Abstract
Background
Quality improvement (QI) is a priority for general practice, and GPs are expected to participate in and provide evidence of QI activity. There is growing interest in harnessing the potential of electronic health records (EHR) to improve patient care by supporting practices to find cases that could benefit from a medicines review.
Aim
To develop scalable and reproducible prescribing safety reports using patient-level EHR data.
Design and setting
UK general practices that contribute de-identified patient data to the Clinical Practice Research Datalink (CPRD).
Method
A scoping phase used stakeholder consultations to identify primary care QI needs and potential indicators. QI reports containing real data were sent to 12 pilot practices that used Vision GP software and had expressed interest. The scale-up phase involved automating production and distribution of reports to all contributing practices that used both Vision and EMIS software systems. Benchmarking reports with patient-level case review lists for two prescribing safety indicators were sent to 457 practices in December 2017 following the initial scale-up (Figure 2).
Results
Two indicators were selected from the Royal College of General Practitioners Patient Safety Toolkit following stakeholder consultations for the pilot phase involving 12 GP practices. Pilot phase interviews showed that reports were used to review individual patient care, implement wider QI actions in the practice, and for appraisal and revalidation.
Conclusion
Electronic health record data can be used to provide standardised, reproducible reports that can be delivered at scale with minimal resource requirements. These can be used in a national QI initiative that impacts directly on patient care.
Keywords: electronic health records, general practice, primary care databases, quality improvement
INTRODUCTION
Quality improvement (QI) is the continuous improvement of healthcare quality with a focus on patient-centred outcomes.1 QI is a priority for UK general practice as outlined in the NHS policy across all four devolved nations.2–5 Prescribing safety is an important focus for QI and current UK initiatives in this area using electronic health record (EHR) data are outlined in Box 1. The need for more research in this area has been highlighted.6 During the development of this project the authors were unable to find any initiatives that addressed all the following needs: benchmarking, individual case-finding, and scalability with minimal resource use to enable sustainability.
Box 1.
QI initiatives in the UK with a focus on prescribing safety
|
EFIPPS = Effective Feedback to Improve Primary Care Prescribing Safety trial. PINCER = pharmacist-led information technology intervention for medication errors. QI = quality improvement.
This article reports on the Royal College of General Practitioners’ (RCGP) QI pilot project that was jointly initiated in 2015 by the RCGP and Clinical Practice Research Datalink (CPRD), a government research service providing de-identified patient data for public health research (www.cprd.com). The project explored the potential of using routinely collected primary care EHR data to support QI in general practice by providing confidential practice-level reports that would enable both benchmarking and individual case-finding with a focus on UK-wide scalability and long-term sustainability.
