Abstract
Background & objectives:
Diabetes mellitus (DM) is an important risk factor for tuberculosis and has received increasing emphasis. However, the reverse association of tuberculosis impacting blood sugar levels has not been well studied. The present study was conducted to evaluate the prevalence of hyperglycemia in patients with tuberculosis and assess its resolution following successful treatment of tuberculosis.
Methods:
In this prospective study, a total of 582 patients with tuberculosis were evaluated for hyperglycaemia [DM or impaired glucose tolerance (IGT)] with random blood sugar (RBS) and all patients with RBS >100 mg/dl were subjected to a 75 g oral glucose tolerance test (OGTT). All patients received thrice weekly intermittent Directly Observed Treatment Short Course (DOTS) for tuberculosis. Patients with hyperglycaemia were re-evaluated at the end of anti-tuberculosis treatment with an OGTT and glycated hemoglobin (HbA1c) levels to assess for glycaemic status.
Results:
In the present study, 41 of the 582 patients were found to have DM [7%, 95% confidence interval (CI) (5.2, 9.4)] while 26 patients were found to have IGT [4.5%, 95% CI (3, 6.5)]. Three patients were lost to follow up. Of the 26 patients with IGT, 17 [65.4%, 95% CI (46.1, 80.7)] reverted to euglycaemic status following successful treatment of tuberculosis, while the blood sugar levels improved in all patients with DM following treatment of tuberculosis.
Interpretation & conclusions:
Our study results show that tuberculosis adversely impacts glycaemic status with improvement in blood sugar levels at the end of successful treatment of tuberculosis. Longitudinal studies with large sample size are required to confirm these findings.
Keywords: Diabetes mellitus, impaired glucose tolerance, pancreatic dysfunction, stress-induced hyperglycaemia, tuberculosis
The adverse impact of diabetes mellitus (DM) on tuberculosis has been well known. Tuberculosis has been found to occur commonly among patients with DM, with general consensus for a unidirectional association between the two conditions since the majority of studies involved patients diagnosed with DM who went on to develop tuberculosis1. A multi-centre study showed a prevalence of 13 per cent of DM in tuberculosis2. DM is not only associated with an increased risk for tuberculosis, but it may also impact clinical presentation and treatment outcomes. While some studies have reported a higher sputum positivity, longer time to sputum conversion and higher rates of cavitation3,4, other studies have reported no difference5,6. This discrepancy may be related to the status of blood sugar control.
The role of tuberculosis in the development of DM was first suspected by Munkner7 and Nichols8 and has since found more favour among researchers with numerous animal and human studies linking a role for tuberculosis in the development of DM9,10,11,12. Human studies assessing the reversibility of impaired glucose tolerance (IGT) with the treatment of tuberculosis are, however, limited. This prospective study was carried out to determine the presence of hyperglycaemia (DM and IGT) in patients with tuberculosis and to assess its resolution following successful treatment of tuberculosis.
Material & Methods
This study was conducted at the All India Institute of Medical Sciences (AIIMS), New Delhi, India. Patients were recruited between May 2012 and December 2013. All patients were followed up for one year after recruitment. All consecutive patients of either sex, aged between 15 and 65 yr presenting to the Directly Observed Treatment, Short-course (DOTS) centre at AIIMS, New Delhi, with a diagnosis of tuberculosis (TB) were enrolled for the study. Both treatment naïve and treatment experienced patients were included. The patients suffering from human immunodeficiency virus (HIV) infection, multidrug-resistant tuberculosis, receiving corticosteroids, aged <15 yr, pregnant women and those not willing for follow up were excluded from the study.
Institutional Ethics Committee of AIIMS, New Delhi, India, approved the study. Written informed consent was obtained from each participant enrolled in the study.
