Abstract
Objectives:
Subtherapeutic drug concentrations contribute to both primary and secondary nonresponse to infliximab in children with Crohn’s disease (CD). The aim of this study was to evaluate treatment outcomes and infliximab concentrations at infusions 2 and 3 with an objective to establish infliximab targets during induction for primary responders.
Methods:
Single-center, prospective cohort of anti-TNF naïve CD patients <22 years old starting infliximab. Clinical response was defined with the weighted pediatric CD activity index at the 4th infusion. Rates of biological response (>50% improvement in fecal calprotectin) and maintenance concentrations ≥5 μg/ml were secondary outcomes.
Results:
We enrolled 72 CD patients with 70/72 receiving infliximab monotherapy. Clinical response, biological response, and start of maintenance concentrations ≥5 µg/ml were achieved in 64%, 54% and 22% respectively. The median (interquartile range) infliximab concentrations at infusion 2 and 3 in clinical responders were 27.8 μg/ml (19.5–40) and 14 μg/ml (8.3–24) compared to 18.8 μg/ml (9.1–23, p<0.001) and 7.8 μg/ml (4–13.2, p<0.01) in nonresponders. Receiver operating characteristic analysis determined that an infliximab concentration ≥15.9 μg/ml at infusion 3 was associated with clinical response (AUC 0.73) while an infusion 3 level ≥18 µg/ml was associated with a start of maintenance concentration >5 µg/ml (AUC 0.85). Independent predictors for infusion 3 levels <18 μg/ml included pre-treatment prednisone, low BMI, elevated ESR and CRP, hypoalbuminemia and an infusion 2 infliximab level <29 µg/ml.
Conclusions:
We found that infusion 2 (≥29 μg/ml) and infusion 3 (≥18 μg/ml) infliximab concentrations were strongly associated with improved early outcomes and higher first maintenance dose levels.
Keywords: therapeutic drug monitoring, inflammatory bowel disease, anti-TNF
Introduction
Early use of treatments targeting tumor necrosis factor-alpha (TNF) in children with Crohn’s disease (CD) have led to significant reductions in penetrating complications, decreases in CD-related hospitalizations and improved rates of sustained remission (1–3). Primary response rates to anti-TNF therapies in pediatrics CD are high and justifies the use of anti-TNF as one of the first-line biologics for children with moderate-severe CD (4, 5). Despite the high rates of primary response, rates of clinical and biological remission at one year are between 50–60% (4, 6). More judicious use of therapeutic drug monitoring (TDM) and subsequent dose escalation, however, have shown to improve long-term rates of anti-TNF response (6, 7).
Subtherapeutic drug concentrations are the leading cause of secondary nonresponse to anti-TNF as low drug levels result in a resurgence of intestinal inflammation and increase the probability of neutralizing anti-drug antibodies (8, 9). Extensive evaluation of infliximab clearance and correlation of drug levels to long-term outcomes such as mucosal healing has informed the development of varying concentration targets during the maintenance phase for infliximab (10–13) with more recent investigations evaluating induction concentrations in primary nonresponders in both adult and pediatric inflammatory bowel disease (IBD) patients (14–16).
With the relative paucity of pediatric-specific guidelines to infliximab intensification strategies during induction for at-risk CD patients, our primary aim was to establish early drug concentration targets that were associated with primary responders. We hypothesized that primary responders to infliximab would have significantly higher drug concentrations at infusions 2 and 3 compared to nonresponders.
Methods
Patient Recruitment
We performed a sub analysis of data from CD patients included in the Clinical and Molecular Signature to Predict Response to Anti-TNF Therapy in Pediatric IBD (PROSE) study. PROSE is a single-center, inception cohort of children and young adults (<22 years old) with IBD who enrolled immediately prior to starting infliximab and were prospectively monitored for treatment response with longitudinal biospecimens collected for one year. All patients were anti-TNF naïve and managed with individual infliximab regimens by multiple clinicians at Cincinnati Children’s Hospital Medical Center between August 2014-March 2018.
Study Outcomes
Clinical response at infusion 4 was defined using the weighted pediatric CD activity index (wPCDAI) (17). The mathematically wPCDAI combines subjective clinical evaluation (abdominal pain, stool frequency, and general well-being), and laboratory tests (albumin and erythrocyte sedimentation rate [ESR]) with physical exam assessments (weight, perirectal disease and evaluation of extraintestinal manifestations) and correlates strongly with mucosal inflammation (18). For the study, the clinical evaluation was calculated with a symptom questionnaire performed prior to each infusion. Baseline laboratory values and patient weights were determined on the same day as each infusion and the perianal examination was recorded as a last observation carried forward from the most recent clinical exam by the primary clinician. Clinical response at infusion 4 was determined by a change of >17.5 points from the baseline wPCDAI and/or a wPCDAI<12.5 with clinical remission defined by a wPCDAI<12.5 (17). In addition, treatment nonresponse was also defined as a failure to receive >4 infliximab infusions, undergoing a CD-related surgery during the first 100 days after starting infliximab or a patient with insufficient data to assess their clinical response (>1 missing wPCDAI item). As infliximab was dosed without a study specific protocol, an infliximab intensification during induction was not considered a treatment failure, however, infusion 4 infliximab levels from the five patients who had a dose intensification were eliminated from the primary analysis. Secondary outcomes included the association between infusion 2 and 3 infliximab concentrations from infusion 4 (a) biological responders (>50% improvement in fecal calprotectin from baseline)(19), (b) biological remission (fecal calprotectin <250 μg/g) (20), (c) normalized CRP (<0.5 mg/dL, (d) combination of clinical and biological response and (e) patients with a drug level >5 μg/ml at infusion 4 (6, 13). Baseline fecal calprotectin was >250 µg/g in all patients who provided a stool sample. All patients receiving prednisone >24 hours prior to the first infliximab dose were classified as prednisone-exposed.
