Abstract
Background
Chagas disease is endemic in Latin America and affects 8 million people worldwide. In 2010, Catalonia introduced systematic public health surveillance to detect and treat congenital Chagas disease.
Aim
The objective was to evaluate the health outcomes of the congenital Chagas disease screening programme during the first 6 years (2010–2015) after its introduction in Catalonia.
Methods
In a surveillance system, we screened pregnant women and newborns and other children of positive mothers, and treated Chagas-positive newborns and children. Diagnosis was confirmed for pregnant women and children with two positive serological tests and for newborns with microhaematocrit and/or PCR at birth or serology at age 9 months.
Results
From 2010 to 2015, the estimated screening coverage rate increased from 68.4% to 88.6%. In this period, 33,469 pregnant women were tested for Trypanosoma cruzi and 937 positive cases were diagnosed. The overall prevalence was 2.8 cases per 100 pregnancies per year (15.8 in Bolivian women). We followed 82.8% of newborns until serological testing at age 9–12 months and 28 were diagnosed with Chagas disease (congenital transmission rate: 4.17%). Of 518 siblings, 178 (34.3%) were tested and 14 (7.8%) were positive for T. cruzi. Having other children with Chagas disease and the heart clinical form of Chagas disease were maternal risk factors associated with congenital T. cruzi infection (p < 0.05).
Conclusion
The increased screening coverage rate indicates consolidation of the programme in Catalonia. The rate of Chagas disease congenital transmission in Catalonia is in accordance with the range in non-endemic countries.
Keywords: Chagas disease, congenital, surveillance system, Trypanosoma cruzi, screening, public health, vertical transmission
Introduction
Chagas disease, a parasitic infection caused by the flagellated protozoan Trypanosoma cruzi, is endemic in Latin America [1]. It is found mainly in rural areas of Central and South America, except on the Caribbean islands, and coincides with the distribution of the vector that belongs to the family of triatomines and is responsible for transmission of the parasite to humans [2]. Other possible mechanisms of transmission are mother-to-child, blood transfusions, transplants of infected organs and tissues and ingestion of contaminated food [3]. There are an estimated 8 million people infected worldwide, of whom up to 30% may develop heart disease, with digestive or nervous system involvement in 10–20% [4-6].
Following migration from endemic areas to other countries, the epidemiological pattern of Chagas disease has changed in recent decades and new cases of congenital transmission and transmission by other mechanisms are detected in non-endemic countries [7]. The last decade (2000–2010) has seen an increase of people from endemic areas migrating to Europe [8]. In 2009, it was estimated that between 68,000 and 122,000 people from endemic countries living in Europe were infected, although the rate of underdiagnosis was 94–96% [8]. European prevalence rates in migrants from endemic areas differ greatly according to the country of origin, with an estimated prevalence rate of 4.2%, which rises to 18.1% in migrants from Bolivia [9].
Rates of congenital transmission in non-endemic countries are lower than those found in endemic countries [10] but the asymptomatic nature of the disease and the lack of knowledge about Chagas disease in non-endemic countries make it difficult to detect new cases [11,12]. Screening newborns of positive mothers is key to the early detection and treatment of possible cases in non-endemic countries [13].
Spain, for cultural reasons, is the European country that has received most migrants from Latin America [14]. Screening for T. cruzi in blood and tissue banks has been mandatory by Royal decree-law since 2005 [15] but legislation on the screening of congenital transmission is still lacking [11].
In Catalonia, estimates of people infected with T. cruzi in 2010 were between 10,000 and 20,000, with between 203 and 387 pregnant women affected and between seven and 16 children with congenital Chagas disease [16]. After confirming the cost-effectiveness of a screening programme for congenital Chagas disease [17] and following the recommendations of the World Health Organization (WHO) in non-endemic countries which had to take appropriate measures to prevent and control vertical transmission [11,18,19], the Deputy director of public health surveillance and response to emergencies of the Public Health Agency of Catalonia (PHAC) has since 2010 progressively introduced and coordinated a protocol to detect, treat and cure cases of congenital Chagas disease [20].
There is no common legislation on the control of the congenital transmission of Chagas disease in Europe, although there are regional initiatives for the early detection and treatment of cases according to WHO recommendations. Official programmes for the detection and treatment of congenital Chagas disease have been introduced in the Valencia (2008) [21], Catalonia (2010) [20] and Galicia (2014) [22] regions in Spain and in Toscana (2012) [23] in Italy. Other regions do not have an official protocol but act locally in hospitals [19,24-26].
The objective of this study was to analyse the epidemiological pattern of congenital Chagas disease in pregnant women from endemic areas and their children in the period from 2010 to 2015 in Catalonia and to evaluate the coverage of the screening programme.
Methods
Surveillance setting
Catalonia is an autonomous community in the north-east of Spain with more than 7.5 million inhabitants. In the study period (2010–2015), ca 450,000 people, 6% of the population, were born in countries where Chagas disease is endemic [27]. There are 45 public and 30 private maternity hospitals in Catalonia and 90% of births in Latin American women occur in public centres [28]. There are also 47 Sexual and Reproductive Health Care centres (Centre d’Atenció a la Salut Sexual I Reproductiva - ASSIR), distributed in 372 maternal assistance points, which form part of the network of public primary care centres. In addition, there are 27 microbiology laboratories able to perform diagnostic tests for Chagas disease [29].
Screening of pregnant women, newborns and their siblings
We introduced a surveillance system to evaluate the impact of congenital Chagas disease in Catalonia. The target population were pregnant women from endemic countries (first or second generation) and pregnant women from other origins (including Spain) who have lived in a rural area of an endemic country for more than one month at any point in their lives.
Serological screening is carried out during the first trimester of pregnancy, although tests done at any time during pregnancy, delivery or after birth are included in the programme (Figure 1) [20]. The tests used for screening are those recommended by the WHO [1]. Samples are collected at the ASSIR centre during pregnancy or in hospitals during or after delivery. If the first test is positive, a second test using a different antigen or serological technique is carried out. If the results between the two tests are discrepant, a third serological test, using a different technique, is carried out. All tests used in the programme follow the WHO recommendation [1] and laboratories choose recommended tests according to their own experience and supplier.
Figure 1.
Congenital Chagas disease screening programme in Catalonia, 2010–2015
a Many advances have been made in molecular biology and expert groups recommend PCR for the diagnosis in infants [28–30]. In case of positive PCR at birth, another PCR at age 1 month is necessary to confirm Chagas disease.
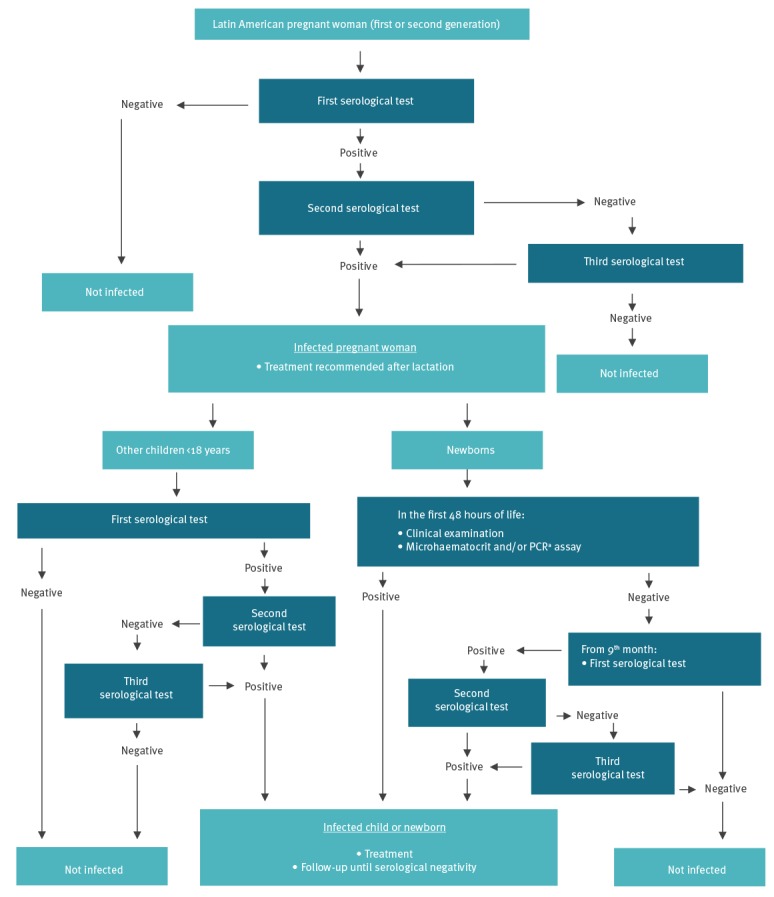
When the diagnosis is confirmed, it is recommended that pregnant women start treatment with trypanocidal drugs (benznidazole or nifurtimox) after birth and lactation, and before a possible new pregnancy. There is no risk of transmitting Chagas disease through breastfeeding.
Immediately after a birth to a mother diagnosed with Chagas disease, a clinical evaluation of the newborn is made in hospital to detect symptoms compatible with Chagas disease. The parasitological tests carried out during the first 48 h of life are the microhaematocrit and/or PCR [20]. If there is a positive PCR at birth, another PCR is carried out 4 weeks later to confirm the diagnosis. If any parasitological test is negative or tests cannot be carried out at birth, the infant is tested with a serological test after 9 months when maternal antibodies have waned. If this test is negative, the follow-up ends and the child is considered not infected; if the test is positive, a second serological test with a different technique is carried out. If the results of the two tests are discrepant, a third serological test, using a different technique, is carried out. If any microhaematocrit at birth, PCR at age 1 month or two serological tests after 9 months old are positive, T. cruzi infection is confirmed and antiparasitic treatment is administered.
