Abstract
Very early onset inflammatory bowel disease (VEO-IBD) represents a diagnostic and treatment challenge. Here we present a case of VEO-IBD secondary to a mutation in BIRC4 gene, which encodes X-linked inhibitor of apoptosis protein (XIAP), in a 17 month-old male with severe failure to thrive, intractable diarrhea and hepatosplenomegaly. Endoscopy and histology identified only mild duodenitis and ileitis, but severe pancolitis with crypt abscesses and epithelium apoptosis. Minimal improvement in symptoms was achieved with total parenteral nutrition (TPN), intravenous (IV) corticosteroids and tacrolimus, whereas induction and maintenance therapy with adalimumab led to complete remission. After 6 months, the patient developed hemophagocytic lymphohistiocytosis (HLH) and eventually died due to multi-system organ failure. A review of the literature revealed that some patients with VEO-IBD secondary to XIAP deficiency develop symptoms that are refractory to medical and surgical management, while initial reports suggest that allogeneic hematopoietic stem cell transplantation (HSCT), with reduced intensity conditioning, can successfully induce long lasting remission and may even be curative. We propose that in patients with XIAP deficiency a constellation of symptoms including colitis at an early age, severe failure to thrive and splenomegaly/hepatosplenomegaly can identify a subgroup of patients at high-risk of experiencing medically-refractory IBD phenotype and increased mortality. Hematopoietic stem cell transplant should be considered early in these high-risk patients, as it may resolve both their intestinal inflammation and a risk of developing life threatening HLH.
Introduction:
Inflammatory bowel disease (IBD) has a bimodal age of distribution and about one quarter of all IBD cases present during childhood. In pediatric patients, location of intestinal involvement, disease severity and response to therapy have age-dependent characteristics. In fact, age of symptom onset has been proposed as a predictor of higher likelihood of an underlying monogenic etiology (1). Based on the discovery of unique disease phenotypes in patients presenting younger than 17 years of age, the Montreal classification defined pediatric-onset IBD as a distinct disease group (2). As dynamic features of pediatric disease phenotype differ in early childhood, even as compared to the adolescents, Pediatric Paris modification further subdivided pediatric-onset IBD into two age groups, one between 10 and 17 years of age and an early onset pediatric IBD group presenting before 10 years of age (3). Subsequently, further sub-classification of early onset pediatric IBD into very early onset (younger than 6 years), infantile (younger than 2 years), and neonatal IBD (first 28 days of age) subtypes has been proposed (1). Moreover, while very early onset IBD (VEO-IBD) currently represents a small fraction of patients with IBD, it is the fastest growing population of IBD patients (4).
VEO-IBD is often characterized by the presence of pancolitis, positive family history and can be associated with medically and surgically refractory disease course and increased mortality. This may be, in part, accounted by increased prevalence of monogenic causes of the disease, often manifesting as a primary immune deficiency. Indeed, genetic defects resulting in dysfunction in epithelial barrier, phagocytic defects, hyperinflammatory disorders, B and T cell defects, and immune dysregulation, including IL10 signaling defects can result in very-early onset IBD (1, 5, 6). However, about 15 percent of all pediatric IBD cases are diagnosed during very early onset period (7) and only a small fraction of these patients have an identified monogenic cause. Defects in more than 50 genes have been reported to result in a monogenic form of VEO-IBD (8–10). This complexity coupled with limited availability of genome wide testing and limitations in the technology to identify all disease causing variants can lead to a delay in initiation of genetic-etiology specific treatments and may contribute to increased morbidity and mortality in this patient population.
