antiplatelet therapy (DAPT) required after stent placement. A 6 French guide catheter was positioned in the cervical internal carotid or vertebral artery for proximal support, which permitted coaxial introduction of the Scepter XC and a coiling microcatheter. The Scepter XC balloon was navigated across the neck of the aneurysm and inflated during coil deployment. An illustrative case is presented in Figure 1.
Figure 1.
Middle-aged woman who presented with a multilobulated, 8-mm ruptured left posterior communicating artery aneurysm. (a) Initial working projection profiles the aneurysm neck, from which arises a large posterior communicating artery (black arrowhead). The Scepter XC was navigated across the neck of the aneurysm over a Synchro-14 standard preshaped microwire. (b) The balloon was inflated while placing the last coil at the aneurysm neck to stabilize the coiling microcatheter (white arrowhead) and prevent coil herniation into the posterior communicating or internal carotid arteries. (c) Final angiogram shows complete occlusion of the aneurysm.
Results
During the study period, 1,358 aneurysms were treated, of which 231 aneurysms in 219 patients were treated with BAC using the Scepter XC. Patient demographics and pertinent comorbidities are listed in Table 1. Aneurysm characteristics are listed in Table 2.
Table 1.
Clinical characteristics.
| Total aneurysms/ total patients | 231/219 | |
| Sex | ||
| Women | 152 (69.4%) | |
| Men | 67 (30.6%) | |
| Mean age | 58.4 ± 12.2 years | |
| Medical comorbidities | ||
| Hypertension | 139/219 (63.5%) | |
| Tobacco use | 105/219 (47.9%) | |
| Hyperlipidemia | 44/219 (20.1%) | |
| Diabetes | 26/219 (11.9%) | |
| Prior stroke | 14/219 (6.4%) | |
| Coronary artery disease | 16/219 (7.3%) |
Table 2.
Aneurysm characteristics.
| Aneurysm dimensions (mean ± standard deviation) | Size 6.1 ± 3.1 mm Neck 3.1 ± 1.3 mm | |
| Wide-neck aneurysms | 179/231 (77.5%) | |
| Acute subarachnoid hemorrhage | ||
| Yes | 92 (39.8%) | |
| No | 139 (60.2%) | |
| Aneurysm location | ||
| AComm | 59 (25.5%) | |
| ACA A1 or A2 | 11 (4.8%) | |
| Pericallosal | 8 (3.5%) | |
| MCA M1 segment | 13 (5.6%) | |
| MCA bifurcation | 32 (13.9%) | |
| MCA M2 or M3 | 10 (4.3%) | |
| Cavernous ICA | 1 (0.4%) | |
| Ophthalmic | 14 (6.1%) | |
| Superior hypophyseal | 5 (2.2%) | |
| Supra-/para-clinoid ICA | 8 (3.5%) | |
| PComm | 27 (11.7%) | |
| Anterior choroidal | 3 (1.3%) | |
| ICA terminus | 10 (4.3%) | |
| Vertebral | 1 (0.4%) | |
| PICA | 6 (2.6%) | |
| Basilar | 12 (5.2%) | |
| SCA | 3 (1.3%) | |
| PCA | 6 (2.6%) | |
| PTA | 1 (0.4%) | |
| PHA | 1 (0.4%) | |
ACA: anterior cerebral artery; AComm: anterior communicating artery; ICA: internal carotid artery; MCA: middle cerebral artery; PCA: posterior cerebral artery; PComm: posterior communicating artery; PHA: persistent hypoglossal artery; PICA: posterior inferior cerebellar artery; PTA: persistent trigeminal artery: SCA: superior cerebellar artery.
