Abstract
Transition metal–catalyzed arene functionalization has been widely used for molecular synthesis over the past century. In this arena, copper catalysis has long been considered a privileged platform due to the propensity of high-valent copper to undergo reductive elimination with a wide variety of coupling fragments. However, the sluggish nature of oxidative addition has limited copper’s capacity to broadly facilitate haloarene coupling protocols. Here, we demonstrate that this copper oxidative addition problem can be overcome with an aryl radical–capture mechanism, wherein the aryl radical is generated through a silyl radical halogen abstraction. This strategy was applied to a general trifluoromethylation of aryl bromides through dual copper-photoredox catalysis. Mechanistic studies support the formation of an open-shell aryl species.
The development of fragment coupling transformations by transition metal catalysis has substantially expanded access to valuable, complex organic molecules (1). Major efforts in this field have been devoted toward the use of aryl halides, stable and highly accessible building blocks, as coupling partners for the construction of functionalized arenes (2). The success of these transformations generally relies on the transition metal catalyst to activate haloarenes through an oxidative addition step and, thereafter, forge the desired bond by a reductive elimination step. This paradigm is exemplified in modern palladium and nickel catalysis, wherein catalyst design over four decades has rendered these elementary steps generically efficient, thereby enabling the development of a vast array of coupling manifolds that convert (hetero)aryl C–X bonds to Csp2-carbon, -nitrogen, -sulfur, and -oxygen bonds (3, 4). In contrast, copper catalysis has been less successful than Pd or Ni with respect to diverse applications in cross-coupling chemistry, despite its potential for economical and operational benefits. Indeed, the most robust copper cross-coupling reactions are limited to transmetalling reagents such as organoboronic acids, specifically to bypass the challenge of oxidative addition (5).
The limited capacity of copper to participate in haloarene functionalizations has been attributed to its relatively low rate of oxidative addition in comparison to nickel and palladium. Formation of the key Cu(III)-arene intermediate has been calculated to possess a high kinetic barrier and is typically the rate-determining step in such catalytic cycles (6–8). Although elegant studies in ligand design have allowed for improvements in the rates of oxidative addition for certain substrate classes, functionalization of aryl halides by copper catalysis remains largely restricted to aryl iodides and activated aryl bromides (9–11). The need to overcome this oxidative addition problem is further underscored by the knowledge that the subsequent reductive elimination step from high-valent Cu(III) complexes is extremely facile for a wide range of coupling partners (12). In many cases, the reductive elimination of highly electronegative groups such as Csp2-CF3, Csp2-F, and Csp2-N bonds is more favorable and more facile with Cu than Pd and Ni (Fig. 1) (5, 13, 14). As such, the invention of a mechanistic paradigm that bypasses the Cu-haloarene oxidative addition problem should enable a range of catalytic cross-coupling reactions of scope and utility that has not been broadly realized using other forms of metal catalysis (including Pd, Ni, or Cu). Here, we describe a silyl radical/halogen abstraction/copper capture mechanism that effectively functions as a surrogate for this elementary oxidative addition step and, in doing so, allows copper-mediated bromoarene functionalization in a general format and under mild conditions (visible light and room temperature). Moreover, this catalytic strategy has been exploited to deliver a general approach to the trifluoromethylation of heteroaryl and aryl bromides through the combination of copper and photoredox catalysis.
Fig. 1. Trifluoromethylation with dual copper/photoredox catalysis.
Aryl radical capture by copper catalysts can bypass oxidative addition. Ar, (hetero)aryl.
