Abstract
Background
Electroacupuncture (EA) has been commonly used to treat stroke in China. However, the underlying mechanism remains largely unknown. The present study investigated the neuroprotective effects of EA in middle cerebral artery occlusion (MCAO) rats and elucidated the possible anti-inflammatory mechanisms.
Material/Methods
In this study, modified neurological severity scoring (mNSS) was used to assess neurological deficits, and TTC staining and brain water content were measured to evaluate the degree of brain damage. HE staining, Nissl staining, and TUNEL staining were employed to evaluate apoptotic neuronal death. Molecular biological methods were used to measure the levels of miR-233, NLRP3, caspase-1, IL-1β, and IL-18 in the peri-infarct cortex.
Results
Our results showed that EA treatment significantly decreased the neurological deficit score and infarct volume of MCAO rats. The level of miR-223 was increased, while the levels of NLRP3, caspase-1, IL-1β, and IL-18 were decreased in the peri-infarct cortex of EA-treated MCAO rats. However, the neuroprotective effect of EA was partially blocked by antagomir-223.
Conclusions
These data suggest that EA treatment can alleviate neuroinflammation by inhibiting the miR-223/NLRP3 pathway, thus playing a neuroprotective role in MCAO in rats.
MeSH Keywords: Brain Ischemia, Electroacupuncture, MicroRNAs, Neurogenic Inflammation
Background
Stroke is a leading cause of death and disability in adults around the world. More than 80% of strokes are of the ischemic type, which has high mortality and morbidity rates [1]. Previous studies have shown that oxidative stress, excitotoxicity, apoptosis, and inflammation contribute to the pathophysiology of ischemic brain damage [2]. In particular, inflammation is one of the key pathological mechanisms, representing a major cause of secondary injury after ischemic stroke [3]. In recent years, mounting evidence has indicated that the inflammasome has critical functions in inflammatory reactions and innate immunity [4]. The nucleotide-binding domain-like receptor family (NLRP3), a pyrin domain-containing inflammasome, is a pattern recognition receptor critical in innate immunity and is a multimolecular protein involved in the inflammatory reactions of cerebral ischemic injury. The NLRP3 inflammasome includes NLRP3, an apoptosis-associated speck-like protein containing an ASC adaptor and pro-caspase-1 [5]. NLRP3 activation induces caspase-1, which controls the biosynthesis of the pro-inflammatory cytokines IL-1β and IL-18, initiating subsequent responses [6].
MicroRNAs (miRNAs) are endogenous, small, noncoding RNA entities (approximately 18–22 nucleotides long) that control mRNA degradation and/or mediate protein translation by binding to specific sites located in the 3′ or 5′-untranslated regions (UTRs) of the target mRNAs [7]. MicroRNAs are involved in multiple physiological and pathophysiological processes of inflammatory diseases, and miR-223, an inflammation-related miRNA, has attracted increasing attention. NLRP3 is inactivated by miR-223 via a conserved binding site in the 3′ UTR [8].
Acupuncture, as an important component of traditional Chinese medicine (TCM), has been clinically used for disease treatment for more than 2000 years [9]. Increasing evidence has shown that electroacupuncture (EA) exerts protective effects on multi-organ systems against ischemic injuries by reducing cell apoptosis via various signal pathways, especially in the brain [10,11] and heart [12,13]. In addition, studies also showed that EA provides a neuroprotective effect by improving cognitive function in neurodegenerative diseases [14]. Recently, it has been proposed that EA alleviates inflammatory responses through various pathways to treat cerebral ischemic injury [15,16]. However, the effect of EA on miRNA-dependent modulators in stroke is largely undefined.
We hypothesized that EA treatment modulates inflammatory reactions in MCAO rats by regulating the miR-223/NLRP3 pathway. In the present study, we assessed the therapeutic effects of EA on neuroinflammation in MCAO rats and explored the underlying molecular mechanisms.
Material and Methods
Animals and experimental design
Adult male Sprague-Dawley rats (220–270 g, purchased from the Experimental Animal Center of Huazhong University of Science and Technology, Wuhan, China) were used in the present study. The rats (4–5/cage) were housed at 24±1°C under a 12-h/12-h light/dark cycle with free access to food and water. After acclimatization to the housing facility for 7 days, the animals were randomized to 5 groups by random sampling method: the Sham (Sham) group, the MCAO model (MCAO) group, the EA treatment (EA+MCAO) group, the EA/Scrambled treatment (EA+MCAO+Scr) group, and the EA/Antagomir-223 treatment (EA+MCAO+Ant-223) group. All animals underwent MCAO with the exception of the Sham group.
Procedures involving animals were approved by the Animal Experimentation Ethics Committee of Tongji Hospital (2017-609), and were performed strictly according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1978).
Intracerebroventricular injection
Intracerebroventricular injection was performed according to a previous report [17]. In brief, the animals were anesthetized and by a sagittal skin incision was made from bregma to lambda. Then, a hand-drill (RWD Life Science, China) was used to make a small hole at the injection site on the skull: anteroposterior −0.8 mm from the bregma, mediolateral 1.5 mm from the midline, and dorsoventral −4.8 mm from the skull. Scrambled-miR (2.5 nmol, 5 μl) or antagomir-223 (2.5 nmol, 5 μl) was microinjected using a 5-μl Hamilton microsyringe at 0.5 μl/min. The needle was slowly removed 5 min after injection. The drilled hole was sealed and the scalp debrided before suturing. Antagomir-223 and scrambled-miR were produced by Ribobio Co. (China) at a final concentration of 0.5 nmol/μl.
Animal surgery
Thirty minutes after right lateral ventricle injection, the middle cerebral artery occlusion (MCAO) model was produced according to methods described in a previous study [18]. In brief, anesthesia was performed with 5% isoflurane-inhalation induction and 2.5% maintenance (RWD Life Science Co., Shenzhen, China), and the right internal carotid artery was inserted by a nylon suture thread to block the middle cerebral artery for 90 min. Then, the nylon suture thread was withdrawn. The Sham group underwent the same surgical process except that the middle cerebral artery was not occluded.