Study design: In this prospective longitudinal study, eligible patients were enrolled after establishing the diagnosis of TB according to previously described criteria. A diagnosis of tuberculosis was made as per the World Health Organization criteria for smear-positive, smear-negative pulmonary TB or extrapulmonary TB13,14. In all patients, random blood sugar (RBS) was checked using a glucometer (Accu-Chek® Active, Roche Diagnostics, Switzerland). All patients with an RBS more than 100 mg/dl underwent a 75 g oral glucose tolerance test (OGTT) in the morning after eight hours overnight fasting. Patients were diagnosed to have IGT as per the WHO criteria15. Patients with IGT were re-evaluated with a 75 g OGTT and glycosylated haemoglobin (HbA1c) at the end of treatment. Patients with persistent IGT were re-evaluated one year after treatment. Patients received category I or category II intermittent thrice weekly anti-tuberculosis therapy from DOTS centre as per the Revised National Tuberculosis Control Programme guidelines16.
Statistical analysis: For all comparisons between patients with IGT and those without, Chi-square test or Fisher's exact test was used for categorical data while Student's t test and Wilcoxon rank-sum test were used for continuous data. The analysis was performed using Stata 11.2 for Windows (Stata Corp, College Station, TX, USA).
Results
A total of 582 patients were recruited in the study. Of these, 35 (6%) patients had type 2 DM. A RBS test was done in the remaining 547 patients, of whom 250 patients had blood sugars above 100 mg/dl and underwent an OGTT. Of these patients, six were newly diagnosed to have DM while 26 patients were found to have IGT, (Fig. 1). Thus, 41 of the 582 patients were found to have DM [7%, 95% confidence interval (CI) (5.2, 9.4)] while 26 patients were found to have IGT [4.5%, 95% CI (3, 6.5)]. Patients with IGT were older than euglycaemic patients (36.5 vs. 31 yr, P<0.05). There were no significant differences between the two groups of patients with respect to body mass index (BMI) or type of tuberculosis. Characteristics of patients with IGT and those with euglycaemic status are described in the Table.
Fig. 1.
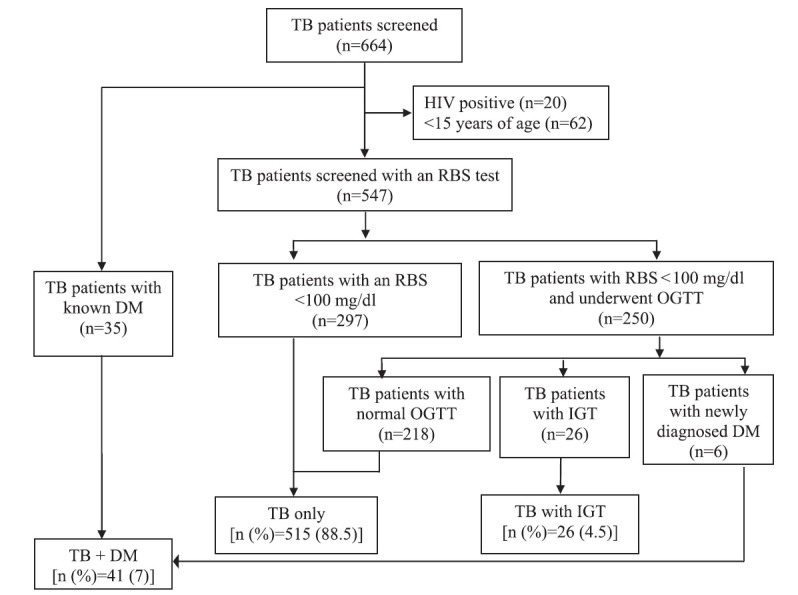
Study profile for the presence of hyperglycaemia in tuberculosis patients. TB, tuberculosis; RBS, random blood sugar level; OGTT, oral glucose tolerance test; DM, diabetes mellitus; IGT, impaired glucose tolerance.
Table.
