Abstract
Background & objectives:
Human telomerase reverse transcriptase (hTERT) is the catalytic subunit of telomerase enzyme that maintains telomere ends by the addition of telomeric repeats to the ends of chromosomal DNA, and that may generate immortal cancer cells. Hence, the activity of telomerase is raised in cancer cells including cervical cancer. The present study aimed to validate the unique siRNA loaded chitosan coated poly-lactic-co-glycolic acid (PLGA) nanoparticle targeting hTERT mRNA to knock down the expression of hTERT in HeLa cells.
Methods:
The siRNA loaded chitosan coated polylactic-co-glycolic acid (PLGA) nanoparticles were synthesized by double emulsion solvent diffusion method. The characterization of nano-formulation was done to determine efficient siRNA delivery. MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay, reverse transcriptase-polymerase chain reaction (RT-PCR) and Western blot were performed to evaluate silencing efficiency of nano-formulation.
Results:
Size, zeta potential and encapsulation efficiency of nanoparticles were 249.2 nm, 12.4 mV and 80.5 per cent, respectively. Sustained release of siRNA from prepared nanoparticle was studied for 72 h by ultraviolet method. Staining assays were performed to confirm senescence and apoptosis. Silencing of hTERT mRNA and protein expression were analyzed in HeLa cells by RT-PCR and Western blot.
Interpretation & conclusions:
The findings showed that biodegradable chitosan coated PLGA nanoparticles possessed an ability for efficient and successful siRNA delivery. The siRNA-loaded PLGA nanoparticles induced apoptosis in HeLa cells. Further studies need to be done with animal model.
Keywords: Apoptosis, cervical cancer, chitosan, human telomerase reverse transcriptase, nanoparticle, siRNA
RNA interference (RNAi) is a process, in which exogenous genetic material [small interfering RNA (siRNA)] is introduced into cells or organism and can activate suppression of specific gene of interest1. It holds promise for the development of therapeutic gene silencing. The major hurdle facing siRNA therapeutics is non-specific target, immune stimulation problems, appropriate delivery and also poor cellular uptake of siRNAs due to their size and negative charge2. The formation of safe delivery vehicle for exogenous gene is a major area of research in gene therapy. Non-viral delivery systems such as organic and inorganic nanoparticles have also been used3.
The US Food and Drug Administration (FDA) approved polylactic-co-glycolic acid (PLGA) as biodegradable polymer because PLGA has biodegradable and biocompatible properties4. PLGA nanoparticles act as a cargo for both siRNA and plasmid DNA delivery. Merits of PLGA-based siRNA delivery are high stability, controlled drug release, lower toxicity, protection from nuclease degradation, high cellular uptake by endocytosis, capacity to reach target site and biodegradability5. The affinity of PLGA nanoarticles towards siRNA is absent due to their negative surface that leads to poor penetration of cell membrane6. To overcome this problem, a cationic excipient was added to the PLGA matrix during encapsulation7. Chitosan used as a cationic excipient confer a positive charge on the nanoparticles; hence, siRNA and PLGA readily bind and condense8. In the present study, the synthesized siRNA-loaded chitosan coated PLGA nanoparticles were designed for protecting and transporting siRNA at the site of action. The siRNA target gene human telomerase reverse transcriptase (hTERT) is a catalytic subunit of telomerase enzyme. Telomeres are maintained by telomerase, a multi-subunit enzyme with an RNA component, which acts as a template for making telomeric repeats and a protein reverse transcriptase component, hTERT, which catalyzes the synthesis of hexameric repeats TTAGGG8. Most of the cancer cells reactivate telomerase activity9, and it may be the target for treating cancers10. Although several studies have documented synthesis of chitosan-coated PLGA-loaded hTERT siRNA nanoparticle, but its effect on cancer cells remains unexplored. Hence, the present study was aimed to explore the silencing efficiency of siRNA-loaded chitosan coated PLGA nanoparticles targeting hTERT mRNA in HeLa cells.
Material & Methods
Cell lines and culture medium: HeLa (human cervical carcinoma) cell line was procured from the National Centre for Cell Sciences, Pune. Cells were propagated with (Dulbecco's modified Eagle's medium) DMEM medium (Sigma-Aldrich, USA) containing 10 per cent foetal bovine serum (FBS) (Gibco Life Technologies, USA), 100 IU/ml penicillin and 10 mg/ml streptomycin (HiMedia Laboratories Pvt. Ltd., Mumbai) in an incubator maintained at 37°C, 5 per cent CO2 humidified atmosphere.
Designing siRNA and synthesis: According to Reynolds criteria11, two different siRNAs were designed to target different regions of the coding sequences of the hTERT mRNA (GenBank AN: AF015950). Selected sequences were submitted to blast search in the Gen Bank to confirm that hTERT was targeted. The designed sequences were as follows: siRNA sense #1 (siRNA-1): 5′-GGAGCAAGUUGCAAAGCAUUG-3′, siRNA sense #2 (siRNA-2):5′ GGAACACCAAGAAGUUCA UCU-3′ and the custom-designed siRNA synthesized products purchased; (VBC-Biotech Service Gmblt, Austria) pre-designed and pre-validated hTERT siRNA (siRNA-3) was also purchased from (Santa Cruz Biotechnology, Inc., Europe).
