Abstract
Although it is well-established that the immune system plays an important role in the development of physiology and behavior, the gut microbiome has recently become of interest in the study of developmental origins of behavior. Studies suggest that the effects of early-life immune activation may not occur until a secondary stressor is introduced, though the precise nature and timing of the stressor may be critical in the response. Further, recent work suggests that the microbiome and the immune system develop in parallel, and therefore any perturbations to one of these systems early in life will likely affect the other. Here, we sought to determine whether early-life activation of the immune system had long-term consequences on how the gut microbiome responds to antibiotic treatment in adulthood and whether those changes influence adult same-sex social behavior. In order to test the hypothesis that an early-life immune challenge makes individuals more vulnerable to the effects of antibiotics, we mimicked an early-life infection by injecting pups at postnatal day 3 and 5 with lipopolysaccharide (LPS; cell wall component of gram-negative bacteria) or saline, and subsequently exposed the same animals to antibiotic treatment (known to influence microbial community composition and behavior) or water in adulthood. We tracked physiology across development, and paired males and females with a novel individual of the same age and sex in adulthood to score same-sex behavior (e.g., aggression, investigation, grooming) before antibiotic treatment, immediately following treatment, and after recovery from antibiotics. LPS-treated females exhibited impaired reproductive physiology and function in adulthood (e.g., smaller ovaries and abnormal estrous cycles), and female and male gut microbial communities were strongly affected by antibiotic treatment in adulthood, but only slightly affected by postnatal LPS alone. Interestingly, LPS-treated males exhibited more robust changes in their behavioral response following adult antibiotic treatment, including decreased investigation and increased grooming, suggestive of changes in anxiety-like behaviors. These data suggest that males may be more vulnerable than females to behavioral abnormalities after being predisposed to an immune challenge early in life. Collectively, these results provide novel evidence that some of the sex-specific behavioral consequences of an early-life immune challenge may not transpire until an individual is faced with a secondary challenge, and the context in which an individual is exposed can greatly influence the response.
Keywords: Development, Gut-brain axis, Immune system, Lipopolysaccharide, Social behavior
1. Introduction
Early-life activation of the immune system can have long-term effects on physiology and behavior, increasing susceptibility to a range of nervous system disorders, including autism spectrum disorders, anxiety, and schizophrenia (Harvey and Boksa, 2012). Treatment with lipopolysaccharide (LPS), a cell wall component of gram-negative bacteria, is commonly employed to induce an immune response in animals, triggering an increase in circulating glucocorticoids (e.g., corticosterone, cortisol) and pro-inflammatory cytokines (Bilbo and Schwarz, 2012; French et al., 2013). LPS mimics a gram-negative bacterial infection, by binding to the toll-like receptor (TLR)-4, stimulating the activation of transcription factors and subsequent pro-inflammatory mediators in the body (e.g., IL-1β, TNF-α). These molecules can then act on the brain to initiate the acute-phase response (APR), including fever, lethargy, decreased food intake, and enhanced pain response (Harvey and Boksa, 2012; Perry, 2004; Quan and Banks, 2007). Previous research in our lab has shown that early-life LPS influences investigation and aggression and affects reproductive physiology in females, but not in males (Sylvia and Demas, 2017).
Interestingly, some of the effects of early-life immune activation may not transpire until a secondary stressor is introduced. For example, neonatal male rats infected with E. coli show no immediate memory changes; however, in adulthood, when infected neonates experienced a secondary LPS challenge, they exhibit impaired recent memory, decreased hippocampal astrocytes, and decreased brain interleukin (IL)-1 (Bilbo et al., 2005). In contrast, in a different study, rats neonatally treated with E. coli show a reduced corticosterone response to inescapable tailshock stress (IS) in adulthood and diminished depression-like symptoms when compared with control-treated individuals, suggesting the type of stressor introduced (e.g., immunological, psychological) can greatly impact the outcome (Bilbo et al., 2008).
Although it is well-established that the immune system plays an important role during development, the gut microbiome (the community of commensal, symbiotic, and pathogenic bacteria, fungi, and viruses that is critical for mammalian survival) has recently become of interest in the developmental origins of behavior (Lee and Mazmanian, 2010). More importantly, the gut-brain axis develops in parallel with the neonatal CNS via the transmission of signals from the vagus nerve to the GI tract. The effects of one of these systems during development, therefore, can pose significant risk to the development of the other throughout prenatal and postnatal periods (Clarke et al., 2014; Cryan and O’Mahony, 2011). For example, in female germ-free (GF) mice, the absence of conventional microbiota beginning at birth facilitates a reduction in response to anxiolytic stimuli, but an increase in circulating corticosterone in adulthood, suggesting that the microbiome may in-fluence the regulation of multiple aspects of important cognitive and emotional behaviors (Neufeld et al., 2011). Further, GF mice exhibit reduced digestive enzyme activity, lymphoid tissue, and resistance to infection (reviewed in Yoon et al. (2014)), therefore, the connections between the immune system and the microbiome appear to be extremely complex. Interestingly, male and female Sprague–Dawley rats treated with LPS as neonates show reduced social contact time, however, when olfactory processing is disrupted, the deficits in social contact are reversed (MacRae et al., 2015). These data suggest that the interactions between the microbiota and the immunomodulatory scent signals may modulate the changes in social interactions following neonatal sickness, however, precisely how the microbiome is affected by neonatal inflammation is not completely understood.
Furthermore, environmental stressors, including antibiotic treatment, diet, and immune activation can greatly affect the gut micro-biome. Recent studies have shown that early-life antibiotics affect the microbial diversity of the gut microbiome and anxiety-like behaviors in several model species (reviewed in Borre et al. (2014)). Our previous work has shown that adult antibiotic treatment affects the microbial diversity of the gut in both sexes and decreases aggression in Siberian hamsters in sex-dependent ways. Specifically, females treated with antibiotics for seven days exhibit decreased aggression, but in males, decreased aggression is only seen after repeated exposure to antibiotics (Sylvia et al., 2016). These data suggest that female behavior may be more strongly affected by changes in the microbiome, though whether these same patterns hold true in the face of multiple stressors at varying times in an individual’s life is not completely known.
Here we aim to determine whether an early-life immune challenge can alter the response to changes in gut microbial communities in adulthood. Specifically, in order to test the hypothesis that early-life activation of the immune system has long-term consequences on how the gut microbiome and behavior respond to antibiotic treatment in adulthood, we mimicked an early-life infection by injecting pups with LPS and subsequently, exposing those same animals to antibiotic treatment in adulthood to target the microbiome directly. In our previous work, we have shown that early-life LPS did not influence same-sex social behavior in either sex, though we found that LPS-treated females exhibited more aggression with and investigation of male conspecifics in a reproductive context compared to saline controls (Sylvia et al., 2018; Sylvia and Demas, 2017). Here, we hypothesized that individuals treated with exogenous LPS in early life would exhibit more robust physiological and behavioral changes following adult antibiotic treatment. These effects would suggest that there are long-term consequences of early-life sickness not only on the immune system, but also on the microbiome, and that some of these effects may not transpire until a secondary challenge in adulthood.
2. Methods
2.1. Animal housing and immune challenge
Adult male and female hamsters were paired (n = 13 pairs) and housed in a 16:8 light:dark photoperiod, in polypropylene cages (28 × 17 × 12 cm). Ambient temperature was maintained at 20 ± 2 °C, and relative humidity was maintained at 55 ± 5%. Hamsters were given ad libitum access to tap water and standard laboratory rodent chow (Lab Diet 5001, PMI Nutrition) throughout the experiment. Pups remained in their litters until weaning (postnatal day24), when they were individually housed for the remainder of the study. On postnatal day (pnd) 3, approximately half of the litters were given a single intraperitoneal (i.p.) injection (100 μL) of 50 μg/kg of lipopolysaccharide (LPS, from Salmonella enterica serotype typhimurium, Sigma-Aldrich, St. Louis, MO, USA), suspended in 0.9% sterile saline (n = 7 litters) and the other half of the litters received i.p. injections of 0.9% sterile saline (n = 6 litters). All pups received a second injection of LPS or saline on pnd5 according to a previously validated protocol, as there is heightened sensitivity of the gonadotropin-releasing hormone (GnRH) pulse generator at these time points (Knox et al., 2009; Sylvia et al., 2018; Sylvia and Demas, 2017). All pups in an individual litter received the same treatment (LPS or saline), and after each injection, we monitored the time it took to return to nursing all pups in the litter. All animals were weighed weekly for the remainder of experimentation. At the conclusion of the study, all animals were euthanized and organs were weighed. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Bloomington Institutional Animal Care and Use Committee (BIACUC) at Indiana University.
2.2. Reproductive physiology
2.2.1. Estimated testis volume
At pnd25, males (n = 14 saline; n = 15 LPS) were lightly anesthetized with isoflurane, and the length and width of the left testis was measured externally (± 0.1 mm) with calipers, as a proxy for reproductive maturity (Gorman, 1995; Sylvia and Demas, 2017). Estimated testis volume (ETV) was calculated as the length × width2, which is directly correlated with testis mass and spermatogenesis (Gorman, 1995; Schlatt et al., 1995). An ETV of 400 mm3 indicates a mass of approximately 200 mg, which is correlated with the critical mass for production of viable spermatids (Gorman, 1995; Schlatt et al., 1995).