METHOD
Setting and recruitment
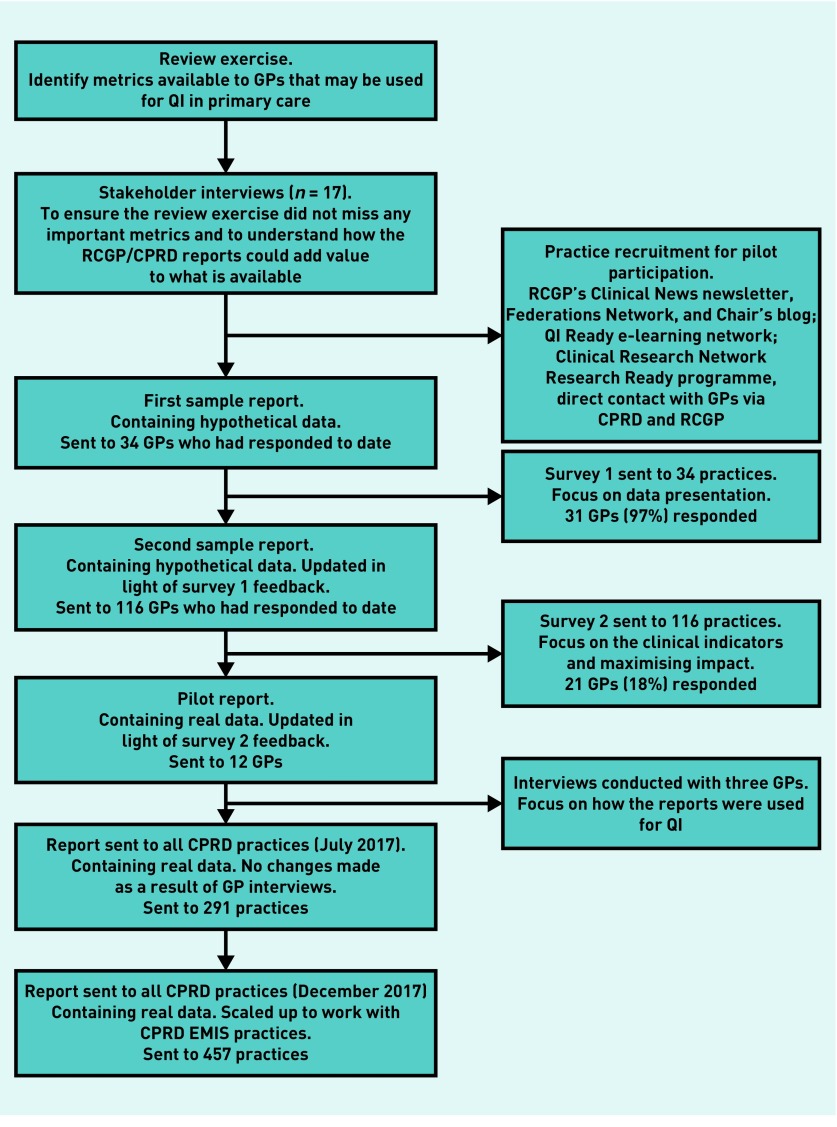
GP practices in the UK contributing data to CPRD were invited to participate in the pilot via channels described in Figure 1 in August 2016.
Figure 1.
Flow diagram of study methodology.
CPRD = Clinical Practice Research Datalink. QI = quality improvement. RCGP = Royal College of General Practitioners.
Scoping phase
A scoping review and stakeholder interviews were conducted to identify existing examples of how routine data were used for QI in primary care and gaps in current provision. Semi-structured interviews (n = 17) were conducted with stakeholders including GPs, RCGP clinical advisers, public health researchers, and epidemiologists and representatives from the Medicines and Healthcare products Regulatory Agency (MHRA), NHS England, the Health Foundation, National Institute for Health Research (NIHR), National Institute for Health and Care Excellence (NICE), and the Health and Social Care Information Centre (HSCIC).
Design phase
The design phase focused on developing a report that was useful, easy to interpret, and helpful in clinical practice. A sample report containing dummy data was developed and sent in two iterations to clinicians with accompanying online surveys. Round 1 focused on data presentation, supporting information that could be included, and the format in which GPs would prefer to receive the report. Round 2 focused on the clinical indicators in the report, case definitions, and accompanying advice to maximise the utility and impact of the final reports.
How this fits in
| Several studies have explored the potential of using electronic health record data in regional quality improvement initiatives, but no single initiative has met all the following needs: benchmarking, individual case-finding, and scalability with minimal resource use to enable sustainability. The current project designed and piloted bespoke reports to allow benchmarking of practice-level prescribing safety indicators with individual case-finding. These reports are provided at no cost to GPs contributing to the Clinical Practice Research Datalink (CPRD) and aim to improve prescribing safety by offering standardised patient-level case-finding with minimal GP workload. The reports were scaled up to multiple GP software systems, and hence additional UK practices, and their production and dissemination automated, thus enabling repeat reporting using minimal resources. |
Implementation phase
After addressing feedback received in the design phase, QI reports containing real data were sent to 12 practices that used Vision GP software and had expressed interest in giving feedback on how the reports were used in practice.
Scale-up phase
The initial programming was extended to include multiple practices using either Vision or EMIS GP software and was tailored to enable feedback over different reporting periods. In addition, the production and dissemination of reports were automated to enable scale-up with minimal additional resource requirements. Manual validation was undertaken for a sample of the automated reports. Figure 1 summarises the pilot study methodology and participation numbers for each phase. Benchmarking reports with patient-level case review lists for two prescribing safety indicators were sent to 457 practices in December 2017 following the initial scale-up.