Trifluoromethylarenes are valuable structural motifs in the area of drug discovery due to the enhancement of desired physicochemical properties upon the introduction of the trifluoromethyl group (15, 16). Among synthetic strategies, transition metal–catalyzed conversion of aryl halides to the corresponding trifluoromethylarenes is considered one of the most attractive approaches (9, 17). Despite extensive effort, nickel catalysis of this transformation has not been achieved, and only a single report of palladium-catalyzed trifluoromethylation of aryl chlorides has been disclosed (18). Such diminished catalytic reactivity in comparison to other well-known coupling reactions is ascribed to the Csp2–CF3 reductive elimination step, a well-documented challenge for both palladium and nickel catalysts (17, 19, 20). In contrast, copper complexes have been shown to generally undergo facile Csp2–CF3 reductive elimination (21, 22). This realization has propelled extensive research efforts to develop copper-catalyzed trifluoromethylation protocols with haloarenes. Although recent examples of copper-mediated (23–25) and copper-catalyzed (26–28) methodologies have provided promising results for the construction of aryl–CF3 motifs, the limited capacity of Cu(I) to participate in oxidative addition has restricted the application of these elegant systems to mostly aryl iodides or activated aryl bromides, often employing elevated temperatures. As such, a mechanistic pathway that can bypass the copper oxidative addition problem, while benefiting from the facility of Cu(III) to engage in difficult reductive elimination, might enable a mild and broadly general strategy toward the trifluoromethylation of aryl bromides.
We recently developed a metallaphotoredox-catalyzed (29) cross-electrophile coupling protocol in which alkyl radicals were generated by a silyl radical–mediated halogen abstraction (30). This manifold was shown to provide facile access to a wide range of alkyl radicals from the corresponding alkyl bromides, a feature that has been found to be remarkably general. Literature precedents indicate that this facile silane-mediated halogen abstraction mechanism should also function broadly with aryl bromides (k ≈ 5 × 106 M−1s−1 to 1.1 × 108 M−1s−1 for bromobenzene with a range of silyl radicals) to generate the corresponding aryl radicals under mild conditions (31, 32). Given that aryl radicals are known to be captured by Cu(II) species at a rate approaching diffusion (33, 34), we questioned whether this light-driven halogen abstraction mechanism might be exploited to forge Cu(III)-arene intermediates from a diverse range of aryl halides at room temperature (Fig. 1), effectively overcoming the copper oxidative addition problem. The successful execution of this mechanistic concept would solve a long-standing challenge in copper catalysis, thereafter providing a general platform to catalytically access trifluoromethylarenes.
Our proposed mechanism for the light-enabled trifluoromethylation protocol is outlined in Fig. 2A. Upon exposure to visible light, photocatalyst Ir(dFFppy)2 (4,4´-dCF3bpy)PF6 [dFFppy = 2-(2,4-difluorophenyl)-5-fluoropyridine; 4,4´-dCF3bpy = 4,4´-di(trifluoromethyl)-2,2´ - bipyridyl)] (1) should produce excited triplet state species 2. This highly oxidizing Ir complex [E1/2red (*IrIII/IrII) = +1.55 V versus saturated calomel electrode (SCE) in CH3CN] can undergo single-electron transfer (SET) with tris-(trimethylsilyl) silanol (4) [supersilanol, Ep (4+•/4) = +1.54 V versus SCE in CH3CN] to give Ir(II) complex 3 (35). We hypothesize that supersilanol 4, upon oxidation and deprotonation, would then undergo rapid silyl migration (Fig. 2B) to yield species 5 as the catalytically relevant silyl radical (36). Subsequent bromine atom abstraction from aryl bromide 6 would then yield the corresponding aryl radical 7. Concurrently, Ir(II) complex 3 [E1/2red (IrIII/IrII) = –0.83 V versus SCE in CH3CN] could facilitate the formation of the CF3 radical (9) through a SET event with electrophilic CF3 reagent 8 [Ep (8/8•–) = –0.52 V versus SCE in CH3CN]. At this time, we believe the Cu(II)–CF3 adduct (11) should be generated through interaction between the CF3 radical (9) and the active Cu(I) complex (10), formed in situ by SET reduction of a Cu(II) catalyst precursor by the photocatalyst. Aryl radical 7 should quickly undergo radical trapping by Cu(II) complex 11 to yield the key aryl–Cu(III)–CF3 adduct 12 (37, 38). Reductive elimination from this high-valent Cu(III) intermediate would yield the desired product 13 and simultaneously regenerate Cu(I) catalyst 10 (39).