Isolation of RNA and reverse transcriptase-(RT)-PCR: HeLa cells were treated with siRNA/RNAi-Max complex for 48 h according to the manufacturer's protocol (Thermo Fisher Scientific, USA) at 37°C for 48 h. The total RNA was isolated using TRIzol reagent (ThermoFishers Scientific, USA) and CDNA was prepared using SuPrime Script RT-PCR Premix (2X) (GENETBIO Inc., Korea). The hTERT and β-actin cDNA were amplified with the following primers; hTERT forward: 5’-GAGAACAAGCTTTGCGGG- 3’, reverse 5’-GGCCGGCATCTGAACAAAAG-3’, β-actin forward 5’-CGGGAAATCGTGCGTGAC-3’ and reverse 5’-CAGGAAGGAAGGCTGGAAGAG- 3’ (Eurofins Genomics, Bangalore). The thermal cycling conditions for amplification of the hTERT (225 bp) and β-actin (120 bp) fragments were as follows: 94° C for 5 min, followed by 35 cycles of 94° C, 45 sec; 60° C, 45 sec and 72° C, 45 sec. The PCR products were separated on a 2 per cent (w/v) agarose gel using 0.5×TAE (tris-acetate-EDTA) buffer and visualized by ethidium bromide staining (Sigma, USA).
Preparation of PLGA-loaded siRNA nanoparticles: The first aqueous phase containing 19 μg siRNA-2 in 300 μl Tris-EDTA was emulsified with organic phase (2 mg/ml of PLGA in 500 μl dichloromethane) by using probe sonicator for 60 sec to form the primary emulsion. The secondary w/o/w (water/ oil/ water) emulsion was formed by emulsification of primary emulsion with secondary aqueous phase [2 ml of 2% PVA (polyvinyl alcohol) containing various amounts of chitosan (0.3, 0.5 and 0.7 mg/ml) in RNase-free water]. The secondary emulsion (diluted with 4 ml of 2% PVA) containing dichloromethane was allowed to evaporate by stirring up to three hours at room temperature. Nanoparticles were collected with the help of centrifugation (Eppendorf Cooling centrifuge 5430 R Hamburg, Germany) at 1581×g and 4°C for 10 min and freeze-dried until use12.
Morphology, particle size, zeta potential and scanning electron microscope (SEM) analysis: Freeze-dried siRNA-2-loaded nanoparticles (1 mg) were suspended in RNAase-free water. Size and surface charge was analyzed by dynamic light scattering (DLS) and zeta potential (ζP) analyzers, respectively. All measurements were recorded in triplicates2. The morphological features of synthesized hTERT-siRNA-2-loaded chitosan coated PLGA nanoparticles were examined through scanning electron microscope (ZEISS EVO18, Carl Zeiss SMT GmbH, Germany).
Measurement of siRNA loading efficiency: The level of siRNA (%) absorbed onto the chitosan-coated PLGA nanoparticles was measured from free siRNA-2 content in the supernatant of the secondary emulsion obtained by centrifugation at 1581×g for 10 min. The free siRNA-2 content was read at 260 nm by ultraviolet-visible spectrophotometer (Touch Duo Spectrophotometer, Bio Drop, England). The per cent encapsulation efficency in the chitosan coated PLGA nanoparticle was given by the following formula13:
siRNA (encapsulated) = siRNA (total) – siRNA (supernatants)
% Encapsulation efficiency = 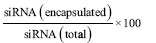
Sustained release of siRNA: One milligram of siRNA-2-loaded nanoparticles was incubated in TE (Tris-EDTA) buffer (pH 7.4) for 90 min at 37°C on a rotary shaker. At each time interval (0, 1, 2, 4, 8, 12, 24, 48, 72 h), 30 μl of nanoparticle suspension in TE buffer was removed to measure free siRNA by UV spectrophotometry12.
Serum stability assay: To determine serum stability of siRNA, the siRNA-2-loaded nanoparticles were incubated at 37°C with DMEM containing 10 per cent FBS. At each prefixed time interval (0, 0.5, 1, 2, 4, 8, 24, 48, 72 h), 30 μl of DMEM was taken out for electrophoresis. siRNA was extracted from nanoparticle by phenol-chloroform method2. Then, 2 per cent agarose gel electrophoresis was performed to determine the siRNA content in nanoparticle12.
Nanoparticle mediated cell transfection: HeLa cells were seeded in a six-well plate at a confluence of 50-60 per cent. Following cell attachment, cells were transfected with PLGA nanoparticles containing hTERTsiRNA-2 at a dose of 60 ng of siRNA-2. Cellular uptake of cy3 (cyanine-3) labelled hTERTsiRNA-2 by nanoparticle was confirmed by fluorescence microscopy (Floid™ Cell Imaging Station, Thermo Fisher Scientific, USA).
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay for cell viability: The comparison of cell inhibiting effects in HeLa cells treated with naked hTERTsiRNA chitosan coated hTERTsiRNA-loaded PLGA nanoparticle and blank nanoparticle were measured by MTT assay. For cellular adherence, cells were seeded in the 96-well plate (100 μl each well) under 5 per cent CO2 at 37°C and 95 per cent O2. The different amount of naked hTERTsiRNA, chitosan-coated hTERTsiRNA-loaded PLGA nanoparticle and blank nanoparticle were separately treated with cells for 48 h. Then, 20 μl MTT (5 mg/ml) was added and incubated for 4 h at 37°C. After aspirating supernatant, 150 μl of DMSO was used to each well, and at 490 nm the absorbance was analyzed using a microplate reader (Bio-Rad, USA).
Cells proliferation assay (trypan blue exclusion assay): Cells 1×104 were seeded per well in a 24 well culture plate and allowed to attach for overnight. On the next day, cells were treated with blank nanoparticle and chitosan-coated hTERTsiRNA-loaded PLGA nanoparticles. After incubation at 12, 24 and 48 h, respectively, cells were trypsinized and counted by Neubauer chamber with trypan blue exclusion method14. All experiments were performed in triplicates.