2.2.2. Vaginal patency and estrous cycling
Beginning at pnd25, and every five days thereafter, all female off-spring (n = 17 saline; n = 12 LPS) were monitored for initial vaginal opening (Adam et al., 2000). Twenty-seven of the twenty-nine females had vaginal openings before pnd71 (beginning of the adult treatment period), and therefore female estrous cycles were monitored in only those females (Carlton et al., 2014; Scotti et al., 2007). We assessed if early-life LPS treatment affect reproductive function, therefore two saline-treated females were excluded from estrous cycle monitoring because they did not have vaginal openings until pnd75 (during the treatment period). Vaginal cell samples were obtained via vaginal lavage. Following lavage, samples were transferred to microscope slides, fixed with methanol, and stained with Giemsa. Samples were then evaluated for estrous stage (diestrus, proestrus, estrus, and metestrus) under 100x magnification (Carlton et al., 2014; Moffatt-Blue et al., 2006; Scotti et al., 2007). We determined estrous stage using the following characteristics: diestrus (presence of many polymorphonuclear leucocytes, some non-nucleated keratinized cells, and some parabasal cells), proestrus (clumps of lightly staining nucleated epithelial cells), estrus (many non-nucleated keratinized cells), and metestrus (flakes of keratinized cells and some leucocytes) (Carlton et al., 2014; Moffatt-Blue et al., 2006; Scotti et al., 2007). Once we determined the stage of the estrous cycle, females exhibiting cycles in which no pattern was seen (no presence of particular cell types or the presence of all one cell type), or those in which the cycle appeared to be incomplete (those not showing more than one stage of the cycle over a five-day period) were considered to be cycling “abnormally.” Siberian hamsters have a cycle lasting approximately four days (McMillan and Wynne-Edwards, 1999), therefore, animals who had not shown evidence of cycling within the five day period were confidently grouped into the “abnormally cycling”group.
2.3. Antibiotic treatment
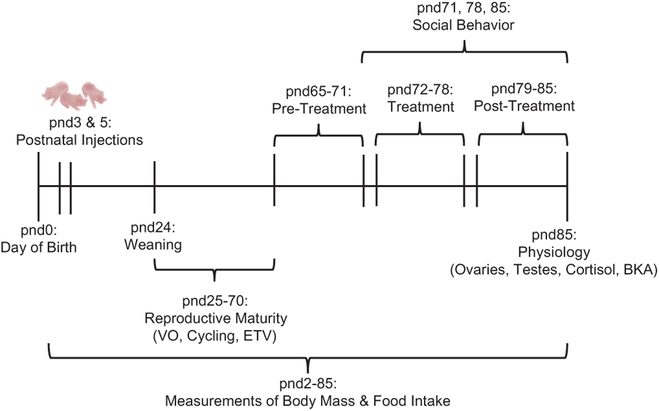
To determine how a secondary challenge (antibiotic treatment) affected social behavior and gut microbial communities in adulthood, at pnd65, approximately half of the animals from LPS-treated litters and half of the animals from saline-treated litters were assigned to either a control group (males: n = 8 LPS-treated and n = 7 saline-treated; females: n = 6 LPS-treated and n = 8 saline-treated), in which animals received sterilized water administered via sterile pipette orally once daily, or an experimental group (males: n = 7 LPS-treated and n = 7 saline-treated; females: n = 6 LPS-treated and n = 9 saline-treated), in which animals received a broad spectrum antibiotic [(Abx): 0.3 μL of enrofloxacin (Baytril) 10% oral solution per gram of body mass] administered via sterile pipette orally once daily (Romick-Rosendale et al., 2009). From pnd65–71 (pre-treatment), all animals were monitored daily, and body mass was measured regularly. During pnd72–78 (treatment), Abx animals received Abx treatment administered via sterile pipette orally once daily and control animals received sterilized water administered via sterile pipette orally once daily, during which the same measurements were taken. During pnd79–85 (post-treatment), both the experimental group and the control group were weighed and monitored (Fig. 1).
Fig. 1.
Experimental timeline demonstrating when treatments were performed and when physiological and behavioral measures were collected. Postnatal day (pnd) 0 represents the time point at which pups were born, and pnd24 represents the time point at which each animal was individually housed for the remainder of the study. Reproductive maturity measurements [Vaginal opening (VO), estrous cycling, and Estimated Testis Volume (ETV)] were assessed before the start of antibiotic treatment (pnd25–70); adult physiology [paired ovarian and testes mass, baseline cortisol, and bacterial killing ability (BKA)] were assessed at the conclusion of the study (pnd85) after all treatments were completed.
2.4. Fecal sampling
On the day of weaning (pnd24), and at the end of the pre-treatment (pnd71), treatment (pnd78), and post-treatment (pnd85) periods, effects on the gut microbiome were assessed by taking fecal samples from each animal. To take fecal samples, animals were removed from their home cage and held over a sterile container once daily, after which samples were stored in −80 °C until processed. All animals were returned to their home cage until the next day of sampling.
2.5. Behavioral trials
On pnd71, pnd78 and pnd85 behavioral testing was conducted during the first 2 h of the dark phase. At least 24 h before behavioral trials, intruder animals had a small patch of fur shaved on the dorsal surface for the purposes of identification. To assess behavior, we used the standard resident-intruder model that is typically used to assess these behaviors in small rodents (Rendon et al., 2015; Sylvia et al., 2016). Specifically, a non-aggressive intruder of the same sex and age was introduced into the home cage of an experimental animal for 5 min and aggression, investigation, and grooming were scored by an unbiased observer, using ODlog™ software (Macropod).
2.6. Tissue collection and blood sampling
At the end of the experiment (D85), all animals were lightly anesthetized with isoflurane vapors, and a terminal blood sample was collected from the retro-orbital sinus, followed by a lethal i.p. injection of a ketamine and xylazine cocktail in 0.9% saline. Following euthanasia, livers, spleens, and reproductive organs were weighed. Blood samples were allowed to clot at room temperature for 1 h, clots were removed, and samples were centrifuged at 4 °C for 30 min at 2500 rpm. Serum was stored at −20 °C until processed.
2.7. Cortisol analysis
Serum cortisol concentrations in males and females were determined in multiple enzyme immunoassays (EIAs) from a commercially prepared kit (Cortisol EIA Kit; Enzo Life Sciences, Inc., Farmingdale, NY, USA) that was previously validated for use in Siberian hamsters (Carlton and Demas, 2015). The assay is highly specific for cortisol, with corticosterone cross-reactivity 27.7% and < 4.0% for other steroid hormones. The sensitivity of the assay is 56.72 pg/mL. Samples were diluted 1:80 with assay buffer and run in duplicate (Carlton and Demas, 2015; Rendon et al., 2015). Male and female samples were run on the same plates. The intra-assay coefficient of variation was 6.44%, and the inter-assay coefficient of variation was6.45%.
2.8. Bacterial killing assay
To determine whether postnatal or adult treatment affect one aspect of the immune system, we used an ex vivo bacterial killing assay (BKA) as a functional assessment of the innate immune system’s ability to clear a relevant pathogen. This assay quantifies the relative number ofEscherichia coli (E. coli) colony forming units (CFU) that grow after incubation with serum. Lyophilized E. coli (Epower™, ATCC #8739, Microbiologics, St. Cloud, MN, USA; 1 pellet = 107 CFU) was added to 40 ml of 1 M sterile PBS and warmed to 37 °C creating a bacterial stock solution. The stock solution (500,000 CFU/ml) was then diluted 1:10 with sterile 1 M PBS to create a 50,000 CFU/ml working solution. Male and female serum samples were diluted 1:20 in glutamine enriched CO2-independent media (Invitrogen Corp., Carlsbad, CA, USA), and for each sample, the bacterial working solution was added at a 1:10 ratio to the diluted serum sample. As a positive control, the bacterial working solution was diluted 1:10 with glutamine enriched CO2-independent media, and as a negative control, we used glutamine enriched CO2-independent media alone. The diluted samples and the controls were incubated for 30 min at 37 °C to induce bacterial killing. After incubation, 50 μL of sample and controls were added to tryptic soy agar plates in duplicate. All plates were covered, inverted, and stored overnight at 37 °C. Following incubation, colony numbers were counted on each plate, and duplicates were averaged. Bactericidal capacity was calculated as a percent of bacteria killed relative to the positive control plates in which no killing occurred. Negative control plates showed no bacterial growth.
2.9. Microbiome analysis
DNA was extracted from fecal samples collected on pnd24 (n = 5 saline × water males; n = 5 saline × Abx males; n = 5 LPS × water males; n = 5 LPS × Abx males; n = 5 saline × water females; n = 5 saline × Abx females; n = 5 LPS × water females; n = 5 LPS × Abx females), pnd71, pnd78, and pnd85 (n = 6 saline × water males; n = 6 saline × Abx males; n = 6 LPS × water males; n = 6 LPS × Abx males; n = 6 saline × water females; n = 6 saline × Abx females; n = 6 LPS × water females; n = 6 LPS × Abx females) using a commercially prepared kit (Promega’s Maxwell® RSC Tissue DNA Kit, Madison, WI). Before homogenizing samples, we added 300 μL of lysis buffer provided in the kit to each sample and then centrifuged each sample at 4 °C for 5 min at 1200 rpm and used the supernatant for extraction. We extracted two different negative control samples simultaneously while processing experimental samples for assessment of any background contamination (a: elution buffer only; and b: TE buffer [supplied by Promega kit] + RNase A Solution [Promega, Madison, WI] + elution buffer).