EA treatment
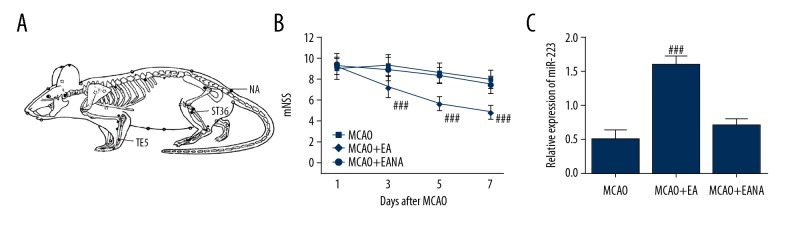
EA was performed 1 day after MCAO. Briefly, insertion of acupuncture needles (0.3 mm diameter, HuaTuo, Suzhou Medical Appliance Company, China) was carried out at a depth of about 3 mm into the acupoints Waiguan (TE5) and Zusanli (ST36) on the paralyzed left limb, followed by electrical stimulation with an electroacupuncture therapeutic apparatus (G6805-II, Shanghai Medical Electronic Apparatus, China) (Figure 1A). Continuous-wave EA (20HZ, 1 mA) was administered to rats for 30 min, once daily, for 7 continuous days. The following experiments were performed by a person blinded to group assignment.
Figure 1.
EA treatment on non-acupoint has no effect on the mNSS scoring and miR-223 expression. (A) The acupoints (TE5, ST36, and NA) of rats were treated by EA in the current study. (B) EA decreased the mNSS scores of the MCAO rats. EA treatment at NA showed no effect on mNSS score compared with the MCAO group. (C) EA increased the miR-223 expression of the MCAO rats. EA treatment at NA showed no effect on miR-223 expression compared with the MCAO group. The data are presented as the mean±SD. (n=8, and error bars represent mean ±SD. ### P<0.001, vs. the MCAO group). EA – electroacupuncture; MCAO – middle cerebral artery occlusion; NA – non-acupoint.
Neurobehavioral evaluation
Neurological deficit scores were evaluated 1, 3, 5, and 7 days after MCAO or sham operation, employing the modified neurological severity scoring (mNSS) system, by a blinded investigator. A higher mNSS score (0, normal; 18, maximal deficit) indicates more severe neurological behavioral impairment.
Infarct volume and brain water content measurements
At the end of the neurobehavioral test, rats (n=5) underwent deep anesthesia and decapitation. Brain samples were obtained, kept at −20°C for 10 min, and cut into 5 coronal 2-mm-thick sections. Staining was performed with 2% 2,3,5-triphenyltetrazolium chloride (TTC) (Sigma, USA; 30 min, 37°C) followed by fixation with paraformaldehyde (4%) overnight at 4°C. Sections were photographed with a digital camera, and ischemic brain injury areas were determined in a blinded manner with an Image-Pro Plus analyzer. Infarct volumes reflected infarct area percentages.
For brain water content measurement, the wet-dry method was employed. Briefly, the brain wet weight was recorded from 25 rats (n=5). Then, the fresh brains were dried and weighed to yield dry weights. Brain water content (%) was derived as (wet weight−dry weight)/wet weight×100%.
Hematoxylin-eosin (HE) staining, Nissl staining, and TUNEL staining
The animals (n=5) were submitted to deep anesthesia and transcardially perfused with pre-cooled heparinized physiological saline and 4% paraformaldehyde. Brain samples were collected, paraffin-embedded, and sectioned at 4-μm coronally. HE staining was carried out according to standard protocols. Toluidine blue (Nissl) staining was performed as previously described [19]. Results were examined under a light microscope (DM2500; Leica Microsystems, Germany). Four randomly selected high-power fields were analyzed, and Nissl-positive cells were calculated by Image-Pro Plus 6.0 (Media Cybernetics, USA) by a person blinded to group assignment. Results are expressed as the number of neurons.
The TUNEL assay was carried out with the In Situ Cell Death Detection Kit (Roche) according to the manufacturer’s directions. The sections underwent treatment with a red fluorescent-tagged enzyme solution and were examined under a fluorescence microscope (DM2500; Leica Microsystems, Germany). TUNEL-positive cells with red fluorescent were measured using Image-Pro Plus version 6.0 (Media Cybernetics, USA) by a person blinded to group assignment. Every sixth section of the coronal cortex samples was processed, and the results are expressed as the number of labeled cells.
Western blot analysis
Peri-infarct cortical tissue samples from each group (n=5) were resected for Western blot analysis using the method detailed in a previous report [19]. Protein amounts were measured by the bicinchoninic acid (BCA) method. Primary antibodies targeting NLRP3 (Abcam, 1: 500), Caspase-1 (Abcam, 1: 5000), and GAPDH (Santa Cruz, 1: 1000) were added and incubated overnight at 4°C, followed by incubation with horseradish peroxidase (HRP)- linked anti-IgG secondary antibodies (1: 1000) for 1 h. Blots were visualized with an enhanced chemiluminescence kit (Amersham Pharmacia, USA). Digital images were obtained and analyzed with BandScan software.
Quantitative real-time PCR
Peri-infarct cortical tissues from 5 rats per group were assessed. Total RNA extraction from the brain tissues was performed with TRIzol reagent (Aidlab) according to the manufacturer’s instructions. For miR-223 quantification, reverse transcription was carried out with a TaqMan MicroRNA Reverse Transcription Kit as directed by the manufacturer. U6 was used as an endogenous control. The primers used in this study included (5′-3′):
U6 (forward) CTCGCTTCGGCAGCACA;
U6 (reverse) AACGCTTCACGAATTTGCGT;
NLRP3 (forward) CTGCCAGGGCTCTGTTCATTG;
NLRP3 (reverse) CCTTCTGGTCCCTTCCTCACG;
rno-miR-223-3p (forward)
ACACTCCAGCTGGGTGTCAGTTTGTCAAATAC;
rno-miR-223-3p (reverse)
TCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGGGGTAT.
