Abstract
Objective:
Fontan surgical planning is an image-based, collaborative effort which is hypothesized to result in improved patient outcomes. A common motivation for Fontan surgical planning is the progression (or concern for progression) of pulmonary arteriovenous malformations (PAVMs). The purpose of this study is to evaluate the accuracy of surgical planning predictions, specifically hepatic flow distribution (HFD, a known factor in PAVM progression), and identify methodological improvements needed to increase prediction accuracy.
Methods:
Twelve single ventricle patients who were enrolled in a surgical planning protocol for Fontan surgery with pre- and post-operative cardiac imaging are included in this study. Computational fluid dynamics were used to compare HFD in both the surgical planning prediction and actual post-operative conditions.
Results:
Overall, HFD prediction error was 17±13%. This error was similar between surgery types (15±18% and 18±10% for revisions vs Fontan completions respectively, p=0.73), but was significantly lower (6±7%, p=0.05) for hepatic to azygous shunts. Y-grafts and extracardiac conduits showed a strong correlation between prediction error and discrepancies in graft insertion points (r=0.99, p<0.001). Improving post-operative anatomy prediction significantly reduced overall HFD prediction error to 9±6% (p=0.03).
Conclusions:
While Fontan surgical planning can offer accurate HFD predictions for specific graft types, methodological improvements are needed to increase overall accuracy. Specifically, improving post-operative anatomy prediction was found to be an important target for future work. Future efforts and refinements to the surgical planning process will benefit from an improved understanding of the current state and will rely heavily on increased follow up data.
Keywords: Fontan, congenital heart defect, surgical planning, hepatic flow distribution, preprocedural planning, pulmonary arteriovenous malformations
Central Message:
Fontan surgical planning accurately predicts hepatic flow distribution for specific graft types and will benefit from improved anatomical predictions.
Introduction
Fontan surgical planning is an image-based, collaborative effort which is hypothesized to result in improved patient outcomes. The general process has been detailed in multiple publications and has been implemented for select cases over the past decade.1–4 Despite its use in dozens of cases, little follow up data has been available to evaluate the accuracy of surgical planning predictions.
A common motivation for Fontan surgical planning is the progression (or concern for progression) of pulmonary arteriovenous malformations (PAVMs). PAVMs are extremely rare in the average population (2/100,000), but are much more common in Fontan patients where a poorly designed total cavopulmonary connection (TCPC) may lead to unbalanced hepatic flow distribution (HFD), a known factor in PAVM formation/progression.5–11 This type of surgical planning can involve both Fontan revision cases (a previous Fontan surgery resulted in PAVMs and therefore must be revised) as well as Fontan completion cases (Stage 2–3, often performed on patients with more complex anatomies and hence the need for added insight to determine the best surgical strategy). In either case, the purpose of Fontan surgical planning is to determine which TCPC design will result in balanced HFD. The goal to optimize HFD has led to a variety of Fontan connections.
Though limited to case studies and relatively short-term follow up data, several previous studies have provided a preliminary understanding of surgical planning prediction accuracy.2 Sundareswaran et al published the first use of surgical planning to correct PAVMs (determined by an increase in oxygen saturation) in 2009.12 Unfortunately, this single patient case report included no post-operative imaging data to compare the predicted and post-operative HFD. Haggerty et al provided the most thorough study to date which included only four patients with short follow up times (2 patients less than one month).13 This study compared HFD and graft resistance and found “sufficient agreement” between the predicted and post-operative states. However, the use of steady flow conditions and fixed outlet flow splits, as well as the small sample size, limit the generalizability of these findings.
The purpose of this study is to evaluate the accuracy of Fontan surgical planning predictions and identify which methodological improvements are needed to improve prediction accuracy. Specifically, this study focuses on the prediction of hepatic flow distribution. Both pre- and post-operative imaging data from patients enrolled in the surgical planning process are used to address these questions. This study offers the first assessment of prediction accuracy and methodological shortcomings for both Fontan revisions and Fontan completion cases using longer term follow up data not available previously.
Methods
Patient Selection
A total of 12 single ventricle patients were included in this study. All patient data were received from the Children’s Hospital of Philadelphia and Children’s Healthcare of Atlanta under IRB approval (H17434 and H09279, respectively) with a waiver of consent. Inclusion criteria were that the patient: (i) was enrolled in the surgical planning process prior to surgery, (ii) patient had pre-and post-operative cardiac magnetic resonance (CMR) and phase contrast CMR imaging, and (iii) imaging quality was sufficient for accurate anatomical segmentation as well as flow segmentation at every TCPC inlet/outlet. This resulted in both Fontan revision cases (n=5) and Fontan completion cases (n=7). All patients in this study were enrolled in surgical planning due to PAVM development or the concern for PAVM development due to atypical vasculature or clinical history. Clinical data including age, gender, body surface area (BSA), imaging and surgery dates, and diagnosis were obtained for each patient.
MRI Acquisition
All CMRs were performed with a Siemens 1.5 T magnetic resonance imaging system (Siemens Medical Solutions, Malvern PA). Patients were scanned supine, head first in the scanner with ECG leads placed. After localizers were obtained, a stack of contiguous, static, diastolic steady state free precession images were obtained from the diaphragm to thoracic inlet to assess anatomy and provide inputs for CFD modeling. Slice thickness was generally 3–4 mm and in plane resolution was 1 × 1 mm.
Through plane, retrospectively gated, phase contrast magnetic resonance (PCMR) was used to assess flows in the cavae, branch pulmonary arteries and across the aortic valve. IVC flow was measured supra-hepatic. Velocity encoding was generally 150 cm/sec for the aorta and 60 cm/sec for the other vessels (SVC, Fontan baffle, RPA and LPA). Slice thickness was generally 3 mm with in plane resolution of 1 × 1 mm. The number of phases was a function of the heart rate and ranged from 20–30. Post-operative CMRs were acquired at the follow up times specified in Table 1.