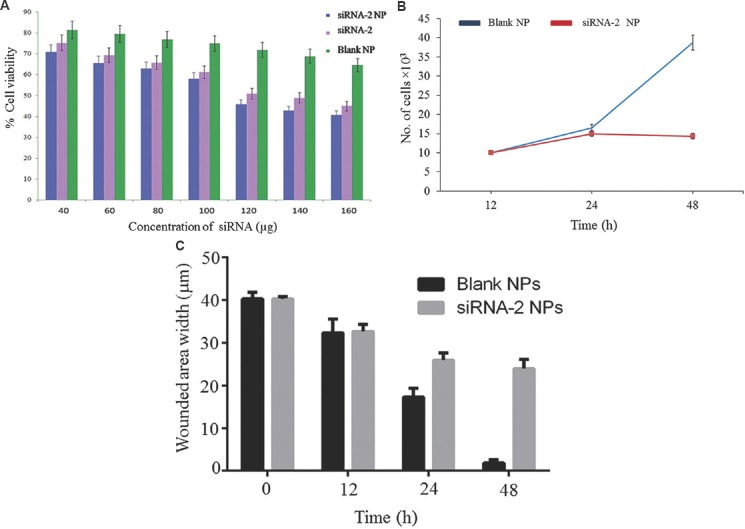
Cell migration assay: HeLa cells were seeded at final concentration of 1×105 per well in a six-well culture plate and treated with siRNA-2-loaded chitosan coated PLGA nanoparticle and blank nanoparticles for 48 h. Then wound was created with sterile sharp pipette tip and was examined (0, 12, 24 and 48 h) under microscope and photographed. The wounded areas were measured and expressed in μM.
Hoechst 33258 DNA staining: The HeLa cells in logarithmic growth phase were seeded at a final concentration of 1×105 in a 6-well culture plate and treated with siRNA-2 loaded chitosan-coated PLGA nanoparticle, blank nanoparticles, and naked siRNA-2 for 48 h. HeLa cells were stained with Hoechst 33258, and the changes in the nuclei of cells were examined and photographed by fluorescence microscopy.
Senescence-associated-β-galactosidase (SA-β-gal) assay: HeLa cells were seeded at a concentration of 1×103 per well and treated with siRNA-2-loaded chitosan coated PLGA nanoparticle, blank nanoparticles and naked siRNA-2 for 48 h. Then, HeLa cells were stained at pH 6.0 with X-Gal (5-bromo-4-chloro-3-ndolyl-β-D-galactopyranoside) as described previously15.
Reverse transcriptase-(RT)-PCR and Western blot: RT-PCR was performed to analyze expression of hTERT mRNA in HeLa cells treated with nanoencapsulated siRNA-2 and naked siRNA-2 along with blank nanoparticles as described by manufacturer's protocol (Thermo Fisher Scientific, USA). Western blot was performed to analyze the expression of hTERT protein after HeLa cells were treated with nanoencapsulated and naked siRNA-2, respectively along with blank nanoparticles. Total proteins were dissociated with trypsin and transferred to 1.5 ml Eppendorf tubes. After washing with phosphate-buffered saline (pH 7.2), cells were lysed with 1 ml radio immune precipitation buffer (RIPA) buffer and centrifuged at 44×g for five minutes, supernatants were collected, and the protein level was measured with the help of Bradford reagent (Sigma, USA). Proteins were separated on 10 per cent sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes followed by blocking overnight at room temperature. The membranes were incubated with primary antibodies (hTERT and β-actin) (Santa Cruz, USA) for two hours. TBST (tris-buffered saline with Tween 20) buffer was added to remove the unbound antibodies and incubated with secondary antibodies labelled with alkaline phosphatase for two hours and coloured with alkaline phosphatase. The gel documentation system (Bio-rad, USA) was used to analyze the results.
Results
The capacity of three independent siRNAs (hTERT SiRNA-1, -2, -3) that downregulate the hTERT mRNA expression in the HeLa cell line, was analyzed. One of the efficient hTERTsiRNA among these three was selected based on the result of RT-PCR as shown in Fig. 1. Knockdown efficiency of hTERTsiRNA-2 was greater than hTERTsiRNA-1 and hTERT siRNA-3. Hence, hTERT siRNA-2 with cy3 tag was selected for the nanoencapsulation, characterization and the silencing of hTERTmRNA.
Fig. 1.
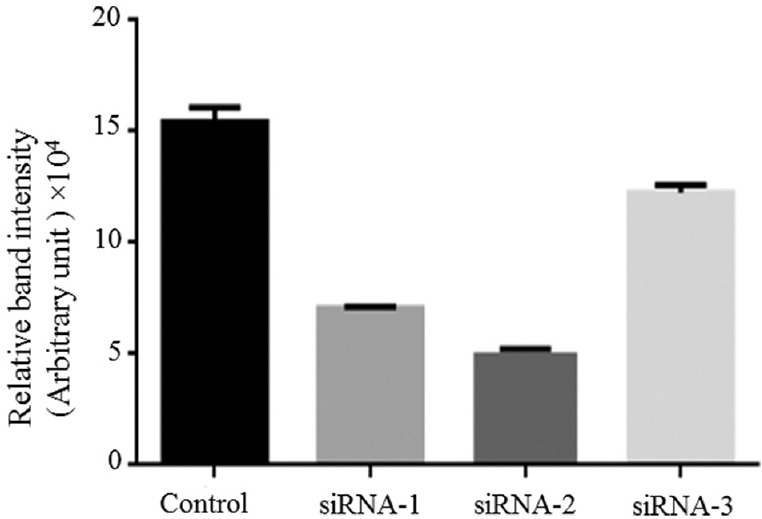
Knockdown the efficiency of siRNAs. Human telomerase reverse transcriptase mRNA expression in HeLa cells.