The programme also includes other older children from positive mothers if they are living in Catalonia, using the same serological testing as for pregnant women. When two serological tests using different technique are positive, T. cruzi infection is confirmed and antiparasitic treatment is administered.
The screening and follow-up of pregnant women, newborns and siblings are included in the public health portfolio and are free of charge.
Epidemiological surveillance
To implement the programme throughout the region, PHAC created the Working Group for Congenital Chagas disease in Catalonia, enrolling a large multidisciplinary group of Chagas disease experts who are responsible for the detection, notification and follow-up of positive pregnant women, newborns and siblings with positive mothers [16]: midwives, obstetricians, gynaecologists, paediatricians, microbiologists, specialists in infectious diseases and internal medicine, community health workers and epidemiologists.
Surveillance includes the mandatory notification of confirmed T. cruzi cases through the Microbiological Reporting System of Catalonia, a network of Catalonian laboratories that collects and reports pathogens of public health importance to the PHAC [30]. Reported cases are included in the Voluntary Registry of Chagas Disease Congenital Cases in Catalonia (VRCH). Sociodemographic, diagnostic and treatment data and epidemiological information about the mothers (years living in Catalonia, the clinical form of Chagas disease and previous treatments for Chagas disease) are voluntarily collected by the Working Group and included in the VRCH.
Laboratories report annually the number of pregnant women screened. To calculate the coverage of the screening of pregnant women, the denominator was estimated taking into account the number of births in women from endemic countries in the Register of Newborns (an official regional registry linked to each maternity hospital, public or private, which collects information on births in Catalonia, including the mothers’ country of origin [31]) and adding an estimation of pregnancies interrupted before giving birth (miscarriages and abortions) and women who moved away from Catalonia before childbirth as reported to the VRCH (13% of total pregnancies). Prevalence rates were calculated on pregnancies and not on pregnant women because the screening is repeated for each new pregnancy. To calculate the prevalence rates by country of origin we applied the distribution of births by maternal country of origin in the Register of Newborns to the total of pregnancies screened.
Statistical analysis
All outcomes are shown in percentages and the annual percentage differences between 2010 and 2015 are shown as a relative change and evaluated using the Z score for two proportions of population.
Maternal epidemiological risk factors were evaluated between newborns with a definitive positive and negative diagnosis of Chagas disease. Continuous variables (age and years living in Catalonia) were transformed into categorical variables, choosing the mean as cut-off point. Statistical significance was established assuming an α error of 0.05. Differences between groups were analysed by simple logistic regression and the results are shown as p value and odds ratio (OR). Multiple logistic regression was used to calculate the adjusted OR (aOR) and variables with a p value < 0.20 in the crude analysis were entered in the model. To avoid the problem of quasi-complete separation, Firth logistic regression was used [32].
The analysis was performed using the Statistical Package for Social Sciences (SPSS v.25 for Windows).
Ethical statement
The study was not submitted for approval by a research ethics committee because the activities described were conducted as part of the legislated mandate of the Health Department of Catalonia, the competent authority for the surveillance of communicable diseases according to Decree 203/2015 of 15 September, which created the epidemiological surveillance network of Catalonia [30]. All the activities studied formed part of public health surveillance and did not require informed consent.
Results
Table 1 shows the overall results for screened pregnant women and follow-up in newborns and siblings.
Table 1. Screening of pregnant women and follow-up of siblings and newborns for Chagas disease, Catalonia, 2010–2015 (n = 40,084).
| Total | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | Difference 2010–2015 |
p value 2010 vs 2015 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | % | ||
| Pregnancies follow-up | ||||||||||||||||
| To be testeda | 40,084 | 7,656 | 7,145 | 7,099 | 6,348 | 6,005 | 5,831 | −23.8 | NA | |||||||
| Tested | 33,469 | 83.5 | 5,238 | 68.4 | 6,107 | 85.5 | 6,324 | 89.1 | 5,524 | 87.0 | 5,108 | 85.1 | 5,168 | 88.6 | 29.5 | < 0.001 |
| Positives | 937 | 2.8 | 128 | 2.4 | 179 | 2.9 | 168 | 2.7 | 163 | 3.0 | 148 | 2.9 | 151 | 2.9 | 19.6 | 0.147 |
| Lost before parturitionb | 38 | 4.1 | 8 | 6.3 | 8 | 4.5 | 11 | 6.5 | 3 | 1.8 | 3 | 2.0 | 5 | 3.3 | −47.0 | 0.381 |
| Miscarriages/abortionsb | 87 | 9.3 | 7 | 5.5 | 16 | 8.9 | 17 | 10.1 | 18 | 11.0 | 14 | 9.5 | 15 | 9.9 | 81.6 | 0.248 |
| Gave birthb | 812 | 86.7 | 113 | 88.3 | 155 | 86.6 | 140 | 83.3 | 142 | 87.1 | 131 | 88.5 | 131 | 86.8 | −1.7 | 0.840 |
| Other children follow-up | ||||||||||||||||
| Siblings to be tested | 519 | 130 | 84 | 92 | 73 | 73 | 67 | −48.5 | NA | |||||||
| Missing information/not tested | 341 | 65.7 | 121 | 93.1 | 42 | 50 | 44 | 47.8 | 49 | 67.1 | 52 | 71.2 | 33 | 49.3 | −47.1 | < 0.001 |
| Tested | 178 | 34.3 | 9 | 6.9 | 42 | 50.0 | 48 | 52.2 | 24 | 32.9 | 21 | 28.8 | 34 | 50.7 | 633.0 | |
| Positives | 14 | 7.9 | 2 | 22.2 | 3 | 7.1 | 2 | 4.2 | 2 | 8.3 | 2 | 9.5 | 3 | 8.8 | −60.3 | 0.596 |
| Negatives | 164 | 92.1 | 7 | 77.8 | 39 | 92.9 | 46 | 95.8 | 22 | 91.7 | 19 | 90.5 | 31 | 91.2 | 17.2 | |
| Newborns follow-up | ||||||||||||||||
| 1. Parasitological control at birth | ||||||||||||||||
| Not tested | 84 | 10.3 | 19 | 16.8 | 30 | 19.4 | 13 | 9.3 | 7 | 4.9 | 6 | 4.6 | 9 | 6.9 | −59.1 | 0.026 |
| Tested | 728 | 89.7 | 94 | 83.2 | 125 | 80.6 | 127 | 90.7 | 135 | 95.1 | 125 | 95.4 | 122 | 93.1 | 12.0 | |
| Tested by PCR | 634 | 87.1 | 83 | 88.3 | 100 | 80.0 | 110 | 86.6 | 122 | 90.4 | 111 | 88.8 | 108 | 88.5 | 0.3 | 0.871 |
| Tested by MH | 419 | 57.6 | 73 | 77.7 | 59 | 47.2 | 69 | 54.3 | 75 | 55.6 | 73 | 58.4 | 70 | 57.4 | −26.1 | 0.003 |
| Positives | 12 | 1.6 | 2 | 2.1 | 2 | 1.6 | 1 | 0.8 | 4 | 3.0 | 3 | 2.4 | 0 | 0 | −100.0 | 0.367 |
| Negatives | 716 | 98.4 | 92 | 97.9 | 123 | 98.4 | 126 | 99.2 | 131 | 97.0 | 122 | 97.6 | 122 | 100 | 2.2 | |
| 2. Serological control at age 9–12 months | ||||||||||||||||
| To be tested | 800 | 111 | 153 | 139 | 138 | 128 | 131 | 18.0 | NA | |||||||
| Not tested | 140 | 17.5 | 21 | 18.9 | 33 | 21.6 | 31 | 22.3 | 26 | 18.8 | 12 | 9.4 | 17 | 13.0 | −31.4 | 0.276 |
| Moved away from Catalonia | 56 | 7.0 | 1 | 0.9 | 13 | 8.5 | 13 | 9.4 | 17 | 12.3 | 9 | 7.0 | 3 | 2.3 | 154.2 | 0.735 |
| Failure to attend the medical visit | 53 | 6.6 | 15 | 13.5 | 9 | 5.9 | 9 | 6.5 | 8 | 5.8 | 2 | 1.6 | 10 | 7.6 | −43.5 | 0.199 |
| Failure of surveillance circuitc | 31 | 3.9 | 5 | 4.5 | 11 | 7.2 | 9 | 6.5 | 1 | 0.7 | 1 | 0.8 | 4 | 3.1 | −32.2 | 0.800 |
| Tested | 660 | 82.5 | 90 | 81.1 | 120 | 78.4 | 108 | 77.7 | 112 | 81.2 | 116 | 90.6 | 114 | 87.0 | 7.3 | 0.276 |
| Mean time to test (months) | 10 ± 4 | 10 ± 3.5 | 10 ± 6 | 10±4 | 10 ± 4 | 10 ± 3 | 9 ± 4 | |||||||||
| Positives | 16 | 2.4 | 4 | 4.4 | 2 | 1.7 | 1 | 0.9 | 4 | 3.6 | 2 | 1.7 | 3 | 2.6 | −40.8 | 0.750 |
| Negatives | 644 | 97.6 | 86 | 95.4 | 118 | 98.3 | 107 | 99.1 | 108 | 96.4 | 114 | 98.3 | 111 | 97.4 | 1.9 | |
| 3. Summary of testing | ||||||||||||||||
| Correctly tested | 672 | 82.8 | 92 | 81.4 | 122 | 78.7 | 109 | 77.9 | 116 | 81.7 | 119 | 90.8 | 114 | 87 | 6.9 | 0.304 |
| Positives | 28 | 4.2 | 6 | 6.5 | 4 | 3.3 | 2 | 1.8 | 8 | 6.9 | 5 | 4.2 | 3 | 2.6 | −59.6 | 0.318 |
| Negatives | 644 | 95.8 | 86 | 93.5 | 118 | 96.7 | 107 | 98.2 | 108 | 93.1 | 114 | 95.8 | 111 | 97.4 | 4.2 | |
| Index | ||||||||||||||||
| Pregnant women screening coverage rate (%) | 83.5 | 68.4 | 85.5 | 89.1 | 87.0 | 85.1 | 88.6 | 29.5 | < 0.001 | |||||||
| Prevalence rate of Chagas disease (%) | 2.80 | 2.44 | 2.93 | 2.66 | 2.95 | 2.90 | 2.92 | 19.6 | 0.147 | |||||||
| Congenital transmission rate (%) | 4.17 | 6.52 | 3.28 | 1.83 | 6.90 | 4.20 | 2.63 | −59.6 | 0.318 | |||||||
NA: not Applicable.
a Number of births from the Newborns Registry plus an estimation (13%) of interrupted pregnancies and women who moved away before childbirth from the Voluntary Registry of Chagas disease congenital cases in Catalonia.
b Among the women who tested positive.
c Missing patient referral or paediatrician’s lack of awareness about Chagas disease.
