Abstract
Context
Cholesterol side-chain cleavage enzyme (P450scc), encoded by CYP11A1, catalyzes the first step of steroidogenesis. Complete P450scc deficiency leads to primary adrenal insufficiency (PAI) and 46,XY disordered sexual development. Partial impairment can cause variable adrenal and gonadal dysfunction.
Objective
Our aim was to evaluate the effects of the CYP11A1 variant p.E314K, identified in patients with PAI, specifically on P450scc enzyme stability and function.
Patients and Methods
We studied four boys from two unrelated families presenting with PAI during childhood (3.6 to 9 years old). All patients were compound heterozygous for c.940G>A (p.E314K), a CYP11A1 nonsynonymous variant likely to be pathogenic by some but not all in silico prediction models, and c.835delA (p.I79Yfs*10), a known pathogenic variant. HEK293T cells were transfected with wild type (WT) and p.E314K mutant vectors, and a cycloheximide chase assay was performed to analyze protein stability. Pregnenolone production was assayed from cells expressing WT and p.E314K-F2 fusion proteins.
Results
Two boys experienced spontaneous puberty but then developed evidence of primary gonadal failure at 14 and 18 years old. Two boys had testicular adrenal rest tumor (TART), detected by ultrasound at ages 8.6 and 16 years. Compared with WT, mutant protein synthesis was reduced (P = 0.0006) with increased protein turnover, and mutant P450scc half-life was decreased by ~50%. p.E314K mutant P450scc retained 60% of WT enzymatic activity (P = 0.007).
Conclusions
The CYP11A1 p.E314K variant impairs P450scc stability and is a possible cause of PAI in childhood. Pathogenic CYP11A1 variants potentially affect both adrenal and gonadal function, and male patients may develop TART.
The CYP11A1 nonsynonymous variant p.E314K impairs P450scc enzyme stability and function and is associated with adrenal insufficiency in childhood and peripubertal gonadal failure.
There are various types of congenital adrenal hyperplasia (CAH), each corresponding to a defect in any one of the steps leading to cortisol biosynthesis. These types of CAH manifest with a wide range of clinical severity with or without aldosterone deficiency and sex steroid imbalance due to effects on steroidogenic pathways. All types of CAH are autosomal recessive, and both classic (severe) and nonclassic (mild) forms have been described (1).
One of the rarest types of CAH is due to deficiency of P450 cholesterol side-chain cleavage enzyme (P450scc, encoded by CYP11A1), which catalyzes the conversion of cholesterol to pregnenolone, the first and rate-limiting step in the steroidogenesis pathway. P450scc deficiency is clinically and biochemically identical to lipoid CAH, which was described before the identification of P450scc deficiency (2). Lipoid CAH is caused by impairment of steroidogenic acute regulatory protein, which transfers cholesterol from the outer to the inner mitochondrial membrane, a key step in the initiation of steroidogenesis (3). Classic P450scc deficiency and classic lipoid CAH are both characterized by deficiency of all steroid hormones, resulting in neonatal adrenal crisis and feminization of the external genitalia in 46,XY affected infants. In 2006, a nonclassic form of lipoid CAH was described with mutations retaining ~20% to 30% of steroidogenic acute regulatory protein activity (4). Most cases were initially misdiagnosed as isolated familial glucocorticoid deficiency or Addison disease (5), and gonadal function ranged from normal to hypergonadotropic hypogonadism. Similarly, nonclassic P450scc deficiency has since been described with variable age of onset of adrenal insufficiency and a range of gonadal effects (6–9). Overall, <40 cases have been reported (1).
Here we describe the deleterious effects of a CYP11A1 nonsynonymous variant, p.E314K, identified in four patients presenting with adrenal insufficiency. We show that the P450scc protein stability and half-life are significantly affected in p.E314K mutant compared with wild type (WT) enzyme, thus characterizing the p.E314K variant as pathogenic. This previously reported missense variant was predicted to be benign by some models, highlighting the importance of performing functional studies.
Patient and Methods
Case reports
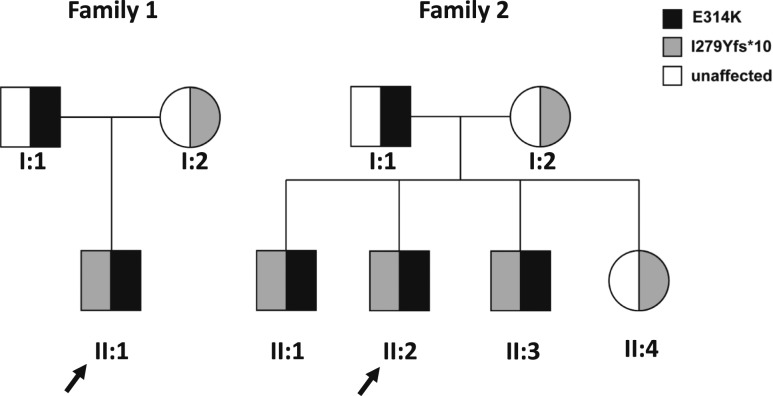
Four patients (three from one kindred, family 2) were evaluated at the National Institutes of Health Clinical Center, Bethesda, Maryland (Fig. 1). All studies were approved by the Eunice Kennedy Shriver National Institute of Child Health and Development Institutional Review Board (NCT00250159). Parents provided written informed consent, and children of ≥7 years of age provided written assent.