Morphology, particle size and zeta potential: The siRNA-2-loaded chitosan coated PLGA nanoparticles were synthesized with PVA as a stabilizer by double emulsion solvent diffusion method (DESE) technique. Particles in size range of 249.2 to 551.3 nm were obtained with polydispersity (PDI) values between 0.2 and 0.5. The zeta potential for all the formulations in this study was found between-1.25 and 12.4 mV. The effect of chitosan on size, PDI, surface charge and encapsulation efficiency of nanoparticles was also measured. The nanoparticle size was decreased when the concentration of chitosan increased. However, the zeta potential of nanoparticle was increased with increase of chitosan up to 0.7 mg/ml as shown in the Table. The optimized nanoformulation used for the preparation of nanoparticle was 0.7 mg/ml of chitosan and 2 mg/ml of PLGA (PLGA/Chitosan ratio: 2.86) and 2 mg/ml of PVA, 19 μg of total siRNA, and sonication time of primary emulsion formation was 60 sec. Zeta potential of +12.4 mV (Fig. 2A) and mean particle size of 249.2 nm in (Fig. 2B), were obtained from the final formulation. The morphology of siRNA-2-loaded chitosan coated PLGA nanoparticles was smooth and spherical (Fig. 2C).
Table.
Effect of chitosan on mean particle size, polydispersive index, zeta potential and per cent encapsulation
| Chitosan concentration (mg/ml) | Mean particle size (nm) | PDI | Zeta potential (mV) | Encapsulation efficiency (%) |
|---|---|---|---|---|
| 0.0 | 551.3 | 0.579 | −1.25 | 49.9 |
| 0.3 | 514.7 | 0.308 | 3.40 | 55.3 |
| 0.5 | 519.3 | 0.218 | 8.28 | 60.3 |
| 0.7 | 249.2 | 0.234 | 12.4 | 80.5 |
PDI, polydispersive index
Fig. 2.
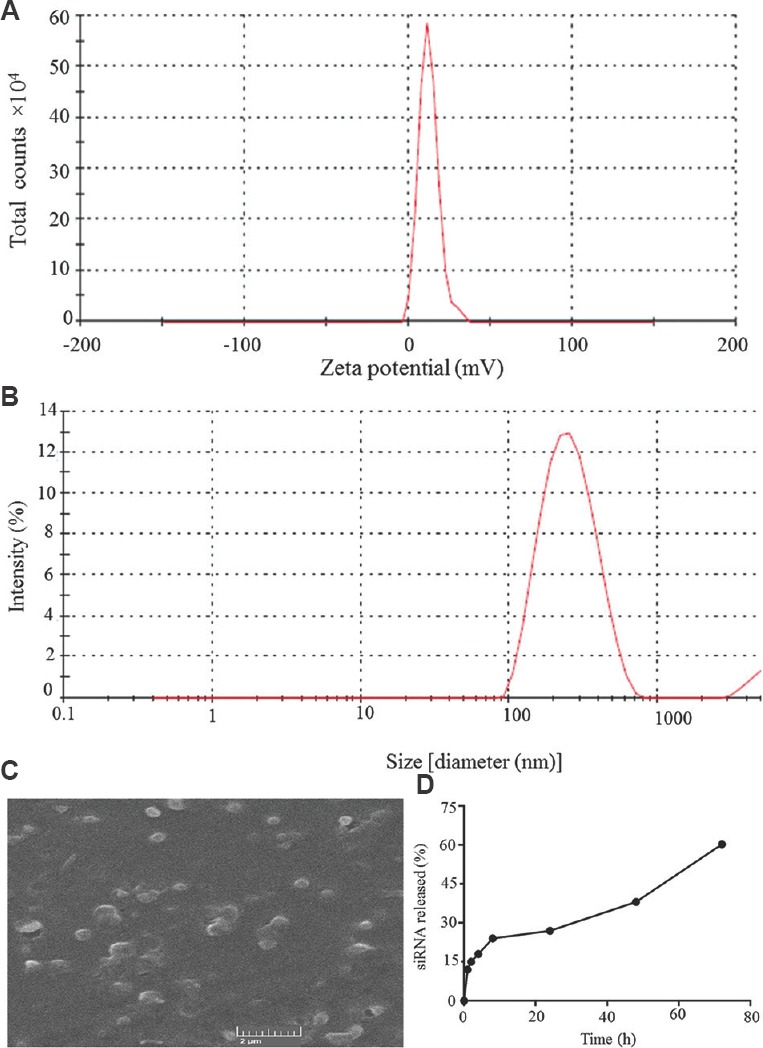
Characterization of siRNA2-loaded chitosan coated poly (D, L-lactide-co-glycolide) nanoparticles. (A) Zeta potential distribution; (B) Size distribution; (C) Scanning electron microscope image of siRNA-2 loaded chitosan coated poly (D, L-lactide-co-glycolide) nanoparticles; (D) Sustained release of siRNA-2 loaded chitosan coated poly (D, L-lactide-co-glycolide) nanoparticles.
Measurement of siRNA loading efficiency: The concentration of siRNA-loaded on the PLGA nanoparticles was calculated with the help of spectrophotometry by comparing with the total initial siRNA-2 concentration (19 μg). The siRNA-2 present in the supernatant (3.7 μg) was removed, and the remaining 15.3 μg present in precipitate was considered, and the siRNA-2 loading efficiency was found to be 80.5 per cent.
Sustained release of siRNA: The final formulation of siRNA-2-loaded nanoparticles releases siRNA-2 constantly for a long period. Around 50 per cent of siRNA was liberated over the period of 60 h (Fig. 2D).
Serum stability assay: Formulations were incubated with 10 per cent FBS to estimate their stability. On visual investigation of the brightness of bands, naked siRNA-2 was found to be intact for up to one hour. Thereafter, partial degradation took place, and complete degradation was observed at six hours (Fig. 3A). siRNA-2 loaded in chitosan coated PLGA nanoparticles was found to be intact for up to four hours (Fig. 3B). The partial degradation was observed at 12 h, and at 48 h siRNA was completely degraded.
Fig. 3.
