Abstract
Background
The aim of this study was to evaluate lysophosphatidic acid receptor-2 (LPA2) and Krüppel-like factor 5 (KLF5) protein expression in gastric adenocarcinoma and their correlation with patient clinicopathological characteristics and prognosis.
Material/Methods
Fifty-one gastric adenocarcinoma tissue samples, 21 gastric intraepithelial neoplasia (GIN) samples, and 13 normal gastric tissue samples were collected to test for LPA2 and KLF5 expression by tissue microarray and immunohistochemistry assay. LPA2 and KLF5 positive expression rate between gastric adenocarcinoma, GIN, and normal gastric tissue were compared. The relationship between LPA2 expression, KLF5 expression, and patients’ clinicopathological characteristics and prognosis were evaluated.
Results
The positive expression rate of LPA2 and KLF5 were statistical different in gastric adenocarcinoma, GIN, and normal gastric tissue (P<0.05). LPA2 positive expression was associated with tumor invasion depth, Lauren type, vascular invasion, local lymph node metastasis, and clinical stage (P<0.05). There was no correlation between LPA2 expression (hazard ratio [HR]=1.84, 95% confidence interval [CI]: 0.89–3.80, P>0.05), KLF5 expression (HR=1.13, 95% CI: 0.53–2.36, P>0.05), and gastric cancer patients’ overall survival.
Conclusions
LPA2 and KLF5 protein expressions were differently expressed in gastric adenocarcinoma, GIN, and normal gastric tissue, and differences were correlated with patients’ clinical characteristic. However, LPA2 and KLF5 expressions were not correlated with the patients’ prognosis.
MeSH Keywords: Prognosis; Receptors, Lysophosphatidic Acid; Stomach Neoplasms
Background
Gastric cancer is one of the most diagnosed malignant tumors and the third most common cause of cancer associated death globally [1]. The general prognosis of gastric cancer is poor because of advanced stages when first diagnosis for most patients [2,3].
The molecular mechanism of the occurrence and development of gastric cancer has not yet been fully elucidated. Recent studies have shown that overexpression of oncogenes and low expression of tumor suppressor genes play an important role in the occurrence and development of gastric cancer [4,5]. It has been reported in the literature that lysophosphatidic acid receptor (LPA) is highly expressed in human malignant tumors and is associated with poor clinical characteristics and prognosis [6–8]. LPA2 protein is encoded by the LPA gene, which is located in the 19.62–19.62 region of human chromosome 19. LPA2 is a G protein-coupled receptor which can bind with its ligand and then activate LPA signaling pathway, thereafter, promote cell proliferation and malignant transformation. It has been reported that LPA2 is highly expressed in solid tumors such as breast cancer and participates in biological behaviors of cancer cells proliferation and invasion [6]. Krüppel-like factor 5 (KLF5), the basic transcription element-binding protein 2 (BTEB2) in eukaryotes, is a zinc finger protein transcription factor, also known as intestinal-enriched KLF (IKLF). KLF5 can further regulate its targets genes expression by activating or inhibiting the transcription of target genes, and plays an important role in cell proliferation, differentiation, and apoptosis [9,10]. However, the expression of LPA2 and KLF5 in gastric cancer and their relationship with the occurrence and development of gastric cancer have rarely been reported.
Material and Methods
Patients
Fifty-one gastric cancer patients seen from March 2010 to March 2016 in Second People’s Hospital of Jiuquan City, Gansu Province were included in this study. All the patients had pathological diagnosed gastric adenocarcinoma. In addition, 13 cases of normal gastric mucosa and 21 cases of gastric intraepithelial neoplasia (GIN) were selected for inclusion, including 12 cases of low-grade GIN and 9 cases of high-grade GIN. The general characteristics of the included cases presented in Table 1. This study was approved by the ethical committee of the Second People’s Hospital of Jiuquan City, Gansu Province. The research related to human use has been complied with all the relevant national regulations, institutional policies, and was performed in accordance with the tenets of the Helsinki Declaration, and was approved by the Second People’s Hospital of Jiuquan City, Gansu Province Institutional Review Board.
Table 1.
General characteristics of the included patients.
