Abstract
Background
Self-monitoring of blood pressure is common but how telemonitoring with a mobile healthcare (mHealth) solution in the management of hypertension can be implemented by patients and healthcare professionals (HCPs) is currently unclear.
Aim
Evaluation of facilitators and barriers to self- and telemonitoring interventions for hypertension within the Telemonitoring and Self-monitoring in Hypertension (TASMINH4) trial.
Design and setting
An embedded process evaluation of the TASMINH4 randomised controlled trial (RCT), in the West Midlands, in UK primary care, conducted between March 2015 and September 2016.
Method
A total of 40 participants comprising 23 patients were randomised to one of two arms: mHealth (self-monitoring by free text/short message service [SMS]) and self-monitoring without mHealth (self-monitoring using paper diaries). There were also15 healthcare professionals (HCPs) and two patient caregivers.
Results
Four key implementation priority areas concerned: acceptability of self- and telemonitoring to patients and HCPs; managing data; communication; and integrating self-monitoring into hypertension management (structured care). Structured home monitoring engaged and empowered patients to self-monitor regardless of the use of mHealth, whereas telemonitoring potentially facilitated more rapid communication between HCPs and patients. Paper-based recording integrated better into current workflows but required additional staff input.
Conclusion
Although telemonitoring by mHealth facilitates easier communication and convenience, the realities of current UK general practice meant that a paper-based approach to self-monitoring could be integrated into existing workflows with greater ease. Self-monitoring should be offered to all patients with hypertension. Telemonitoring appears to give additional benefits to practices over and above self-monitoring but both need to be offered to ensure generalisability.
Keywords: blood pressure, hypertension, qualitative research
INTRODUCTION
Mobile health care (mHealth), defined as the use of mobile and wireless technologies for health,1 has the potential to improve access to, and use of, health services. Digital health interventions that can be delivered by mobile phone offer scalable, potentially cost-effective ways to improve medication-taking behaviours and include promising tools for supporting hypertension self-management.2
Hypertension or high blood pressure (BP)3 is the most significant risk factor globally for cardiovascular diseases, such as heart attack or stroke, and lowering BP reduces these outcomes.4–7 In England, approximately 30% of adult males and females have hypertension, with little recent change in prevalence, but many remain uncontrolled.8
Self-monitoring, with or without additional support such as provision of educational materials, telecounselling, or telemonitoring (electronic transmission of BP data), has been shown to lower BP, with greater intensity of co-intervention associated with greater effect on BP.9
Evidence for the use of BP self-monitoring values by GPs to titrate antihypertensive medication in primary care has until recently been equivocal,10,11 but this has changed with the Telemonitoring and Self-management in Hypertension (TASMINH4) trial.12
TASMINH4, a national randomised controlled trial (RCT) in 138 general practices, was designed to evaluate clinician antihypertensive titration using self-monitored BP values either sent to clinicians by free short message service (SMS: telemonitoring) or manually posted to surgeries via paper diaries (self-monitoring alone). After 1 year, those in both self-monitoring groups had significantly lower systolic BP than those whose medication was adjusted using clinic readings.12
The telemonitoring group had more rapid BP reductions and both groups were prescribed more antihypertensive medication. No significant changes were detected in adherence to antihypertensive medication or to lifestyle factors.
In this study the researchers evaluated the trial processes to understand how the self-monitoring interventions used in TASMINH4 for BP management were implemented by patients and healthcare professionals (HCPs), by identifying any facilitators and barriers promoting or inhibiting implementation.
METHOD
Participants
The study population for this qualitative study included patients, their carers (defined as a spouse/friend/relative who identified themselves as helping patients with any aspect of hypertension management), and HCPs employed in practices based in the West Midlands taking part in the TASMINH4 RCT (ISRCTN 83571366), registered 17 July 2014.13
How this fits in
| Self-monitoring of blood pressure is common but the routine implementation of telemonitoring by healthcare professionals and patients is currently unclear. This embedded study of the TASMINH4 trial highlights that telemonitoring delivered by mobile phone was convenient and easy to implement in daily practice. Healthcare professionals and patients valued the ease of communication from telemonitoring and the automated calculation of average blood pressure, but found that paper-based recording integrated better with current workflows in UK general practice. Telemonitoring using an mHealth solution is a promising tool and should be offered for supporting hypertension self-management alongside traditional paper-based recording. |
The TASMINH4 trial commenced in November 2014 and phased recruitment of patients to the present qualitative study commenced between March 2015 and September 2016. Patients aged >35 years with uncontrolled hypertension <140/90 mmHg were eligible for this process evaluation.12 Patients not agreeing to participate were excluded. For practical reasons, all interviews were conducted in central England.
Study processes
The authors consulted established criteria in the reporting of the present qualitative study.14 Full details of the TASMINH4 interventions have been published previously.12,13 In brief, participants were randomised to intervention and control (usual care) groups.