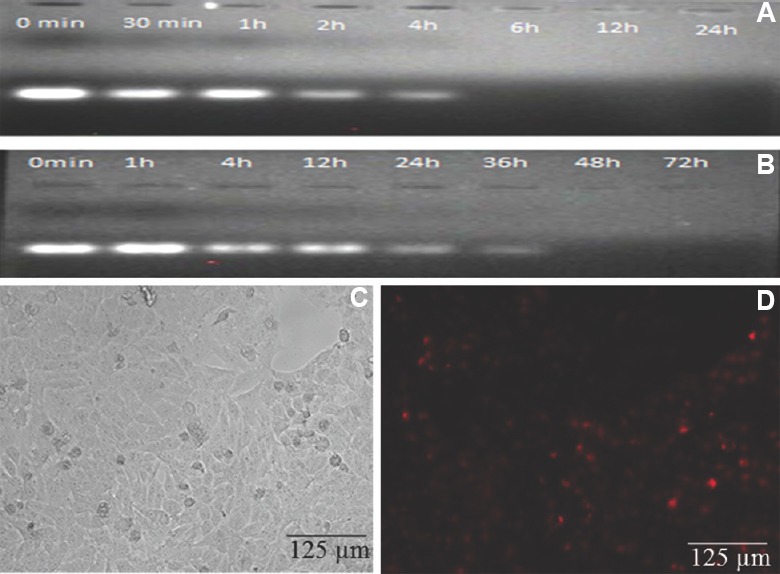
Serum stability of siRNA (A & B) and nanoparticles mediated transfection (C & D): (A) Serum stability of naked siRNA2; (B) Serum stability of siRNA2-loaded chitosan coated polylactic-co-glycolic acid (PLGA) nanoparticles incubated in 10 per cent of foetal bovine serum over a period of time; (C) Chitosan-coated PLGA nanoparticle-mediated siRNA2 transfection in HeLa cells (Phase contrast); (D) Chitosan-coated PLGA nanoparticle-mediated siRNA2 transfection in HeLa cell emitted fluorescence due to cy3 labelling with siRNA.
Nanoparticle mediated cell transfection: With the intracellular uptake of hTERTsiRNA-2-loaded chitosan coated PLGA nanoparticles in HeLa cells (Fig. 3C) the cells appeared red due to the presence of Cy3 in siRNA-2-treated HeLa cells (Fig. 3D).
MTT assay for cell viability: Cytotoxicity of the siRNA-loaded chitosan coated PLGA nanoparticles was measured by MTT method in HeLa cells. The siRNA-2-loaded chitosan coated PLGA nanoparticles, blank nanoparticles, naked siRNA-2-treated HeLa cells were incubated for 48 h. About 72-75 per cent cells were viable after 48 h treatment with the blank formulations. Whereas 29-38 per cent viability of cells was observed following treatment with siRNA-loaded chitosan coated PLGA nanoparticles as shown in Fig. 4A. There was no cytotoxicity observed in cells treated with chitosan, PLGA nanoparticles.
Fig. 4.
(A) In vitro cytotoxicity of the siRNA-2 loaded chitosan coated poly-lactic-co-glycolic acid of different concentration on HeLa cells as measured by MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay for cell viability. Cytotoxicity effect of chitosan-coated siRNA-2-loaded polylactic-co-glycolic acid (PLGA) nanoparticles (NP), naked siRNA-2, chitosan-coated PLGA nanoparticles (blank) in HeLa cells. The data describe the means ±SD of five independent experiments; (B) cell proliferation assay; (C) cell migration assay.
Cell proliferation assay: The hTERTsiRNA-2-loaded chitosan coated PLGA nanoparticle downregulated the cell proliferation rate when compared to blank nanoparticle-treated HeLa cells (Fig. 4B).
Cell migration assay: The hTERTsiRNA-2-loaded chitosan coated PLGA nanoparticle reduced the cell migration when compared to blank nanoparticle-treated cells. The wound was almost closed after 48 h in blank nanoparticle-treated cells. However, in hTERTsiRNA-2-loaded chitosan coated PLGA nanoparticle-treated cells, the size of the wound was 24 μm after 48 h (Fig. 4C).
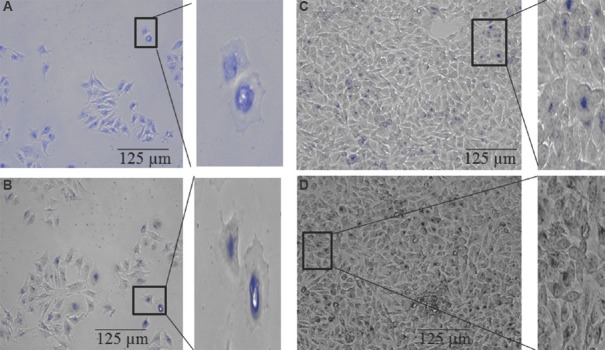
Senescence-associated-β-galactosidase (SA-β-gal) assay: Senescence-associated-β-galactosidase (SA-β-gal) activity was determined by incubating the HeLa cells with chromogenic substrate (X-Gal at pH 6.0) followed by bright-field microscopy, 48 h after treatment with siRNA-loaded chitosan coated PLGA nanoparticles and naked siRNA, blank nanoparticles. Intense blue staining indicated SA-β-gal activity and senescence. The siRNA-loaded chitosan loaded PLGA nanoparticles-treated cells displayed flattened morphology and intense blue staining (Fig. 5A). Naked siRNA-treated cell showed faint background staining (Fig. 5B), blank nanoparticles-treated cells and control cells were not stained due to the absence of SA-β-gal activity (Fig. 5C & D).
Fig. 5.
Induction of a senescence (A) siRNA2-loaded chitosan coated polylactic-co-glycolic acid (PLGA) nanoparticle-treated cells; (B) Naked siRNA2-treated cells; (C) Blank nanoparticle-treated cells; (D) Untreated cells from a separate inhibition experiment were stained for β-galactosidase activity at pH 6.
