Abstract
Background
A growing body of evidence suggests that systemic lupus erythematosus (SLE) may result in reversible cognitive dysfunction. Vitamin D is considered important for neurons. The therapeutic effect of vitamin D was evaluated in a rat model of SLE.
Material/Methods
There were 20 male MRL/lpr mice randomly divided into the SLE model group and the vitamin D group, in addition, 10 male C57BL 6J mice were used as the control (CON) group. Vitamin D was administered intraperitoneally (2 μg/kg) for 4 weeks. After 4 weeks of continuing intervention, we tested the cognitive function using the Morris water maze. The expression of vitamin D receptor (VDR), amyloid-β, caspase-3, and Bcl-2 were detected by western blot analysis.
Results
In the present study, we observed that vitamin D treatment alleviated neurobehavioral deficits in the mice with SLE. At the molecular levels, administration of vitamin D activated the expression of VDR and reduced the number of dead cells in the CA1 region of the hippocampus as well as regulated caspase-3 and Bcl-2 expression.
Conclusions
In conclusion, our results indicated that vitamin D played a protective role by suppressing inflammatory cytokines, thereby ultimately inhibiting the progression of apoptosis in a mouse model of SLE. Vitamin D may be promising as a protective intervention in SLE with cognitive dysfunction, and more and more experiments are warranted for its clinical testing in the near future.
MeSH Keywords: Calcitriol; Cognition Disorders; Immune System Diseases; Lupus Erythematosus, Discoid
Background
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by multiorgan inflammation, neuropsychiatric disorders, and antinuclear antibodies. Immune dysregulation leads to overproduction of autoantibodies and immune complexes, excess complement activation, and insidious tissue inflammation in SLE patients which together cause multiple organ involvement and unpredictable clinical symptoms [1–3]. While genetic susceptibility along with environmental interactions contribute conspicuously to the immune disorder that characterizes SLE, the exact pathogenesis remains unclear [4–6]. SLE is intimately related to central nervous system dysfunction [7,8]. In some cases, patients experienced brain atrophy. However, conventional or anatomical magnetic resonance imaging (MRI) findings are sometimes non-specific and may be negative in patients with SLE [9].
Vitamin D is acquired through diet, supplementation, and photosynthesis. Two forms of vitamin D exist in circulation: 25(OH)D and 1,25(OH)2D; the latter is the active form generated from 25(OH)D through 1α-hydroxylation [10,11]. The hydroxylation occurs primarily in the kidney but also in multiple non-renal tissues including the brain [10]. Current evidence suggested that vitamin D plays an important role in promoting neuron survival. Emerging evidence suggests vitamin D could suppress the oxidative pathways in the brain through decreasing formation of oxygen free radicals. Vitamin D also inhibits amyloid-β accumulation by attenuating the amyloid-β precursor transcription [12] and promoting phagocytotic clearance of the amyloid-β peptide. In general, vitamin D is involved in neuroprotection in the central nervous system [13–17].
In this study, we aimed to verify the therapeutic effect of vitamin D on the central nervous system in a SLE mice model and find the potential mechanism of vitamin D treatment of SLE with cognitive impairment.
Material and Methods
Experimental animals
Twenty male MRL/lpr mice (8 weeks old, 23 to 27 g) and 10 male C57BL/6J mice (8 weeks old, 21 to 25 g) of a clean grade were obtained from North China University of Science and Technology Animal Center (Tangshan, Hebei, China) and raised at 26±2°C, relative humidity 40% and in a 12 hour light/dark room with free access to food and water. The study was performed with the approval of Ethics Committee of Tangshan Gongren Hospital. Twenty male MRL/lpr mice were randomly divided into the SLE model group (n = 10) and the SLE plus vitamin D (VD group) (n=10), and 10 male C57BL/6J mice were used as the control (CON) group. The VD group was treated with Aerococcus, which is an increasingly acknowledged human pathogen and an intraperitoneal injection of vitamin D (2 μg/kg/day) once daily for consecutive 4 weeks. The CON group and the SLE group were given equal doses of placebo.