Intervention groups comprised:
self-monitoring alone (self-monitoring plus recording readings on paper diaries and posting these to the practice); and
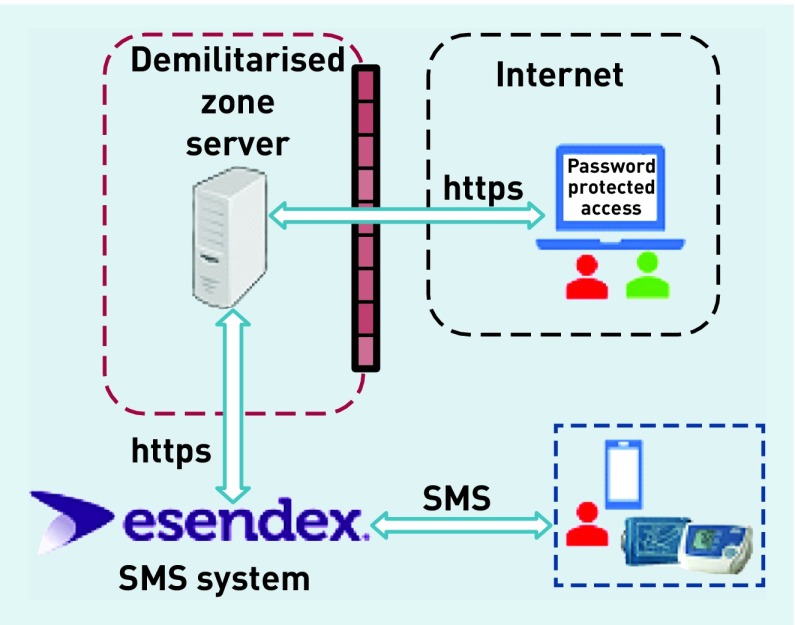
self-monitoring with telemonitoring (self-monitoring plus sending readings via an SMS text-based telemonitoring service with web-based data entry back up — mHealth solution) (Figure 1).
Figure 1.
Overall architecture deployed for the telemonitoring arm of the randomised controlled study.
Following randomisation, all participants were asked to attend their own GP for a medication review. GPs used self-monitored BP to titrate antihypertensive medication in both self-monitoring groups. Participants randomised to control groups (usual care) were managed with titration of antihypertensive treatment based on clinic BP measurements at the discretion of their attending HCP. Box 1 shows participant training and further details of the interventions. Participants randomised to the self-monitoring interventions self-monitored BP for 12 months.
Box 1.
Participant training and intervention description
| Patients |
| Patients randomised to self-monitoring were shown how to use a validated automated electronic sphygmomanometer at enrolment stage. They were asked to monitor their own BP in their non-dominant arm, twice each morning and evening, for the first week of every month using standard recommendations, and their GPs were asked to use the self-monitored measurements for titration of antihypertensive medication. |
| Self-monitoring alone using paper-based diaries |
| For participants randomised to self-monitoring using a paper-based diary, a simple colour chart was used to train participants to attend their practice for BP checks in the light of very high or very low readings. At the end of each monitoring week they were asked to record their readings on paper and send them for review to their practice in a reply-paid envelope. |
| Self-monitoring with telemonitoring |
| Participants randomised to self-monitoring using telemonitoring (mHealth) were trained to send readings via a simple free SMS text-based telemonitoring service with web-based data entry back-up. The telemonitoring system incorporated an algorithm that alerted participants to contact their surgery in the light of very high or very low readings, reminded them if insufficient readings were transmitted, prompted them to contact their practice if their average BP was above target, and presented readings to attending clinicians via a web interface. This secure web page automatically calculated mean BP for each monitoring week, highlighted very high or very low readings, and presented a graphical display of BP measurements. |
| Healthcare professionals |
| Healthcare professionals and relevant practice staff were trained in study procedures at the beginning of the trial by a standardised presentation delivered by the study research team or local clinical network facilitators. This included the methods by which patients in different intervention groups reported their BP readings. The package included a discussion of the key aspects of data monitoring and management according to participation in an RCT. Healthcare professionals were given autonomy to tailor their implementation of the self-monitoring interventions alongside routine clinical practice, titrating and prescribing medication as appropriate. |
BP = blood pressure. RCT = randomised controlled trial.