Hoechst 33,258 DNA staining: The hTERT siRNA-2 loaded chitosan coated PLGA nanoparticles induced apoptosis markedly but only a few apoptotic cells were seen after treatment with naked siRNA-2, blank nanoparticles and control. The siRNA-2 loaded chitosan coated PLGA nanoparticle-treated cell had elevated level (88.7%) of apoptotic cells and exhibited bright blue colour as shown in Fig. 6A, while naked siRNA-treated cells had a low level of apoptotic bodies as shown in Fig. 6B. Control cells and blank nanoparticle-treated cells (Fig. 6C and D) were seen with uniformly light blue nuclei under fluorescence microscope.
Fig. 6.
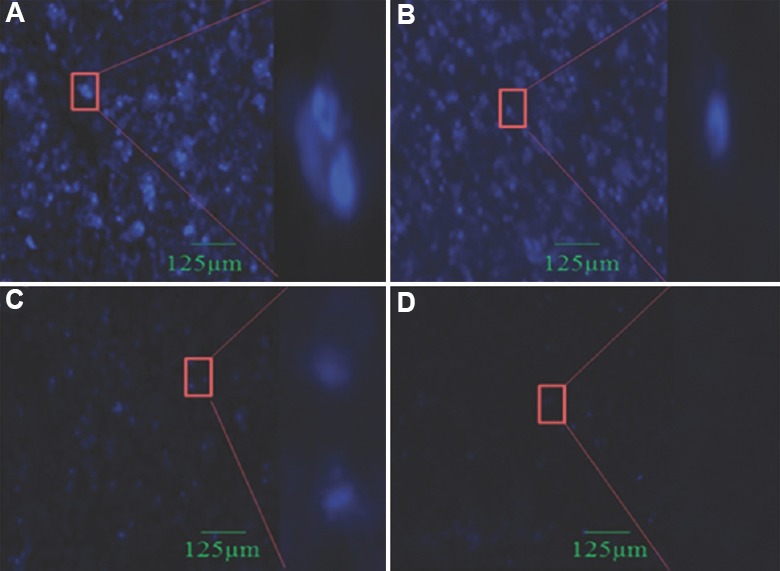
Hoechst 33258 staining: Apoptotic bodies are visualized in HeLa cells. Cells were treated with siRNA-2 loaded chitosan coated PLGA nanoparticle, naked siRNA-2 and blank nanoparticle. After 48 h incubation, cells were stained with Hoechst 33258 DNA staining and morphological changes were observed using fluorescence microscope (20×). (A) siRNA2 loaded chitosan coated polylactic-co-glycolic acid nanoparticle-treated cells; (B) Naked siRNA2-treated cells; (C) Blank nanoparticle-treated cells; (D) Untreated cells.
RT-PCR and Western blot analyses: The levels of hTERTmRNA and protein were reduced (53 and 42%) in cells treated with siRNA-loaded chitosan coated PLGA nanoparticles than naked siRNA-2-treated cell (Figs 7 & 8). Blank nanoparticles had no impact on the hTERTmRNA and protein levels. The level of hTERT protein reduction was greater in siRNA-2-loaded chitosan coated PLGA nanoparticle-treated cells when compared to both the blank and the naked siRNA-2-treated cells (42%), suggesting that the siRNA-2-loaded chitosan coated PLGA-treated cells could effectively reduce the hTERT protein level.
Fig. 7.
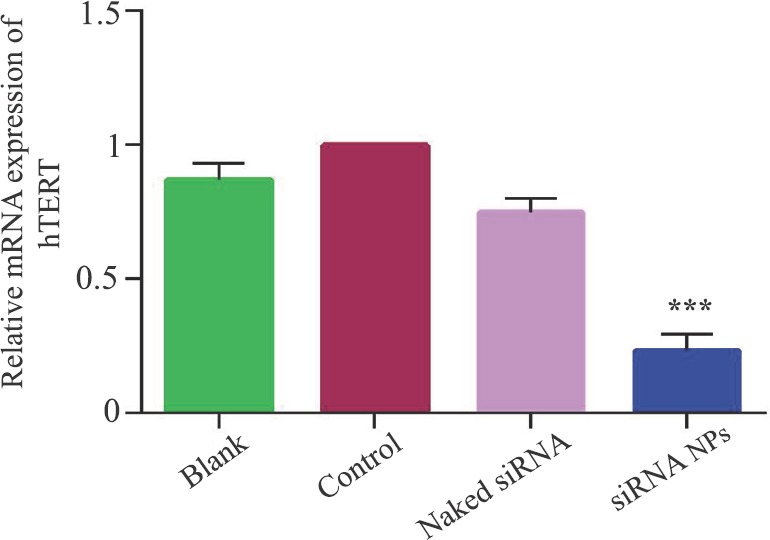
Human telomerase reverse transcriptase mRNA expression measured by reverse transcriptase-polymerase chain reaction in HeLa cells. Values are mean ± standard deviation of five independent tests. ***P<0.001 compared to control. NPs, nanoparticles.
Fig. 8.