The relative miR-223 and NLRP3 expression levels were assessed by the 2−ΔΔCT method.
Enzyme-linked immunosorbent assay (ELISA)
Rats were sacrificed 7 days after MCAO, and amounts of IL-18 and IL-1β in peri-infarct cortical tissues were measured with specific ELISA kits (E-EL-R0567c and E-EL-R0012c, respectively; Elabscience, China) as directed by the manufacturer. The results are shown as pictograms per milligram (pg/mg).
Statistical analysis
GraphPad Prism 7.0 software was employed for data analyses. Values are expressed as mean ±SD. The Kaplan-Meier method was used for survival analysis, with the log-rank test used for comparisons. The mNSS scores were assessed by two-way ANOVA, while one-way ANOVA was used for the remaining data. Bonferroni correction was performed for multiple comparisons as needed. P<0.05 indicated statistical significance.
Results
EA increases survival rates and improves neurological outcome after MCAO
Our preliminary work first evaluated the EA treatment on non-acupoints (located at the base of the tail) on MCAO rats (MCAO+EANA). After 7 days of treatment, the effect was evaluated by mNSS scoring, and rats were sacrificed for miR-223 detection. We found that EA treatment on non-acupoints showed no effect on the mNSS scoring or miR-223 expression on MCAO rats (Figure 1).
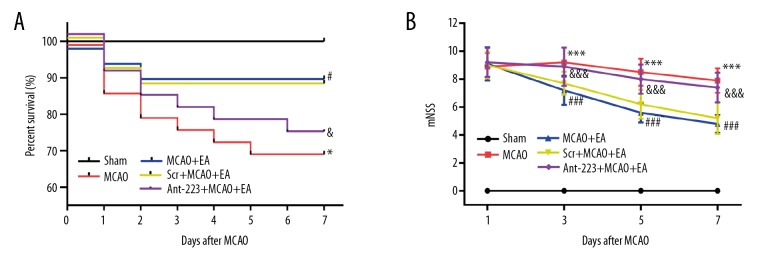
We initially included 108 rats in the study, and no deaths were recorded in the Sham group. Rat survival within 7 days of MCAO was markedly higher in the EA treatment group (91.7%) in comparison with the MCAO model (70%) and EA+MCAO+Ant-223 (73.3%) groups. The survival rate in the EA+MCAO+Scr group was 87.5%, comparable with that of the EA+MCAO group (Figure 2A). These findings suggested that EA treatment protected from MCAO-related death, and this neuroprotective effect was blocked by intracerebral injection of Antagomir-223.
Figure 2.
EA treatment increases the survival rate and improves neurological deficits in MCAO rats. (A) The Kaplan-Meier method was used for survival evaluation 7 days after the Sham or MCAO operation. The data are presented as percentages (n=20, 30, 24, 24 and 30 in the Sham, MCAO, EA+MCAO, EA+MCAO+Scr, EA+MCAO+Ant-223 groups, respectively). (B) The mNSS scores were markedly higher in the MCAO model compared with the Sham group, whereas EA decreased the mNSS scores of the MCAO rats; this effect was partially weakened by Ant-223. The data are presented as the mean ±SD. (n=10, and error bars represent mean ±SD. * P<0.05, vs. the Sham group; *** P<0.001, vs. the Sham group; # P<0.05, vs. the MCAO group; ### P<0.001, vs. the MCAO group; & P<0.05, vs. the EA+MCAO group; &&& P<0.001, vs. the EA+MCAO group). EA – electroacupuncture; MCAO – middle cerebral artery occlusion.
To explore whether EA improved neurological function recovery, mNSS was performed 1, 3, 5, and 7 days after modeling (Figure 2B). The mNSS score was markedly increased in MCAO rats in comparison with the sham group (P<0.001); meanwhile, EA treatment significantly decreased the mNSS scores in MCAO animals (P<0.001). Interestingly, intracerebral injection of antagomir-223 partially reversed the treatment effect, with higher mNSS scores in EA-treated MCAO rats, whereas scramble had no effect on the mNSS scores in EA-treated MCAO rats. These findings indicated that EA could efficiently improve neurological function after cerebral ischemia-reperfusion in this model. Moreover, antagomir-223 could partially suppress the EA-associated neurological function recovery.
EA treatment ameliorated cerebral infarction volume
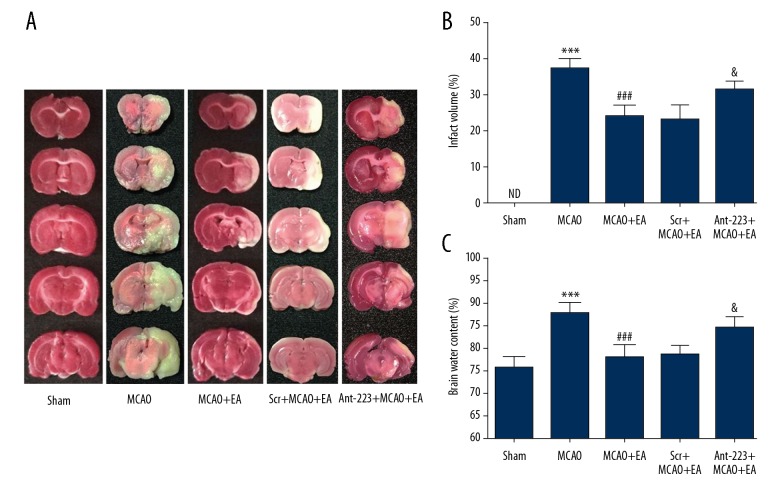
To assess the effect of EA on infarct volume, we performed TTC staining. The normal brain tissue appeared red, with infarct loci stained white (Figure 3A, 3B). Infarct volumes of the cerebral cortex were remarkably larger in MCAO rats (36.80±2.86%) compared with the Sham group (P<0.001). However, EA greatly reduced the cortical infarct volume in MCAO rats (23.60±3.36%). Intracerebral ventricular microinjection of antagomir-223 significantly reversed the reduction of infarct volume in EA-treated MCAO rats to 31.40±2.07%, while scramble had no obvious effects on infarct volume in EA-treated MCAO rats (cerebral infarct volume of 22.60±4.39%). As shown in Figure 3C, the water content of the brain tissue in MCAO rats (87.60±2.61%) was significantly higher than in the Sham group (75.60±2.61%). Meanwhile, brain water content was markedly lower in the EA treatment group (77.80±2.78%) in comparison with MCAO model rats (P<0.001). Antagomir-223 administration significantly reversed the reduction of brain water content in EA-treated MCAO rats to 84.40±2.51%. Scramble did not affect brain water content in EA-treated MCAO rats (brain water content of 78.40±2.41%). These results suggested that the MCAO model was successfully established, and EA treatment markedly reduced infarct volumes and brain water contents in MCAO rats. Moreover, the protective effects of EA were suppressed by antagomir-223.