Sampling strategy
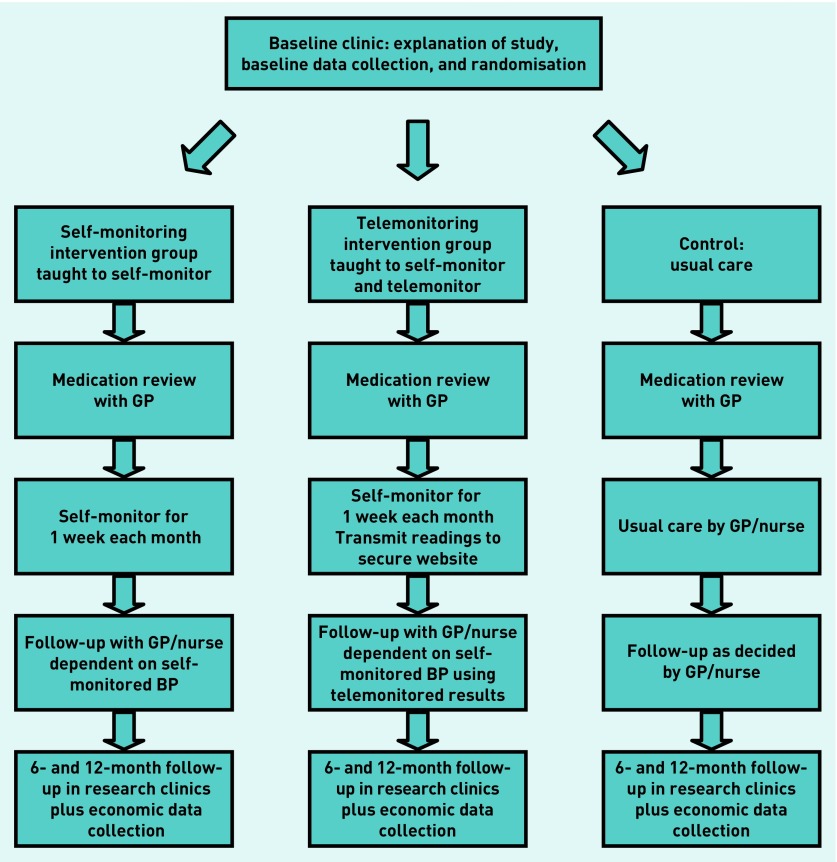
Participants were recruited from a convenience sample of two areas: Birmingham and the Black Country (BBC); and West Midlands South (WMS), both regions within central England. This area was chosen because together they cover a diverse range of patients in terms of levels of social deprivation and urban/rural diversity. Participants were purposefully sampled15 to reflect a range of deprivation levels16 and to ensure a range of views based on sex, participant type (HCP or patient), and randomisation arm. Usual-care participants were interviewed to add further context; however, as the present study focused on understanding the implementation of the self-monitoring interventions in management of hypertension, their views were not reported here. Caregivers identified as assisting with self-monitoring were asked to provide consent and then interviewed separately in their homes. HCPs were interviewed at their respective practices. The flow of trial participants through TASMINH4 is outlined in Figure 2.
Figure 2.
Patient flow through TASMINH4 Trial 1.
A total of 40 participants were included, of which 23 patients were randomised to one of the two arms: mHealth (self-monitoring by free text/SMS) and self-monitoring without mHealth (self-monitoring using paper diaries). The remaining participants comprised 15 HCPs and two patient caregivers.
Design and data collection
Interviews were carried out between November 2015 and September 2016, parallel to trial data collection, recruiting participants after a minimum of 6 months of trial experience. The interviews were conducted by multiple researchers whose backgrounds and disciplines included health psychology, sociology, and nursing. Structured topic guides modified to suit each intervention arm were used, informed by a previous self-management study.17 Interview question topic guides for patients in both interventions (paper or text message-based monitoring) and for HCPs are available from the authors on request. Each interview lasted approximately 1 hour, and was audiorecorded and transcribed verbatim. Recruitment continued until data saturation for implementation themes was reached within patient and HCP groups separately.18 In line with the authors’ analysis approach, the authors sought perspectives from three key informants involved directly in the trial, that is, patients, their carers, and HCPs.
Data analysis
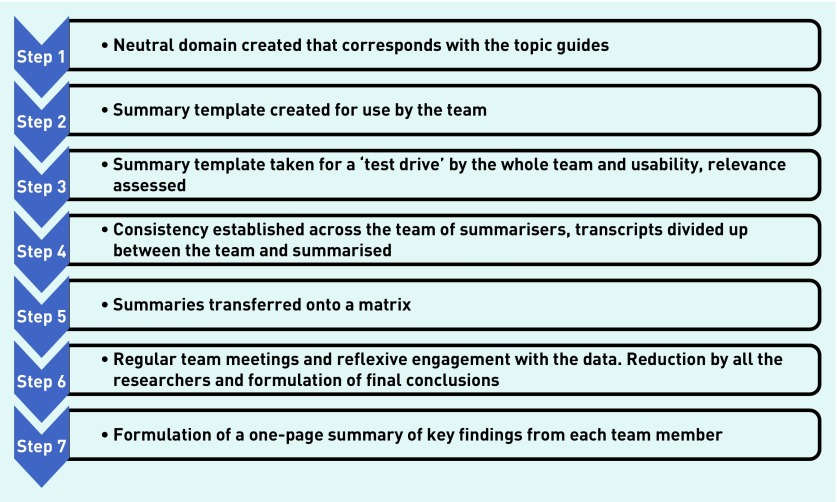
Hamilton’s rapid-analysis approach was used to understand how patients and HCPs adopted the interventions.19 This is a ‘tailored approach’ of an application of information and strategies for rapid-cycle projects from the ‘rapid assessment process’ pioneered by John Beebe in 2001. This approach has been used in many different fields by various individuals. Although the TASMINH4 trial was not in itself a rapid project, qualitative interviews were conducted alongside the trial, and analysis of incoming data was required to be assessed rapidly as part of the process evaluation. Assessment of data through team-based qualitative inquiry involving multiple researchers in data collection and analysis enables intensive triangulated qualitative inquiry to iteratively provide understanding from the ‘insider’s’ perspective.19,20 Distinct from other conventional approaches, this form of qualitative inquiry and method is designed to give a preliminary understanding of key themes arising out of data designed for situations where information is needed within a short timeframe, for example, to inform a trial or where service change needs to be implemented quickly, rather than a more in-depth understanding. Importantly it uses methods that give a systematic approach in so doing.19 Figure 3 outlines the processes involved in rapid analysis using templates (the templates are available from the authors on request) developed by four of the researchers based on the topic guides’ contents and derived for HCPs and patients separately. These templates were subsequently refined after a period of ‘road testing’19 and the domains were reclassified through a number of phases to yield four key areas.