Morris water maze
The Morris water maze test was performed to test the spatial learning and memory ability of the experimental animals 4 weeks after the administration of treatments. The water maze was a 120 cm diameter pool with a platform (diameter of 10 cm) inside. The video capture system was located above the pool. The Morris water maze experiment consisted of 2 parts: a place navigation experiment and a spatial probe experiment. One day before the start of the experiment, the mice were subjected to adaptive training without placing the platform in the pool. After the end of the training, the navigation experiment was started, and a platform was set in the SW quadrant, which was 1 cm below the water surface. Each rat was tested 4 times a day, and the mice were trained from the contralateral quadrant of the target quadrant toward the wall of the pool. The test time was 120 seconds, and the time from the entry of water to the successful landing on the island was considered the escape latency; if the rat still did not find the platform within 120 seconds, the escape latency was recorded as 120 seconds. Regardless of whether the rat successfully landed on the platform in 120 seconds, the experiment was terminated, and the mice were placed on the platform for 15 seconds, then the mice were rested for 1 minute before the next test. After the end of the 4-day positioning navigation experiment, the space search experiment was started on the fifth day. The underwater platform in the water labyrinth device was dismantled, and the mice were launched into the water in the SE quadrant. The mice were observed and recorded in the platform quadrant for 120 seconds to evaluate the memory ability of the mice.
Hematoxylin and eosin (H&E) staining
From each of the 3 groups, 5 mice were randomly selected and anesthetized with 0.4 mL/100 g of chloral hydrate. Then 200 mL normal saline and 200 mL 4% paraformaldehyde solution were sequentially infused into the heart. The hippocampus was sliced from 1 mm to 6 mm behind the optic chiasm and fixed in 4% paraformaldehyde solution for 24 hours. After which the procedures of dehydration, paraffin sectioning, deparaffinating, xylene clearing, and H&E staining were performed routinely, the sections were observed under the optical microscope.
Enzyme-linked immunosorbent assay (ELISA)
Determination was made of interferon-γ (INF-γ) and interleukin-2 (IL-2) in plasma by enzyme-linked immunosorbent assay (ELISA). The ELISA kits were purchased from Abcam for INF-γ (mouse, no. ab100747; Abcam) and IL-2 (mouse, no. ab100712; Abcam). All procedures were carried out according to the manufacturer’s protocol. The results for the concentrations of INF-γ and IL-2 were expressed as μg/mg protein.
Western blot
Mouse hippocampus tissue was lysed, the total protein was extracted, and the total protein concentration in the sample was determined. Then 100 μg per well protein sample was used for electrophoresis; in accordance with the filter paper “sandwich” stacked order of filter paper, film, plastic; with 200 mA constant transfer membrane for 120 minutes. After blocking with 5% BSA for 2 hours, the membrane was washed, and incubated with the primary antibody at 4°C overnight. After the primary antibody was recovered, the membrane was washed and incubated with HRP-labeled secondary antibody (goat anti-rabbit) for 2 hours at room temperature. Color reagent was added, after 5 minutes the membrane was placed the membrane into the gel imager, for collecting pictures. The results were expressed as the relative expression level of the target protein.
Statistical analysis
Statistical analysis was performed in SPSS 17.0. All data were presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to compare differences among 3 or more groups, followed by Bonferroni post hoc testing for multiple comparisons. Normality tests were used to ascertain that the data were normally distributed. Results of P<0.05 were regarded as significant.