Figure 3.
EA treatment reduces the brain infarct volume and alleviates cerebral edema upon MCAO. (A) Representative micrographs of TTC-stained brain sections in the 5 groups. (B) Infarct volume quantitation. (C) Brain water content quantitation (n=5, and error bars represent mean ±SD. *** P<0.001, vs. the Sham group; ### P<0.001, vs. the MCAO group; & P<0.05, vs. the EA +MCAO group). ND – not detected.
EA treatment alleviates apoptotic neuronal death
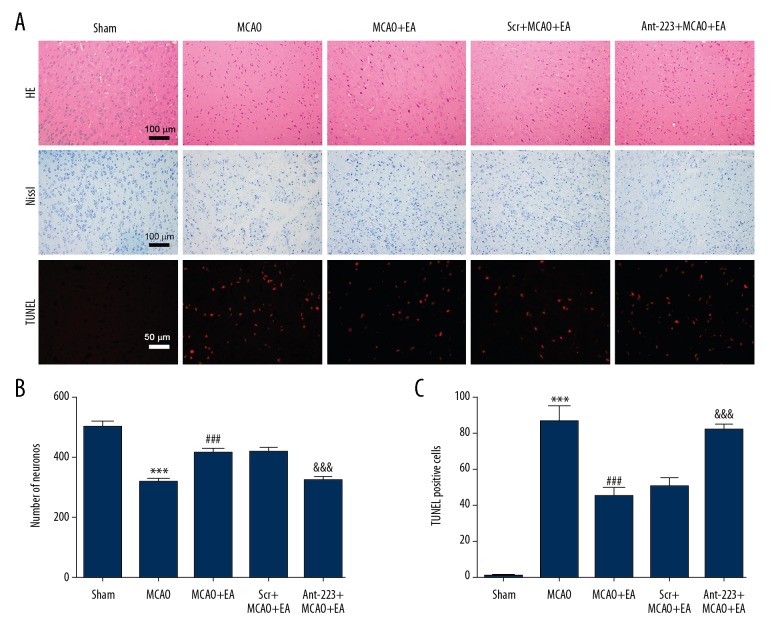
HE staining was used to assess histological changes in the brain. As shown in Figure 4A (first row), in the Sham operation group, histopathology was normal and no neuronal necrosis was observed. On the other hand, in the MCAO model group, most cells were arranged disorderly and neuronal necrosis was observed. However, EA treatment ameliorated these pathological abnormalities. The morphological changes in the EA+MCAO+Ant-223 group were more severe than in the EA+MCAO+Scr group.
Figure 4.
EA treatment rescues apoptotic neuronal death upon MCAO. (A) Representative micrographs of H&E staining, Nissl staining and TUNEL staining (scale bar=100 μm for H&E staining and Nissl staining; scale bar=50 μm for TUNEL staining). EA treatment increased the number of viable neurons and decreased the number of TUNEL-positive neurons after MCAO. (B) Quantitation of Nissl-stained neurons. (C) Quantitation of TUNEL-positive neurons. (n=5, and error bars represent mean ±SD. *** P<0.001, vs. the Sham group; ### P<0.001, vs. the MCAO group; &&& P<0.001, vs. the EA+MCAO group).
Next, the numbers of viable cortical neurons were evaluated. Histological analysis (Figure 4A, second row and Figure 4B) indicated that cerebral ischemia injury caused a marked decrease in numbers of viable neurons, while EA treatment protected the neurons. Markedly fewer Nissl-positive neurons were found in the MCAO model group (318.2±10.2) in comparison with Sham animals (499.6±19.0) (P<0.001), whereas EA treatment increased the numbers of Nissl-positive neurons (412.0±18.0) compared with the MCAO model group (P<0.001). Nissl-positive neurons in the EA+ MCAO+Scr group (416.6±12.8) was much more numerous than those of the EA+MCAO+Ant-223 group (321.4±13.4) (P<0.001).
We further examined the effect of EA treatment on brain injury after cerebral ischemia-reperfusion, evaluating neuronal apoptosis in the cortex (Figure 4A, third row and Figure 4C. Limited numbers of TUNEL-positive neurons were observed in Sham rats, while markedly more TUNEL-positive neurons were found in MCAO model animals (86.0±8.5). EA treatment (44.6±5.1) significantly decreased the number of TUNEL-positive neurons in MCAO rats; scramble microinjection (50.0±5.2) had no effect on the number of TUNEL-positive neurons in EA-treated MCAO rats, while antagomir-223 microinjection (81.0±3.5) significantly increased the number of TUNEL-positive neurons in EA-treated MCAO rats (P<0.001). These findings indicated that EA inhibited apoptotic neuronal death after MCAO in rats, and this effect was blocked by antagomir-223.