Table 1.
Demographic and surgical data.
| Patient ID | Gender | Diagnosis | Surgery Type | PAVMs* | Surgical Option | Age at surgery (years) | Age at follow up (years) | Follo w up (mont hs) | HFD prediction (%LPA) | HFD post-op (%LPA) | HFD Error |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | F | H, PA | Revision | Left | Hep to AZ | 4.7 | 12.8 | 97.8 | 60 | 56 | 4 |
| Patient 2 | F | H | Revision | Left | Y-graft | 19.0 | 19.0 | 0.3 | 38 | 45 | 7 |
| Patient 3 | M | H, D | Revision | Right | Hep to AZ | 11.6 | 15.0 | 40.4 | 100 | 100 | 0 |
| Patient 4 | F | U | Revision | Right | Y-graft | 12.7 | 12.8 | 1.6 | 53 | 32 | 21 |
| Patient 5 | M | H, PA | Revision | Left | ECC | 17.5 | 18.3 | 9.0 | 26 | 71 | 45 |
| Patient 6 | M | HLHS | Fontan completeon | - | ECC | 1.3 | 4.3 | 35.9 | 17 | 51 | 34 |
| Patient 7 | M | H, HLHS, | Fontan completeon | - | Y-graft | 2.6 | 2.6 | 0.2 | 27 | 48 | 21 |
| Patient 8 | F | PA, TH | Fontan completeon | - | Y-graft | 3.0 | 8.3 | 63.7 | 60 | 77 | 17 |
| Patient 9 | M | PA, DILV | Fontan completeon | - | ECC | 2.2 | 2.2 | 0.3 | 73 | 80 | 7 |
| Patient 10 | F | H | Fontan completion | - | Hep to AZ | 1.1 | 1.1 | 0.3 | 51 | 56 | 5 |
| Patient 11 | F | H, DORV | Fontan completion | - | Hep to AZ | 3.2 | 4.2 | 11.7 | 71 | 87 | 16 |
| Patient 12 | F | H, HLHS | Fontan completion | - | Hep to Inn | 1.4 | 1.5 | 2.1 | 48 | 25 | 23 |
The pulmonary arteriovenous malformations (PAVMs) column indicates which lung contained the malformations. Diagnoses are abbreviated as heterotaxy (H), pulmonary atresia (PA), dextrocardia (D), hypoplastic left heart syndrome (HLHS), tricuspid hypoplasia (TH), double inlet left ventricle (DILV), unbalanced canal (U) and double outlet right ventricle (DORV). Surgical options are abbreviated as hepatic to azygous shunt (Hep to AZ), extracardiac conduit (ECC) and hepatic to innominate vein (Hep to Inn). HFD is hepatic flow distribution.
Anatomic Reconstruction and Blood Flow Segmentation
Patient-specific anatomies were reconstructed from axial CMR images using methods previously developed.14,15 Geomagic Studio® (Geomagic Inc., Research Triangle Park, NC) was used to fit a surface around the reconstructed point-cloud and export the surface for mesh generation. Patient-specific blood flow waveforms were segmented from PCMR images for all vessels of interest using previously validated methods.16,17 Pulmonary flow distribution (PFD) is calculated as where Q is flow rate. Changes in flows pre- to post-operative are calculated as to show absolute magnitude changes in flow rates.
Surgical Planning Prediction
For complex Fontan cases, surgical planning has been used to compare fundamentally different surgical options (extracardiac conduit vs Y-graft vs hepatic to azygous etc.). In addition, this process is used to determine which location on the pulmonary arteries the graft should be anastomosed. SURGEM III, a solid modeling software designed specifically for Fontan surgical planning, was used to generate the surgical planning anatomy prediction.18 First, the pre-operative TCPC (for Fontan revisions) or bidirectional Glenn (for Fontan completion) and hepatics were imported. With input from the respective clinician, the desired surgical option was then created and exported as a surface mesh. Pre-operative flow waveforms reconstructed from PC-MRI were directly used as the “predicted” flow waveforms. This technique makes the simplifying assumption that post-operative flows will be identical to pre-operative flows. It is important to mention that during the surgical planning process, multiple surgical options are created. However, for the sake of comparing surgical planning predictions with post-operative data, only the prediction of the actual implemented surgical option is considered here.
Computational Fluid Dynamics
The 3D anatomy (from either the pre-op scan, surgical planning prediction, or postoperative scan) was imported into ANSYS workbench, where vessel extensions of length 10*(vessel diameter) were added to overcome entrance effects and establish an appropriate velocity profile. A polyhedral mesh of approximately DIVC /20 mm elements was used in order to achieve mesh independent results, where DIVC is the diameter of the IVC.19 All simulations were performed using ANSYS Fluent (Release 17.1) which is a finite volume pressure-based NavierStokes solver. Blood was modeled as a single-phase Newtonian fluid (μ=0.04 g/(cm·s), ρ=1.06 g/cm3). The appropriate patient specific blood flow waveforms extracted from PC-MRI were used as boundary conditions for each TCPC inlet and outlet19,20. Twenty cardiac cycles were simulated to overcome transition effects and achieve period stability, using the final cycle for data analysis.
To investigate the potential accuracy of surgical planning if methodological improvements allowed for more accurate anatomy and flow predictions, two additional simulations were run representing either “improved” anatomy or flow predictions. This resulted in four simulations for each patient:
Predicted (simulation uses predicted anatomy and predicted flows)
Actual post-operative (simulation uses post-operative anatomy and post-operative flows)
“Improved” anatomy prediction (simulation uses actual post-operative anatomy and predicted flows) The post-operative anatomy represents a perfect anatomical prediction from the surgical planning process.