Characteristics of patients with impaired glucose tolerance and diabetes mellitus (DM)
| Characteristics | IGT + TB (n=26) | TB only (n=515) | TB + DM (n=41) |
|---|---|---|---|
| Age (yr), | 36.5±12.2* | 31±11.1 | 45.6±8.6† |
| Sex | |||
| Male | 13 (50) | 285 (55.3) | 27 (65.9) |
| Female | 13 (50) | 230 (44.7) | 14 (34.2) |
| BMI (kg/m2) | 20.5±3.2 | 20.4±4 | 22.5±2.6† |
| Type of TB | |||
| Pulmonary TB | 13 (50) | 200 (38.8) | 21 (51.1) |
| Extrapulmonary TB | 13 (50) | 315 (61.2) | 20 (48.9) |
| Type of pulmonary TB | |||
| Sputum positive | 8 (61.5) | 103 (51.5) | 8 (38) |
| 1+ | 8 | 100 | 8 |
| 2+ | 0 | 3 | 0 |
| 3+ | 0 | 0 | 0 |
| Sputum negative | 5 (38.5) | 97 (48.5) | 13 (62) |
| Type of extrapulmonary TB | |||
| Lymph node | 9 (69.2) | 215 (67.9) | 10 (50)‡ |
| Pleural effusion | 1 (7.7) | 39 (12.4) | 7 (35)‡ |
| Pericardial effusion | 1 (7.7) | 1 (0.3) | 1 (5) |
| Peritonitis | 1 (7.7) | 5 (1.6) | 0 |
| Genitourinary | 43 (13.7) | 2 (10) | |
| Musculoskeletal | 1 (7.7) | 6 (1.9) | 0 |
| Meningitis | 0 | 2 (0.6) | 0 |
| Cutaneous | 0 | 4 (1.2) | 0 |
Age and BMI data have been expressed as mean±SD and sex, type of TB, type of pulmonary TB and type of extrapulmonary TB as number (%). *P<0.05 compared to TB only; †<0.001 compared to TB only; ‡P<0.05. TB, tuberculosis; IGT, impaired glucose tolerance; BMI, body mass index; SD, standard deviation
Patients with DM were older than non-diabetic patients (45.6 vs. 31 yr, P<0.001) and were found to have a higher BMI (22.5 vs. 20.4 kg/m2). There was no significant difference between the two groups with respect to type of tuberculosis (Table).
Of the 26 patients with IGT, three patients were lost to follow up, while a 75 g OGTT was repeated and a HbA1c was done at the end of treatment in the remaining 23 patients. All patients with DM were followed up with HbA1c measurements every three monthly and were treated with lifestyle modification measures and oral hypoglycaemic agents (OHA) and/or insulin as appropriate.
Of the 23 patients with IGT, eight patients (34.8%) were found to have persistent IGT while 15 patients were found to be euglycaemic at the end of treatment. Of the eight patients with persistent IGT at the end of treatment, five were lost to follow up while two patients reverted to euglycaemic status one year after completion of treatment and one patient died likely due to severe dengue infection during the dengue outbreak. Thus, 17 of the 26 patients with IGT reverted to euglycaemic status one year after the completion of treatment [65.4%, 95% CI (46.1, 80.7)] (Fig. 2). Of the 41 diabetic patients, six (14.6%) newly detected diabetic patients continued to remain diabetic one year after the completion of treatment.
Fig. 2.
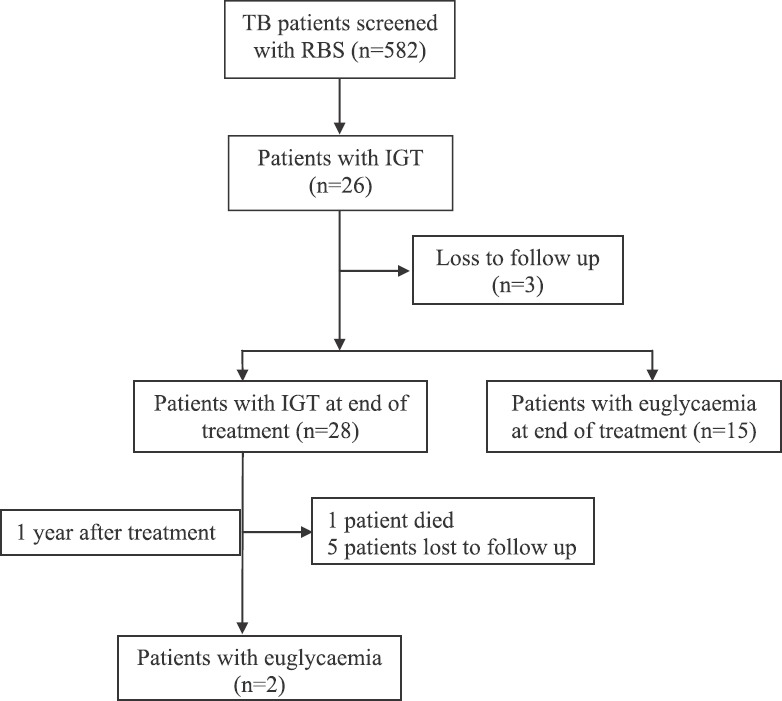
Follow up of patients with impaired glucose tolerance (IGT) at the end of treatment and on one-year post-treatment follow up. Abbreviations are as given in Fig.1.
