Abstract
Background:
Prenatal cocaine exposure (PCE) is associated with arousal dysregulation, but interactions between exposure and age are rarely investigated directly with longitudinal study designs. Our previous study had examined task-elicited emotional arousal and noted persistently high amygdala activations in the development of adolescents with PCE. However, while externally imposed emotional arousal could be considered a “state” effect depending on specific task stimuli, it is still unclear whether similar developmental alterations extend to intrinsic functional connectivity (FC), reflecting more of a “trait” effect.
Methods:
We used a longitudinal design and analyzed resting-state functional magnetic resonance imaging data acquired twice from 25 adolescents with PCE and 16 non-exposed controls. Both groups were each scanned first at the mean age of 14.3 and then again at 16.6 years. Seeding in bilateral amygdalae and comparing the 2nd scan with the 1st, we examined the interaction effect between PCE and age on FCs in the emotional network.
Results:
Compared with the younger age, we observed a generally decreased FC in the emotional network of the control group at the older age, but these FCs were generally increased at the older age in this same network of the PCE group. Additionally, this interaction effect of exposure by age in the right fusiform was positively correlated with the emotional interference imposed by external task stimuli.
Conclusions:
These results provided additional data directly characterizing developmental changes in the emotional network of adolescents with PCE, complementing and extending the notion of a PCE-associated long-term teratogenic effect on arousal regulation.
Keywords: Prenatal Cocaine Exposure, Adolescent, Longitudinal Design, Resting-State Functional Magnetic Resonance Imaging, Amygdala, Functional Connectivity
1. Introduction
Prenatal cocaine exposure (PCE) has been reported to affect arousal regulation in different stages of child development ranging from neonates (Bauer et al., 2005; Salzwedel et al., 2016; Salzwedel et al., 2015), infants (Bard et al., 2000; Eiden et al., 2009a; Eiden et al., 2009b; Schuetze and Eiden, 2006; Schuetze et al., 2007), toddlers (Chaplin et al., 2009; Eiden et al., 2015; Schuetze et al., 2014), and preschoolers (Bada et al., 2007; Bandstra et al., 2001; Dennis et al., 2006; Richardson et al., 2009) to grade-schoolers (Accornero et al., 2011; Kable et al., 2008; Lester et al., 2010) and teens (Chaplin et al., 2010; Greenwald et al., 2011; Li et al., 2009; Li et al., 2016; Li, Z. et al., 2013; Li et al., 2011; Zakiniaeiz et al., 2017). Specifically, and more relevant to older children and adolescents, individuals with PCE appear to be oversensitive to salient but task-irrelevant distractions (Harvey, 2004; Mayes, 2002; Mayes et al., 1998); consequently, processing of these distractions may compromise other ongoing and resource-demanding mental procedures such as working memory (Li et al., 2009), response inhibition (Accornero et al., 2007), language (Mentis, 1998), or more generally, executive function (Minnes et al., 2016). This PCE effect of imbalanced stimulus gating has been reported not only in human but also in animal studies (Chae and Covington, 2009; Gendle et al., 2003; Romano and Harvey, 1998) that preclude potential confounds of socioeconomic status or poly-drug exposure.
Arousal regulation concerns how stimulation is gated to different cortical regions, reflecting one's capability to adjust and allocate mental resources for distinct yet interactive streams of information processing (Damasio, 1995). It provides an excitatory/inhibitory balancing mechanism that protects the central executive brain system from excessive stimulation and also facilitates coordination among multiple cortical systems involved in an ongoing task (Mayes, 2002). For example, there is evidence for excitatory/inhibitory balancing between brain regions mediating emotional arousal and cognitive activity (Drevets and Raichle, 1998) as well as for balancing between intrinsic or spontaneous mentation (known as “default mode” function) and stimulus-oriented thoughts (Raichle et al., 2001). For arousal dysregulation, our previous neuroimaging studies have shown inefficient suppression of task-irrelevant activations in the amygdala in adolescents with PCE, reflecting excessive emotional arousal (Li et al., 2009; Li, Z. et al., 2013), as well as inefficient suppression of internal mentation, reflecting excessive default-mode arousal (Li et al., 2011). Unsuppressed peripheral arousal may lead to increased cognitive effort in adolescents with PCE as a compensatory mechanism to achieve task performance comparable to their non-exposed peers (Li et al., 2009; Sheinkopf et al., 2009). Consistent with this behavioral teratogenic model (Mayes, 2002), recent studies have also reported elevated functional connectivity in the amygdala-frontal (Salzwedel et al., 2015) and thalamo-frontal (Salzwedel et al., 2016) networks of neonatal brain, delineating the earliest network alterations in human infants with PCE.
While all these previous findings are informative, and most studies have inferred a “longterm” effect for PCE, most previous neuroimaging studies are not longitudinal in design, so direct inferences for the interaction between neurodevelopment and PCE are still lacking. Given that adolescence is the period during which developmental and social problems can become acute for children of substance-abusing parents (Smith and Jonides, 1998), and of cocaine abusing parents in particular (Delaney-Black et al., 2011; Greenwald et al., 2011), within-subject and longitudinal studies are desirable and necessary in adolescents with PCE to improve characterization of associated brain alterations and risks. Particularly, although it is known that the ability to regulate emotion and attention increases substantially during adolescence (Crone and Dahl, 2012), there is still a lack of longitudinal evidence showing if and how this typical brain maturation is affected by PCE.
To address this question, our previous study analyzed task-state functional magnetic resonance imaging (tfMRI) data acquired twice from the same group of adolescents when they were perceiving the same set of emotionally arousing pictures at the mean ages of 14.3 and 16.7 years (Li et al., 2016). Comparing the older age with the younger longitudinally, the PCE group exhibited persistently high activation in the amygdala even though they had viewed those pictures before in the same task and even though those pictures were task-irrelevant. This earlier study demonstrated that the ~2 years of development did not offset the imbalanced stimulus gating, which in turn may pose a long-term challenge for effective attention allocation in adolescents with PCE.
This previous study with tfMRI, however, does not inform us whether the observed developmental effect is stimulus-dependent. In other words, the persistently high emotional arousal could be elicited only by externally imposed stimuli in a specific task, and thus may only reflect a “state” effect. But what if there is no specific task involvement? Could PCE also be associated with a long-term high emotional arousal in intrinsic functional activities, reflecting more of a “trait” effect that may manifest itself even without a specific and externally imposed challenge?
