Autologous hematopoietic stem cell transplant (autoHSCT) remains standard of care for fit patients with multiple myeloma[1]. The use of granulocyte colony stimulating factor (G-CSF) after autoHSCT accelerates neutrophil recovery by 1 – 6 days without improving platelet recovery, incidence neutropenic fever, or intravenous (IV) antibiotic exposure using multiple conditioning regimens[2,3]. The American Society of Clinical Oncology (ASCO) guidelines recommend that G-CSF should be initiated 1–5 days after administration of high-dose chemotherapy and should be continued until the absolute neutrophil count (ANC) is 2000 – 3000/μL[4]. Earlier administration of G-CSF post-transplant accelerates neutrophil recovery, but the clinical implications were thought to be modest [5]. The duration of severe neutropenia, rather than the time to neutrophil engraftment, has rarely been analyzed in patients with myeloma, nor has the G-CSF schedule been studied in terms of its effect on safety with multiple schedules. After identifying three cohorts that received G-CSF (Neupogen) starting on day +1, day +5, and day +7, we report on the influence of G-CSF administration timing on the duration of severe neutropenia, safety, and disease control.
Three patient cohorts were identified that all received high dose melphalan (140 or 200 mg/m2) on day −2 over a 30-minute infusion, followed by autologous stem cell rescue (day 0), and G-CSF was administered daily 5 mcg/kg. Three cohorts (N=221) received G-CSF starting day+1 (n=43), day+5 (n=75), or day+7 (n=103) until the absolute neutrophil count (ANC) was >1500/μL or white blood cell count >5000/μL. This study was approved by The Ohio State University Cancer Institutional Review Board (NCT01653106), and the different G-CSF treatment schedules resulted from changes in institutional standard practices at different time periods. The duration of severe neutropenia (grade 4) was calculated using the estimated start and end time in hours during which the ANC was below 500/μL, including the time when the total white blood cell count was less than 500/μL and differential was not performed. Most patients (223 out of 226) received levofloxacin, acyclovir and fluconazole as prophylaxis. We evaluated the duration of severe neutropenia (ANC<500), the onset of bacteremia, World Health Organization (WHO) grade 2–4 mucositis, time to neutrophil engraftment, the incidence of febrile neutropenia, bacteremia (excluding coagulase-negative staphylococcus & microbacterium) the time to biochemical progression and overall survival.
The influence of the G-CSF treatment schedule, either day +1, +5, or +7, on each outcome was controlled for baseline characteristics through inverse probability weighting methodology (IPW)[6]. We estimated the IPW through a multinomial regression model which included age, race, gender, melphalan dose, and cytogenetic risk group. Linear, logistic and Cox regression models, weighted by the estimated stabilized IPW, were utilized to control confounding, and along with robust variance estimators, to test the influence of treatment schedule on each outcome of interest [7]. Duration of severe neutropenia, the number of hospital days, and time to neutrophil engraftment were heavily right skewed, and were natural log transformed for analysis. Time to relapse was determined as the time from transplant until the earliest of the following time points: progressive disease, clinical relapse, or relapse from CR as defined by the International Myeloma Working Group (IMWG). Patients without known progression were censored at the date of last follow up. Overall survival was defined as the time from transplant until death; patients were censored when lost to follow up or on the last date known to be alive through January 2017. All reported p-values and confidence intervals were two-sided, and reported at the nominal level; all analyses were performed using Stata 13.0.
Patient characteristics are given in Table 1. Patient characteristics were generally similar between the three G-CSF cohorts. The most notable difference was observed between groups in the proportion of patients receiving 200 mg/m2 of melphalan. The duration of severe neutropenia was significantly increased for patients receiving G-CSF on day +7 (geometric mean (GM): 151.4, 95% CI: 145.6– 157.1) and in those receiving G-CSF on day +5 (GM: 119.3, 95% CI 114–126.1) compared to those receiving it day +1 (GM: 104.2, 95% CI: 98.85–110.0, IPW adjusted estimates and Wald test: day +7 vs. day +1: p-value < 0.001, day+5 vs. day +1: p-value=0.001, day +7 vs. day +5: p-value<0.001) (Figure 1a). Moreover, time to neutrophil engraftment was substantially longer for those in the day +7 cohort and the day +5 cohort compared to the day +1 cohort (GM: day +7: 12.8 days, 95% CI: 12.6–13.0; day +5: 12.3, 95% CI: 12.2–12.5; day +1: 11.2 days, 95% CI: 10.9–11.5; day +7 vs. day +1: p-value <0.001, day +7 vs. day +5: p-value <0.001, day +7 vs. day +5: p-value<0.001) (Figure 1b).