“Improved” flow prediction (simulation uses predicted anatomy and actual postoperative flows) The post-operative flows represent a perfect flow prediction from the surgical planning process.
In addition, pre-operative simulations (pre-op anatomy and pre-op flows) were also run for Fontan revision cases in order to investigate the relationship between pre-operative HFD and PAVM progression.
Hepatic Flow Distribution
Hepatic flow distribution was quantified by seeding massless particles at the IVC and calculating the total flux of particles leaving the left and right pulmonary arteries. Hepatic flow distribution is defined as where θ is the total flux of particles throughout a cardiac cycle. The error in HFD prediction is defined as .
Anatomy Comparison
To compare the predicted and post-operative anatomies, the TCPCs were first registered to account for differences in imaging coordinate systems. A mesh comparison software (CloudCompare, version 2.10) was then used to quantify average and maximum deviations between the surfaces of the two TCPCs. This was done for both the full TCPC and the graft alone. Graft insertion offset was calculated by measuring the distance between the predicted and post-operative anastomosis locations (distance between center points of each anastomosis). For Y-grafts, the largest insertion offset of the two branches was used.
Statistical Analysis
SPSS (IBM Corp., Version 25, Armonk, NY) was used for statistical analyses. The Shapiro-Wilk test was used to determine normality for each parameter. Pearson’s and Spearman’s correlations were used to investigate bivariate correlations between HFD prediction error and clinical, hemodynamic and anatomic parameters for linear and monotonic relationships respectively. Depending on normality, either a Wilcoxon rank sum test or a two-sample t-test was used to test for equal medians between the revision and Fontan completion groups, as well as between various surgical connection types. A paired-sample t-test was used to test for differences between surgical planning methodologies (current vs improved anatomy/flow). Statistical significance was determined using p<0.05. Values are shown as average ± standard deviation [median, IQR].
Results
Clinical Data
Clinical and surgical data are given in Table 1. The cohort consisted of 5 Fontan revisions and 7 Fontan completion cases. Implemented surgical options included 4 hepatic to azygous shunts, 4 Y-grafts, 3 traditional extracardiac conduits and 1 hepatic to innominate vein connection. Average follow up time was 22±32 [6, 39] months.
Revisions vs Fontan completions
Age at surgery and age at follow up were significantly different between the revision (13.1±5.7 [12.7, 10.1] and 15.6±2.9 [15.0, 5.8] years) and Fontan completion (2.1±0.9 [2.2, 1.7] and 3.5±2.5 [2.6, 2.8] years) cases respectively (p<0.001 for both, Table 2). Follow up time was not significantly different between the revision and Fontan completion cases (30±41 [9, 68] and 16±25 [2, 64] months respectively, p=0.49, Table 2). No significant differences in pre- to postoperative changes in flow rates were seen between the revision and Fontan completion cases (Table 2). Additionally, no flow rates (grouped by surgery type or vessel) showed consistent directionality in flow rate changes. Significant differences in geometric variations (between the predicted and actual post-operative anatomy) were seen between the revision and Fontan completion cases both in terms of the TCPC as a whole and the graft alone (Table 2).
Table 2.
Fontan revision and Fontan completion comparison.
| Revision | Fontan completion | p-value | |
|---|---|---|---|
| Age at surgery (yrs) | 13.1 ±5.7 [12.7, 10.1] | 2.1±0.9 [2.2, 1.7] | <0.001 |
| Age at follow up (yrs) | 15.6±2.9 [15.0, 5.8] | 3.5±2.5 [2.6, 2.8] | <0.001 |
| Follow up time (months) | 30±41 [9, 68] | 16±25 [2, 64] | 0.492 |
| HFD prediction error | 15±18 [7, 31] | 18±10 [17, 16] | 0.795 |
| IVC flow change (L/min) | 0.22±0.16 [0.15, 0.32] | 0.25±0.19 [0.17, 0.33] | 0.782 |
| SVC flow change (L/min) | 0.35±0.13 [0.4, 0.24] | 0.44±0.32 [0.34, 0.56] | 0.573 |
| AZ flow change (L/min) | 0.61±0.45 [0.63, 0.82] | 0.20±0.17 [0.2, 0.32] | 0.104 |
| LPA flow change (L/min) | 0.57±0.19 [0.57, 0.32] | 0.48±0.37 [0.35, 0.67] | 0.638 |
| RPA flow change (L/min) | 0.80±0.60 [0.91, 1.13] | 0.25±0.22 [0.14, 0.39] | 0.052 |
| IVC flow change (%) | 42±42 [35, 62] | 41±26 [32, 35] | 0.955 |
| SVC flow change (%) | 36±21 [35, 40] | 48±29 [52, 44] | 0.462 |
| AZ flow change (%) | 49±30 [51, 58] | 40±28 [48, 52] | 0.656 |
| LPA flow change (%) | 38±21 [30, 41] | 59±35 [64, 38] | 0.274 |
| RPA flow change (%) | 62±40 [55, 58] | 41±53 [19, 32] | 0.471 |
| PFD change (%) | 9±9 [4, 14] | 14±6 [13, 12] | 0.261 |
| TCPC deviation (mm) | 3.3±0.8 [3, 1.4] | 1.6±0.5 [1.5, 0.9] | 0.001 |
| TCPC max deviation (mm) | 17.6±2.0 [19, 3.5] | 9.2±3.7 [9.4, 4.1] | 0.004 |
| Graft deviation (mm) | 5.6±2.8 [6.3, 5.1] | 2.8±1.9 [2.7, 3.0] | 0.062 |
| Graft max deviation (mm) | 15.3±4.4 [15.5, 8.3] | 9.3±4.5 [9.4, 6.2] | 0.046 |
| Graft insertion offset (mm) | 13.5±8.3 [15.0, 16.1] | 5.8±4.5 [6.8, 7.5] | 0.063 |
Change represents absolute difference between pre-operative and post-operative flows. Hepatic flow distribution (HFD); inferior and superior vena cava (IVC and SVC); left and right pulmonary artery (LPA and RPA); azygous vein (AZ); pulmonary flow distribution (PFD); total cavopulmonary connection (TCPC).
