Abstract
In recent years, we found that Hishimonus lamellatus Cai et Kuoh is a potential vector of jujube witches’-broom phytoplasma. However, little is known about the anatomy and histology of this leafhopper. Here, we examined histology and ultrastructure of the digestive system of H. lamellatus, both by dissecting and by semi- and ultrathin sectioning techniques. We found that the H. lamellatus digestive tract consists of an esophagus, a filter chamber, a conical midgut and midgut loop, Malpighian tubules, an ileum, and a rectum. Furthermore, both the basal region of the filter chamber epithelium and the apical surface of the midgut epithelium have developed microvilli. We also identify the perimicrovillar membrane, which ensheaths the microvilli of midgut loop enterocyte, and the flame-like luminal membrane, which covers the microvilli of the conical midgut epithelium. In addition, H. lamellatus has the principal and accessory salivary glands. Our observations also showed that the endoplasmic reticulum, mitochondria, and secretory granules were all highly abundant in the secretory cells of the principal salivary glands, while the accessory glands consist of only one ovate or elbow-like acinus. We also briefly contrast the structure of the gut of H. lamellatus with those of other leafhopper species. These results intend to offer help for the future study on the histological and subcellular levels of phytopathogen–leafhopper relationships, including transmission barriers and the binding sites of pathogens and other microorganisms within their leafhopper vectors.
Keywords: leafhopper, digestive system, Malpighian tubule, ultrastructure, histology
Leafhoppers are herbivorous insects belong to the family to the old suborder Auchenorrhyncha. More than 25,000 species of leafhoppers are known worldwide (Dietrich 1997), of which over 1,200 are present in China (Li et al. 2012). Some of the leafhoppers must be mesophyll feeders and hence do not tap into plant vasculature to suck plant sap, thereby directly harming plants. Moreover, many leafhoppers are responsible for the transmission of plant pathogens—including viruses and bacteria—thus causing serious losses of production in the agriculture and forestry industries (Nault and Ammar 1989, Bové et al. 2003). To date, 118 species of leafhopper are known vectors (Weintraub and Wilson 2010). In East Asia, the pathogen of rice dwarf disease is spread by leafhoppers, which is the reason for the sharp decline in rice production every year (Ruan et al. 1985, Sivamani et al. 1999). Furthermore, mulberry dwarf disease phytoplasma, which is transmitted by Hishimonus sellatus (Hemiptera: Cicadellidae), has caused a severe decline of mulberry tree (Kawakita et al. 2000, Mitsuhashi et al. 2002).
Jujube witches’-broom (JWB) phytoplasma is known to be transmitted by leafhoppers, and the resulting disease results in economic losses that reach hundreds of millions of dollars annually (Liu et al. 2010). Early studies suggested that the transmission of the JWB phytoplasma occurred mainly by insect vectors such as H. sellatus (La and Woo 1980, Sun et al. 1988). Hishimonus lamellatus was first reported in Huolu County, Hebei Province, China (Cai et al. 1995). Both leafhopper species were found to be carrying JWB phytoplasma in vivo, but vector competence of H. lamellatus was not empirically tested. (Hao et al. 2015).
Phytoplasmas, the single-celled prokaryotes, are parasitic on the phloem of plants. Insects that feed on phloem can acquire and transmit phytoplasmas (Lee et al. 2000, Weintraub and Beanland 2006), and it is generally believed that phytoplasmas circulating in insects need to pass through the midgut and salivary glands before transmission to healthy plants via interface and salivary secretions during feeding (Weintraub et al. 2004, Weintraub and Beanland 2006, Ammar et al. 2011).
To date, few researchers have conducted detailed analyses of the digestive systems of leafhopper vectors. Gil-Fernandez and Black (1965) observed the general structure of the digestive tract of Agallia constricta Van Duzee (Hemiptera: Cicadellidae) (Gil-Fernandez and Black 1965). In addition, Lindsay and Marshall (1980) described the morphology and ultrastructure of the Eurymela distincta Signoret (Hemiptera: Eurymelidae) filter chamber (Lindsay and Marshall 1980), Cheung and Purcell (1993) revealed the ultrastructure of the digestive system of Euscelidius variegatus (Kirschbaum) (Hemiptera: Cicadellidae) (Cheung and Purcell 1993), and Wayadande et al. (1997) compared the general morphology of the digestive tracts of Circulifer tenellus (Hemiptera: Cicadellidae) and Dalbulus maidis (Hemiptera: Cicadellidae) (Wayadande et al. 1997). More recently, Zhang et al. (2012) observed the ultrastructure of the digestive tract of Psammotettix striatus (Linnaeus) (Hemiptera: Cicadellidae) (Zhang et al. 2012), and Utiyama et al. (2016) studied similar features in Bucephalogonia xanthophis (Hemiptera: Cicadellidae) (Utiyama et al. 2016). Taken together, the results of these analyses suggest that there are important differences in the morphology and ultrastructure of the digestive tracts and salivary glands of different leafhopper species (Sōgawa 1965, Wayadande et al. 1997). Globally, there are 41 species of Hishimonus, of which there are 17 Hishimonus in China (Li and Wang 2004). However, little information is currently available regarding the structural characteristics of the digestive systems of Hishimonus insects. Actually, there are few research on the histology and ultrastructure of the digestive tract and salivary glands of H. lamellatus.
Therefore, the purpose of this study was to characterize the structure of the digestive system of H. lamellatus using optical and transmission electron microscopy (TEM), and to understand the ultrastructural characteristics of the digestive tract, the Malpighian tubule (MT) system, and the salivary glands.
Materials and Methods
Leafhopper Rearing
Hishimonus lamellatus individuals were raised in the insect breeding room of the Beijing Key Laboratory of New Technology in Agricultural Application. Leafhoppers were originally collected in Liucun, Changping District, Beijing in August 2010. A laboratory population was established in the insect breeding room and identified by Professor Cai Ping. The H. lamellatus population was reared on jujube (Ziziphus jujuba Mill (Rhamnales: Rhamnaceae)) seedlings and placed in a 40-cm-high cylindrical transparent plastic worm cage, which was sealed with 40 × 40 mesh gauze. The feeding temperature was maintained at 25 ± 1°C, the relative humidity ranged between 50 and 70%, and the photoperiod was 16 L:8 D (Hao et al. 2015).
Sample Preparation for Light Microscopy
Hishimonus lamellatus samples were chilled at −20°C for 20 min and then each sample was then placed on a grooved glass slide (SAIL BRAND, 7103 Single Concave). Under a stereoscopic microscope (Motic, K Series), the wings and feet of leafhoppers were removed with forceps (Dumont, 0208-5-po), and the side line of the abdomen stalk was cut with scissors. A drop of phosphate buffer solution was added dropwise to this cut, and an anatomical needle was used to extract the digestive tract from abdominal segments 1–7. The salivary glands, located at the top of the head, were gently separated using forceps. The salivary glands were dissected and stained with toluidine blue solution and photographed using a stereomicroscope (Zeiss SteREO Discovery.V20) (Gil-Fernandez and Black 1965, Sōgawa 1965). About 80 leafhopper individuals were examined by light microscopy.