| Characteristics | Poor differentiation (n=19) | Moderate differentiation (n=16) | Well differentiation (n=16) | High grade GIN (n=9) | Low grade GIN (n=12) | Normal (n=13) |
|---|---|---|---|---|---|---|
| Age | 57.3±7.7 | 62.1±5.9 | 63.2±10.3 | 58.9±21.7 | 59.1±8.5 | 58.3±6.6 |
| Gender | ||||||
| Male | 18 | 15 | 14 | 7 | 10 | 12 |
| Female | 1 | 1 | 2 | 2 | 2 | 1 |
| Clinical stage | ||||||
| I | 5 | 5 | 12 | NA | NA | NA |
| II | 5 | 6 | 2 | NA | NA | NA |
| III | 6 | 4 | 2 | NA | NA | NA |
| IV | 3 | 1 | 0 | NA | NA | NA |
| Tumor location | ||||||
| Cardia | 6 | 9 | 13 | NA | NA | NA |
| Body of stomach | 3 | 3 | 3 | NA | NA | NA |
| Pylorus | 10 | 4 | 0 | NA | NA | NA |
| Invasion depth | ||||||
| Mucosa or submucosa | 2 | 2 | 7 | NA | NA | NA |
| Muscularis | 3 | 4 | 3 | NA | NA | NA |
| Serosa | 14 | 10 | 6 | NA | NA | NA |
| Lauren type | ||||||
| Intestinal type | 10 | 16 | 16 | NA | NA | NA |
| Diffuse type | 9 | 0 | 0 | NA | NA | NA |
| Vascular invasion | NA | NA | NA | |||
| Yes | 8 | 5 | 0 | NA | NA | NA |
| No | 11 | 11 | 16 | NA | NA | NA |
| Lymph node metastasis | ||||||
| Yes | 11 | 9 | 3 | NA | NA | NA |
| No | 8 | 7 | 13 | NA | NA | NA |
| Tumor diameter | ||||||
| ≤5 cm | 6 | 13 | 12 | NA | NA | NA |
| >5 cm | 13 | 3 | 4 | NA | NA | NA |
Methods
Instruments and equipment
The following instruments and reagents were used in this study: 1) rabbit anti-human LPA2 polyclonal antibody (Beijing Booshen Biotechnology Company, dilution number: bs-2881R, 1: 500); 2) anti-human KLF5 polyclonal antibody (Abcam, UK, 1: 600 dilution number: ab 24331); (3) instant SP9001 Kit (Beijing Zhongshan Jinqiao Biotechnology Company); 4) DAB enzyme substrate color reagent Kit (Beijing Zhongshan Jinqiao Biotechnology Company); 5) paraffin slicer (Leica Camera AG; Model: SHANDONAS-325); 6) OLYMPUSBX-40 microscope (Olympus); 7) constant-temperature bath (Grant Instruments), and 8) water purification system (Millipore Corporation).
Tissue microarray
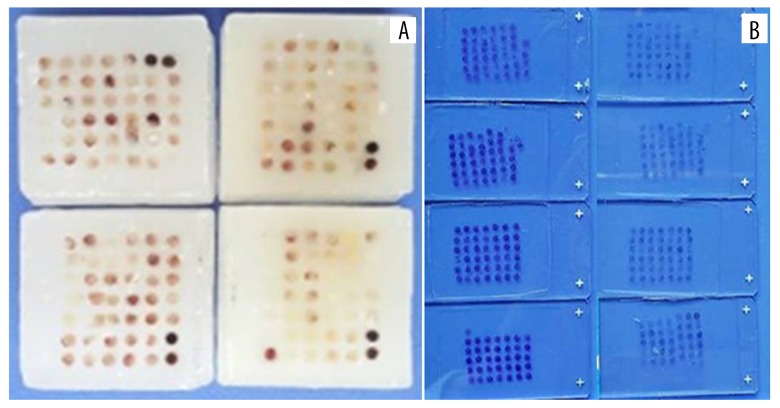
The pathology slices were reviewed, the tissues were located under the microscope, and the location of the wax blocks was marked to determine the sampling site (Figure 1). A 2-mm hollow wax pattern was made with a tissue chip die. The tissue wax core was taken out at the marker site of the wax block by a tissue chip sampler with an inner diameter of 2 mm. The wax core was placed into the hole of the hollow wax pattern. A 6×7 tissue microarray was made, and the wax block of the tissue chip was sliced continuously at a thickness of 4 microns. The wax core was attached to the APES anti-stripping slide (Figure 2).
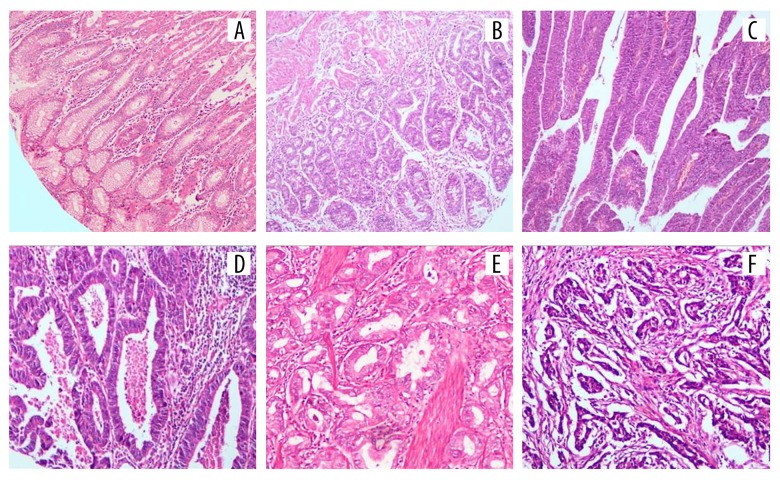
Figure 1.