EA treatment increases miR-223 levels and decreases NLRP3 amounts
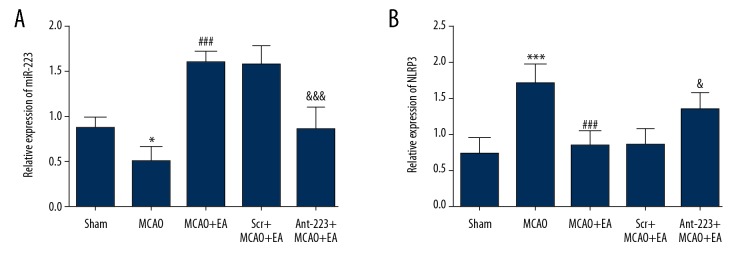
The expression of miR-223 was assessed by RT-PCR. Figure 5A shows that miR-223 expression was obviously lower in the MCAO control group (0.50±0.16) in comparison with Sham rats (0.87±0.13) (P<0.001), while miR-223 levels in the EA treatment group (1.60±0.13) were significantly higher than in MCAO model rats (P<0.001). Antagomir-223 administration significantly reduced the elevation of miR-223 induced by EA treatment (P<0.001) (0.86±0.25), while scramble had no effect on changes in level of miR-223 in EA-treated MCAO rats.
Figure 5.
Effects of EA treatment on miR-223 and NLRP3 levels in the peri-infarct cortex. (A) Statistical results of relative expression levels of miR-223 in the peri-infarct cortex; (B) Quantitative results of relative expression levels of NLRP3 in the peri-infarct cortex (n=5, and error bars represent mean ±SD. * P<0.05, vs. Sham group; *** P<0.001, vs. Sham group; ### P<0.001, vs. MCAO group; & P<0.05, vs. EA+MCAO group; &&& P<0.001, vs. EA+MCAO group).
The expression of NLRP3 was detected by RT-PCR. As shown in Figure 5B, NLRP3 expression levels in MCAO control rats (1.71±0.30) were markedly higher compared with those of Sham rats (0.74±0.24) (P<0.001). NLRP3 amounts in the EA treatment group (0.85±0.21) were greatly reduced in comparison with those of the MCAO control group (P<0.001). Antagomir-223 administration significantly increased NLRP3 elevation induced by EA treatment (1.35±0.23), while scramble had no effect on changes of NLRP3 level in EA-treated MCAO rats. The above findings indicated that EA promoted miR-223 activation and suppressed the expression of NLRP3 in the cortex of rats.
EA treatment ameliorates neuroinflammation
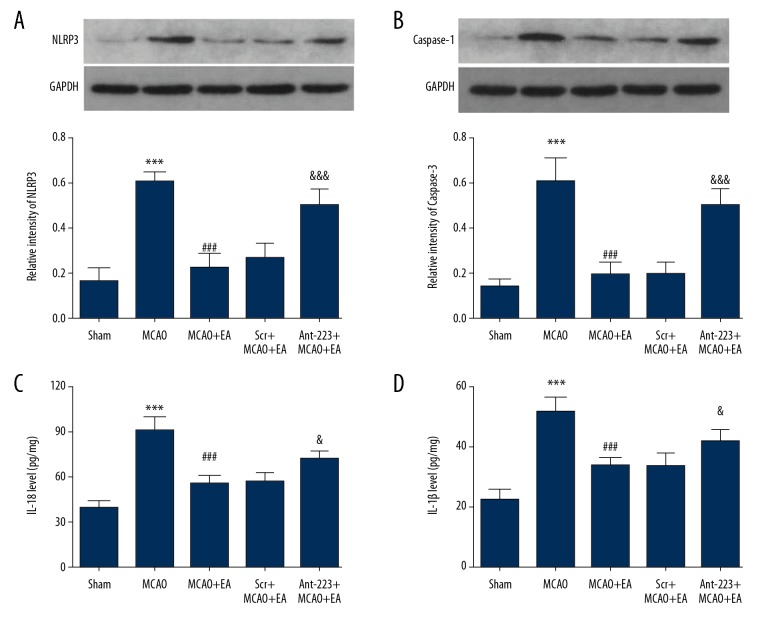
NLRP3 and Caspase-1 protein amounts were assessed by Western blot analysis. As illustrated in Figure 6A, 6B, NLRP3 levels were remarkably increased in the MCAO control group (0.61±0.05) in comparison with Sham animals (0.17±0.07) (P<0.001). EA decreased NLRP3 levels to 0.23±0.06. However, the effects of EA were reversed by antagomir-223 administration, with NLRP3 levels in the EA+MCAO+Ant-223 group of 0.50±0.08, while scramble had no effect on NLRP3 levels in EA-treated MCAO rats (0.27±0.06). Similarly, Caspase-1 levels were remarkably higher in the MCAO control group (0.60±0.11) in comparison with Sham animals (0.14±0.03) (P<0.001), and this effect was reversed by EA treatment to 0.19±0.06. Antagomir-223 treatment resulted in increased Caspase-1 levels in EA-treated MCAO rats (0.49±0.08), while scramble had no effect (0.20±0.05).
Figure 6.
Effects of EA treatment on NLRP3, Caspase-1, IL-1β, and IL-18 levels in the peri-infarct cortex. (A, B) Representative images and quantitative assessment of NLRP3 and caspase-1 in the cerebral cortex; (C, D) Quantitative evaluation of IL-1β and IL-18 levels in the cerebral cortex (n=5, and error bars represent mean ±SD. *** P<0.001, vs. Sham group; ### P<0.001, vs. MCAO group; & P<0.05, vs. EA+MCAO group, &&& P<0.001, vs. EA+MCAO group).