Figure 3.
Flow diagram of the rapid-analysis approach. Modified version adapted from Hamilton.19
RESULTS
Of the 18 practices selected, 15 agreed to participate. Of the 59 trial patients listed within these 15 practices, 39 were approached, six did not respond, and three declined to participate, resulting in 30 interviewed patient participants (including seven in usual care). In addition, two caregivers and 15 HCPs were also interviewed. Characteristics of the study population and participating practices are detailed in Table 1.
Table 1.
Summary of participant and practice characteristics, N = 40
| Participant characteristic | Participants, n |
|---|---|
| Healthcare professionals, N= 15 | |
| GP | 11 |
| Practice nurse | 4 |
|
| |
| Sex | |
| Male | 7 |
|
| |
| Patients/carers, N= 25a | |
| Ethnicity | |
| White | 21 (and 2 carers) |
| Black/Asian/mixed/other | 2 |
|
| |
| Sex | |
| Male | 18 |
|
| |
| Intervention | |
| Self-monitoring plus text (telemonitoring) | 13 (and 2 carers) |
| Self-monitoring plus post (paper diary) | 10 |
|
| |
| Practice location | |
| BBC, number of practices = 12 | 30 (10 HCPs, 19 patients, 1 carer) |
| WMS, number of practices = 3 | 10 (5 HCPs, 4 patients, 1 carer) |
Total includes two carers from self-monitoring plus text arm. BBC = Birmingham and the Black Country. HCPs = healthcare professionals. WMS = West Midlands South.
Four key priority areas emerged that related to how the interventions were applied within participating practices. The facilitators and barriers to self-monitoring and telemonitoring are summarised in Box 2, classified by priority area.
Box 2.
Facilitators and barriers to self-monitoring using paper-based diaries and telemonitoring interventions (mHealth), classified by key implementation priority area
| Intervention group | |||
|---|---|---|---|
|
| |||
| Self-monitoring with mHealth | Self-monitoring without mHealth | ||
|
|
|
||
| Facilitators | Barriers | Facilitators | Barriers |
| Acceptability | |||
| • Simple, quick, and easy to use, technology widely available | • May not suit all people across the wider population, for example, less technologically-minded patients | • Non-technical alternative, more usable across a wider population | • Paperwork unwieldy |
| • Active patient engagement, empowerment to take control of ‘own’ BP | • Not all patients want to be actively engaged with BP | • Active patient engagement empowerment to take control of ‘own’ BP | • Not all patients want to be actively engaged with BP |
|
| |||
| Managing data | |||
| • Easily accessible online portal for HCP to view monthly BP readings | • Separate website to log into, not linked to practice’s clinical system to enter average BP calculations | • Hard copies/written record of BP data for every patient, easily scanned to practice’s clinical system | • Extra workload for health professional/other practice staff to process the paperwork (BP readings), for example, scanning/data entry/averaging |
| • Automatic calculation of average BP reading | • Average BP value does not automatically import to the practice’s clinical system | • Easy view of the range of BP readings across the monitoring week | • Risk of human error while manually calculating a weekly average and entering monthly BP readings for each patient |
| • Web-based visual metric of monthly average BP | • May require help of others to make the system work, such as partner assistance, using relative’s phone | • Once scanned in, manual written log was integral to the electronic health record | _ |
| • Encryption on own mobile phone device keeps data secure | • Confidentiality and security concerns if medical advice is missed/not read, or others, for example, caregivers, required to help patient use system | _ | _ |
|
| |||
| Communication | |||
| • Patients liked timely reminder feedback texts to send in BP readings | • Potential increase in face-to-face appointments if uncertain of texting back | _ | • Potential increase in patients making extra appointments while at the practice to deliver |
|
| |||
| Integrating self-monitoring in hypertension management (structured care) | |||
| • Schedule for home monitoring BP provided | • Time consuming, too rigid protocol for some, not suitable for everyone | • Schedule for home monitoring of BP provided | • Time consuming, too rigid protocol for some, not suitable for everyone |
| • Rapid clinical decision making reduced clinical inertia through a trusted reliable database of home-monitored BP readings | • Lack of reminder system for HCPs to check BP readings | _ | • Lack of reminder system for HCPs to check BP readings |
BP = blood pressure. HCP = healthcare professional.