Semi-Thin Section Sample Preparation for Light Microscopy
The selected adult H. lamellatus individuals, anesthetized with ether, were placed on single concave slides. We then added 2.5% glutaraldehyde fixative and quickly dissected the digestive tract of the leafhopper using tweezers. Next, the digestive tract was then placed in a centrifuge tube containing 1.5 ml of glutaraldehyde fixative. The digestive tract was fixed for 48 h in the dark at room temperature. Next, fixed tracts were washed with phosphate-buffered saline (PBS) for 3 h. Afterward, we added 1% osmium tetroxide to the digestive tracts for 90 min before rinsing with phosphate buffer. Then, the samples were washed with PBS for 30 min. The samples were dehydrated in a series of ethanol concentrations of 30, 50, 70, 80, 90, and 100% for 6 min, respectively, and again in that of 100% ethanol for 30 min. Samples were then infiltrated with a 1:2 mixture of ethanol and epoxy resin 618 for 2 h and pure epoxy resin 618 for 12 h. The samples were transferred to plastic flat embedding mold (Electron Microscopy Sciences) and polymerized for 24 h at 37°C, 12 h at 45°C, and 48 h at 60°C. The embedded block was trimmed to the appropriate size and the sample was cut into 2-μm-ultrathin sections with a glass knife of Leica UC6 ultrathin slicer. The sections were placed in a saturated solution of NaOH in absolute ethanol for 3 min. The samples were infiltrated in a series of ethanol concentrations of 100, 95, 80, 70, 50, and 35% for 5 min. Slides were immersed in a staining jar containing 2% hydrochloric acid for 5 min, and then in a staining jar containing distilled water for 2 min. The samples were stained in Harris’s hematoxylin for 20 min, and washed in tap water. Slides were immersed in a staining jar containing acid alcohol (0.5:99 v/v) differentiation solution for 30 s, and then in a staining jar containing tap water for 15 min, and counterstained in 1% aqueous eosin for 2 min. Slides were immersed in a staining jar containing tap water for 5 min. The samples were dehydrated in a series of ethanol concentrations of 80 and 95% for 1 min and twice in 100% for 1 min. The samples were sealed with a drop of neutral balsam, capped with a cover slip, and then kept in drying oven. The treated samples then were observed with a Zeiss microscope Imager A1 (Aparicio and Marsden 1969).
Sample Preparation for TEM
To prepare samples for analysis using TEM, we used the same method as described in ‘Semi-Thin Section Sample Preparation for Light Microscopy’. At least five embedded blocks were made for each sample to be observed. The ultrathin resin sections (60 nm) were cut with an ultramicrotome (Leica UC6) using a glass knife, transferred to copper grids, and stained with 2% (w/v) uranyl acetate for 15 min (in dark), and then washed in distilled water. The sections were stained with lead citrate for 20 min, and then washed in distilled water again. The sections were kept in a desiccator (Ghanim et al. 2016, Ammar et al. 2017). Then ultrathin sections were examined at 80 kV using an Hitachi H-7500 transmission electron microscope at the electron microscope laboratory of the Institute of Food Science and Technology, Chinese Academy of Agricultural Sciences. About 30 leafhopper individuals were examined by TEM.
Results
Histology and Morphology Features of the Digestive Tract
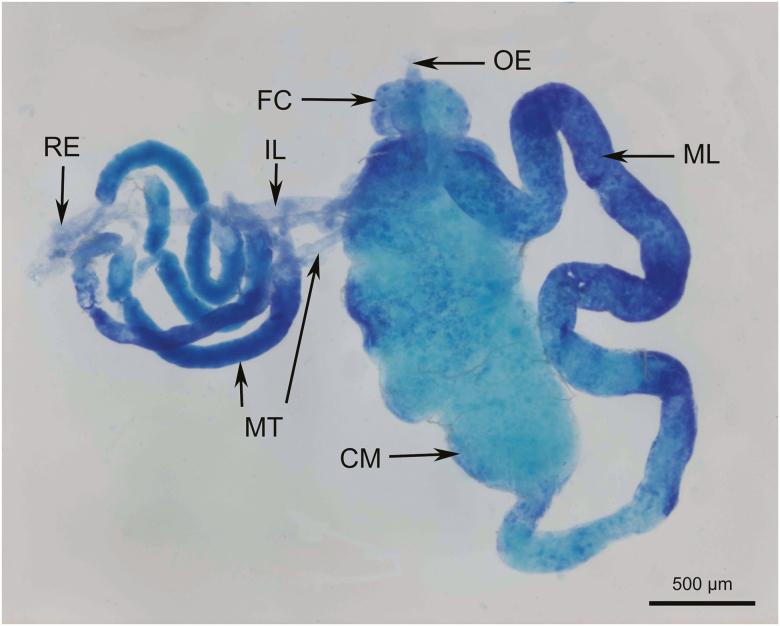
The foregut, midgut, and hindgut (Fig. 1) are the three major parts of the digestive tract of adult H. lamellatus.
Fig. 1.
Light micrograph of the alimentary canal of H. lamellatus. CM, conical midgut; FC, filter chamber; IL, ileum; ML, midgut loop; MT, Malpighian tubule; OE, esophagus; RE, rectum.
Foregut
The esophagus is a narrow, slender tube. The esophagus is approximately 50 μm in diameter and is translucent or pale white in color (Fig. 1).
Filter Chamber
The filter chamber (FC) has a half-moon shape, a diameter of 300–400 μm, and is light milky white in color. The FC is composed of the anterior segment of the midgut, the posterior segment of the midgut, the base of the Malpighian tube, and the basal hindgut. It is also connected to the conical midgut (CM).
Midgut
The midgut is divided into a CM and a midgut loop (ML). The spherical or long vesicular CM is about 1,200 μm long and 700 μm wide. The surface of the CM is translucent and sometimes contains white particulate matter. The apical of the CM, which has a thick basement membrane, rapidly collapses into the ML. The ML has a diameter of about 400–600 μm and is only slightly smaller than the diameter of the rest of the midgut (Fig. 1). Its surface is rough and its color is yellowish. The basement membrane is relatively thin, and the columnar epithelium cells (CECs) found there are large and are located near the basement membrane (Fig. 2).
Fig. 2.
Semi-thin section of the digestive tract of H. lamellatus. BM, basement membrane; CEC, columnar epithelial cell; CM, conical midgut; ML, midgut loop; N, nucleus.
Malpighian tubule
: Hishimonus lamellatus has four MTs that extend out of the same side of the ileum as the FC. Each of the malpighian tubes is divided into four parts. The first part is connected to the FC, which is short and has a smooth surface. The second part is wavy, smooth, translucent, and is not easily stained with toluidine blue. The diameter of this stage is about 60 μm. The third part is larger in diameter (about 100 μm) and is thicker than the first segment. Its surface is rough. The fourth part is a short translucent tube with a smooth surface that is also not easily stained with toluidine blue. Its diameter is about 50 μm, and it merges with the rectum to form a complex (Fig. 1).