Table 1.
Patient characteristics of those treated with G-CSF starting Day +1, Day +5 and Day +7.
| Baseline Characteristics | GCSF day +1 (n = 43) |
GCSF day +5 (n = 75) |
GCSF day +7 (n = 103) |
p- valuea |
|---|---|---|---|---|
|
Age Mean (sd) Median (min – max) < 60(%) 60 – 65(%) > 65(%) |
57.7 (8.1) 57 (40 – 72) 23 (53.5) 15 (34.9) 5 (11.6) |
58.5 (7.4) 59 (37–75) 41 (54.7) 22 (29.3) 12 (16.0) |
58.3 (7.4) 59 (35 – 70) 52 (50.5) 35 (34.0) 16 (15.5) |
0.88 0.93 |
|
Gender Female(%) Male(%) |
16 (37.2) 27 (62.8) |
26 (34.7) 49 (65.3) |
40 (38.8) 63 (61.2) |
0.87 |
|
Race White(%) Other(%) |
37 (86.1) 6 (14.0) |
64 (85.3) 11 (14.7) |
90 (87.4) 13 (12.6) |
0.90 |
|
Melphalan dose 140 mg/m2(%) 200 mg/m2(%) |
9 (20.9) 34 (79.1) |
14 (18.7) 61 (81.3) |
14 (13.6) 89 (86.4) |
0.48 |
|
Risk group Standard(%) Intermediate(%) High(%) Missing(%) |
27 (62.8) 11 (25.6) 4 (9.3) 1 (2.3) |
39 (52.0) 24 (32.0) 12 (16.0) 0 |
62 (60.2) 23 (22.3) 15 (14.6) 3 (2.9) |
0.55 |
|
Number of infused cells Mean (sd) Median (min – max) |
5.2 (2.2) 4.7 (2.4–11.7) |
5.1 (2.7) 4.6 (1.6–18.3) |
4.4 (2.1) 4.0 (1.9–15.7) |
0.08 |
| Outcomesb | ||||
|
Duration (hours) of severe neutropenia Mean (sd) Adjusted geometric mean (95% CI) |
104.8 (19.7) 104.2 (98.5–110.0) |
120.8 (27.6) 119.3 (112.5–126.1) |
153.4 (36.9) 151.4 (145.6–157.1) |
< 0.001 |
|
Length of Stay Mean (sd) Adjusted geometric mean (95% CI) |
13.9 (2.8) 13.8 (13.0–14.6) |
17.7 (5.6) 17.0 (16.2–17.9) |
15.2 (2.8) 15.0 (14.5–15.5) |
< 0.001 |
|
Mucositis grade 0–1(%) 2–3(%) |
41 (95.3) 2 (4.7) |
71 (94.7) 4 (5.3) |
85 (82.5) 18 (17.5) |
0.01 |
|
Bacteremiac No(%) Yes(%) |
34 (79.1) 9 (20.9) |
66 (88.0) 9 (12.0) |
76 (73.8) 27 (26.2) |
0.06 |
|
Time to Neutrophil Engraftment
Mean (sd) Adjusted geometric mean (95% CI) |
10.2 (1.0) 11.2 (10.9–11.5) |
11.3 (0.7) 12.3 (12.2–12.5) |
11.8 (1.1) 12.8 (12.6–13.0) |
<0.001 |
|
Febrile Neutropenia
No(%) Yes(%) |
15 (34.9) 28 (65.1) |
26 (34.7) 49 (65.3) |
12 (11.7) 91 (88.4) |
0.002 |
|
Platelet transfusion No(%) Yes(%) |
3 (7.0) 40 (93.0) |
5 (6.7) 70 (93.3) |
8 (7.8) 95 (92.2) |
0.91 |
|
Red cell transfusion No(%) Yes(%) |
22 (51.2) 21 (48.8) |
49 (65.3) 26 (34.7) |
49 (47.6) 54 (52.4) |
0.06 |
Comparisons between baseline characteristics were made by Fisher’s exact test for categorical variables or by linear regression for continuous variables.
