Abstract
Cancer treatments can damage the ovaries, causing primary ovarian insufficiency (POI), a condition associated with numerous sequelae that impact long-term quality of life. This article systematically reviews the literature on the prevalence, surveillance, and treatment of POI in survivors of pediatric and adolescent and young adult (AYA) cancers. A systematic review of the literature was conducted in January 2018 through a search of Medline, Embase, Web of Science, and SCOPUS, alongside the screening of relevant reference lists. An initial search identified 746 potentially relevant studies. A total of 36 studies were included in the final review. Studies were categorized into one of the following categories: incidence/prevalence of POI, measurement of ovarian reserve, and other. Depending on patient characteristics, cancer diagnosis, and treatment, the prevalence of POI ranged from 2.1% to 82.2%. Risk factors for POI included exposure to alkylating agents and abdominal/pelvic radiation. POI may be associated with a number of complications, including low bone mineral density and poor cardiovascular health. Radiotherapy and chemotherapy are known to cause gonadal damage in female survivors of pediatric and AYA cancers. Acute or chronic effects depend on the dose of treatment, age of the individual, radiotherapy field, and ovarian reserve of the individual. Some women experience short-term loss of reproductive function and then may resume menstrual cycles, months or even years later. Although protecting fertility through banking of mature eggs, embryos, and tissue samples has become standard of care, additional steps need to be taken to ensure that patients have adequate hormone levels to maintain whole-body health, including life expectancy, bone health, cardiovascular health, quality of life, sexual and genitourinary function, and neurologic function. Surveillance and management of each of these comorbidities is critically important to survivor health.
Introduction
Modern cancer treatment regimens are highly effective, with up to 88.8% of patients with cancer aged <45 years surviving 5 years after diagnosis.1 With this success comes the need to think beyond life-and-death decision-making and toward long-term quality-of-life (QoL) considerations. Fertility-sparing options are available to many patients, and whether patients opt for these interventions depends on their family planning expectations.2–7 Survivors who are not provided with fertility options experience anxiety, which can be a comorbidity in its own right.8 Beyond fertility, patients with cancer also risk loss of endocrine function, leading to primary ovarian insufficiency (POI), a syndrome characterized by amenorrhea, sex hormone deficiency, and elevated serum follicle-stimulating hormone (FSH) levels on at least 2 occasions, tested at least 1 month apart in women aged <40 years.9 The cessation of episodic endocrine hormones may result in menopausal symptoms, such as hot flashes, vaginal dryness, and sexual dysfunction, as well as increased morbidity associated with a reduction in endogenous estrogen, including bone mineral density (BMD) and cardiovascular changes. Thus, surveillance of the endocrine health of adolescent and young adult (AYA) cancer survivors is critical to their overall health.
This article systematically reviews the literature on prevalence and management of POI in survivors of pediatric and AYA cancers (aged 0–24 years). All peer-reviewed literature and qualitative and quantitative data reporting on prevalence, surveillance, and management of POI-related symptoms in cancer survivors are examined. To gain a full understanding of short-term and long-term treatment effects, data were not restricted by time from diagnosis. This inclusive approach allowed for the identification of POI symptoms that may emerge late after diagnosis and treatment. Thus, this review will help determine the scope of POI-related symptoms and their management at various time points throughout the cancer journey for pediatric and AYA cancer survivors.
Methods
Inclusion Criteria and Search
This systematic review considered all studies that discussed prevalence and management of sequalae related to POI in female survivors of pediatric and AYA cancers. We excluded studies that focused specifically on fertility or family planning. To meet inclusion criteria, patient age needed to be within the range of 0 to 24 years or have a mean sample age <25 years. Studies that focused on symptoms of POI during or after cancer treatment were included. Studies needed to be in English, published in a peer-reviewed journal, and of sound quality, as assessed using a validated quality assessment tool.10 No publication date restrictions were imposed.
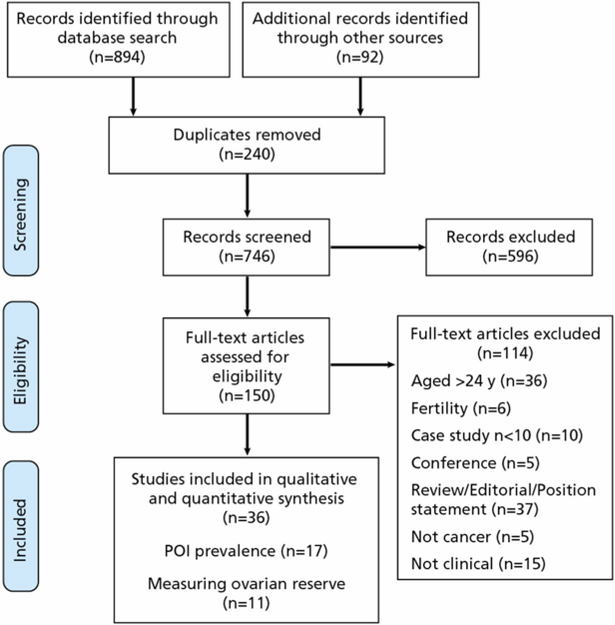
A comprehensive literature search was performed in January 2018, with suitable studies identified through the electronic databases Medline, Embase, PsycInfo, Web of Science, and Scopus, alongside the screening of reference lists. Search terms were tailored to individual databases in order to map terms to database subject headings and take an inclusive approach (Table 1). The search across electronic databases and reference lists identified 746 potentially relevant studies after the deletion of duplicates. All titles and abstracts were screened bya single reviewer, who then assessed the full text of the remaining 150 studies to determine eligibility for inclusion. A total of 36 studies remained eligible for further analysis (Figure 1).
Table 1.
Search Terms
| Cancer | Primary Ovarian Insufficiency | Fertility | ||
|---|---|---|---|---|
| [Neoplasm] or [oncology] or [cancer survivor] or [cancer survival] or [childhood cancer] or [cancer therapy] | AND | [Menopause] or [menopause, premature] or [primary ovarian insufficiency] | NOT | [Fertility] or [infertility, female] or [fertility preservation] |
Figure 1.
Flowchart of inclusion/exclusion process.
Abbreviation: POI, primary ovarian insufficiency.
Quality Analysis and Extraction
The quality of the final studies was assessed using the Mixed Methods Appraisal Tool (MMAT).10 Scores on the MMAT vary from 25% (1 criterion met) to 100% (all criteria met). Quality was assessed against criteria related to either qualitative or quantitative enquiry. All 36 final studies were of sound quality (75%–100%) and were then analyzed for data by one reviewer. Data regarding sample size, patient characteristics (age, sex, cancer diagnosis, treatment), and descriptions and details of POI-related sequelae were extracted from each paper.