Hindgut
The hindgut consists of an elongated tubular ileum (Fig. 1) and an enlarged rectum (RE; see Fig. 1). The ileum is connected to the rectum through four MTs (Fig. 1). Its surface is smooth, translucent, and is not easily stained with toluidine blue; its diameter is approximately 100 microns. The ileal apical swells into the rectum, and the end of the ileum sac is open to the anal tube.
Ultrastructure of the Digestive Tract
Filter Chamber and Conical Midgut
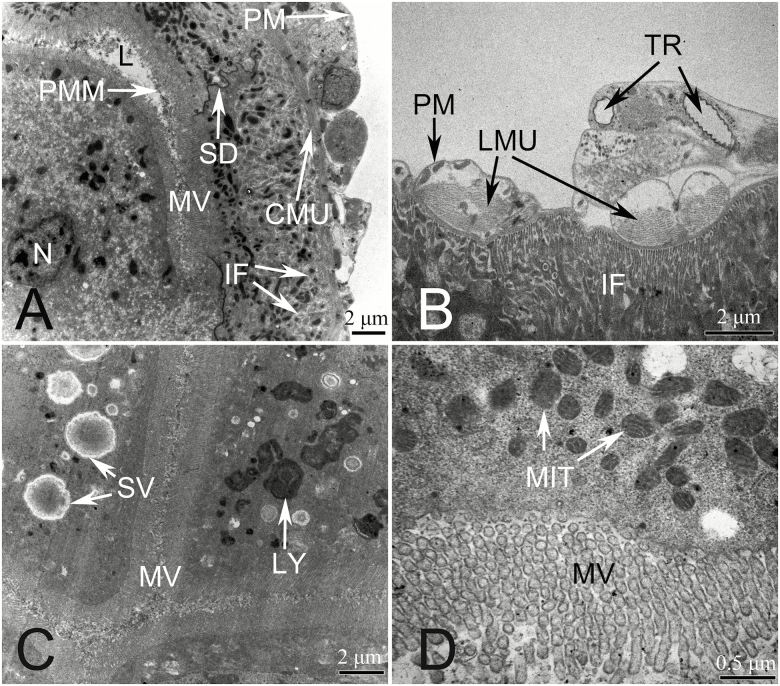
The FC has a thin membrane known as the peritoneal membrane (PM), which is adjacent to the longitudinal muscles (LMU). Cells of these tissues include large numbers of mitochondria, which distributed around developed muscle fibers (Fig. 3C). The circular muscles (CMU) lie on the inside of the LMU surround the basement membrane. The basement membrane has channels, formed by infolding (IF), which gives it a network-like and highly developed appearance. The intima is highly specialized into microvilli (MV) which are dense and regularly arranged. Many mitochondria were also observed in the MV-containing cells. Furthermore, many well-developed MV extend into the lumen (L) (Fig. 3D). Moreover, there are also a large number of secretory vesicles (SVs) in the cytoplasm of many of these cells in these tissues (Fig. 3A and B), and many cells also contain a highly developed rough endoplasmic reticulum (RER) connecting the SVs and the basement membrane (Fig. 3B). In many cases, there are also distinct septal desmosomes (SDs) between the cells (Fig. 3D).
Fig. 3.
Transmission electron microscopy (TEM) photographs of the filter chamber and conical midgut of H. lamellatus. (A) Cross-section of the filter chamber (partial). (B) Secretory vesicles in the cells are amplified. (C) Basement membrane infolding in the filter chamber cells. (D) Microvilli developing in the basement membrane. (E) Cross-section of the conical segment (partial). (F) Conical midgut cells are attached to a flame-like luminal membrane (black arrow). CMU, circular muscle; FLM, flame-like luminal membrane; IF, infolding; L, lumen; LMU, longitudinal muscles; MIT, mitochondria; MV, microvilli; N, nucleus; PM, peritoneal membrane; RER, rough endoplasmic reticulum; SD, septate desmosomes; SV, secretory vesicle.
The basement membrane of the CM was found to be thinner than that of the FC, and we also found that the basement membrane has one or more IFs. Mitochondria are present between the basement membrane and the IF (Fig. 3E). The intima is highly specialized into dense and regularly arranged MV (Fig. 3E and F). In addition, its uniform and well-aligned flame-like luminal membrane (FLM) is covered in MV (Fig. 3).
Midgut Loop
The midgut’s PM is relatively thick (Fig. 4A and B) and is wrapped with developed LMU (Fig. 4B). There is a tracheole (TR) distributed between the PM and the LMU (Fig. 4B), and the LMU are found adjacent to the CMU (Fig. 4A). The basement membrane has channels formed by IF (Fig. 4A), and these are network-like and highly developed; in addition, there are a large number of mitochondria distributed therein (Fig. 4A). Many well-developed MV extend into the lumen of the midgut (Fig. 4A, C, and D), and these MV are generally covered with a perimicrovillar membrane (PMM) (Fig. 4A and C). Moreover, many mitochondria were observed near the developing MV (Fig. 4D). SVs in the cytoplasm (Fig. 4A) were found to differ in size and shape. We also found unknown inclusions present in the SVs (Fig. 4C), and there were SDs between the cells (Fig. 4A). In addition, many lysosomes (LY) were observed in the cytoplasm of ML cells. These cells also show internal organelle fragments (Fig. 4C).
Fig. 4.
Transmission electron microscopy (TEM) photographs of the midgut loop of H. lamellatus. (A) Midgut loop (partial) cross-section. (B) Longitudinal muscle at the peritoneal membrane. (C) Lysosome in the cytoplasm. (D) Microvilli at the lumen of the midgut. CMU, circular muscle; L, lumen; LMU, longitudinal muscle; LY, lysosome; MIT, mitochondria; MV, microvilli; PM, peritoneal membrane; PMM, perimicrovillar membrane; SD, septate desmosomes; SV, secretory vesicle; TR, tracheole.
Ileum
The nucleus of ileal cells is relatively large. N1 has a distinct heterochromatin, whereas N2 is not obvious (Fig. 5A). The ileum PM is thin, and is adjacent to the developed circular muscle (Fig. 5B and C). The cytoplasm of the cells of the ileum PM is filled with SVs and mitochondria that are circular, oval, or clavate. We also identified a developed RER around mitochondria (Fig. 5C). An elliptical bacterial-like structure is present in the ileum cytoplasm of 50% leafhopper individuals (Fig. 5C).
Fig. 5.
Transmission electron microscopy (TEM) photographs of the ileum of H. lamellatus. (A) Ileum cross-section. (B) A section of panel A is enlarged to show an abundance of mitochondria. (C) Microorganisms (black arrows) are present in ileum cells. N1 and N2, nucleus; CMU, circular muscle; L, lumen; MIT, mitochondria; RER, rough endoplasmic reticulum; SV, secretory vesicle.