Data analysis
Stakeholder interviews and free text GP responses were analysed using thematic analysis.12 Descriptive statistics (percentages) to summarise online survey responses were obtained using the in-built statistical analysis feature in SurveyMonkey, which was used to administer the online surveys. Initial programming for the QI reports was undertaken using Stata (version 14), and SAS (version 9.4) was used for the automation of production and dissemination of reports.
RESULTS
Scoping phase
The review identified existing primary care data reports from a range of sources (including national and regional bodies, and health analytic companies) covering topics that included service use metrics, referrals to secondary care, medicine prescribing and costs, and adherence to guidelines. A clear preference was expressed for a focus on prescribing safety in the 17 stakeholder interviews. Key gaps in QI reports available to GPs were: lack of information at a patient-level, non-user-friendly formats, and regional differences in availability. Based on the findings of the review exercise and the stakeholder interviews, a set of general principles for guiding development of the reports was developed (Box 2). The RCGP/CPRD QI project steering committee then agreed decision criteria to guide selection of safety indicators for inclusion in the reports (Box 3).
Box 2.
General principles for guiding development of QI reports based on scoping interviews
|
QI = quality improvement.
Box 3.
Criteria used for the selection of indicators to be included in the pilot QI reports
|
CPRD = Clinical Practice Research Datalink. MHRA = Medicines and Healthcare products Regulatory Agency. QI = quality improvement.
Two indicators from the RCGP Patient Safety Toolkit relating to prescribing in patients with heart failure were selected for use in the pilot study: prescribing of non-steroidal anti-inflammatory drugs (NSAIDs); and prescribing of thiazolidinediones (glitazones). Two case definitions were used to identify patients with heart failure using the Read code hierarchy: a narrow one based on the Quality and Outcomes Framework (QOF) prevalence definition,13 and a broad definition that included all relevant codes identified following a code review by the project team comprising epidemiologists, data experts, and GPs. Both heart failure definitions were included in the final report based on survey results. A sample report is available from the authors on request.
Design phase
Survey responses were received from a total of 31 (round 1) and 21 (round 2) clinicians in response to the sample report containing dummy data. All survey responders fed back that the report was useful for their clinical practice. In terms of content, key elements identified as being useful were benchmarking of an individual practice’s prescribing rate against the other practices in the network and case-finding, which meant that reports could be used not just for peer comparison, but also directly for patient care by enabling individual patient review.
In round 1, most responders (n = 18, 58%) preferred bar charts over funnel or box plots for presentation of benchmarking data. The report template included a ‘what next’ section with more guidance on best practice for the topic of the data report. Subsections included ‘why this topic is important’, ‘suggested actions’, ‘alternative treatment’, and ‘links to further resources’. Responders felt all subsections were relevant (n = 25, 81%) and 18 (86%) of round 2 responders felt there was the ‘right amount of information’ in this section. The reports were felt to be ‘about right’ in terms of length by most responders (n = 26, 84%) in round 1.