HFD Prediction Error
The predicted and post-operative HFD can be found in Table 1. Overall, HFDprediction error was 17±13 [17, 17]%, and was not significantly different between revisions (15±18 [7, 31]%) and Fontan completion (18±10 [17, 16]%) cases (p=0.73, Table 2). Fontan completion predictions underestimated HFD in 6/7 cases, while revisions were evenly split between overestimations and underestimations. CFD results comparing the predicted and post-operative streamlines for all Fontan revisions and Fontan completion cases are shown in Figures 1–2 respectively. In addition, Figure 1 shows the pre-operative HFD for all Fontan revisions, confirming a lack of hepatic flow to the lung with PAVMs. Overall, no significant correlations were found between HFD prediction error and age at surgery, age at follow up, or follow up time. Moderate correlations were seen between the percent change in IVC flow rate (r=0.60, p=0.04) and the change in PFD (r=0.60, p=0.04) from pre- to post-op with HFD prediction error.
Figure 1:
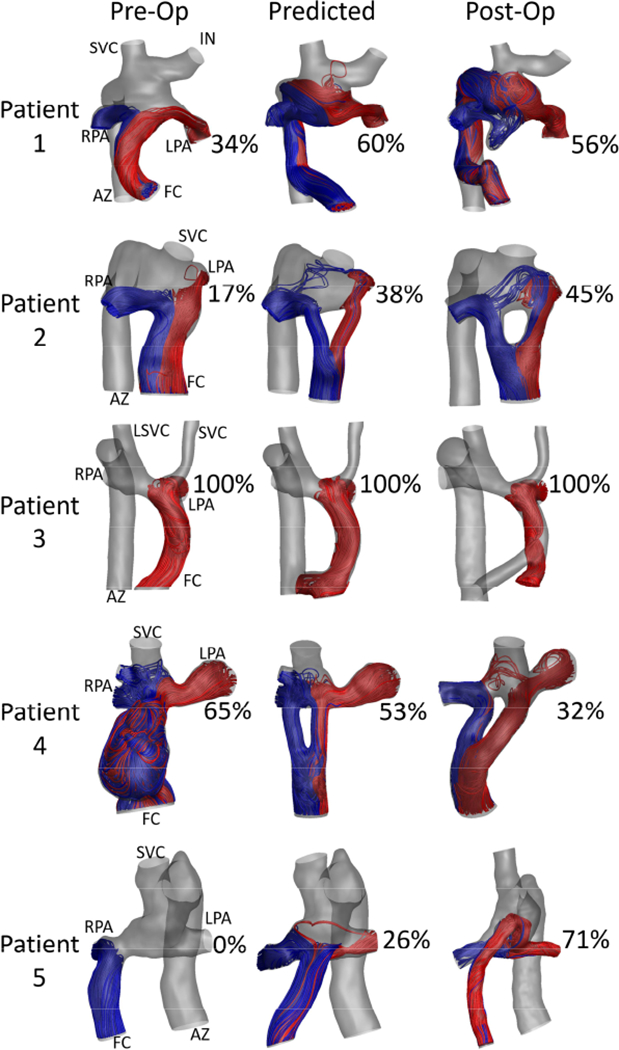
Streamline comparison for the pre-operative, predicted, and post-operative states for all Fontan revision cases. Patients 1, 2 and 5 were diagnosed with left-sided pulmonary arteriovenous malformations (PAVMs), and Patients 3 and 4 with right-sided PAVMs. PAVMs regressed in each case where the revision resulted in increased hepatic flow to the affected lung. Hepatic flow distribution (HFD) is noted as the percent of HFD to the left pulmonary artery. Vessels are labeled as FC: Fontan conduit, SVC: superior vena cava, LSVC: left superior vena cava, AZ: azygous vein, LPA and RPA: left and right pulmonary artery, IN; innominate vein.
Figure 2:
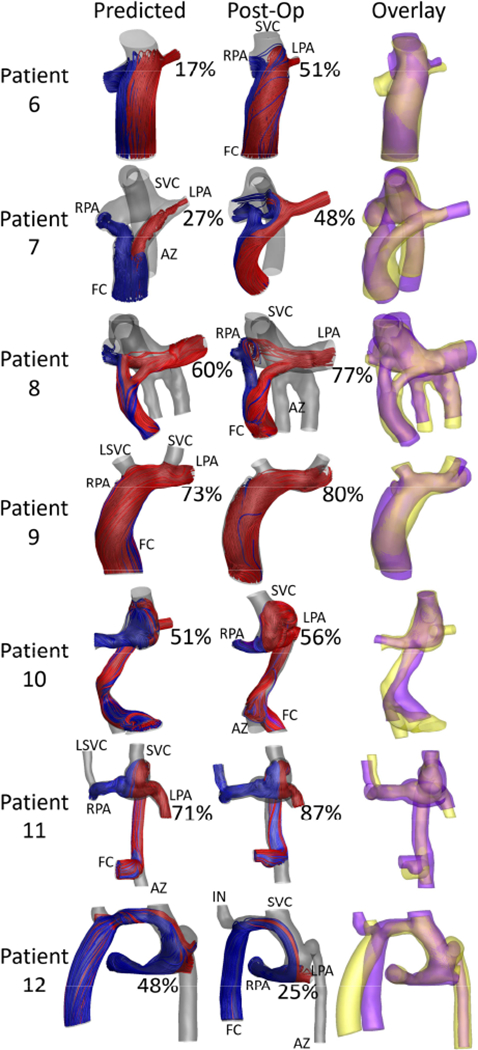
Streamline comparison between the predicted and post-operative states for all Fontan completion patients. Hepatic flow distribution (HFD) is noted as the percent of HFD to the left pulmonary artery. The overlay column compares the predicted (yellow) and post-operative (purple) anatomies. Vessels are labeled as FC: Fontan conduit, SVC: superior vena cava, LSVC: left superior vena cava, AZ: azygous vein, LPA and RPA: left and right pulmonary artery, IN: innominate vein.