Fig. 2. Reaction design.
(A) Proposed mechanism for the trifluoromethylation of aryl bromides with dual copper/photoredox catalysis. (B) Proposed mechanism for the generation of the active silyl radical. (C) Optimized conditions. (D) Reagent dMesSCF3 and its solid-state structure. Ellipsoids are drawn at 50% probability. The triflate counterion is omitted. Me, methyl; LED, light-emitting diode; SET, single-electron transfer; Mes, 2,4,6-trimethylphenyl; –OTf, trifluoromethanesulfonate.
A survey of various combinations of different electrophilic CF3 reagents, copper sources, and solvents revealed that blue light-emitting diode (LED) irradiation of a mixture of 4-bromobenzonitrile, supersilanol (4), dMesSCF3 (8), CuBr2•2LiBr [20 mole % (mol %)], and Ir(III) photocatalyst 1 (0.25 mol %) in acetone at room temperature afforded the desired trifluoromethylarene in 89% yield (Fig. 2C). The use of supersilanol in place of hydrosilanes was found to be crucial, because it minimizes unproductive protodehalogenation of the bromoarene, which presumably proceeds through hydrogen atom abstraction by aryl radicals from weak Si–H bonds (31). In addition, a variety of diaryl sulfonium CF3 salts were found to be compatible with the coupling protocol. However, the dimesityl sulfonium triflate salt (8) provided the highest efficiency of the desired product. We attribute this efficiency to suppression of unproductive CF3 radical addition to the corresponding diaryl sulfide (40). No reactivity was observed between sulfonium 8 and a variety of Cu(I) and Cu(II) complexes, which further supports a photoredox-catalyzed generation of a CF3 radical. Electrophilic CF3 reagent 8 is a bench-stable, crystalline solid, which allows for ease in purification and handling (Fig. 2D). Moreover, optimization in the preparation procedure allowed for a one-step, scalable synthesis of this now-commercial trifluoromethylating reagent.
With the optimized conditions in hand, we sought to examine the scope of the trifluoromethylation protocol (Fig. 3). A broad range of electronically differentiated parasubstituted bromoarenes were found to give good to excellent yields of the desired product (15 to 20, 78 to 96% yield). Functionalities with coupling capability, such as chloro and pinacolborato, remain intact, potentially allowing for subsequent orthogonal functionalization (17 and 19, 96 and 78% yield). Substituents at the meta and ortho positions were also well tolerated (21 and 22, 81 and 70% yield). Without the implementation of extensive optimization studies, the functionalization of dibromobenzenes provided the bis(trifluoromethyl)arene adduct product in good yield with the para-case and moderate to low yields with the ortho and meta adducts (figs. S28 to S30). The monotrifluoromethylated product could be achieved in good yield for all cases using our standard conditions. Aryl bromides with fused cyclic motifs were converted to the desired trifluoromethylarenes in good yields (23 to 26, 75 to 84% yield). Additional examples of bromoarene trifluoromethylation can be found in fig. S31. Regarding the scope of heteroarylbromides, pyridine-derived substrates were functionalized in good to excellent yields (27 to 36, 65 to 91% yield), and the trifluoromethylation of bromopyrazines, -pyrimidines, and -pyridazines was also accomplished in generally high efficiency (37 to 42, 64 to 91% yield). We were pleased to find that a wide range of five-membered heteroaryl bromides, such as imidazoles, pyrazoles, and thiazoles, were competent substrates, providing the desired products in useful to good yields (43 to 50, 38 to 64% yield). Finally, the compatibility of the trifluoromethylation protocol with biorelevant molecules was also examined. Indeed, CF3-derivatives of Celebrex, Lopid, Skelaxin, and Rupatadine were readily synthesized from the corresponding aryl bromide precursors (51 to 54, 42 to 77% yield).
Fig. 3. Synthesis of trifluoromethyl(hetero)arenes.