We verified the purity of the DNA collected from each sample, and using Bioo Scientific’s NEXTflex™ 16S V4 Amplicon-Seq Library PrepKit 2.0 (Austin, TX), multiplexed amplicon libraries spanning the V4 hypervariable domain of microbial 16S ribosomal RNA (rRNA) gene were prepared. Samples were cleaned using Agencourt AMPure XP Magnetic Beads, amplified using the supplied customized PCR primers that target the V4 domain, and sequence information was determined using the Illumina MiSeq v3 (600 cycle) platform in the Center for Genomics and Bioinformatics (CGB). Filtering, error correction, and removal of chimeras were completed, and sequences were then identified using Swarm and matched against the Silva database to identify operational taxonomic units (OTUs) (Armanhi et al., 2016; Mahé et al., 2014). For experimental samples, we found a mean of 60,718 sequences per sample. For negative control samples, we found a mean of 15 sequences per sample, suggesting little to no contamination of samples. We ran all analyses with negative OTUs included and without, and we did not find a significant difference between analyses, therefore all analyses were run with all OTUs included.
3. Statistical analyses
All statistical analyses were performed in R v. 3.3.3 (R Core Team, 2017), and we attributed statistical significance at p < 0.05 after controlling for false discovery rate (FDR) when performing multiple comparisons (Verhoeven et al., 2005). Data were checked for normality and homogeneity of variance and data that could not be transformed to attain normality were analyzed using non-parametric tests. If a model reported a significant effect, two-tailed t-tests or Tukey-HSD tests were run to determine pair-wise comparisons. Measures of reproductive maturity and organ mass were analyzed using a generalized linear mixed effects model (GLMM), including the fixed effects of the model (e.g., treatments) as well as the random effect of litter in a hierarchical experimental design, enabling us to take into account the fact that individual pups from the same litter may not have truly been independent samples. Differences in repeated measures (i.e., body mass, food intake, behavior) were assessed via repeated-measures GLMMs with individual included as a random effect as well as litter. Models included LPS treatment, antibiotic treatment, day, and all of their interactions. Because all males in this study reached reproductive maturity by pnd25, we compared the values of estimated testis volume at pnd25 between LPS- and saline-treated males using a GLMM as well. To test whether there was a difference in time to return to nursing after injection 1 and injection 2 we ran Mann-Whitney U tests to compare groups. We found including litter as a random effect in a GLMM for assessing the effects of LPS treatment on estrous cycling was not appropriate, and when tested, litter did not significantly affect the outcome of the model. Therefore, the effect of LPS treatment on estrous cycling was assessed with Fisher’sExact Tests.
Only one randomly chosen individual from each litter, per sex and treatment group was assessed for changes in microbial communities, therefore, litter effects were not included in the analyses. Two-way analyses of variance (ANOVAs) were used to compare the effects of postnatal treatment (saline or LPS), adult treatment (water or antibiotic), and time (Pre-Treatment, Treatment, and Post-Treatment) on the bacterial phyla and families present in the microbiome across groups. Principle coordinate analysis (PCoA) was performed on the microbial communities to visualize differences between groups and time (Sze et al., 2014). To determine alpha diversity, we calculated the Shannon-Wiener index and ran two-way analyses of variance (ANOVAs) to determine statistically significant changes in the alpha diversity (Hill, 1973; Jost, 2006). Further, to determine beta diversity, we calculated Bray-Curtis dissimilarity scores across groups and time points. A Bray-Curtis dissimilarity score of zero represents groups that are similar in composition, and a score of 1 represents groups that do not share microbial composition. We also converted Bray-Curtis dissimilarity scores to percent differences between groups for a clearer comparison. Finally, to determine if overall bacterial community composition was affected by postnatal treatment (saline vs. LPS), adult treatment (antibiotic vs. water), time (pre-treatment, treatment, or post-treatment), or the interactions between them, we used multivariate nonparametric ANOVA of dissimilarities (PERMANOVA) with 999 permutations, using the Adonis function with the Hellinger transformation in the Vegan package in R (Oksanen, 2015; Oksanen et al., 2007; Schriever and Lytle, 2016) based on Euclidean distance.
4. Results
4.1. Pre-weaning measures
4.1.1. Early-life immune activation did not affect litter physiology
The total number of pups in each litter did not differ across treatment groups (t11 = 1.974, p = 0.074). Further, the time to retrieve pups and return to nursing after the first injection (W = 15, p = 0.445) and after the second injection (W = 19, p = 0.836) was not affected by treatment. The mean offspring mass from pnd2 through pnd24 in LPS-and saline-treated litters was not affected by treatment (t15 = −0.040, p = 0.969), however, litter mass increased over time in saline- and LPS-treated litters (t128 = 27.324, p < 2e−16).
4.1.2. Early-life LPS did not affect food intake or body mass before weaning
In females, food intake increased over time (t136 = −2.728, p = 0.007) but was independent of LPS treatment (t96 = −0.388, p = 0.699). Additionally, body mass also increased over time (t164 = 15.932, p < 0.001) but was not affected by LPS treatment (t45 = 0.16, p = 0.874). Similarly, in males, food intake increased over time (t162 = 17.664, p < 0.001) but was not affected by LPS treatment (t134 = 1.092, p = 0.277); and body mass increased over time in males (t162 = 17.664, p = 2.000E−16), independent from LPS (t67 = −0.081, p = 0.935).
4.1.3. LPS treatment did not affect microbial community composition in adolescent females and males
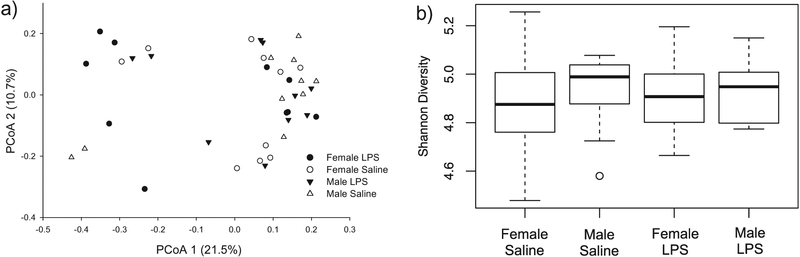
Gut microbial communities in adolescent females and males were not significantly different from each other (F1,39 = 12.975, p = 0.254), therefore all further analyses on the gut microbiome during adolescence were completed on both sexes combined. Overall gut bacterial community composition during adolescence was not significantly affected by LPS treatment (F1,39 = 26.815, p = 0.086), though there was a trend towards significance. The relative abundance of OTUs in females and males is plotted in the Principle Coordinates Analysis (PCoA) plots in Fig. 2a, showing that the bacterial communities across treatment groups and sex were very similar to one another.
Fig. 2.
Principle Coordinates Analysis (PCoA) of the microbiome in adolescent female and male hamsters (a); white circles represent saline-treated females; white triangles represent saline-treated males; black circles represent LPS-treated females; and black triangles represent LPS-treated males. Shannon-Wiener diversity across treatment groups and sex during adolescence (pnd24) (b). Shannon diversity did not significantly differ across treatment or sex (p > 0.05 in all cases).
4.1.4. LPS treatment did not affect the diversity of the gut microbiome in adolescent females and males
Shannon diversity in males and females was not affected by LPS treatment (F1,36 = 0.015, p = 0.903; Fig. 2b). Further, according to theBray-Curtis dissimilarity scores, LPS-treated animals were 16.887% different from saline-treated animals, suggesting they were similar in beta diversity.
4.1.5. LPS treatment affected some families, but did not affect any phyla in the adolescent gut microbiome
In males and females, there were 13 different phyla present in the gut microbiome. No individual phylum was significantly different across postnatal LPS and saline treatment groups (p > 0.05 in all cases; see Table 1 in Supplemental Materials for further detail). There were 43 families present in the adolescent male and female microbiome, and of those families, three were significantly different across treatment groups. Specifically, Porphyromonadaceae (F1,39 = 16.773, p = 0.009),Brachyspiraceae (F1,39 = 9.909, p = 0.046), and one family designated “Other” (F1,39 = 14.175, p = 0.012) were significantly decreased inLPS-treated adolescents when compared to saline-treated adolescents. All other families were not significantly different across groups (p > 0.05 in all cases; see Table 2 in Supplemental Materials for further detail).
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.bbi.2018.07.001.
4.2. Post-weaning measures
4.2.1. Neither LPS nor antibiotic treatment affected body mass or food intake post-weaning
Body mass in females did not significantly change in adulthood (from pnd65 through pnd85) (t591 = 1.075, p = 0.283), and neitherLPS nor antibiotic treatment affected body mass throughout the course of the study (p > 0.05 in all cases). However, post-weaning male body mass increased over time (t591 = −2.391, p = 0.017) but was not affected by either treatment (p > 0.05 in all cases). Food intake significantly differed from pnd65 through pnd85 in both sexes. Specifically, food intake increased over time in females (t585 = −3.748, p < 0.001) and males (t589 = −4.446, p < 0.001), but was independent of LPS or antibiotic treatment (p > 0.05 in all cases).