Malpighian Tubule
The cells of the MT contained many SVs (Fig. 6A) in which a large number of brochosomes (BRs) were found. The cell showed the widespread RER (Fig. 6B). Mitochondria were located near SVs (Fig. 6C). The outer wall of each BR was honeycomb-shaped. BRs were observed: Somes had high central electron density and appeared dark (Fig. 6C; white arrow); while other BRs were centrally transparent (BR2), embedded (BR1), or showed multiple small cavities (BR3) (Fig. 6C). This may be mitochondria at different developmental stages. SVs with low electron density were scattered around the nuclei (Fig. 6A). Sparse MV were observed on the outside of the intima (Fig. 6D).
Fig. 6.
Transmission electron microscopy (TEM) photograph of a Malpighian tubule of H. lamellatus. (A) Cross-section of the cell tip region. (B) A developed rough endoplasmic reticulum near the secretory vesicles. (C) A section of panel A is enlarged to show different forms of brochosomes. Also visible is an inclusion in the central cavity of BR1, the fact that the cavity of BR2 is transparent, and that the center of BR3 is a plurality of small cavities. Brochosomes in the opaque center (white arrow). (D) Sparse microvilli. BR, brochosomes; BV, brochosomes vesicles; MIT, mitochondria; MV, microvilli; RER, rough endoplasmic reticulum; SV, secretory vesicle.
Morphology of Salivary Glands
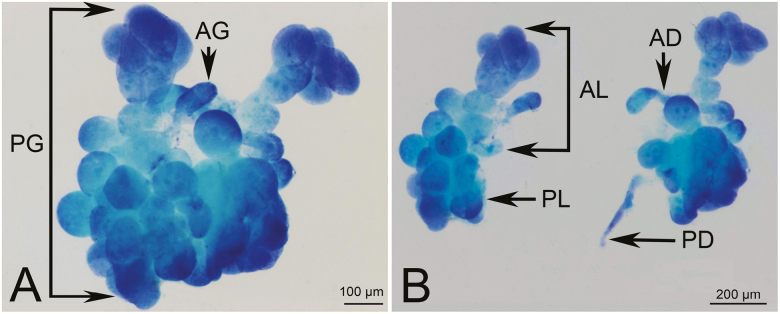
The salivary glands of H. lamellatus are located in the head cavity. The salivary gland complex consists of pairs of principal glands (PGs) and accessory glands (AGs) (Fig. 7).
Fig. 7.
Light microscopy photograph of the salivary glands of H. lamellatus. (A) Overall morphology of the salivary gland. (B) Unilateral salivary gland morphology. AD, accessory duct; AG, accessory gland; AL, anterior lobe; PD, principal duct; PG, principal gland; PL, posterior lobe.
Principal Gland
The vesicular PG is a major part of the salivary glands (Fig. 7A). The PG includes both an anterior lobe (AL) and posterior lobe (PL) (Fig. 7B). The distal and proximal regions of the AL consist of small, closely packed acini that have a smooth surface. In addition, we identified five larger acini in the middle region that are loosely arranged. The PL consists of about 10 acini that are divided into two types: the first type is a group of about five relatively small acini that are closely arranged in a petal shape and have a rough surface. The second type refers to about five larger acini present in a loose pattern around the periphery of the petal-like acinus (Fig. 7B). The acini of the PL are slightly larger than the acini of the middle region of the AL. Finally, the PGs are connected via the lateral salivary ducts which converge to form the common salivary duct (Fig. 7B).
Accessory Glands
The AGs (Fig. 7A) found in the salivary gland complex were rod- or elliptically-shaped. These are connected to the PG by a short accessory duct. AGs are simply constructed and have only one acinus, which is similar in structure to the acini of the PL. AGs are free on both sides of PG.
Ultrastructure of the PG
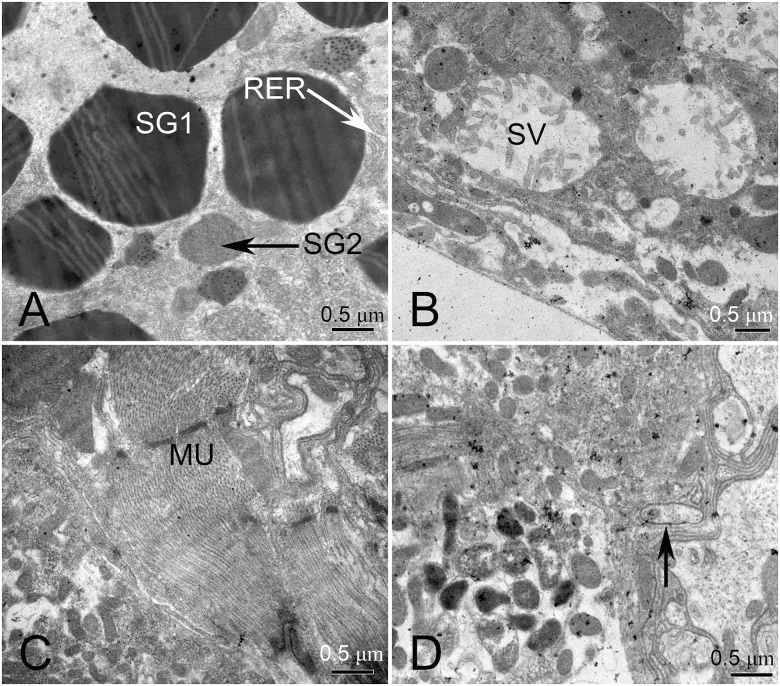
The nucleus of secretory cells contained a variety of heterochromatin (Fig. 8A), and was generally located in the basement membrane region, as were the TRs (Fig. 8B). Unknown inclusions were also present in the salivary duct (Fig. 8C, see asterisk [*]). The atrium is lined with a cuticle, which is surrounded by IFs of the apical plasma membrane (Fig. 8C).
Fig. 8.
Transmission electron microscopy (TEM) photograph of the principal gland of H. lamellatus. (A) Salivary gland cells are highly enlarged to show the multicellular nucleus. (B) The tracheole, present near the basement membrane. (C) A cross-section of the salivary duct. AMP, apical plasma membrane; CU, cuticle; MIT, mitochondria; N, nucleus; RER, rough endoplasmic reticulum; SD, salivary duct; SG, secretory granule; SV, secretory vesicle; TR, tracheole; *The contents of the lumen.
We observed the presence of many secretory granules (SGs) within the cells (Fig. 9A). There are differences between the SGs, SG1 has a transparent core and filaments are attached to its inner edge, and the core of SG2 is opaque and has no filaments (Fig. 9A). We also identified dispersed SVs that have MV-like protrusions along the inner edge of the core (Fig. 9B). In the salivary gland cells, significant muscles were observed (Fig. 9C). We observed the presence of rod-shaped bacteria-like structures in the cytoplasm of salivary gland cells (Fig. 9D).
Fig. 9.
Transmission electron microscopy (TEM) photograph of the principal gland of H. lamellatus. (A) Secretory granules are placed at higher magnification. (B) Secretory vesicles are placed at higher magnifications. (C) Muscle tissue in salivary glands. (D) Microorganisms (black arrows) in the host cells. MU, muscle; RER, rough endoplasmic reticulum; SG1 and SG2, secretory granule; SV, secretory vesicle.