Mutations in BIRC4, the gene that encodes X-lined inhibitor of apoptosis (XIAP) protein, were first reported in 2006 to be associated with immune deficiency (11), and the first case of VEO-IBD secondary to a defect in this protein was reported in 2011 (12). The link between XIAP and IBD has been further strengthened by a study showing that, among 96 male patients with pediatric onset Crohn’s disease, 4 were found to have a deficiency in XIAP (13). While deficiency in XIAP protein can cause a severe form of VEO-IBD, only 17% of the patients with XIAP deficiency develop IBD-like symptoms (14). Due to variable disease penetrance in patients with XIAP deficiency, some present with severe, treatment-refractory intestinal inflammation early in childhood, while others present in mid-adulthood, with only mild intestinal symptoms. Currently, correlation between specific gene mutations and disease severity has not been recognized (19) and there are no clinical indicators to predict which patients will develop severe IBD manifestations. Furthermore, there are no standard treatment recommendations for aggressive IBD phenotype associated with this genetic mutation, even though HSCT is becoming more accepted therapy.
Here we present a male patient with VEO-IBD secondary to XIAP deficiency, who developed a severe form of VEO-IBD and died 21 months after presentation. Our review of the literature identified very early onset of colitis, severe failure to thrive and splenomegaly as poor prognostic indicators. In this specific subgroup of patients with XIAP deficiency, HSCT should be considered early in a disease course in order to prevent mortality and improve disease specific morbidity.
Case Presentation
A 17-month old male presented to our emergency department in 2012, with severe watery, but non-bloody diarrhea, profound failure to thrive (weight: 3.9kg, z-score −10.9; length: 58.0cm, z-score −8.6), and hepatosplenomegaly. He was a full-term infant, with birth weight of 2.9 kg and signs of non-immune hydrops. Diarrhea commenced at 3 months of age and was treated with trials of different formulas. By 6 months of age, he was noted to have hepatosplenomegaly and was briefly admitted for treatment of anemia and thrombocytopenia, requiring multiple transfusions. At 10 months, he started losing weight and was admitted several times for failure to thrive, dehydration, anemia, and thrombocytopenia. The patient did not have perianal fistulas/abscesses, skin infections, arthritis or fevers.
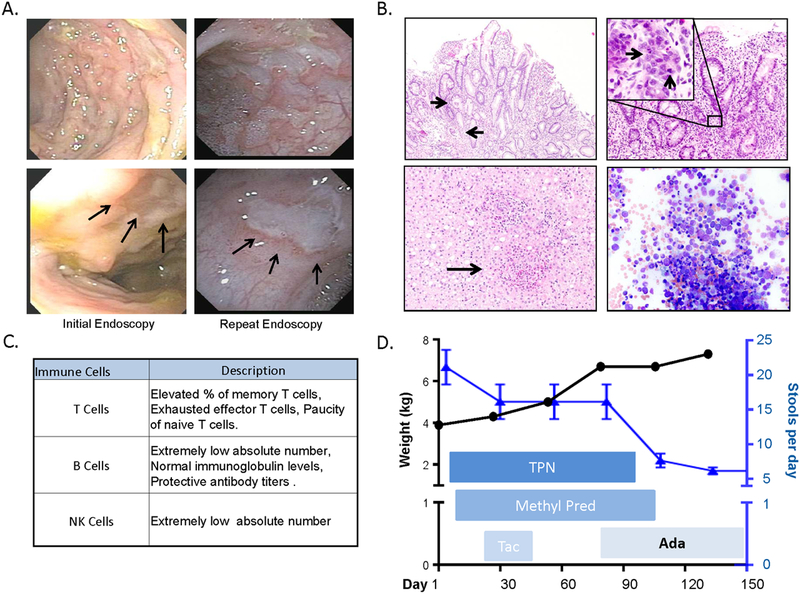
On presentation to our hospital, lab results revealed anemia and thrombocytopenia, normal liver enzymes, but low albumin and total protein. Serum inflammatory markers were elevated (Suppl. Table 1). An extensive work up revealed no infectious or metabolic etiologies. Stool studies documented elevated lactoferrin, with no evidence of carbohydrate, fat or protein malabsorption (Suppl. Table 2). Immunoglobulin levels were normal, with absence of known disease specific auto-antibodies (Suppl. Table 3). Endoscopy identified mild duodenitis, ileitis and severe pancolitis with crypt abscesses and epithelium apoptosis (Figures 1A, 1B). Treatment with total parenteral nutrition (TPN) and IV steroids was initiated, and a trial of tacrolimus was also begun, but the patient continued to have severely active disease.