4.2.2. Early-life LPS, but not antibiotic treatment, affected female reproductive physiology
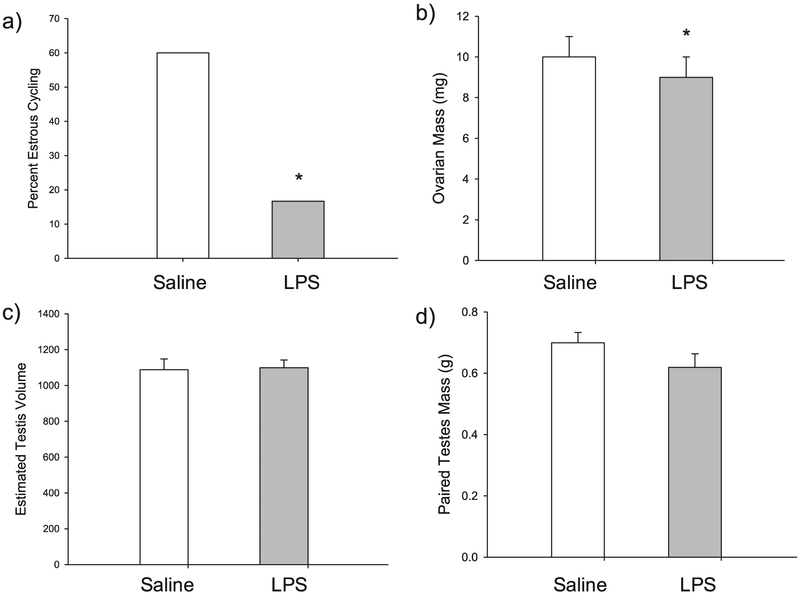
Though postnatal LPS did not significantly affect the timing of female vaginal opening (t10 = 0.516, p = 0.617), postnatal LPS disrupted female estrous cycles. Specifically, only 16.7% of LPS-treated females exhibited normal 4–5 day estrous cycles, whereas 60.0% of the saline-treated females displayed normal estrous cycles. This difference in estrous cycling between saline- and LPS-treated females was significant (P = 0.047) (Fig. 3a). Further, LPS-treated females had significantly smaller ovaries when compared with saline-treated females (t10 = 2.280, p = 0.046) (Fig. 3b), but there was no effect of antibiotic treatment (t15 = 0.093, p = 0.927). Further, uterine horn mass was not affected by LPS treatment (t10 = 0.013, p = 0.990) or antibiotic treatment (t15 = 1.023, p = 0.323).
Fig. 3.
(a) LPS-treated females (gray bars) had a lower percentage of normal estrous cycles when compared with saline-treated females (white bars); (b) LPS-treated females had significantly smaller ovaries when compared with saline-treated females; (c) male estimated testis volume (ETV) at pnd25 was not significantly different across groups; and (d) male paired testes mass was not affected by LPS treatment. Bar heights represent mean ± SEM. An asterisk (*) indicates a statistically significant effect of treatment (LPS vs. saline) with the random effect of litter included (p < 0.05).
In contrast, postnatal LPS did not affect any measures of reproductive physiology taken in males. Estimated testis volume (ETV) at pnd25 did not differ between LPS- and saline-treated males (t10 = −0.146, p = 0.887)(Fig. 3c). Further, paired testes mass in adulthood did not differ between LPS-treated males and saline-treated males (t10 = 0.512, p = 0.620)(Fig. 3d), and there was no effect of antibiotic treatment (t15 = 0.389, p = 0.703) on paired testes mass as well.
4.2.3. Neither LPS nor antibiotic treatment affected baseline cortisol
LPS nor antibiotic treatment affected the baseline concentration ofcortisol in females (LPS: t10 = 0.606, p = 0.558; antibiotics: t15 = 0.752, p = 0.464) or males (LPS: t14 = 0.587, p = 0.566; antibiotics: t14 = 0.266, p = 0.794).
4.2.4. Neither LPS nor antibiotic treatment affected bacterial killing ability
LPS nor antibiotic treatment affected bacterial killing ability in females (LPS: t10 = 0.672, p = 0.517; antibiotics: t15 = 0.246, p = 0.809) or males (LPS: t10 = −1.350, p = 0.207; antibiotics: t15 = −1.292, p = 0.216).
4.2.5. Antibiotic treatment affected microbial community composition in adult females and males
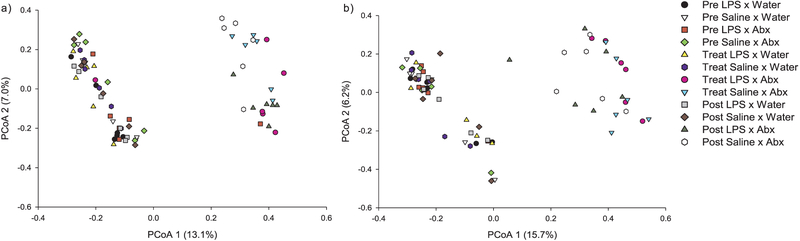
Although gut microbial communities in females and males were not significantly different from each other (F1,143 = −70.557, p = 0.974), in order to make important connections between the gut microbiome and behavior, we completed all microbiome analyses on each sex independently. In males and females, the composition of gut bacterial communities was significantly different across treatments and time, however, postnatal treatment alone did not significantly affect bacterial community composition in either sex (p > 0.05). The relative abundance of OTUs in females and males is plotted in the Principle Coordinates Analysis (PCoA) plots in Fig. 4a & b, respectively. Water and antibiotic groups were separated in opposite directions during treatment and recovery in both sexes, indicating that antibiotic treatment altered gut microbial composition.
Fig. 4.
Principle Coordinates Analysis (PCoA) of female (a) and male (b) hamsters. Black circles represent the Pre-Treatment time point in LPS × water treated hamsters; white triangles represent the Pre-Treatment time point in saline × water treated hamsters; red squares represent the Pre-Treatment time point in LPS × Abx treated hamsters; green diamonds represent the Pre-Treatment time point in saline × Abx treated hamsters; yellow triangles represent the Treatment time point in LPS × water treated hamsters; blue circles represent the Treatment time point in saline × water treated hamsters; pink circles represent the Treatment time point in LPS × Abx treated hamsters; aqua triangles represent the Treatment time point in saline × Abx treated hamsters; gray squares represent the Post-Treatment time point in LPS × water treated hamsters; brown diamonds represent the Post-Treatment time point in saline × water treated hamsters; green triangles represent the Post-Treatment time point in LPS × Abx treated hamsters; and white circles represent the Post-Treatment time point in saline × Abx treated hamsters. In both females and males, water and antibiotic groups were separated in opposite directions during treatment and recovery, which indicates that antibiotic treatment altered microbial composition of the gut. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4.2.6. Antibiotic treatment affected the diversity of the gut microbiome in adult females and males
Shannon-Wiener index, as a measure of alpha diversity, was affected by adult antibiotic treatment but not by postnatal LPS (see Fig. 2 in Supplementary Materials for more detail). Specifically, in females, Shannon diversity was significantly reduced after antibiotic treatment when compared with water treatment (F1,22 = 34.261, p < 0.001), and it remained significantly reduced in antibiotic-treated females after the seven-day recovery period (Fig. 5a). Further, according to the Bray-Curtis dissimilarity scores, before treatment, females were 78.05% similar to one another; following the treatment period, antibiotic- and water-treated female similarity was reduced to 48.78%; and following the recovery period, the groups were 51.78% similar to each other (see Table 3 in Supplemental Materials for more details).
Fig. 5.
Box and whisker plots of Shannon-Wiener diversity across adult treatment groups (water vs. antibiotics) and time (pre-treatment, treatment, and post-treatment) in female (a) and male (b) hamsters. Boxes represent the median and quartiles; whiskers represent the minimum and maximum. Outliers are represented as single open circles. Shannon diversity was significantly reduced after antibiotic treatment in both females and males when compared to water treatment, and it remained significantly reduced in antibiotic-treated animals after the seven-day recovery period. Postnatal LPS treatment had no effect on Shannon diversity in males or females. An asterisk (*) indicates statistically significant differences between groups (p < 0.05).
Similarly, in males, Shannon diversity was significantly reduced after antibiotic treatment when compared with water treatment (F1,20 = 40.446, p < 0.001), and it remained significantly reduced in antibiotic-treated males after the seven-day recovery period (Fig. 5b). Before the treatment period, males in both treatment groups were78.48% similar; following the treatment period, antibiotic- and water-treated male similarity was reduced to 41.84% similarity; and following the recovery period, the groups were 52.54% similar to each other (see Table 4 in Supplemental Materials for more details).