Discussion
The Ultrastructure and Function of the Tubular Midgut in H. lamellatus
The ML of H. lamellatus is divided into two parts. In the front is the cone, which is connected to the FC. The tubular midgut is also connected to the FC, thus forming the ‘midgut ring’. The presence of this structure is consistent with previous reports of other leafhoppers (Tsai and Perrier 1996, Zhong et al. 2014). Our results also confirmed that the epithelial cells of the tubular midgut of the digestive tract of H. lamellatus were present in the shape of a column, and that the endoplasmic reticulum and SVs were abundant in the cells. We found many well-organized and developed brush-like borders of MV, which have a specialized function during digestion (Silva et al. 1995). As observed in the midguts of other leafhoppers (Zhong et al. 2014), these MV play an important role in the absorption of digested nutrients including carbohydrates, amino acids, and water.
The midguts of most insects have a peritrophic matrix, which is mainly used to protect midgut cells from pathogens and food particles, and has a compartmentalization effect on the midgut (Terra 1990). However, there is no peritrophic membrane in the midgut of Hemiptera insects. Instead, a layer of lipoprotein membranes covered with MV tips is present, termed the PMM (Terra 1988). This membrane is derived from the inner membrane of a double-membrane vesicle, which are produced by budding from the Golgi region (Werner et al. 1991, Silva et al. 1995). Here, we identified an obvious PMM in the tubular midgut of H. lamellatus. Its morphology is similar to that found in the midgut cells of Dysdercus peruvianus (Hemiptera: Pyrrhocoridae), Mahanarva posticata (Hemiptera: Cercopidae), and Cicadella viridis (Hemiptera: Cicadellidae) (Fonseca et al. 2010, Zhong et al. 2014). The PMM and MV form a closed space, i.e., the perimicrovillar space that mediates the digestion and absorption of nutrients in the midgut (Terra 1990).
Doi et al. (1967) first discovered phytoplasmas in plants infected with yellow-type diseases (e.g., aster yellows disease), and similar pathogens were found to be present in the insect digestive tract (Doi et al. 1967, Maramorosch et al. 1968, Granados 1969). Later, Raine and Forbes (1969) also found that phytoplasmas were present in salivary sheaths (i.e., in saliva) secreted by leafhoppers (Raine and Forbes 1969). Wayadande et al. (1997) showed that the pathogen must pass through the basal layer, the basal plasma membrane, and the apical plasma membrane before they can be ejected with the saliva (Wayadande et al. 1997). In general, when phytoplasma circulate through the digestive tract and salivary glands of insects, they must adhere to the intestinal epithelial cells of the insect vectors. Recently, several variable membrane proteins were used by the phytoplasma to bind to the PMM—which covers the MV of leafhopper gut cells have been identified. These membrane proteins may promote the degradation of PMM protein components, so that phytoplasma reaching the MV apical membrane region of the midgut epithelium adhere and can colonize gut cells (Arricau-Bouvery et al. 2018). This binding transport mechanism was present in Trypanosoma cruzi (Trypanosomatina: Trypanosomatidae), which adheres to the PMM of the Chagas disease vector bug Rhodnius prolixus (Hemiptera: Reduviidae) (Gutiérrez-Cabrera et al. 2016). Taken together, we believe that our data improve our understanding of the cellular and subcellular structure of the H. lamellatus gut, which is important because it is vital to improve our understanding of the nature of pathogenic microbial interaction with the cells of insect vectors to mitigate the impact of plant diseases.
The Morphology and Function of Salivary Glands in Leafhoppers
The salivary glands of Cicadellidae insects are composed of the PG, the AG, and its salivary duct. Importantly, the morphology of the principal and AGs are significantly different (Ammar et al. 1985). Our observations revealed that the salivary glands of H. lamellatus, including both the principal and AGs, are lightly cream-colored or translucent. The former is bulky, has a more complex structure, and consists of an aggregate body containing many acini. In contrast, the latter is a short rod-shaped tubular gland with a structure similar to that of salivary gland of H. sellatus (Sōgawa 1965). However, the salivary glands of H. lamellatus are morphologically distinct from those found in other leafhoppers, such as P. striatus (L.), where the AGs are more slender than those of H. lamellatus (Zhang et al. 2012). Moreover, the AGs of C. tenellus and D. maidis are both relatively large (Wayadande et al. 1997), and the PGs of Nephotettix cincticeps (Hemiptera: Cicadellidae) and Chlorita flavescens (Hemiptera: Cicadellidae) have a small number of acini and a relatively simple structure (Sōgawa 1965)
We also observed that the PG is composed of two parts: an AL and a PL. The AL is itself divided in two parts, i.e., the head and tail portions. The ALs of H. sellatus are quite different from those found in Graminella nigrifrons (Hemiptera: Cicadellidae) or D. maidis (Sōgawa 1965, Tsai and Perrier 1993). Moreover, as mentioned by Sōgawa (1965), the size, number, and shape of the ALs vary from species to species in the Cicadellidae. The PL consists of approximately 13 acini arranged in a multilayered petal-like shape similar to the shape and structure of the principal salivary glands of the subfamily Deltocephalinae (Sōgawa 1965). The principal salivary gland is a complex gland that contains at least two secretory systems, one that secretes precursors of the salivary sheath and another that produces water saliva that contains several enzymes (Sōgawa 1965). The AGs secrete mucus and phenolic enzymes which, together with proteins secreted by the principal salivary gland, constitute the salivary sheath (Miles 1964, 1965). In general, the salivary sheath of the leafhoppers contains lipids and neutral mucus substances (Sōgawa 1965, 1967). Saliva secreted by leafhoppers can damage host plants due to the presence of toxins and anticoagulants, and saliva containing pathogenic microorganisms may transmit them during mouthpart penetration (Raine and Forbes 1969, Sauer 1977). A variety of plant pathogens have been reported in the salivary glands of the leafhoppers (Ghanim and Medina 2007, Ammar et al. 2009), and the phytoplasma associated with mulberry dwarf disease has been observed in the salivary glands of H. sellatus and Hishimonoides sellatiformis (Hemiptera: Cicadellidae) (Kawakita et al. 2000). In addition, we used molecular data to show that the JWB phytoplasma was present in some leafhoppers (Hao et al. 2015). Moreover, recent studies of the relationship between microorganisms and their insect vectors have suggested that the primary glands are beneficial to the survival of microorganisms, since they are very sensitive to nutritional quality in the intracellular environment (Crotti et al. 2009, 2010). Kwaik (1996) found that the vesicular RER and its SVs may provide a rich environment replete with essential nutrients for microorganisms. This is thought to be due to the fact that there is a lot of RER, mitochondria, and numerous SGs within leafhopper PG cells (Kwaik 1996). Here, we found rod-shaped or oval microorganisms in the PGs of the leafhoppers, although these glands are known to contain phytopathogens in other leafhopper species (Gonzalez 2016). Although to date there has been no detailed investigation of the saliva of H. lamellatus, our histological and ultrastructure results suggest that the salivary glands of H. lamellatus play an important role in the transmission of plant pathogens (Reis et al. 2003).