The overall symptomatic neurologic complication rate was 8.6% (20/231), including a 4.8% (11/231) rate of thromboembolic events and 3.9% (9/231) rate of intraoperative aneurysm rupture. Characteristics of procedures with these complications are detailed in Tables 3 and 4. The major procedure-related neurologic complication rate was 0.9% (2/231) per treated aneurysm and 0.9% (2/219) per treated patient. The first of these cases was a middle-aged man with hypertension and current tobacco use who presented with Hunt-Hess 1, Fisher 2 SAH due to a ruptured 4.7 mm Acomm aneurysm with a 2.4 mm neck. Intraoperative rupture occurred during deployment of the first coil without balloon inflation. The rupture then prompted balloon inflation to arrest blood flow to the aneurysm while subsequent coils were placed. This patient ultimately died due to complications related to elevated intracranial pressure. The second patient was a middle-aged man with hypertension, hyperlipidemia, type 2 diabetes mellitus, and active tobacco use who suffered a thromboembolic left corona radiata infarct after elective treatment of a 10.5 mm internal carotid artery (ICA) terminus aneurysm with a 5.9 mm neck that resulted in right hemiparesis and dysarthria. The patient was discharged to a rehabilitation facility and made a full recovery. The non-neurologic complication rate was 2.2% (5/231), including two femoral pseudoaneurysms, one retroperitoneal hematoma, and two instances of leg ischemia due to a closure device.
Excluding patients who died, angiographic follow up was available for 85.3% (191/224) of aneurysms. During a mean follow up of 17.4 ± 13.0 months (range 1.7–66.5 months) after initial treatment, Raymond-Roy 1 and 2 occlusion rates were 56.5% (108/191) and 35.6% (68/191), respectively. The retreatment rate was 12.6% (24/191) (Table 5).
Table 3.
Characteristics of procedures with thromboembolic complications.
| Patients | 11 |
| Major complications | 1 (9%) |
| Sex | |
| Women | 9 (82%) |
| Men | 2 (18%) |
| Mean age | 59.1 ± 9.7 years |
| Aneurysm dimensions (mean ± standard deviation) | Size 7.9 ± 5.2 mm Neck 4.1 ± 1.9 mm |
| Wide-neck aneurysms | 10 (91%) |
| Acute subarachnoid hemorrhage | |
| Yes | 8 (73%) |
| No | 3 (27%) |
| Aneurysm location | |
| AComm | 1 (9%) |
| MCA bifurcation | 3 (27%) |
| Ophthalmic | 1 (9%) |
| Pcomm | 5 (45%) |
| ICA terminus | 1 (9%) |
AComm: anterior communicating artery; ICA: internal carotid artery; MCA: middle cerebral artery; PComm: posterior communicating artery.
Table 4.
Characteristics of procedures complicated by intraoperative rupture.
| Patients | 9 |
| Major complications | 1 (11%) |
| Sex | |
| Women | 5 (56%) |
| Men | 4 (44%) |
| Mean age | 55.2 ± 13.2 years |
| Aneurysm dimensions (mean ± standard deviation) | Size 6.3 ± 3.2 mm Neck 2.6 ± 0.8 mm |
| Wide-neck aneurysms | 6 (67%) |
| Acute subarachnoid hemorrhage | |
| Yes | 6 (67%) |
| No | 3 (33%) |
| Aneurysm location | |
| AComm | 4 (44%) |
| SHA | 1 (11%) |
| Pcomm | 3 (33%) |
| MCA bifurcation | 1 (11%) |
AComm: anterior communicating artery; MCA: middle cerebral artery; PComm: posterior communicating artery; SHA: superior hypophyseal artery.
Table 5.
Characteristics of cases with aneurysm recurrences.
| Retreated aneurysms | 24 |
| Sex | |
| Women | 15 (63%) |
| Men | 9 (37%) |
| Mean age | 54.5 ± 13.6 years |
| Medical comorbidities | |
| Hypertension | 14/24 (58%) |
| Tobacco use | 13/24 (54%) |
| Hyperlipidemia | 6/24 (25%) |
| Diabetes | 2/24 (8.3%) |
| Prior stroke | 0/24 (0%) |
| Coronary artery disease | 2/24 (8.3%) |
| Aneurysm dimensions (mean ± standard deviation) | Size 8.0 ± 4.2 mm Neck 3.5 ± 1.5 mm |
| Wide-neck aneurysms | 13 (54%) |
| Acute subarachnoid hemorrhage | |
| Yes | 16 (67%) |
| No | 8 (33%) |
| Aneurysm location | |
| AComm | 6 (25%) |
| ACA A1 or A2 | 1 (4.2%) |
| MCA M1 segment | 1 (4.2%) |
| MCA bifurcation | 3 (13%) |
| MCA M2 or M3 | 1 (4.2%) |
| Ophthalmic | 2 (8.3%) |
| Pcomm | 4 (17%) |
| ICA terminus | 2 (8.3%) |
| PICA | 1 (4.2%) |
| Basilar tip | 3 (13%) |
| Imaging follow up (mean ± standard deviation) | 11.4 ± 9.5 months |
ACA: anterior cerebral artery; AComm: anterior communicating artery; ICA: internal carotid artery; MCA: middle cerebral artery; PComm: posterior communicating artery; PICA: posterior inferior cerebellar artery.