Substrate scope for the metallaphotoredox-catalyzed trifluoromethylation of (hetero)aryl bromides. All yields are isolated unless noted otherwise. See the supplementary materials for experimental details and more examples. *With photocatalyst 14. †With photocatalyst 1. ‡Yield determined by 19F or proton nuclear magnetic resonance (1H NMR) of crude reaction mixture with respect to an internal standard. §With 20 mol % 1,10-phenanthroline. ||Copper(I)-thiophene-2-carboxylate as catalyst. ¶With 20 mol % 4,7-dimethoxy-1,10-phenanthroline. Ac, acetyl; Pin, pinacolato; Boc, tert-butoxycarbonyl; Et, ethyl; Ts, 4-toluenesulfonate.
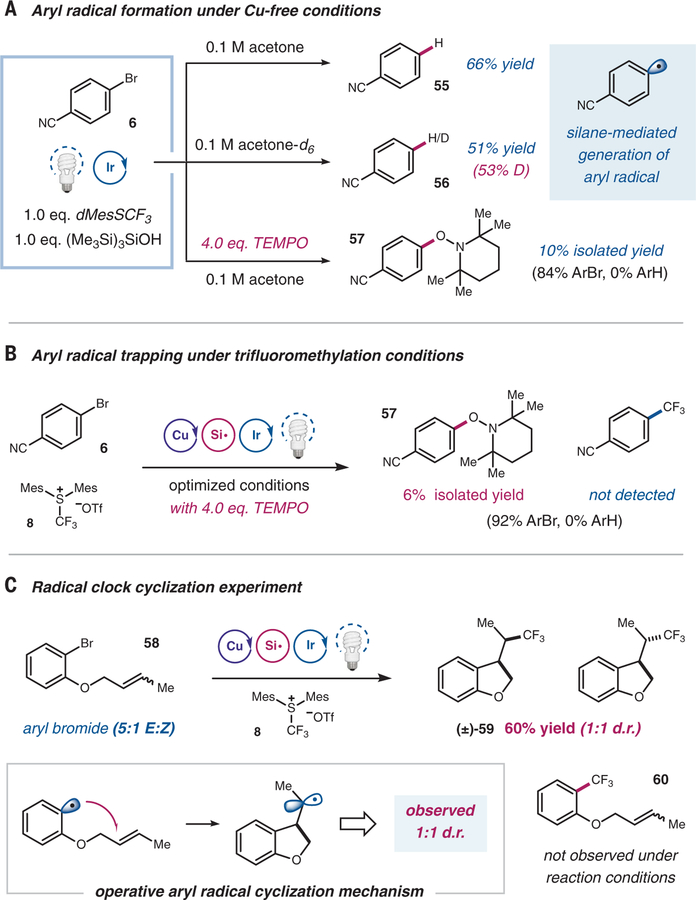
We next turned our attention to investigating the intermediacy of an aryl radical under this dual copper/photoredox platform. First, generation of an aryl radical under copper-free conditions was examined by exposure of a mixture of bromoarene (6), supersilanol (4), dMesSCF3 reagent (8), and photocatalyst (1) to blue LEDs (Fig. 4A). When acetone was employed as the reaction medium, 66% yield of the protodehalogenation product (55) was observed. This product is proposed to arise from a hydrogen atom transfer event between an aryl radical and a solvent molecule. When the same reaction was carried out in acetone-d6, a similar yield was observed with 53% deuterium incorporation at the para position (56, 51% yield). Furthermore, conducting the identical reaction in the presence of 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO) resulted in complete shutdown of formation of the reduced product, and, more important, aryl-trapped TEMPO adduct 57 was isolated, further supporting the intermediacy of an aryl radical (41, 42). A similar result was observed when a TEMPO-trapping experiment was carried out with the optimized trifluoromethylation conditions. As shown in Fig. 4B, aryl-TEMPO adduct 57 was isolated, whereas the formation of the desired trifluoromethylarene was completely suppressed. Control experiments indicated that the aryl radical formation is not mediated by direct SET reduction of the aryl bromide by the photocatalyst (fig. S41). To lend additional support to our proposed mechanism, we next employed a radical clock experiment with ortho-O-(3-methyl-allyl)bromobenzene (5:1 trans:cis mixture) (58) as the substrate (Fig. 4C). Subjecting the olefin mixture to the standard conditions yielded exclusively the cyclized alkyl-CF3 product in good yield (59, 60% yield). The 1-to-1 diastereomeric ratio of the cyclized product 59, along with the absence of the CF3-arene product 60, further support the hypothesis of aryl radical generation during the course of the reaction (33, 34). Previously reported copper-mediated aryl halide trifluoromethylation protocols did not exhibit aryl radical characteristics when similar radical clock experiments were used as a mechanistic probe (24, 25). In addition, whereas aryl radical generation has previously been shown to be operative under light-irradiated copper catalysis (33), the open-shell aryl species under our optimized conditions is generated by a silyl radical halogen abstraction, independent of the copper catalyst (43).