Discussion
Tuberculosis and DM have a bi-directional relationship with each condition adversely impacting the other. There are several mechanisms by which tuberculosis can cause hyperglycaemia: the stress of disease leads to increased release of hormones such as cortisol which increase blood sugars; also release of various cytokines, chemokines and tubercular proteins may cause pancreatic dysfunction, the so-called ‘concomitant pancreatitis’17. This dysfunction is likely due to increased deposition of amylin within the pancreas consequent to increased beta cell function due to the transient hyperglycaemia at the onset of disease it may also occur due to direct invasion of the pancreas by the mycobacteria which often goes unrecognized9,17.
In the present study, seven per cent patients were found to have DM. The prevalence of DM varies from as high as 19.5 per cent in Kerala to 6.1 per cent in the Kashmir valley18,19, A large multi-centric study found 13 per cent prevalence of DM among tuberculosis across India2.
IGT was found in 4.5 per cent patients. This was lower compared to other studies which reported the IGT between 10.3 and 14 per cent20,21. However, these studies have been done in different parts of India and the prevalence IGT has regional variation22. In addition, our study had a higher proportion of patients with extrapulmonary TB while the association with hyperglycaemia was higher with pulmonary TB.
The limitation of the present study was that all patients with IGT received advice regarding lifestyle modification which could have also contributed to the improvement in glycaemic status. IGT is considered a risk factor for future development of DM. Although IGT in patients with TB may be related to the stress of infection and often reverts following successful treatment of TB, these patients remain at risk for future development of DM23. Further, the progression to DM increases the risk of recurrence of TB. Therefore, patients diagnosed with IGT should receive lifestyle modification and close follow up of blood sugar status and not be neglected as mere cases of transient stress hyperglycaemia.
In conclusion, our study showed the adverse impact of tuberculosis on glycaemic status. Given the high prevalence of active and latent tuberculosis infection in our country and the increasing prevalence of DM, further analyzing this association may contribute to filling the knowledge gap regarding the pathogenesis of DM and eventually enable a permanent cure for this condition. Long-term studies with large sample size are required after completion of anti-TB treatment to determine the reversible nature of these metabolic changes.
Acknowledgment:
The authors thank the State Tuberculosis Officer, Delhi for his valuable guidance in the conduct of this study. The authors would also like to thank all the participating patients and Vineet, Rohini, Vinod, Mikashmi and Jigyasa for facilitating the study.
Footnotes
Financial support & sponsorship: The study was supported by the JC Bose National Fellowship awarded to the second author (SKS) (SB/S2/JCB-04/2013), from the Department of Science & Technology, Ministry of Science and Technology, Government of India, New Delhi.
Conflicts of Interest: None.