Acceptability of self-monitoring/telemonitoring to patients and healthcare professionals
Regular home monitoring was preferred by patients to visiting the GP surgery for BP measurement. Irrespective of the self-monitoring arm that patients were randomised into, patients felt ‘looked after’ and found either method of communicating self-measured BP manageable (Box 2). Patients who telemonitored described the process as a ‘slick operation’ while HCPs found the data provided electronically as ‘brilliant’ for quickly accessing a monthly view of readings, and the graphing ‘awesome’ in contrast to dealing with the paper-based records, which one GP described as ‘unwieldy’ (Box 2). Among the telemonitoring group, patients liked being able to use their own mobile phone for sending BP readings electronically, resulting in wider acceptance of the intervention among the more technophobic participants. Similarly, HCPs favoured the rapid and direct mHealth solution for reviewing patients’ BP readings over what they felt was the more time-consuming process of calculating means from the paper record. Patients and HCPs recognised that telemonitoring may not be a suitable way of sending readings for all patients, such as patients who were older, and so felt a conventional paper record option was an important alternative (Box 2 and 3).
Box 3.
Participant quotations
| Acceptability of self-monitoring/telemonitoring to patients and healthcare professionals |
|
‘They’re looking after me … I text them my results and they text me back. If it’s high they’ll tell me to see my GP and if it’s low they tell me it’s all right.’ (Patient [P] 0328, male [M], mHealth) ‘I think the only hesitation with that [texting process] for me is that in terms of what sort of target group you’re aiming at because in this study there are a lot of not elderly but more retired people and perhaps less confident at using mobile phones and texting is perhaps a younger population.’ (Practice nurse 11014, female [F]) |
|
|
| Managing data: getting started with self-monitoring/telemonitoring |
|
‘We initially had a [practice manager] working the averages out, then we had a receptionist when she left and it’s a bit clunky … I haven’t had to contact them. But if I did have to contact them, it would be much more time-consuming.’ (GP 11001, F) ‘It’s tedious to enter it in, isn’t it? [entering BP values into the clinical system] As I’ve lost my HCA [healthcare assistant] who was trained on that training day and left us, I actually ended up doing it all myself … with the texting business which I then have to look up on the website … it puts the onus on me. I would not do this in my general day-to-day practice. It is up to me to go into an inbox to look at what patients may have communicated.’ (GP 11008, M) ‘The only thing is, potentially, the confidentiality, if you’re texting back on the phone, as to who could potentially read it … mine have been okay, so I’ve said, “Thank you. Repeat them in a month and continue as you are.” So, it’s been a bit non-committal, I think if you were wanting to make some changes … you shouldn’t be doing it over a text anyway.’ (GP 11004, F) |
|
|
| Communication |
|
‘Yeah … my doctor’s very good, Dr [X] phones me every month to see if I’m satisfied and if I have any problems … I’ve had more contact … I feel easier asking her questions and talking to her now…’ (P 2286, F, self-monitoring without mHealth) ‘Well I quite like the idea that blood pressure is being monitored and I take more interest in the blood pressure with my own doctor, you know I’ll take the figures and talk to her.’ (P 3421, M, self-monitoring with mHealth) ‘One guy was on ramipril so he needed blood tests doing and then we’d reviewed the results. You really could do with talking to them.’ (GP 11001, F) ‘I do think they feel a lot more confident in partnership with the clinician, so I think it’s actually enhanced the doctor–patient relationship and I think the adherence to treatment is probably greater … It’s just great having the access there on your desktop all the time, not scrambling to find it.’ (GP 11006, M) |
|
|
| Integrating self-monitoring in hypertension management (structured care) |
|
‘It meant that I was more intensely watching their numbers than I would normally have done for someone whose blood pressure is well controlled. I would do an annual review and, with this system, I was getting monthly readings and so that’s more intense follow-up’ (GP 11009, M) ‘It’s fantastic. I love it. It’s just so easy to access it quick and you don’t have to rely on finding the paper and getting it scanned, or the quality of the scan, or patients remembering to bring it, or whatever. It’s just really useful.’ (GP 11006, M) |
Managing data
Each practice had autonomy regarding their management of patients and how self-monitoring was implemented within their organisation. The trial specified that patients undertook self-monitoring following a standard schedule and posted or sent readings electronically (Box 1). For manual recordings, GPs nominated a member of staff, usually the practice nurse or manager, to handle the paperwork, calculate monthly BPs, and enter these into the practice clinical system for GP review. Although the paper-based records integrated better within existing clinical systems via scanning documentation, HCPs favoured the rapid and direct mHealth solution over what they felt was the more time-consuming paper record. Both self-monitoring interventions, however, ultimately required human effort to input the average monthly BP into the clinical system, which could have increased the likelihood of human error.