The Structure of the Hindgut of the Leafhopper and Its Microorganisms
Ultrastructural observations showed that cells of the ileum (hindgut) of H. lamellatus possessed apical IFs associated with SVs as well as an abnormal abundance of mitochondria. These IFs were delicately formed by the invaginations of apical plasma membrane. Similar IFs have been described in the intestines of the leafhoppers P. striatus (L.) and Cic. viridis (Zhang et al. 2012, Zhong et al. 2014), and the IFs were found to increase the contact surface with food in the luminal space. In addition, we identified oval and short rod-shaped microorganisms embedded within the epithelial cells of the hindgut wall in H. lamellatus. These microorganisms are likely to be prokaryotic microbes (i.e., bacteria), and resemble those found in the intestines of other leafhopper vectors including Cic. viridis and A. constricta (Van Duzee) (Gil-Fernandez and Black 1965, Zhong et al. 2014). The microbes present in insect intestines and gut cells are probably involved in digestion processes (Caetano et al. 2009), and may participate in many different metabolic pathways. Thus, the presence of microorganisms is thought to be generally beneficial to host insects (Ishikawa 2003). These microorganisms were observed to be surrounded by the endoplasmic reticulum, mitochondria, and cytoplasm of the host cells, which again is likely beneficial for leafhoppers digestion and essential nutrient uptake (Ishikawa 2003, Salehi et al. 2007). Because the leafhoppers feed on phloem sap, which contains few amino acids, they are likely to rely on microbes to supply certain nutrients that are lacking in their food. However, more research is needed to explore the identity and characteristics of these microbes.
Structure and Function of FC and Cone Segment
Our results also showed that the muscles of the FC of H. lamellatus were well developed. The MV of the epithelial cells of the FC were tubular, uniform in length, and regularly arranged. Many insects in the Cicadomorpha that feed on plant juices have developed LMU, which power the contraction of the FC, allowing a large amount of water to move directly from the anterior midgut into the rear end of the midgut and the MT. This in turn concentrates sap in the xylem and phloem before absorption, and the FC then acts as a waterway (Cheung and Marshall 1973). Studies on the fine structure of the FC in the leafhopper E. distincta showed that the cells in the anterior midgut had a storage and secretion function, while the cells in the posterior midgut had an ion-secretion function, and the cells in the FC were similar to the cells in the anterior midgut. There is only one type of enterocyte in the inner tubule, and this is related to the passive diffusion and active transport of ions from the hemolymph to the lumen; the inner ileum thereby facilitates the absorption of water (Lindsay and Marshall 1980).
We observed that the anatomy of the CM of the H. lamellatus is different from that of the leafhopper P. striatus (L.) (Zhang et al. 2012), which itself is similar to the gross morphology of the xylem-feeding leafhopper Cic. viridis (Zhong et al. 2014). The basement membrane is deeply deepened and forms IFs associated with mitochondria and openings of the basal lamina. In addition, the RER around the mitochondria is clearly visible, as is the apical membrane of the epithelial cells, which is tightly arranged into MV. The mitochondria at the base of the MV of enterocytes provide a large amount of energy for transport, and we also identified a large number of SVs in the cells, which play an important role in the absorption, storage, and secretion of metabolites (Silva et al. 2004). Thus, the absorption of nutrients and ions occurs in the CM and the anterior midgut (Cheung and Marshall 1973). In addition, the MV are covered with a filamentous membrane complex similar to the FLM found in the CM of the leafhopper B. xanthophis. This membrane complex originates from intestinal epithelial cells, and does not secrete product directly. The MV tips of the enterocytes are often constricted, thereby forming a membrane that projects into the midgut lumen and remains associated with MV (Utiyama et al. 2016). However, unlike the PMM, they may be anchored during intestinal digestion. Enzymes such as cathepsin and a-glucosidase prevent excretion and binding to amino acids, thereby constricting and concentrating them at the absorption site and enhance to effect of absorption (Cristofoletti et al. 2003). In the end, further studies are needed to explore the relationship between this FLM complex and intestinal microorganisms.
Acknowledgments
We sincerely appreciate Prof. P. Cai (Soochow University, China) for his work identifying leafhopper species. We thank Dr. Zhang Qing and Yang Liu at Beijing Key Laboratory of New Technology in Agricultural Application for their help with photomicrography. This research was supported by grants from the Beijing Municipal Natural Science Foundation, and the Beijing Municipal Education Commission Science and Technology Plan Key Project (KZ201810020026), the Beijing Municipal Natural Science Foundation (6182002), the National Key R&D Program of China (2017YFD020030703), the Beijing Municipal Science & Technology Commission (Z15100002115030-2), the National Natural Science Foundation of China (31272099, 31170602), and the Beijing Agricultural Commissioner Project. We acknowledge TopEdit LLC for linguistic editing and proofreading during the preparation of this manuscript.
References Cited
- Ammar E. D., Nault L. R., and Rodriguez J. G.. . 1985. Internal morphology and ultrastructure of leafhoppers and planthopper, pp. 127–162. In L. R. Nault and J. G. Rodriguez (eds.), Leafhoppers & planthoppers. John Wiley & Sons, New York. [Google Scholar]
- Ammar e. l.-.D., Gargani D., Lett J. M., and Peterschmitt M.. 2009. Large accumulations of maize streak virus in the filter chamber and midgut cells of the leafhopper vector Cicadulina mbila. Arch. Virol. 154: 255–262. [DOI] [PubMed] [Google Scholar]
- Ammar E., Shatters R. G., and Hall D. G.. . 2011. Localization of Candidatus Liberibacter asiaticus, associated with citrus huanglongbing disease, in its psyllid vector using fluorescence in situ hybridization. J. Phytopathol. 159: 726–734. [Google Scholar]