Figure 1.
A. Endoscopic images of sigmoid colon (upper panels) and close up images of sigmoid colon ulcers (lower panels, arrows) during initial and repeat colonoscopies. B. Histological examination showed neutrophilic crypt abscesses (top left, arrows) and epithelial apoptosis (top right, arrows). Liver biopsy showed lobular lymphocytic inflammation, occasional non-necrotizing granulomas and marked portal fibrosis (bottom left, arrow points to granuloma). Bone marrow biopsy (bottom right) did not show hemophagocytosis. C. Summary of serum inflammatory cell subpopulations. {See supplemental table 4 for more details) D. Response to treatments in terms of weight gain {black circles) and stool output (blue triangles).
Further work-up including liver biopsy showed T cell infiltration, occasional non-necrotizing granulomas and marked portal fibrosis (Figure 1B). Cellular inclusion bodies were found on bone marrow aspirate without hemophagocytosis, excluding hemophagocytic lymphohistiocytosis (HLH) (Figure 1B). Immunological workup revealed extremely low B and NK cell levels, with normal immunoglobulin levels and protective antibody titers (Figure 1C and Suppl. Table 4). T cell profiling was notable for an elevated percentage of memory and exhausted effector T cells and a paucity of naive T cells. Regulatory T cells quantification and dihydrorhodamine assays were normal. Pro-inflammatory cytokines TNF-α, IL-6 and IL-8 were increased (Suppl. Table 4).
In agreement with serum inflammatory profile, chronic liver inflammation was characterized by predominance of CD3 positive cells, including CD4 and CD8 positive T-lymphocytes, whereas CD20 positive B-lymphocytes were remarkably absent (Suppl. Fig. 1A). Similarly, there was a marked absence of CD20 positive B-lymphocytes on intestinal biopsy despite presence of high numbers of CD3 positive inflammatory cells, including CD4 and CD8 positive T-lymphocytes (Suppl. Fig. 1B).
Three months of treatment with systemic corticosteroids and TPN failed to produce clinical improvement or evidence of intestinal mucosal healing on repeat endoscopy (Figure 1A). Due to elevation of serum TNF-α, adalimumab was chosen to address the ongoing IBD-like intestinal inflammation. Dramatic improvement was observed within 7 days of treatment, with decreased stool frequency from 10–15 to 3–5 times per day and the patient was able to tolerate full enteral nutrition within two weeks (Figure 1D).
Targeted polymerase chain reaction (PCR)-based sequence analysis of BIRC4 gene identified a frameshift mutation (p.951_961 del 11, W317fs*318) leading to premature termination in the BIR3 domain. The patient was therefore diagnosed with X-linked lymphoproliferative disorder type 2 (XLP2). Stem cell transplant was discussed and a plan made to send HLA typing and for the patient to return to Boston in 3 months for transplant planning. In spite of significant weight gain at 22 months of age, the patient’s weight was still only 7.38kg (z-score of −5.04). The patient was discharged home and returned to Puerto Rico on every two week adalimumab therapy, with instructions to return for HSCT.
Three months post-discharge, the patient was admitted to a hospital in Puerto Rico with severe diarrhea, pneumonia, and respiratory distress secondary to respiratory syncytial virus infection. Due to several out-of-state healthcare issues and the family’s work-related difficulties the patient was not able to return to our hospital soon after symptom flared up. Seven months after initial discharge, the patient was transferred to Boston Children’s Hospital with acute respiratory failure, cavitary lung lesions, gross hematuria, hemoccult positive diarrhea, increased abdominal girth and elevated liver enzymes. A repeat evaluation for HLH was performed due to its association with XLP2 and the patient’s critical illness. He met clinical HLH diagnostic criteria based on genetically proven XLP2 (BIRC4 mutation) and by the following: hepatosplenomegaly, cytopenia, elevated ferritin, high triglycerides and evidence of hemophagocytosis on bone marrow biopsy.