To further investigate the influence of EA on neuroinflammation, ELISA was performed to evaluate IL-18 and IL-1β levels in the peri-infarct cortex. As shown in Figure 6C, 6D, IL-18 levels were clearly elevated in the MCAO control group (90.88±8.78 mg) compared with Sham rats (38.85±4.98 mg), whereas EA treatment significantly reversed this effect (IL-18 levels of 55.50±5.73 mg). Antagomir-223 treatment (71.47±6.31 mg) increased IL-18 levels in EA-treated MCAO rats, while scramble microinjection had no effect (IL-18 levels of 56.35±6.07 mg). Similarly, IL-1β levels were remarkably higher in the MCAO control group (51.49±5.01 mg) in comparison with Sham rats (22.44±3.41 mg) (P<0.001), whereas EA treatment significantly reversed IL-1β levels to 33.56±2.67 mg. Antagomir-223 treatment increased IL-1β levels in EA-treated MCAO rats (41.74±3.91 mg), while scramble microinjection had no effect (33.65±4.33 mg). These results suggested that EA treatment downregulated NLRP3, Caspase-1, IL-18, and IL-1β levels in the cortex of rats. Moreover, miR-223 was essential for the ability of EA to inhibit the NLRP3 inflammasome. The above-mentioned results revealed that EA treatment inhibited inflammatory reactions via the miR-223/NLRP3 pathway.
Discussion
EA, based on traditional acupuncture and electrotherapy, is used as a prevention and treatment tool in stroke. Clinically, EA treatment is commonly performed via the acupoints of Waiguan (TE5) and Zusanli (ST36) in stroke patients [20]. Several experimental studies have shown that EA stimulation at acupoints TE5 or ST36 provides neuroprotective effects in cerebral ischemia rats [21,22]. In this study, we selected the acupoints TE5 and ST36 to assess the effects of EA treatment in the MCAO rat model and to explore the underlying mechanisms.
MicroRNAs are known to be associated with the regulation of stroke-related networks [23]. miR -223 is a biomarker of a wide variety of human metabolic ailments; it is also involved in inflammatory, autoimmune, and cardiovascular diseases, as well as cancer and other pathologies [24]. A previous study revealed that miR-223 participates in adipocyte inflammation related to obesity [25]. Meanwhile, miR-223 regulates the NLRP3 inflammasome, which is involved in inflammation after intracerebral hemorrhage [26]. In addition, miR-223 can also protect the brain from damage by glutamate excitotoxicity [27]. However, miR-223 involvement in the inflammatory reaction after ischemia/reperfusion injury has not been assessed. It was previously suggested that the neuroprotective effects of EA are related to the regulation of miRNAs [28,29]. Liu and colleagues demonstrated that EA treatment increases the levels of miR-214 and prevents neuronal apoptosis via Bax regulation and downregulates the sodium channel Nav1.3 in rats after spinal cord injury [30]. Zhou and collaborators demonstrated that miRNAs participate in the response to EA treatment after cerebral ischemia, with EA treatment alleviating brain damage by targeting NCS-1 through miR-191a-5p [31]. As shown above, miR-223 was associated with the inflammatory response and was significantly downregulated in brain tissue after cerebral ischemia/reperfusion injury, while miR-223 was upregulated after EA.
Extensive evidence indicates that inflammatory responses play crucial roles in brain injury following ischemia-reperfusion injury [32]. The NLRP3 inflammasome is found at high levels in the central nervous system, and it has thus attracted considerable attention [33]. The NLRP3 inflammasome is indeed critical in mediating inflammation in ischemic brain injury [34]. NLRP3, ASC, and pro-caspase-1 are the key components [35]. Upon stimulation by endogenous or exogenous activating irritants, the complete assembly of the NLRP3 inflammasome is activated [36]. Activated NLRP3 induces caspase-1, which in turn promotes IL-1β and IL-18 biosynthesis [37] and drives inflammatory reactions via multiple downstream signaling pathways, thus causing neuronal damage [38]. Research has shown that EA treatment attenuates neuroinflammation in the hippocampus by inhibiting the expression of the NLRP3 inflammasome [39]. Gao and colleagues demonstrated that EA treatment decreases the levels of the NLRP3 inflammasome through CB2 receptors in inflammatory pain [40]. As shown above, NLRP3 levels were elevated in MCAO rats. Moreover, NLRP3 amounts were decreased upon EA. NLRP3 is a direct miR-223 target [41]. In the present study, miR-223 levels were markedly higher after EA treatment. The increased expression of miR-223 downregulated NLRP3 in brain tissue. In addition, to investigate whether EA reduces NLRP3, caspase-1, IL-1β, and IL-18 levels in the MCAO model rats by upregulating miR-223, an intracerebroventricular injection of antagomir-223 or scrambled-miR was used. The results showed that miR-223 expression was essential for the inhibitory effect of EA on NLRP3. In addition, NLRP3 was shown to have a major role in neuronal death and behavioral deficits in cerebral ischemia/reperfusion injury [42]. Therefore, miR-223 may be a critical mediator of EA in an inflammatory state in brain ischemia/reperfusion injury.
In the central nervous system (CNS), astrocytes and microglia cells play an important and functionally role in regulating the inflammatory response. Accumulating studies have shown that activation of astrocytes and microglia cells was closely related to the neuroinflammation induced by neuro-injuries and neurodegenerative diseases [43–45]. It has been shown that, in the astrocytic α-synuclein mutant mouse model, which exhibit extensive neuroinflammatory and motor phenotypes of neurodegenerative disorders, EA stimulation at ST36 and SP6 enhanced both anti-inflammatory and antioxidant activities and suppressed aberrant glial activation in the diseased sites of brains [46]. In the central poststroke pain rat model, EA treatment at ST36 and GV20 could inhibit cyclooxygenase-2 (COX-2) expression to attenuate the inflammation and inhibit neuronal apoptosis and aberrant astrocyte activation [47]. Our current study focused more on the EA treatment than on the neuron protection and inhibition of neuroinflammation, which is a limitation of our research. Our future work will pay more attention to effects of EA treatment on the astrocyte and microglia cell functions, as well as the regulation mechanisms.
Another limitation of our study is that we only used a single EA parameter (20 HZ, 1 mA) for the MCAO rat treatment, as in previous studies [48–50]. Recent research has shown that high-frequency EA treatment was more effective in improving cognitive impairment in Alzheimer rats [51]. Our future work will focus more on the effect of different EA parameters on the CNS disease models.