Figure 1.
Pedigrees of two families with CYP11A1 mutations. Affected individuals in both families (dark filled symbols) were compound heterozygous for the indicated mutations. Unaffected parents and a healthy sibling have heterozygous mutations (half-filled symbols). Gray symbols indicate c.835delA (I279Yfs*10). Black symbols indicate c.940G>A (p.E314K). Arrows indicate the probands.
All patients were born at term with normal-appearing male genitalia; one patient (family 2, II:1) was large for gestational age (Fig. 1, Table 1). At the time of initial diagnosis, all patients presented with vomiting and hypoglycemia, and two had additional electrolyte findings (family 2, II:2, Na 120 mEq/L; family 2, II:3, Na 129 mEq/L and K 5.4 mEq/L) suggestive of adrenal insufficiency. The probands (family 1, II:1; family 2, II:2) had prior history of hypoglycemia during febrile illnesses. Adrenal antibodies were undetectable, and very long chain fatty acids were within the normal range. Three patients were diagnosed with primary adrenal insufficiency (PAI) of unknown etiology between 3 and 5 years of age, and one patient (family 2, II:1) was diagnosed with PAI at age 9. Past medical history included autism spectrum disorder in two siblings (family 2, II:1 and II:3), and one of them also had failure to thrive and short stature (height 1st percentile, family 2, II:3). All parents were healthy, and there was no family history of consanguinity, miscarriage, sudden death, or endocrine disease.
Table 1.
Clinical and Laboratory Findings of 4 Patients (2 Families) With Nonclassic P450scc Deficiency
| Family 1, Patient II:1 (Proband) | Family 2, Patient II:1 | Family 2, Patient II:2 (Proband) | Family 2, Patient II:3 | ||
|---|---|---|---|---|---|
| Gestational age, wk | 41 | 38 | 38 | 37 | |
| Birth weight, g | 3100 | 4280 | 3540 | 3400 | |
| External genitalia | Normal male | Normal male | Normal male | Normal male | |
| Age at presentation, y | 3.6 | 9 | 5 | 4.8 | |
| Presentation | Vomiting, lethargy, hypoglycemia (32 mg/dL) | Vomiting, lethargy, hypoglycemia (39 mg/dL) | Vomiting, abdominal pain, hypoglycemia (20 mg/dL), hyponatremia | Vomiting, abdominal pain, lethargy, hypoglycemia (30 mg/dL), hyponatremia hyperkalemia | |
| Age at evaluation, y | 18a | 13.5 | 8.6 | 5.5 | |
| Tanner stage | 5 | 3 | 1 | 1 | |
| Medications at evaluation | Hydrocortisone, mg/m2/day | 11.9 | 9.2 | 9.4 | 11.3 |
| Fludrocortisone, µg/day | 100 | 50 | 50 | 50 | |
| ACTH, pg/mL (rr: 5–46) | >2500 | 455 | 1049 | 429 | |
| Cortisol, µg/dL | Basal | 3 | 2 | 4.1 | 3.9 |
| 60 minb | 2.5 | 1.7 | 4.1 | 4.1 | |
| Aldosterone, ng/dL (rr: 10–33) | Basal | <3 | 6.9 | <4 | <4 |
| 60 minb | <3 | 6.5 | <4 | <4 | |
| 17-OH pregnenolone, ng/dL (rr: 55–455) | <16 | <16 | <16 | <16 | |
| 17-OH progesterone, ng/dL (rr: 13–120) | 60 | <13 | <13 | <13 | |
| 11-deoxycortisol, ng/dL (rr: 0–89.9) | 46.9 | 21.8 | 50.9 | 22.5 | |
| Androstenedione, ng/dL | 44 (rr: 65–210) | <13 | <13 | <13 | |
| DHEA-S, μg/dL | 16 (rr: 89–457) | <15 | <15 | <15 | |
| PRA, ng/mL/h | 1.1 (rr: 1.2–2.4) | 1.4 (rr: 1.2–2.4) | 3.8 (rr: 0.8–2) | 2.8 (rr: 1.5–3.5) | |
| FSH, mIU/mL | 20.4 (rr: 2.6–11) | 5.5 (rr: 1.2–5.8) | 1.2 (rr: 0.26–3.0) | 0.6 (rr: 0.26–3.0) | |
| LH, mIU/mL | 11.3 (rr: 1–8) | 2.1 (rr: 0.2–5.0) | 0.08 (rr: 0.02–0.3) | 0.094 (rr: 0.02–0.3) | |
| Total testosterone, ng/dL | 370 (rr: 350–970) | 92.9 (rr: 100–320) | <20 | <20 | |
| Scrotal ultrasound | Right testis: 3 hypoechoic lesions: 21 × 10 mm, 12 × 10 mm, 4 × 3 mm. Left testis: 2 hypoechoic lesions: 20 × 10 mm, 13 × 10 mm | Normal | Left testis: 4 × 3 mm hypoechoic lesion | Normal | |
| Adrenal imaging | CT: Bilaterally atrophic adrenal glands | MRI: Bilateral normal adrenal glands without evidence of hyperplasia | Not done | Not done | |
Laboratory evaluation performed at ~0800 h before medication administration. Age- and sex-specific normal ranges specified in parentheses.