HLH therapy was initiated with etoposide and dexamethasone. In spite of continued respiratory failure requiring tracheostomy placement and ventilatory dependence, the patient responded well to HLH therapy and underwent unrelated, allogenic, hematopoietic stem cell transplant (HSCT) following reduced intensity conditioning with alemtuzumab, fludarabine and melphalan. Engraftment was achieved 23 days post-transplant. Respiratory failure required ongoing ICU treatment on high-frequency ventilator support, total parenteral nutrition with trophic enteral feeds, as well as broad spectrum antibiotic/antifungal coverage. The patient eventually died from multi-organ failure at 38 months of age.
Literature review
Along with our patient, we have identified 5 additional case reports in the literature of XIAP deficiency presenting with VEO-IBD. In all of these patients, symptoms developed during very early onset period, most patients had severe failure to thrive and evidence of splenomegaly (Table 1). Medically-refractory IBD was the presenting phenotype in all of the patients, as evidenced by a steroid-refractory course and minimal, time-limited, or no symptomatic improvement following treatment with TNF-α inhibitors. Specifically, 3/6 patients required colectomy, as their symptoms could not be managed with medical therapy alone. HSCT was performed in 5/6 patients and of those 5 it was curative in 4 patients.
Table 1:
Case reports of XIAP with colitis presenting during infancy
| Publication | n | Symptom onset (months) | Weight (z-score) | HSM | Mutation | Immunosuppression | Colectomy | HSCT | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S.T.A.C | Infliximab | Adalimumab | |||||||||
| Our Case | 1 | 3 | −10.98 | HSM | p.951_961 del 11 | Yes | No | TLR | No | Yes | Died at 38 mo |
| (Beser et al., 2016) | 1 | 3 | −3.4 | HSM | p.Ala459Glyfs*472 | Yes | TLR | NR | No | No | Died at 7 years |
| (Worthey et al., 2011) | 1 | 15 | −2.5 | HSM | p.C203Y | Yes | NCR | TLR | Yes | Yes | Rem ind- 42 days |
| (Tsuma et al., 2015) & (Zhao et al., 2010) | 1 | 20 | Normal | SM | p.R238X | Yes | No, but tocilizumab was tried | No, colitis developed at 7 yo | Yes | Healthy 11 mo f/u | |
| (Girardelli et al., 2015) | 1 | 2 | FTT | HSM | p.N341YfsX7 | Yes | NR | TLR | Yes | Yes | Rem ind- 6 mo, healthy 15 mo f/u |
| (Kelsen et al., 2015) | 1 | 1 | Profound growth failure | SM | Whole gene deletion | Yes | NCR | NCR | Yes | Yes | Healthy, 3 years f/u |
Ada-Adalimumab, FTT-failure to thrive, HMS-hepatosplenomegaly, HSCT-hematopoetic steam cell transplant, Inf-Infliximab, n-number of patients, NCR-no clinical response, NR-not reported, PR-partial response, Rem-remission induced, S.T.A.C –steroids, or tacrolimus, or azathioprine, cyclosporine; SM-splenomegaly, TLR-time limited response,
We also identified 6 case series, and report outcomes in total, on 44 patients with XIAP deficiency and IBD. The age at onset of intestinal symptoms ranged from 1 month to 41 years. Similar to the outcomes observed in case reports, these patients generally developed medically-refractory IBD. In 92% of the patients, IBD was refractory to treatment with some combination of steroids, tacrolimus, azathioprine or cyclosporine. Anti-TNF-α therapy was initiated on 58% of the patients. Forty-four percent of the patients required surgical interventions. The most common surgery performed was colectomy, while at least one patient underwent diverting ileostomy. On the other hand, a few mild cases were also described including a 39 year-old who was successfully treated with mesalamine therapy alone (Table 2).