The present study attempts to examine this “trait” hypothesis with functional connectivity (FC) analysis of resting-state functional magnetic resonance imaging (rfMRI) data acquired longitudinally from the same sample of adolescents with or without PCE. Instead of regional brain activation, this study focuses on FC of the emotion network seeded in the amygdala. In this network, based on (i) its central role in representing emotional arousal (Kim et al., 2011), (ii) its typical maturational changes of FC reported previously (such as increasing FC in the medial prefrontal cortex and decreasing FC in the insula) (Gabard-Durnam et al., 2014), and (iii) its PCE-associated activation changes probed by emotional stimuli (Li et al., 2009; Li et al., 2016; Li, Z. et al., 2013), we hypothesize a deviant developing pattern of intrinsic signal synchronization in adolescents with PCE. More specifically, based on the persistently high emotional arousal for the PCE group in the task state (Li et al., 2016), we hypothesize a similar pattern of PCE effect across ages in resting state with a persistently high FC in this emotion network of the exposed adolescents.
2. Materials and Methods
2.1. Participants
Forty-one adolescents (25 with PCE and 16 non-exposed controls) were included in the present study. They were from a larger sample for assessing developmental effect of PCE (Deshpande et al., 2010; Kable et al., 2008; Li, K. et al., 2013; Li et al., 2009; Li, Z. et al., 2013; Li et al., 2011) with usable rfMRI data available at 2 scanning visits about 2.3 years apart. The sample size for rfMRI was N=56 at the first imaging visit (time_1) (Li et al., 2011), but this sample was reduced to N=41 at the second imaging visit (time_2) due to loss of participants during follow-up (N=11), installation of dental braces (N=3), and scanner malfunction (N=1) (Li et al., 2016). However, there were no significant (all p>0.3) differences on birth weight, head circumference, gestational age, Apgar scores, birth mother's age, amount of prenatal drug and alcohol use, child's age at time_1 imaging, family monthly income level, verbal performance, or full-scale IQ between the presently remaining and analyzed sample (N=41) and those lost to attrition (N=15),
The participating adolescents, predominantly African-American and from low income backgrounds (demographic characteristics shown in table 1), were on average 14.3 (SD = 2.0) years of age at the 1st imaging visit and 16.6 (SD = 2.1) at the 2nd imaging visit. The ~2.3 years between these imaging visits was determined practically, as that was the time spent for completing recruitment and assessment (neurobehavioral and neuroimaging) for the entire sample at time_1 (Li et al., 2009). Detailed information regarding the determination of substance use, participants’ inclusion criteria, and classification of participants into experimental groups have been described extensively and repetitively in our previous reports (Brown et al., 1998; Coles et al., 1992; Li et al., 2009; Li et al., 2016; Li, Z. et al., 2013; Li et al., 2011). Briefly, PCE was determined by maternal self-report and/or positive urine screen at recruitment postpartum. Mothers (maternal background shown in table 2) were 19 years of age or older without major medical complications. Infants were either singleton or firstborn of multiple births, and they were either healthy full term or preterm (no less than 30 weeks) without major medical complications.
Table 1.
Characteristics of teens.
| Variable | CON (n = 16)a |
PCE (n = 25)a |
p-valueb |
|---|---|---|---|
| Age - Time_1 (SD) | 14.2 (2.3) | 14.4 (1.8) | 0.79 |
| Age - Time_2 (SD) | 16.4 (2.3) | 16.7 (2.1) | 0.68 |
| Duration Between Time_1 and Time_2 Day (SD) | 791.8 (131.9) | 851.3 (134.2) | 0.17 |
| Gender, No. (%) | 0.53 | ||
| Male | 7(43.8) | 14 (56.0) | |
| Female | 9 (56.3) | 11 (44.0) | |
| Total monthly household income,$ (SD) (n = 38) | 1930 (1098) | 1356 (1133) | 0.13 |
| Birth Weight, g (SD) | 2774(656) | 2746 (622) | 0.89 |
| Gestational Age, week (SD) | 38.1 (3.3) | 38.0 (2.6) | 0.86 |
| WASI Full scale IQ (SD) | 89.6 (11.1) | 86.1 (12.1) | 0.52 |
| WASI Verbal IQ (SD) | 85.4 (10.2) | 85.3 (12.8) | 0.98 |
| WASI Performance IQ (SD) | 84.8 (12.6) | 89.2 (12.1) | 0.27 |
CON: control group; PCE: prenatal cocaine exposure group.
If data are not available for all participants, the n used for the analysis is stated next to the variable name.
Independent sample t-tests were completed for the continuous variables; chi square analyses were completed for categorical variables.
Table 2.
Maternal characteristics at child’s birth.
| Variable | CON (n = 16)a | PCE (n = 25)a |
p-valueb |
|---|---|---|---|
| Age (SD) | 26.3 (4.8) | 28.4(4.0) | 0.17 |
| Marital status, No. (%) | 0.40 | ||
| Married | 3 (18.8) | 2(8.0) | |
| Single/divorced/separated/widowed | 13 (81.3) | 23 (92.0) | |
| Other substance use in pregnancy | |||
| Tobacco – cigarettes/week (n = 39) | 10.1 (35.0) | 58.3 (48.8) | 0.002 |
| Alcohol – oz. absolute alcohol/week(n = 40) | 0.0 (0.0) | 1.3 (2.1) | 0.020 |
| Marijuana – Joints/week | 0.0 (0.0) | 1.9 (3.3) | 0.032 |
CON: control group; PCE: prenatal cocaine exposure group.
If data are not available for all participants, the n used for the analysis is stated next to the variable name.
Independent sample t-tests were completed for the continuous variables; chi square analyses were completed for categorical variables.
Urine sample drug screens for amphetamines, barbiturates, benzodiazepines, marijuana, cocaine, opiates, and phencyclidine were available for 38 of the adolescents at time_1 and for all the 41 adolescents at time_2. Of the 266 tests (38 adolescents × 7 drugs) completed at time_1, only 4 were positive (2 for amphetamines and 2 for marijuana; both these adolescents were from the PCE group). Of the 287 tests (41 adolescents × 7 drugs) completed at time_2, only 10 were positive. Nine of these positive screens were from the PCE group with 1 adolescent positive for amphetamines, 7 for marijuana, and 1 from these 7 also positive for cocaine. The other 1 positive screen was for marijuana from a control adolescent.