Screening of pregnant women
It was estimated that 40,084 pregnant women should have been tested in Catalonia between 2010 and 2015. Of these, 33,469 (83.5%) were actually screened, an annual mean of 5,578 tests (Table 1). No positive cases were detected in pregnant women who were second-generation migrants or travellers.
A total of 818 women were diagnosed with T. cruzi during pregnancy between 2010 and 2015: 707 (86%) became pregnant once, 103 twice (13%) and eight (1%) three times. In total, 937 pregnancies in positive women were followed between 2010 and 2015.
Screening coverage of pregnant women increased mainly between 2010 (68.4%) and 2011 (85.5%), when the logistics of the programme were introduced in all areas. The coverage gradually increased further until 2015 (88.6%) (p < 0.001 between 2010 and 2015).
The highest density of Chagas-positive women was seen in the Barcelona health area (717 cases; 87.7%), especially in the Baix Llobregat (270 cases; 37.7%) and Barcelona (233 cases; 32.5%) areas (Figure 2).
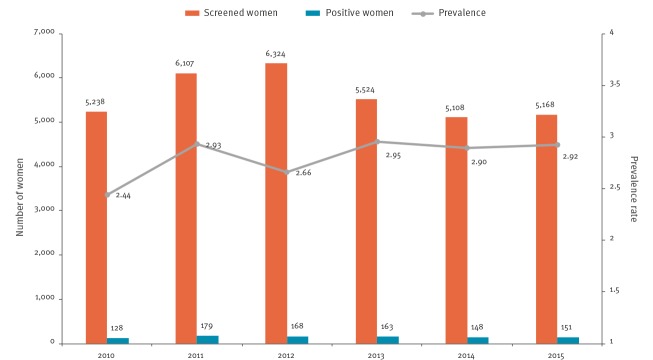
Figure 2.
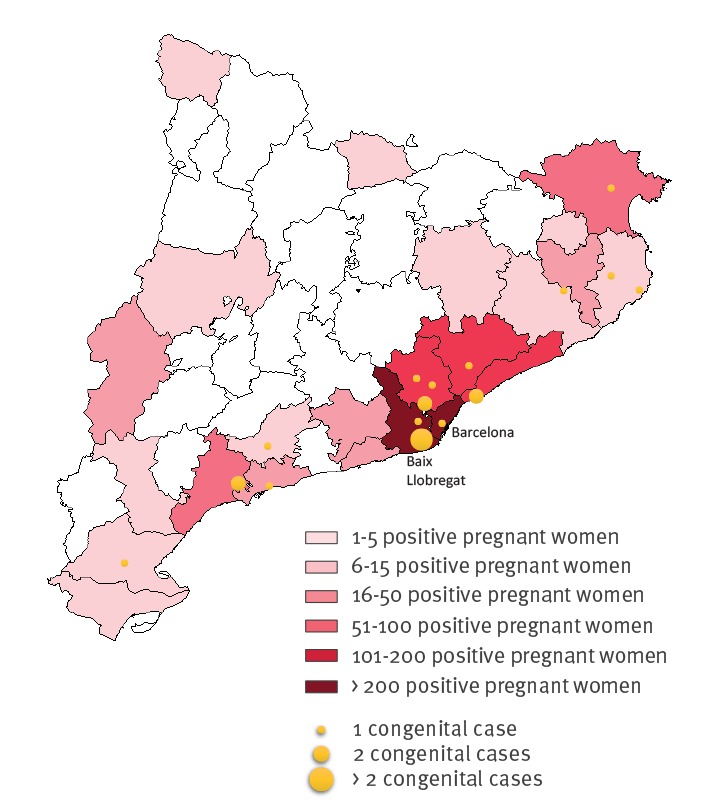
Geographical distribution of Trypanosoma cruzi-positive pregnant women and cases of congenital transmission, Catalonia, 2010–2015 (n = 818)
During the study period, the prevalence rate was 2.8 positive cases per 100 pregnancies screened (Figure 3). The rates were highest in women from Bolivia (15.79), El Salvador (1.41) and Paraguay (1.24) (Table 2).
Figure 3.
Annual number of screened women and Trypanosoma cruzi-positive pregnant women, Catalonia, 2010–2015 (n = 33,469)
Table 2. Epidemiological characteristics, prevalence rates by endemic country of origin and maternal risk factors for congenital Chagas disease, Catalonia, 2010–2015 (n = 818).
| Maternal risk factors | Positive pregnant women (n = 818) | Prevalence rates for country of origina | Completed follow-up negative in newborns (n = 644) | Completed follow-up positive in newborns (n = 28) | Crude p value | Crude OR (CI) | Adjusted p valueb | Adjusted OR (CI)b | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||||||
| Age | |||||||||||
| < 33 years | 389 | 47.6 | 305 | 47.4 | 17 | 60.7 | 0.166 | 1.71 (0.79–3.72) |
0.693 | 1.30 (0.35–5.28) |
|
| ≥ 33 years | 429 | 52.4 | 339 | 51.6 | 11 | 39.3 | Ref | ||||
| Previous treatmentc | |||||||||||
| Yes | 115 | 26.0 | 107 | 29.9 | 2 | 8.3 | Ref | ||||
| No | 328 | 74.0 | 251 | 70.1 | 22 | 91.7 | 0.033 | 3.85 (1.02–14.49) |
0.093 | 6.67 (0.78–876.89) |
|
| Country of birthd | |||||||||||
| Bolivia | 755 | 92.5 | 15.79 | 598 | 92.9 | 27 | 96.4 | Ref | |||
| Paraguay | 20 | 2.5 | 1.24 | 17 | 2.6 | 1 | 3.6 | 0.801 | 1.30 (0.17–10.15) |
NA | |
| Argentina | 13 | 1.6 | 0.52 | 11 | 1.7 | 0 | 0 | NA | |||
| Ecuador | 7 | 0.9 | 0.10 | 4 | 0.6 | 0 | 0 | NA | |||
| Honduras | 6 | 0.7 | 0.26 | 4 | 0.6 | 0 | 0 | NA | |||
| Chile | 5 | 0.6 | 0.50 | 1 | 0.2 | 0 | 0 | NA | |||
| El Salvador | 4 | 0.5 | 1.41 | 3 | 0.5 | 0 | 0 | NA | |||
| Peru | 4 | 0.5 | 0.11 | 4 | 0.6 | 0 | 0 | NA | |||
| Nicaragua | 1 | 0.1 | 0.57 | 1 | 0.2 | 0 | 0 | NA | |||
| Colombia | 1 | 0.1 | 0.02 | 1 | 0.2 | 0 | 0 | NA | |||
| Clinical form of Chagas diseasee | |||||||||||
| Indeterminate | 524 | 94.1 | 432 | 94.9 | 23 | 88.5 | Ref | ||||
| Heart | 21 | 3.8 | 15 | 3.3 | 3 | 11.5 | 0.047 | 3.76 (1.02–13.90) |
0.009 | 14.40 (2.11–87.67) |
|
| Digestive | 9 | 1.6 | 8 | 1.8 | 0 | 0 | NA | ||||
| Mixed | 3 | 0.5 | 0 | 0 | 0 | 0 | NA | ||||
| Siblings completing follow-upf | |||||||||||
| Negative | 131 | 92.3 | 150 | 97.4 | 4 | 40 | Ref | ||||
| Positive | 11 | 7.7 | 4 | 2.6 | 6 | 60 | < 0.001 | 56.25 (11.26–280.9) |
0.001 | 22.79 (3.75–161.54) |
|
| Years living in Cataloniag | |||||||||||
| ≤ 7 years | 254 | 57.9 | 186 | 49.7 | 12 | 75 | 0.059 | 3.03 (0.96–9.57) |
0.453 | 1.76 (0.42–10.05) |
|
| > 7 years | 185 | 42.1 | 188 | 50.3 | 4 | 25 | Ref | ||||
CI: confidence interval; NA: Not Applicable; OR: odds ratio; Ref: reference value.
a Number of positive newborns per 100 births, adjusted by pregnant women screening coverage rate.
b Firth multiple logistic regression.
c Unknown data: n = 375 (45.8%).
d Unknown data: n = 2 (0.2%).
e Unknown data: n = 261 (31.9%).
f Mothers without other children and with untested other children: n = 676 (82.6%).
g Unknown data: n = 379 (46.3%).