Table2:
Summary of reported cases series of XIAP with colitis case.
| Publication | n | Age (Years) | S.T.A.C | Anti-TNF-a | Colectomy | HSCT | Outcome |
|---|---|---|---|---|---|---|---|
| (Speckmann et al., 2013) | 25 XIAP/6 IBD | 1–18 | 5/6 | 2/6 1-Rem | 3/6 | 4/6 | 4 well, 1 symp 1 died |
| (Pachlopnik Schmid et al., 2011) | 30 XIAP/5 IBD | 4–41 | 5/5 | 1/5 | 1/5 | 5-NK | 2 symp 3 died |
| (Yang et al., 2012) | 9 XIAP/2 IBD | 0.3–0.5 | 1/1 1-NK | 1/2 1-Rem | No | No | 1 well 1 died |
| (Zeissig et al., 2015) | 96 Ped IBD/4XIAP | 0.7–16 | 3/3 1-NK | 4/4 | 2/4 | No | 3 symp 1 died |
| (Dziadzio et al., 2015) | 4 XIAP with IBD | 0.1–12 | 4/4 | 1/4 | 2/4 | 2/4 | 2 died |
| (Aguilar et al., 2014) | 17 XIAP with IBD | 0.3–41 | 12/14 3-NK | 10/14 3-NK | 7/14 3-NK | 2/14 3-NK | 3 died |
| Individual case reports* | 6 XIAP with IBD | 0.1–3 | 6/6 | 5/6 | 3/6 | 4/6 | 2 died |
| Total | 44 | 0.1–41 | 36/39 (92%) | 24/41 (58%) | 18/41 (44%) | 12/36 (33%) | 13/44 died (29%) |
Colectomy-colectomy or other surgical interventions; NK-not known; n-number of patients; Rem- in remission; S.T.A.C-Steroids, tacrolimus, azathioprine, cyclosporine; symp –symptomatic;
individual case reports from table 1.
Discussion
Mutations in BIRC4, leading to XIAP deficiency is a well-recognized cause of VEO-IBD, with variable disease penetrance and severity. We report a patient with XIAP deficiency who developed severe colitis, had profound failure to thrive and presence of hepatosplenomegaly in infancy. He experienced severe multisystem disease and ultimately developed HLH and died at 38 months of age. In patients with XIAP deficiency and severe colitis, HSCT can successfully treat intestinal inflammation and ameliorate the risk of developing HLH. In these high-risk patients, we believe that HSCT should be considered early in a disease course, prior to development of life threatening HLH and severe multi-system disease.
X-linked lymphoproliferative syndrome (XLP) is an inherited immunodeficiency estimated to affect approximately one in one million males (15). The classical form of the disease, XLP1, was first described in 1974, as immunodeficiency resulting in defective NK cell-mediated toxicity secondary to inactivating mutations in SH2D1A, encoding SLAM-associated protein (SAP). XLP1 is associated with development of HLH, dysgammaglobulimemia and lymphoma. In 2006, Rigaud et al. demonstrated that mutations in BIRC4, which encodes XIAP protein, result in a closely related X-linked lymphoproliferative syndrome, XLP2 (11). Patients with XLP2 may also develop HLH and dysgammaglobulinemia. While only XLP1 patients seem to develop lymphoma, XLP2 patients are uniquely at risk of developing an IBD-like phenotype (14). In both groups, the highest risk of mortality is associated with development of HLH, and over 80% of XLP2 patients developed HLH by 20 years of age (14).