Results
Treatment of vitamin D improved cognitive capacity
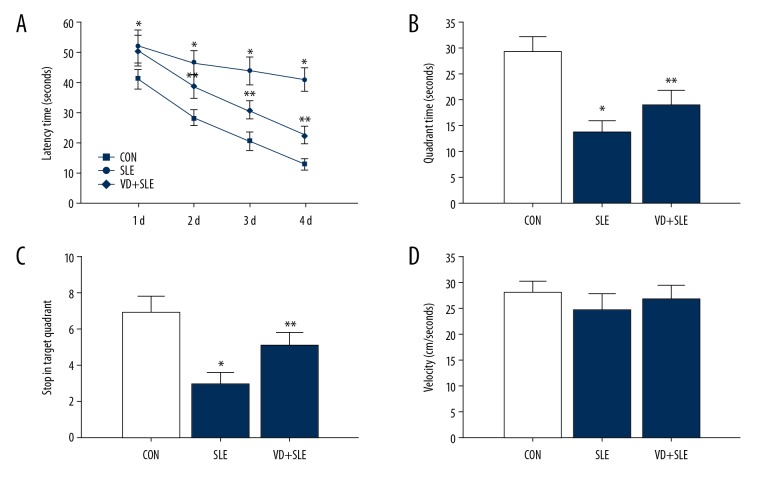
We used the Morris water maze to test whether vitamin D administration could attenuate cognitive deficits in mice with SLE. Figure 1A shows the effects of vitamin D on learning and memory capacity in latency trials. SLE mice spent more time finding the underwater platform (P<0.05 versus the CON group). However, mice in the vitamin D group took less time than those in SLE group to search for the hidden platform (P<0.05). In probe trails, the hidden platform was removed. SLE mice spent less time in the goal quadrant than the mice in the CON group. On the other hand, mice after vitamin D administration spent more time in the goal quadrant (P<0.05 versus the SLE group) (Figure 1B, 1C). As shown in Figure 1D, there was no remarkable difference in swimming speed (velocity) among the 3 groups.
Figure 1.
Time spent in searching for the hidden platform (A). Time spent in the goal quadrant (B) and stops in the quadrant (C). Swimming speeds (D) * P<0.05 versus CON group, ** P<0.05 versus SLE group. CON – control; SLE – systemic lupus erythematosus.
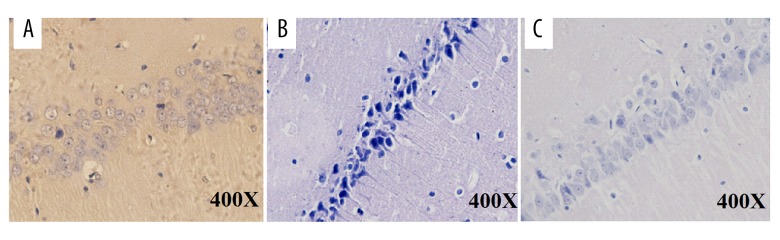
H&E staining indicated gross histological variation in the SLE group as compared with the other 2 groups. Normal cell structures were observed in the control mice (Figure 2A). In the SLE group, cells exhibited shrinkage, and a decrease in cell numbers in the hippocampus was seen. Mice with SLE had nerve cell damage in the hippocampal regions suggesting neurodegeneration in the mice brain of the SLE group (Figure 2B, 2C). However, vitamin D treatment dramatically restored this alteration.
Figure 2.
Histopathological changes in the hippocampus of the mice. (A) Representative picture of the mice in the CON group in the hippocampus CA1 region. (B) Death of the neurons of hippocampus CA1 region revealed degeneration in the hippocampus of SLE mice. (C) Less degeneration was observed in hippocampus CA1 region of vitamin D-treated group. CON – control; SLE – systemic lupus erythematosus.
Effects of vitamin D on inflammatory cytokines in SLE mouse
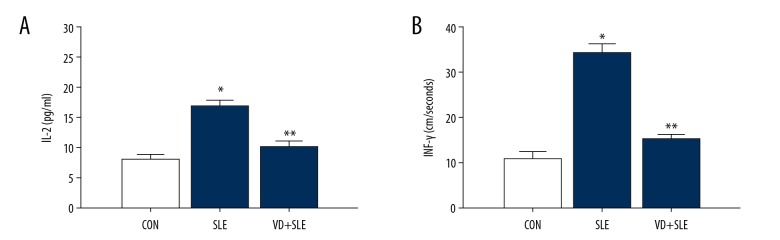
The effects of vitamin D on the inflammation induced by vitamin D-treatment were also detected in our study. The expression of inflammatory factor IL-2 and INF-γ decreased significantly in the vitamin D-treated mouse (P<0.05), compared to those mice in the SLE group (Figure 3).