Results
Study Characteristics
Tables 2, 3 and 4 reflect the key features of each study. Table 2 includes the 17 studies that report the prevalence of POI in female survivors of pediatric and AYA cancers11–27; Table 3 summarizes the data from 11 studies related to measurement of ovarian reserve16,24,28–36; and Table 4 lists the remaining studies identified in this literature review.37–44 The number of subjects in Tables 2, 3 and 4 reflects only the female cancer survivors in each study; some of the studies included male survivors and/or healthy controls, but these numbers are not reflected. Studies were conducted across 9 countries; the sample size range varied from 15 to 3,749 participants. Among the papers, 3 reported on acute lymphoblastic leukemia only,37–39 1 reported only on Hodgkin lymphoma,13 and 1 only on Ewing sarcoma,22 whereas the other studies reported on a range of cancer diagnoses; 7 studies did not report the specific cancer diagnoses.12,19,24,30,33,37,41
Table 2.
POI Prevalence Data Extracted by Study
| Study Details | Age, y | na | Cancer | Treatment | Method | Prevalence | Risk Factors | Notes | MMAT (%) |
|---|---|---|---|---|---|---|---|---|---|
| Livesey et al,11 1990 (UK) | Median age at treatment, 6.7 (range, 1.3–15); median age at study, 9.6 | 67 | Brain tumor | 100% cranial RT and some also received spinal Some also received adjuvant chemo |
Hormone measurement | 26.8% | Cranial RT combined with either spinal RT OR adjuvant chemo | No child treated with cranial RT alone experienced gonadal dysfunction | 75 |
| Byrne et al,12 1992 (US) | At diagnosis, age range: 13–19 | 954 | Various | RT and/or chemo | Self-report/questionnaire | 42.0% (by age 31 y) | Cancer diagnosis after age 12 years (RR, 2.32; 95% Cl, 1.63–3.29) RT alone (RR, 3.7) Alkylating agents alone (RR, 9.2) Both RT and alkylating agents (RR, 27.39; 95% Cl, 2.67–31.49) |
75 | |
| Mackie et al,13 1996 (UK) | Mean age at diagnosis, 13.0 (range, 9.0–15.2) | 32 | HL | ChlVPP | Hormone measurement | 31,25% | 60% of survivors with POI were taking HRT | 100 | |
| Teinturier eta 1,14 1998 (France) | Median age at treatment, 9 (range, 2–17); age at study, 14.5 (range, 11.5–21) | 21 | 85.7% solid tumors; 14.3% hematologic | High-dose chemo and autologous BMT without abdominal/pelvic RT | Hormone measurement | 57.1% | B us u If an | 100% of girls treated with busulfan had evidence of POI vs 27% of those who did not receive it | 100 |
| Chlarelli et al,151999 (Canada) | Age at diagnosis < 20; median age at study, 28 (range, 18–49) | 719 | 60.1 % solid tumors; 39.9% hematologic | Chemo, surgery, and/or RT | Self-report/questionnaire | 8.8% | Alkylating agents and abdominal/pelvic RT {RR, 2.58; 95% G, 1.14–5.80) | 75 | |
| Larsen et al,16 2003 (Denmark) | Median age at diagnosis, 5.4 (range, 0.1–15.3); median age at study, 25.7 (range, 18.5–44.4) | 100 | 37% solid tumors; 63% hematologic | 100% chemo; 56% RT; 12% BMT’ 37% surgery | Hormone measurement and transvagina I sonography | 17% | 100 | ||
| Sklar et al,17 2006 (US, Canada) | Median age at diagnosis, 7 (range, 0–20); age at study, 29 (range, 18–50) | 2,819 | 45% solid tumors; 55% hematologic | 10% surgery only; 10% chemo only; <1 % RT only; 17% chemo + RT; 20% surgery + chemo; 8% surgery + RT; 33% surgery + RT + chemo; 1% stem cell transplant | Self-report/questionnaire | 15% (RR, 1.05; 95% Cl, 1.04–1.07 vs siblings); 8% nonsurgical POI | Older age at diagnosis and follow-up Diagnosis of HL Exposure to alkylating agents Exposure to abdominal/pelvic radiation |
100 | |
| Mansky et al,18 2007 (US) | Median age at treatment, (range, 7.1–34.2); median age at study, (range, 17.5–55.4) | 15 | Sarcomas | Chemo + RT | Self-report/questionnaire | 49% | 75 | ||
| Green et al,19 2009 (US, Canada) | All cancers diagnosed at age<18 | 2,819 | Not described | Not described | Self-report/questionnaire | 8% | Attained age Exposure to increasing doses of radiation to the ovaries Increasing alkylating agent exposure Diagnosis of HL |
75 | |
| Thomas- Teinturier et al,20 2013 (France) | Median age at diagnosis, 4 (range, 0–17); median age at study, 36 (range, 21–66) | 706 | 85% solid tumors; 15% hematologic | 9% no chemo or RT; 29.5% chemo; 13% RT; 48.5% chemo + RT | Self-report/questionnaire | 2.1% | Alkylating agents (RR, 9; 95% Cl, 2.7–18) Radiation to ovaries Procarbazine dose Cyclophosphamide dose Unilateral oophorectomy |
75 |
Abbreviations: aHSCT, autologous hematopoietic stem cell transplantation; ALL, acute lymphoblastic leukemia; BMT, bone marrow transplant; CED, cyclophosphamide equivalent dose; chemo, chemotherapy; ChlVPP, chlorambucil/vinblastine/procarbazine/prednisolone; CNS, central nervous system; HL, Hodgkin lymphoma; HR, hazard ratio; HRT, hormone replacement therapy; MMAT, Mixed Methods Appraisal Tool; POI, primary ovarian insufficiency, RR, relative risk; RT, radiotherapy.
The number of patients reflects only the female cancer survivors in each study; some of these studies included male survivors and/or healthy controls, but these numbers are not reflected.
Table 3.