Discussion
Our experience demonstrates that the Scepter XC is a safe and effective tool for BAC of cerebral aneurysms. The Scepter XC was used to treat ruptured and unruptured aneurysms in a variety of locations in both the anterior and posterior circulation with a very low rate of procedure-related neurologic morbidity and an acceptable angiographic occlusion rate. Our results with the Scepter XC compare favorably with other publications describing BAC with other balloon catheters.7,8 There was only one major thromboembolic complication from which the patient made a full recovery. The only fatal complication resulted from an intraoperative rupture that did not occur during balloon inflation. Notably, the other eight intraoperative ruptures in this series caused no morbidity, potentially in part because the perforation site could be quickly tamponaded with the balloon.
The Scepter XC has several advantages compared to traditional single-lumen balloon catheters. The second lumen accommodates 0.014 inch microwires, which are more torquable than the 0.010 inch wires of single-lumen balloon catheters. The larger-diameter microwires can also provide greater support for navigating the Scepter XC through tortuous vessels. Additionally, the microwire cannot be removed from a single-lumen balloon catheter after it has been introduced into the body, because retrograde blood flow into the balloon can interfere with balloon inflation or deflation. Because the microwire and balloon lumens are separate in the Scepter XC, the microwire can be removed and reshaped or exchanged without losing the catheter position. The Scepter C has the same advantages, but we prefer the Scepter XC because the more compliant balloon better conforms to the parent artery.
Limitations of this study include its retrospective design, non-independent assessment of angiographic outcomes by the treating physician, and use of both DSA and MRA for angiographic follow up.
Conclusion
Our experience using the coaxial dual-lumen Scepter XC for BAC demonstrates acceptable aneurysm occlusion and complication rates.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JEDA and EAS are consultants for Microvention.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Moret J, Cognard C, Weill A, et al. The “remodelling technique” in the treatment of wide neck intracranial aneurysms. Angiographic results and clinical follow-up in 56 cases. Interv Neuroradiol J Peritherapeutic Neuroradiol Surg Proced Relat Neurosci 1997; 3: 21–35. [DOI] [PubMed] [Google Scholar]
- 2.Malek AM, Halbach VV, Phatouros CC, et al. Balloon-assist technique for endovascular coil embolization of geometrically difficult intracranial aneurysms. Neurosurg 2000; 46: 1397–1406. discussion 1406–1407. [DOI] [PubMed] [Google Scholar]
- 3.Moon K, Albuquerque FC, Ducruet AF, et al. Balloon remodeling of complex anterior communicating artery aneurysms: Technical considerations and complications. J NeuroInterventional Surg 2015; 7: 418–424. [DOI] [PubMed] [Google Scholar]
- 4.Baldi S, Mounayer C, Piotin M, et al. Balloon-assisted coil placement in wide-neck bifurcation aneurysms by use of a new, compliant balloon microcatheter. Am J Neuroradiol 2003; 24: 1222–1225. [PMC free article] [PubMed] [Google Scholar]
- 5.Ross IB, Dhillon GS. Balloon assistance as a routine adjunct to the endovascular treatment of cerebral aneurysms. Surg Neurol 2006; 66: 593–601. [DOI] [PubMed] [Google Scholar]
- 6.Mascitelli JR, Moyle H, Oermann EK, et al. An update to the Raymond–Roy occlusion classification of intracranial aneurysms treated with coil embolization. J NeuroInterventional Surg 2015; 7: 496–502. [DOI] [PubMed] [Google Scholar]
- 7.Spiotta AM, Miranpuri A, Hawk H, et al. Balloon remodeling for aneurysm coil embolization with the coaxial lumen Scepter C balloon catheter: Initial experience at a high volume center. J NeuroInterventional Surg 2013; 5: 582–585. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro M, Babb J, Becske T, et al. Safety and efficacy of adjunctive balloon remodeling during endovascular treatment of intracranial aneurysms: A literature review. Am J Neuroradiol 2008; 29: 1777–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]