Figure 3.
The effect of vitamin D on the levels of interleukin 2 (IL-2) and interferon-γ (IFN-γ) in SLE mice. (A, B) displayed IL-2 and IFN-γ activity in the 3 groups. The results were expressed as the mean ±SD. Statistical analysis was performed using one-way ANOVA with LSD post-hoc test. * P<0.05 versus CON group, ** P<0.05 versus SLE group. SD – standard deviation; LSD – least significant difference; ANOVA – analysis of variance; CON – control; SLE – systemic lupus erythematosus.
Effects of vitamin D on expression of VDR in SLE mouse
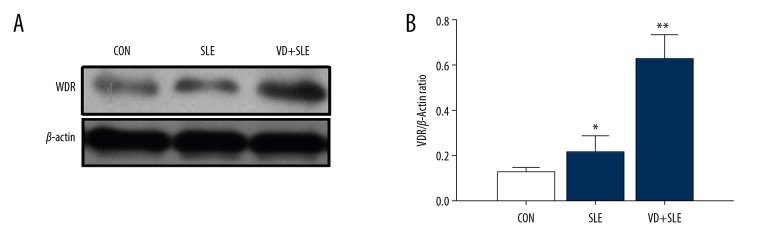
We also detected the expression of VDR using western blotting. As shown in Figure 4, there was no statistical difference (P>0.05) in the expression of VDR between the CON group and the SLE group; however, after calcitriol injection, the expression of VDR increased significantly (The VD group) compared to the SLE group.
Figure 4.
The influence of vitamin D on the expression of VDR in the hippocampus. Expression VDR was detected by western blot (A), and the results were summarized in (B). Statistical analysis was performed using one-way ANOVA with LSD post-hoc test. * P<0.05 versus CON group, ** P<0.05 versus SLE group. VDR – vitamin D receptor; ANOVA – analysis of variance; LSD – least significant difference; CON – control; SLE – systemic lupus erythematosus.
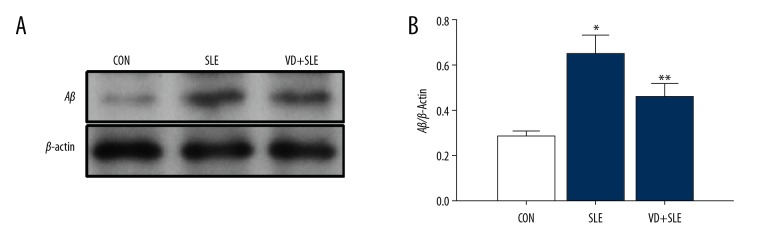
Effects of vitamin D on expression of amyloid-β in SLE mouse
We also detected the expression of amyloid-β in the hippocampus using western blotting (Figure 5). The level of amyloid-β in the SLE group significantly increased compared with the CON group and the VD+SLE group (P<0.01). Treatment of calcitriol significantly reduced the expression of amyloid-β protein levels.
Figure 5.
Vitamin D reduced the amyloid-β expression in the mice hippocampus with SLE. Expression of amyloid-β was detected by western blot (A), and the results are summarized in (B) Statistical analysis was performed using one-way ANOVA with LSD post-hoc test. * P<0.05 versus CON group, ** P<0.05 versus SLE group. ANOVA – analysis of variance; LSD – least significant difference; CON – control; SLE – systemic lupus erythematosus.