Hematoxylin and eosin (H&E) staining results of tissues of different groups: (A) normal gastric mucosa tissue; (B) low grade GIN; (C) high grade GIN; (D) well differentiation gastric adenocarcinoma tissue; (E) moderate differentiation gastric adenocarcinoma tissue; (F) poor differentiation gastric adenocarcinoma tissue); magnification 10x. GIN, gastric intraepithelial neoplasia.
Figure 2.
Tissue microarray for detection LPA2 and KLF5 expression: (A) paraffin blocks of tissue microarray; (B) sections of tissue microarray. LPA2 – lysophosphatidic acid receptor-2; KLF5 – Krüppel-like factor 5.
Immunohistochemistry
After dewaxing and gradient alcohol hydration, pH 6.0 citric acid was used to repair antigen under high temperature and high pressure. Then 3% hydrogen peroxide was used to remove endogenous enzyme activity for 10 minutes. Reagent A was applied and sealed at room temperature for 10 minutes. Primary and secondary antibodies were added by dripping after the antigen was retrieved. The tissues were developed and mounted for microscopic examination.
Determination of positive results
Two pathologists read the slices independently. Five visual fields were selected under high power microscopy to calculate the proportion of LPA positive cells in each visual field. The main positive cells were brown-yellow granules in cytoplasm/membrane. Results were qualitatively described as follows: positive cells <5% (−), positive cells 5~25% (+), positive cells 25~50% (++), positive cells (++), positive cells (++) and positive cells >50% (+++).
Statistical analysis
Data in the present work was analyzed through Stata 11.0 statistical software (Stata Corporation, College Station, TX, USA). Measurement data and counted data expressed as mean ± standard deviation and rate. Comparisons were made by Fisher’s exact test and F test. Differences were considered significant at P<0.05.
Results
LPA 2 expression in different groups
LPA2 was mainly expressed in the cytoplasm and cell membrane with brown granular (Figure 3). The positive expression rate was 89.5% (17 out of 19), 81.3% (13 out of 16), 25.0% (4 out of 16), 33.3% (3 out of 9), 25.0% (3 out of 12), and 23.1% (3 out of 13) for poor, moderate, well differentiation gastric adenocarcinoma, high grade GIN, low grade GIN, and normal gastric tissue respectively with statistical difference (P<0.05) (Table 2). The mRNA relative expression level of LPA2 was examined by real-time polymerase chain reaction (PCR) assay. The results showed that the LPA2 mRNA level of gastric adenocarcinoma and GIN were 0.045±0.018 and 0.021±0.009 respectively with statistical difference (P<0.05).
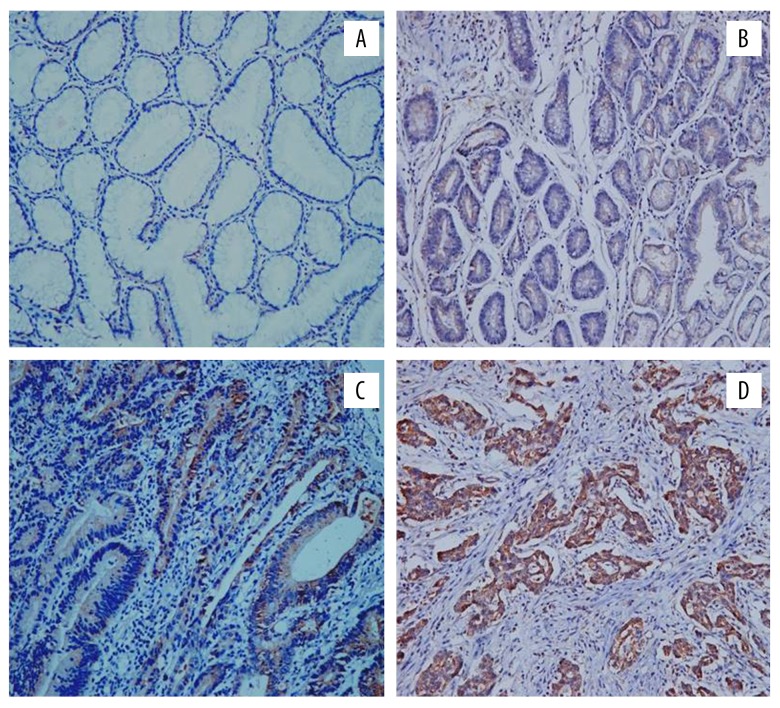
Figure 3.
Immunohistochemical staining results of LPA2 expression of different groups: (A) positive expression of LPA2 in normal gastric mucosa tissue; (B) positive expression of LPA2 in low grade GIN; (C) positive expression of LPA2 in high grade GIN; (D) positive expression of LPA2 in gastric adenocarcinoma tissue); magnification 20×. LPA2 – lysophosphatidic acid receptor-2; GIN – gastric intraepithelial neoplasia.