Connection types
HFDprediction error was found to be associated with surgical connection type. A comparison of HFDprediction error between graft types can be seen in Figure 3. Hepatic to azygous shunts had significantly lower prediction errors than other connection types (6±7 [5, 12]% vs 22±13 [21, 22]% respectively, p=0.05, Figure 3b). In addition, a strong, positive correlation was seen between HFD prediction error and graft insertion offset within the Y-graft and ECC groups (r=0.99, p<0.001, Figure 4d). Example cases of low, moderate and high graft insertion offsets are shown in Figure 4a-4c.
Figure 3:
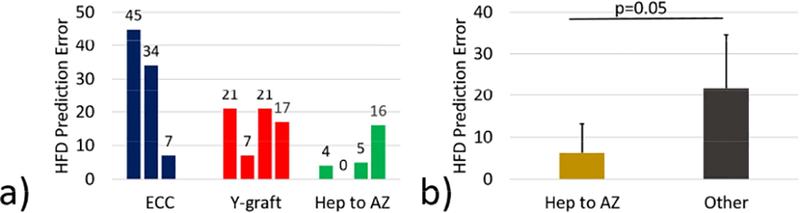
Effect of connection type on hepatic flow distribution (HFD) prediction error. (a) High variability in prediction error was observed within and across graft types. (b) Hepatic to azygous (Hep to AZ) shunts showed significantly lower HFD prediction errors than other connection types. ECC: extracardiac conduit.
Figure 4:
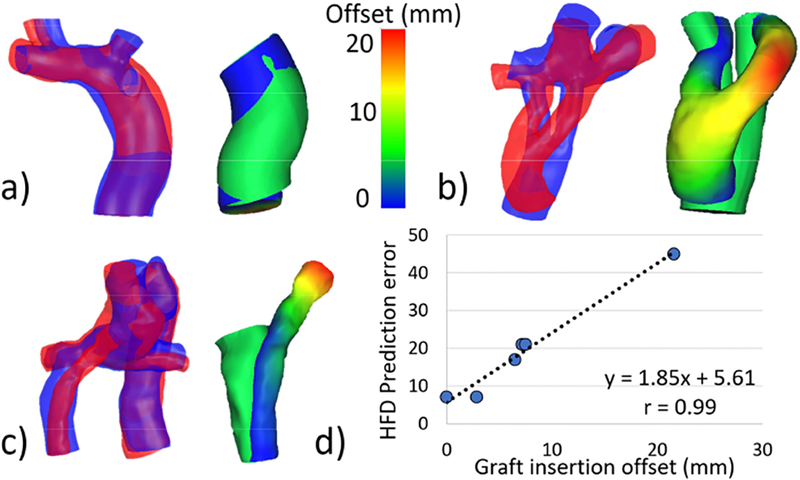
Relationship between hepatic flow distribution (HFD) prediction error and graft insertion offset for extracardiac conduit and Y-graft Fontan connections. Graft insertion offset describes the distance between the predicted and implemented graft insertion locations. Representative cases are shown for (a) low, (b) moderate, and (c) high graft insertion offsets. A strong correlation (d) was seen between prediction error and offset for these connection types. The overlay figures (panels a-c) compare the predicted (blue) and post-operative (red) TCPCs on the left, and show a colormap of the offset between the predicted and post-operative grafts on the right for each representative case. All cases use the same color scale. TCPC: total cavopulmonary connection.
Potential methodological improvements
The HFD prediction error associated with the “improved” post-operative anatomy and flow predictions and the current methodology is shown in Table 3. While a reduced or identical prediction error was seen for the majority of patients using either methodological improvement, (8/12 and 7/12 for the improved anatomy and improved flow scenarios respectively), a more substantial reduction in error was seen when using the improved anatomy. The current HFD prediction error (17±13 [17, 17]%) was significantly reduced by improving anatomy prediction (9±6 [9, 12]%, p=0.03, paired-sample t-test), but remained nearly the same when using only an improved flow prediction (18±17 [14, 24]%, p=0.73, paired-sample t-test). When comparing the two potential methodological improvements, improved post-operative anatomy prediction resulted in equivalent or more accurate HFD predictions for 9/12 patients when compared with improved flow prediction.
Table 3.
Comparison of HFD prediction errors between the current surgical planning process and potential methodological improvements.
| HFD Prediction Error | ||||||
|---|---|---|---|---|---|---|
| Patient ID | Surgery Type | Vessels present | Connection Type | Current | Improved anatomy prediction | Improved flow prediction |
| Patient 1 | Revision | AZ, INN | Hep to AZ | 4 | 6 | 11 |
| Patient 2 | Revision | AZ | Y-graft | 7 | 13 | 1 |
| Patient 3 | Revision | AZ, LSVC | Hep to AZ | 0 | 0 | 0 |
| Patient 4 | Revision | - | Y-graft | 21 | 10 | 29 |
| Patient 5 | Revision | AZ | ECC | 45 | 16 | 58 |
| Patient 6 | Fontan completion | - | ECC | 34 | 18 | 18 |
| Patient 7 | Fontan completion | AZ | Y-graft | 21 | 7 | 38 |
| Patient 8 | Fontan completion | AZ | Y-graft | 17 | 6 | 14 |
| Patient 9 | Fontan completion | LSVC | ECC | 7 | 13 | 5 |
| Patient 10 | Fontan completion | AZ | Hep to AZ | 5 | 2 | 3 |
| Patient 11 | Fontan completion | AZ, LSVC | Hep to AZ | 16 | 3 | 23 |
| Patient 12 | Fontan completion | AZ, INN | Hep to Inn | 23 | 18 | 13 |
| Average | - | - | - | 17±13 [17, 17] | 9±6 [9, 12]* | 18±17 [14, 24] |
The presence of any non-standard vessels (in addition to the inferior/superior vena cava and left/right pulmonary artery) is indicated in the vessels present column. Hepatic flow distribution (HFD); hepatic to azygous shunt (Hep to AZ); extracardiac conduit (ECC); hepatic to innominate connection (Hep to Inn); left superior vena cava (LSVC). Asterisk indicates statistically significant difference (paired sample t-test) from the current method.