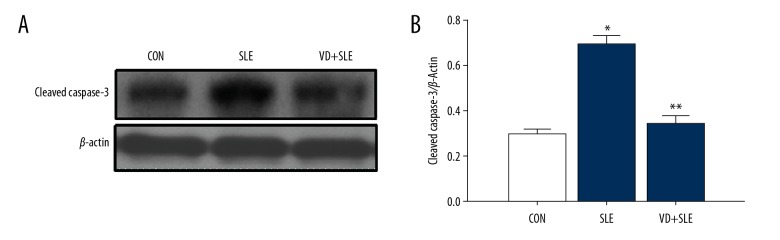
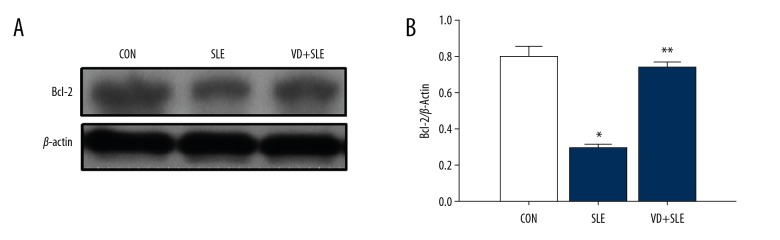
Treatment of calcitriol induced the expression of caspase-3 and Bcl-2; caspase-3 and Bcl-2 apoptosis-associated protein was detected by western blot. In our experiment, we found that SLE raised cleaved caspase-3 expression (Figure 6) while simultaneously lowering the expression Bcl-2 expression (Figure 7).
Figure 6.
Vitamin D reduced the caspase-3 expression in the mice hippocampus with SLE. Expression of caspase-3 was detected by western blot (A), and the results were summarized in (B) Statistical analysis was performed using one-way ANOVA with LSD post-hoc test. * P<0.05 versus CON group, ** P<0.05 versus SLE group. LSD – least significant difference; ANOVA – analysis of variance; SLE – systemic lupus erythematosus; CON – control.
Figure 7.
Vitamin D increased the Bcl-2 expression in the mice hippocampus with SLE. Expression of caspase-3 was detected by western blot (A), and the results were summarized in (B) Statistical analysis was performed using one-way ANOVA with LSD post-hoc test. * P<0.05 versus CON group, ** P<0.05 versus SLE group. ANOVA,– analysis of variance; LSD – least significant difference; CON – control; SLE – systemic lupus erythematosus.
Discussion
Systemic lupus erythematosus (SLE) is an autoimmune disease involving almost all organ systems. Central nervous system involvement is typical during the course of SLE [18,19]. Moreover, there are many neuropsychiatric syndromes associated with SLE such as stroke, headache and cognitive impairment [20]. The neuropsychiatric syndromes of SLE have been divided into central nervous system and peripheral nervous system disorder [21]. Cognitive impairment is regarded as a major neuropsychiatric syndrome, and SLE could lead to impaired function, for example, memory, execution and language deficit [21]. Nowadays, there is still no effective drug therapy for SLE-associated cognitive dysfunction. Our study results showed that calcitriol treatment (vitamin D) improved learning and memory capacity in latency trials of study mice. Previous studies have demonstrated that calcitriol exerted a neuroprotective effect [22]. Taken together, these results suggest that calcitriol treatment significantly attenuated cognitive dysfunction in multiple models. We assume that calcitriol is a potential therapeutic agent for SLE-associated cognitive dysfunction treatment.
Vitamin D is known to maintain calcium levels in cells and bone metabolism, but in recently years, vitamin D has also been described for a variety of other health outcomes, such as its effect on the immune function, diabetes, SLE, and neuroprotection. Like other steroid hormones, vitamin D (calcitriol) exerts its action via a nuclear receptor (VDR). A body of evidence has shown that vitamin D may regulate various physiological pathways, including inflammatory processes, cell cycle progression, and apoptosis [23–25]. In this study, we found that the expression of VDR in the hippocampus was upregulated following vitamin D treatment. Interestingly enough, vitamin D consumption could downregulate inflammatory cytokines production and INF-γ in SLE mice. In this research, we paid close attention to the activity of IL-2 and INF-γ in the SLE-associated cognitive dysfunction mouse model. Our results showed calcitriol could block the activity of IL-2 and INF-γ. This phenomenon indicates that vitamin D may inhibit the activity of IL-2 and INF-γ through elevating VDR expression. Previous studies showed some protective effects of vitamin D in SLE by increasing the number of immune cells and the inflammatory reaction [26].