Table 2.
LPA2 Expression in different groups (n).
| Histological category | Negative (n) | Positive (n) | Positive (%) |
|---|---|---|---|
| Gastric adenocarcinoma | |||
| Poor (n=19) | 2 | 17 | 89.5 |
| Moderate (n=16) | 3 | 13 | 81.3 |
| Well (n=16) | 12 | 4 | 25.0 |
| GIN | |||
| High grade GIN (n=9) | 6 | 3 | 33.3 |
| Low grade GIN (n=12) | 9 | 3 | 25.0 |
| Normal gastric tissue (n=13) | 10 | 3 | 23.1 |
LPA2 – lysophosphatidic acid receptor-2; GIN – gastric intraepithelial neoplasia.
Correlation between LPA2 expression and clinicopathological features
LPA2 positive expression was not correlated with age, sex, location, diameter, or histological grade of the patients (P>0.05), but was correlated with depth of invasion, Lauren classification, vascular invasion, lymph node metastasis, and clinical stage (P<0.05) (Table 3).
Table 3.
Correlation between LPA2 expression and clinicopathological characteristics (n).
| Index | N=51 | LPA2 | Chi-square | P | |
|---|---|---|---|---|---|
| + (n=34) | − (n=17) | ||||
| Gender | 0.17 | 0.68 | |||
| Male | 47 | 32 | 15 | ||
| Female | 4 | 2 | 2 | ||
| Age (years) | 0.30 | 0.59 | |||
| <50 | 8 | 6 | 2 | ||
| ≥50 | 43 | 28 | 15 | ||
| Tumor location | 0.96 | 0.62 | |||
| Cardia | 28 | 17 | 11 | ||
| Body of stomach | 10 | 7 | 3 | ||
| Pylorus | 13 | 10 | 3 | ||
| Lauren type | 5.46 | 0.02 | |||
| Intestinal type | 42 | 25 | 17 | ||
| Diffuse type | 9 | 9 | 0 | ||
| Vascular invasion | 5.16 | 0.02 | |||
| No | 38 | 22 | 16 | ||
| Yes | 13 | 12 | 1 | ||
| Lymph node metastasis | 7.76 | 0.01 | |||
| No | 28 | 14 | 14 | ||
| Yes | 23 | 20 | 3 | ||
| Clinical stage | 8.71 | 0.03 | |||
| I | 22 | 10 | 12 | ||
| II | 13 | 11 | 2 | ||
| III | 12 | 9 | 3 | ||
| IV | 4 | 4 | 0 | ||
| Invasion depth | 11.63 | 0.00 | |||
| Mucosa or submucosa | 11 | 3 | 8 | ||
| Muscularis | 10 | 6 | 4 | ||
| Serosa | 30 | 25 | 5 | ||
| Tumor diameter | 0.02 | 0.89 | |||
| ≤5 cm | 35 | 23 | 12 | ||
| >5 cm | 16 | 11 | 5 | ||
| Histological grade | 0.00 | 0.96 | |||
| Well | 16 | 4 | 12 | ||
| Moderate | 16 | 13 | 3 | ||
| Poor | 19 | 17 | 2 | ||
LPA2 – lysophosphatidic acid receptor-2.
KLF5 expression in different groups
KLF5 was mainly expressed in the cytoplasm, brown granular (Figure 4). The positive expression rate was 78.9% (15 out of 19), 75.0% (12 out of 16), 75.0% (12 out of 16), 66.7% (6 out of 9), 58.3% (7 out of 12), and 38.5% (5 out of 13) for poor, moderate, well differentiation gastric adenocarcinoma, high grade GIN, low grade GIN, and normal gastric tissue respectively with statistical difference (P<0.05) (Table 4). The mRNA of relative expression level of KLF5 was examined by real-time PCR assay. The results showed that the KLF5 mRNA level of gastric adenocarcinoma and GIN were 0.64±0.21 and 0.62±0.20 respectively without statistical difference (P>0.05).