All participating families consented for this study according to a protocol approved by Emory University’s Institutional Review Board. Adolescents provided written assent, and adults, including both caregivers and participants aged 18+, provided informed consent to participate.
2.2. Imaging Data Acquisition
Using a 3 Tesla Siemens magnetic resonance imaging scanner (Malvern, PA), rfMRI data were acquired from each adolescent with identical imaging parameters at both time_1 and time_2. Specifically, a T2*-weighted echo-planar imaging sequence was used with repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 90°, field of view (FOV) = 192 × 192 cm2, volume measurements = 210, slices/thickness/gap = 20/4 mm/0 mm, and matrix = 64 × 64. Participants were instructed to simply keep their eyes fixated on a central cross during the rfMRI scan. For anatomical references and brain normalization, T1-weighted 3D structural images were also acquired with an isotropic spatial resolution of 1 mm3.
2.3. Imaging Data Analysis
The present rfMRI data were analyzed with the software package of “Analysis of Functional NeuroImages” (AFNI, http://afni.nimh.nih.gov). Preprocessing steps included outlier (~5.5 times median absolute deviation) detection, despiking, slice timing correction, motion correction, anatomical-to-functional image co-registration (Saad et al., 2009), nuisance signal (6 head motion parameters and their derivatives, cerebral spinal fluid, and white matter) regression, band pass filtering (0.009 < f < 0.08 Hz), and 5 mm full-width half-maximum spatial smoothing. Time points with more than 10% of voxels identified as temporal outliers and with excessive head motion (Euclidean displacement > 0.3 mm, and its previous volume) were excluded (or “censored” in AFNI terms) from the subsequent connectivity analysis (Power et al., 2014). Excluded time points were less than 31 (15% of the total volumes) in all the 41 adolescents analyzed. After this preprocessing pipeline, individuals’ 4D rfMRI data were transformed into the standard Talairach space (Talairach and Tournoux, 1988) in 3 mm isotropic spatial resolution.
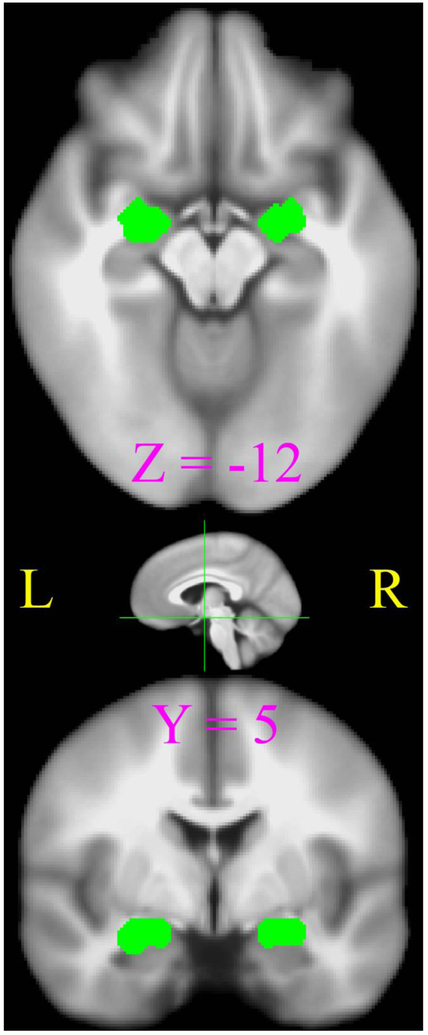
The individual-level analysis of FC was performed by seeding in bilateral amygdalae (Fig.1), which were localized functionally by the group-level activation contrast of perceiving negative vs. neutral pictures in a separate scan of tfMRI. Details of this tfMRI are not directly related to the present study and have been reported previously (Li et al., 2009; Li et al., 2016; Li, Z. et al., 2013; Li et al., 2011). To avoid potential bias on group and/or imaging visit in seed definition, this activation contrast involved 48 tfMRI datasets from equal numbers of 12 PCE and 12 control adolescents scanned at both time_1 and time_2. Here the “12” included all participants available in the control group with tfMRI data from both scanning visits (Li et al., 2016). Bilateral amygdala activations (Talairach coordinateleft = 23.4 5.4 −12.8, volumesleft = 1809 mm3; coordinatesright = −22.6 5.1 −12.2, volumeright = 1539 mm3) were jointly used as a single seed region of interest (ROI) in the present FC analysis. For each adolescent at each scanning visit, preprocessed rfMRI time series were averaged across all voxels in the seeding ROI, and this mean time series was correlated with all voxels in the brain, yielding individual FC maps of the emotion network. Pearson correlation coefficients (r) in all these FC maps were then converted into Fisher’s Z-values () for subsequent group analysis.
Figure 1.
The seeding ROIs of bilateral amygdala shown in axial (top) and coronal (bottom) views. The position of these two slices are depicted as green lines in the sagittal view.
To examine the FC interaction between GROUP (PCE vs. non-exposed controls) and TIME* (time_1 vs. time_2), Fisher’s z-values were submitted to analysis of variance (ANOVA) conducted at both voxel and ROI levels. These analyses are complementary to each other with the voxel-wise analysis superior on spatial resolution and the ROI-wise analysis superior on signal-to-noise ratio. The voxel-level analysis was conducted with AFNI’s 3dMVM (3-dimensional Multi-Variate Modeling, https://afni.nimh.nih.gov/MVM) and subsequently corrected for multiple comparison with a voxel-wise threshold of p < 0.001 combined with a cluster threshold of 459 mm3. This cluster threshold was determined by Monte Carlo simulations with a non-Gaussian spatial auto-correlation function for achieving a corrected threshold of p < 0.05 (Cox et al., 2017). In the ROI level analysis, the ROIs were spheres (radius = 9 mm) defined at 10 local maxima in the group connectivity map derived from the rfMRI scans from equal numbers of 16 PCEs and 16 controls at both time_1 and time_2. These ROIs (described more in the results below and in Supplementary Figure 1) were widely distributed in the functional network of the amygdala thus probing comprehensively in different locations of this network. The ROI-level analyses of these 10 ROIs were conducted in SPSS (https://www.ibm.com/products/spss-statistics) with repeated measures (for TIME) in general linear modeling (MANOVA) and Bonferroni correction.