Ovarian Reserve Data Extracted by Study (cont.)
| Study Details | Age, y | na | Cancer | Treatment | Method | Results | MMAT (%) |
|---|---|---|---|---|---|---|---|
| Thomas-Teinturier et al,36 2015 (France) | Median age at diagnosis, 9.3 (range, 0.04–17.7); median age at study, 25 (range, 17–40) | 105 | 61% solid tumors; 39% hematologic | 100% alkylating agents; 18% subdiaphragmatic RT | Hormone measurement Transvaginal ultrasound |
Survivors had lower OSA (3.5 vs 4.4 cm2; P=.0004) and AMH levels (10.7 vs 22 pmol/L; P=.003) vs controls, but AFC was not different (12 vs 11; P=.8) OSA, AMH, and AFC were lower in patients who received high-dose vs conventional-dose alkylating agents (P=.01 for OSA; P=.002 for AMH; P<.00001 for AFC) HL survivors had the greatest reduction in ovarian parameters, and procarbazine (not cyclophosphamide or ifosfamide) dose was associated with reduced OSA, AFC, and AMH, and higher FSH; however, the individual impacts of procarbazine and HL on ovarian reserve could not be dissociated AFC was correlated with AMH and, to a lesser extent, OSA |
100 |
| Salih etal.24 2015 (US) | Median age at diagnosis, 7 (range, 0–20); age at last follow-up, 14.0 (range, 2–34) | 222 | Various (25% ALL) | Chemo. RT. BMT. or combination 74% chemo; 23% RT; 7% BMT | Hormone measurement | 69% had normal ovarian reserve (FSH <10 IU/L), 17% had DOR (FSH. 10–40 IU/L) 14% had POI (FSH >40 IU/L) |
75 |
Abbreviations: AFC, antral follicle count; ALL, acute lymphoblastic leukemia; AMH, anti-Mullerian hormone, BMT, bone marrow transplant; CED, cyclophosphamide equivalent dose; chemo, chemotherapy; COCPs, combined oral contraceptive pills; DOR. diminished ovarian reserve; FSH, follicle-stimulating hormone; HL, Hodgkin lymphoma; MMAT, Mixed Methods Appraisal Tool; MOPP, mechlorethamine/vincristine/procarbazine/prednisone; OCPs, oral contraceptive pills; OSA, ovarian surface area; POI, primary ovarian insufficiency; RT, radiotherapy; TBI, total body irradiation.
The number of patients reflects only the female cancer survivors in each study; some of these studies included male survivors and/or healthy controls, but these numbers are not reflected.
Table 4.
Data From Remaining Studies
| Study Details | Age, y | na | Cancer | Treatment | Results | MMAT (%) |
|---|---|---|---|---|---|---|
| Wilson et al,37 2016 (US) | Median age at diagnosis, 5.0 (range, 0.2–19.5); median age at study, 31.3 (range, 18.4–59.7) | 436 | ALL | RT, high-dose MTX. cyclophosphamide, glucocorticoids | Women with growth hormone deficiency (OR, 2.18; 95% Cl, 1.26–3.78) or moderate alcohol consumption (OR, 2.09, 95% Cl, 1.14–3.83) had increased odds of low BMD No association between POI and low BMD or frailty |
100 |
| Gurney et al,38 2014 (US) | Median age at diagnosis 5; median age at study 31 | 429 | ALL | RT, MTX, glucocorticoids, anthracydine, etoposide, vincristine | No significant association between POI and BMD z score (P=.24) The cumulative prevalence of those with low BMD (z score ≤−1 ) at age 40 years was 28.3% (95% Cl, 21.9%–34.9%) |
100 |
| Mandel et al,39 2004(Canada) | Mean age at diagnosis, 5.8 (range, 1.0–17.1); mean age at study, 15.9 | 62 | ALL | 50% cranial RT; 81 % standard dose of MTX, 7% high-dose MTX, and 12% very high-dose MTX | No difference was observed in BMD between survivors of childhood ALL and controls Those with low femoral BMD were more likely to have received high-dose MTX or higher-dose corticosteroids vs remainder of the group |
100 |
| Hesseling et al,40 1998 (South Africa) | Median age at diagnosis, 4.5; median age at follow-up, 14.9 | 46 | 60.5% solid tumors; 39.5% hematologic | 42% corticosteroids; 35% cranial RT | Low BMD was observed in 45% of children Significant osteopenia (BMD z score <−2) in 13.4% of children, and osteopenia (−2 < z score < −1) in 32% Low BMD was associated with increasing doses of cranial RT Height for age at follow-up correlated significantly with BMD z score |
75 |
| Wilson et al,41 2012 (US) | Median age at diagnosis, 6.9 (range, 0–21); median age at study, 36.2 (range, 21.2–58.8). | 3,749 | Various | Alkylating agents, MTX, glucocorticoids, cranial irradiation, pelvic RT | Prevalence of fractures among female survivors and sibling controls was comparable Risk factors for fractures in female survivors were increasing age at follow-up, white race, MTX treatment, and balance difficulties |
100 |
| Gurney et al,42 2003 (US) | Age at diagnosis <20 | 734 | Brain tumors | 25.8% surgery only; 42.4% surgery + RT; 27.8% surgery + RT + chemo; 4.0% other treatments | 43% reported ≥1 endocrine condition RR for osteoporosis, 24.7 (95% Cl, 9.9–61.4) | 75 |
| Ness et al,43 2013 (US) | Mean age at study, 33.6 ± 8.1 | 956 | 37.3% solid tumors; 2.7% hematologic | RT, chemo, surgery | Prevalence of prefrailty and frailty were 31.5% and 13.1%, respectively, among women; similar to rates seen in the population aged >65 years Increasing age and cranial RT were the only factors associated with frailty |
75 |
| Tomioka et al,44 2017 (Japan) | Median age at diagnosis. 7 (range, birth–15); median age at study, 25 (range, 20–41) | 49 | 30.6% solid tumors; 69.4% hematologic | 65.2% RT (13 TBI, 8 cranial); 36.7% HSCT | 40.8% experienced gonadal dysfunction 20.4% experienced endocrine dysfunction 8.2% experienced muscle/bone damage 6.1 % experienced cardiovascular dysfunction 4.1% experienced neurocognitive dysfunction or mental problems |
75 |
Abbreviations: ALL, acute lymphoblastic leukemia; BMD, bone mineral density; chemo, chemotherapy; HSCT, hematopoietic stem cell transplantation; MMAT, Mixed Methods Appraisal Tool; MTX, methotrexate; OR, odds ratio; POI, primary ovarian insufficiency; RR, relative risk; RT, radiotherapy; TBI, total body irradiation.
The number of patients reflects only the female cancer survivors in each study; some of these studies included male survivors and/or healthy controls, but these numbers are not reflected.