The mean age of positive women at pregnancy was 33 years, which increased from 32 years in 2010 to 34 years in 2015. Bolivian women represented 92.5% of the positive cases in whom the country of birth could be identified, followed by women from Paraguay (2.5%), Argentina (1.6%), Ecuador (0.9%), Honduras (0.7%), Chile (0.6%), El Salvador (0.5%), Peru (0.5%), Nicaragua (0.1%) and Colombia (0.1%). Almost half of the cases (47.6%) had arrived in Catalonia between 2005 and 2006, and the mean number of years from arrival to pregnancy was 7 years (Table 2).
The main clinical form of Chagas disease was indeterminate (94.1%). Women with heart clinical form represented 3.8% of cases, while digestive and mixed pathologies (heart and digestive) accounted for 1.6% and 0.5% of cases, respectively. Only 26% of pregnant women received treatment with benznidazole or nifurtimox before pregnancy. This percentage increased from 7.8% (7/90 cases with data about treatment) for pregnant women diagnosed in 2010 to 46.5% for those diagnosed in 2015 (33/71 cases with data about treatment) (p < 0.001).
Pregnancies were interrupted in 9.3% (n = 87) of pregnancies. More than half were miscarriages (65.5%), followed by abortions (23%) and cases where the reason for interruption was missing (11.5%), while 4.1% of pregnant women left Catalonia before childbirth, which meant that follow-up of the newborn was not possible.
There were 812 births from 937 pregnancies in 818 T. cruzi-positive women in Catalonian maternity hospitals (Table 1).
Follow-up of siblings
For 674 of the 818 T. cruzi-positive women (82.4%) detected by the programme, it was possible to determine whether they had other children born before the current pregnancy and living in Catalonia, and 359 (53.3%) had at least one. The mean of other children per mother was 0.8. We identified 519 children for screening. In most cases, the children had not been tested or testing information not notified by the working group (341 cases; 65.7%), ranging from 93.1% in 2010 to 49.3% in 2015 (p < 0.001). Of the 178 children who were successfully screened and reported (34.3%), 14 were positive (7.9%) (Table 1). The median age of those 14 children was 10 years (range: 3–18 years) and 12 were male. Five of them were born in Catalonia between 2005 and 2008 but were not tested during the first year of life, while nine arrived in Catalonia during childhood. All 14 cases started treatment with benznidazole, but treatment was interrupted in two cases because of side effects such as neutropenia and toxicoderma and was not resumed, although the follow-up continued. In seven of the 14 cases, the follow-up was not completed with the required serological test. None of the seven children who continued the follow-up had negative serological tests after treatment, with a median follow-up of 4 years (range: 1–6 years) (Table 3).
Table 3. Positive Trypanosoma cruzi diagnostic tests, treatment and follow-up in newborns and their siblings, Catalonia, 2010–2015 (n = 42).
| ID | Microhaematocrit | PCR | Serology | Country of birth | Age of arrival | Age at diagnosis (months) | Age at treatment start (months) | Symptoms compatible with Chagas disease | Adverse reactions to treatment | Completed treatment | Serological negativisation | Follow-up after treatment end (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Newborns | ||||||||||||
| 1 | + | + | NA | NA | NA | 0 | 2 | No | No | Yes | Yes | 1 |
| 2 | + | + | NA | 0 | 0 | Yes | Yes | No | Yes | 9 | ||
| 3 | + | + | NA | 0 | 0 | Yes | No | Yes | Yes | 9 | ||
| 4 | − | + | NA | 0 | 1 | No | No | Yes | Yes | 1 | ||
| 5 | + | NP | NA | 0 | 0 | Yes | No | Yes | Yes | 6 | ||
| 6 | NP | + | NA | 0 | 0 | No | No | Yes | Yes | 21 | ||
| 7 | − | + | NA | 1 | 2 | No | No | Yes | Yes | 6 | ||
| 8 | NP | + | NA | 1 | 4 | No | No | Yes | Yes | 6 | ||
| 9 | − | + | NA | 1 | 1 | No | No | Yes | No | 34 | ||
| 10 | + | NP | NA | 1 | 3 | No | No | Yes | Yes | 3 | ||
| 11 | − | + | NA | 2 | 15 | No | No | Yes | No | 11 | ||
| 12 | NP | + | NA | 6 | 6 | No | No | Yes | Yes | 12 | ||
| 13 | NP | − | + | 9 | 9 | No | No | Yes | Yes | 5 | ||
| 14 | − | NP | + | 9 | 10 | No | No | Yes | Losta | NA | ||
| 15 | NP | NP | + | 9 | 9 | No | No | Yes | Yes | 0 | ||
| 16 | − | NP | + | 9 | 11 | No | No | Yes | Yes | 9 | ||
| 17 | NP | − | + | 9 | 9 | No | Yes | Yes | No | 24 | ||
| 18 | − | − | + | 10 | 11 | No | No | Yes | Losta | NA | ||
| 19 | − | − | + | 11 | 12 | No | No | Yes | No | 33 | ||
| 20 | NP | NP | + | 11 | 11 | No | No | Yes | No | 59 | ||
| 21 | − | − | + | 12 | 13 | No | Yes | Yes | Yes | 18 | ||
| 22 | − | NP | + | 12 | 12 | Yes | No | No | Losta | NA | ||
| 23 | − | − | + | 13 | 12 | No | No | Yes | No | 35 | ||
| 24 | NP | − | + | 15 | 16 | No | No | Yes | Yes | 16 | ||
| 25 | NP | NP | + | 20 | 24 | No | No | Yes | No | 28 | ||
| 26 | NP | NP | + | 20 | 21 | No | No | Yes | No | 30 | ||
| 27 | − | − | + | 23 | 23 | No | No | Yes | No | 4 | ||
| 28 | NP | − | + | 27 | 28 | No | Yes | Yes | No | 27 | ||
| Median (interquartile range) | 9 (11) | 9.5 (10.75) | 11.5 (21.75) | |||||||||
| Siblings | ||||||||||||
| 1 | NA | NA | + | Spain | NA | 3 | 3 | No | No | Yes | No | 4 |
| 2 | + | Spain | NA | 4 | 5 | No | No | Yes | No | 4 | ||
| 3 | + | Spain | NA | 4 | 4 | No | No | Yes | No | 1 | ||
| 4 | + | Bolivia | 7 | 5 | 5 | No | No | Yes | No | 6 | ||
| 5 | + | Spain | NA | 7 | 7 | No | Yes | No | Losta | NA | ||
| 6 | + | Bolivia | 1 | 7 | 8 | No | Yes | No | Losta | NA | ||
| 7 | + | Spain | NA | 9 | 9 | No | No | Yes | No | 3 | ||
| 8 | + | Bolivia | 9 | 11 | 11 | No | No | Yes | Losta | NA | ||
| 9 | + | Bolivia | 9 | 11 | 11 | No | No | Yes | Losta | NA | ||
| 10 | + | Bolivia | 6 | 11 | 11 | No | No | Yes | No | 2 | ||
| 11 | + | Bolivia | 10 | 12 | 12 | No | No | Yes | Losta | NA | ||
| 12 | + | Bolivia | 13 | 13 | 14 | No | No | Yes | Losta | NA | ||
| 13 | + | Bolivia | 9 | 16 | 16 | No | No | Yes | No | 5 | ||
| 14 | + | Bolivia | 15 | 18 | 18 | No | No | Yes | Losta | NA | ||
| Median (interquartile range) | 10 (7.5) | 10 (7.5) | 4 (3) | |||||||||
+: positive; −: negative; ID: identification number; NP: not performed; NA: not applicable.
a Lost to follow-up before serological control.
Follow-up of newborns
Of the 812 newborns, 728 (89.7%) were tested for T. cruzi parasite at birth. The most frequent tests were PCR (87.1%) and microhaematocrit (57.6%). In 84 of 812 cases (10.3%) the newborn was not tested at birth (16.8% in 2010 and 6.9% in 2015; p = 0.029). Testing after age 9 months was carried out in 672 of 812 newborns (82.8%). Of these, 95.8% (n = 644) tested negative. The median age at screening was 10 months (Table 1).
A total of 140 newborns (17.5%) did not complete the follow-up. The main reason was the departure of the family from Catalonia before the newborn was 9 months old (7.0%), followed by failure to attend the medical visit (6.6%) and failure of the surveillance circuit (3.9%).
Twenty-eight cases were diagnosed with T. cruzi infection acquired through congenital transmission (4.2%). In 27 cases, the mother was from Bolivia and in one case from Paraguay. Twelve infants were diagnosed by parasitological tests before age 9 months and 16 infants with serological tests after age 9 months (Table 3). In four of 28 cases, the newborn presented symptoms compatible with Chagas disease, including splenomegaly (3/4), hepatomegaly (3/4) and jaundice (3/4).
All 28 positive cases were treated with benznidazole. Treatment was suspended because of failure to attend follow-up visits in one case and because of an adverse reaction in one case. Overall, four of 28 newborns had adverse reactions, including increased transaminases (n = 1), pancytopenia (n = 1), cessation of weight gain (n = 1) and anorexia (n = 1). Serology after treatment was negative in 15 cases, with a mean time between treatment end and serology of 8.1 months (range: 0–21 months). Two newborns treated before age 12 months did not become seronegative: the first was diagnosed by PCR 1 month after birth and remained positive 1 year after treatment. Treatment was repeated 4 years later and the subsequent PCR was negative, but serological testing was not carried out. The second child had a negative PCR at birth, but positive PCR and serology at 9 months. Treatment was stopped after 10 days because of pancytopenia and was resumed 2 months later. Two years later serology remained positive.
Recovery rates were 89% for newborns treated before 6 months of age, 80% for those treated between 6 and 12 months of age and 20% after 12 months of age. Taking the serological diagnosis after 9 months as the gold standard, the sensitivity of the microhaematocrit and PCR was 29.4% and 52.6%, respectively, and the specificity 100% and 99.2% (in four cases, PCR was positive at birth but negative after 1 month).