Biologic assays
Trough infliximab concentrations were determined with IDKmonitor® (Immundiagnostik, Germany) from stored plasma samples collected at each infusion. The sandwich enzyme-linked immunosorbent assay (ELISA) has an upper detection limit of 45 μg/ml, lower detection limit of 0.7 μg/ml at 1:200 dilution and an intra-assay coefficient variation (CV) of 1.8–9.7% (21). We did not test for the presence of antibodies to infliximab. Fecal calprotectin was measured from a subset (n=43) of patients who collected stool samples prior to (up to 48 hours) infusions 1 and 4 utilizing an ELISA kit with an intra-assay CV of 2.6–10.5% (Buhlmann, Switzerland) (22).
Statistical analysis
Continuous variables are represented as means with standard deviations (SD) or medians with interquartile range (IQR) depending on data distribution. Infliximab concentrations at each infusion were compared between infliximab responders and nonresponders (clinical and biological) using the Mann-Whitney test. The optimal infliximab concentration cut-point at infusions 2 and 3 were determined for all infusion 4 outcomes using the Youden index from the receiver operating characteristic (ROC) curve. The area under the ROC (AUROC) curve with 95% confidence intervals (CI), sensitivity, specificity, positive predictive value (PPV) and negative predictive values (NPV) for infliximab concentrations were determined for the outcome measures. Rates of response at infusion 4 were also compared between selected independent variables by the Fisher’s exact test with odd’s ratios (OR) calculated. Pre-infliximab (baseline) categorical variables were assessed for significance for treatment outcomes with a univariate logistic regression analysis. After the univariate analysis, all variables with a P value <0.05 were tested for significance in two separate multivariate logistic regression models (concentrations below the new infusion 2 and 3 targets, respectively). Multivariate linear regression analysis was performed to evaluate significant covariates for infusion 3 infliximab concentration with the final model assessed for multicollinearity. A P value <0.05 was considered statistically significant. All statistical analyses was performed using PRISM version 7 (GraphPad, San Diego, CA) and R version 3.4.3 (R Development Core Team, Austria).
Ethical Considerations
The PROSE study was approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center.
Results
We evaluated the infliximab concentrations of 72 consecutive anti-TNF naïve CD patients enrolled in our PROSE cohort. The mean (SD) age of study participants was 13.6 years (±4) with 90% white race, 65% male and 3% on concomitant immunomodulator (Table 1). The median (IQR) time from diagnosis to infliximab start was 51 days (17–362) with 61% and 38% initiating infliximab ≤90 and ≤30 days following diagnosis, respectively. Sixty-three (88%) subjects were receiving standard infliximab dosing (5 mg/kg, rounding up to nearest 100 mg) with limited group-wise variation from standardized dosing regimens as the median (IQR) time for infusion 2 was 14 days (14–15) from infusion 1, the median time for infusion 3 was 28 days (27–28) from infusion 2 and the median time for infusion 4 was 56 days (53–57) from infusion 3. The rates of clinical response and remission at infusion 4 were 64% and 50%. We found no significant differences in baseline clinical characteristics or laboratory tests between clinical responders and nonresponders (data not shown). By infusion 4, biological response and remission were achieved in 23/43 (54%) and 13/43 (30%).
Table 1.
Clinical characteristics and baseline laboratory results.
| Number of patients, n | 72 |
| Female, n (%) | 25 (35%) |
| White race, n (%) | 65 (90%) |
| Age at infusion 1, years (mean, SD) | 13.6 (4) |
| Disease duration, days (median, IQR) | 51 (17-362) |
| <90 days, n (%) | 44 (61%) |
| Previous surgery, n (%) | 4 (5.6%) |
| Concomitant IMM, n (%) | 2 (3%) |
| Concomitant prednisone, n (%) | 44 (61%) |
| Time on prednisone, days (median, IQR) | 18 (7-43) |
| Crohn’s location | |
| Ileal only, n | 6 |
| Colon only, n | 9 |
| Ileocolonic, n | 57 |
| Crohn’s behavior | |
| Inflammatory | 61 |
| Stricturing | 7 |
| Penetrating | 3 |
| Both stricturing/penetrating | 1 |
| Perianal Crohn’s, n (%) | 11 (15%) |
| Starting dose, mg/kg (median, IQR) | 5.8 (5.2-6.6) |
| BMI kg/m2 (median, IQR) | 17.6 (15.4-20.9) |
| BMI z-score (median, IQR) | −0.69 (−1.4 to 0.17) |
| wPCDAI (mean, SD) | 46 (28) |
| ESR mm/hr. (median, IQR) | 18 (10-38) |
| CRP mg/dL (median, IQR) | 1.1 (0.28-2.1) |
| Albumin g/dL (mean, SD) | 3.3 (0.6) |
| Fecal calprotectin μg/g (median, IQR) | 2160 (1009-2501) |
Imm, immunomodulator; BMI, body mass index; wPCDAI, weighted Crohn’s disease activity index; ESR, erythrocyte sedimentation rate; CRP, c-reactive protein.