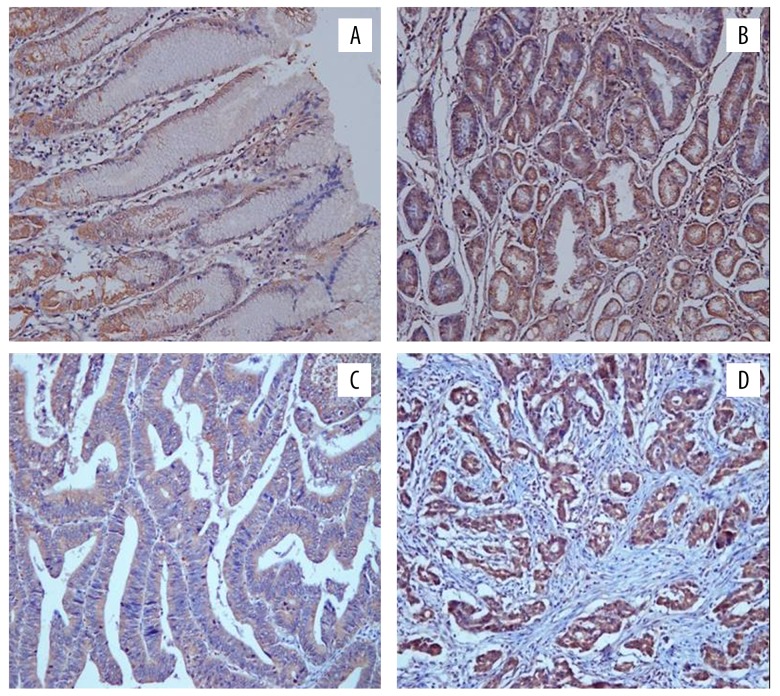
Figure 4.
Immunohistochemical staining results of KLF5 expression of different groups: (A) positive expression of KLF5 in normal gastric mucosa tissue; (B) positive expression of KLF5 in low grade GIN; (C) positive expression of KLF5 in high grade GIN; (D) positive expression of KLF5 in gastric adenocarcinoma tissue); magnification 20×. KLF5 – Krüppel-like factor 5; GIN – gastric intraepithelial neoplasia
Table 4.
KLF5 expression in different groups (n).
| Histological category | Negative (n) | Positive (n) | Positive (%) |
|---|---|---|---|
| Gastric adenocarcinoma | |||
| Poor (n=19) | 4 | 15 | 78.9 |
| Moderate (n=16) | 4 | 12 | 75.0 |
| Well (n=16) | 4 | 12 | 75.0 |
| GIN | |||
| High grade GIN (n=9) | 3 | 6 | 66.7 |
| Low grade GIN (n=12) | 5 | 7 | 58.3 |
| Normal gastric tissue (n=13) | 8 | 5 | 38.5 |
KLF5 – Krüppel-like factor 5; GIN – gastric intraepithelial neoplasia.
Correlation between KLF5 expression and clinicopathological features
The positive expression of KLF5 protein was not correlated with age, sex, tumor location, tumor diameter, histological grade, depth of invasion, Lauren classification, vascular invasion, regional lymph node metastasis, or clinical stage (P>0.05), (Table 5).
Table 5.
Correlation between KLF5 expression and clinicopathological characteristics (n).
| Index | N=51 | KLF5 | Chi-square | P | |
|---|---|---|---|---|---|
| + (n=39) | − (n=12) | ||||
| Gender | 0.01 | 0.94 | |||
| Male | 47 | 36 | 11 | ||
| Female | 4 | 3 | 1 | ||
| Age (years) | 0.64 | 0.42 | |||
| <50 | 8 | 7 | 1 | ||
| ≥50 | 43 | 32 | 11 | ||
| Tumor location | 0.46 | 0.79 | |||
| Cardia | 28 | 22 | 6 | ||
| Body of stomach | 10 | 8 | 2 | ||
| Pylorus | 13 | 9 | 4 | ||
| Lauren type | 0.01 | 0.92 | |||
| Intestinal type | 42 | 32 | 10 | ||
| Diffuse type | 9 | 7 | 2 | ||
| Vascular invasion | 0.51 | 0.48 | |||
| No | 38 | 30 | 8 | ||
| Yes | 13 | 9 | 4 | ||
| Lymph node metastasis | 0.88 | 0.35 | |||
| No | 28 | 20 | 8 | ||
| Yes | 23 | 19 | 4 | ||
| Clinical stage | 5.41 | 0.14 | |||
| I | 22 | 16 | 6 | ||
| II | 13 | 12 | 1 | ||
| III | 12 | 7 | 5 | ||
| IV | 4 | 4 | 0 | ||
| Invasion depth | 1.28 | 0.53 | |||
| Mucosa or submucosa | 11 | 7 | 4 | ||
| Muscularis | 30 | 24 | 6 | ||
| Serosa | 10 | 8 | 2 | ||
| Tumor diameter | 1.59 | 0.21 | |||
| ≤5 cm | 37 | 30 | 7 | ||
| >5 cm | 14 | 9 | 5 | ||
| Histological grade | 0.07 | 0.78 | |||
| Well | 16 | 12 | 4 | ||
| Moderate | 16 | 12 | 4 | ||
| Poor | 19 | 15 | 4 | ||
KLF5 – Krüppel-like factor 5.