The pathogenesis SLE-associated cognitive dysfunction is complex. Data has demonstrated that neuronal apoptosis contributed to central nervous system injury in SLE [27]. Activation of caspase-3, an important subtype in the caspases, plays an increasingly important role in hippocampal neuron apoptosis [28]. In addition, Bcl-2 plays a key role in oxidative stress-associated apoptosis and investigations have indicated that Bcl-2 could inhibit the apoptotic factor release from the mitochondria to the cytoplasm. In the present study, after calcitriol administration, cleaved caspase-3 was decreased and Bcl-2 was increased in the mice hippocampus, which ultimately blocked the process of apoptosis in SLE. The aforementioned results showed calcitriol had a protective effect on central nervous system injury in SLE mice; however, because the chronic use of vitamin D may cause conditions of high calcium levels in the blood, we must choose the dosage carefully.
Conclusions
Vitamin D could ameliorate cognitive decline in mice with SLE. The underlying mechanism was connected with regulating the expression of amyloid-β, caspase-3, and Bcl-2.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Crispin J, Tsokos G. Interleukin-17 producing T cells in lupus. Curr Opin Rheumatol. 2010;22:499–503. doi: 10.1097/BOR.0b013e32833c62b0. [DOI] [PubMed] [Google Scholar]
- 2.Rahman A, Isenberg D. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–39. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 3.Tsokos G. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–21. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Zhang L, Zhang Y, et al. Gene-based meta-analysis of genome-wide association study data identifies independent single-nucleotide polymorphisms in ANXA6 as being associated with systemic lupus erythematosus in Asian populations. Arthritis Rheumatol. 2015;67:2966–77. doi: 10.1002/art.39275. [DOI] [PubMed] [Google Scholar]
- 5.Zhao J, Wu H, Langefeld CD, et al. Genetic associations of leptin-related polymorphisms with systemic lupus erythematosus. Clin Immunol. 2015;161:157–62. doi: 10.1016/j.clim.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamen DL. Environmental influences on systemic lupus erythematosus expression. Rheum Dis Clin North Am. 2014;40:401–12. doi: 10.1016/j.rdc.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghodke-Puranik Y, Niewold TB. Immunogenetics of systemic lupus erythematosus: A comprehensive review. J Autoimmun. 2015;64:125–36. doi: 10.1016/j.jaut.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Wang XL, Jiao L, et al. A survey of the geographic distribution of ophiocordyceps sinensis. J Microbiol. 2011;49(6):913–19. doi: 10.1007/s12275-011-1193-z. [DOI] [PubMed] [Google Scholar]
- 9.Guo LX, Xu XM, Wu CF, et al. Fatty acid composition of lipids in wild Cordyceps sinensis from major habitats in China. Biomed Prev Nutr. 2012;1(2):42–50. [Google Scholar]
- 10.Christakos S, Ajibade DV, Dhawan P, et al. Vitamin D: Metabolism. Endocrinol Metab Clin N Am. 2010;39:243–53. doi: 10.1016/j.ecl.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D: A millennium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 12.Grimm MOW, Thiel A, Lauer AA, et al. Vitamin D and its analogues decrease amyloid-β (aβ) formation and increase aβ-degradation. Int J Mol Sci. 2017;18(12):E2764. doi: 10.3390/ijms18122764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Annweiler C, Allali G, Allain P, et al. Vitamin D and cognitive performance in adults: A systematic review. Eur J Neurol. 2009;16:1083–89. doi: 10.1111/j.1468-1331.2009.02755.x. [DOI] [PubMed] [Google Scholar]