Prevalence of and Risk Factors for POI in Survivors
Depending on patient factors, cancer diagnosis, and treatment exposures, the prevalence of POI in survivors of pediatric and AYA cancer ranged from 2.1% to 82.2% (Table 2). A recent report from the St. Jude Lifetime Cohort reported the prevalence of POI to be 10.9%.27 In this study, POI status was determined with hormone measurements (estradiol level <17 pg/mL and FSH level >30 IU/L before age 40 years), and therefore provides a more accurate figure for POI prevalence than previous studies that relied on self-report of menopausal status, in which true rates of POI can be obscured by factors such as use of combined oral contraceptive pills (COCPs). Multiple studies in different cohorts have reported the following risk factors for POI: cancer diagnosis after puberty12,17; alkylating agents,12,15,17,19,20,27,45especially busulfan,14 procarbazine,20 and cyclophosphamide20; abdominal/pelvic radiation12,15,17,19,20,22,27; and diagnosis of Hodgkin lymphoma.17,19 Given that these studies were performed in cohorts treated >20 years ago (before risk-adapted protocols and new, potentially gonadotoxic agents, such as tyrosine kinase inhibitors and PD-L1 inhibitors), it is difficult to predict how the prevalence of POI will compare in today’s and tomorrow’s survivors of childhood cancer.
Management of Perimenopause and Menopause in Survivors
POI results in the cessation of menses and postmenopausal levels of sex hormones and gonadotropins. Regardless of the cause (spontaneous menopause vs treatment-induced POI), menopause is characterized by a constellation of symptoms, including vasomotor symptoms (hot flashes and night sweats), osteoporosis with associated fracture risk, and vaginal dryness and dyspareunia.46 In addition to these symptoms, women experiencing perimenopause and menopause often complain of anxiety, depression, irritability, loss of libido, palpitations, skin dryness, and fatigue, although it is debated whether these symptoms can be attributed to menopause.46 Importantly, many of these menopause symptoms mirror common chemotherapy and radiation side effects. It may be easy to dismiss these symptoms as side effects, but they may be a harbinger of ovarian damage. Careful history taking and physical examination, supplemented with appropriate laboratory tests, may help patients have more realistic expectations regarding their ovarian function and their risks of morbidity/mortality. For women, there is social value in ovarian function and fertility, such that ovarian failure and menopause may impose psychosocial burden.8 Regardless, it is important for providers to recognize the distress these symptoms cause patients and manage the symptoms using a combination of pharmacologic treatments, behavioral interventions, and communication. The following sections review recommendations for comprehensive perimenopause/menopause (or POI) care in young female cancer survivors.
Perimenopause:
Perimenopause is the transition between regular cycling and menopause, often characterized by heavy, irregular vaginal bleeding and irregular ovulations, which can increase the risk of unintended pregnancy. Contraception counseling is necessary for all women of childbearing age, even those who have POI. Iatrogenic POI may be transient, and hence an instance of ovarian dysfunction does not assure complete and permanent infertility.47 For example, recovery of ovarian function was observed in 38.5% of female survivors of primary central nervous system embryonal tumors treated with craniospinal radiation followed by adjuvant cyclophosphamide and stem cell rescue.23 Many options for contraception are available, including hormonal and nonhormonal methods, with the latter more appropriate for survivors of hormone-dependent cancers. To set realistic expectations, providers should clearly explain that future ovarian function and fertility after cancer treatments are uncertain and that fertility preservation should be pursued as early as possible, ideally before cancer treatment, if it is desired.
Various methods have been used to determine ovarian reserve in female cancer survivors, including serum anti-Müllerian hormone (AMH), antral follicle count (AFC), and ovarian volume or surface area (Table 3). Nielsen et al31 found that serum AMH level and AFC were highly correlated (r=0.83; P<.001) in a study of 71 Danish survivors of childhood cancer. Determination of AFC and ovarian volume or surface area require the use of transvaginal sonography and are thus invasive. Measurement of AMH level, although controversial, is a less invasive test. Moreover, in a prospective study of the effect of cancer treatment on ovarian reserve, Brougham et al30 assessed AMH levels at various points throughout treatment in a cohort of 22 patients with cancer, ranging from 0.3 to 15 years of age, and showed that AMH could be detected at all ages. Although still controversial, measurement of serum AMH levels may be the most appropriate test for some patients and can help guide fertility counseling and decision-making. AMH level correlates with the degree of ovarian damage after cancer treatment48 and measures ovarian reserve, although it does not necessarily predict the likelihood of future live births.49
Does POI Lead to Increased Morbidity/Mortality?
POI affects not only fertility, but a broad range of organ systems, including cardiovascular, musculoskeletal, and neurologic systems. The best evidence for long-term effects of POI is from the Mayo Clinic Cohort Study of Oophorectomy and Aging-1. In this cohort, women who had bilateral oophorectomy before age 45 years had increased mortality compared with age-matched control women (hazard ratio [HR], 1.67; 95% CI, 1.16–2.40; P=.006).50 Primary causes of death were cardiovascular disease and osteoporotic fracture, and the study also reported cognitive impairment, dementia, Parkinsonism, sexual dysfunction, and reduced psychological well-being. Other large observational studies have also investigated morbidity/mortality in women with iatrogenic POI caused by surgical removal of the ovaries. The Nurses’ Health Study found increased mortality (HR, 1.12; 95% CI, 1.03–1.21) among women who underwent total abdominal hysterectomy (TAH) with bilateral oophorectomy (TAHBSO) compared with those who underwent TAH alone, with 24 years of follow-up.51 The Women’s Health Initiative (WHI) Observational Study found no difference in mortality between TAHBSO and TAH, but had <8 years of follow-up.52 Several epidemiologic studies also support the notion that POI is associated with increased mortality. In a Dutch cohort of 12,000 women followed over 17 years, life expectancy of women with POI was 2 years less than that of controls.53 Similar results have been seen in non-Caucasian populations, including Japanese,54 Korean,55 and Chinese women.56
Influence of Estrogen Replacement on Mortality After POI
No long-term prospective studies have examined the safety and efficacy of estrogen replacement therapy in terms of mortality in patients with POI.57 Evidence regarding the use of hormone replacement therapy (HRT) in survivors of childhood cancer is even more limited. However, evidence suggests that women who do not receive HRT have increased mortality compared with those who receive treatment. Shuster et al58 reported increased mortality in women experiencing menopause before age 45 years who did not recieve estrogen replacement. The WHI randomized controlled trials of HRT versus placebo did not show a protective effect of HRT within 10 years of menopause against cardiovascular disease or death (HR, 0.76; 95% CI, 0.50–1.16; absolute excess risk: 6/10,000 woman-years).59 Caution must be exercised regarding the timing of initiation of estrogen replacement, because the protective effects are considered to be optimal with its introduction in relatively atheroma-free vessels compared with atheromatous vessels, in which it is considered a have a prothrombotic effect.60,61
Bone Health Interventions and Monitoring
Studies of interventions to improve bone health have generally examined surrogate markers for fracture risk, such as DEXA scans. Lifestyle interventions, such as smoking cessation, regular weight-bearing exercise, increased intake of calcium and vitamin D, moderate alcohol consumption, and maintenance of a healthy body weight, are likely to improve BMD and therefore fracture risk in women with POI.37,67 Estrogen replacement has shown clear benefits for postmenopausal women in increasing BMD and reducing fracture risk. In women with POI, estrogen replacement improves BMD as assessed by DEXA, but there is insufficient evidence of its effect on fracture risk. COCPs are widely used to support bone health in women with POI, but a crossover randomized controlled trial showed that transdermal 17β-estradiol and micronised progesten HRT, which provided physiologic levels of sex hormones, outperformed COCPs in terms of lumbar bone density, bone turnover markers, and bone formation markers, although the sample size was low.68 Currently, there are no trials on the uses of non-hormonal pharmacotherapies for women with POI, such as bisphosphonates, selective estrogen receptor modulators, or strontium/denosumab. Bisphosphonates are especially problematic for women desiring subsequent pregnancy. Regarding surveillance of bone health, guidelines from the Children’s Oncology Group grecommend a baseline DEXA scan when survivors enter long-term follow-up (generally at 2 years after completion of therapy)69; no consensus exists on the optimal intervals for repeated screening, both in the cancer survivor population and in postmenopausal women.70 Scans should therefore be repeated as clinically indicated based on individual risk factors, such as exposure to glucocorticoids, cranial radiation, methotrexate, or hematopoetic stem cell transplantation, or if DEXA results would alter treatment.71 Decreases in BMD should prompt consideration of changes in treatment.