4.2.7. Antibiotics affected phyla in the gut microbiome in adult females and males
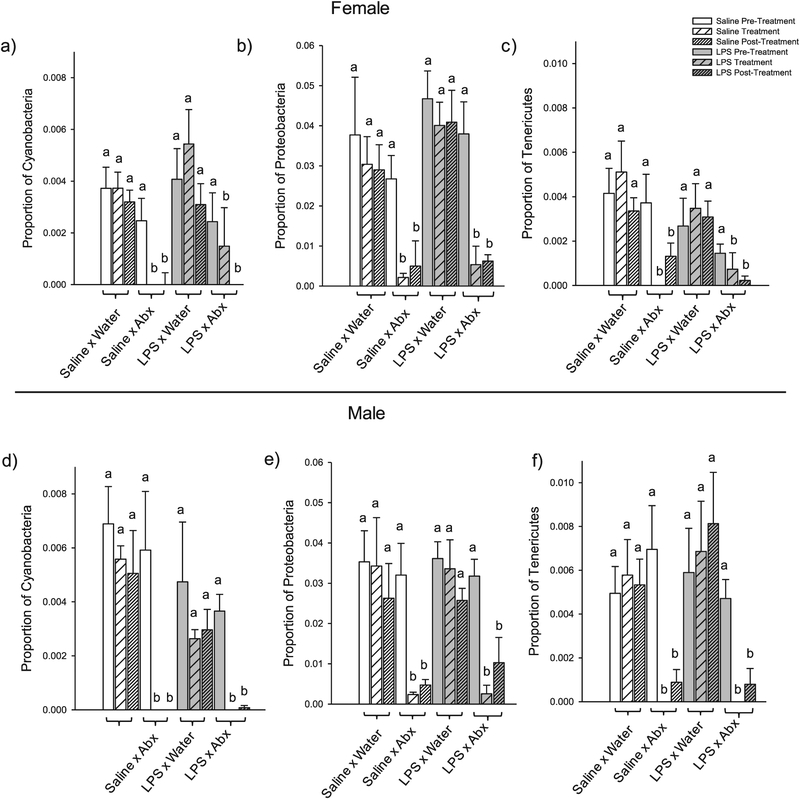
In females, adult antibiotic treatment decreased the relative abundance of three phyla: Cyanobacteria (F1,20 = 20.298, p = 0.009), Proteobacteria (F1,20 = 32.719, p = 0.001), and Tenericutes (F1,20 = 13.515, p = 0.033) (Fig. 6 and Supplementary Table 5); and time affected the Proteobacteria phylum (F3,45 = 6.016, p = 0.033) (Supplementary Material Table 5). There was no effect of treatment, time, or their interaction for the other nine phyla in the female microbiome: (p > 0.05 in all cases; Supplementary Table 5).
Fig. 6.
Phyla in the female (a–c) and male (d–f) microbiome that were affected by antibiotic treatment. In females, adult antibiotic treatment reduced the relative abundance of three phyla: Cyanobacteria (a), Proteobacteria (b), and Tenericutes (c). Similarly, in males, adult antibiotic treatment reduced the abundance of relative abundance of the same three phyla: Cyanobacteria (d), Proteobacteria (e), and Tenericutes (f). Bar heights represent mean ± SEM. Bars with different letters within each separate graph represent statistically different means (p < 0.05), and bars with the same letter represent means that are not significantly different from one another (p > 0.05).
Similarly, in males, adult antibiotic treatment decreased the relative abundance three phyla: Cyanobacteria (F1,20 = 19.325, p = 0.004), Proteobacteria (F1,20 = 19.775, p = 0.004), and Tenericutes (F1,20 = 10.025, p = 0.046) (Fig. 6 and Supplementary Table 6); time affected four phyla: Cyanobacteria (F2,40 = 11.729, p = 0.003), Proteobacteria (F2,40 = 9.974, p = 0.004), Saccharibacteria (F2,40 = 16.306, p = 0.003), and Tenericutes (F2,40 = 7.109, p = 0.024) (Supplementary Table 6); and there was an interaction between antibiotic treatment and time for two phyla: Saccharibacteria (F2,40 = 9.299, p = 0.006) and Tenericutes (F2,40 = 15.333, p = 0.003) (Supplementary Table 6). There were no effects of treatment, time, or their interaction for the other eight phyla in the male microbiome: (p > 0.05 in all cases; Supplementary Table 6).
4.2.8. Treatment affected families in the gut microbiome in adult females and males
In females, adult antibiotic treatment reduced the relative abundance of 16 families: Bacteroidaceae, Bacteroidales S24–7, Brachyspiraceae, Clostridiales vadinBB60, Corynebacteriaceae, Desulfovibrionaceae, Family XIII, Helicobacteraceae, Mycoplasmataceae, Oxalobacteraceae, Porphyromonadaceae, Prevotellaceae, Rikenellaceae, two families designated as “Other,” and one designated as “Uncultured rumen bacterium”(p < 0.05 in all cases; Supplementary Table 7). Postnatal treatment increased the relative abundance of the Ruminococcaceae family independent of the time point in adulthood; and the relative abundance of bacteria in the Corynebacteriaceae family was reduced in LPS treated-females before the adult treatment period (p < 0.05; Supplementary Table 7). The relative abundance of the Peptococcaceae family decreased after the treatment period in adulthood, independent of treatment group (p < 0.05 in all cases; Supplementary Table 7). There was no effect of postnatal or adult treatment for the other 26 families in the female microbiome: (p > 0.05 in all cases).
Similarly, in males, adult antibiotic treatment reduced the relative abundance of 12 families: Bacteroidales RF16, Clostridiales vadinBB60, Family XIII, Helicobacteraceae, Mycoplasmataceae, Oxalobacteraceae, Peptococcaceae, Porphyromonadaceae, Prevotellaceae, two families designated as “Other, and one family designated as an “Uncultured rumen bacterium” (p < 0.05 in all cases; Supplementary Table 8), but postnatal treatment did not affect the relative abundance of any families in the male gut microbiome. The relative abundance of Campylobacteraceae, Rhodospirillaceae, Rikenellaceae, and 3 families designated as “Other” decreased over time independent of treatment (p < 0.05 in all cases; Supplementary Table 8). There was no effect of treatment, time, or their interaction for the other 25 families in the male microbiome: (p > 0.05 in all cases).
4.2.9. Treatment differentially affected behavior
4.2.9.1. LPS and antibiotic treatment affected investigation in males.
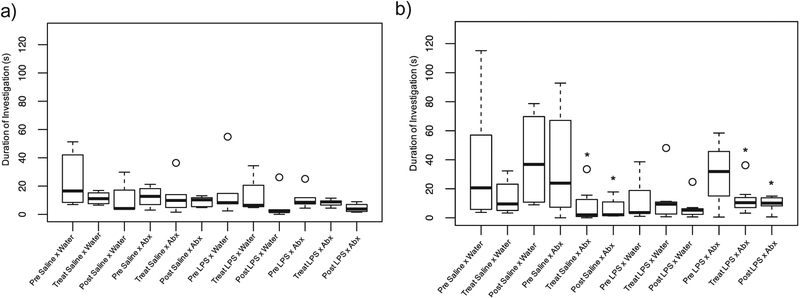
In males, investigation was differentially affected by treatment. Specifically, the frequency of nose-to-nose investigation was not affected by postnatal or adult treatment (F9,45.44 = 0.88, p = 0.550). The duration of nose-to-noseinvestigation, however, was affected by the interaction among LPS treatment, antibiotic treatment, and day (F9,47.92 = 2.675, p = 0.013;Fig. 7). Pairwise comparisons determined that LPS-treated males who received antibiotic treatment as adults (LPS × antibiotics) had a reduction in the duration of nose-to-nose investigation when compared with controls immediately following the 7-day treatment (D78; p = 0.047) and at D85 (p = 0.032). Males who received neonatal saline and antibiotics in adulthood (saline × antibiotics) also showed a decrease in the duration of nose-to-nose investigation compared to controls at D78 (p = 0.032) and at D85 (p = 0.032). The frequency (F9,46.85 = 0.500, p = 0.867) and duration (F9,47.08 = 1.298, p = 0.264) of nose-to-anogenital investigation was notaffected by postnatal or adult treatment.
Fig. 7.
Box and whisker plots of the duration of nose-to-nose investigation in female and male hamsters. Boxes represent the median and quartiles; whiskers represent the minimum and maximum. Outliers are represented as single open circles. An asterisk (*) indicates a statistically significant effect of treatment with the random effect of litter included (p < 0.05). There were no significant effects of either treatment on the duration of nose-to-nose investigation in females (a); but the duration of nose-to-nose investigation was affected by antibiotic treatment in both LPS- and saline-treated males (b).
In females, postnatal and adult treatment did not affect investigation. Specifically, the frequency (F9,34.58 = 0.380p = 0.937) and duration (F9,30.42 = 1.319, p = 0.268; Fig. 7) of nose-to-nose investigation was notaffected by either treatment; and the frequency (F9,37.21 = 0.716, p = 0.691) and duration (F9,32.20 = 1.908, p = 0.0865) of nose-to-anogenital investigation was not affected by either treatment.
4.2.9.2. LPS and antibiotic treatment affected grooming in males.
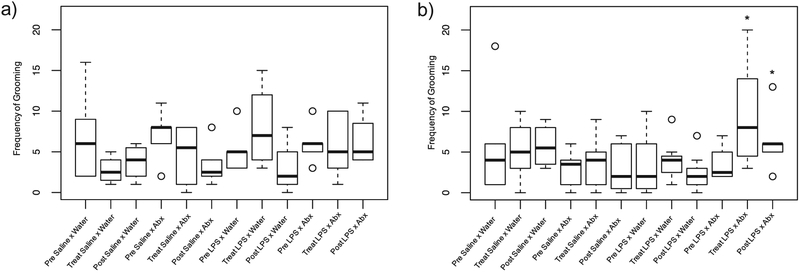
In males, the frequency of grooming was affected by postnatal and adult treatment (F9,49.12 = 2.465, p = 0.021; Fig. 8). Specifically, LPS × antibiotics males showed an increase in the frequency of grooming when compared with LPS-treated neonate controls immediately following treatment (at D78; p = 0.001) and after the recovery period (D85; p = 0.037); LPS × antibiotic males also showed an increase in the frequency of grooming when compared with saline-treated neonate controls at D78 (p = 0.029). Further, at D78, saline × antibiotic males (p = 0.037) showed a significantly lower frequency of grooming when compared with LPS × antibiotic males at the same time point; and at D85, LPS × water (p = 0.003), saline × antibiotic (p = 0.029), and saline × water (p = 0.029) males showed a significantly lower frequency of grooming when compared with LPS × antibiotic males. The duration of grooming in males, however, was not affected by postnatal or adult treatment (F9,52.47 = 1.653, p = 0.124).