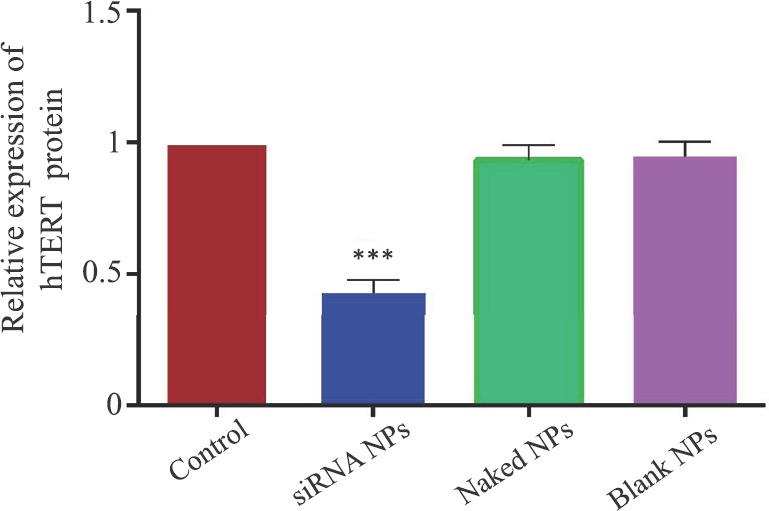
Human telomerase reverse transcriptase protein expression measured by Western blotting in HeLa cells. Values are mean±standard deviation of five independent tests. ***P<0.001, compared to control. NPs, nanoparticles.
Discussion
The present study showed that the successful use of siRNA was dependent on the development of a delivery vehicle. Non-toxic, biodegradable nanoparticle PLGA used as in vitro RNAi delivery vehicle. However, negative charge of PLGA nanoparticle was unfavourable for cellular uptake. To overcome these challenges, cationic surface modification was done in PLGA by chitosan. Previously, during encapsulation in PLGA particles, BSA was used as stabilizing agents, which reduced diffusion of siRNA into the outer water phase16. In addition, some cationic complexing agents (polyethylamine and polyL-lysine) may increase surface charge, and encapsulation efficiency as also the potential toxicity17,18. However, PVA and PLGA are nontoxic to cells. Chitosan has the ability to release the drug slowly from nanoparticles and also stabilizes the drug, and it gives a positive charge to PLGA19. Chitosan-coated siRNA-2 loaded PLGA nanoparticles enhances transfection into mucosal surfaces and favours siRNA delivery. The size of the particle and surface charge of nanoparticles are directly correlated with chitosan concentration2. Non-coated PLGA has a negative surface charge, but it is shifted to a positive charge by chitosan coating.
The alteration of chitosan concentration in the DESE method could decrease the size, but increased zeta potential and encapsulation efficiency of nanoparticles. When chitosan was used at 0.7 mg/ml concentration, the resulting siRNA-loaded chitosan coated PLGA nanoparticles had an appropriate size (249.2 nm) with the biological activity of the loaded siRNA. The siRNA-loaded chitosan coated PLGA nanoparticles had a spherical and smooth surface structure, nanoparticle diameter of 249.2 nm, good stability, zeta potential of 12.4 mV and a PDI (Polydispersity Index) index of 0.2, encapsulation efficiency of 80.5 per cent. Encapsulation efficiency found in our study was higher than the previous report8.
Release rates depend on the distribution of siRNA present in the nanoparticle matrix20. In vitro release profile of final formulation showed that about 35 per cent of siRNA released from nanoparticles over a period of 45 h and there was no connection between release profile and the level of siRNA loading. From the stability experiments, it was observed that the integrity of the siRNA was preserved up to 36 h under 10 per cent FBS which was harsh condition for siRNA. During the preparation of the nanoparticles by DESE, the siRNA degradation was not detectable.
Replicative senescence of cells showed increased intrinsic fluorescence caused by the acquisition of oxidatively destructed proteins and lipids. HeLa cells treated with siRNA loaded chitosan coated PLGA became large in size, contained multiple nuclei, had vacuolated cytoplasm and showed signs of SA-β-gal activity. Cell shrinkage, apoptotic bodies formation and chromatin condensation are the indication of apoptosis5. Bright blue spherical beads in HeLa cells treated with siRNA loaded chitosan coated PLGA nanoparticles were observed due to the presence of a large number of apoptotic cells, but cells treated with naked siRNA were stained with light blue fluorescence due to the presence of only a small number of apoptotic cells. Untreated and blank nanoparticle-treated cells were not stained with Hoechst 33,258 denoting that the cells were intact.
The levels of hTERT mRNA and protein expression were significantly reduced in cells treated with chitosan-coated siRNA-2 loaded PLGA nanoparticles compared to those treated with blank nanoparticles and naked siRNA-2. Bcl2 and caspase-3 are important regulators of mitochondrial-mediated apoptosis20. Bcl2 suppresses apoptosis and lengthens cell survival in cancer cells. After the binding of Bax with Bcl2 protein, cell death is induced. Caspase enzymes inactivate the anti-apoptotic function of Bcl2, and further, it enhances the cell death through non-Bax/Bcl2-dependent pathway21. The decrease of hTERT expression in HeLa cells may trigger the expression of apoptosis-related proteins. In the present study, decreased level of anti-apoptotic Bcl2 protein with elevated level of proapoptotic protein Bax and caspase-3 in HeLa cells treated with chitosan-coated siRNA-loaded PLGA nanoparticles was observed. This indicated that PLGA nanoparticles loaded with hTERT-targeting siRNA induced apoptosis through Bax/Caspase-3-dependent pathway.
In conclusion, siRNA-loaded PLGA nanoparticles were synthesized for targeting hTERT gene. The inclusion of chitosan in PLGA nanoparticles preparations significantly improved the loading efficiency of siRNA for sustained release of siRNA from nanoparticles and reduced degradation of siRNA by serum enzymes. Moreover, chitosan coated siRNA loaded PLGA nanoparticles were found to be non-toxic. Besides, chitosan coated PLGA nanoparticle enhances siRNA delivery in HeLa cells. The decrease of hTERT expression in HeLa cells triggers senescence and apoptosis. Shi et al22 have reported that knockdown of hTERT by siRNA inhibits cervical cancer cell growth through PI3k/Akt pathway to a certain extent. However, the present results showed that the siRNA-loaded chitosan coated PLGA nanoparticle targeting hTERT induced apoptosis in cervical cancer cells.