Cardiovascular Risk and Monitoring Cardiovascular Health
Women with POI have increased risk of cardiovascular disease compared with age-matched controls,72 because decreased endogenous estrogen production leads to changes in lipid profiles that result in a more atherogenic milieu. In a study of 49 female cancer survivors in Japan, 6.1% experienced cardiovascular dysfunction.44 Of these women, 40.8% had undergone HRT. In a study of 18 women aged 19 to 39 years with POI, physiologic estrogen replacement with transdermal 17β-estradiol resulted in lower blood pressure, improved renal function, and decreased activation of the renin-angiotensin system.73 To date, there no are established guidelines for managing cardiovascular risk in women with POI, but this study demonstrates the protective effect of estrogen in maintaining cardiovascular health in young female cancer survivors. The importance of regular screening for cardiovascular risk factors should be emphasized, including monitoring of blood pressure, lipids, fasting glucose, and body mass index, and counseling for smoking cessation and maintaining a balanced diet and regular physical activity.
Genitourinary Function
Women with POI often experience vaginal dryness, causing dyspaurenia, or discomfort with intercourse. Our systematic review did not uncover any studies related to genitourinary symptoms in survivors of pediatric and AYA cancers experiencing POI. We therefore searched the literature on breast cancer survivors, and found recent evidence showing that genitourinary symptoms are rarely assessed or treated in this population, revealing a missed opportunity to improve QoL.74 In addition to physical discomfort, hormonal changes in survivors with POI are also associated with alterations in libido and arousal. Libido may be affected by the emotional burden of disease and its ongoing sequalae. Patients may experience anxiety and depression, sleep disturbance, pain, loss of skin sensation, changes in body image or weight, and strained intimate partner relationships that may compromise sexual function.75 Interventions to address genitourinary function include systemic estrogen replacement, local vaginal estrogen replacement, and androgen replacement, and nonmedical strategies, including pelvic floor exercises, clitoral therapy devices, individual or couple’s therapy, and use of vaginal lubricants.76,77
Neurologic Function
Evidence suggests that POI may be associated with impairments in neurologic function. Women with POI after hysterectomy and oophorectomy without estrogen replacement have shown an acute decline in verbal memory and increased risk of dementia and Parkinson disease.78,79 A systematic review showed that the relationship between neurologic function and iatrogenic POI after breast cancer treatment was inconclusive, partly because most studies did not consider menopausal status a potential contributor to neurologic dysfunction.80 A Japanese study of 49 female childhood cancer survivors reported that 4.1% experienced neurocognitive dysfunction.44 Further studies with larger sample sizes and more diverse patient populations are needed to establish the link between cancer-related POI and cognitive dysfunction.
It is possible that estrogen replacement may improve neurologic function in POI. Strong evidence suggests that estrogen exerts protective effects on the aging brain, but limited data show improvements in cognitive function with HRT,63 with some evidence suggesting that there is a “window of opportunity” for estrogen to exert its neuroprotective effects.81
Vasomotor Symptoms
Vasomotor symptoms, such as hot flashes and night sweats, are the most common and among the most burdensome menopause symptoms, affecting up to 80% of women.46 Hot flashes are a significant driver of decreased QoL in postmenopausal women and are also at least partially responsible for menopause-related sleep disturbance. Behavioral modifications, such as dressing in layers, keeping living spaces cool, and avoiding food triggers (alcohol and spicy foods), may help reduce the severity and frequency of hot flashes. Obesity is associated with more severe hot flashes, and therefore maintenance of a healthy weight is recommended to relieve vasomotor symptoms and for general health.
Stress reduction through yoga and meditation has also been shown to improve vasomotor symptoms.82 Although behavioral modifications carry little to no risk and may provide some symptom relief, pharmacologic treatments are also available. HRT remains the most effect treatment for vasomotor symptoms, although it may not be appropriate for all patients. There are absolute and relative contraindications to HRT, including significant cardiovascular risk factors and history of breast cancer. Children exposed to chest radiotherapy during cancer treatment are at an increased risk for developing breast cancer in adulthood. Among these patients, survivors treated with HRT for menopause at age <20 years had a lower breast cancer risk than premenopausal survivors (HR, 0.47; 95% CI, 0.23–0.94), indicating that exogenous hormones do not fully mirror the role of endogenous hormones in the development of breast cancer.83
Using shared decision-making, providers and patients should weigh individual risks and benefits when deciding whether to begin, continue, or discontinue HRT. If HRT is to be prescribed in a woman with an intact uterus, it should contain both estrogen and progesterone, because this can decrease the risk of endometrial hyperplasia and cancer. Even so, women should be screened regularly for irregular, heavy bleeding, which can indicate endometrial hyperplasia and cancer. As an added bonus, combined estrogen/progesterone therapy not only treats vasomotor symptoms but also serves as a contraceptive.47 For women without a uterus, estrogen can be given alone as HRT. In women for whom HRT is inappropriate or unacceptable and behavioral interventions do not provide sufficient symptom relief, non-hormonal pharmacologic options include serotonin reuptake inhibitors, norepinephrine reuptake inhibitors, gabapentin, and pregabalin.46
Discussion
The goal of this article was to systematically review the literature on surveillance and management of POI in young female cancer survivors. Because few of these studies were found, the search was broadened to idiopathic POI, surgically induced early menopause, and management of menopause symptoms in adult cancer survivors, and results were extrapolated to the pediatric and AYA cancer population. Although these studies likely provide valuable information on the management of menopause symptoms, they do not take into account the additional burden of disease process and other comorbidities associated with treatment that are experienced by pediatric and AYA cancer survivors. The Children’s Oncology Group publishes Long-Term Follow-Up Guidelines,71 which recommend screening and management of specific conditions, such as POI or osteopenia, for survivors with exposures that place them at high-risk for these conditions. However, Hudson et al84 screened childhood cancer survivors based on their exposure risk and uncovered a substantial number of previously undiagnosed problems, indicating that systematic risk-based medical assessments are underused. Moreover, a recent study that examined the prevalence of endocrinopathies in the Childhood Cancer Survivor Study cohort showed that even survivors treated with “low-risk” therapies experience an increased risk for endocrine disorders compared with sibling controls.45 This observation highlights the need for long-term surveillance and individualized screening practices for all survivors of pediatric and AYA cancers regardless of predicted risk of treatment exposure.