Early infliximab target concentrations for clinical and biological responses
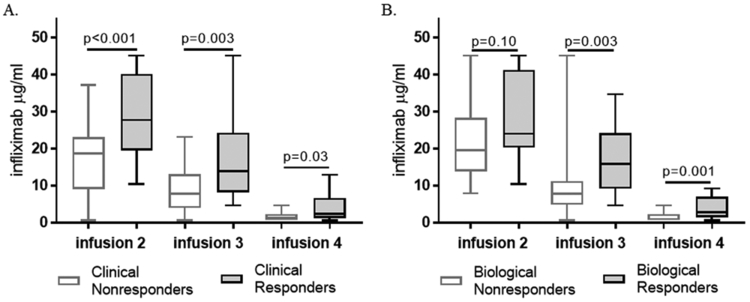
The median (IQR) infliximab concentration for clinical responders was 27.8 μg/ml (19.5–40) and 14 μg/ml (8.3–24) at infusions 2 and 3 respectively (Table, Supplemental Digital Content 1). Clinical responders had significantly higher infliximab trough concentrations compared to nonresponders at infusion 2, 3 and 4 while biological responders had higher drug levels at infusion 3 and 4 compared to nonresponders (Figure 1). We found an infliximab concentration ≥26.7 μg/ml at infusion 2 was 56% sensitive, 91% specific with a 92% PPV and 50% NPV for end of induction clinical response (AUROC 0.76, 95% CI 0.64–0.88, p<0.01). Infusion 3 infliximab concentration ≥15.9 μg/ml was 49% sensitive, 86% specific with a 88% PPV and 45% NPV for end of induction clinical response (AUROC 0.73, 95% CI 0.6–0.86, p<0.01). In contrast, the optimal cut-point for end of induction biological response was ≥20.7 μg/ml (AUROC 0.66, p=0.10) at infusion 2 and ≥13.9 μg/ml (AUROC 0.78, p=0.003) at infusion 3. We also found the median infliximab concentrations at infusion 3 were significantly higher for patients with a normal CRP and fecal calprotectin <250 µg/g at infusion 4, respectively (Figure, Supplemental Digital Content 2).
Figure 1. Infliximab trough concentrations for primary and second outcomes.
(A) Clinical response at infusion 4 was determined by improvement in the baseline wPCDAI (delta >17.5) and remaining on infliximab without surgery. (B) Biological response at infusion 4 was defined by >50% improvement from the baseline fecal calprotectin. Drug concentrations at each infusion were compared with the Mann-Whitney test.
Optimal infliximab induction targets
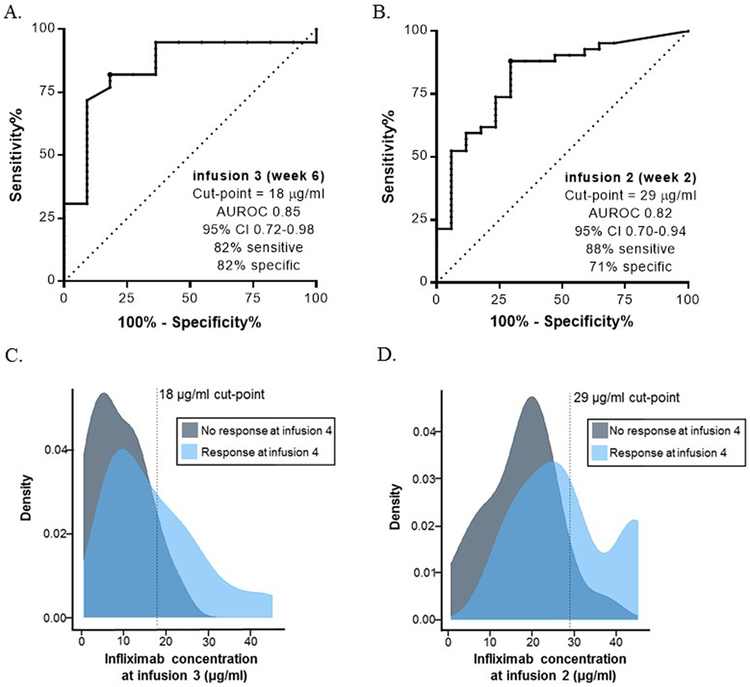
The median infliximab concentration for the entire cohort at the start of maintenance (infusion 4) was 2.1 μg/ml (1.1–4.3). As recent studies have found infliximab concentrations >5 µg/ml at the start of maintenance are associated with improved outcomes (6, 23, 24), we investigated the ideal infliximab concentration at infusions 2 and 3 for patients with levels >5 µg/ml at the 4th infusion. We found an infusion 3 infliximab concentration ≥18 μg/ml was 82% sensitive and 82% specific with a 56% PPV and a 94% NPV (AUROC 0.85, 95% CI 0.72–0.98, p<0.001, Figure 2a) for therapeutic level at the 4th infusion. Patients attaining the infusion 3 target of ≥18 μg/ml had a mean infusion 2 infliximab concentration of 34 μg/ml (SD 10.4) compared to a mean of 21.2 μg/ml (SD 9.8) in patients below this target (p<0.001). As previously noted, infliximab ≥26.7 μg/ml at infusion 2 was the target level for clinical response, however, an infusion 2 level of ≥29 μg/ml was the optimal cut-point to achieve our novel target (≥18 µg/ml) at infusion 3 (AUROC 0.82, 95% CI 0.70–0.94, p<0.001, Figure 2b).