Acknowledgment:
The authors thank the DST-FIST for the instrument facility in the department of Biochemistry, School of Life Sciences, Bharathidasan University, Tiruchirappalli.
Footnotes
Financial support & sponsorship: This work was supported by the Women Scientists Scheme-A, Department of Science and Technology, Government of India [Grant DST: SR/WOS-A/LS-37/2013 dated: 26/11/2013 (2013-2017)] received by the first author (ALN).
Conflicts of Interest: None.
References
- 1.Voorhoeve PM, Agami R. Knockdown stands up. Trends Biotechnol. 2003;21:2–4. doi: 10.1016/s0167-7799(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Li Z, Han Y, Liang LH, Ji A. Nanoparticle-based delivery system for application of siRNA in vivo . Curr Drug Metab. 2010;11:182–96. doi: 10.2174/138920010791110863. [DOI] [PubMed] [Google Scholar]
- 3.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8:526–33. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singha K, Namgung R, Kim WJ. Polymers in small-interfering RNA delivery. Nucleic Acid Ther. 2011;21:133–47. doi: 10.1089/nat.2011.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koganti S, Jagani HV, Palanimuthu VR, Mathew JA, Rao MC, Rao JV. In vitro and in vivo evaluation of the efficacy of nanoformulation of siRNA as an adjuvant to improve the anticancer potential of cisplatin. Exp Mol Pathol. 2013;94:137–47. doi: 10.1016/j.yexmp.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Tahara K, Sakai T, Yamamoto H, Takeuchi H, Kawashima Y. Establishing chitosan coated PLGA nanosphere platform loaded with wide variety of nucleic acid by complexation with cationic compound for gene delivery. Int J Pharm. 2008;354:210–6. doi: 10.1016/j.ijpharm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura TM, Cech TR. Reversing time: Origin of telomerase. Cell. 1998;92:587–90. doi: 10.1016/s0092-8674(00)81123-x. [DOI] [PubMed] [Google Scholar]
- 8.Meyerson M. Role of telomerase in normal and cancer cells. J Clin Oncol. 2000;18:2626–34. doi: 10.1200/JCO.2000.18.13.2626. [DOI] [PubMed] [Google Scholar]
- 9.Zisuh AV, Han TQ, Zhan SD. Expression of telomerase & its significance in the diagnosis of pancreatic cancer. Indian J Med Res. 2012;135:26–30. doi: 10.4103/0971-5916.93420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A. Rational siRNA design for RNA interference. Nat Biotechnol. 2004;22:326–30. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- 11.Cun D, Foged C, Yang M, Frøkjaer S, Nielsen HM. Preparation and characterization of poly(DL-lactide-co-glycolide) nanoparticles for siRNA delivery. Int J Pharm. 2010;390:70–5. doi: 10.1016/j.ijpharm.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 12.Jagani HV, Josyula VR, Palanimuthu VR, Hariharapura RC, Gang SS. Improvement of therapeutic efficacy of PLGA nanoformulation of siRNA targeting anti-apoptotic Bcl-2 through chitosan coating. Eur J Pharm Sci. 2013;48:611–8. doi: 10.1016/j.ejps.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Mukerjee A, Shankardas J, Ranjan AP, Vishwanatha JK. Efficient nanoparticle mediated sustained RNA interference in human primary endothelial cells. Nanotechnology. 2011;22:445101. doi: 10.1088/0957-4484/22/44/445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mauro MO, Sartori D, Oliveira RJ, Ishii PL, Mantovani MS, Ribeiro LR. Activity of selenium on cell proliferation, cytotoxicity, and apoptosis and on the expression of CASP9, BCL-XL and APC in intestinal adenocarcinoma cells. Mutat Res. 2011;715:7–12. doi: 10.1016/j.mrfmmm.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo . Proc Natl Acad Sci U S A. 1995;92:9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanco D, Alonso MJ. Protein encapsulation and release from poly(lactide-co-glycolide) microspheres: Effect of the protein and polymer properties and of the co-encapsulation of surfactants. Eur J Pharm Biopharm. 1998;45:285–94. doi: 10.1016/s0939-6411(98)00011-3. [DOI] [PubMed] [Google Scholar]
- 17.Patil Y, Panyam J. Polymeric nanoparticles for siRNA delivery and gene silencing. Int J Pharm. 2009;367:195–203. doi: 10.1016/j.ijpharm.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blum JS, Saltzman WM. High loading efficiency and tunable release of plasmid DNA encapsulated in submicron particles fabricated from PLGA conjugated with poly-L-lysine. J Control Release. 2008;129:66–72. doi: 10.1016/j.jconrel.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawashima Y, Handa T, Kasai A, Takenaka H, Lin SY, Ando Y. Novel method for the preparation of controlled-release theophylline granules coated with a polyelectrolyte complex of sodium polyphosphate-chitosan. J Pharm Sci. 1985;74:264–8. doi: 10.1002/jps.2600740308. [DOI] [PubMed] [Google Scholar]
- 20.Jeyamohan S, Moorthy RK, Kannan MK, Arockiam AJ. Parthenolide induces apoptosis and autophagy through the suppression of PI3K/Akt signaling pathway in cervical cancer. Biotechnol Lett. 2016;38:1251–60. doi: 10.1007/s10529-016-2102-7. [DOI] [PubMed] [Google Scholar]
- 21.Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, et al. Conversion of bcl-2 to a bax-like death effector by caspases. Science. 1997;278:1966–8. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 22.Shi YA, Zhao Q, Zhang LH, Du W, Wang XY, He X, et al. Knockdown of hTERT by siRNA inhibits cervical cancer cell growth in vitro and in vivo . Int J Oncol. 2014;45:1216–24. doi: 10.3892/ijo.2014.2493. [DOI] [PubMed] [Google Scholar]