HCPs set up personal reminder systems to review patients’ readings but, in some cases, where the designated nominated staff member was not present, GPs would have to deal with the paperwork personally (Box 2). Though HCPs had to spend extra time logging into a separate web portal, the automatic calculation of average BP by the system meant GPs generally favoured telemonitoring over the manual written log. Data confidentiality, security, and the potential risk of important medical advice being received by the wrong person, or easily missed, were among concerns raised by some GPs over telemonitoring (Box 3).
Communication
A key aspect of the interventions within TASMINH4 was for HCPs to manage and titrate medication using self-monitored BP. Medication changes were made for patients in the telemonitoring arm only. For those requiring a change, and where BP values were seen out of normal range on the system, GPs were prompted to initiate contact. They felt this improved communication around BP, resulting in more rapid control (Box 2 and 3). For the few GPs using the text-back facility some felt complete advice was not always possible within one text and there was a need to safeguard confidentiality by keeping communication non-committal, therefore, in such cases, face-to-face follow-up appointments were sometimes felt necessary. Irrespective of the method by which patients sent in readings (whether post or text), patients felt empowered from engaging in their own BP monitoring. Those within the telemonitoring arm valued timely interaction with the system (and by extension their GP) and, although text acknowledgement messages were automated when patients sent readings, they were nevertheless reassured from this instant feedback.
Integrating self-monitoring into hypertension management (structured care)
HCPs and patients adapted integration of self-monitoring into their BP management, and this was illustrated within the telemonitoring arm. If patients could not use their existing mobile phone, though the study supplied patients with a phone, they borrowed a mobile phone or asked their partner or caregiver to send the SMS message. Patients and HCPs found both self-monitoring systems and schedules easy to use. Minor technical problems experienced with the mHealth system were alleviated after brief consultation with the study research team. Conventionally, GPs would undertake annual reviews of patients with hypertension, but both self-monitoring interventions enabled more intense monitoring and follow-up with further intervention where needed or reassurance where not. Clinicians felt any decisions about medication changes for patients who were telemonitoring were based on a reliable database of BP readings (Box 2 and 3).
DISCUSSION
Summary
The present qualitative process aimed to evaluate the facilitators and barriers to self-monitoring and telemonitoring within the TASMINH4 trial. HCPs managed patients’ medications based on self-monitored readings as they would routinely, regardless of the mode of transfer.
Telemonitoring of BP was convenient and therefore acceptable to most patients and HCPs, with notably a few stating it was time consuming. Telemonitored data facilitated regular communication between clinicians and patients relating to BP and supported rapid clinical decisions about intensifying medication for patients. The paper-based option, however, integrated better with practice records offering a simple scan and storage process, directly matching the readings to the patient within the GP practice’s clinical system. Integration has previously been documented as a requirement for accepting telehealth systems in the long term.21,22 Patients and HCPs agreed that telemonitoring may not suit all people across a wider population. The benefits of structured care provided by both self-monitoring methods over standard clinical BP management were perhaps as important as the method of monitoring communication.
Some concerns were raised over data confidentiality by clinicians, as previously reported with mobile data usage;23 these concerns could be reduced by limiting the advice given to the character allowance of one SMS and booking an additional face-to-face appointment in the event that medication change was required, but clear advice to this effect would be necessary. This may reduce the potential savings in time associated with telemonitoring.
Strengths and limitations
This study was embedded within a large RCT24,25 with flexibility regarding the implementation of mHealth within practices, avoiding the need for HCPs to adhere to strict protocols. Qualitative approaches are ideal for exploring the mechanisms of adoption of such interventions and therefore important in maximising future dissemination.26
Rapid analysis is designed to enable a prompt preliminary understanding of key priority areas and key features of interventions when considering implementation in wider practice.19,27 Therefore, the authors ensured a range of expertise within their team of researchers who were also responsible for data analysis to facilitate this rapid process evaluation. The present analysis provides suggestions of the key areas relating to implementation to focus a deeper inductive analysis in the future by other researchers.20, 27
Although purposive sampling was carried out in the present study with equal representation of males and females across the HCP and patient population, like the TASMINH4 national RCT there was under-representation of non-white ethnic minorities across the sample. The present findings and conclusions could be different if other medical practices had participated in the trial.