2.4. Control of Confounding Factors
Based on an examination of demographic and background characteristics of teens and mothers (Tables 1, 2), the present analyses considered confounding factors associated with prenatal multi-drug exposure and socioeconomic status. Specifically, 4 variables, including prenatal exposures to (i) tobacco (cigarettes/week), (ii) alcohol (ounces/week), (iii) marijuana (joints/week), and (iv) total monthly household income ($) were included as covariates in statistical models whenever a GROUP effect was involved in the analysis. Additionally, because urine test results of current marijuana use were higher in the PCE group at time_2 than at time_1, effects of current marijuana used were removed from connectivity values (FZ’s) with regression analysis before these values were fed into the ANOVA model.
3. Results
3.1. Voxel-Level Analysis
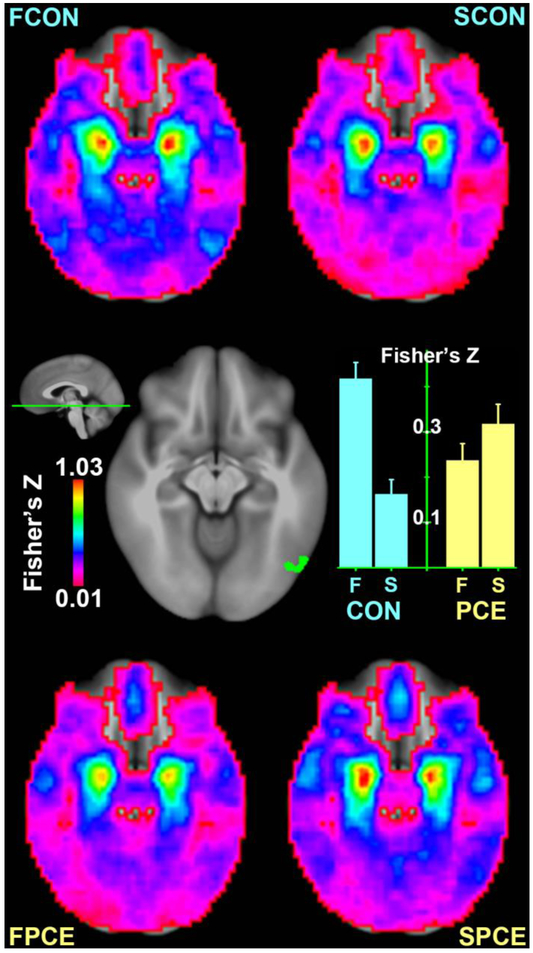
Seeding in the bilateral amygdala, the present analysis identified a functional network typically involved in affective perception and emotion regulation (Figure 2). In this network, the 2-way ANOVA revealed a significant interaction of GROUP × TIME (FZs: Control1st=0.42, Control2nd=0.16, PCE1st=0.24, PCE2nd=0.32; p = 0.006) in the right fusiform area (BA19, centroid coordinates: −49.5, 67.3 −11.2, volume: 486 mm3). As shown in Figure 2, the FC in this region decreased at time_2 in comparison to time_1 in the control group, but this developmental effect reversed in the PCE group with an increased FC at time_2. Besides this significant cluster, a visual inspection of Figure 2 suggests that similar interactions may also be evident in other brain regions, as areas in blue and cyan are increasing with age in the controls but decreasing in the PCE group. However, interaction effects of GROUP × TIME beyond the right fusiform did not survive the statistical threshold, justifying additional analyses at the ROI level in complementing the limited sensitivity at the voxel level.
Figure 2.
Comparison of amygdala connectivity between groups and scanning visits in a ventral slice. Connectivity maps are shown for controls’ first visit (FCON, upper left), controls’ second visit (SCON, upper right), PCEs’ first visit (FPCE, bottom left), and PCEs’ second visit (SPCE, bottom right) in the four corners, respectively. A significant interaction of GROUP × TIME is identified in the right fusiform area shown in the middle with its FZ values visualized in the bar graph.
3.2. ROI-Level Analysis
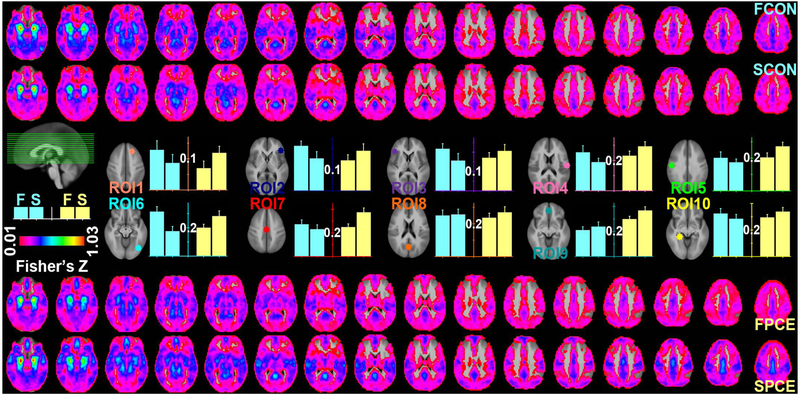
Based on the mean connectivity map derived from both groups at both scanning visits, the 10 ROIs identified at local maxima in the emotion network include the right superior frontal gyrus (ROI1), right inferior frontal gyrus (ROI2), left inferior frontal gyrus (ROI3), right postcentral gyrus (ROI4), left precentral gyrus (ROI5), right middle occipital gyrus (ROI6), paracentral lobule (ROI7), left precuneus (ROI8), left medial frontal gyrus (ROI9), and left parahippocampal gyrus (ROI10) (Figure 3; Supplementary Figure 1; Supplementary Table 1). Amygdala connectivity of these ROIs jointly exhibited a significant interaction of GROUP × TIME (FZs: Control1st=0.26, Control2nd=0.19, PCE1st=0.23, PCE2nd=0.25; p = 0.014) with the connectivity generally decreased in the control group but increased in the PCE group at time_2 versus time_1. The single variable test also showed that this interaction was significant in the ROI3 (FZs: Control1st=0.22, Control2nd=0.16, PCE1st=0.17, PCE2nd=0.21; p = 0.021) and ROI6 (FZs: Control1st=0.31, Control2nd=0.18, PCE1st=0.20, PCE2nd=0.28; p = 0.042). These ROI results are better appreciated in Figure 3 together with voxel-wise whole brain rendering; areas in the color gradient blue-cyan-green were generally decreased at the older age of the controls but increased in the PCE group. Notably, the amygdala FCs were not unanimously decreasing with age in the control group. Their FCs increased with age in the ROI8 and ROI9.