- Ammar E. D., Hall D. G., and Shatters R. G. Jr. 2017. Ultrastructure of the salivary glands, alimentary canal and bacteria-like organisms in the Asian citrus psyllid, vector of citrus huanglongbing disease bacteria. J. Microsc. Ultrastruct. 5: 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio S. R., and Marsden P.. . 1969. Application of standard micro-anatomical staining methods to epoxy resin-embedded sections. J. Clin. Pathol. 22: 589–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arricau-Bouvery N., Duret S., Dubrana M., Batailler B., Desqué D., Beven L., Danet J., Monticone M., Bosco D., Malembic-Maher S., . et al. 2018. Variable membrane protein a of flavescence dorée phytoplasma binds the midgut perimicrovillar membrane of Euscelidius variegatus and promotes adhesion to its epithelial cells. Appl. Environ. Mocrob. 84: e02487-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bové J. M., Renaudin J., Saillard C., Foissac X., and Garnier M.. 2003. Spiroplasma citri, a plant pathogenic molligute: relationships with its two hosts, the plant and the leafhopper vector. Annu. Rev. Phytopathol. 41: 483–500. [DOI] [PubMed] [Google Scholar]
- Caetano F. H., Bution M. L., and Zara F. J.. 2009. First report of endocytobionts in the digestive tract of ponerine ants. Micron. 40: 194–197. [DOI] [PubMed] [Google Scholar]
- Cai P., Cui S. Y., and Ge Z. L.. . 1995. A new species of Hishimonus injurious to Ziziphus jujuba (Homoptera: Cicadellidae, Euscelidae). Acta Entomol. Sin. 38: 217–219. [Google Scholar]
- Cheung W. W., and Marshall A. T.. . 1973. Studies on water and ion transport in homopteran insects: ultrastructure and cytochemistry of the cicadoid and cercopoid midgut. Tissue Cell 5: 651–669. [DOI] [PubMed] [Google Scholar]
- Cheung W. W. K., and Purcell A. H.. . 1993. Ultrastructure of the digestive system of the Le Fhopper Euscelidius variegatus Kirshbaum (Homoptera: Cicadellidae), with and without congenital bacterial infections. Int. J. Insect Morphol. Embryol. 22: 49–61. [Google Scholar]
- Cristofoletti P. T., Ribeiro A. F., Deraison C., Rahbé Y., and Terra W. R.. 2003. Midgut adaptation and digestive enzyme distribution in a phloem feeding insect, the pea aphid Acyrthosiphon pisum. J. Insect Physiol. 49: 11–24. [DOI] [PubMed] [Google Scholar]
- Crotti E., Damiani C., Pajoro M., Gonella E., Rizzi A., Ricci I., Negri I., Scuppa P., Rossi P., Ballarini P., et al. 2009. Asaia, a versatile acetic acid bacterial symbiont, capable of cross-colonizing insects of phylogenetically distant genera and orders. Environ. Microbiol. 11: 3252–3264. [DOI] [PubMed] [Google Scholar]
- Crotti E., Rizzi A., Chouaia B., Ricci I., Favia G., Alma A., Sacchi L., Bourtzis K., Mandrioli M., Cherif A., et al. 2010. Acetic acid bacteria, newly emerging symbionts of insects. Appl. Environ. Microbiol. 76: 6963–6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich C. H. 1997. The role of grasslands in the diversification of leafhoppers (Homoptera: Cicadellidae): a phylogenetic perspective. InWarwick C., (ed.), Fifteenth North American Prairie Conference, Bend, Oregon. http://digicoll.library.wisc.edu/cgi-bin/EcoNatRes/EcoNatRes-idx?id=EcoNatRes.NAPC15. [Google Scholar]
- Doi Y., Teranaka M., Yora K., and Asuyama H.. . 1967. Mycoplasma- or PLT group-like microorganisms found in the phloem elements of plants infected with mulberry dwarf, potato witches’ broom, aster yellows, or paulownia witches’ broom. Japan. J. Phytopathol. 33: 259–266. [Google Scholar]
- Fonseca F. V., Silva J. R., Samuels R. I., DaMatta R. A., Terra W. R., and Silva C. P.. 2010. Purification and partial characterization of a midgut membrane-bound alpha-glucosidase from Quesada gigas (Hemiptera: Cicadidae). Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 155: 20–25. [DOI] [PubMed] [Google Scholar]
- Ghanim M., and Medina V.. . 2007. Localization of tomato yellow leaf curl virus in its whitefly vector Bemisia tabaci. InCzosnek H. (ed.), Tomato yellow leaf curl virus disease. Springer, Dordrecht, the Netherlands. [Google Scholar]
- Ghanim M., Fattah-Hosseini S., Levy A., and Cilia M.. 2016. Morphological abnormalities and cell death in the Asian citrus psyllid (Diaphorina citri) midgut associated with Candidatus Liberibacter asiaticus. Sci. Rep. 6: 33418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Fernandez C., and Black L. M.. . 1965. Some aspects of the internal anatomy of the leafhopper Agallia constricta (Homoptera: Cicadellidae). Ann. Entomol. Soc. Am. 58: 275–284. [DOI] [PubMed] [Google Scholar]
- Gonzalez J. G. 2016. Interactions of maize bushy stunt phytoplasma with the leafhopper vector, Dalbulus maidis (Delong and Wolcott) (Hemiptera: Cicadellidae) and associated microbiota. University of São Paulo, Piracicaba. [Google Scholar]
- Granados R. R. 1969. Electron microscopy of plants and insect vectors infected with the corn stunt disease agent. Contr. Boyce Thompson Inst. PI. Res. 24: 173–188. [Google Scholar]
- Gutiérrez-Cabrera A. E., Córdoba-Aguilar A., Zenteno E., Lowenberger C., and Espinoza B.. . 2016. Origin, evolution and function of the hemipteran perimicrovillar membrane with emphasis on Reduviidae that transmit Chagas disease. Bull. Entomol. Res. 106: 279–291. [DOI] [PubMed] [Google Scholar]
- Hao S. D., Chen Y. Q., Wang J. Z., Wang H., Tao W. Q., Zhang Z. Y., Shi X. Y., and Zhou S.. . 2015. Multiplex-PCR for identification of two Hishimonus species (Hemiptera: Cicadellidae) in jujube orchards and detection of jujube witches’ broom JWB phytoplasma in their bodies. Acta Entomol. Sin. 58: 264–270. [Google Scholar]
- Ishikawa H. 2003. Insect symbiosis: an introduction, pp. 1–22. InBourtzis K. and Miller T. A. (eds.), Insect symbiosis. CRC Press, Boca Raton, FL. [Google Scholar]
- Kawakita H., Saiki T., Wei W., Mitsuhashi W., Watanabe K., and Sato M.. 2000. Identification of mulberry dwarf phytoplasmas in the genital organs and eggs of leafhopper Hishimonoides sellatiformis. Phytopathology 90: 909–914. [DOI] [PubMed] [Google Scholar]
- Kwaik Y. A. 1996. The phagosome containing Legionella pneumophila within the protozoan Hartmannella vermiformis is surrounded by the rough endoplasmic reticulum. Appl. Environ. Microbiol. 62: 2022–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Y. J., and Woo K. S.. . 1980. Transmission of jujube witches’ broom mycoplasma by the leaf hopper Hishimonus sellatus Uhler. J. Korean For. Soc. 48: 29–39. [Google Scholar]
- Lee I. M., Davis R. E., and Gundersen-Rindal D. E.. 2000. Phytoplasma: phytopathogenic mollicutes. Annu. Rev. Microbiol. 54: 221–255. [DOI] [PubMed] [Google Scholar]