Like all women, modifiable lifestyle factors likely contribute to symptom severity, and young female cancer survivors should be advised to adopt healthier habits. Additionally, psychological factors likely play a large role in QoL among young female cancer survivors. Women with cancer-related POI have higher levels of depression, higher perceived stress, and lower levels of self-esteem and life satisfaction than healthy controls. Cognitive behavioral therapy (CBT) and physical exercise have been shown to be cost-effective interventions for alleviating cancer-related menopause symptoms.85 Recently, an internet-based CBT intervention showed promising results for the treatment of menopausal symptoms in breast cancer survivors.86 Unless contraindicated, estrogen-containing hormone therapy should be initiated and continued until the estimated age of natural menopause.46 Hormone treatment improves the physical symptoms of women with POI, including vasomotor symptoms, vaginal dryness, and dyspareunia, and preserves BMD and cardiovascular health, but these hormonal treatments have a limited effect on QoL. Open communication, validation, and psychological interventions play a role in improving overall QoL.
An understanding of the patient’s values and attitudes toward menopause will guide culturally competent, whole-woman healthcare. Compared with 71% of oncologists, only 15% of primary care physicians are aware of the risk of premature menopause following exposure to alkylating agents.87 This disparity in awareness of late effects of cancer treatments highlights the need for a multidisciplinary team comprising gynecologists, endocrinologists, primary care providers, psychologists, social workers, physical therapists, and nurses to provide comprehensive care to cancer survivors.76,88 A team-based approach will ensure that providers address all aspects and consequences of POI during follow-up visits.
Acknowledgments
This work was supported by the NIH National Center for Translational Research in Reproduction and Infertility (NCTRI) Center for Reproductive Health After Disease (P50HD076188).
Footnotes
The authors have disclosed that they have no financial interests, arrangements, affiliations, or commercial interests with the manufacturers of any products discussed in this article or their competitors.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. , eds. SEER Cancer Statistics Review, 1975–2014, National Cancer Institute. Bethesda, MD. Available at: https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017. Accessed August 2, 2018.
- 2.Jeruss J, Woodruff T. Preservation of fertility for cancer patients. N Engl J Med 2009;360:902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet 2014;384:1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson EK, Finlayson C, Rowell EE, et al. Fertility preservation for pediatric patients: current state and future possibilities. J Urol 2017;198:186–194. [DOI] [PubMed] [Google Scholar]
- 5.Woodruff TK. Reproductive endocrinology: fertility in female survivors of childhood cancer. Nat Rev Endocrinol 2013;9:571–572. [DOI] [PubMed] [Google Scholar]
- 6.Woodruff TK. The Oncofertility Consortium—addressing fertility in young people with cancer. Nat Rev Clin Oncol 2010;7:466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodruff TK. Preserving fertility during cancer treatment. Nat Med 2009;15:1124–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sobota A, Ozakinci G. Fertility and parenthood issues in young female cancer patients—a systematic review. J Cancer Surviv 2014;8:707–721. [DOI] [PubMed] [Google Scholar]
- 9.De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet 2010;376:911–921. [DOI] [PubMed] [Google Scholar]
- 10.Pluye P, Gagnon MP, Griffiths F, Johnson-Lafleur J. A scoring system for appraising mixed methods research, and concomitantly appraising qualitative, quantitative and mixed methods primary studies in Mixed Studies Reviews. Int J Nurs Stud 2009;46:529–546. [DOI] [PubMed] [Google Scholar]
- 11.Livesey EA, Hindmarsh PC, Brook CG, et al. Endocrine disorders following treatment of childhood brain tumours. Br J Cancer 1990;61:622–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrne J, Fears TR, Gail MH, et al. Early menopause in long-term survivors of cancer during adolescence. Am J Obstet Gynecol 1992;166:788–793. [DOI] [PubMed] [Google Scholar]
- 13.Mackie EJ, Radford M, Shalet SM. Gonadal function following chemotherapy for childhood Hodgkin’s disease. Med Pediatr Oncol 1996;27:74–78. [DOI] [PubMed] [Google Scholar]
- 14.Teinturier C, Hartmann O, Valteau-Couanet D, et al. Ovarian function after autologous bone marrow transplantation in childhood: high-dose busulfan is a major cause of ovarian failure. Bone Marrow Transplant 1998;22:989–994. [DOI] [PubMed] [Google Scholar]
- 15.Chiarelli AM, Marrett LD, Darlington G. Early menopause and infertility in females after treatment for childhood cancer diagnosed in 1964–1988 in Ontario, Canada. Am J Epidemiol 1999;150:245–254. [DOI] [PubMed] [Google Scholar]
- 16.Larsen EC, Muller J, Schmiegelow K, et al. Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab 2003;88:5307–5314. [DOI] [PubMed] [Google Scholar]
- 17.Sklar CA, Mertens AC, Mitby P, et al. Premature menopause in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst 2006;98:890–896. [DOI] [PubMed] [Google Scholar]
- 18.Mansky P, Arai A, Stratton P, et al. Treatment late effects in long-term survivors of pediatric sarcoma. Pediatr Blood Cancer 2007;48:192–199. [DOI] [PubMed] [Google Scholar]
- 19.Green DM, Sklar CA, Boice JD, et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J Clin Oncol 2009;27:2374–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas-Teinturier C, El Fayech C, Oberlin O, et al. Age at menopause and its influencing factors in a cohort of survivors of childhood cancer: earlier but rarely premature. Hum Reprod 2013;28:488–495. [DOI] [PubMed] [Google Scholar]
- 21.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 2013;309:2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raciborska A, Bilska K, Filipp E, et al. Ovarian function in female survivors after multimodal Ewing sarcoma therapy. Pediatr Blood Cancer 2015;62:341–345. [DOI] [PubMed] [Google Scholar]