Figure 3.
Voxel-level and ROI-level comparisons of amygdala connectivity between groups and scanning visits. Results for the control (CON) and exposed (PCE) groups are labeled in cyan and yellow, respectively, with “F” and “S” indicating the first and second scanning visits. Voxel-level results are shown in the top two rows for the control and bottom two rows for the PCE group, respectively. Results for the 10 ROIs are shown in the middle rows with their FZ values visualized in the bar graphs.
All results reported above involved statistical control of poly-drug exposures and monthly household income. However, because these variables are part of the overall phenotype of prenatal drug exposure, statistical results without considering these confounding factors are also reported in the supplementary material (Supplementary Table 2), showing effects of these factors in the present statistical model.
3.3. Correlations Between Regional FCs and Emotional Regulation
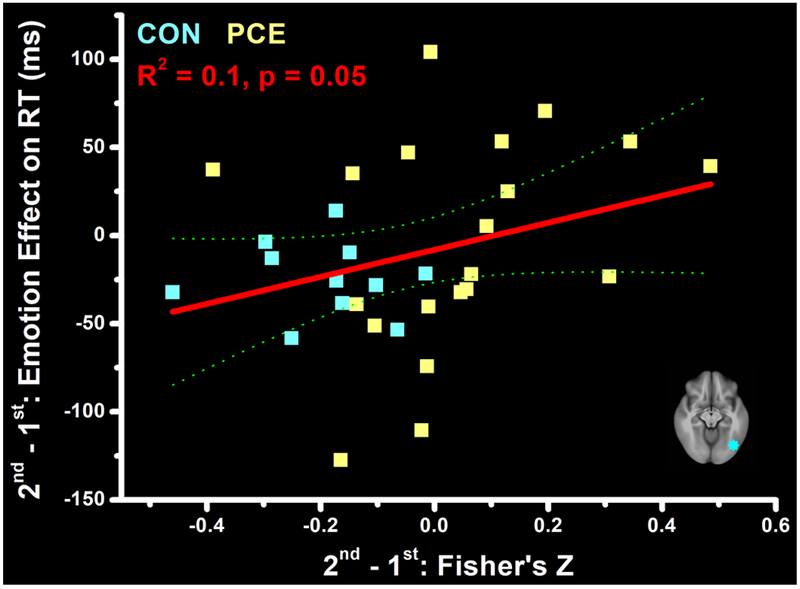
To further investigate whether the observed developmental effects on FC were associated with behavioral measures of emotion regulation, we conducted a post-hoc regression analysis examining this potential relationship. Specifically, we previously tested this same adolescent sample using a memory task with distracting pictures. Participants’ reaction time (RT) in that task was longer when distracted by emotionally negative than neutral pictures, reflecting the emotional interference on cognition (Li et al., 2016). Here, based on this emotional modulation on RT and using behavioral data we previously collected from this sample, we calculated a Developmental Effect on the Emotional Interference (DEEI) as DEEI = (RTnegative – RTneutral)time_2 – (RTnegative – RTneutral)time_1 and then regressed this DEEI value on developmental effect of Fisher’s Z (FZtime_2 – FZtime_1) derived from the right fusiform (from the voxel-level analysis, Figure 2) and the other 10 ROIs (from the ROI-level analysis, Figure 3). This analysis was conducted with stepwise regression (to avoid potential collinearity) with 31 adolescents (11 controls and 20 PCEs) who had both behavioral and rfMRI data. This regression model showed 2 regions— the right fusiform (p = 0.049) and the ROI6 (p = 0.012)— that jointly predicted (p = 0.034) the DEEI value well. Since these 2 regions are very close in spatial location, we considered them the same functional area and plotted the relation of FC and behavioral changes only using the data from the ROI6. As shown in Figure 4, a higher FZ difference in the right fusiform predicted a higher DEEI, indicating higher emotional interferences are associated with stronger amygdala FC. However, while changes of the FZ and emotional arousal were generally negative in the control group, meaning decreased FC and emotional interference at the older age, increasing FC and emotional interference (positive values on either axis) were noted in many adolescents with PCE, suggesting less suppressed emotional arousal even at the older age.
Figure 4.
Correlations between the over-time changes (2nd - 1st) of the emotion interference on reaction time (RT, ms) and the over-time changes of functional connectivity (Fisher’s Z) in the right fusiform. This ROI is depicted in the bottom right corner.
4. Discussion
While ample behavioral studies on animals and humans have associated PCE with arousal dysregulation (Chaplin et al., 2010; Mayes, 2002), neuroimaging studies examining the underlying neurobiological mechanisms have lagged behind (Salzwedel et al., 2015). Particularly, neuroimaging studies with a longitudinal design that directly examine the developmental effect are still rare (Akyuz et al., 2014; Li et al., 2016) due to complexities in data acquisition and analysis (Ackerman et al., 2010; Buckingham-Howes et al., 2013; Derauf et al., 2009). The present study attempts to fill in this gap with a specific focus on intrinsic FCs associated with the amygdala. Comparing rfMRI data acquired at the mean age of 16.6 years to that at 14.3, we observed increased FCs widely distributed in the emotion network of the PCE group, but these FCs were generally decreased in the control group. Representative interaction effect of GROUP × TIME was identified in the right fusiform region, whose developmental changes of FC with amygdala in resting state also predicted developmental changes of emotional arousal elicited by affective pictures in task state.