Fig. 4. Evidence for an aryl radical mechanism.
(A) Under copper-free conditions, silane-mediated generation of aryl radical was observed through hydrogen/deuterium trapped product and TEMPO-trapped adduct. (B) Similar behavior was observed under the optimized reaction condition when TEMPO was used as a radical trap. (C) Exclusive cyclized product formation, along with the observed diastereoselectivity, served as strong indications for an aryl radical mechanism being operative. TEMPO, (2,2,6,6-Tetramethylpiperidin-1-yl)oxyl; d.r., diastereomeric ratio.
Use of aryl radical trapping to circumvent the sluggish nature of conventional oxidative addition in copper catalysis has allowed for the development of a general dual copper/photoredox-catalyzed trifluoromethylation protocol for aryl bromides. We expect this method to be widely adopted by the synthetic community as a complementary tool to existing trifluoromethylation platforms. Perhaps most important, we see this halide abstraction/radical capture mechanism (at room temperature) as an approach to overcome the copper oxidative addition problem and in doing so provide a general paradigm to the development of many, previously unknown, copper-catalyzed coupling reactions.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank C. Kraml, N. Byrne, and L. Wilson (Lotus Separations) for compound purification and P. Jeffrey for assistance with x-ray structure determination.
Funding: Research reported in this publication was supported by the NIH National Institute of General Medical Sciences (R01 GM103558-03) and gifts from Merck, Bristol-Myers Squibb, Eli Lilly, Genentech, Pfizer, and Johnson & Johnson. C. L. thanks Bristol-Myers Squibb for a graduate fellowship.
Footnotes
Competing interests: The authors declare no conflicts of interest.
Data and materials availability: Crystallographic parameters for compound 8 are available free of charge from the Cambridge Crystallographic Data Centre under CCDC 1833832. Data are available in the supplementary materials.
SUPPLEMENTARY MATERIALS
REFERENCES AND NOTES
- 1.Hegedus LS, Söderberg BCG, Transition Metals in the Synthesis of Complex Organic Molecules (University Science Books, United States, ed. 3, 2010). [Google Scholar]
- 2.Petrone DA, Ye J, Lautens M, Chem. Rev 116, 8003–8104 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Tsuji J, Palladium Reagents and Catalysts: New Perspectives for the 21st Century (John Wiley & Sons, 2005). [Google Scholar]
- 4.Tasker SZ, Standley EA, Jamison TF, Nature 509, 299–309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evano G, Blanchard N, Copper-Mediated Cross-Coupling Reactions (John Wiley & Sons, 2014). [Google Scholar]
- 6.Jones GO, Liu P, Houk KN, Buchwald SL, J. Am. Chem. Soc 132, 6205–6213 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu H-Z, Jiang Y-Y, Fu Y, Liu L, J. Am. Chem. Soc 132, 18078–18091 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Giri R et al. , J. Am. Chem. Soc 140, 793–806 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amal Joseph PJ, Priyadarshini S, Org. Process Res. Dev 21, 1889–1924 (2017). [Google Scholar]
- 10.Systems competent for aryl chloride oxidative addition haverecently been reported. See (11).