- 23.DeWire M, Green DM, Sklar CA, et al. Pubertal development and primary ovarian insufficiency in female survivors of embryonal brain tumors following risk-adapted craniospinal irradiation and adjuvant chemotherapy. Pediatr Blood Cancer 2015;62:329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salih SM, Elsarrag SZ, Prange E, et al. Evidence to incorporate inclusive reproductive health measures in guidelines for childhood and adolescent cancer survivors. J Pediatr Adolesc Gynecol 2015;28:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elchuri SV, Patterson BC, Brown M, et al. Low anti-Müllerian hormone in pediatric cancer survivors in the early years after gonadotoxic therapy. J Pediatr Adolesc Gynecol 2016;29:393–399. [DOI] [PubMed] [Google Scholar]
- 26.Wilson CL, Chemaitilly W, Jones KE, et al. Modifiable factors associated with aging phenotypes among adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol 2016;34:2509–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chemaitilly W, Li Z, Krasin MJ, et al. Premature ovarian insufficiency in childhood cancer survivors: a report from the St. Jude Lifetime Cohort. J Clin Endocrinol Metab 2017;102:2242–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bath LE, Wallace WHB, Shaw MP, et al. Depletion of ovarian reserve in young women after treatment for cancer in childhood: detection by anti-Müllerian hormone, inhibin B and ovarian ultrasound. Hum Reprod 2003;18:2368–2374. [DOI] [PubMed] [Google Scholar]
- 29.Lie Fong S, Laven JS, Hakvoort-Cammel FG, et al. Assessment of ovarian reserve in adult childhood cancer survivors using anti-Müllerian hormone. Hum Reprod 2009;24:982–990. [DOI] [PubMed] [Google Scholar]
- 30.Brougham MF, Crofton PM, Johnson EJ, et al. Anti-Mullerian hormone is a marker of gonadotoxicity in pre- and postpubertal girls treated for cancer: a prospective study. J Clin Endocrinol Metab 2012;97:2059–2067. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen SN, Andersen AN, Schmidt KT, et al. A 10-year follow up of reproductive function in women treated for childhood cancer. Reprod Biomed Online 2013;27:192–200. [DOI] [PubMed] [Google Scholar]
- 32.Krawczuk-Rybak M, Leszczynska E, Poznanska M, et al. Anti-Müllerian hormone as a sensitive marker of ovarian function in young cancer survivors. Int J Endocrinol 2013;2013:125080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krawczuk-Rybak M, Leszczynska E, Poznanska M, et al. The progressive reduction in the ovarian reserve in young women after anticancer treatment. Horm Metab Res 2013;45:813–819. [DOI] [PubMed] [Google Scholar]
- 34.Charpentier AM, Chong AL, Gingras-Hill G, et al. Anti-Müllerian hormone screening to assess ovarian reserve among female survivors of childhood cancer. J Cancer Surviv 2014;8:548–554. [DOI] [PubMed] [Google Scholar]
- 35.Lunsford AJ, Whelan K, McCormick K, McLaren JF. Antimullerian hormone as a measure of reproductive function in female childhood cancer survivors. Fertil Steril 2014;101:227–231. [DOI] [PubMed] [Google Scholar]
- 36.Thomas-Teinturier C, Allodji RS, Svetlova E, et al. Ovarian reserve after treatment with alkylating agents during childhood. Hum Reprod 2015;30:1437–1446. [DOI] [PubMed] [Google Scholar]
- 37.Wilson CL, Chemaitilly W, Jones KE, et al. Modifiable factors associated with aging phenotypes among adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol 2016;34:2509–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurney JG, Kaste SC, Liu W, et al. Bone mineral density among long-term survivors of childhood acute lymphoblastic leukemia: results from the St. Jude Lifetime Cohort study. Pediatr Blood Cancer 2014;61:1270–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandel K, Atkinson S, Barr RD, Pencharz P. Skeletal morbidity in childhood acute lymphoblastic leukemia. J Clin Oncol 2004;22:1215–1221. [DOI] [PubMed] [Google Scholar]
- 40.Hesseling PB, Hough SF, Nel ED, et al. Bone mineral density in long‐term survivors of childhood cancer. Int J Cancer 1998;78:44–47. [PubMed] [Google Scholar]
- 41.Wilson CL, Dilley K, Ness KK, et al. Fractures among long-term survivors of childhood cancer. Cancer 2012;118:5920–5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gurney JG, Kadan-Lottick NS, Packer RJ, et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors. Cancer 2003;97:663–673. [DOI] [PubMed] [Google Scholar]
- 43.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort study. J Clin Oncol 2013;31:4496–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomioka A, Maru M, Kashimada K, et al. Physical and social characteristics and support needs of adult female childhood cancer survivors who underwent hormone replacement therapy. Int J Clin Oncol 2017;22:786–792. [DOI] [PubMed] [Google Scholar]
- 45.Mostoufi-Moab S, Seidel K, Leisenring WM, et al. Endocrine abnormalities in aging survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2016;34:3240–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015;100:3975–4011. [DOI] [PubMed] [Google Scholar]
- 47.Britton LE. Unintended pregnancy: a systematic review of contraception use and counseling in women with cancer. Clin J Oncol Nurs 2017;21:189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Visser JA, Schipper I, Laven JS, Themmen AP. Anti-Müllerian hormone: an ovarian reserve marker in primary ovarian insufficiency. Nat Rev Endocrinol 2012;8:331–341. [DOI] [PubMed] [Google Scholar]
- 49.Iwase A, Nakamura T, Osuka S, et al. Anti-Müllerian hormone as a marker of ovarian reserve: what have we learned, and what should we know? Reprod Med Biol 2016;15:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rocca WA, Grossardt BR, de Andrade M, et al. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol 2006;7:821–828. [DOI] [PubMed] [Google Scholar]
- 51.Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol 2009;113:1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacoby VL, Grady D, Wactawski-Wende J, et al. Oophorectomy vs ovarian conservation with hysterectomy: cardiovascular disease, hip fracture, and cancer in the Women’s Health Initiative Observational Study. Arch Intern Med 2011;171:760–768. [DOI] [PubMed] [Google Scholar]
- 53.Ossewaarde ME, Bots ML, Verbeek AL, et al. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology 2005;16:556–562. [DOI] [PubMed] [Google Scholar]