Fig. 8.
Box and whisker plots of the frequency of grooming in female and male hamsters. Boxes represent the median and quartiles; whiskers represent the minimum and maximum. Outliers are represented as single open circles. An asterisk (*) indicates a statistically significant effect of treatment with the random effect of litter included (p < 0.05). Though females showed an overall significant effect of postnatal and adult treatment, there were no significant differences among any individual groups or time points (a); males that received LPS as neonates and antibiotics in adulthood (male LPS × antibiotics), however, showed an increase in the frequency of grooming when compared with all other groups (b).
In females, there was a slight overall effect of postnatal and antibiotic treatment on the frequency (F9,30.38 = 2.169, p = 0.054; Fig. 8) and duration (F9,26.40 = 2.87, p = 0.048) of grooming, however, pairwise comparisons determined that there were no significant differences among any of the individual time points or groups (p > 0.10 in all cases).
4.2.9.3. LPS and antibiotic treatment did not affect aggression.
Female hamsters displayed no significant change in the duration (F9,36.60 = 1.403, p = 0.222) of attacks, the frequency (F9,33.16 = 1.122, p = 0.375) andduration (F9,32.77 = 0.950, p = 0.498) of chases, or the latency to firstattack (F9,43.89 = 0.857, p = 0.569) across postnatal treatment or adulttreatment. The frequency of attacks (F9,40.65 = 2.055, p = 0.057), thoughnot significantly affected, was trending towards a significant change. Specifically, saline × antibiotics females showed a slight decrease in the number of attacks from D71 to D85 (Supplementary Table 9).
Male hamsters displayed no change in aggression across groups. Specifically, the frequency (F9,41.54 = 1.656, p = 0.131) and duration(F9,35.55 = 1.966, p = 0.074) of attacks, the frequency (F9,49.85 = 0.953, p = 0.489) and duration (F9,50.79 = 1.016, p = 0.440) of chases, and thelatency to first attack (F9,49.68 = 1.212, p = 0.309) were not significantlydifferent across postnatal treatment or adult treatment (Supplementary Table 10).
5. Discussion
Previous work in our lab and others has shown that there are sexspecific effects of both early-life and adult stress on physiology and behavior, and females are often more robustly affected by those stressors. Here we determined that an early-life immune challenge (i.e., LPS) did not severely affect baseline HPA axis activity, one measure of innate immunity, or microbial communities in either sex in adulthood. However, we found that LPS was associated with small changes in the gut microbiome in adolescent males and females; antibiotics robustly decreased the diversity of gut microbial communities in both sexes; and interestingly, males seem to be more vulnerable than females to behavioral abnormalities in response to antibiotics after being predis-posed to sickness early in life, regardless of the robust microbial response to adult antibiotic treatment in both sexes. Together, these results provide evidence of sex-specific behavioral consequences of an early-life immune challenge that do not emerge until an individual is faced with a secondary challenge to the gut microbiome (i.e., antibiotics) and that studies focusing on the role of sex in the gut-brain axis should consider the previous and current context in which the investigation is taking place. Additionally, our data suggest that the sex specific behavioral changes we found may be, in part, mediated by the gut-brain axis, though further investigation into precisely how these systems are working in concert to modulate behavior is needed.
5.1. Sex differences in physiological and behavioral responses
Many studies suggest that sexually dimorphic responses to stress may be more severe in the face of an early-life stressor (Bekhbat and Neigh, 2018; Dalla et al., 2010), which could lead to more robust physiological and behavioral changes in adulthood. For example, following maternal deprivation, male, but not female adolescent albino Wistar rats exhibit increased IL-1 receptor levels at the synapse of hippocampal neurons (Viviani et al., 2014), suggesting that early-life stress can influence the neural response to specific pro-inflammatory cytokines later in life in a sex-dependent manner. Moreover, there are vast sexually-dimorphic behavioral responses to stress as well. For example, in response to prenatal restraint stress, adult male Sprague-Dawley rats exhibit increased anxiety-like behavior in the elevated plus maze, with females showing reduced levels of anxiety-like behavior in this same test and improved learning in the Morris water maze. Males also show an increase in the levels of brain-derived neurotrophic factor (BDNF) in the hippocampus, though females do not show the same changes (Zuena et al., 2008). In a different study, male and female Long-Evans rats prenatally injected with propionic acid (PPA), a short chain fatty acid and important bacteria byproduct, exhibit impaired nest-seeking behavior early in life, and males show increased novel object approaching and locomotor activity in adolescence (Foley et al., 2014). Similarly, male Long-Evans rats treated with intraventricular infusions of PPA exhibit hyperactivity, repetitive, full body turning, and forelimb or hindlimb repeated adduction and extension (Macfabe et al., 2007). These studies and others (Lyte, 2013) may suggest that byproducts (or neurochemicals) that are often produced by gut microbes can act on the brain, and in turn, may influence behavioral responses later in life, and importantly, they may do so in a sex-dependent manner. Collectively, these studies along with our current work, provide evidence that there are sex-dependent responses to stress. Importantly, the idea that early-life stress, whether psychological or immunological, can differentially affect the direction of behavioral outcomes opens the door for possible clinical implications in the treatment of psychological disorders.
5.2. The timing of a stressor mediates the response
In our previous work, we found marked differences in aggressive behavior in males after two antibiotic treatments and in females after a only a single treatment (Sylvia et al., 2016). In the current study, however, we found that male, but not female behavior was significantly altered after just one seven-day period of antibiotics. Interestingly, male investigation and grooming behaviors were affected, but we saw no change in aggression. Though we saw similar results between these two studies in terms of changes in microbial composition, the conflicting behavioral results may be due to the age that the individuals received antibiotic treatment in each study. Siberian hamsters are naturally aggressive animals (Wynne-Edwards, 2003), though younger adults, such as those in the present study, tend to show less aggression than older adults (personal observation). It is likely, therefore, that we would find less aggression overall and no changes across all treatment groups in the current study.
Other studies have provided evidence that the precise timing of a stressor is important as well. For example, C57BL/6 mice exposed to prenatal polyinosinic:polycytidylic acid (poly I:C) and a series of sub-chronic stress (e.g., electric foot shock, restraint stress) at peri-puberty or adolescence found that prenatal sickness increased the vulnerability of both sexes to the neuropathological effects of subsequent stress (e.g., increased hippocampal dopamine, TNF-α, and IL1-β levels)during the peri-pubertal period, but not lasting through adulthood (Giovanoli et al., 2013). Further, guinea pigs exposed to prenatal psychological stress at varying times during gestation exhibited varying responses to spatial learning and memory tests at different ages. Specifically, the later the stress occurred during gestation, the less effect it had on spatial memory (Kapoor et al., 2009), suggesting the earlier a stressful event happens, the more likely the stress will have long-lasting effects on the individual.
In the current study, though we did not see any differences in baseline cortisol levels or in bacterial killing ability in either sex, it is likely that there would be a difference in stress-induced levels of cortisol and measures of innate immunity across groups at different time points. Further, we do not know if cortisol was significantly increased in pups immediately following injections, which may play a role in adult responsivity. Future studies will look at how the immune system affects the HPA axis more thoroughly at various time points throughout life. The results of these studies and others like them provide evidence that there are different mechanisms working on the same systems at varying times in an individual’s life, and that it is important to investigate how the response to stress may differ during the course of a lifetime.
5.3. Secondary responses to stressors
Many studies suggest that early-life stress can influence behavior in adulthood, however, much of the empirical work in this area has investigated the response to a single stressor or multiple stressors of the same kind, with the microbiome often studied in isolation (reviewed in Bekhbat and Neigh (2018)). For example, exposure to LPS increases anxiety-like behavior and hippocampal microglial activation in adult male Fischer 344 rats (Sominsky et al., 2012; Walker et al., 2004). In contrast, in females, early-life LPS results in reduced anxiety-like behavior in the elevated plus maze, reduced hippocampal volume, and increased inflammatory responses in Sprague–Dawley rats (Wang et al., 2013), as well as increased social investigation and aggression in a reproductive context in Siberian hamsters (Sylvia and Demas, 2017).
Fewer studies have investigated precisely how a secondary stressor may influence these physiological and behavioral responses. Of the studies that have investigated the influence of secondary stressors on physiology and behavior, many have focused on only one sex or have provided mixed results. In one such study, neonatal infection was associated with exaggerated acoustic startle responses, increased circulating corticosterone, and altered locomotor activity in Long Evans male rats, but only following exposure to social isolation and restraint stress (Walker et al., 2008). In a different study, C57BL/6 male and female mice exposed to prenatal poly I:C followed by restraint stress exhibited disrupted Prepulse Inhibition (PPI) of the acoustic startle response, as well as dopaminergic and GABAergic abnormalities in the prefrontal cortex and striatum (Deslauriers et al., 2013). These studies may suggest that a neonatal immune challenge predisposes individuals to stress-associated behavioral abnormalities in adulthood.