Figure 2. Early induction infliximab targets and kernel density plots of the drug targets as predictors for clinical response.
ROC curve analysis was performed to define (A) the optimal infusion 3 drug concentration to achieve an infliximab level ≥5 μg/ml at infusion 4 and (B) the optimal infusion 2 drug concentration to achieve an infliximab level (≥18 μg/ml) at infusion 3. The optimal cut-points were defined by the Youden index. The density plot represents the distribution of infliximab concentrations at (C) infusion 3 in patients with clinical response and infliximab concentrations at (D) infusion 2 in patients with clinical response. The vertical line in the density plot denotes the threshold established using the ROC analysis. The density plot illustrates a large percentage of treatment nonresponders were below the newly established targets. AUROC, area under the receiver operating characteristic curve.
We found patients with an infusion 3 trough concentration ≥18 μg/ml had a higher pre-infliximab albumin, lower ESR and a lower frequency of prednisone exposure compared to those with a concentration below this target (Table Supplemental Digital Content 3). Additionally, a trough ≥18 µg/ml was associated with additional end of induction clinical and biological outcomes (Table, Supplemental Digital Content 4) including a median infusion 4 concentration of 6.6 μg/ml (2.5–7.5) compared to 1.5 µg/ml (0.84–2.6, p<0.001) with higher proportion of patients with a clinical response at this target (Figure 2c). Moreover, patients with an infusion 2 infliximab >29 μg/ml, were more likely to achieve clinical response (94% vs. 56.5%, OR 13, p<0.01, Figure 2d) and had a higher end of induction level with a median of 4.3 μg/ml (2.4–7.1) compared to a median of 1.54 μg/ml (0.85–3.2, p=0.004) in patients with a level <29 μg/ml at infusion 2. The 9 patients (12%) receiving high-dose infliximab had higher infusion 4 concentrations (median 7.1 vs 2.1 µg/ml, p=0.009) but no difference in drug concentrations at earlier time-points and no difference in outcomes compared to patients receiving standard doses.
Infliximab targets for combined clinical and biological response
We found 44% (19/43) of the cohort (patients with a fecal calprotectin at infusions 1 and 4) achieved the combination of clinical and biological response with a median infusion 4 infliximab concentration of 3.5 µg/ml (1.8–7.1) compared to 1.1 µg/ml (0.7–2.4, p<0.001). The ideal infusion 2 and 3 infliximab Youden cut-points for this combined outcome were 28 µg/ml (AUROC 0.68, 95% CI 0.50–0.85) and 14 µg/ml (AUROC 0.74, 95% CI 0.58–0.9), respectively.
Baseline factors associated with infliximab concentrations and rates of response
Patient baseline clinical factors and laboratory biomarkers were evaluated as predictors of treatment outcomes by univariate logistic regression. In our univariate regression analysis, we found pre-infliximab prednisone-exposure was associated with biological nonresponse, infusion 2 level <29 µg/ml and an infusion 3 level <18 µg/ml (Table 2). The regression analysis also found that an infusion 2 level <29 μg/ml was strongly predictive of a subtherapeutic infusion 3 level (OR 17.8, p<0.001). Of the outcomes listed in Table 2, we performed a multivariate logistic regression analysis for the significant predictors (univariate p<0.05) associated with an infusion 3 infliximab concentration <18 µg/ml and found ESR ≥20 mm/hr. and pre-infliximab prednisone-exposure were significant independent predictors. Similarly, we found both prednisone-exposure and body mass index (BMI) <18 kg/m2 (not with BMI z-score) were independent predictors for an infusion 2 level <29 µg/ml. Targeting an infusion 3 infliximab level ≥18 μg/ml was also found to be a significant predictor of a drug concentration >5 µg/ml at infusion 4 (OR 20.6, 95% CI 4.2–157, p<0.001).
Table 2.