Analysis of maternal risk factors
In an analysis of maternal risk factors for vertical transmission of the infection, we saw significant differences between positive and negative siblings (aOR = 22.79; 95% confidence interval (CI): 3.75–161.54) and between heart and indeterminate clinical forms (aOR = 14.4; 95% CI: 2.11–87.67) (Table 2). Differences between untreated and treated mothers showed crude statistical significance (p = 0.033) but significance was lost after adjusting for multivariate logistic regression (aOR = 6.67; 95% CI: 0.78–876.89). Other risk factors analysed, such as the mother’s age, country of origin or time living in Catalonia (≤ 7 years) had no significant influence on the likelihood of vertical transmission.
Discussion
The congenital Chagas disease prevention and control programme in Catalonia is one of few screening programmes for the control of congenital Chagas disease launched by public health authorities in a non-endemic region [9].The observed prevalence of Chagas disease (2.8 cases/100 pregnancies) was similar to that found in other studies in pregnant women in Catalonia [33, 34]. The prevalence in Bolivian pregnant women was lower (15.8%) than in a similar programme in Bolivia (23.3%) [35]. Studies in other regions in Spain show higher prevalence rates in Valencia (34.1%) [36] and Vizcaya (22%) [37] but lower rates in Madrid (11.4%) [38] and Almeria (12.5%) [39]. Other non-endemic countries show lower rates in Bolivian pregnant women living in Italy (8.7%) [25] and Switzerland (8.8%) [24]. These differences may be due, in part, to methodological differences in estimating the rates. The prevalence rates observed in our programme in women from other endemic countries such as Paraguay, Argentina, Ecuador, Honduras, Chile, el Salvador, Peru, Nicaragua and Colombia (range: 0.02–1.41), were much lower than those detected in other Spanish studies (range: 0.2–7.4) [36,40] or in studies from the endemic countries themselves (range: 3.2–12.7) [41-45].
The rate of congenital transmission in Catalonia (4.17%) was within the range detected in endemic (range: 1.7–5) [35,46-50] and non-endemic countries (range: 0–7.3) [25,33,34,36-38].
The estimated screening coverage rate in pregnant women was 83.5%, which is lower than the rate found in Valencia (94.5%) [36]. This may be due, in part, to the greater centralisation and smaller number of centres included in the Valencia programme (three maternity hospitals) compared with Catalonia (45 public maternity hospitals and 372 primary health centres with midwife care, including all public health centres).
Screening of the newborns’ siblings is widely neglected in gestational screening programmes and there are few studies of this subgroup [51-53]. A prevalence study conducted in Catalonia in children younger than 18 years with a Chagas disease-positive mother [53] found a slightly higher rate (10.9%) than ours (7.3%), and a clinical study of children in Catalonia and Switzerland identified a higher percentage of adverse effects during treatment (36%) than our programme (14.3%) and a recovery rate at age 2 years of 17.2%, compared with 0% in our programme [51]. Although the screening of other children improved significantly between 2010 (6.9%) and 2015 (50.7%), the high percentage of missing cases (352 cases, 66.4%), and the missing follow-up in positive cases (50%) demonstrate a lack of a well-established notification and follow-up circuit for this subgroup.
Parasitological testing at birth improved significantly between 2010 (83.2%) and 2015 (93.1%). PCR was used more than the microhaematocrit (87.1% and 57.6%, respectively), and the microhaematocrit was less frequent in 2015 than in 2010 (57.4% vs 77.7%). This confirms the greater practicality of PCR in our region. Even if PCR is widely accepted for the early diagnosis of Chagas disease [54-56], false positive (four cases) and false negative results (nine cases) indicate that a standardised PCR technique with higher sensitivity is required [57]. Currently, it is still necessary to wait until age 1 month to validate the diagnosis by PCR or to perform a serological test after 9 months for PCR-negative cases [58].
We detected some delay between diagnosis and start of treatment for positive newborns. There are several possible explanations: the presence of other pathologies that require other incompatible treatments, difficulty in obtaining the medication (there was a significant lack of supply of Benznidazole a few years ago) or a decision made by the patient's family.
The recovery rates observed in treated newborns are provisional data because newborns with positive serology will be followed until serology is negative and the results could therefore change in the future. Sometimes problems with treatment compliance or adverse reactions can affect seronegativisation in post-treatment follow-up. However, although our results are based on very few cases, the current results suggest that it is very important to detect the infection before age 12 months to achieve a probability of cure of more than 80%. Schijman et al. found a 100% recovery rate when treatment is started before age 6 months compared with 88.9% in our study [59].
With respect to maternal epidemiological risk factors for congenital transmission, we found three studies that showed an increased risk of congenital transmission in untreated women [60-62]. In our study, women untreated before pregnancy had an almost sevenfold greater probability of congenital infection but the adjusted significance was weak (p = 0.093). Having the heart clinical form of Chagas disease rather than the indeterminate clinical form and having other infected children increased the risk of congenital transmission 14 and 23 times, respectively. These findings demonstrate the importance of recommending treatment of women of childbearing age before a new pregnancy, especially in those who already have infected children or those with the heart clinical form of the disease.
Other studies in Catalonia found a higher proportion of the digestive clinical form (up to 21% vs 1.6%) [5,6]. Our results may be an underestimate because infected women diagnosed during pregnancy could not undergo specific radiological tests to detect possible digestive disorders.
The main challenge of our programme was to calculate the coverage of screening for pregnant women and the prevalence rate by country of origin, because the protocol did not plan for quantifying the target population and collecting epidemiological information on pregnant women with negative results. To solve this limitation, we used the Register of Newborns as a source. It will be necessary to involve the ASSIR centres in reporting all cases, negative or positive, or create an improved data collection system to provide this information. Another limitation of the programme were the 10.5% missing numbers in the follow-up at age 9–12 months owing to failures in the follow-up circuit such as a lack of awareness about Chagas disease among paediatricians and patients, or a missing patient referral. The percentage lost to follow-up was smaller in 2013 (6.5%) and 2014 (2.4%) because of a specific community health action to redirect lost cases [63]. It is therefore necessary to improve primary healthcare circuits to control the newborns and other children of positive mothers and to add community health actions to the surveillance of congenital Chagas disease.
Conclusion
The results of the congenital Chagas disease programme in Catalonia show that systematic control of the congenital transmission of Chagas disease by an integrated public health surveillance system is possible in a non-endemic region and the increase in the estimated screening coverage rate indicates its consolidation in Catalonia.
Prevalence and congenital transmission rates were within the ranges detected in other studies conducted in non-endemic settings. Having previous children with Chagas disease and presenting the heart clinical disease form of the disease were risk factors for the congenital transmission of T. cruzi. Treatment of women of childbearing age with these characteristics is recommended in order to improve the treatment of Chagas disease in non-endemic countries.
Acknowledgements
We are grateful to Sonia Broner from the Public Health Agency of Catalonia for the statistical support and recommendation.
Funding: ARM and JG belongs to the RICET, a Tropical Disease Cooperative Research Network in Spain (RD16/0027/0004) co-funded by ISCIII and the Fondo Europeo de Desarrollo Regional (FEDER).
This work was supported by the Catalan Agency for the Management of Grants for University Research, Spain (AGAUR; grant numbers 2017/SGR 924 and 2017/SGR 1342).
Members of the Working Group of Congenital Chagas disease in Catalonia
Alba Cebollero. CLILAB Diagnòstics, Consorci del Laboratori Intercomarcal.
Alicia Carrascon. ASSIR Izquierdo (Barcelona ciudad), Institut Català de la Salut (ICS).
Ana Moreira. Hospital Sant Joan de Déu de Martorell.
Ana Requena-Mendez. ISGlobal, Hospital Clínic de Barcelona, Universitat de Barcelona.
Ana Torres Soto. Hospital Residència Sant Camil.
Andrea Martín. Hospital Universitari Vall d’Hebron.
Anna Ballester. Corporació de Salut del Maresme i la Selva.
Anna Bragulat. Hospital de la Cerdanya.
Anna Soligó. ASSIR Consorci Sanitari de Terrassa.
Anna Vilamala Bastarras. Consorci Hospitalari de Vic.
Antoni Payà. Hospital del Mar.
Antoni Soriano-Arandes. Hospital Universitari Vall d’Hebron.
Antoni Sorni. Hospital Verge de la Cinta de Tortosa.
Antonia Calvo. Hospital Clínic de Barcelona.
Antonio Mur. Hospital del Mar.
Assumpta Colomer. Consorci Hospitalari de Vic.
Begoña Carral. ASSIR Baix Llobregat Litoral, ICS.
Begoña Treviño. Unitat de Salut Internacional Drassanes, Hospital Universitari Vall d’Hebron.
Borja Guarch. Hospital Universitari Dr Josep Trueta de Girona.
Carles Orta. Hospital Comarcal de l'Alt Penedès.
Carlos Rodrigo. Hospital Universitari Germans Trias i Pujol.
Carme Gallés. Corporació de Salut del Maresme i la Selva.
Carme Garrido. Hospital de la Santa Creu i Sant Pau.
Carme Molina. Hospital Joan XXIII de Tarragona.
Carme Mora. Hospital de Figueres.
Carmen Muñoz. Hospital de la Santa Creu i Sant Pau.
Carmina Martí. Hospital General de Granollers.
Carmina Sanjosé. CLILAB Diagnòstics, Consorcio del Laboratorio Intercomarcal.
Celia Guardia. Laboratori Barcelonès Nord i Vallés Oriental.
Cristina Cortes. Hospital General de l'Hospitalet.
Cristina Gutierrez. Hospital Universitari Joan XXIII de Tarragona.