Discussion
Previously limited by a lack of post-operative data, the current study offers the first assessment of prospective Fontan surgical planning accuracy for both Fontan revisions and Fontan completions using medium-term post-operative data. This study incorporates a unique data set resulting from more than a decade of surgical planning experience and provides a methodological assessment necessary for the improvement of surgical planning accuracy.
Though an exact “cut-off” for HFD to prevent PAVMs is currently unknown, and may vary between patients, the Fontan revision results (Table 1, Figure 1) confirm a lack of hepatic flow to the lung with PAVMs in each case. Furthermore, PAVMs regressed in each case where the revision resulted in increased hepatic flow to the affected lung. In combination with previous studies, these results emphasize the importance of achieving a balanced hepatic flow distribution through appropriate TCPC design.
In this study, HFD prediction error averaged 17±13 [17, 17]% across all patients. Prediction error was similar between revision and Fontan completion cases, but differed across connection types. Intuitively, hepatic to azygous connections are more robust to variations in surgical implementation since all hepatic flow will join the azygous flow and then travel through the entire azygous vein before interacting with other flows regardless of exact placement of the shunt. No colliding flows from multiple vessels are present locally in hepatic to azygous connections, in contrast with Y-graft and ECC connections where slight offsets may substantially change the interactions between various inlets and therefore stray from predicted results (Figure 4d).21 Therefore, hepatic to azygous predictions were found to be quite accurate, while predictions for other connection types were more varied.
Capitalizing on the available post-operative data, various simulations were run using the post-operative anatomy or flows as a surrogate for an “improved” prediction. While it is unlikely that anatomy or flow prediction techniques will ever produce exact matches to postoperative outcomes, this analysis is instructive by showing the full potential of surgical planning accuracy if methodological improvements in either of these areas offered extremely accurate predictions. To reiterate, improvements in anatomy prediction led to a significant (p=0.03) reduction in HFD prediction error. Interestingly, improvements in flow prediction did not result in similar error reduction (Table 3). These findings stress two critical points: (i) post-operative anatomy prediction is a primary factor in HFD prediction, and (ii) anatomy prediction methods must improve in order for Fontan surgical planning to provide more accurate HFD predictions.
Again, improved flow prediction alone did not result in more accurate HFD predictions (p=0.73, Table 3). In general, HFD is primarily driven by graft placement.22 In a complex connection such as the TCPC, relatively small offsets in graft placement and angulation can largely alter the collisions and interactions among flows from various vessels.22 These variations can affect the preferential streaming of inlet flows including hepatic flow, which in turn will determine hepatic flow distribution. Naturally, severe changes in individual flow rates can affect HFD prediction; however, this was not observed in this cohort.
Though it is common to use indexed flow rates (normalized by BSA) in pediatric studies involving changes over time, raw flow rates are shown in this study (Table 2) to emphasize the changes in actual inputs to the surgical planning process. While an indexed flow rate may remain constant over a several year follow up, the actual flow rate (and therefore the flow rate that needs to be predicted) does not. This raw data better represents how boundary conditions for the surgical planning process change over time.
Multiple methods exist to predict post-operative Fontan anatomies and flows, ranging from simple (basic CAD software and using pre-operative flows as the “predicted” post-op flows) to more sophisticated (designated surgical planning software and lumped parameter modeling) methods.18,23 As the methods have progressed in complexity, anatomy prediction methods have become faster (software is designed specifically for Fontan surgical planning) and flow prediction methods have become slower (more complex calculations and “full body” modeling). Meeting the clinical timeline for most surgical planning cases requires accelerated analysis.1 Conveniently, the present results indicate that anatomy prediction is a primary shortcoming, which can hopefully be improved without lengthening the surgical planning process.
Accurate anatomy prediction involves both predicting a viable surgical option and accurately implementing that option. Modeling a viable surgical option is heavily dependent on high quality imaging data, clinician involvement and inclusion of relevant anatomical landmarks. Current methods include the heart, aorta and pulmonary circulation, but future efforts could potentially add additional organs and the process of chest closure. Once a surgeon has selected the surgical option to implement, closely replicating that option in vivo may be challenging. Little intra-operative guidance is currently offered as part of the surgical planning process. Some efforts have explored 3D printing and augmented/virtual reality as planning/guidance tools, but further refinements are needed.24–27 In addition, growth is another difficult factor to model that may be necessary to improve surgical planning results.28,29 It is possible that “variations” between a predicted and post-operative anatomy are due to growth rather than “imperfect” surgical implementation.
Finally, Patient 3 is a unique case which deserves attention. As shown in Figure 1, this patient had 100% HFD to the left lung in the pre-operative state. This led to right-sided PAVMs and the need for a Fontan revision. Also shown in Figure 1, the surgical option implemented during the Fontan revision similarly resulted in 100% HFD to the left lung. This was predicted during the surgical planning process but implemented nonetheless due to other concerns. Importantly, not all surgical options for Patient 3 resulted in 100% HFD to the left lung. This case illustrates the importance of being able to predict which options will perform poorly in addition to which ones perform well. This case also shows that surgical planning predictions are not always the main determinant for decision making in current clinical practice. Assessing how surgical planning affects clinical decision-making and patient outcomes is an important next step once surgical planning predictions are known to offer accurate results.