In patients with XIAP deficiency, the presence of an IBD-like phenotype has also been associated with poor outcomes. Development of intestinal inflammation is not surprising, as XIAP is involved in regulation of apoptosis and inflammatory responses. On a molecular level, XIAP is required for nucleotide binding and oligomerization domain (NOD) 1 and 2 receptor signaling (16), the latter of which is the strongest susceptibility gene associated with Crohn’s disease. Further association between XIAP deficiency and IBD has been established through epidemiologic studies, as 4% of male patients with early onset IBD have been found to have a mutation in BIRC4 (13). IBD has also been diagnosed in female carriers of BIRC4 mutation, due to random inactivation of X chromosome (17). In spite of a strong genetic risk and epidemiologic association, only 17% of XIAP deficient patients develop IBD (14). Furthermore, severity of IBD in patients with XIAP deficiency is highly variable, both, in terms of clinical presentation, and the natural course of disease. Some patients develop symptoms in the fourth decade of life and have only a mild disease, whereas others present in early childhood with severe colitis, which is often refractory to medical and surgical therapy.
Children who develop IBD at a very young age can have a more severe disease course (7), and often presents with predominantly colonic involvement. These patients also experience malnutrition and growth failure at a much higher rate, even as compared to older children with IBD (18). Malnutrition in these patients is somewhat unexpected, as the colon is not involved in nutrient absorption. Rather, malnutrition may be a sign of severe inflammation, leading to high inflammatory cells turnover and, thus, increased energy expenditure. Furthermore, malnutrition has independent negative effects on immune function and may add to the severity of immune dysregulation. Therefore, it is reasonable to postulate that, in patients with XIAP deficiency, early onset colitis and failure to thrive are associated with a high degree of intestinal inflammation and thus a severe IBD phenotype.
Additionally, patients with XIAP deficiency who develop symptoms of immunodeficiency at an early age are often reported to have splenomegaly or hepatosplenomegaly. In fact, some centers recommend screening all patients with unexplained splenomegaly for XIAP deficiency (19). Splenomegaly is found in 57% of patients with XIAP deficiency (20) and is thought to be a product of immune activation, even in patients who do not meet full HLH diagnostic criteria. Thus, splenomegaly and/or hepatosplenomegaly in these patients may be another sign of wide-spread extra intestinal immune system dysregulation. Indeed, our patient developed hepatosplenomegaly, with the liver and intestine sharing similar inflammatory characteristics. Liver biopsy identified the presence of granuloma, which is a pathognomonic characteristic of Crohn’s disease, when found on intestinal biopsies. Indeed, patients with Crohn’s disease have been reported to develop hepatic granulomas, although this is a rare finding (21, 22).
Taken together, our data suggest that XIAP deficiency associated with early onset of colitis, failure to thrive and splenomegaly/hepatosplenomegaly may thus identify patients with high degree of immune system dysregulation, who are at risk of experiencing medically-refractory IBD. We identified 6 case reports, including ours, of patients presenting in infancy with severe colitis. Five out of 6 patients had failure to thrive and all had splenomegaly or hepatosplenomegaly. These patients were noted to have a severe VEO-IBD phenotype, as evident by the use of biologic immunosuppressive drugs, a high rate of surgical interventions and 33% overall mortality. In these case reports, the patients that had HSCT generally achieved resolution of intestinal inflammation (Table 1).
Favorable outcomes have been reported after HSCT in patients with XLP-1 and familial HLH, with greatly improved survival as compared to the medical treatment (23). Much less is known concerning outcomes of HSCT for patients with XIAP deficiency. Recent evidence suggests that myeloablative conditioning regimens in patients with XIAP deficiency are associated with poor outcomes, with one in 7 chance of survival following HSCT, while 6 out of 11 patients survived transplant with reduced intensity conditioning regiment (24). Further analysis revealed that 4 out of 5 deaths in reduced intensity conditioning group were not in full remission from HLH and died from multi-organ failure (25). Initial reports also suggest that HSCT is curative treatment for intractable colitis (14, 26, 27). Thus, in high-risk patients with VEO-IBD secondary to XIAP deficiency, who have not yet developed HLH and may be primarily treated by a gastroenterologist, early referral to a hematologist for consideration of HSCT may successfully treat their intestinal inflammation and mitigate their risk of developing life-threatening HLH. Our patient received HSCT after developing HLH and respiratory failure requiring tracheostomy placement and chronic ventilation. The HSCT was performed in the ICU and the patient never recovered and eventually succumbed to multi-organ failure.