Cristina Martinez. ASSIR Barcelona Ciutat, ICS.
Cristina Riera. Facultat de Farmàcia. Universitat de Barcelona.
Cristina Soler. Hospital de Santa Caterina.
Dolors Gonzalez. CAP Rambla Nova. ICS Camp de Tarragona.
Dolors Guix. ASSIR Granollers, ICS.
Eduard Solé Mir. Hospital Universitari Arnau de Vilanova, IRBLleida.
Eduardo Padilla. Laboratori de Referència de Catalunya.
Elena Sulleiro. Hospital Universitari Vall d’Hebron.
Elisa Llurba. Hospital de la Santa Creu i Sant Pau.
Elisabeth Del Amo. ASSIR Litoral (Barcelona ciudad), ICS.
Elisenda Moliner. Hospital de la Santa Creu i Sant Pau.
Emma Padilla Esteba. Catlab-Centre Analítiques Terrassa, AIE.
Engràcia Coll. ASSIR Hospital Mutua Terrassa, ICS.
Enrique Rodriguez. Hospital General de l'Hospitalet.
Enrnesto Monaco. Hospital Residencia Sant Camil.
Ester Muñoz. Hospital de la Cerdanya.
Esther Freixas. ASSIR l’Hospitalet, ICS.
Eva Dopico. Laboratori Clínic L’Hospitalet.
Eva Sardá. ASSIR Muntanya (Barcelona ciudad), ICS.
Federic Ballester. Hospital Universitari Sant Joan de Reus.
Federic Gomez. Hospital Joan XXIII de Tarragona.
Ferran Barranco. Hospital de la Cerdanya.
Francesc Ripoll. Hospital Santa Caterina.
Francesc-Josep Fargas. ASSIR Camp de Tarragona, ICS.
Gemma Falguera. ASSIR Direcció d’Atenció Primària Metropolitana Nord, ICS.
Gema Fernández-Rivas. Microbiology Department, Clinical Laboratory North Metropolitan Area, Hospital Universitari Germans Trias i Pujol.
Gemma Ginovart. Hospital de la Santa Creu i Sant Pau.
Gemma Navarro. Hospital Universitari Parc Taulí.
Graciano García-Pardo. Hospital Joan XXIII de Tarragona.
Gustavo Lorenzo. Hospital de Mollet del Vallès.
Hakima Ouaarab. Unitat de Salut Internacional Drassanes, Hospital Universitari Vall d’Hebron.
Imma Caubet. Espitau Vall d’Aran.
Isabel Claveria. Unitat de Salut Internacional Drassanes, Hospital Universitari Vall d’Hebron
Isabel Sanfeliu. Hospital Universitari Parc Taulí.
Israel Molina. Hospital Universitari Vall d’Hebron.
Jesus Blanch. Hospital Residencia Sant Camil.
Joan Agullo Martí. Hospital de Palamós.
Joan Farré. Hospital Universitari Arnau Vilanova de Lleida.
Joaquim Gascón. ISGlobal, Hospital Clínic, Universitat de Barcelona.
Jordi Costa. Hospital Universitari Parc Taulí.
Jordi Gomez i Prat. Unitat de Salut Internacional Drassanes, Hospital Universitari Vall d’Hebron.
Jose Fulquet. Hospital de Figueres.
Josefa Perez Jove. Catlab-Centre Analítiques Terrassa, AIE.
Josep Armengol. Hospital de la Santa Creu i Sant Pau.
Juan Carlos Riera. ASSIR Gironès Pla de l'Estany, ICS.
Judith Villar. Parc de Salut Mar.
Laia Sanchez Torrent. Hospital General del Parc Sanitari Sant Joan de Déu (Sant Boi de Llobregat).
Leonardo De La Torre. ISGlobal, Hospital Clínic de Barcelona.
Lluis Delgado. Hospital Comarcal de l’Alt Penedès.
Lluis Valerio. Unitat de Salut Internacional Metropolitana Nord, ICS.
Lourdes Montsant. Hospital de la Cerdanya.
Luca Basile. Agència de Salut Pública de Catalunya.
Luis Mayol. Hospital Universitario de Girona Doctor Josep Trueta
M. Eugenia Valls. ISGlobal, Hospital Clínic de Barcelona.
MªAngels Vives. Hospital de Terrassa.
MªGoretti Sauca. Hospital de Mataró.
Maite Coll. Hospital General de Granollers.
MªJesus Pinazo. ISGlobal, Hospital Clínic de Barcelona.
MªJosé Ferri. Hospital Universitari Dr Josep Trueta de Girona.
MªJosé Vidal. Agència de Salut Pública de Catalunya.
MªLuz Villegas. Hospital General de l'Hospitalet.
Manuel Andres Samper Anquela. PIUS Hospital de Valls.
Manuel Monsonis. Hospital Maternoinfantil Sant Joan de Déu.
MªPilar Blasco. ASSIR Santa Coloma de Gramenet, ICS.
Mar Olga Pérez-Moreno. Hospital Verge de la Cinta de Tortosa.
Margarida Curriu Sabates. Hospital Sant Bernabé.
Maria Mèndez. Hospital Germans Trias i Pujol
Maria Navarro. Consorci Hospitalari de Vic.
Marisa Urcola. Hospital de Santa Caterina.
Marta Lora. Hospital Universitari Dr Josep Trueta de Girona.
Marta Navarro. Hospital Universitari Parc Taulí.
Merce Almirall. Hospital Universitari Arnau Vilanova de Lleida.
Mercè Arasa. ASSIR Terres de l'Ebre, ICS.
Meritxell Vidal Alaball. ASSIR Baix Empordà, Hospital de Palamós.
Mireia Carulla. Hospital de Sant Pau i Santa Tecla.
Mireia Jané. Agència de Salut Pública de Catalunya.
Monica Ribell-Bachs. Hospital General de Granollers.
Montse Abella. ASSIR Sabadell, ICS.
Montserrat Gallego. ISGlobal, Hospital Clínic de Barcelona.
Neus Prat. ASSIR Baix Llobregat, ICS.
Neus Rius. Hospital Universitario Sant Joan de Reus
Nuria Prim Bosh. Laboratori de Referència de Catalunya.
Pablo Garcia. Hospital Comarcal Móra d’Ebre.
Paloma Araujo. Hospital de Terrassa.
Pepa Solé. Hospital de Mataró.
Pilar Ciruela. Agència de Salut Pública de Catalunya.
Pilar Villalobos. Hospital de Figueres.
Rosa Almirall. ASSIR Izquierdo (Barcelona ciudad), ICS.
Rosa Diaz. ASSIR Mataró, ICS.
Rosa Puigarnau. Hospital Mútua Terrassa.
Rosa Serra. ASSIR Gironès Pla de l'Estany.
Roser Diez. Hospital de Mataró.
Sara Torrent Bosch. Hospital Universitario Dr Josep Trueta de Girona.
Silvia Franch. Hospital de Sant Pau i Santa Tecla.
Sonia Vega. Hospital de Figueres.
Susanna Garcia Mani. ASSIR Baix Llobregat Nord, ICS.
Teresa Juncosa. Hospital Maternoinfantil Sant Joan de Déu.
Valentí Pineda. Hospital Parc Taulí de Sabadell
Vicky Fumado. Hospital Maternoinfantil Sant Joan de Déu.
Xavier Urquizu. Hospital Mutua de Terrassa.
Conflict of interest: None declared.
Authors’ contributions: Programme design and implementation: LB, PC, ARM, ED, AMN, ES, JG, MJ and Working Group of Congenital Chagas disease in Catalonia.
Data collection: Working Group of Congenital Chagas disease in Catalonia.
Data analysis: LB.
Original draft: LB, PC.
Draft review and edition: ARM, MJV, ED, AMN, ES, JG, MJ.