- 11.Bhunia S, Pawar GG, Kumar SV, Jiang Y, Ma D, Angew. Chem. Int. Ed 56, 16136–16179 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Casitas A, Ribas X, Chem. Sci 4, 2301–2318 (2013). [Google Scholar]
- 13.Furuya T, Kamlet AS, Ritter T, Nature 473, 470–477 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hickman AJ, Sanford MS, Nature 484, 177–185 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uneyama K, Organofluorine Chemistry (Blackwell, Oxford, UK, 2006). [Google Scholar]
- 16.Hagmann WK, J. Med. Chem 51, 4359–4369 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Tomashenko OA, Grushin VV, Chem. Rev 111, 4475–4521 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Cho EJ et al. , Science 328, 1679–1681 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bour JR, Camasso NM, Sanford MS, J. Am. Chem. Soc 137, 8034–8037 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Alonso C, de Marigorta EM, Rubiales G, Palacios F, Chem. Rev 115, 1847–1935 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Konovalov AI, Lishchynskyi A, Grushin VV, J. Am. Chem. Soc 136, 13410–13425 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Jover J, ACS Catal 4, 4389–4397 (2014). [Google Scholar]
- 23.Dubinina GG, Furutachi H, Vicic DA, J. Am. Chem. Soc 130, 8600–8601 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Zanardi A, Novikov MA, Martin E, Benet-Buchholz J, Grushin VV, J. Am. Chem. Soc 133, 20901–20913 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Morimoto H, Tsubogo T, Litvinas ND, Hartwig JF, Angew. Chem. Int. Ed 50, 3793–3798 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Q-Y, Wu S-W, J. Chem. Soc. Chem. Commun 11, 705–706 (1989). [Google Scholar]
- 27.Oishi M, Kondo H, Amii H, Chem. Commun. (Camb.) 14, 1909–1911 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Schareina T et al. , Top. Catal 55, 426–431 (2012). [Google Scholar]
- 29.Twilton J et al. , Nat. Rev. Chem 1, 0052 (2017). [Google Scholar]
- 30.Zhang P, Le CC, MacMillan DWC, J. Am. Chem. Soc 138, 8084–8087 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chatgilialoglu C, Organosilanes in Radical Chemistry (Wiley, Chichester, UK, 2014). [Google Scholar]
- 32.Devery III JJ, Nguyen JD, Dai C, Stephenson CRJ, ACS Catal 6, 5962–5967 (2016). [Google Scholar]
- 33.Creutz SE, Lotito KJ, Fu GC, Peters JC, Science 338, 647–651 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Johnson MW, Hannoun KI, Tan Y, Fu GC, Peters JC, Chem. Sci 7, 4091–4100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stern-Volmer studies confirmed quenching interaction between supersilanol 4 and the excited photocatalyst. See fig. S40.
- 36.Lucarini M, Marchesi E, Pedulli GF, Chatgilialoglu C, J. Org. Chem 63, 1687–1693 (1998). [Google Scholar]
- 37.For reviews on radical-radical cross-coupling in the presence ofa transition metal catalyst, see (38).
- 38.Yi H et al. , Chem. Rev 117, 9016–9085 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Lishchynskyi A, Berthon G, Grushin VV, Chem. Commun. (Camb.) 50, 10237–10240 (2014). [DOI] [PubMed] [Google Scholar]
- 40.See the supplementary materials for optimization studies.
- 41.Xia Z, Zhu Q, Org. Lett 15, 4110–4113 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Leas DA, Dong Y, Vennerstrom JL, Stack DE, Org. Lett 19, 2518–2521 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Studies were carried out to rule out an aryl bromide activation by an excited Cu(I)-CF3 species as the major pathway under the standard conditions. See the supplementary materials.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.