The amygdala is considered a critical region in mediating affective experience and emotional arousal (Aggleton, 1993; Phelps, 2006). In typical neurodevelopment from childhood through adulthood, age-dependent changes in amygdala FC have been reported in the medial prefrontal cortex (increasing with age), in the insula/temporal/parietal regions (decreasing with age), and in the cerebellar/occipital/parahippocampal regions (decreasing with age) (Gabard-Durnam et al., 2014). The present observations in the non-exposed group are consistent with these typical development features showing increasing FC with age in the medial prefrontal cortex (ROI9, though not statistically significant) but decreasing FC in a broader distribution of other brain areas. However, while increasing FC was also observed in the medial prefrontal cortex of the PCE group, decreasing FCs in other brain areas were generally absent in these cocaine-exposed adolescents. Implications of these finding are twofold. On one hand, given the critical role for the connection between amygdala and medial prefrontal cortex in regulations of emotion and physiological arousal (Perlman and Pelphrey, 2011; Zhang et al., 2014), the strengthening of this connection suggested an increased ability for these regulations in both groups of adolescents. However, on the other hand, because the amygdala represents a critical nexus for enhanced sensitivity toward emotional perception (Anderson, 2007), opposite age dependence of FC in other brain regions suggested a reduction of resting-state emotion sensitivity in the development of the non-exposed adolescents but a long-term persistence of high emotional arousal in the development of adolescents with PCE.
The present group differences were observed in resting state without any specific and externally imposed task challenge. However, these developmental changes of amygdala FC in resting state emerged in parallel with developmental changes of amygdala activation in task state. Considered together with our previous study examining task-state emotional arousal in this same sample (Li et al., 2016), it was noted that both task activations and most functional connections of the amygdala decreased with age in the control group, but these activations and FCs increased with age in the exposed group. Additionally, both activations and FCs of the amygdala explained a significant amount of inter-subject variance in behavioral measures of emotional arousal. These results are consistent with the previous findings from healthy adults that the amygdala’s task-dependent activity and task-free connectivity both contribute to feelings of emotional arousal (Touroutoglou et al., 2014). In this study, we extended these findings in a longitudinal sense, demonstrating a parallel change in these contributions during adolescence. Amygdalae’s connectivity at rest may set the basal tone (or trait) of the autonomic nervous system in response to salient stimuli, and its activation on task (or its state) may add to this basal tone in realizing a specific affective experience. Our previous findings on task state (Li et al., 2016) and the present findings on resting state may reflect a longitudinal PCE effect on both the basal tone and specific arousal responses in the autonomic system. This effect could derive from the PCE-associated alterations in “fetal programming” of the hypothalamic-pituitary-adrenal axis (Lester and Padbury, 2009) and contribute to different issues reported in this population such as social escape (Greenwald et al., 2011), heightened stress response (Chaplin et al., 2010), and teen substance use (Delaney-Black et al., 2011).
Significant interactions between GROUP and TIME were identified in the fusiform and anterior insular (ROI3) cortices in the present study. Together with the seeding area of the amygdala, these regions serve as prominent nodes in the “social context network” (SCN) in supporting individual’s emotional awareness and social interaction (Adolphs, 2002; Amoruso et al., 2011; Gu et al., 2013). Specifically, the fusiform area percepts socially and emotionally relevant visual stimuli such as facial expressions (Haxby et al., 2002), the amygdala recognizes these signals (Baxter and Croxson, 2012), and the anterior insular integrates this information to generate current emotional awareness as well as to provide descending signals for autonomic reflexes (Gu et al., 2013). Given the trait characteristics of internalizing (e.g., social withdrawal) and externalizing (e.g., delinquency) problems reported in children with PCE (Greenwald et al., 2011; Lambert and Bauer, 2012; Minnes et al., 2010), as well as the elevated FCs in the SCN of individuals with social anxiety disorder (Frick et al., 2013), the presently noted FC increases in the SCN of the PCE group may indicate more risks for behavioral dysregulation in adolescent development for the exposed teens. This may be especially relevant in situations where heightened social stress and anxiety occur (Chaplin et al., 2010; Min et al., 2017). In other words, individuals with PCE may experience a stronger emotional influence on cognition and behavior during adolescence than occurs in a non-exposed population at this time of development.
5. Conclusions
When compared to their non-exposed peers, adolescents with prenatal cocaine exposure largely exhibit different patterns of amygdala functional connectivity in relation to age during this developmental period. This observation in resting state appears in parallel with developmental group differences in task state. In the emotion network anchored in the bilateral amygdala, task-state activation and resting-state functional connectivity may represent state and trait alterations, respectively, and jointly contribute into the long-term teratogenic effect of arousal dysregulation in adolescents with PCE.
Supplementary Material
Highlights.
Prenatal cocaine exposure (PCE) is associated with long-term arousal dysregulation.
PCEs and controls were scanned with rfMRI at the mean ages of 14.3 and 16.6 years.
Amygdala connectivity changed oppositely with age in the PCE and control groups.
Amygdala connectivity in rest predicted emotional interference in task state.
PCE may contribute to increased emotional arousal in adolescent development.
Acknowledgements
This study was supported by the Natural Science Foundation of China and Shenzhen (grants to ZL: 31671169, 31530031, and 000099) as well as Georgia research alliance and the National Institute of Health (grants to XH: RO1 DA17795 and RO1 DA033393).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Capitalized words GROUP and TIME are specifically used to refer ANOVA factors in this entire paper.
Conflict of Interest
No conflict declared.