References
- 1. WHO Expert Committee Control of Chagas disease. World Health Organ Tech Rep Ser. 2002;905:i-vi, 1-109, back cover. [PubMed] [Google Scholar]
- 2.Silveira AC, Rojas de Arias A, Segura E, Guillén G, Russomando G, Schenone H, et al. El control de la enfermedad de Chagas en los países del Cono Sur de América. Historia de una iniciativa internacional 1991/2001. [The control of Chagas disease in the countries of the Southern Cone of America. History of an international initiative 1991/2001]. Washington: Pan American Health Organization; 2002. Spanish. Available from: http://www1.paho.org/spanish/ad/dpc/cd/dch-historia-incosur.PDF
- 3. Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375(9723):1388-402. 10.1016/S0140-6736(10)60061-X [DOI] [PubMed] [Google Scholar]
- 4. Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis. 2001;1(2):92-100. 10.1016/S1473-3099(01)00065-2 [DOI] [PubMed] [Google Scholar]
- 5. Pinazo MJ, Lacima G, Elizalde JI, Posada EJ, Gimeno F, Aldasoro E, et al. Characterization of digestive involvement in patients with chronic T. cruzi infection in Barcelona, Spain. PLoS Negl Trop Dis. 2014;8(8):e3105. 10.1371/journal.pntd.0003105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muñoz J, Gómez i Prat J, Gállego M, Gimeno F, Treviño B, López-Chejade P, et al. Clinical profile of Trypanosoma cruzi infection in a non-endemic setting: immigration and Chagas disease in Barcelona (Spain). Acta Trop. 2009;111(1):51-5. 10.1016/j.actatropica.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 7. Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 2010;115(1-2):14-21. 10.1016/j.actatropica.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 8. Basile L, Jansa JM, Carlier Y, Salamanca DD, Angheben A, Bartoloni A, et al. Chagas disease in European countries: the challenge of a surveillance system. Euro Surveill. 2011;16(37):19968. [PubMed] [Google Scholar]
- 9. Requena-Méndez A, Aldasoro E, de Lazzari E, Sicuri E, Brown M, Moore DA, et al. Prevalence of Chagas disease in Latin-American migrants living in Europe: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2015;9(2):e0003540. 10.1371/journal.pntd.0003540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Howard EJ, Xiong X, Carlier Y, Sosa-Estani S, Buekens P. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG. 2014;121(1):22-33. 10.1111/1471-0528.12396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Requena-Méndez A, Albajar-Viñas P, Angheben A, Chiodini P, Gascón J, Muñoz J, et al. Health policies to control Chagas disease transmission in European countries. PLoS Negl Trop Dis. 2014;8(10):e3245. 10.1371/journal.pntd.0003245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oliveira I, Torrico F, Muñoz J, Gascon J. Congenital transmission of Chagas disease: a clinical approach. Expert Rev Anti Infect Ther. 2010;8(8):945-56. 10.1586/eri.10.74 [DOI] [PubMed] [Google Scholar]
- 13. Carlier Y, Torrico F, Sosa-Estani S, Russomando G, Luquetti A, Freilij H, et al. Congenital Chagas disease: recommendations for diagnosis, treatment and control of newborns, siblings and pregnant women. PLoS Negl Trop Dis. 2011;5(10):e1250. 10.1371/journal.pntd.0001250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jackson Y, Angheben A, Carrilero Fernandez B, Jansa i Lopez del Vallado JM, Jannin JG, Albajar-Viñas P. [Management of Chagas disease in Europe. Experiences and challenges in Spain, Switzerland and Italy]. Bull Soc Pathol Exot. 2009;102(5):326-9. French. [PubMed] [Google Scholar]
- 15.Ministry of Health. Real Decreto 1088/2005, de 16 de septiembre, por el que se establecen los requisitos técnicos y condiciones mínimas de la hemodonación y de los centros y servicios de transfusión. [Royal decree 1088/2005 of September 16 which establishes the technical requirements and minimum conditions of blood donation and of transfusion centres and services]. Madrid: Ministeri de Sanitat y Consum; 2005:31288-304. Spanish. Available from: http://www.boe.es/boe/dias/2005/09/20/pdfs/A31288-31304.pdf
- 16. Basile L, Oliveira I, Ciruela P, Plasencia A, Working Group For Developing The Catalonian Screening Programme For Congenital Transmission Of Chagas Disease The current screening programme for congenital transmission of Chagas disease in Catalonia, Spain. Euro Surveill. 2011;16(38):19972. 10.2807/ese.16.38.19972-en [DOI] [PubMed] [Google Scholar]
- 17. Sicuri E, Muñoz J, Pinazo MJ, Posada E, Sanchez J, Alonso PL, et al. Economic evaluation of Chagas disease screening of pregnant Latin American women and of their infants in a non endemic area. Acta Trop. 2011;118(2):110-7. 10.1016/j.actatropica.2011.02.012 [DOI] [PubMed] [Google Scholar]
- 18. Organització Mundial de la Salut Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. 2015;90(6):33-43. 10.2147/IBPC.S70402 [DOI] [PubMed] [Google Scholar]
- 19. Soriano-Arandes A, Angheben A, Serre-Delcor N, Treviño-Maruri B, Gómez I Prat J, Jackson Y. Control and management of congenital Chagas disease in Europe and other non-endemic countries: current policies and practices. Trop Med Int Health. 2016;21(5):590-6. 10.1111/tmi.12687 [DOI] [PubMed] [Google Scholar]
- 20.Autonomous government of Catalonia. Protocol for screening and diagnosing Chagas disease in pregnant Latin American women and their newborns. Barcelona: Generalitat de Catalunya; 2010. Available from: https://scientiasalut.gencat.cat/bitstream/handle/11351/1173/protocol_cribratge_diagnostic_malaltia_chagas_embarassades_2010_ang.pdf
- 21.A Bayón Rueda, R Borrás Salvador, E Calabuig Muñoz, MT Fraile Fariña, MJ Giménez Martí, E Montesinos Sanchis, Elena, et al. Enfermedad de Chagas importada: protocolo de actuación en la Comunitat Valenciana. [Imported Chagas Disease. Protocol of actions in the Valencian Community]. Valencia: Conselleria de Sanitat. Generalitat Valenciana; 2009. Spanish. Available from: http://publicaciones.san.gva.es/publicaciones/documentos/V-5243-2008.pdf
- 22.Autonomous Government of Galicia. Protocolo de cribado da enfermidade de Chagas en mulleres embarazadas. [Chagas disease screening protocol in pregnant women]. Santiago de Compostela: Xunta de Galicia; 2014. Spanish. Available from: https://www.sergas.es/Asistencia-sanitaria/Protocolo-de-cribado-da-enfermidade-de-chagas-en-mulleres-embarazadas-Actualizacion-2014
- 23.Bartoloni A, Strohmeyer M, Mantella A, Zammarchi L, Trotta M, Rossolini GM, et al. Programma regionale per la prevenzione e il controllo della malattia di Chagas congenita: indicazioni per l’assistenza in gravidanza. [Regional program for the prevention and control of congenital Chagas disease: indications for assistance during pregnancy]. Firenze: Regione Toscana; 2012. Italian. Available from: http://www.regione.toscana.it/documents/10180/13329059/Allegato+parere+n.+46-2015+Prev+e+conrollo+malattia+di+Chagas.pdf/6e153700-0d7b-4d70-b098-faba975c6de8?version=1.0
- 24. Martinez de Tejada B, Jackson Y, Paccolat C, Irion O, Groupe Chagas Congénital Genéve [Congenital Chagas disease in Geneva: diagnostic and clinical aspects]. Rev Med Suisse. 2009;5(222):2091-2, 2094-6. French. [PubMed] [Google Scholar]
- 25. Rodari P, Angheben A, Gennati G, Trezzi L, Bargiggia G, Maino M, et al. Congenital Chagas disease in a non-endemic area: Results from a control programme in Bergamo province, Northern Italy. Travel Med Infect Dis. 2018;25:31-4. 10.1016/j.tmaid.2018.04.011 [DOI] [PubMed] [Google Scholar]
- 26. Merino FJ, Martínez-Ruiz R, Olabarrieta I, Merino P, García-Bujalance S, Gastañaga T, et al. [Control of Chagas disease in pregnant Latin-American women and her children]. Rev Esp Quimioter. 2013;26(3):253-60. Spanish. [PubMed] [Google Scholar]
- 27.Institut d’Estadística de Catalunya. [Statistical Institute of Catalonia]. Població estrangera resident a Catalunya segons la seva nacionalitat 2016. [Foreign population resident in Catalonia by nationality]. Barcelona: Generalitat de Catalunya. [Accessed: 13/06/2019]. Catalan. Available from: http://www.idescat.cat/poblacioestrangera/
- 28.Agència de Salut Pública de Catalunya. [Public Health Agency of Catalonia]. Xifres i dades de salut maternoinfantil 2018. [Figures and data on maternal and child health 2018]. Barcelona: Generalitat de Catalunya. [Accessed: 13 Jun 2019]. Catalan. Available from: http://salutpublica.gencat.cat/ca/ambits/promocio_salut/Embaras-part-i-puerperi/Xifres-i-dades/
- 29.Subdirecció general de vigilància i resposta a emergencies en salut pública, Agència de Salut Pública de Catalunya. [Deputy director of public health surveillance and response to emergencies, Public Health Agency of Catalonia]. Centres collaboradors del Programa de prevenció i control de la malaltia de Chagas congènita a Catalunya. [Collaborating Centers of the Congenital Chagas disease Screening Program in Catalonia]. Barcelona: Generalitat de Catalunya; 2019. Catalan. Available from: http://canalsalut.gencat.cat/web/.content/_A-Z/C/chagas/arxius/llistatcentres.pdf
- 30.Departament de Salut. [Department of Health]. Decret 203/2015, de 15 de setembre, pel qual es crea la Xarxa de Vigilància Epidemiológica i es regulen els sistemes de notificació de malalties de declaració obligatòria i brots epidémics. [Decree 203/2015, of September 15, which creates the Epidemiological Surveillance Network and regulates the reporting systems for compulsory declaration diseases and epidemic outbreaks]. Barcelona: Generalitat de Catalunya; 2015. Catalan. Available from: http://dogc.gencat.cat/ca/pdogc_canals_interns/pdogc_resultats_fitxa/?action=fitxa&documentId=702922&language=ca_ES