LPA2, KLF5 expression and prognosis
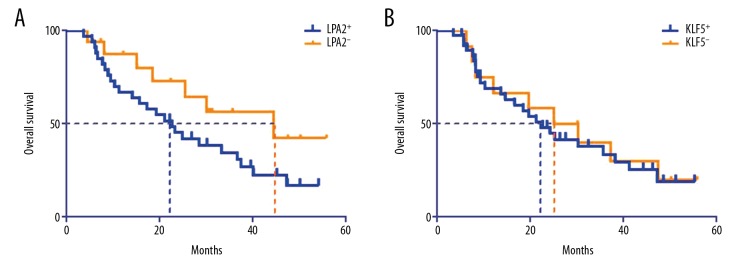
There was no correlation between LPA2 (HR=1.84, 95% CI: 0.89–3.80, P>0.05), KLF5 (HR=1.13, 95% CI: 0.53–2.36, P>0.05) expression and patients’ overall survival (Figure 5). For disease free survival (DFS), the difference was also not statistical different between LPA2 (HR=1.34, 95% CI: 0.68–3.12), KLF5 (HR=1.05, 95% CI: 0.46–2.14) positive and negative groups.
Figure 5.
Survival curves f gastric adenocarcinoma patients between LPA2, KLF5 positive and negative groups: (A) LPA2; (B) KLF5. LPA2 – lysophosphatidic acid receptor-2; KLF5 – Krüppel-like factor 5.
Discussion
Gastric cancer is one of the most diagnosed malignant tumor of digestive system and the third leading cause of cancer related death [11]. The development of gastric cancer is a multi-factor, multi-gene and multi-step process [12,13]. It is also the result of the interaction of genetic and environmental factors. It involves the activation of oncogene and inactivation of anti-oncogene, activation of telomere and gene instability. In the process of tumorigenesis and development, although the activation of oncogenes is one of the main factors to promote tumorigenesis, the inactivation of tumor suppressor genes may play a more important role [14,15].
Generally, canceration seldom occurs directly from normal gastric mucosal epithelium. In most cases, the occurrence and development of gastric cancer is closely related to benign chronic gastric diseases and dysplasia of gastric mucosa. Precancerous diseases of gastric cancers refer to a number of clinical situations in which the risk of gastric cancer is significantly increased. Precancerous disease of gastric cancer includes chronic atrophic gastritis with or without intestinal metaplasia and malignant anemia, chronic gastric ulcer, remnant stomach, gastric polyp and gastric mucosal giant plica disease (Menetrier disease) after operation, and precancerous lesions of gastric cancer include gastric cancer-related diseases and dysplasia of gastric mucosa [16–19]. Gastric mucosal dysplasia is currently recognized as a precancerous lesion, especially moderate to severe dysplasia. Gastric intraepithelial neoplasia (GIN) is synonymous with dysplasia.
LPA is a multifunctional “messenger phospholipid”, which can promote cell proliferation and change cell morphology. LPA is a normal component of serum, but its level in normal human plasma is extremely low [20]. As a lipid signaling molecule, LPA can mediate various signal transduction pathways and plays its biological role by activating specific G protein-coupled receptors. There are 6 G protein-coupled receptors (GPCRs) in LPA, namely LPA1-6. Classical LPA receptors, including LPA1, LPA2, and LPA3, belong to the endothelial differentiation gene receptor (EDG) family, namely LPA1/EDG2, LPA2/EDG4, and LPA3/EDG7. LPA2 protein is encoded by the LPA gene, which is located in the 19.62–19.62 region of human chromosome 19. LPA2 is a G protein-coupled receptor. LPA2 binding with its ligand can activate LPA signaling pathway, thereafter, promote cell proliferation and malignant transformation [6]. Human LPA2 protein contains 351 amino acid residues with molecular weight of 39 100. There are 3 exons and 2 introns in the coding region of LPA2 gene. LPA1, LPA2 and LPA3 can bind to Gai/o and Gaq to regulate the signal transduction pathway of LPA.
Studies have shown that LPA2 is closely related to the occurrence of various solid tumors, such as breast cancer, ovarian cancer, colorectal cancer, pancreatic cancer, etc. [21,22]. LPA2 is abnormally expressed in these tumors, and has a certain correlation with the tumor occurrence, invasion, metastasis, and prognosis.
In the present work, we found that the positive rate of LPA2 in gastric cancer tissue was significantly higher than that of GIN and normal gastric mucosa (P<0.001), which was in accordance with a precious study [23]. However, LPA2 positive expression was not correlated with age, sex, tumor location, tumor diameter, or histological grade of gastric adenocarcinoma (P>0.05), but was significantly correlated with depth of invasion, Lauren classification, vascular invasion, lymph node metastasis, and clinical stage (all P<0.05). The expression of LPA2 protein increased with the depth of invasion, regional lymph node metastasis, vascular invasion, Lauren’s diffuse type, and clinical stage of gastric adenocarcinoma, which preliminarily indicated that the gastric adenocarcinoma with high expression of LPA2 might be more invasive, and LPA2 might be involved in the development and metastasis of gastric adenocarcinoma, which played a role in the invasion, metastasis, and progression of gastric adenocarcinoma. However, long-rank survival analysis did not find overall and DFS difference between LPA2 positive and negative groups.