In contrast, both maternal separation and juvenile stress independently increased anxiety-like behavior in Sprague Dawley rats, but together, they do not significantly influence the behavioral response (Yee et al., 2011). Further, peripubertal poly I:C and social isolation are independently associated with deficits in PPI and novel recognition, however, combining the viral challenge with social isolation does not exacerbate the behavioral deficits (Lukasz et al., 2013). The results of these studies suggest that the precise timing and type of stressor may influence the response to a secondary challenge, and it is particularly important to focus on how varying types of stimuli might interact with one another. In our current study, we suggest that the immune system may interact with the gut microbiome early in life predisposing individuals to gut-mediated behavioral abnormalities in adulthood. Together, these studies may suggest that the early life environment could be particularly important in the behavioral response to a secondary stressor. Further work should investigate the precise mechanisms regulating the crosstalk between the microbiome and the immune system, not simply in terms of immediate response, but also the long-term consequences of these interactions.
5.4. Social behavior in the context of the gut-brain axis
Many previous studies investigating the role of the gut-brain axis on behavior have been performed in GF animals in an isolated social environment. While GF animals provide an important tool to explore the consequences of a complete lack of microbes, these experimental designs are not consistent with a natural environment, since organisms are not born in completely sterile environments, nor are they likely to grow up in such an environment. Further, many studies aim to investigate anxiety-like behavior through isolated tests such as the elevated plus maze, open field test, or forced swim test (reviewed in Mayer et al. (2015)). These tests, however, lack the ability to determine how an individual would act in a social context, which is vital for the survival of most species, and thus should be investigated in the context of the gut-brain axis. For example, GF mice spend significantly more time in the open arms of an elevated plus maze when compared with control specific pathogen free (SPF) mice, which may suggest reduced anxiety in GF mice (Neufeld et al., 2011), however precisely how these GF animals might react in different types of social environments is not completely known. The design of the present study, however, provides a more natural picture of how the gut-brain axis may be mediating social behavior to supplement the vast research in the area of GF models. In particular, we found sex-specific effects of antibiotic treatment on social anxiety-like behaviors (e.g., investigation and grooming), which may provide key information on how an individual defends a territory, finds a mate, and reproduces (Archie and Theis, 2011). Previous work on GF mice provide further evidence that the behavioral changes we found in antibiotic-treated males are suggestive of increased anxiety-like behavior. For example, GF mice exhibit repetitive self-grooming and decreased investigation in a social context, which the authors suggest could be correlated with deficits in social motivation, poor social and communication skills, and repetitive behavior of autistic patients (Desbonnet et al., 2014). The mechanisms by which these sex-specific behavioral consequences take place, however, are still not known. Future studies should look further into the pathways of the gut-brain axis to determine the role that sex might play in the social behavioral consequences of a stressor.
5.5. Mechanisms mediating gut-brain-behavior crosstalk
The present study illustrates that some of the sex-specific effects of an early-life immune challenge may not transpire until an individual is exposed to a secondary challenge later in life. More specifically, our data suggest that the interactions between the immune system and the microbiome early in life may be modulating some behavioral responses to stimuli in adulthood. Precisely how these systems are influencing one another, however, is still unknown. Some evidence suggests that neurotransmitters may play a large role in regulating communication between the gut microbiome and the brain (reviewed in Forsythe et al. (2010)). Specifically, male GF mice exhibit significant increases in hippocampal concentrations of 5-HT (5-hydrotryptamine) and 5-HIAA (5-hydroxyindoleacectic) when compared with controls. More importantly, because neurons in the serotonergic system are among the first to develop and are at high risk to early life disturbance, it seems plausible that serotonin and its precursors may have played a role in modulating behavior in the present study (Gaspar et al., 2003).
Additionally, male GF mice exhibit a significant decrease in BDNF expression in the hippocampus compared to control animals (Clarke et al., 2012), suggesting changes in the expression of BDNF may regulate the gut-brain-behavior axis. Furthermore, mice that are vagotomized prior to treatment with the probiotic, Lactobacillus rhamnosus, do not exhibit neurochemical (e.g., changes in GABA mRNA expression in the brain) or behavioral changes (e.g., reduced anxiety- and depression- like behaviors) seen in control animals treated with the probiotic, suggesting that the vagus nerve is likely a key mediator of the gut-brain communication pathway as well (Bravo et al., 2011). These findings and others suggest that there likely is not a single mechanism mediating the effects of the gut-brain axis but rather a series of mechanisms working in parallel.
6. Conclusions
The precise manner by which the gut microbiome may act on the brain to elicit behavior remains to be determined, but the connections among the gut microbiome, neuroendocrine system, and behavior appear vast. Our work here suggests that males may be more vulnerable than females to behavioral abnormalities associated with the gut-brain axis after being predisposed to an immune challenge early in life. Further work investigating precisely how the microbiome and the immune system interact early in life is necessary to more fully understand how an immune challenge might influence the developing gut microbial communities. Determining the exact role that the gut microbiome plays in maintaining homeostasis in the body across the sexes will help us to better understand how behavior is mediated and how psychological disorders may be prevented and treated. Our results provide insight into the connections between the immune system and the microbiome early in life and specifically how sex might modulate both short-term and long-term physiological and behavioral responses.
Supplementary Material
Acknowledgements
The authors thank L. Beck, D. Boyes, S. Henderson, C. Logan, L. Mroz, and E.A. St. John for assistance in behavioral filming, necropsies, and general animal procedures, and Jang Dong (Jd) Seo and Thomas Olmstead for statistical support. This work was supported by the National Institute of Child Health and Human Development (T32HD49336); the National Science Foundation, Division of Integrative Organismal Systems (1656414); and Indiana University.
Footnotes
Conflict of interest
The authors declare no conflict of interest, financial or otherwise.
References
- Adam CL, Moar KM, Logie TJ, Ross AW, Barrett P, Morgan PJ, Mercer JG, 2000. Photoperiod regulates growth, puberty and hypothalamic neuropeptide and receptor gene expression in female siberian hamsters. Endocrinology 141, 4349–4356. 10.1210/endo.141.12.7807. [DOI] [PubMed] [Google Scholar]
- Archie EA, Theis KR, 2011. Animal behaviour meets microbial ecology. Anim. Behav 82, 425–436. 10.1016/j.anbehav.2011.05.029. [DOI] [Google Scholar]
- Armanhi JSL, de Souza RSC, de Araújo LM, Okura VK, Mieczkowski P, Imperial J, Arruda P, 2016. Multiplex amplicon sequencing for microbe identification in community-based culture collections. Sci. Rep 6, 29543 10.1038/srep29543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M, Neigh GN, 2018. Sex differences in the neuro-immune consequences of stress: focus on depression and anxiety. Brain. Behav. Immun 67, 1–12. 10.1016/j.bbi.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF, 2005. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav. Neurosci 119, 293–301. 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM, 2012. The immune system and developmental programming of brain and behavior. Front. Neuroendocrinol 33, 267–286. 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Yirmiya R, Amat J, Paul ED, Watkins LR, Maier SF, 2008. Bacterial infection early in life protects against stressor-induced depressive-like symptoms in adult rats. Psychoneuroendocrinology 33, 261–269. 10.1016/j.psyneuen.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF, 2014. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol. Med 1–10. 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG,Bienenstock J, Cryan JF, 2011. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U.S.A 108, 16050–16055. 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton ED, Cooper CL, Demas GE, 2014. Metabolic stressors and signals differentially affect energy allocation between reproduction and immune function. Gen. Comp. Endocrinol 208, 21–29. 10.1016/j.ygcen.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton ED, Demas GE, 2015. Body mass affects seasonal variation in sickness intensity in a seasonally-breeding rodent. J. Exp. Biol 1667–1676. 10.1242/jeb.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF, 2012. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Clarke G, O’Mahony SM, Dinan TG, Cryan JF, 2014. Priming for health: gut microbiota acquired in early life regulates physiology, brain and behaviour. ActaPaediatr. Int. J. Paediatr 103, 812–819. 10.1111/apa.12674. [DOI] [PubMed] [Google Scholar]