Comparison with existing literature
These findings are contrary to previous research investigating the use of self-management mHealth technology: a Swedish study of a mobile phone-base support system or platform28 and an Irish study by Morrissey et al of a smartphone application29 found participants expressed difficulty using the mobile platforms. Patients telemonitoring in the present study did not report such difficulties, suggesting an advantage of using SMS (texts), enabling compatibility with patients’ existing environments, and ease of delivering BP readings, key elements of telehealth interventions that ensure successful implementation.30 Furthermore, the authors’ recommendation of the availability of an equally cost-effective paper-based method of recording and sending readings is an additional way to facilitate wider appeal.31 In a recent meta-ethnography of digital health interventions across wider health conditions, Morton et al conclude that engagement with such tools provides reassurance from the insight patients receive of their health.32 This is both motivating and empowering for patients, supporting the findings of the present study and the conclusions of other studies relating specifically to populations with hypertension.33, 34
Effective communication between patients with hypertension and GPs has been emphasised across several previous studies32,34,35 and was identified as a key priority area for implementation. The mobile texting system potentially enabled opportunity for discussion via consultation concordant with findings by Hallberg et al28 and two recent systematic reviews showing that technology-based strategies that prompt and promote user engagement are more likely to be effective.36,37
Implications for practice
The present study suggests self-monitoring, whether it is using a mobile text-based system or a diary paper-based record, is relatively simple, cost-effective,31 and potentially easy to adopt for managing hypertension in primary care. A system whereby HCPs can be easily alerted to patients in whom intensification of anti-hypertensive BP medication is necessary appears favourable over conventional paper-diary methods, though the latter is recommended as a required alternative option to suit the broader population. Overall, a system easily accessed by patients using their existing non-smartphone mobile phones makes this an acceptable form of telemonitoring.
Acknowledgments
The authors would like to thank Alice Tompson, Lucy Hughes, and Siobhan Milner for interviewing participants for the study, and Karen Biddle for administrative assistance.
Funding
The TASMINH4 trial is funded by an National Institute for Health Research (NIHR) Programme Grant for Applied Heath Research (grant reference: RP-PG-1209-10051) and by an NIHR professorship awarded to Richard J McManus (reference number: NIHR-RP-R2-12-015), the chief investigator. FD Richard Hobbs acknowledges his part-funding from the NIHR School for Primary Care Research, the NIHR Collaboration for Leadership in Applied Health Research and Care (CLARHC) Oxford, the NIHR Oxford Biomedical Research Centre (OxBRC), and the NIHR Oxford Medtech and In-Vitro Diagnostics Co-operative (MIC).
Ethical approval
Ethical Approval was given on 7 July 2015 by South Central Oxford B Research Ethics Committee (reference number: 14/SC/0218, AM02 05/06/2015).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Agarwal S, LeFevre AE, Lee J, et al. Guidelines for reporting of health interventions using mobile phones: mobile health (mHealth) evidence reporting and assessment (mERA) checklist. BMJ. 2016;352:i1174. doi: 10.1136/bmj.i1174. [DOI] [PubMed] [Google Scholar]
- 2.Band R, Bradbury K, Morton K, et al. Intervention planning for a digital intervention for self-management of hypertension: a theory-, evidence- and person-based approach. Implement Sci. 2017;12(1):25. doi: 10.1186/s13012-017-0553-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute of Health and Care Excellence. Hypertension in adults: diagnosis and management CG127. 2011. http://guidance.nice.org.uk/CG127 (accessed 25 Jun 2019) [PubMed]
- 4.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010 a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 6.Neal B, MacMahon S, Chapman N. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration. Lancet. 2000;356:1955–1964. doi: 10.1016/s0140-6736(00)03307-9. [DOI] [PubMed] [Google Scholar]
- 7.Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA. 1997;277(9):739–745. [PubMed] [Google Scholar]
- 8.NHS Digital Health Survey for England, 2015: trend tables. 2016. http://content.digital.nhs.uk/pubs/hse2015trend (accessed 25 Jun 2019)
- 9.Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14(9):e1002389. doi: 10.1371/journal.pmed.1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verberk WJ, Kroon AA, Lenders JW, et al. Self-measurement of blood pressure at home reduces the need for antihypertensive drugs: a randomized, controlled trial. Hypertension. 2007;50(6):1019–1025. doi: 10.1161/HYPERTENSIONAHA.107.094193. [DOI] [PubMed] [Google Scholar]
- 11.Staessen JA, Den Hond E, Celis H, et al. Antihypertensive treatment based on blood pressure measurement at home or in the physician’s office: a randomized controlled trial. JAMA. 2004;291(8):955–964. doi: 10.1001/jama.291.8.955. [DOI] [PubMed] [Google Scholar]