The human KLF5 gene is encoded in the 13q21 region of human chromosome. The KLF5 protein contains 457 amino acids with a molecular weight of 55 kDa. Its CDS coding region contains an activation domain and a DNA binding domain. KLF5 contains many target genes, such as nuclear factor κB (NF-κB), peroxisome proliferator-activated receptor (PPAR), platelet-derived growth factor a (pDGFa) and T cell antigen receptor (TCR), which have been confirmed in different cells. As a zinc finger transcription factor, KLF5 further participates in the regulation of cell proliferation, cell differentiation, cell apoptosis, and individual development by regulating the expression of GC-rich promoter region.
Publications have proven that KLF5 plays an important role in many malignant tumors, such as bladder cancer [24], prostate cancer [25], breast cancer [26], colon cancer, thyroid cancer [27], etc., but the biological behavior of KLF5 is different in different tumors [28]. Nandan et al. [29] found that KLF5 was highly expressed in the proliferative and active crypt epithelium of normal intestinal tissues. By stimulating cyclinB1, cyclinD1 and Cdc2, KLF5 accelerated cell cycle and promoted cell growth. KLF5 can promote the growth of normal cells, while KLF5 can inhibit the growth of some cancer cells, which is incomprehensible. Studies have reported that KLF5 is highly expressed in gastric adenocarcinoma [30], but studies have also shown that the expression of KLF5 in gastric adenocarcinoma is significantly downregulated [31]. In our study, we didn’t find any correlation between KLF5 expression and patients’ clinicopathological features and prognosis.
Conclusions
LPA2 and KLF5 were different expressed in gastric adenocarcinoma, GIN, and normal gastric tissue. LPA2 protein is highly expressed in gastric adenocarcinoma tissues. Its positive expression is closely related to the depth of invasion, Lauren classification, vascular invasion, regional lymph node metastasis, and clinical stage. This suggests that LPA2 might be involved in the development of gastric adenocarcinoma and could be used as an important biological marker for prognosis and biological behavior of gastric cancer. However, the sample size of our study was small, and the statistical power was limited. The conclusion should be further proven by large sample sized high quality studies.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Fang H, Zhang Y, Wu Z, et al. Regional hyperthermia combined with chemotherapy in advanced gastric cancer. Open Med (Wars) 2019;14:85–90. doi: 10.1515/med-2019-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinoshita J, Fushida S, Kaji M, et al. A randomized controlled trial of postoperative intravenous acetaminophen plus thoracic epidural analgesia vs. thoracic epidural analgesia alone after gastrectomy for gastric cancer. Gastric Cancer. 2019;22:392–402. doi: 10.1007/s10120-018-0863-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Zhang M, Zheng X. High Expression of NLRC5 is associated with prognosis of gastric cancer. Open Med (Wars) 2018;13:443–49. doi: 10.1515/med-2018-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun L, Zhang Y, Zhang C. Distinct expression and prognostic value of MS4A in gastric cancer. Open Med (Wars) 2018;13:178–88. doi: 10.1515/med-2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M, Xiao D, Zhang J, et al. Expression of LPA2 is associated with poor prognosis in human breast cancer and regulates HIF-1α expression and breast cancer cell growth. Oncol Rep. 2016;36:3479–87. doi: 10.3892/or.2016.5206. [DOI] [PubMed] [Google Scholar]
- 7.Tao K, Guo S, Chen R, et al. Lysophosphatidic acid receptor 6 (LPAR6) expression and prospective signaling pathway analysis in breast cancer. Mol Diagn Ther. 2019;23:127–38. doi: 10.1007/s40291-019-00384-3. [DOI] [PubMed] [Google Scholar]
- 8.Ishii S, Tsujiuchi T, Fukushima N. Functional characterization of lysophosphatidic acid receptor 1 mutants identified in rat cancer tissues. Biochem Biophys Res Commun. 2017;486:767–73. doi: 10.1016/j.bbrc.2017.03.118. [DOI] [PubMed] [Google Scholar]
- 9.Ma Y, Wang Q, Liu F, et al. KLF5 promotes the tumorigenesis and metastatic potential of thyroid cancer cells through the NF-κB signaling pathway. Oncol Rep. 2018;40:2608–18. doi: 10.3892/or.2018.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Q, Jia J, Hui K, et al. KLF5 promotes apoptosis induced by phorbol ester as an effector of the autocrine factor TNFα in LNCaP prostate cancer cells. Oncol Lett. 2017;14:1847–54. doi: 10.3892/ol.2017.6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 12.Huang T, Song C, Zheng L, et al. The roles of extracellular vesicles in gastric cancer development, microenvironment, anti-cancer drug resistance, and therapy. Mol Cancer. 2019;18:62. doi: 10.1186/s12943-019-0967-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto MP, Owen GI, Retamal I, Garrido M. Angiogenesis inhibitors in early development for gastric cancer. Expert Opin Investig Drugs. 2017;26:1007–17. doi: 10.1080/13543784.2017.1361926. [DOI] [PubMed] [Google Scholar]