Univariate regression for selected treatment outcomes.
| Variables | Odd’s Ratio | 95% CI | p-value |
|---|---|---|---|
| Clinical non-response | |||
| Pre-infliximab prednisone | 2.3 | 0.83-6.8 | 0.12 |
| <29 μg/ml at infusion 2 | 13.1 | 2.4-246 | 0.016 |
| <18 μg/ml at infusion 3 | 6.2 | 1.5-42 | 0.024 |
| Biological non-response | |||
| Pre-infliximab prednisone | 3.9 | 1.1-15.5 | 0.04 |
| <29 μg/ml at infusion 2 | 1.9 | 0.47-8.4 | 0.39 |
| <18 μg/ml at infusion 3 | 11 | 1.8-218 | 0.03 |
| <29 μg/ml at infusion 2 | |||
| Pre-infliximab prednisone | 4 | 1.3-13.1 | 0.018 |
| Pre-infliximab BMI <18 kg/m2 | 4.9 | 1.6-17.5 | 0.01 |
| Pre-infliximab albumin ≤3.5 g/dL | 2.7 | 0.82-8.8 | 0.1 |
| <18 μg/ml at infusion 3 | |||
| Pre-infliximab prednisone | 4.8 | 1.6-16 | 0.008 |
| Pre-infliximab BMI <18 kg/m2 | 3.6 | 1.2-11.9 | 0.029 |
| Pre-infliximab ESR ≥20 mm/hr. | 3.9 | 1.1-15.9 | 0.04 |
| Pre-infliximab CRP ≥0.5 mg/dL | 3.9 | 1.1-15.4 | 0.04 |
| Pre-infliximab albumin ≤3.5 g/dL | 5.4 | 1.6-19.1 | 0.007 |
| <29 μg/ml at infusion 2 | 17.8 | 4.7-80 | <0.001 |
Pre-infliximab prednisone, 84% of all patients were receiving a daily dose >0.5 mg/kg up to 40 mg.
Prednisone exposure and infliximab clearance
As noted, 61% (n=44) of the patients were receiving prednisone prior to the first infliximab dose (median of 18 days [7–43] with 37/44 receiving >0.5 mg/kg or 40 mg daily dosing) and weaned during induction per the treating physician. We found the infliximab trough concentrations at infusions 2 and 3 were significantly higher in the prednisone-free group (Figure, Supplemental Digital Content 5) with 48% of the prednisone–free patients reaching an infusion 3 concentration ≥18 μg/ml compared to only 16% in the prednisone-exposed group (p=0.0121). As we did not predict prednisone-exposure to influence early infliximab concentrations, the following sensitivity analysis was post-hoc. We postulated the observed differences in drug concentrations between prednisone-exposed (61%) patients and unexposed (39%) was directly related to disease severity. However, we found there was no statistical difference in baseline clinical factors, wPCDAI and non-invasive inflammatory biomarkers other than an elevated (expected) white blood cell count in the prednisone-exposed group (Table, Supplemental Digital Content 6). We produced additional ROC curves for the 28 patients who received infliximab monotherapy (prednisone-unexposed) during induction of which 75% had a clinical response and 33% had an infusion 4 concentration ≥5 µg/ml. The infliximab concentration cut-points for clinical response was 23.2 µg/ml (AUC 0.8, 95% CI 0.57–1) at infusion 2 and 6.6 µg/ml (AUC 0.79, 95% CI 0.58–0.99) at infusion 3. For an infusion 4 level >5 µg/ml, the infusion 2 cut-point was 36.8 µg/ml (AUC 0.61, 95% CI 0.31–0.90) while the infusion 3 cut-point was 24.8 µg/ml (AUC 0.75, 95% CI 0.53–0.97). In a multivariate linear regression analysis, prednisone-exposure and pre-infliximab hypoalbuminemia (<3.5 g/dL) were significant, independent predictors for the infusion 3 infliximab concentration (adjusted R-squared 0.23, p<0.001).
Discussion
With our real-world pediatric CD cohort, we evaluated the relationship of infliximab concentrations during the induction phase with multiple treatment outcomes. In this study, we found that an infliximab concentration at infusion 3 (week 6) ≥18 μg/ml was strongly associated with early clinical and biological responses as well as higher rates of infliximab levels >5 µg/ml at infusion 4. We also found an infusion 2 (week 2) concentration ≥29 μg/ml was strongly associated with improved rates of clinical response, a higher infusion 4 (week 14) drug concentration and a higher likelihood of achieving our newly established infusion 3 infliximab target concentration (≥18 μg/ml).
Despite the universal practice of weight-based dosing (starting at 5 mg/kg), there are limited infliximab PK and pharmacodynamic studies in children (25). The largest pediatric PK study (25) included study participants receiving infliximab in combination with an immunomodulator in the REACH clinical trial (4), which may not be reflective of real-world practice with recent data showing a decline in the use of combination therapy in CD (26). Additionally, achieving more consistent drug concentrations within a pre-specified range using TDM may reduce the need for combination anti-TNF/immunomodulator with a post-hoc analysis of the SONIC trial finding the improved outcomes were likely attributable to the higher infliximab concentrations from patients on combination therapy (11).