- 54.Amagai Y, Ishikawa S, Gotoh T, et al. Age at menopause and mortality in Japan: the Jichi Medical School cohort study. J Epidemiol 2006;16:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong JS, Yi SW, Kang HC, et al. Age at menopause and cause-specific mortality in South Korean women: Kangwha Cohort Study. Maturitas 2007;56:411–419. [DOI] [PubMed] [Google Scholar]
- 56.Wu X, Cai H, Kallianpur A, et al. Impact of premature ovarian failure on mortality and morbidity among Chinese women. PLoS One 2014;9:e89597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manson JE, Woodruff TK. Reproductive health as a marker of subsequent cardiovascular disease. JAMA Cardiol 2016;1:776–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shuster LT, Rhodes DJ, Gostout BS, et al. Premature menopause or early menopause: long-term health consequences. Maturitas 2010;65:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007;297:1465–1477. [DOI] [PubMed] [Google Scholar]
- 60.Reslan OM, Khalil RA. Vascular effects of estrogenic menopausal hormone therapy. Rev Recent Clin Trials 2012;7:47–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang XP, Reckelhoff JF. Estrogen, hormonal replacement therapy and cardiovascular disease. Curr Opin Nephrol Hypertens 2011;20:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Langdahl B, Ferrari S, Dempster DW. Bone modeling and remodeling: potential as therapeutic targets for the treatment of osteoporosis. Ther Adv Musculoskelet Dis 2016;8:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI, Webber L, Davies M, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod 2016;31:926–937. [DOI] [PubMed] [Google Scholar]
- 64.Ratcliffe MA, Lanham SA, Reid DM, Dawson AA. Bone mineral density (BMD) in patients with lymphoma: the effects of chemotherapy intermittent corticosteroids and premature menopause. Hematol Oncol 1992;10:181–187. [DOI] [PubMed] [Google Scholar]
- 65.Castañeda S, Carmona L, Carvajal I, et al. Reduction of bone mass in women after bone marrow transplantation. Calcif Tissue Int 1997;60:343–347. [DOI] [PubMed] [Google Scholar]
- 66.Popat VB, Calis KA, Vanderhoof VH, et al. Bone mineral density in estrogen-deficient young women. J Clin Endocrinol Metab 2009;94:2277–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Christianson MS, Shen W. Osteoporosis prevention and management: nonpharmacologic and lifestyle options. Clin Obstet Gynecol 2013;56:703–710. [DOI] [PubMed] [Google Scholar]
- 68.Crofton PM, Evans N, Bath LE, et al. Physiological versus standard sex steroid replacement in young women with premature ovarian failure: effects on bone mass acquisition and turnover. Clin Endocrinol (Oxf) 2010;73:707–714. [DOI] [PubMed] [Google Scholar]
- 69.Blatt J, Meacham LR. Keeping Your Bones Healthy After Childhood Cancer. Available at: http://www.survivorshipguidelines.org/pdf/healthlinks/English/bone_health_Eng.pdf. Accessed August 2, 2018.
- 70.Preventive US Services Task Force. Screening for osteoporosis: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2011;154:356–364. [DOI] [PubMed] [Google Scholar]
- 71.Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. Children’s Oncology Group website. Available at: www.survivorshipguidelines.org. Accessed August 2, 2018.
- 72.Muka T, Oliver-Williams C, Kunutsor S, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality. JAMA Cardiol 2016;1:767–776. [DOI] [PubMed] [Google Scholar]
- 73.Langrish JP, Mills NL, Bath LE, et al. Cardiovascular effects of physiological and standard sex steroid replacement regimens in premature ovarian failure. Hypertension 2009;53:805–811. [DOI] [PubMed] [Google Scholar]
- 74.Cook ED, Iglehart EI, Baum G, et al. Missing documentation in breast cancer survivors. Menopause 2017;24:1360–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lindau ST, Abramsohn EM, Matthews AC. A manifesto on the preservation of sexual function in women and girls with cancer. Am J Obstet Gynecol 2015;213:166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pereira N, Schattman GL. Fertility preservation and sexual health after cancer therapy. J Oncol Pract 2017;13:643–651. [DOI] [PubMed] [Google Scholar]
- 77.Carter J, Stabile C, Seidel B, et al. Vaginal and sexual health treatment strategies within a female sexual medicine program for cancer patients and survivors. J Cancer Surviv 2017;11:274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 2007;69:1074–1083. [DOI] [PubMed] [Google Scholar]
- 79.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology 2008;70:200–209. [DOI] [PubMed] [Google Scholar]
- 80.Vearncombe KJ, Rolfe M, Wright M, et al. Predictors of cognitive decline after chemotherapy in breast cancer patients. J Int Neuropsychol Soc 2009;15:951–962. [DOI] [PubMed] [Google Scholar]
- 81.Scott EL, Zhang QG, Vadlamudi RK, Brann DW. Premature menopause and risk of neurological disease: basic mechanisms and clinical implications. Mol Cell Endocrinol 2014;389:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cramer H, Rabsilber S, Lauche R, et al. Yoga and meditation for menopausal symptoms in breast cancer survivors—a randomized controlled trial. Cancer 2015;121:2175–2184. [DOI] [PubMed] [Google Scholar]
- 83.Moskowitz CS, Chou JF, Sklar CA, et al. Radiation-associated breast cancer and gonadal hormone exposure: a report from the Childhood Cancer Survivor Study. Br J Cancer 2017;117:290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 2013;309:2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mewes JC, Steuten LM, Duijts SF, et al. Cost-effectiveness of cognitive behavioral therapy and physical exercise for alleviating treatment-induced menopausal symptoms in breast cancer patients. J Cancer Surviv 2015;9:126–135. [DOI] [PubMed] [Google Scholar]
- 86.Atema V, van Leeuwen M, Oldenburg HS, et al. An internet-based cognitive behavioral therapy for treatment-induced menopausal symptoms in breast cancer survivors. Menopause 2017;24:762–767. [DOI] [PubMed] [Google Scholar]
- 87.Nekhlyudov L, Aziz NM, Lerro C, Virgo KS. Oncologists’ and primary care physicians’ awareness of late and long-term effects of chemotherapy: implications for care of the growing population of survivors. J Oncol Pract 2014;10:e29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cohen PA, Brennan A, Marino JL, et al. Managing menopausal symptoms after breast cancer—a multidisciplinary approach. Maturitas 2017;105:4–7. [DOI] [PubMed] [Google Scholar]