As shown above, EA treatment at TE5 and ST36 acupoints significantly improved neurological functional recovery and reduced cerebral infarct. Furthermore, EA markedly increased miR-223 levels, and this effect was accompanied by decreased NLRP3, caspase-1, IL-1β, and IL-18 levels in the peri-infarct cortex, which resulted in alleviated brain ischemia/reperfusion-associated inflammatory injury. Taken together, the current findings suggest that EA has protective effects in cerebral injury upon ischemia/reperfusion, at least partly via miR-223/NLRP3 regulation.
Conclusions
EA treatment significantly improves neurological functional recovery and inhibits inflammatory reactions. The underlying mechanism may include miR-223/NLRP3 regulation. The present findings suggest a novel mechanism of EA treatment for cerebral ischemic injury.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (no. 81572238, 81071601 and 81774404)
References
- 1.Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120:439–48. doi: 10.1161/CIRCRESAHA.116.308413. [DOI] [PubMed] [Google Scholar]
- 2.Brouns R, De Deyn PP. The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg. 2009;111:483–95. doi: 10.1016/j.clineuro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Chen YJ, Nguyen HM, Maezawa I, et al. The potassium channel KCa3.1 constitutes a pharmacological target for neuroinflammation associated with ischemia/reperfusion stroke. J Cereb Blood Flow Metab. 2016;36:2146–61. doi: 10.1177/0271678X15611434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santoni G, Cardinali C, Morelli MB, et al. Danger- and pathogen-associated molecular patterns recognition by pattern-recognition receptors and ion channels of the transient receptor potential family triggers the inflammasome activation in immune cells and sensory neurons. J Neuroinflammation. 2015;12:21. doi: 10.1186/s12974-015-0239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: Master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 6.Liu D, Zeng X, Li X, et al. Role of NLRP3 inflammasome in the pathogenesis of cardiovascular diseases. Basic Res Cardiol. 2017;113:5. doi: 10.1007/s00395-017-0663-9. [DOI] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauernfeind F, Rieger A, Schildberg FA, et al. NLRP3 inflammasome activity is negatively controlled by miR-223. J Immunol. 2012;189:4175–81. doi: 10.4049/jimmunol.1201516. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Wan Y. Acupuncture mechanisms: Anesthesia, analgesia and protection on organ functions. World J Tradit Chin Med. 2015;1:59–66. [Google Scholar]
- 10.Zhang Y, Lan R, Wang J, et al. Acupuncture reduced apoptosis and up-regulated BDNF and GDNF expression in hippocampus following hypoxia–ischemia in neonatal rats. J Ethnopharmacol. 2015;172:124–32. doi: 10.1016/j.jep.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 11.Xing Y, Zhang M, Li W-B, et al. Mechanisms involved in the neuroprotection of electroacupuncture therapy for ischemic stroke. Front Neurosci. 2018;12:929. doi: 10.3389/fnins.2018.00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu S-f, Huang Y, Wang N, et al. Cardioprotective effect of electroacupuncture pretreatment on myocardial ischemia/reperfusion injury via antiapoptotic signaling. Evid Based Complement Alternat Med. 2016;2016 doi: 10.1155/2016/4609784. 4609784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Li J, Chen Z, et al. The influence of PC6 on cardiovascular disorders: A review of central neural mechanisms. Acupunct Med. 2012;30:47–50. doi: 10.1136/acupmed-2011-010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu H-P, Lin J-G. Acupuncture elicits neuroprotective effect by ameliorating cognitive deficits. Experimental Acupuncturology. 2018:151–68. [Google Scholar]
- 15.Han D, Liu Z, Wang G, et al. Electroacupuncture improves cognitive deficits through increasing regional cerebral blood flow and alleviating inflammation in CCI Rats. Evid Based Complement Alternat Med. 2017;2017 doi: 10.1155/2017/5173168. 5173168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu W, Wang X, Zheng Y, et al. Electroacupuncture inhibits inflammatory injury by targeting the miR-9-mediated NF-kappaB signaling pathway following ischemic stroke. Mol Med Rep. 2016;13:1618–26. doi: 10.3892/mmr.2015.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo F, Han X, Zhang J, et al. Repetitive transcranial magnetic stimulation promotes neural stem cell proliferation via the regulation of MiR-25 in a rat model of focal cerebral ischemia. PLoS One. 2014;9:e109267. doi: 10.1371/journal.pone.0109267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 19.Zhang B, Wei K, Li X, et al. Upregulation of Cdh1 signaling in the hippocampus attenuates brain damage after transient global cerebral ischemia in rats. Neurochem Int. 2018;112:166–78. doi: 10.1016/j.neuint.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Yang JS, Gao X, Sun R, et al. Effect of electroacupuncture intervention on rehabilitation of upper limb motor function in patients with ischemic stroke. Acupunct Res. 2015;40:489–92. [PubMed] [Google Scholar]
- 21.Lin D, Lin LL, Sutherland K, Cao CH. Manual acupuncture at the SJ5 (Waiguan) acupoint shows neuroprotective effects by regulating expression of the anti-apoptotic gene Bcl-2. Neural Regen Res. 2016;11:305–11. doi: 10.4103/1673-5374.177740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li QQ, Shi GX, Yang JW, et al. Hippocampal cAMP/PKA/CREB is required for neuroprotective effect of acupuncture. Physiol Behav. 2015;139:482–90. doi: 10.1016/j.physbeh.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Soreq H, Wolf Y. NeurimmiRs: MicroRNAs in the neuroimmune interface. Trends Mol Med. 2011;17:548–55. doi: 10.1016/j.molmed.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Taibi F, Metzinger-Le Meuth V, Massy ZA, Metzinger L. miR-223: An inflammatory oncomiR enters the cardiovascular field. Biochim Biophys Acta. 2014;1842:1001–9. doi: 10.1016/j.bbadis.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Zhuang G, Meng C, Guo X, et al. A novel regulator of macrophage activation: MiR-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125:2892–903. doi: 10.1161/CIRCULATIONAHA.111.087817. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z, Zhong L, Xian R, Yuan B. MicroRNA-223 regulates inflammation and brain injury via feedback to NLRP3 inflammasome after intracerebral hemorrhage. Mol Immunol. 2015;65:267–76. doi: 10.1016/j.molimm.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Harraz MM, Eacker SM, Wang X, et al. MicroRNA-223 is neuroprotective by targeting glutamate receptors. Proc Natl Acad Sci USA. 2012;109:18962–67. doi: 10.1073/pnas.1121288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng B, Bai F, Zhou H, et al. Electroacupuncture enhances rehabilitation through miR-181b targeting PirB after ischemic stroke. Sci Rep. 2016;6:38997. doi: 10.1038/srep38997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu W, Wu J, Huang J. Electroacupuncture regulates hippocampal synaptic plasticity via miR-134-,ediated LIMK1 function in rats with ischemic stroke. Neural Plast. 2017;2017 doi: 10.1155/2017/9545646. 9545646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Wu Y. Electro-acupuncture-modulated miR-214 prevents neuronal apoptosis by targeting Bax and inhibits sodium channel Nav1.3 expression in rats after spinal cord injury. Biomed Pharmacother. 2017;89:1125–35. doi: 10.1016/j.biopha.2017.02.077. [DOI] [PubMed] [Google Scholar]
- 31.Zhou H, Yang C, Bai F, et al. Electroacupuncture alleviates brain damage through targeting of neuronal calcium sensor 1 by miR-191a-5p after ischemic stroke. Rejuvenation Res. 2017;20:492–505. doi: 10.1089/rej.2017.1920. [DOI] [PubMed] [Google Scholar]
- 32.Mizuma A, Yenari MA. Anti-inflammatory targets for the treatment of reperfusion injury in stroke. Front Neurol. 2017;8:467. doi: 10.3389/fneur.2017.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agostini L, Martinon F, Burns K, et al. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–25. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 34.Lammerding L, Slowik A, Johann S, et al. Poststroke inflammasome expression and regulation in the peri-infarct area by gonadal steroids after transient focal ischemia in the rat brain. Neuroendocrinology. 2016;103:460–75. doi: 10.1159/000439435. [DOI] [PubMed] [Google Scholar]
- 35.Song L, Pei L, Yao S, et al. NLRP3 Inflammasome in neurological diseases, from functions to therapies. Front Cell Neurosci. 2017;11:63. doi: 10.3389/fncel.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He Q, Li Z, Wang Y, et al. Resveratrol alleviates cerebral ischemia/reperfusion injury in rats by inhibiting NLRP3 inflammasome activation through Sirt1-dependent autophagy induction. Int Immunopharmacol. 2017;50:208–15. doi: 10.1016/j.intimp.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 37.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–31. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 38.Fink SL, Bergsbaken T, Cookson BT. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc Natl Acad Sci USA. 2008;105:4312–17. doi: 10.1073/pnas.0707370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang J, Ding N. Electroacupuncture could influence the expression of IL-1beta and NLRP3 inflammasome in hippocampus of Alzheimer’s disease animal model. Evid Based Complement Alternat Med. 2018;2018 doi: 10.1155/2018/8296824. 8296824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao F, Xiang HC, Li HP, et al. Electroacupuncture inhibits NLRP3 inflammasome activation through CB2 receptors in inflammatory pain. Brain Behav Immun. 2018;67:91–100. doi: 10.1016/j.bbi.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Zhao G, Jiang K, Yang Y, et al. The potential therapeutic role of miR-223 in bovine endometritis by targeting the NLRP3 inflammasome. Front Immunol. 2018;9:1916. doi: 10.3389/fimmu.2018.01916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teng X, Chen W, Liu Z, et al. NLRP3 inflammasome is involved in Q-VD-OPH induced necroptosis following cerebral ischemia-reperfusion injury. Neurochem Res. 2018;43:1200–9. doi: 10.1007/s11064-018-2537-4. [DOI] [PubMed] [Google Scholar]
- 43.Kaur D, Sharma V, Deshmukh R. Activation of microglia and astrocytes: A roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology. :2019. doi: 10.1007/s10787-019-00580-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 44.Stephenson J, Nutma E, van der Valk P, Amor S. Inflammation in CNS neurodegenerative diseases. Immunology. 2018;154:204–19. doi: 10.1111/imm.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jassam YN, Izzy S, Whalen M, et al. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron. 2017;95:1246–65. doi: 10.1016/j.neuron.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng J, Lv E, Yang J, et al. Electroacupuncture remediates glial dysfunction and ameliorates neurodegeneration in the astrocytic α-synuclein mutant mouse model. J Neuroinflammation. 2015;12:103. doi: 10.1186/s12974-015-0302-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian G-H, Tao S-S, Chen M-T, et al. Electroacupuncture treatment alleviates central poststroke pain by inhibiting brain neuronal apoptosis and aberrant astrocyte activation. Neural Plast. 2016;2016 doi: 10.1155/2016/1437148. 1437148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han X, Huang X, Wang Y, Chen H. A study of astrocyte activation in the periinfarct region after cerebral ischemia with electroacupuncture. Brain Inj. 2010;24:773–79. doi: 10.3109/02699051003610482. [DOI] [PubMed] [Google Scholar]
- 49.Han X, Zhao X, Lu M, et al. Electroacupuncture ameliorates learning and memory via activation of the CREB signaling pathway in the hippocampus to attenuate apoptosis after cerebral hypoperfusion. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/156489. 156489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng C-X, Lu M, Guo Y-B, et al. Electroacupuncture ameliorates learning and memory and improves synaptic plasticity via activation of the PKA/CREB signaling pathway in cerebral hypoperfusion. Evid Based Complement Alternat Med. 2016;2016 doi: 10.1155/2016/7893710. 7893710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu C-C, Wang Y, Shen F, et al. High-frequency (50 Hz) electroacupuncture ameliorates cognitive impairment in rats with amyloid beta 1-42-induced Alzheimer’s disease. Neural Regen Res. 2018;13:1833–41. doi: 10.4103/1673-5374.238620. [DOI] [PMC free article] [PubMed] [Google Scholar]