- 31.Jané M, Vidal MJ, Tomas Z, Maresma M, Mayoral LK. Indicators of perinatal health in Catalonia 2016. Executive Report. Barcelona: Sub-directorate General for Epidemiological Surveillance and Public Health Emergency Response. Secretariat for Public Health; 2018. Available from: http://canalsalut.gencat.cat/web/.content/_Professionals/Vigilancia_epidemiologica/documents/arxius/Perinatal_Indicator_Catalonia_2016.pdf
- 32. Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80(1):27-38. 10.1093/biomet/80.1.27 [DOI] [Google Scholar]
- 33. Muñoz J, Coll O, Juncosa T, Vergés M, del Pino M, Fumado V, et al. Prevalence and vertical transmission of Trypanosoma cruzi infection among pregnant Latin American women attending 2 maternity clinics in Barcelona, Spain. Clin Infect Dis. 2009;48(12):1736-40. 10.1086/599223 [DOI] [PubMed] [Google Scholar]
- 34. Otero S, Sulleiro E, Molina I, Espiau M, Suy A, Martín-Nalda A, et al. Congenital transmission of Trypanosoma cruzi in non-endemic areas: evaluation of a screening program in a tertiary care hospital in Barcelona, Spain. Am J Trop Med Hyg. 2012;87(5):832-6. 10.4269/ajtmh.2012.12-0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alonso-Vega C, Billot C, Torrico F. Achievements and challenges upon the implementation of a program for national control of congenital Chagas in Bolivia: results 2004-2009. PLoS Negl Trop Dis. 2013;7(7):e2304. 10.1371/journal.pntd.0002304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barona-Vilar C, Giménez-Martí MJ, Fraile T, González-Steinbauer C, Parada C, Gil-Brusola A, et al. Prevalence of Trypanosoma cruzi infection in pregnant Latin American women and congenital transmission rate in a non-endemic area: the experience of the Valencian Health Programme (Spain). Epidemiol Infect. 2012;140(10):1896-903. 10.1017/S0950268811002482 [DOI] [PubMed] [Google Scholar]
- 37. Avila Arzanegui O, Liendo Arenaza P, Martinez Indart L, Martinez Astorkiza T, Pocheville Guruceta MI, Egurbide Arberas MV. [Prevalence of Trypanosoma cruzi infection and vertical transmission in Latin-American pregnant women in a health area of Biscay.] Enferm Infecc Microbiol Clin. 2013;31(4):210-6. Spanish. 10.1016/j.eimc.2012.01.029 [DOI] [PubMed] [Google Scholar]
- 38. Flores-Chavez MD, Merino FJ, Garcia-Bujalance S, Martin-Rabadan P, Merino P, Garcia-Bermejo I, et al. Surveillance of Chagas disease in pregnant women in Madrid, Spain, from 2008 to 2010. Euro Surveill. 2011;16(38):19974. 10.2807/ese.16.38.19974-en [DOI] [PubMed] [Google Scholar]
- 39. Muñoz-Vilches MJ, Salas J, Cabezas T, Metz D, Vázquez J, Soriano MJ. [Chagas screening in pregnant Latin-American women. Experience in Poniente Almeriense (Almeria, Spain)]. Enferm Infecc Microbiol Clin. 2012;30(7):380-2. Spanish. 10.1016/j.eimc.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 40. Ramos JM, Milla A, Rodríguez JC, López-Chejade P, Flóres M, Rodríguez JM, et al. Chagas disease in Latin American pregnant immigrants: experience in a non-endemic country. Arch Gynecol Obstet. 2012;285(4):919-23. 10.1007/s00404-011-2081-9 [DOI] [PubMed] [Google Scholar]
- 41. Russomando G, Almirón M, Candia N, Franco L, Sánchez Z, de Guillen I. [Implementation and evaluation of a locally sustainable system of prenatal diagnosis to detect cases of congenital Chagas disease in endemic areas of Paraguay]. Rev Soc Bras Med Trop. 2005;38(Suppl 2):49-54. Spanish. [PubMed] [Google Scholar]
- 42. Kolliker-Frers RA, Insua I, Razzitte G, Capani F. Chagas disease prevalence in pregnant women: migration and risk of congenital transmission. J Infect Dev Ctries. 2016;10(9):895-901. 10.3855/jidc.7118 [DOI] [PubMed] [Google Scholar]
- 43. Castellanos-Domínguez YZ, Cucunubá ZM, Orozco LC, Valencia-Hernández CA, León CM, Florez AC, et al. Risk factors associated with Chagas disease in pregnant women in Santander, a highly endemic Colombian area. Trop Med Int Health. 2016;21(1):140-8. 10.1111/tmi.12634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Apt W, Zulantay I, Arnello M, Oddó D, González S, Rodríguez J, et al. Congenital infection by Trypanosoma cruzi in an endemic area of Chile: a multidisciplinary study. Trans R Soc Trop Med Hyg. 2013;107(2):98-104. 10.1093/trstmh/trs013 [DOI] [PubMed] [Google Scholar]
- 45. Carrera Vargas C, Narváez AO, Muzzio Aroca J, Shiguango G, Robles LM, Herrera C, et al. Seroprevalence of Trypanosoma cruzi infection in schoolchildren and in pregnant women from an Amazonian region in Orellana Province, Ecuador. Am J Trop Med Hyg. 2015;93(4):774-8. 10.4269/ajtmh.14-0807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martins-Melo FR, Lima M da S, Ramos ANJ, Jr, Alencar CH, Heukelbach J. Prevalence of Chagas disease in pregnant women and congenital transmission of Trypanosoma cruzi in Brazil: a systematic review and meta-analysis. Trop Med Int Health. 2014;19(8):943-57. 10.1111/tmi.12328 [DOI] [PubMed] [Google Scholar]
- 47. Howard EJ, Xiong X, Carlier Y, Sosa-Estani S, Buekens P. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG. 2014;121(1):22-33. 10.1111/1471-0528.12396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Salas Clavijo NA, Postigo JR, Schneider D, Santalla JA, Brutus L, Chippaux J-P. Prevalence of Chagas disease in pregnant women and incidence of congenital transmission in Santa Cruz de la Sierra, Bolivia. Acta Trop. 2012;124(1):87-91. 10.1016/j.actatropica.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 49. Torrico F, Alonso-Vega C, Suarez E, Rodriguez P, Torrico M-C, Dramaix M, et al. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg. 2004;70(2):201-9. 10.4269/ajtmh.2004.70.201 [DOI] [PubMed] [Google Scholar]
- 50. Luquetti AO, Tavares SB, Siriano LR, Oliveira RA, Campos DE, de Morais CA, et al. Congenital transmission of Trypanosoma cruzi in central Brazil. A study of 1,211 individuals born to infected mothers. Mem Inst Oswaldo Cruz. 2015;110(3):369-76. 10.1590/0074-02760140410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rodriguez-Guerineau L, Posfay-Barbe KM, Monsonis-Cabedo M, Juncosa-Morros T, Diana A, Wyler-Lazarevic CA, et al. Pediatric Chagas disease in Europe: 45 cases from Spain and Switzerland. Pediatr Infect Dis J. 2014;33(5):458-62. 10.1097/INF.0000000000000139 [DOI] [PubMed] [Google Scholar]
- 52. Russomando G, Cousiño B, Sanchez Z, Franco LX, Nara EM, Chena L, et al. Chagas disease: national survey of seroprevalence in children under five years of age conducted in 2008. Mem Inst Oswaldo Cruz. 2017;112(5):348-53. 10.1590/0074-02760160407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fumadó V, Juncosa T, Posada E, Fisa R, Gállego M, Gascón J. [Paediatric Chagas in a non-endemic area.] Enferm Infecc Microbiol Clin. 2014;32(5):293-6. Spanish. 10.1016/j.eimc.2013.04.024 [DOI] [PubMed] [Google Scholar]
- 54. Virreira M, Torrico F, Truyens C, Alonso-Vega C, Solano M, Carlier Y, et al. Comparison of polymerase chain reaction methods for reliable and easy detection of congenital Trypanosoma cruzi infection. Am J Trop Med Hyg. 2003;68(5):574-82. 10.4269/ajtmh.2003.68.574 [DOI] [PubMed] [Google Scholar]
- 55. Schijman AG, Bisio M, Orellana L, Sued M, Duffy T, Mejia Jaramillo AM, et al. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl Trop Dis. 2011;5(1):e931. 10.1371/journal.pntd.0000931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bua J, Volta BJ, Perrone AE, Scollo K, Velázquez EB, Ruiz AM, et al. How to improve the early diagnosis of Trypanosoma cruzi infection: relationship between validated conventional diagnosis and quantitative DNA amplification in congenitally infected children. PLoS Negl Trop Dis. 2013;7(10):e2476. 10.1371/journal.pntd.0002476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Abras A, Muñoz C, Ballart C, Berenguer P, Llovet T, Herrero M, et al. Towards a new strategy for diagnosis of congenital Trypanosoma cruzi infection. J Clin Microbiol. 2017;55(5):1396-407. 10.1128/JCM.02248-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Volta BJ, Perrone AE, Rivero R, Scollo K, Bustos PL, Bua J. Some limitations for early diagnosis of congenital Chagas infection by PCR. Pediatrics. 2018;141(Suppl 5):S451-5. 10.1542/peds.2016-3719 [DOI] [PubMed] [Google Scholar]
- 59. Schijman AG, Altcheh J, Burgos JM, Biancardi M, Bisio M, Levin MJ, et al. Aetiological treatment of congenital Chagas’ disease diagnosed and monitored by the polymerase chain reaction. J Antimicrob Chemother. 2003;52(3):441-9. 10.1093/jac/dkg338 [DOI] [PubMed] [Google Scholar]
- 60. Álvarez MG, Vigliano C, Lococo B, Bertocchi G, Viotti R. Prevention of congenital Chagas disease by Benznidazole treatment in reproductive-age women. An observational study. Acta Trop. 2017;174:149-52. 10.1016/j.actatropica.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 61. Fabbro DL, Danesi E, Olivera V, Codebó MO, Denner S, Heredia C, et al. Trypanocide treatment of women infected with Trypanosoma cruzi and its effect on preventing congenital Chagas. PLoS Negl Trop Dis. 2014;8(11):e3312. 10.1371/journal.pntd.0003312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Murcia L, Simón M, Carrilero B, Roig M, Segovia M. Treatment of infected women of childbearing age prevents congenital Trypanosoma cruzi infection by eliminating the parasitemia detected by PCR. J Infect Dis. 2017;215(9):1452-8. 10.1093/infdis/jix087 [DOI] [PubMed] [Google Scholar]
- 63. Soriano-Arandes A, Basile L, Ouaarab H, Clavería I, Gómez i Prat J, Cabezos J, et al. Controlling congenital and paediatric chagas disease through a community health approach with active surveillance and promotion of paediatric awareness. BMC Public Health. 2014;14(1):1201. 10.1186/1471-2458-14-1201 [DOI] [PMC free article] [PubMed] [Google Scholar]