- 14.Molaei F, Forghanifard MM, Fahim Y, Abbaszadegan MR. Molecular signaling in tumorigenesis of gastric cancer. Iran Biomed J. 2018;22:217–30. doi: 10.22034/ibj.22.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molaei F, Forghanifard MM, Fahim Y, Abbaszadegan MR. Molecular signaling in tumorigenesis of gastric cancer. Iran Biomed J. 2018;22:217–30. doi: 10.22034/ibj.22.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang HP, Yang S, Chen WH, et al. The diagnostic value of confocal laser endomicroscopy for gastric cancer and precancerous lesions among Asian population: A system review and meta-analysis. Scand J Gastroenterol. 2017;52:382–88. doi: 10.1080/00365521.2016.1275770. [DOI] [PubMed] [Google Scholar]
- 17.Watari J, Chen N, Amenta PS, et al. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J Gastroenterol. 2014;20:5461–73. doi: 10.3748/wjg.v20.i18.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang HP, Yang S, Chen WH, et al. The diagnostic value of confocal laser endomicroscopy for gastric cancer and precancerous lesions among Asian population: A system review and meta-analysis. Scand J Gastroenterol. 2017;52:382–88. doi: 10.1080/00365521.2016.1275770. [DOI] [PubMed] [Google Scholar]
- 19.Langner C. [Precursors of gastric cancer: Dysplasia and adenoma]. Pathologe. 2017;38:67–74. doi: 10.1007/s00292-017-0270-4. [DOI] [PubMed] [Google Scholar]
- 20.Han SG, Baek SI, Son TJ, et al. Preparation of functional human lysophosphatidic acid receptor 2 using a P9* expression system and an amphipathic polymer and investigation of its in vitro binding preference to Gα proteins. Biochem Biophys Res Commun. 2017;487:103–8. doi: 10.1016/j.bbrc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 21.Dutta S, Wang FQ, Wu HS, et al. The NF-κB pathway mediates lysophosphatidic acid (LPA)-induced VEGF signaling and cell invasion in epithelial ovarian cancer (EOC) Gynecol Oncol. 2011;123:129–37. doi: 10.1016/j.ygyno.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Lee SJ, Ritter SL, Zhang H, et al. MAGI-3 competes with NHERF-2 to negatively regulate LPA2 receptor signaling in colon cancer cells. Gastroenterology. 2011;140:924–34. doi: 10.1053/j.gastro.2010.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamashita H, Kitayama J, Shida D, et al. Differential expression of lysophosphatidic acid receptor-2 in intestinal and diffuse type gastric cancer. J Surg Oncol. 2006;93:30–35. doi: 10.1002/jso.20397. [DOI] [PubMed] [Google Scholar]
- 24.Du C, Gao Y, Xu S, et al. KLF5 promotes cell migration by up-regulating FYN in bladder cancer cells. FEBS Lett. 2016;590:408–18. doi: 10.1002/1873-3468.12069. [DOI] [PubMed] [Google Scholar]
- 25.Guan C, Zhang L, Wang S, et al. Upregulation of microRNA-21 promotes tumorigenesis of prostate cancer cells by targeting KLF5. Cancer Biol Ther. 2019 doi: 10.1080/15384047.2019.1599659. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin Y, Liu R, Zhao P, et al. Discovery of novel mifepristone derivatives via suppressing KLF5 expression for the treatment of triple-negative breast cancer. Eur J Med Chem. 2018;146:354–67. doi: 10.1016/j.ejmech.2018.01.056. [DOI] [PubMed] [Google Scholar]
- 27.Ma Y, Wang Q, Liu F, et al. KLF5 promotes the tumorigenesis and metastatic potential of thyroid cancer cells through the NF-κB signaling pathway. Oncol Rep. 2018;40:2608–18. doi: 10.3892/or.2018.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma D, Chang LY, Zhao S, et al. KLF5 promotes cervical cancer proliferation, migration and invasion in a manner partly dependent on TNFRSF11a expression. Sci Rep. 2017;7:15683. doi: 10.1038/s41598-017-15979-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nandan MO, Yoon HS, Zhao W, et al. Krüppel-like factor 5 mediates the transforming activity of oncogenic H-Ras. Oncogene. 2004;23:3404–13. doi: 10.1038/sj.onc.1207397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soon MS, Hsu LS, Chen CJ, et al. Expression of Krüppel-like factor 5 in gastric cancer and its clinical correlation in Taiwan. Virchows Arch. 2011;459:161–66. doi: 10.1007/s00428-011-1111-0. [DOI] [PubMed] [Google Scholar]
- 31.Kwak MK, Lee HJ, Hur K, et al. Expression of Krüppel-like factor 5 in human gastric carcinomas. J Cancer Res Clin Oncol. 2008;134:163–67. doi: 10.1007/s00432-007-0265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]