As more frequent TDM is being utilized in the management of patients receiving biologic therapies, there is a crucial need for personalized dosing schemes with pre-defined (and validated) targets as we have shown in this study. In a comparable report in adult IBD patients by Bar-Yoseph et al, ROC curve analysis determined the optimal infliximab cut-point for primary nonresponse at week 2 (infusion 2) was <6.8 μg/ml and a week 6 (infusion 3) level <3.5 μg/ml (16). Additionally, Ungar et al. reported infliximab targets of >9.2 µg/ml at week 2 and >7.2 µg/ml at week 6 for end of induction clinical remission in a pediatric IBD cohort (27). While our primary outcome was clinical response in a CD-only cohort, we found the target infliximab concentrations for clinical responders at infusion 2 and 3 were ≥26.7 μg/ml and ≥15.9 μg/ml respectively which are more consistent with infliximab concentrations (week 2, 28.3 μg/ml; week 6, 15 μg/ml) that were previously shown to correlate with short-term mucosal healing in adult-onset ulcerative colitis patients (28). The variation in drug levels seen by the Bar-Yoseph et al and Ungar et al. studies may be reflective of differences in the drug assay utilized (29), outcomes assessed, the population (IBD vs CD patients) studied or rates of immunogenicity in the cohort (16, 27).
Real-world primary nonresponse to infliximab in both pediatric and adult-onset CD vary between 10–30% (16, 30). The primary clinical nonresponse rate of 36% in our study is higher than expected, however, the majority of our cohort was receiving monotherapy (no immunomodulator) and clinical response was determined with the wPCDAI (>17.5 point improvement) at infusion 4 (REACH study evaluated the change in PCDAI at week 10; ≥15 point improvement). The wPCDAI was chosen in our study as it more suitable for observational studies then the full PCDAI (17). However, it’s possible our cohort represented more severe patients (mean wPCDAI of 46 [±28], median calprotectin of 2160 [1009–2501] μg/g) with an accelerated use of infliximab (61% started infliximab less than 90 days from diagnosis) who had less exposure to prior treatments (all REACH patients were on combination therapy) and therefore, a potential for delayed response to infliximab.
We unexpectedly discovered that our prednisone-exposed patients had significantly lower infliximab concentrations at infusions 2 and 3. We suspected this was secondary to disease severity but found no significant differences when comparing the exposed/unexposed groups independently. In our linear regression analysis, we found baseline hypoalbuminemia and prednisone-exposure were independently predictive of infusion 3 infliximab concentrations. To our knowledge, differences in infliximab clearance secondary to prednisone-exposure has not been previously published and will require further evaluation in future studies. It is noteworthy that we found the ideal infusion 3 cut-point was 24.8 µg/ml for patients unexposed to prednisone who achieved end of induction drug levels >5 µg/ml (compared to an infusion concentration of 18 µg/ml for all patients). Although speculative, this could suggest that higher infliximab exposure would be required to achieve similar concentration targets secondary to a higher inflammatory (TNF) burden in patients who are receiving steroid-sparing therapy during induction.
The strengths of the study include enrolling a large, prospectively monitored cohort of children and young adults with CD who predominantly received infliximab monotherapy in a real-world setting. We also evaluated infliximab targets for multiple outcome measures. Our study, however, had two limitations as we did not measure anti-drug antibodies and did not perform endoscopy at the end of induction.
While development of neutralizing anti-drug antibodies is noted to increase drug clearance (31), immunogenicity has been less studied during induction. Papamichael et al., utilizing a drug-tolerant ELISA, found 5% of adult patients with ulcerative colitis developed anti-drug antibodies during induction while Singh et al. reported 10% of children with IBD had anti-drug antibodies during infliximab induction (using a homogenous mobility shift assay) (6, 28). As this was a known limitation, our main conclusions are centered on infusion 2 and 3 infliximab concentration targets when the incidence of anti-drug antibodies are predicted to be lower.
The gold-standard to evaluate infliximab response would have been to obtain a pre/post-treatment colonoscopy. Aside from a clinical trial, repeat endoscopy is not feasible and led us to explore rates of biological response and remission with fecal calprotectin in a subset of patients. As the lack of validated fecal calprotectin cut-points for response (19) and remission (20) will continue to be a limitation for future studies, it is vital to develop optimal cut points while continuing to explore novel, blood pharmacodynamic biomarkers to better classify treatment response.
In conclusion, we have found an infliximab concentration of ≥29 μg/ml at infusion 2 and ≥18 µg/ml at infusion 3 was associated with improved outcomes. Although future studies will need to validate these targets, clinicians could consider these drug levels as a guideline when proactive TDM is utilized in CD patients at-risk for accelerated infliximab clearance during induction.
Supplementary Material
Figure, Supplemental Digital Content 5. Infliximab concentrations by pre-infliximab prednisone exposure. 84% of all prednisone-exposed patients were receiving a daily dose >0.5 mg/kg up to 40 mg. Drug concentrations at each infusion were compared with the Mann-Whitney test.
Figure, Supplemental Digital Content 2. Infliximab trough concentrations and secondary outcomes. We evaluated infliximab concentrations at each infusion between (A) patients with a normal CRP at infusion 4 and (B) patients with a fecal calprotectin <250 µg/g at infusion 4. Drug concentrations at each infusion were compared with the Mann-Whitney test.