Conclusion
In patients with XIAP deficiency and IBD, development of colitis at an early age, presence of severe failure to thrive and evidence of splenomegaly/hepatosplenomegaly may identify a subgroup of patients at high risk of developing treatment refractory IBD phenotype. In this selected subgroup of patients, HSCT should be considered early in the disease course.
Supplementary Material
Acknowledgments:
The authors would like to thank the patient’s family for their persistence, positive attitude and relentless optimism. This case was presented as part of Gastroenterology Longwood Case Conference. The authors would like to thank doctors, John Watkins, MD and Richard Grand, MD for organizing and leading this case conference over the last 30 years, which has significantly enriched education of GI fellows in our institution and far beyond.
References:
- 1.Uhlig HH, Schwerd T, Koletzko S, Shah N, Kammermeier J, Elkadri A, et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology 2014;147(5):990–1007 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19 Suppl A:5A–36A. [DOI] [PubMed] [Google Scholar]
- 3.Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011;17(6):1314–21. [DOI] [PubMed] [Google Scholar]
- 4.Benchimol EI, Bernstein CN, Bitton A, Carroll MW, Singh H, Otley AR, et al. Trends in Epidemiology of Pediatric Inflammatory Bowel Disease in Canada: Distributed Network Analysis of Multiple Population-Based Provincial Health Administrative Databases. Am J Gastroenterol 2017;112(7):1120–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelsen JR, Baldassano RN, Artis D, Sonnenberg GF. Maintaining intestinal health: the genetics and immunology of very early onset inflammatory bowel disease. Cell Mol Gastroenterol Hepatol 2015;1(5):462–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandrakasan S, Venkateswaran S, Kugathasan S. Nonclassic Inflammatory Bowel Disease in Young Infants: Immune Dysregulation, Polyendocrinopathy, Enteropathy, X-Linked Syndrome, and Other Disorders. Pediatr Clin North Am 2017;64(1):139–160. [DOI] [PubMed] [Google Scholar]
- 7.Heyman MB, Kirschner BS, Gold BD, Ferry G, Baldassano R, Cohen SA, et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr 2005;146(1):35–40. [DOI] [PubMed] [Google Scholar]
- 8.Uhlig HH. Monogenic diseases associated with intestinal inflammation: implications for the understanding of inflammatory bowel disease. Gut 2013;62(12):1795–805. [DOI] [PubMed] [Google Scholar]
- 9.Uhlig HH, Booth C. A Spectrum of Genetic Variants Contributes to Immune Defects and Pathogenesis of Inflammatory Bowel Diseases. Gastroenterology 2018;154(8):2022–2024. [DOI] [PubMed] [Google Scholar]
- 10.Charbit-Henrion F, Parlato M, Hanein S, Duclaux-Loras R, Nowak J, Begue B, et al. Diagnostic Yield of Next-Generation Sequencing in Very Early-Onset Inflammatory Bowel Diseases: A Multicenter Study. J Crohns Colitis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rigaud S, Fondaneche MC, Lambert N, Pasquier B, Mateo V, Soulas P, et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature 2006;444(7115):110–4. [DOI] [PubMed] [Google Scholar]
- 12.Worthey EA, Mayer AN, Syverson GD, Helbling D, Bonacci BB, Decker B, et al. Making a definitive diagnosis: successful clinical application of whole exome sequencing in a child with intractable inflammatory bowel disease. Genet Med 2011;13(3):255–62. [DOI] [PubMed] [Google Scholar]