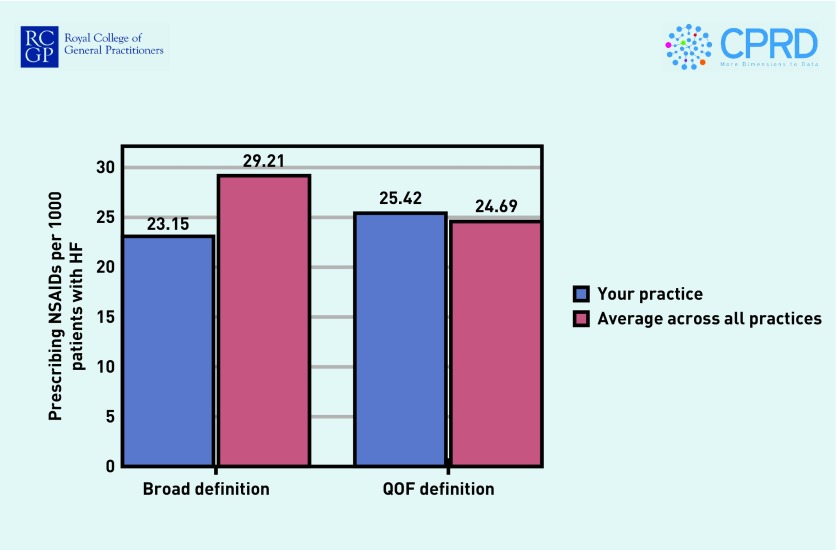
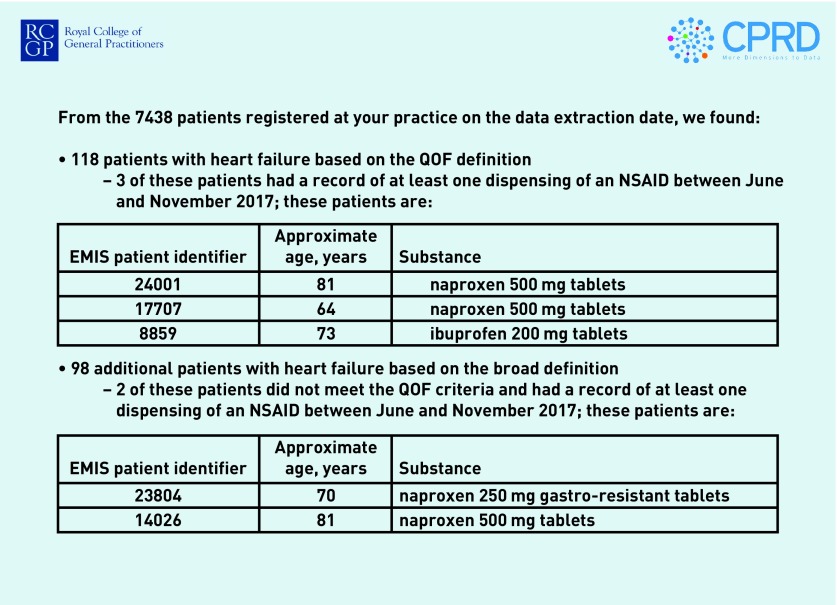
In round 1 there was some variation in the preferred frequency of the reports, with 17 (55%) of responders suggesting quarterly reports would be best, whereas 6 (19%) preferred 6-monthly reports and 5 (16%) monthly reports. Most responders expressed a preference for pdf reports sent by email (n = 26, 84%), with 5 (16%) preferring to log in to an online portal or receive a pop-up notification in the practice system, and only 1 (3%) preferred a paper report sent by post. Figures 2 and 3 show the benchmarking and case-finding elements of the reports, using hypothetical data.
Figure 2.
Benchmarking of practice prescribing rates (hypothetical data).
HF = heart failure. NSAIDs = non-steroidal anti-inflammatory drugs. QOF = Quality and Outcomes Framework.
Figure 3.
Case-finding table (hypothetical data).
NSAIDs = non-steroidal anti-inflammatory drugs. QOF = Quality and Outcomes Framework.
Implementation phase
Interviews were conducted with three GPs to discuss the utility of the reports. Use of the reports fell into three broad categories:
Individual patient review
For patients with heart failure who were identified as having been prescribed NSAIDs using the reports, the following actions were taken after review: no further action required for acute (one-off) prescriptions; repeat prescriptions for chronic pain were inactivated and meetings arranged with patients to discuss their ongoing pain management; patients moved to other pain medication; some patients decided that the benefits of NSAIDs outweighed the risks. In the last case, NSAID prescription continued but was altered to low-dose naproxen or ibuprofen:
‘I reviewed patients as a result of the report, then took it to the partner’s meeting. One patient (on NSAIDs) had a flag put on, so prescriptions could be reviewed with the patient because the risk–benefit was quite balanced.’
(GP 1, England)
Practice-level quality improvement actions
Flags were added to the notes of all patients with heart failure suggesting the avoidance of NSAIDs and glitazones; all clinicians within participating practices were made aware of the identified issues with prescribing and patient safety:
‘[We] have flagged up NSAIDs on relevant notes — and have been changing practice as a result.’