Table, Supplemental Digital Content 1. Baseline biomarkers and median infliximab trough values for selected outcome measures.
Table, Supplemental Digital Content 4. Infusion 3 infliximab targets and end of induction outcomes.
Table, Supplemental Digital Content 3. Clinical characteristics and baseline laboratory results for infusion 3 trough concentrations.
Table, Supplemental Digital Content 6. Disease characteristics and baseline laboratory results for prednisone-exposed and prednisone-free
What is known:
Subtherapeutic drug concentrations during maintenance contribute to infliximab loss-of-response.
Optimal infliximab target concentrations during induction for primary responders have not been established in pediatric Crohn’s disease.
What is new:
An infliximab concentration ≥18 μg/ml at infusion 3 (week 6) was strongly associated with clinical and biological response as well as infliximab levels >5 µg/ml at start of maintenance.
Baseline hypoalbuminemia (≤3.5 g/dL), elevated erythrocyte sedimentation rate (>20 mm/hr.) and c-reactive protein (≥0.05 mg/dL), low body mass index (<18 kg/m2), and prednisone-exposure were risk factors for infliximab levels below this new infusion 3 target (<18 μg/ml).
Acknowledgments
Conflicts of Interest and Source of Funding: The authors have no financial, professional, or personal arrangement(s) with a company whose product figures prominently in the submitted manuscript or with a company making a competing product.
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health [K23 DK105229 to PM, K23 DK094832 to MJR] and by the Cincinnati Children’s Research Foundation Trustee Award Program (PM).
Footnotes
Conference: We have presented the abstract from this study at the 2017 North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) Annual Meeting in Las Vegas, Nevada.
References
- 1.Kugathasan S, Denson LA, Walters TD, et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study. Lancet 2017;389(10080):1710–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walters TD, Kim MO, Denson LA, et al. Increased effectiveness of early therapy with anti-tumor necrosis factor-alpha vs an immunomodulator in children with Crohn’s disease. Gastroenterology 2014;146(2):383–91. [DOI] [PubMed] [Google Scholar]
- 3.Church PC, Guan J, Walters TD, et al. Infliximab maintains durable response and facilitates catch-up growth in luminal pediatric Crohn’s disease. Inflamm Bowel Dis 2014;20(7):1177–86. [DOI] [PubMed] [Google Scholar]
- 4.Hyams J, Crandall W, Kugathasan S, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology 2007;132(3):863–73; quiz 1165–6. [DOI] [PubMed] [Google Scholar]
- 5.Merrick VM, Mortier K, Williams LJ, et al. Real-life Anti-tumor Necrosis Factor Experience in More Than 500 Patients: High Co-immunosuppression Rates But Low Rates of Quantifying Treatment Response. J Pediatr Gastroenterol Nutr 2018;66(2):274–80. [DOI] [PubMed] [Google Scholar]
- 6.Singh N, Rosenthal CJ, Melmed GY, et al. Early infliximab trough levels are associated with persistent remission in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 2014;20(10):1708–13. [DOI] [PubMed] [Google Scholar]
- 7.Papamichael K, Chachu KA, Vajravelu RK, et al. Improved Long-term Outcomes of Patients With Inflammatory Bowel Disease Receiving Proactive Compared With Reactive Monitoring of Serum Concentrations of Infliximab. Clin Gastroenterol Hepatol 2017;15(10):1580–88 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanai H, Lichtenstein L, Assa A, et al. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol 2015;13(3):522–30 e2. [DOI] [PubMed] [Google Scholar]
- 9.Steenholdt C, Brynskov J, Thomsen OO, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63(6):919–27. [DOI] [PubMed] [Google Scholar]
- 10.Vande Casteele N, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol 2013;108(6):962–71. [DOI] [PubMed] [Google Scholar]
- 11.Reinisch W, Colombel JF, Sandborn WJ, et al. Factors associated with short- and long-term outcomes of therapy for Crohn’s disease. Clin Gastroenterol Hepatol 2015;13(3):539–47 e2. [DOI] [PubMed] [Google Scholar]
- 12.Ungar B, Levy I, Yavne Y, et al. Optimizing Anti-TNF-alpha Therapy: Serum Levels of Infliximab and Adalimumab Are Associated With Mucosal Healing in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2016;14(4):550–57 e2. [DOI] [PubMed] [Google Scholar]
- 13.Feuerstein JD, Nguyen GC, Kupfer SS, et al. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology 2017;153(3):827–34. [DOI] [PubMed] [Google Scholar]
- 14.Davidov Y, Ungar B, Bar-Yoseph H, et al. Association of Induction Infliximab Levels With Clinical Response in Perianal Crohn’s Disease. J Crohns Colitis 2017;11(5):549–55. [DOI] [PubMed] [Google Scholar]
- 15.Liefferinckx C, Minsart C, Toubeau JF, et al. Infliximab Trough Levels at Induction to Predict Treatment Failure During Maintenance. Inflamm Bowel Dis 2017;23(8):1371–81. [DOI] [PubMed] [Google Scholar]