If Fontan surgical planning is to be used in clinical practice, the importance and necessity of follow up data and validation studies such as this cannot be overstated. Understanding the current accuracy and methodological shortcomings is imperative in order to correctly use the results and progress the field. Future efforts and refinements to the surgical planning process will greatly benefit from an improved understanding of the current state-of-the-art.
Limitations
Though important conclusions can be drawn from this study, a more complete understanding of surgical planning accuracy and methodological needs with increased statistical power will require a substantial amount of data most likely through a multi-center study. Additionally, the current study includes a broad range of follow up times and offers only a “snapshot” of the patients’ post-operative hemodynamics. If available, the inclusion of serial data in a similar study could offer a better understanding of hemodynamic changes over time. The predicted results in this study are representative of the specific surgical planning process used. Results may vary with other prediction techniques. However, this study employs one of the most advanced anatomy prediction methods and still concludes that post-operative anatomy prediction is a limiting factor, which is unlikely to change based on prediction technique. Finally, two experienced surgeons were involved in these surgical planning cases. While we saw no differences in prediction accuracy between the two surgeons, it is possible that results may vary based on the surgeon involved. Finally, the results from this study do not indicate how the surgical strategy changed due to the surgical planning process. A surgical strategy was not developed prior to the surgical planning process for each case to determine how surgical planning influenced the original plan. Therefore, these results report prediction accuracy and do not quantify the impact on clinical decision making.
Conclusions
Overall, HFD prediction error was 17±13%. This error was similar between Fontan revisions and Fontan completions, but varied across surgical connection types. While Fontan surgical planning can offer accurate HFD predictions for specific graft types, methodological improvements are needed to increase overall accuracy. Specifically, improving post-operative anatomy prediction was found to be an important target for future efforts that would substantially improve flow field modeling, and therefore increase HFD prediction accuracy. Future efforts and refinements to the surgical planning process will greatly benefit from an improved understanding of the current state-of-the art and will rely heavily on increased follow up data.
Supplementary Material
Central picture legend:
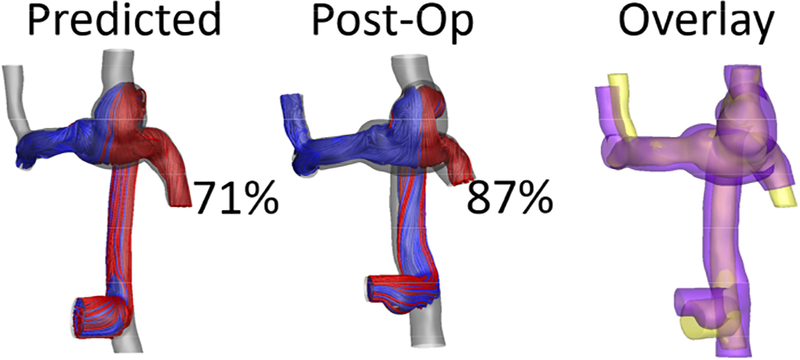
Comparison of predicted and post-op hepatic flow distribution (indicated by percentage) for a representative patient. The overlay shows predicted (yellow) and postoperative (purple) anatomies.
Video legend:
Discussion of surgical planning predictions, accuracy, and implications by Dr. Timothy Slesnick.
Perspective Statement:
Fontan surgical planning can provide accurate predictions of hepatic flow distribution for specific graft types. Anatomical prediction was found to be a key methodological shortcoming in the surgical planning process. With continued improvements, surgical planning may be a useful tool to avoid pulmonary arteriovenous malformations in Fontan patients.
Acknowledgements
The authors acknowledge the use of ANSYS software which was provided through an Academic Partnership between ANSYS, Inc. and the Cardiovascular Fluid Mechanics Lab at the Georgia Institute of Technology. We also acknowledge Dr. Jarek Rossignac from the College of Computing at Georgia Tech for his contributions to the development of the SURGEM III platform.
Sources of Funding:
This work was partially supported by NIH grants R01 HL067622and HL098252, as well as an American Heart Association Predoctoral Fellowship 17PRE33630117.
Abbreviations:
- AZ
azygous vein
- CFD
computational fluid dynamics
- CMR
cardiac magnetic resonance
- HFD
hepatic flow distribution
- IVC
inferior vena cava
- LPA
left pulmonary artery
- PAVM
pulmonary arteriovenous malformation
- PFD
pulmonary flow distribution
- RPA
right pulmonary artery
- SVC
superior vena cava
- TCPC
total cavopulmonary connection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
None of the authors have potential conflicts of interest in relation to the presented work.
Disclosures:
Phillip Trusty - none; Alan Wei - none; Timothy Slesnick – none; Kirk Kanter – none; Thomas Spray – none; Mark Fogel – Research grant, modest; Ajit Yoganathan – none.