(GP 2, Scotland)
Appraisal and revalidation
One participant described how they had used their QI report to review and alter the care of relevant patients within their practice. They then reported this as evidence for their annual appraisal and revalidation with the General Medical Council (Domain 2 — Safety and Quality).
No further amendments were made to the design of the report in response to interviews conducted as part of the implementation phase.
Scale-up phase
Reports were sent to 291 CPRD Vision practices in July 2017 covering prescriptions in the previous financial year. Updated reports were sent to 271 Vision and 186 EMIS practices in December 2017 covering the subsequent 6-month period.
DISCUSSION
Summary
This pilot study demonstrates how routine EHRs can be used to implement a QI initiative that has the capacity to directly impact patient care. The production and dissemination of reports was automated to enable UK-wide scalability and sustainability over time, thus allowing GPs to observe change in their practice. The involvement of GPs in the design phase ensured that the reports were acceptable and clinically meaningful. Unlike the schemes identified in the scoping review and wider literature, the RCGP/CPRD QI reports provide patient-level feedback, allow re-identification of patients for case-finding, enable comparison with peers nationally, do not require GPs to implement their own searches, and are free of charge.
Strengths and limitations
QI initiatives, clinical audits, and learning event analysis are longstanding within primary care, but are often unsustainable due to a high administrative burden14,15 and many initiatives may never reach the scientific literature. The review exercise highlighted that many of the resources currently used by GPs for QI are not produced primarily for that purpose and are often limited to aggregate data at practice-level or larger geographies. The authors’ use of patient-level EHR data that are collected daily from general practices enables production of timely, standardised QI reports enabling both benchmarking and individual case-finding.
The structure of CPRD data is a strength, allowing queries to be reproduced at regular intervals as demonstrated in the pilot study. The reports were scaled up to cover a larger number of GP practices across two GP software systems. This is made possible by automation of production, including publication of the reports and their dissemination to recipients via email. The reports used in this study have the potential to facilitate a continuous and sustainable approach to QI, which is consistent across GP practices that contribute data to CPRD.
A potential limitation of using de-identified EHR data that do not contain details such as names or NHS numbers is that individual case-finding is usually not possible. For this initiative, case-finding was made possible by providing the practices with pseudonymised identifiers, which could be used by GPs to re-identify their own patients via entry into the individual practice’s computer system. This ensured patient confidentiality while reducing the burden on practice staff reviewing the highlighted patients. However, these data governance considerations mean that these reports can only be generated in collaboration with CPRD.
Another potential limitation is that the reports rely on the validity of EHR data for accurate identification of patients triggering the indicators. Recording of primary care data may be impacted by different factors including practice characteristics, pay for performance and QI schemes such as QOF, and differences in practice software systems.16 The validity of diagnoses in CPRD have been investigated with generally positive findings.17 However, expert clinical input when designing case definitions in any EHR data is always advisable and was sought during the development of the indicators in this pilot. The pilot study focused on a condition (heart failure) that has been included in QOF since its onset in 2004. Nevertheless, to overcome any potential misclassification issues, the researchers used two code lists to identify patients, one based on QOF, and the second based on a broader definition that would prioritise sensitivity over specificity. The recording of non-QOF conditions will need to be considered in the development of future indicators and clinical input sought to ensure that the indicators are implemented in a clinically meaningful way using EHR data.18,19 Partnership with the RCGP offered a high degree of clinical input into development of the indicators, and the study drew on RCGP expertise in quality improvement and patient safety.20 During the pilot study there were poor response rates to the second questionnaire and GP interview components. The authors have not been able to explore the reasons for this, but research of this nature requires a pragmatic approach and they used the feedback obtained to tailor the reports.
Comparison with existing literature
Since the inception of this project other similar initiatives have been trialled and evaluated. In Scotland, the Data-driven Quality Improvement in Primary Care (DQIP) study21,22 and Effective Feedback to Improve Primary Care Prescribing Safety (EFIPPS) trial11 used similar techniques to provide practices with benchmarking data and search tools for case identification. These studies incorporated more intensive interventions than the present project but were based on one-off interventions over a period of 1 year and acknowledged the importance of repeatability for sustained effect and of scalability for large-scale implementation, both of which the RCGP/CPRD QI reports allow with minimal additional resource.