- 12.McManus RJ, Mant J, Franssen M, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391:949–959. doi: 10.1016/S0140-6736(18)30309-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franssen M, Farmer A, Grant S, et al. Telemonitoring and/or self-monitoring of blood pressure in hypertension (TASMINH4): protocol for a randomised controlled trial. BMC Cardiovasc Disord. 2017;17(1):58. doi: 10.1186/s12872-017-0494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357. doi: 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 15.Palys T. Purposive sampling. In: Given LM, editor. Sage encyclopedia of qualitative research methods. Los Angeles, CA: Sage; 2008. pp. 697–698. [Google Scholar]
- 16.Department for Communities and Local Government. The English Indices of Deprivation 2015. 2015. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/465791/English_Indices_of_Deprivation_2015_-_Statistical_Release.pdf (accessed 25 Jun 2019)
- 17.McManus RJ, Mant J, Bray EP, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet. 2010;376:163–172. doi: 10.1016/S0140-6736(10)60964-6. [DOI] [PubMed] [Google Scholar]
- 18.Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res. 2015;26(13):1753–1760. doi: 10.1177/1049732315617444. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton AB. Qualitative methods in rapid turnaround health services research. VA HSR&D Cyberseminar Spotlight on Women’s Health. 2013. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/780-notes.pdf (accessed 25 Jun 2019)
- 20.Beebe J. Rapid assessment process: an introduction. Lanham, MD: AltaMira Press; 2001. [Google Scholar]
- 21.Davidson E, Simpson CR, Demiris G, et al. Integrating telehealth care-generated data with the family practice electronic medical record: qualitative exploration of the views of primary care staff. Interact J Med Res. 2013;2(2):e29. doi: 10.2196/ijmr.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanley J, Pinnock H, Paterson M, McKinstry B. Implementing telemonitoring in primary care: learning from a large qualitative dataset gathered during a series of studies. BMC Fam Pract. 2018;19(1):118. doi: 10.1186/s12875-018-0814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meingast M, Roosta T, Sastry S. Security and privacy Issues ith healthcare information technology. Conf Proc IEEE Eng Med Biol Soc. 2006;1:5453–5458. doi: 10.1109/IEMBS.2006.260060. [DOI] [PubMed] [Google Scholar]
- 24.McManus RJ, Franssen M, Mant J, Nickless A. Telemonitoring and/or self-monitoring of blood pressure in hypertension (TASMINH4): a randomised controlled trial. J Hypertens. 2018;36:e5. [Google Scholar]
- 25.Franssen M, Farmer A, Grant S, et al. Telemonitoring and/or self-monitoring of blood pressure in hypertension (TASMINH4): protocol for a randomised controlled trial. BMC Cardiovasc Disord. 2017;17(1):58. doi: 10.1186/s12872-017-0494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewin S, Glenton C, Oxman AD. Use of qualitative methods alongside randomised controlled trials of complex healthcare interventions: methodological study. BMJ. 2009;339:b3496. doi: 10.1136/bmj.b3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Utarini A, Winkvist A, Pelto GH. Appraising studies in health using rapid assessment procedures (RAP): eleven critical criteria. Hum Org. 2001;60(4):390–400. [Google Scholar]
- 28.Hallberg I, Ranerup A, Kjellgren K. Supporting the self-management of hypertension: patients’ experiences of using a mobile phone-based system. J Hum Hypertens. 2015;30(2):141–146. doi: 10.1038/jhh.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrissey EC, Casey M, Glynn LG, et al. Smartphone apps for improving medication adherence in hypertension: patients’ perspectives. Patient Prefer Adherence. 2018;12:813–822. doi: 10.2147/PPA.S145647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vassilev I, Rowsell A, Pope C, et al. Assessing the implementability of telehealth interventions for self-management support: a realist review. Implement Sci. 2015;10(1):59. doi: 10.1186/s13012-015-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monahan M, Jowett S, Nickless A, et al. Cost-effectiveness of telemonitoring and self-monitoring of blood pressure for antihypertensive titration in primary care (TASMINH4). Hypertension. 2019;73(6):1231–1239. doi: 10.1161/HYPERTENSIONAHA.118.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morton K, Dennison L, May C, et al. Using digital interventions for self-management of chronic physical health conditions: a meta-ethnography review of published studies. Patient Educ Couns. 2017;100(4):616–635. doi: 10.1016/j.pec.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grant S, Greenfield SM, Nouwen A, McManus RJ. Improving management and effectiveness of home blood pressure monitoring: a qualitative UK primary care study. Br J Gen Pract. 2015. [DOI] [PMC free article] [PubMed]
- 34.Fletcher BR, Hinton L, Hartmann-Boyce J, et al. Self-monitoring blood pressure in hypertension, patient and provider perspectives: a systematic review and thematic synthesis. Patient Educ Couns. 2016;99(2):210–219. doi: 10.1016/j.pec.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 35.Jolles EP, Clark AM, Braam B. Getting the message across: opportunities and obstacles in effective communication in hypertension care. J Hypertens. 2012;30(8):1500–1510. doi: 10.1097/HJH.0b013e32835476e1. [DOI] [PubMed] [Google Scholar]
- 36.Palmer MJ, Barnard S, Perel P, Free C. Mobile phone-based interventions for improving adherence to medication prescribed for the primary prevention of cardiovascular disease in adults. Cochrane Database Syst Rev. 2018;6:CD012675. doi: 10.1002/14651858.CD012675.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alkhaldi G, Hamilton FL, Lau R, et al. Effectiveness of prompts to promote engagement with digital interventions: a systematic review. J Med Internet Res. 2016;18(1):e6. doi: 10.2196/jmir.4790. [DOI] [PMC free article] [PubMed] [Google Scholar]