- Li Z. Z., and Wang L. M.. . 2004. Notes on Chinese species of Hishimonus with descriptions of two new species (Homoptera, Cicadellidae, Euscelinae). Acta Zootaxon. Sin. 29: 486–490. [Google Scholar]
- Li H., Dai R. H., and Li Z. Z.. . 2012. DNA barcoding technique and its application in Cicadellidae (Insecta, Hemiptera, Cicadelloidae) research. J. Mount. Agricult. Biol. 31: 432–438. [Google Scholar]
- Lindsay K. L., and Marshall A. T.. . 1980. Ultrastructure of the filter chamber complex the alimentary canal of Eurymela distincta Signoret (Homoptera, Eurymelidae). Int. J. Insect Morphol. Embryol. 9: 179–198. [Google Scholar]
- Liu M. J., Zhao J., and Zhou J. Y.. . 2010. Jujube witches’ broom disease. China Agriculture Press, Beijing, China. [Google Scholar]
- Maramorosch K., Shikata E., and Granados R. R.. . 1968. Structures resembling mycoplasma in diseased plants and in insect vectors. Transact. NY Acad. Sci. 30: 841–855. [Google Scholar]
- Miles P. W. 1964. Studies on the salivary physiology of plant bugs: the chemistry of formation of the sheath material. J. Insect Physiol. 10: 147–160. [DOI] [PubMed] [Google Scholar]
- Miles P. W. 1965. Studies on the salivary physiology of plant-bugs: the salivary secretions of aphids. J. Insect Physiol. 11: 1261–1268. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi W., Saiki T., Wei W., Kawakita H., and Sato M.. 2002. Two novel strains of Wolbachia coexisting in both species of mulberry leafhoppers. Insect Mol. Biol. 11: 577–584. [DOI] [PubMed] [Google Scholar]
- Nault L. R., and Ammar E. D.. . 1989. Leafhopper and planthopper transmission of plant viruses. Ann. Rev. Entomol. 34: 503–529. [Google Scholar]
- Raine J., and Forbes A. R.. . 1969. Mycoplasma-like bodies in the saliva of the leafhopper Macrosteles fascifrons (Stål) (Homoptera: Cicadellidae). Can. J. Microbiol. 15: 1105–1107. [DOI] [PubMed] [Google Scholar]
- Reis M. M., Meirelles R. M., and Soares M. J.. 2003. Fine structure of the salivary glands of Triatoma infestans (Hemiptera: Reduviidae). Tissue Cell. 35: 393–400. [DOI] [PubMed] [Google Scholar]
- Ruan Y. L., Chen S. X., and Jin D. D.. . 1985. Dynamics and chemical control of the leafhopper vector: Nephotettix cincticeps, which spread rice virus disease. Insect Knowledge 3: 54–57. [Google Scholar]
- Salehi M., Izadpanah K., Siampour M., Bagheri A., and Faghihi S. M.. 2007. Transmission of ‘Candidatus Phytoplasma aurantifolia’ to Bakraee (Citrus reticulata hybrid) by feral Hishimonus phycitis leafhoppers in Iran. Plant Dis. 91: 466. [DOI] [PubMed] [Google Scholar]
- Sauer J. R. 1977. Acarine salivary glands–physiological relationships. J. Med. Entomol. 14: 1–9. [DOI] [PubMed] [Google Scholar]
- Silva C. P., Ribeiro A. F., Gulbenkian S., and Terra W. R.. . 1995. Organization, origin and function of the outer microvillar (perimicrovillar) membranes of Dysdercus peruvianus (Hemiptera) midgut cells. J. Insect Physiol. 41: 1093–1103. [Google Scholar]
- Silva C. P., Silva J. R., Vasconcelos F. F., Petretski M. D., Damatta R. A., Ribeiro A. F., and Terra W. R.. 2004. Occurrence of midgut perimicrovillar membranes in paraneopteran insect orders with comments on their function and evolutionary significance. Arthropod Struct. Dev. 33: 139–148. [DOI] [PubMed] [Google Scholar]
- Sivamani E., Huet H., Shen P., Ong C. A., Kochko A. D., Fauquet C., and Beachy R. N.. . 1999. Rice plant (Oryza sativa L.) containing rice tungro spherical virus (RTSV) coat protein transgenes are resistant to virus infection. Mol. Breed. 5: 177–185. [Google Scholar]
- Sōgawa K. 1965. Studies on the salivary glands of rice plant leafhoppers. I. Morphology and histology. Japan. Soc. Appl. Entomol. Zool. 9: 275–290. [Google Scholar]
- Sōgawa K. 1967. Chemical nature of the sheath materials secreted by leafhoppers (Homoptera). Appl. Entomol. Zoolog. 2: 13–21. [Google Scholar]
- Sun S. M., Zhang F. W., and Tian X. D.. . 1988. Studies on the biology and control of Hishimonus sellatus Uhler: a vector of jujube witches’-broom disease. Acta Phytophyl. Sin. 15: 173–177. [Google Scholar]
- Terra W. R. 1988. Physiology and biochemistry of insect digestion: an evolutionary perspective. Braz. J. Med. Biol. Res. 21: 675–734. [PubMed] [Google Scholar]
- Terra W. R. 1990. Evolution of digestive systems of insects. Ann. Rev. Entomol. 35: 181–200. [Google Scholar]
- Tsai J. H., and Perrier J. L.. . 1996. Morphology of the digestive and reproductive systems of Dalbulus maidis and Graminella nigrifrons (Homoptera: Cicadellidae). Fla. Entomol. 79: 563–578. [Google Scholar]
- Tsai J. H., and Perrier J. L.. . 1993. Morphology of the digestive and reproductive systems of Peregrinus maidis (Homoptera: Delphacidae). Fla. Entomol. 76: 428–436. [Google Scholar]
- Utiyama A. H., Terra W. R., and Ribeiro A. F.. . 2016. The digestive system of the leafhopper Bucephalogonia xanthophis (Hemiptera, Cicadellidae): the organization of the luminal membrane complex. J. Entomol. Res. 40: 339–346. [Google Scholar]
- Weintraub P. G., and Beanland L.. . 2006. Insect vectors of phytoplasmas. Annu. Rev. Entomol. 51: 91–111. [DOI] [PubMed] [Google Scholar]
- Weintraub P. G., and Wilson M. R.. . 2010. Control of phytoplasma diseases and vectors, pp. 233–249. InWeintraub P. G. and Jones P. (eds.), Phytoplasmas: genomes, plant hosts and vectors. CABI, Wallingford, United Kingdom. [Google Scholar]
- Weintraub P. G., Pivonia S., Rosner A., and Gera A.. . 2004. A new disease in Limonium hybrids. II. Insect vectors. HortScience 39: 1060–61 [Google Scholar]
- Wayadande A. C., Baker G. R., and Fletcher J.. . 1997. Comparative ultrastructure of the salivary glands of two phytopathogen vectors, the beet leafhopper, Circulifer tenellus (Baker), and the corn leafhopper, Dalbulus maidis DeLong and Wolcott (Homoptera: Cicadellidae). Int. J. Insect Morphol. Embryol. 26: 113–120. [Google Scholar]
- Werner K., Moutairou K., and Werner G.. . 1991. Formation and structure of the surface coat in the midgut of a waterstrider, Gerris najas Deg. (Heteroptera: Gerridae). Int. J. Insect Morphol. Embryol. 20: 69–77. [Google Scholar]
- Zhang F. M., Zhang C. N., Dai W., and Zhang Y. L.. . 2012. Morphology and histology of the digestive system of the vector leafhopper Psammotettix striatus (L.) (Hemiptera: Cicadellidae). Micron 43: 725–738. [DOI] [PubMed] [Google Scholar]
- Zhong H. Y., Zhang Y. L., and Wei C.. . 2014. Morphology of the alimentary canal of the leafhopper Cicadella viridis (Hemiptera: Cicadellidae). Ann. Entomol. Soc. Am. 108: 57–69. [Google Scholar]