Outcome measures are compared through inverse probability weighted regression models, adjusting for age, race. melphalan dose, and risk group.
Does not include coagulase-negative staphylococcus, microbacterium, and rare skin specific strep species.
Figure 1.
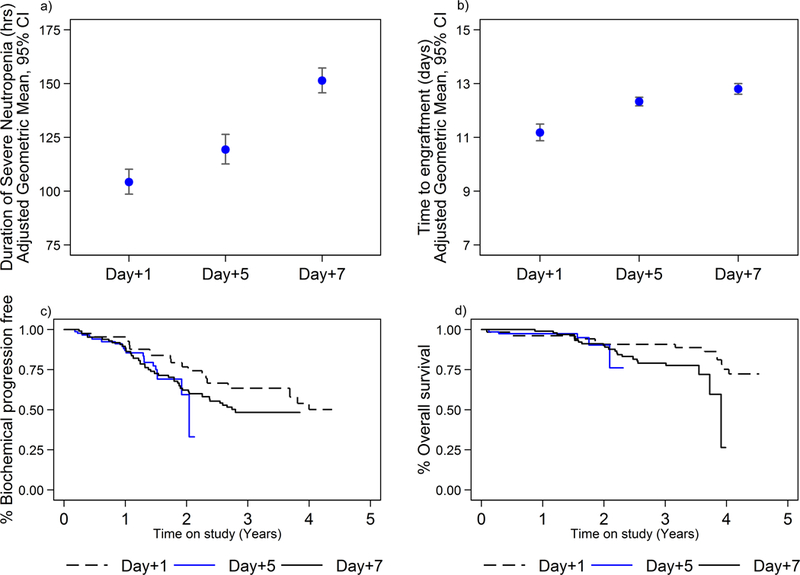
a. Duration of severe neutropenia in hours (ANC <500) in patients who received G-CSF on day +1, day + 5, or day + 7 from ASCT. IPW adjusted geometric mean estimates: day +7 vs. day +1: p-value < 0.001, day+5 vs. day +1: p-value=0.001, day +7 vs. day +5: p-value<0.001) .
b. Time to neutrophil engraftment in patients who received G-CSF on day +1, day + 5, or day + 7 from ASCT. IPW adjusted geometric mean estimates: day +7 vs. day +1: p-value, day+5 vs. day +1:, day +7 vs. day +5: p-value) .
c. Time to biochemical relapse comparing the patients who received G-CSF on day +1 to those patients who received G-CSF on day +5 and +7. Eighty-one subjects relapsed during this time (17 treated with G-CSF day +1, 20 treated with G-CSF day +5, 44 treated with G-CSF day +7). Day +7 vs. day +1: aHR: 1.6, 95% CI: 0.9– 2.8, p-value = 0.143; day +5 vs. day +1: aHR: 1.7, 95% CI: 0.8– 3.5, p-value = 0.146).
d. Overall survival in patients who received G-CSF on day +1, day + 5, or day + 7 from ASCT. 41 patients died (10 in G-CSF day +1 cohort, 6 in G-CSF day +5 cohort, 25 in G-CSF day +7 cohort). G-CSF on day +7 versus day +1, aHR: 2.8, 95% CI: 1.0–7.8, day +5 vs. day +1: 2.8, 95% CI: 0.6–12.4 .
Annotations:
ANC: Absolute neutrophil count; G-CSF: Granulocyte-colony stimulating factor; autoHSCT: autologous hematopoietic stem cell transplant.