- 16.Bar-Yoseph H, Levhar N, Selinger L, et al. Early drug and anti-infliximab antibody levels for prediction of primary nonresponse to infliximab therapy. Aliment Pharmacol Ther 2018;47(2):212–18. [DOI] [PubMed] [Google Scholar]
- 17.Turner D, Griffiths AM, Walters TD, et al. Mathematical weighting of the pediatric Crohn’s disease activity index (PCDAI) and comparison with its other short versions. Inflamm Bowel Dis 2012;18(1):55–62. [DOI] [PubMed] [Google Scholar]
- 18.Turner D, Levine A, Walters TD, et al. Which PCDAI Version Best Reflects Intestinal Inflammation in Pediatric Crohn Disease? Journal of Pediatric Gastroenterology and Nutrition 2017;64(2):254–60. [DOI] [PubMed] [Google Scholar]
- 19.Zubin G, Peter L Predicting Endoscopic Crohn’s Disease Activity Before and After Induction Therapy in Children: A Comprehensive Assessment of PCDAI, CRP, and Fecal Calprotectin. Inflamm Bowel Dis 2015;21(6):1386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roblin X, Boschetti G, Duru G, et al. Distinct Thresholds of Infliximab Trough Level Are Associated with Different Therapeutic Outcomes in Patients with Inflammatory Bowel Disease: A Prospective Observational Study. Inflamm Bowel Dis 2017;23(11):2048–53. [DOI] [PubMed] [Google Scholar]
- 21.Guiotto C, Daperno M, Frigerio F, et al. Clinical relevance and inter-test reliability of anti-infliximab antibodies and infliximab trough levels in patients with inflammatory bowel disease. Dig Liver Dis 2016;48(2):138–43. [DOI] [PubMed] [Google Scholar]
- 22.Louis E Fecal calprotectin: towards a standardized use for inflammatory bowel disease management in routine practice. J Crohns Colitis 2015;9(1):1–3. [DOI] [PubMed] [Google Scholar]
- 23.Vaughn BP, Martinez-Vazquez M, Patwardhan VR, et al. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis 2014;20(11):1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148(7):1320–9 e3. [DOI] [PubMed] [Google Scholar]
- 25.Fasanmade AA, Adedokun OJ, Blank M, et al. Pharmacokinetic properties of infliximab in children and adults with Crohn’s disease: a retrospective analysis of data from 2 phase III clinical trials. Clin Ther 2011;33(7):946–64. [DOI] [PubMed] [Google Scholar]
- 26.Berkowitz JC, Stein-Fishbein J, Khan S, et al. Declining use of combination infliximab and immunomodulator for inflammatory bowel disease in the community setting. World J Gastrointest Pharmacol Ther 2018;9(1):8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ungar B, Glidai Y, Yavzori M, et al. Association Between Infliximab Drug and Antibody Levels and Therapy Outcome in Pediatric Inflammatory Bowel Diseases. J Pediatr Gastroenterol Nutr 2018. June 12 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Papamichael K, Van Stappen T, Vande Casteele N, et al. Infliximab Concentration Thresholds During Induction Therapy Are Associated With Short-term Mucosal Healing in Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol 2016;14(4):543–9. [DOI] [PubMed] [Google Scholar]
- 29.Vande Casteele N, Buurman DJ, Sturkenboom MG, et al. Detection of infliximab levels and anti-infliximab antibodies: a comparison of three different assays. Aliment Pharmacol Ther 2012;36(8):765–71. [DOI] [PubMed] [Google Scholar]
- 30.Ding NS, Hart A, De Cruz P Systematic review: predicting and optimising response to anti-TNF therapy in Crohn’s disease - algorithm for practical management. Aliment Pharmacol Ther 2016;43(1):30–51. [DOI] [PubMed] [Google Scholar]
- 31.De Bie CI, Hummel TZ, Kindermann A, et al. The duration of effect of infliximab maintenance treatment in paediatric Crohn’s disease is limited. Aliment Pharmacol Ther 2011;33(2):243–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure, Supplemental Digital Content 5. Infliximab concentrations by pre-infliximab prednisone exposure. 84% of all prednisone-exposed patients were receiving a daily dose >0.5 mg/kg up to 40 mg. Drug concentrations at each infusion were compared with the Mann-Whitney test.
Figure, Supplemental Digital Content 2. Infliximab trough concentrations and secondary outcomes. We evaluated infliximab concentrations at each infusion between (A) patients with a normal CRP at infusion 4 and (B) patients with a fecal calprotectin <250 µg/g at infusion 4. Drug concentrations at each infusion were compared with the Mann-Whitney test.
Table, Supplemental Digital Content 1. Baseline biomarkers and median infliximab trough values for selected outcome measures.
Table, Supplemental Digital Content 4. Infusion 3 infliximab targets and end of induction outcomes.
Table, Supplemental Digital Content 3. Clinical characteristics and baseline laboratory results for infusion 3 trough concentrations.
Table, Supplemental Digital Content 6. Disease characteristics and baseline laboratory results for prednisone-exposed and prednisone-free