References
- 1.Trusty PM, Slesnick TC, Wei ZA, et al. Fontan Surgical Planning : Previous Accomplishments, Current Challenges, and Future Directions. Journal of Cardiovascular Translational Research. 2018;1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fogel MA, Khiabani RH, Yoganathan A. Imaging for preintervention planning pre- and post-fontan procedures. Circ Cardiovasc Imaging. 2013;6:1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slesnick TC, Yoganathan AP. Computational modeling of Fontan physiology: At the crossroads of pediatric cardiology and biomedical engineering. Int J Cardiovasc Imaging. 2014;30:1073–1084. [DOI] [PubMed] [Google Scholar]
- 4.de Zélicourt DA, Marsden A, Fogel MA et al. Imaging and patient-specific simulations for the Fontan surgery: Current methodologies and clinical applications. Prog Pediatr Cardiol. 2010;30:31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan BW, Desai S. Pulmonary arteriovenous malformations after cavopulmonary anastomosis. Ann Thorac Surg [Internet]. 2003. [cited 2014 Sep 12];76:1759–1766. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0003497503004508 [DOI] [PubMed] [Google Scholar]
- 6.Khurshid I, Downie GH. Pulmonary arteriovenous malformation. Postgrad Med J. 2002;78:191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srivastava D, Preminger T, Lock JE et al. Hepatic Venous Blood and the Development of Pulmonary Arteriovenous Malformations in Congenital Heart Disease. Circulation. 1995;92:1217–1222. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein HS, Brook MM, Silverman NH et al. Development of Pulmonary Arteriovenous Fistulae in Children After Cavopulmonary Shunt. Circulation. 1995;92:309 LP–314. [DOI] [PubMed] [Google Scholar]
- 9.Shinohara T, Yokoyama T. Pulmonary Arteriovenous Malformation in Patients with Total Cavopulmonary Shunt: What Role Does Lack of Hepatic Venous Blood Flow to the Lungs Play? Pediatr Cardiol. 2001;22:343–346. [DOI] [PubMed] [Google Scholar]
- 10.McElhinney DB, Marshall AC, Lang P et al. Creation of a brachial arteriovenous fistula for treatment of pulmonary arteriovenous malformations after cavopulmonary anastomosis. Ann Thorac Surg. 2005;80:1604–1609. [DOI] [PubMed] [Google Scholar]
- 11.Vettukattil JJ. Pathogenesis of pulmonary arteriovenous malformations: role of hepatopulmonary interactions. Heart. 2002;88:561–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundareswaran KS, de Zélicourt D, Sharma S et al. Correction of Pulmonary Arteriovenous Malformation Using Image-Based Surgical Planning. JACC Cardiovasc Imaging [Internet]. 2009. [cited 2014 Sep 12];2:1024–1030. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3698243&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haggerty CM, de Zélicourt DA, Restrepo M et al. Comparing Pre- and Post-operative Fontan Hemodynamic Simulations: Implications for the Reliability of Surgical Planning. Ann Biomed Eng. 2012;40:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martins T, Barbara A, Silva G et al. InVesalius: three-dimensional medical reconstruction software. Virtual rapid Manuf. 2007;135–141. [Google Scholar]
- 15.de Moraes Thiago F, Amorim PH. InVesalius-An open-source imaging application. Comput Vis Med Image Process. 2011;405. [Google Scholar]
- 16.Heiberg E, Sjögren J, Ugander M et al. Design and validation of Segment - freely available software for cardiovascular image analysis. BMC Med Imaging. 2010;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bidhult SL, Carlsson M, Steding-Ehrenborg K et al. A new method for vessel segmentation based on a priori input from medical expertise in cine phase-contrast Magnetic Resonance Imaging. J Cardiovasc Magn Reson. 2014;16:P355. [Google Scholar]
- 18.Luffel M, Sati M, Rossignac J et al. SURGEM: A solid modeling tool for planning and optimizing pediatric heart surgeries. Comput Des. 2015; [Google Scholar]
- 19.Wei ZA, Tree M, Trusty PM et al. The Advantages of Viscous Dissipation Rate over Simplified Power Loss as a Fontan Hemodynamic Metric. Ann Biomed Eng [Internet]. 2017;Available from: http://link.springer.com/10.1007/s10439-017-1950-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei ZA, Trusty PM, Tree M et al. Can time-averaged flow boundary conditions be used to meet the clinical timeline for Fontan surgical planning? J Biomech [Internet]. 2017;50:172–179. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0021929016311939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Restrepo M, Luffel M, Sebring J et al. Surgical Planning of the Total Cavopulmonary Connection: Robustness Analysis. Ann Biomed Eng [Internet]. 2015;43:1321–1334. Available from: http://link.springer.com/10.1007/s10439-014-1149-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang E, Restrepo M, Haggerty CM et al. Geometric Characterization of Patient-Specific Total Cavopulmonary Connections and its Relationship to Hemodynamics. JACC Cardiovasc Imaging [Internet]. 2014;7:215–224. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1936878X14000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marsden A, Esmaily-Moghadam M. Multiscale Modeling of Cardiovascular Flows for Clinical Decision Support. Appl Mech Rev [Internet]. 2015;67:1–11. Available from: http://appliedmechanicsreviews.asmedigitalcollection.asme.org/article.aspx?doi=10.1115/1.4029909 [Google Scholar]
- 24.Sodian R, Weber S, Markert M et al. Pediatric cardiac transplantation: Three-dimensional printing of anatomic models for surgical planning of heart transplantation in patients with univentricular heart. J Thorac Cardiovasc Surg. 2008;136:1098–1099. [DOI] [PubMed] [Google Scholar]
- 25.Ventola CL. Medical Applications for 3D Printing: Current and Projected Uses. P T. 2014;39:704–711. [PMC free article] [PubMed] [Google Scholar]
- 26.McCloy R, Stone R. Science, medicine, and the future. Virtual reality in surgery. BMJ. 2001;323:912–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seymour NE, Gallagher a G, Roman SA et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg. 2002;236:454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Restrepo M, Mirabella L, Tang E et al. Fontan pathway growth: A quantitative evaluation of lateral tunnel and extracardiac cavopulmonary connections using serial cardiac magnetic resonance. Ann Thorac Surg [Internet]. 2014;97:916–922. Available from: 10.1016/j.athoracsur.2013.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Restrepo M, Tang E, Haggerty CM et al. Energetic Implications of Vessel Growth and Flow Changes Over Time in Fontan Patients. Ann Thorac Surg [Internet]. 2015;99:163–170. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0003497514017482 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