References
- 1.Root HF. The association of diabetes and tuberculosis. N Eng J Med. 1934;210:1–13. [Google Scholar]
- 2.India Tuberculosis-Diabetes Study Group. Screening of patients with tuberculosis for diabetes mellitus in India. Trop Med Int Health. 2013;18:636–45. doi: 10.1111/tmi.12084. [DOI] [PubMed] [Google Scholar]
- 3.Magee MJ, Kempker RR, Kipiani M, Gandhi NR, Darchia L, Tukvadze N, et al. Diabetes mellitus is associated with cavities, smear grade, and multidrug-resistant tuberculosis in Georgia. Int J Tuberc Lung Dis. 2015;19:685–92. doi: 10.5588/ijtld.14.0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tatar D, Senol G, Alptekin S, Karakurum C, Aydin M, Coskunol I. Tuberculosis in diabetics: Features in an endemic area. Jpn J Infect Dis. 2009;62:423–7. [PubMed] [Google Scholar]
- 5.Bacakoǧlu F, Başoǧlu OK, Cok G, Sayiner A, Ateş M. Pulmonary tuberculosis in patients with diabetes mellitus. Respir Int Rev Thorac Dis. 2001;68:595–600. doi: 10.1159/000050578. [DOI] [PubMed] [Google Scholar]
- 6.Alisjahbana B, Sahiratmadja E, Nelwan EJ, Purwa AM, Ahmad Y, Ottenhoff TH, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis. 2007;45:428–35. doi: 10.1086/519841. [DOI] [PubMed] [Google Scholar]
- 7.Munkner T. Incidence of pulmonary tuberculosis among diabetics in the county of Vejle in 1944-1951. Acta Tuberc Scand. 1953;28:355–64. [PubMed] [Google Scholar]
- 8.Nichols GP. Diabetes among young tuberculous patients; a review of the association of the two diseases. Am Rev Tuberc. 1957;76:1016–30. doi: 10.1164/artpd.1957.76.6.1016. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz P. Amyloid degeneration and tuberculosis in the aged. Gerontologia. 1972;18:321–62. doi: 10.1159/000211943. [DOI] [PubMed] [Google Scholar]
- 10.Snider WR. Tuberculosis in canine and feline populations. Review of the literature. Am Rev Respir Dis. 1971;104:877–87. doi: 10.1164/arrd.1971.104.6.877. [DOI] [PubMed] [Google Scholar]
- 11.Engelbach K. Transitory diabetes mellitus in two tuberculotics. Beitr Klin Tuberk Spezif Tuberkuloseforsch. 1954;110:470–3. [PubMed] [Google Scholar]
- 12.Karachunskiĭ MA, Balabolkin MI, Beglarian NR. Changes in carbohydrate metabolism in patients with tuberculosis. Vestn Ross Akad Med Nauk. 1995;7:18–21. [PubMed] [Google Scholar]
- 13.Stop TB Department & Department of HIV/AIDS. Improving the diagnosis and treatment of smear-negative pulmonary and extrapulmonary tuberculosis among adults and adolescents. Geneva: World Health Organization; 2006. [Google Scholar]
- 14.World Health Organization. Guidelines for treatment of tuberculosis. 4th ed. Geneva: WHO; 2010. [Google Scholar]
- 15.World Health Organization. Geneva: WHO; 1999. Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO consultation. Part 1, diagnosis and classification of diabetes mellitus. [Google Scholar]
- 16.Revised National Tuberculosis Control Programme. Technical and operational guidelines for tuberculosis control. New Delhi: Central TB Division Directorate General of Health Services Ministry of Health and Family Welfare; 2005. [Google Scholar]
- 17.Broxmeyer L. Diabetes mellitus, tuberculosis and the mycobacteria: Two millenia of enigma. Med Hypotheses. 2005;65:433–9. doi: 10.1016/j.mehy.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Menon VU, Kumar KV, Gilchrist A, Sugathan TN, Sundaram KR, Nair V, et al. Prevalence of known and undetected diabetes and associated risk factors in central Kerala - ADEPS. Diabetes Res Clin Pract. 2006;74:289–94. doi: 10.1016/j.diabres.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 19.Zargar AH, Khan AK, Masoodi SR, Laway BA, Wani AI, Bashir MI, et al. Prevalence of type 2 diabetes mellitus and impaired glucose tolerance in the Kashmir valley of the Indian subcontinent. Diabetes Res Clin Pract. 2000;47:135–46. doi: 10.1016/s0168-8227(99)00110-2. [DOI] [PubMed] [Google Scholar]
- 20.Jain MK, Baghel PK, Agrawal R. Study of impaired glucose tolerance in pulmonary tuberculosis. Indian J Community Med. 2006;31:117–214. [Google Scholar]
- 21.Ramesh B, Nagaraj VT, Kumar A. A study of oral glucose tolerance in pulmonary tuberculosis. Sch J App Med Sci. 2013;1:739–44. [Google Scholar]
- 22.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian council of medical research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54:3022–7. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 23.Kapur A, Harries AD. The double burden of diabetes and tuberculosis - Public health implications. Diabetes Res Clin Pract. 2013;101:10–9. doi: 10.1016/j.diabres.2012.12.001. [DOI] [PubMed] [Google Scholar]