- Cryan JF, O’Mahony SM, 2011. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol. Motil 23, 187–192. 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z, 2010. Sex differences in animal models of depression and antidepressant response. Basic Clin. Pharmacol. Toxicol 106, 226–233. 10.1111/j.1742-7843.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF, 2014. Microbiota is essential for social development in the mouse. Mol. Psychiatry 19, 146–148. 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslauriers J, Larouche A, Sarret P, Grignon S, 2013. Combination of prenatal immune challenge and restraint stress affects prepulse inhibition and dopaminergic/GABAergic markers. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 45, 156–164. 10.1016/j.pnpbp.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Foley KA, Macfabe DF, Vaz A, Ossenkopp K-P, Kavaliers M, 2014. Sexually dimorphic effects of prenatal exposure to propionic acid and lipopolysaccharide on social behavior in neonatal, adolescent, and adult rats: implications for autism spectrum disorders. Int. J. Dev. Neurosci 1–11. 10.1016/j.ijdevneu.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J, 2010. Mood and gut feelings. Brain. Behav. Immun 24, 9–16. 10.1016/j.bbi.2009.05.058. [DOI] [PubMed] [Google Scholar]
- French SS, Chester EM, Demas GE, 2013. Maternal immune activation affects litter success, size and neuroendocrine responses related to behavior in adult offspring. Physiol. Behav 119, 175–184. 10.1016/j.physbeh.2013.06.018. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L, 2003. The developmental role of serotonin: news from mouse molecular genetics. Nat. Rev. Neurosci 4, 1002–1012. 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, Winter C, Riva MA, Mortensen PB, Feldon J, Schedlowski M, Meyer U, 2013. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice sandra. Science (80-) 339, 1095–1099. 10.1126/science.1206034. [DOI] [PubMed] [Google Scholar]
- Gorman MR, 1995. Seasonal adaptations of Siberian hamsters. I. Accelerated gonadal and somatic development in increasing versus static long day lengths. Biol. Reprod 53, 110–115. [DOI] [PubMed] [Google Scholar]
- Harvey L, Boksa P, 2012. Prenatal and postnatal animal models of immune activation: relevance to a range of neurodevelopmental disorders. Dev. Neurobiol 72, 1335–1348. 10.1002/dneu.22043. [DOI] [PubMed] [Google Scholar]
- Hill MO, 1973. Diversity and evenness: a unifying notation and its consequences.Ecology 54, 427–432. [Google Scholar]
- Jost L, 2006. Entropy and diversity. Oikos 113, 363–375. 10.1111/j.2006.0030-1299.14714.x. [DOI] [Google Scholar]
- Kapoor A, Kostaki A, Janus C, Matthews SG, 2009. The effects of prenatal stress on learning in adult offspring is dependent on the timing of the stressor. Behav. Brain Res 197, 144–149. 10.1016/j.bbr.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Knox AM, Li XF, Kinsey-Jones JS, Wilkinson ES, Wu XQ, Cheng YS, Milligan SR, Lightman SL, O’Byrne KT, 2009. Neonatal lipopolysaccharide exposure delays puberty and alters hypothalamic Kiss1 and Kiss1r mRNA expression in the female rat. J. Neuroendocrinol 21, 683–689. 10.1111/j.1365-2826.2009.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Mazmanian SK, 2010. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science (80-) 330, 1768–1773. 10.1126/science.1195568.Has. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasz B, O’Sullivan NC, Loscher JS, Pickering M, Regan CM, Murphy KJ, 2013Peripubertal viral-like challenge and social isolation mediate overlapping but distinct effects on behaviour and brain interferon regulatory factor 7 expression in the adult Wistar rat. Brain Behav. Immun 27, 71–79. 10.1016/j.bbi.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Lyte M, 2013. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 9, 9–11. 10.1371/journal.ppat.1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfabe DF, Cain DP, Rodriguez-capote K, Franklin AE, Hoffman JE, Boon F, Taylor AR, Kavaliers M, Ossenkopp K, 2007. Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav. Brain Res 176, 149–169. 10.1016/j.bbr.2006.07.025. [DOI] [PubMed] [Google Scholar]
- MacRae M, Kenkel WM, Kentner AC, 2015. Social rejection following neonatal in-flammation is mediated by olfactory scent cues. Brain Behav. Immun 49, 43–48. 10.1016/j.bbi.2015.02.026. [DOI] [PubMed] [Google Scholar]
- Mahé F, Rognes T, Quince C, de Vargas C, Dunthorn M, 2014. Swarm: robust and fast clustering method for amplicon-based studies. PeerJ 2, e593 10.7717/peerj.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Tillisch K, Gupta A, 2015. Gut/brain axis and the microbiota. J. Clin. Invest 125, 926–938. 10.1172/JCI76304.Several. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan HJ, Wynne-Edwards KE, 1999. Divergent reproductive endocrinology of the estrous cycle and pregnancy in dwarf hamsters (phodopus). Comp. Biochem. Physiol. A: Mol. Integr. Physiol 124, 53–67. [DOI] [PubMed] [Google Scholar]
- Moffatt-Blue CS, Sury JJ, Young KA, 2006. Short photoperiod-induced ovarian regression is mediated by apoptosis in Siberian hamsters (Phodopus sungorus). Reproduction 131, 771–782. 10.1530/rep.1.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J, Foster JA, 2011. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil 23, 255–265. 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- Oksanen J, 2015. Multivariate analysis of ecological communities in R. Doi: 10.1016/0169-5347(88)90124-3. [DOI] [Google Scholar]
- Oksanen J, Kindt R, Legendre P, O’Hara B, Stevens MHH, 2007. The VeganPackage. [Google Scholar]
- Perry VH, 2004. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav. Immun 18, 407–413. 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Quan N, Banks WA, 2007. Brain-immune communication pathways. Brain. Behav. Immun 21, 727–735. 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Rendon NM, Rudolph LM, Sengelaub DR, Demas GE, 2015. The agonistic adrenal: melatonin elicits female aggression via regulation of adrenal androgens. Proc. R. Soc. B: Biol. Sci 282, 20152 10.1098/rspb.2015.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romick-Rosendale LE, Goodpaster AM, Hanwright PJ, Patel NB, Wheeler ET, Chona DL, Kennedy MA, 2009. NMR-based metabonomics analysis of mouse urine and fecal extracts following oral treatment with the broad-spectrum antibiotic enrofloxacin (Baytril). Magn. Reson. Chem 47, S36–S46. 10.1002/mrc.2511. [DOI] [PubMed] [Google Scholar]
- Schlatt S, De Geyter M, Kliesch S, Nieschlag E, Bergmann M, 1995. Spontaneous recrudescence of spermatogenesis in the photoinhibited male Djungarian hamster, Phodopus sungorus. Biol. Reprod 53, 1169–1177. [DOI] [PubMed] [Google Scholar]
- Schriever TA, Lytle DA, 2016. Convergent diversity and trait composition in temporary streams and ponds. Ecosphere 7, 1–12. 10.1002/ecs2.1350 [DOI] [Google Scholar]
- Scotti M-AL, Place NJ, Demas GE, 2007. Short-day increases in aggression are independent of circulating gonadal steroids in female Siberian hamsters (Phodopus sungorus). Horm. Behav 52, 183–190. 10.1016/j.yhbeh.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Sominsky L, Walker AK, Ong LK, Tynan RJ, Walker FR, Hodgson DM, 2012. Increased microglial activation in the rat brain following neonatal exposure to a bacterial mimetic. Behav. Brain Res 226, 351–356. 10.1016/j.bbr.2011.08.038. [DOI] [PubMed] [Google Scholar]
- Sylvia KE, Báez Ramos P, Demas GE, 2018. Sickness-induced changes in physiology do not affect fecundity or same-sex behavior. Physiol. Behav 184, 68–77. 10.1016/j.physbeh.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvia KE, Demas GE, 2017. Overcoming neonatal sickness: sex-specific effects of sickness on physiology and social behavior. Physiol. Behav 179, 324–332. 10.1016/j.physbeh.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvia KE, Jewell CP, Rendon NM, St. John EA, Demas GE, 2016. Sex-specific modulation of the gut microbiome and behavior in Siberian hamsters. Brain Behav. Immun 60, 51–62. 10.1016/j.bbi.2016.10.023. [DOI] [PubMed] [Google Scholar]
- Sze MA, Tsuruta M, Yang SWJ, Oh Y, Man SFP, Hogg JC, Sin DD, 2014. Changes in the bacterial microbiota in gut, blood, and lungs following acute LPS instillation into mice lungs. PLoS One 9, 3–10. 10.1371/journal.pone.0111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven KJF, Simonsen KL, McIntyre LM, 2005. Implementing false discovery rate control: increasing your power. Oikos 108, 643–647. 10.1111/j.0030-1299.2005.13727.x. [DOI] [Google Scholar]
- Viviani B, Boraso M, Valero M, Gardoni F, Marco EM, Llorente R, Corsini E, Galli CL, Di Luca M, Marinovich M, López-Gallardo M, Viveros MP, 2014. Early maternal deprivation immunologically primes hippocampal synapses by redistributing interleukin-1 receptor type I in a sex dependent manner. Brain Behav. Immun 35, 135–143. 10.1016/j.bbi.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Walker FR, Knott B, Hodgson DM, 2008. Neonatal endotoxin exposure modifies the acoustic startle response and circulating levels of corticosterone in the adult rat but only following acute stress. J. Psychiatr. Res 42, 1094–1103. 10.1016/j.jpsychires.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Walker FR, March J, Hodgson DM, 2004. Endotoxin exposure in early life alters the development of anxiety-like behaviour in the Fischer 344 rat. Behav. Brain Res 154, 63–69. 10.1016/j.bbr.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Wang KC, Fan LW, Kaizaki A, Pang Y, Cai Z, Tien LT, 2013. Neonatal lipopolysaccharide exposure induces long-lasting learning impairment, less anxiety-like response and hippocampal injury in adult rats. Neuroscience 234, 146–157. 10.1016/j.neuroscience.2012.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne-Edwards KE, 2003. From Dwarf Hamster to daddy: the intersection of ecology, evolution, and physiology that produces paternal behavior. Adv. Study Behav 32, 207–261. 10.1016/S0065-3454(03)01005-2. [DOI] [Google Scholar]
- Yee N, Ribic A, de Roo CC, Fuchs E, 2011. Differential effects of maternal immune activation and juvenile stress on anxiety-like behaviour and physiology in adult rats: no evidence for the “double-hit hypothesis”. Behav. Brain Res 224, 180–188. 10.1016/j.bbr.2011.05.040. [DOI] [PubMed] [Google Scholar]
- Yoon MY, Lee K, Yoon SS, 2014. Protective role of gut commensal microbes against intestinal infections. J. Microbiol 52, 983–989. 10.1007/s12275-014-4655-2. [DOI] [PubMed] [Google Scholar]
- Zuena AR, Mairesse J, Casolini P, Cinque C, Alemà GS, Morley-Fletcher S,Chiodi V, Spagnoli LG, Gradini R, Catalani A, Nicoletti F, Maccari S, 2008. Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS One 3 10.1371/journal.pone.0002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.