References
- Accornero VH, Amado AJ, Morrow CE, Xue L, Anthony JC, Bandstra ES, 2007. Impact of prenatal cocaine exposure on attention and response inhibition as assessed by continuous performance tests. J. Dev. Behav. Pediatr. 28, 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accornero VH, Anthony JC, Morrow CE, Xue L, Mansoor E, Johnson AL, McCoy CB, Bandstra ES, 2011. Estimated effect of prenatal cocaine exposure on examiner-rated behavior at age 7 years. Neurotoxicol. Teratol. 33, 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman JP, Riggins T, Black MM, 2010. A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics 125, 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, 2002. Neural systems for recognizing emotion. Curr. Opin. Neurobiol. 12, 169–177. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, 1993. The contribution of the amygdala to normal and abnormal emotional states. Trends Neurosci. 16, 328–333. [DOI] [PubMed] [Google Scholar]
- Akyuz N, Kekatpure MV, Liu J, Sheinkopf SJ, Quinn BT, Lala MD, Kennedy D, Makris N, Lester BM, Kosofsky BE, 2014. Structural brain imaging in children and adolescents following prenatal cocaine exposure: Preliminary longitudinal findings. Dev. Neurosci. 36, 316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoruso L, Couto B, Ibanez A, 2011. Beyond extrastriate body area (EBA) and fusiform body area (FBA): Context integration in the meaning of actions. Front. Hum. Neurosci. 5, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, 2007. Feeling emotional: The amygdala links emotional perception and experience. Soc. Cogn. Affect. Neurosci. 2, 71–72. [Google Scholar]
- Bada HS, Das A, Bauer CR, Shankaran S, Lester B, LaGasse L, Hammond J, Wright LL, Higgins R, 2007. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics 119, E348–E359. [DOI] [PubMed] [Google Scholar]
- Bandstra ES, Morrow CE, Anthony JC, Accornero VH, Fried PA, 2001. Longitudinal investigation of task persistence and sustained attention in children with prenatal cocaine exposure. Neurotoxicol. Teratol. 23, 545–559. [DOI] [PubMed] [Google Scholar]
- Bard KA, Coles CD, Platzman KA, Lynch ME, 2000. The effects of prenatal drug exposure, term status, and caregiving on arousal and arousal modulation in 8-week-old infants. Dev. Psychobiol. 36, 194–212. [PubMed] [Google Scholar]
- Bauer CR, Langer JC, Shankaran S, Bada HS, Lester B, Wright LL, Krause-Steinrauf H, Smeriglio VL, Finnegan LP, Maza PL, Verter J, 2005. Acute neonatal effects of cocaine exposure during pregnancy. Arch. Pediatr. Adolesc. Med. 159, 824–834. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Croxson PL, 2012. Facing the role of the amygdala in emotional information processing. Proc. Natl. Acad. Sci. U.S.A. 109, 21180–21181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JV, Bakeman R, Coles CD, Sexson WR, Demi AS, 1998. Maternal drug use during pregnancy: Are preterm and full-term infants affected differently? Dev. Psychol. 34, 540–554. [DOI] [PubMed] [Google Scholar]
- Buckingham-Howes S, Berger SS, Scaletti LA, Black MM, 2013. Systematic review of prenatal cocaine exposure and adolescent development. Pediatrics 131, e1917–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae SM, Covington CY, 2009. Biobehavioral outcomes in adolescents and young adults prenatally exposed to cocaine: Evidence from animal models. Biol. Res. Nurs. 10, 318–330. [DOI] [PubMed] [Google Scholar]
- Chaplin TM, Fahy T, Sinha R, Mayes LC, 2009. Emotional arousal in cocaine exposed toddlers: Prediction of behavior problems. Neurotoxicol. Teratol. 31, 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Freiburger MB, Mayes LC, Sinha R, 2010. Prenatal cocaine exposure, gender, and adolescent stress response: A prospective longitudinal study. Neurotoxicol. Teratol. 32, 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Smith I, James ME, Falek A, 1992. Effects of cocaine and alcohol-use in pregnancy on neonatal growth and neurobehavioral status. Neurotoxicol. Teratol. 14, 23–33. [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA, 2017. FMRI Clustering in AFNI: False-positive rates redux. Brain Connect 7, 152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE, 2012. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat. Rev. Neurosci. 13, 636–650. [DOI] [PubMed] [Google Scholar]
- Damasio AR, 1995. On some functions of the human prefrontal cortex. Ann. N.Y. Acad. Sci. 769, 241–251. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Chiodo LM, Hannigan JH, Greenwald MK, Janisse J, Patterson G, Huestis MA, Partridge RT, Ager J, Sokol RJ, 2011. Prenatal and postnatal cocaine exposure predict teen cocaine use. Neurotoxicol. Teratol. 33, 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis T, Bendersky M, Ramsay D, Lewis M, 2006. Reactivity and regulation in children prenatally exposed to cocaine. Dev. Psychol. 42, 688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derauf C, Kekatpure M, Neyzi N, Lester B, Kosofsky B, 2009. Neuroimaging of children following prenatal drug exposure. Semin. Cell Dev. Biol. 20, 441–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Li Z, Santhanam P, Coles CD, Lynch ME, Hamann S, Hu X, 2010. Recursive cluster elimination based support vector machine for disease state prediction using resting state functional and effective brain connectivity. PLoS One 5, e14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME, 1998. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cogn. Emot. 12, 353–385. [Google Scholar]
- Eiden RD, Godleski S, Schuetze P, Colder CR, 2015. Prenatal substance exposure and child self-regulation: Pathways to risk and protection. J. Exp. Child Psychol. 137, 12–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, McAuliffe S, Kachadourian L, Coles C, Colder C, Schuetze P, 2009a. Effects of prenatal cocaine exposure on infant reactivity and regulation. Neurotoxicol. Teratol. 31, 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, Veira Y, Granger DA, 2009b. Prenatal cocaine exposure and infant cortisol reactivity. Child Dev. 80, 528–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A, Howner K, Fischer H, Kristiansson M, Furmark T, 2013. Altered fusiform connectivity during processing of fearful faces in social anxiety disorder. Transl. Psychiatry 3, e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, Hare T, Tottenham N, 2014. The development of human amygdala functional connectivity at rest from 4 to 23 years: A cross-sectional study. Neuroimage 95, 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendle MH, Strawderman MS, Mactutus CF, Booze RM, Levitsky DA, Strupp BJ, 2003. Impaired sustained attention and altered reactivity to errors in an animal model of prenatal cocaine exposure. Brain Res. Dev. Brain Res. 147, 85–96. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Chiodo LM, Hannigan JH, Sokol RJ, Janisse J, Delaney-Black V, 2011. Teens with heavy prenatal cocaine exposure respond to experimental social provocation with escape not aggression. Neurotoxicol. Teratol. 33, 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Hof PR, Friston KJ, Fan J, 2013. Anterior insular cortex and emotional awareness. J. Comp. Neurol. 521, 3371–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey JA, 2004. Cocaine effects on the developing brain: Current status. Neurosci. Biobehav. Rev. 27, 751–764. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI, 2002. Human neural systems for face recognition and social communication. Biol. Psychiatry 51, 59–67. [DOI] [PubMed] [Google Scholar]