Patients who received G-CSF starting day +7 suffered an increased incidence of grade 2 or higher mucositis compared to patients that started G-CSF day +5 (adjusted odds ratio (aOR) 4.4, CI 1.4–13.6, p-value=0.01) and on day +1 (aOR 4.1, CI: 0.9–18.8, p-value=0.07). Similarly, the proportion of patients with bacteremia was significantly increased in those treated on day +7 compared to those on day +5 (aOR: 2.7, 95% CI: 1.2–6.3, p-value: 0.019), but not between day +7 and day +1. Febrile neutropenia was more common in patients receiving GCS-F on day +7 compared to those receiving it on day +5 or day +1 (aOR vs. day +1: 3.6, 95% CI: 1.5–8.7, p-value=0.006; aOR vs. day +5: 3.8, 95% CI: 1.7–8.3, p-value=0.001). These findings suggest that starting G-CSF on day +1 following autoHSCT decreases the duration of severe neutropenia, time to neutrophil engraftment, incidence of neutropenic fever, and mucositis compared to starting G-CSF on days +5 and +7.
Subjects were followed for a median duration of 20.0 months (maximum 52 months); 81 subjects relapsed during this time (17 treated with G-CSF day +1, 20 treated with G-CSF day +5, 44 treated with G-CSF day +7) and 41 patients died (110 G-CSF day +1, 6 G-CSF day +5, 25 G-CSF day +7). While time to biochemical relapse does not appear to be influenced by treatment day with G-CSF (IPW adjusted hazard ratio (aHR): day +7 vs. day +1: 1.6, 95% CI: 0.9– 2.8, p-value = 0.143; day +5 vs. day +1: 1.7, 95% CI: 0.8– 3.5, p-value = 0.146), there is some indication of worse overall survival in those receiving G-CSF on day +7 versus day +1 (aHR 2.8, 95% CI: 1.0–7.8, p-value=0.049; day +5 vs. day +1: aHR 2.8, 95% CI: 0.6–12.3, p-value=0.17 (Figure 1c & 1d)
Our analysis fits well with other reports that compared different start times for G-CSF administration after autologous transplant. The only phase 3 trial which randomized G-CSF starting with transplant versus placebo demonstrated clear benefits in terms of a reduction in the duration of neutropenia, number of infections, and length of stay[3] in the G-CSF arm, but this does not address whether there is a dose-response relationship. The first randomized study to test different schedules of G-CSF examined starting G-CSF at day +1 and day +5 was performed in just 86 patients and found starting G-CSF on day+1 shortened the period of severe neutropenia but few other differences were significant, although analysis of the effect on mucositis was not reported[5]. The second prospective trial[8] that tested different G-CSF schedules (no G-CSF vs day +1 vs day +5) in 240 patients with a variety of hematologic malignancies and found a statistically longer period of neutropenia without G-CSF (13 days) than with G-CSF (9 days starting day +1 and 10 days starting day +5); the conclusions of this trial are difficult to generalize given the mix of conditioning regimens and cancers. More modern comparisons were retrospective, analyzing large patient populations at the expense of patient heterogeneity. Comparing G-CSF starting day +7 vs day +14 (n=117) showed a longer period of neutropenia, longer treatment with intravenous antibiotics, and longer hospital stay with delayed G-CSF[9]. The influence of G-CSF on the length of hospital stay is controversial in the literature with some reports showing longer length of stay and more neutropenic fevers with earlier administration of G-CSF (n=664)[10], but because most reports were retrospective analyses, the institutional standards regarding prophylactic antibiotic administration and infection detection policies differed within the cohorts studied.
We have illustrated that starting G-CSF injections the day after stem cell infusion (day +1) decreases the duration of severe neutropenia, incidence of neutropenic fever and significant mucositis in comparison to day +5 and day +7 in patients receiving autologous transplant for MM. The finding that starting G-CSF earlier after transplant decreases the period of severe neutropenia is expected, and decreasing mucositis follows as mucositis correlates with neutropenia[11]. Downstream measurements of the incidence of bacteremia and length of hospital stay clearly relate to time to engraftment, but multiple variables, including melphalan exposure (area under the curve)[12], and hematopoietic cell transplantation-specific comorbidity index (HCT-CI) can influence these measures. Instead of a costly prospective trial to confirm our findings, the next step could be a cost-effective multi-center retrospective analysis. This multicenter trial could identify subgroups most likely to benefit[13,14], justifying the increased cost of more G-CSF. Until then, starting G-CSF day +1 to decrease the incidence of neutropenic fever and significant mucositis is appealing.