The pharmacist-led information technology intervention for medication errors (PINCER) comprised a pharmacist-led intervention in combination with patient-level prescribing feedback to reduce inappropriate prescribing in primary care. This cluster randomised controlled trial demonstrated a reduction in hazardous prescribing for the intervention arm over the control arm of feedback alone, which could be considered similar to the current study.9 The PINCER intervention has been successfully scaled up in the East Midlands since the trial, and is planned for national roll-out by NHS England. The PINCER tool interfaces directly with the practice’s system and allows staff to interrogate the data to identify patients at risk. At the time of writing this article, a small annual licence fee was required for access to the tool.23 By contrast, the RCGP/CPRD QI tool is produced externally, with pdf reports emailed to practices free of charge as per the expressed preference of GPs participating in the present pilot. Benchmarking in the reports is against all practices in the CPRD network across the UK.
Implications for practice
CPRD has incorporated the RCGP/CPRD reports into business-as-usual activities as part of its GP engagement strategy with the aim of sending out two to four reports per year, with new indicators added each year. The next research output from this project will be an impact evaluation to determine whether the reports have resulted in changes to prescribing for the relevant indicators.
System-level changes, such as the migration to Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT) coding and associated Read code retirement in primary care from 2018 and the move away from QOF in Scotland, will need to be considered when developing future indicators to ensure comparability over time. Including a range of code lists alongside the original ones will allow benchmarking over time in a clinically meaningful way.24 Code lists used in the reports are available to GPs receiving the report and can be obtained on request.
The NHS England clinical pharmacist pilot scheme is now complete and is due to be extended nationally, providing an additional 2000 pharmacists in general practice by 2020.4,25 The RCGP/CPRD QI reports could prove a valuable tool alongside the clinical pharmacist scheme in initiating relevant QI actions within the practices that receive them.
Acknowledgments
Wilhelmine Meeraus provided valuable input to the design of the indicators during the early pilot phase.
Funding
No funding was obtained to conduct this work.
Ethical approval
This pilot study was conducted under a research ethics exemption from the NHS Health Research Authority as it was categorised as a clinical audit with a service evaluation component.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
CPRD is jointly sponsored by the UK government’s Medicines and Healthcare products Regulatory Agency and the National Institute for Health Research (NIHR). As a not-for-profit UK government body, CPRD seeks to recoup the cost of delivering its research services to academic, industry, and government researchers through research user licence fees. Helen Booth, Arlene Gallagher, Lucy Carty, Shivani Padmanabhan, Puja Myles, Stephen Welburn, and Janet Valentine are employees of CPRD.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Royal College of General Practitioners. Quality improvement guide for general practice. 2015. https://www.rcgp.org.uk/clinical-and-research/our-programmes/quality-improvement/quality-improvement-guide-for-general-practice.aspx (accessed 25 Jun 2019)
- 2.Royal College of General Practitioners. The 2022 GP: a vision for general practice in the future NHS. 2013. https://www.rcgp.org.uk/policy/rcgp-policy-areas/general-practice-2022.aspx (accessed 25 Jun 2019)
- 3.Welsh Government. Our plan for a primary care service for Wales up to March 2018. 2015. https://gov.wales/docs/dhss/publications/150218primaryen.pdf (accessed 25 Jun 2019)
- 4.NHS England General practice forward view. 2016. https://www.england.nhs.uk/wp-content/uploads/2016/04/gpfv.pdf (accessed 25 Jun 2019)
- 5.Scottish Government. Improving together: a national framework for quality and GP clusters in Scotland. 2017. https://www.gov.scot/publications/improving-together-national-framework-quality-gp-clusters-scotland/ (accessed 25 Jun 2019)