- Kable JA, Coles CD, Lynch ME, Platzman K, 2008. Physiological responses to social and cognitive challenges in 8-year olds with a history of prenatal cocaine exposure. Dev. Psychobiol. 50, 251–265. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ, 2011. The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behav. Brain Res. 223, 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert BL, Bauer CR, 2012. Developmental and behavioral consequences of prenatal cocaine exposure: A review. J. Perinatol. 32, 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Lagasse LL, Shankaran S, Bada HS, Bauer CR, Lin R, Das A, Higgins R, 2010. Prenatal cocaine exposure related to cortisol stress reactivity in 11-year-old children. J. Pediatr. 157, 288–295 e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Padbury JF, 2009. Third pathophysiology of prenatal cocaine exposure. Dev. Neurosci. 31, 23–35. [DOI] [PubMed] [Google Scholar]
- Li K, Zhu D, Guo L, Li Z, Lynch ME, Coles C, Hu X, Liu T, 2013. Connectomics signatures of prenatal cocaine exposure affected adolescent brains. Hum. Brain Mapp. 34, 2494–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Coles CD, Lynch ME, Hamann S, Peltier S, LaConte S, Hu X, 2009. Prenatal cocaine exposure alters emotional arousal regulation and its effects on working memory. Neurotoxicol. Teratol. 31, 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Coles CD, Lynch ME, Luo Y, Hu X, 2016. Longitudinal changes of amygdala and default mode activation in adolescents prenatally exposed to cocaine. Neurotoxicol. Teratol. 53, 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Santhanam P, Coles CD, Ellen Lynch M, Hamann S, Peltier S, Hu X, 2013. Prenatal cocaine exposure alters functional activation in the ventral prefrontal cortex and its structural connectivity with the amygdala. Psychiatr. Res. 213, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZH, Santhanam P, Coles CD, Lynch ME, Hamann S, Peltier S, Hu XP, 2011. Increased "default mode" activity in adolescents prenatally exposed to cocaine. Hum. Brain Mapp. 32, 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes LC, 2002. A behavioral teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotoxicol. Teratol. 24, 385–395. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Grillon C, Granger R, Schottenfeld R, 1998. Regulation of arousal and attention in preschool children exposed to cocaine prenatally. Ann. N.Y. Acad. Sci. 846, 126–143. [PubMed] [Google Scholar]
- Mentis M, 1998. In utero cocaine exposure and language development. Semin. Speech Lang. 19, 147–165. [DOI] [PubMed] [Google Scholar]
- Min MO, Minnes S, Kim JY, Yoon M, Singer LT, 2017. Association of prenatal cocaine exposure, childhood maltreatment, and responses to stress in adolescence. Drug Alcohol Depend. 177, 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Min MO, Short EJ, Wu M, Lang A, Yoon S, Singer LT, 2016. Executive function in children with prenatal cocaine exposure (12-15 years). Neurotoxicol. Teratol. 57, 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Singer LT, Kirchner HL, Short E, Lewis B, Satayathum S, Queh D, 2010. The effects of prenatal cocaine exposure on problem behavior in children 4-10 years. Neurotoxicol. Teratol. 32, 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Pelphrey KA, 2011. Developing connections for affective regulation: Age-related changes in emotional brain connectivity. J. Exp. Child Psychol. 108, 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, 2006. Emotion and cognition: Insights from studies of the human amygdala. Annu. Rev. Psychol. 57, 27–53. [DOI] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE, 2014. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 84, 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL, 2001. A default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 98, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Willford J, 2009. Continued effects of prenatal cocaine use: Preschool development. Neurotoxicol. Teratol. 31, 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano AG, Harvey JA, 1998. Prenatal cocaine exposure: Long-term deficits in learning and motor performance. Ann. N.Y. Acad. Sci. 846, 89–108. [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW, 2009. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage 44, 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzwedel AP, Grewen KM, Goldman BD, Gao W, 2016. Thalamocortical functional connectivity and behavioral disruptions in neonates with prenatal cocaine exposure. Neurotoxicol. Teratol. 56, 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzwedel AP, Grewen KM, Vachet C, Gerig G, Lin W, Gao W, 2015. Prenatal drug exposure affects neonatal brain functional connectivity. J. Neurosci. 35, 5860–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze P, Eiden RD, 2006. The association between maternal cocaine use during pregnancy and physiological regulation in 4- to 8-week-old infants: An examination of possible mediators and moderators. J. Pediatr. Psychol. 31, 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze P, Eiden RD, Coles CD, 2007. Prenatal cocaine and other substance exposure: Effects on infant autonomic regulation at 7 months of age. Dev. Psychobiol. 49, 276–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze P, Eiden RD, Molnar DS, Colder CD, 2014. Empathic responsivity at 3 years of age in a sample of cocaine-exposed children. Neurotoxicol. Teratol. 42, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinkopf SJ, Lester BM, Sanes JN, Eliassen JC, Hutchison ER, Seifer R, Lagasse LL, Durston S, Casey BJ, 2009. Functional MRI and response inhibition in children exposed to cocaine in utero. Preliminary findings. Dev. Neurosci. 31, 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J, 1998. Neuroimaging analyses of human working memory. Proc. Natl. Acad. Sci. U.S.A. 95, 12061–12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P, 1988. Co-planar stereotaxic atlas of the human brain. Thieme Medical Publishers, Inc., New York. [Google Scholar]
- Touroutoglou A, Bickart KC, Barrett LF, Dickerson BC, 2014. Amygdala task-evoked activity and task-free connectivity independently contribute to feelings of arousal. Hum. Brain Mapp. 35, 5316–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakiniaeiz Y, Yip SW, Balodis IM, Lacadie CM, Scheinost D, Constable RT, Mayes LC, Sinha R, Potenza MN, 2017. Altered functional connectivity to stressful stimuli in prenatally cocaine-exposed adolescents. Drug Alcohol Depend. 180, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hu S, Chao HH, Ide JS, Luo X, Farr OM, Li CS, 2014. Ventromedial prefrontal cortex and the regulation of physiological arousal. Soc. Cogn. Affect. Neurosci. 9, 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.