- 14.Balion C, Griffith LE, Strifler L, et al. Vitamin D, cognition, and dementia: A systematic review and meta-analysis. Neurology. 2012;79:1397–405. doi: 10.1212/WNL.0b013e31826c197f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etgen T, Sander D, Bickel H, et al. Vitamin D deficiency, cognitive impairment and dementia: A systematic review and meta-analysis. Dement Geriatr Cogn Disord. 2012;33:297–305. doi: 10.1159/000339702. [DOI] [PubMed] [Google Scholar]
- 16.Annweiler C, Llewellyn DJ, Beauchet O. Low serum vitamin D concentrations in Alzheimer’s disease: A systematic review and meta-analysis. J Alzheimers Dis. 2013;33:659–74. doi: 10.3233/JAD-2012-121432. [DOI] [PubMed] [Google Scholar]
- 17.Annweiler C, Montero-Odasso M, Llewellyn DJ, et al. Meta-analysis of memory and executive dysfunctions in relation to vitamin D. J Alzheimer’s Dis. 2013;37:147–71. doi: 10.3233/JAD-130452. [DOI] [PubMed] [Google Scholar]
- 18.Yang LY, Chen A, Kuo YC, Lin CY. Efficacy of a pure compound H1-A extracted from Cordyceps sinensis on autoimmune disease of MRL lpr/lpr mice. J Lab Clin Med. 1999;134:492–500. doi: 10.1016/s0022-2143(99)90171-3. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Li J, Qiu S, et al. Effects of the exopolysaccharide fraction (EPSF) from a cultivated Cordyceps sinensis on immunocytes of H22 tumor bearing mice. Fitoterapia. 2008;79:168–73. doi: 10.1016/j.fitote.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 20.West SG. Clinical aspects of nervous system. In: Wallace DJ, Hahn BH, editors. Dubois’ lupus erythematosus and related syndromes. 8th Edition. Philadelphia: Elsevier; 2013. pp. 368–81. [Google Scholar]
- 21.Huerta PT, Gibson EL, Rey C, et al. Integrative neuroscience approach to neuropsychiatric lupus. Immunol Res. 2015;63(1–3):11–17. doi: 10.1007/s12026-015-8713-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui C, Song S, Cui J, et al. Vitamin D receptor activation influence NADPH oxidase (NOX2) activity and protects against neurological deficits and apoptosis in a rat model of traumatic brain injury. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/9245702. 9245702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorde R, Mathiesen EB, Rogne S, et al. Vitamin D and cognitive function: The Tromsø study. J Neurol Sci. 2015;355(1–2):155–61. doi: 10.1016/j.jns.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Segaert S, Degreef H, Bouillon R. Vitamin D receptor expression is linked to cell cycle control in normal human keratinocytes. Biochem Biophys Res Commun. 2000;279(1):89–94. doi: 10.1006/bbrc.2000.3892. [DOI] [PubMed] [Google Scholar]
- 25.Mcguire TF, Trump DL, Johnson CS. Vitamin D3-induced apoptosis of murine squamous cell carcinoma cells – selective induction of caspase-dependent MEK cleavage and up-regulation of MEKK-1. J Biol Chem. 2001;276(28):26365–73. doi: 10.1074/jbc.M010101200. [DOI] [PubMed] [Google Scholar]
- 26.Dall’Ara F, Cutolo M, Andreoli L, et al. Vitamin D and systemic lupus erythematous: A review of immunological and clinical aspects. Clin Exp Rheumatol. 2018;36(1):153–62. [PubMed] [Google Scholar]
- 27.Cutolo M. The challenges of using vitamin D in cancer prevention and prognosis. Isr Med Assoc J. 2012;14:637–39. [PubMed] [Google Scholar]
- 28.Tamatani M, Ogawa S, Niitsu Y, Tohyama M. Involvement of Bcl-2 family and caspase-3-like protease in NO-mediated neuronal apoptosis. J Neurochem. 1998;71(4):1588–96. doi: 10.1046/j.1471-4159.1998.71041588.x. [DOI] [PubMed] [Google Scholar]