- 6.Verbakel NJ, Langelaan M, Verheij TJ, et al. Improving patient safety culture in primary care: a systematic review. J Patient Saf. 2016;12(3):152–158. doi: 10.1097/PTS.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 7.Akbarov A, Kontopantelis E, Sperrin M, et al. Primary care medication safety surveillance with integrated primary and secondary care electronic health records: a cross-sectional study. Drug Saf. 2015;38(7):671–682. doi: 10.1007/s40264-015-0304-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams R, Keers R, Gude WT, et al. SMASH! The Salford medication safety dashboard. J Innov Health Inform. 2018;25(3):183–193. doi: 10.14236/jhi.v25i3.1015. [DOI] [PubMed] [Google Scholar]
- 9.Avery AJ, Rodgers S, Cantrill JA, et al. A pharmacist-led information technology intervention for medication errors (PINCER): a multicentre, cluster randomised, controlled trial and cost-effectiveness analysis. Lancet. 2012;379(9823):1310–1319. doi: 10.1016/S0140-6736(11)61817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margham T, Symes N, Hull SA. Using the electronic health record to build a culture of practice safety: evaluating the implementation of trigger tools in one general practice. Br J Gen Pract. 2018. [DOI] [PMC free article] [PubMed]
- 11.Guthrie B, Kavanagh K, Robertson C, et al. Data feedback and behavioural change intervention to improve primary care prescribing safety (EFIPPS): multicentre, three arm, cluster randomised controlled trial. BMJ. 2016;354:i4079. doi: 10.1136/bmj.i4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun V, Clark V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. [Google Scholar]
- 13.NHS Digital Condition prevalence. 2018. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/general-practice-data-hub/condition-prevalence (accessed 25 Jun 2019)
- 14.Glew S, Sornalingam S, Crossman T. Audit: time to review the cycle. Br J Gen Pract. 2014. [DOI] [PMC free article] [PubMed]
- 15.Thomas N, Gallagher H, Jain N. A quality improvement project to improve the effectiveness and patient-centredness of management of people with mild-to-moderate kidney disease in primary care. BMJ Qual Improv Rep. 2014;3(1) doi: 10.1136/bmjquality.u201337.w825. pii: u201337.w825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verheij RA, Curcin V, Delaney BC, McGilchrist MM. Possible sources of bias in primary care electronic health record data use and reuse. J Med Internet Res. 2018;20(5):e185. doi: 10.2196/jmir.9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrett E, Thomas SL, Schoonen WM, et al. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;69(1):4–14. doi: 10.1111/j.1365-2125.2009.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guthrie B, Tang J. Series. Literature Review. What did we learn from 12 years of QOF? 2016 http://www.sspc.ac.uk/media/media_486342_en.pdf (accessed 25 Jun 2019) [Google Scholar]
- 19.Roland M, Guthrie B. Quality and Outcomes Framework: what have we learnt? BMJ. 2016;354:i4060. doi: 10.1136/bmj.i4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Royal College of General Practitioners. Quality improvement. http://www.rcgp.org.uk/clinical-and-research/our-programmes/quality-improvement.aspx (accessed 25 Jun 2019)
- 21.MacBride-Stewart S, Marwick C, Houston N, et al. Evaluation of a complex intervention to improve primary care prescribing: a phase IV segmented regression interrupted time series analysis. Br J Gen Pract. 2017. [DOI] [PMC free article] [PubMed]
- 22.Willis TA, West R, Rushforth B, et al. Variations in achievement of evidence-based, high-impact quality indicators in general practice: an observational study. PLoS One. 2017;12(7):e0177949. doi: 10.1371/journal.pone.0177949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.University of Nottingham PINCER. https://www.nottingham.ac.uk/pincer/pincer.aspx (accessed 25 Jun 2019)
- 24.Williams R, Kontopantelis E, Buchan I, Peek N. Clinical code set engineering for reusing EHR data for research: a review. J Biomed Inform. 2017;70:1–13. doi: 10.1016/j.jbi.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Mann C, Anderson C, Avery AJ, et al. Clinical pharmacists in general practice: pilot scheme Independent Evaluation Report: Full Report. 2018. https://www.nottingham.ac.uk/pharmacy/documents/generalpracticeyearfwdrev/clinical-pharmacists-in-general-practice-pilot-scheme-full-report.pdf (accessed 25 Jun 2019)