- 13.Zeissig Y, Petersen BS, Milutinovic S, Bosse E, Mayr G, Peuker K, et al. XIAP variants in male Crohn’s disease. Gut 2015;64(1):66–76. [DOI] [PubMed] [Google Scholar]
- 14.Pachlopnik Schmid J, Canioni D, Moshous D, Touzot F, Mahlaoui N, Hauck F, et al. Clinical similarities and differences of patients with X-linked lymphoproliferative syndrome type 1 (XLP-1/SAP deficiency) versus type 2 (XLP-2/XIAP deficiency). Blood 2011;117(5):1522–9. [DOI] [PubMed] [Google Scholar]
- 15.Sumegi J, Huang D, Lanyi A, Davis JD, Seemayer TA, Maeda A, et al. Correlation of mutations of the SH2D1A gene and epstein-barr virus infection with clinical phenotype and outcome in X-linked lymphoproliferative disease. Blood 2000;96(9):3118–25. [PubMed] [Google Scholar]
- 16.Krieg A, Correa RG, Garrison JB, Le Negrate G, Welsh K, Huang Z, et al. XIAP mediates NOD signaling via interaction with RIP2. Proc Natl Acad Sci U S A 2009;106(34):14524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dziadzio M, Ammann S, Canning C, Boyle F, Hassan A, Cale C, et al. Symptomatic males and female carriers in a large Caucasian kindred with XIAP deficiency. J Clin Immunol 2015;35(5):439–44. [DOI] [PubMed] [Google Scholar]
- 18.Al-Hussaini A, El Mouzan M, Hasosah M, Al-Mehaidib A, K AL, Saadah OI, et al. Clinical Pattern of Early-Onset Inflammatory Bowel Disease in Saudi Arabia: A Multicenter National Study. Inflamm Bowel Dis 2016;22(8):1961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Speckmann C, Lehmberg K, Albert MH, Damgaard RB, Fritsch M, Gyrd-Hansen M, et al. X-linked inhibitor of apoptosis (XIAP) deficiency: the spectrum of presenting manifestations beyond hemophagocytic lymphohistiocytosis. Clin Immunol 2013;149(1):133–41. [DOI] [PubMed] [Google Scholar]
- 20.Latour S, Aguilar C. XIAP deficiency syndrome in humans. Semin Cell Dev Biol 2015;39:115–23. [DOI] [PubMed] [Google Scholar]
- 21.Rojas-Feria M, Castro M, Suarez E, Ampuero J, Romero-Gomez M. Hepatobiliary manifestations in inflammatory bowel disease: the gut, the drugs and the liver. World J Gastroenterol 2013;19(42):7327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patedakis Litvinov BI, Pathak AP. Granulomatous hepatitis in a patient with Crohn’s disease and cholestasis. BMJ Case Rep 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Booth C, Gilmour KC, Veys P, Gennery AR, Slatter MA, Chapel H, et al. X-linked lymphoproliferative disease due to SAP/SH2D1A deficiency: a multicenter study on the manifestations, management and outcome of the disease. Blood 2011;117(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh RA, Rao K, Satwani P, Lehmberg K, Muller I, Li D, et al. Allogeneic hematopoietic cell transplantation for XIAP deficiency: an international survey reveals poor outcomes. Blood 2013;121(6):877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuma Y, Imamura T, Ichise E, Sakamoto K, Ouchi K, Osone S, et al. Successful treatment of idiopathic colitis related to XIAP deficiency with allo-HSCT using reduced-intensity conditioning. Pediatr Transplant 2015;19(1):E25–8. [DOI] [PubMed] [Google Scholar]
- 26.Yang X, Kanegane H, Nishida N, Imamura T, Hamamoto K, Miyashita R, et al. Clinical and genetic characteristics of XIAP deficiency in Japan. J Clin Immunol 2012;32(3):411–20. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen OH, LaCasse EC. How genetic testing can lead to targeted management of XIAP deficiency-related inflammatory bowel disease. Genet Med 2017;19(2):133–143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.