ACKNOWLEDGEMENTS
This research was supported by Multiple Myeloma Opportunities for Research and Education (MMORE) [CCH, MAP, YKC], a Pelotonia IDEA award (46050-502048) [MAP, CCH, MP], the Ohio State University Comprehensive Cancer Center Core Grant (P30 CA016058), and an Eli-Lilly fellowship [YKC]. The content is solely the responsibility of the authors and does not represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
DISCLOSURE
The authors confirm that neither the submitted manuscript nor any similar manuscript, in whole or in part, other than an abstract, is under consideration, in press, or published elsewhere.
CONFLICTS of INTEREST
None of the authors have a relevant conflict of interest to report.
REFERENCES
- 1.Attal M, Lauwers-Cances V, Hulin C, et al. . Autologous Transplantation for Multiple Myeloma in the Era of New Drugs: A Phase III Study of the Intergroupe Francophone Du Myelome (IFM/DFCI 2009 Trial). Blood 2015;126:391–391. [Google Scholar]
- 2.Klumpp TR, Mangan KF, Goldberg SL, Pearlman ES, Macdonald JS. Granulocyte colony-stimulating factor accelerates neutrophil engraftment following peripheral-blood stem-cell transplantation: a prospective, randomized trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 1995;13:1323–1327. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz N, Ljungman P, Cordonnier C, et al. . Lenograstim after autologous peripheral blood progenitor cell transplantation: results of a double-blind, randomized trial. Bone Marrow Transplant 2004;34:955–962. [DOI] [PubMed] [Google Scholar]
- 4.Smith TJ, Bohlke K, Lyman GH, et al. . Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015;33:3199–3212. [DOI] [PubMed] [Google Scholar]
- 5.de Azevedo AM, Nucci M, Maiolino A, et al. . A randomized, multicenter study of G-CSF starting on day +1 vs day +5 after autologous peripheral blood progenitor cell transplantation. Bone Marrow Transplant 2002;29:745–751. [DOI] [PubMed] [Google Scholar]
- 6.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–560. [DOI] [PubMed] [Google Scholar]
- 7.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valteau-Couanet D, Faucher C, Auperin A, et al. . Cost effectiveness of day 5 G-CSF (Lenograstim) administration after PBSC transplantation: results of a SFGM-TC randomised trial. Bone Marrow Transplant 2005;36:547–552. [DOI] [PubMed] [Google Scholar]
- 9.Cox JE, Campos S, Wu J, et al. . Efficacy of deferred dosing of granulocyte colony-stimulating factor in autologous hematopoietic transplantation for multiple myeloma. Bone Marrow Transplant 2014;49:219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gertz MA, Gastineau DA, Lacy MQ, et al. . SCT without growth factor in multiple myeloma: engraftment kinetics, bacteremia and hospitalization. Bone Marrow Transplant 2011;46:956–961. [DOI] [PubMed] [Google Scholar]
- 11.Wardley AM, Jayson GC, Swindell R, et al. . Prospective evaluation of oral mucositis in patients receiving myeloablative conditioning regimens and haemopoietic progenitor rescue. British Journal of Haematology 2000;110:292–299. [DOI] [PubMed] [Google Scholar]
- 12.Cho YK, Sborov DW, Lamprecht M, et al. . Associations of high-dose melphalan pharmacokinetics and outcomes in the setting of a randomized cryotherapy trial. Clin Pharmacol Ther 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbert K, Ritchie DS. The Goldilocks conundrum: how much granulocyte colony-stimulating factor following autologous stem cell transplant is ‘just right’? Leuk Lymphoma 2011;52:548–549. [DOI] [PubMed] [Google Scholar]
- 14.Khot A, Dickinson M, Stokes K, et al. . A risk-adapted protocol for delayed administration of filgrastim after high-dose chemotherapy and autologous stem cell transplantation. Clin Lymphoma Myeloma Leuk 2013;13:42–47. [DOI] [PubMed] [Google Scholar]