Abbreviations: DHEA-S, dehydroepiandrosterone sulfate; PRA, plasma renin activity; rr, reference range, adjusted for age or Tanner stage as needed.
Scrotal ultrasound obtained at age 16.
60 min after cosyntropin 250 µg.
During their initial encounter at the National Institutes of Health, all patients were normotensive, and they were receiving thrice-daily hydrocortisone (average dosage 10.4 ± 1.1 mg/m2/day) and once-daily fludrocortisone. Early morning laboratory evaluation before medication administration revealed low levels of all adrenal steroids and elevated ACTH (Table 1). Two patients had ultrasonographic evidence of testicular adrenal rest tumor (TART), and the oldest patient had biochemical evidence hypergonadotropic hypogonadism (age 18, family 1, II:1). Additional follow-up revealed evidence of early gonadal failure at age 14 in the second oldest patient (family 2, II:1), as evidenced by elevated gonadotropins (FSH 12.0 mIU/mL, LH 7.4 mIU/mL) and low normal testosterone (109 ng/dL).
Mutational analysis
Whole exome sequencing was performed in family trio [GeneDX (XomeDx), Gaithersburg, MD], and the identified variants were confirmed by targeted Sanger sequencing (Fulgent Genetics, Temple City, CA).
In silico analysis of P450scc variants
We sought to predict how the c.940G>A (p.E314K) variant in the CYP11A1 would affect the P450scc protein conformation by using a structural prediction database and predictor software. The CYP11A1 gene sequence was taken from the National Center for Biotechnology Information reference accession number NM_000781.2, NP_000772.2; rs6161, with a minor allele frequency of f = 0.002422 (Broad Exome Dataset: http://exac.broadinstitute.org/gene/ENSG00000140459). In silico analyses of this sequence were performed with Polymorphism Phenotyping version 2 (PolyPhen2) (10), Sorting Intolerant From Tolerant (SIFT) (11), Protein Variation Effect Analyzer (PROVEAN) (12), Mutation Taster (13), and DUET protein prediction server (14) to predict the effects of the p.E314K variant on protein stability.
Protein expression and functional studies
Construction of P450scc expression vectors
FLAG-CYP11A1-V5 expression vector was constructed by PCR amplification of a commercially available FLAG-CYP11A1 cDNA clone (Sino Biological Inc., Beijing, China) followed by its subcloning into a pcDNA 3.1TM D/V5-HIS-TOPO expression vector (Invitrogen, Carlsbad, CA). PCR reaction was performed with the forward and reverse primers 5′-CACCATGGATTACAAGGATGACGACGAT-3′ and 5′-CTGCTGGGTTGCTTCCTGG-3′, respectively, and the Platinum SuperFi Green PCR Master Mix (Thermo Fisher Scientific, Waltham, MA). A mutant clone of FLAG-CYP11A1 (E314K)-V5 was generated with the Quick Change Multi-Mutagenesis kit (Agilent Technologies, Santa Clara, CA) and the primer 5′-CAGCAAGATGTCCTTCAAGGACATCAAGGCCAACG-3′. A cassette of human cDNAs for P450scc, ferredoxin reductase, and ferredoxin was assembled to form a fusion protein expressing F2 plasmid (NH2-P450scc-ferredoxin reductase-ferredoxin-COOH), which was previously engineered (15) (kindly provided by Dr. W. L. Miller, Department of Pediatrics, University of California, San Francisco, CA) and served as a template to generate the mutant p.E314K-F2 plasmid clone by using a Quick Change Multi-Mutagenesis kit (Agilent Technologies) with a primer of 5′-cagcaagatgtccttcAaggacatcaaggccaacg-3′. The cDNA sequences of both vectors were confirmed by Sanger sequencing.
Functional studies of P450scc enzyme stability
HEK293T cells were maintained in DMEM medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin solution and incubated at 37°C with 5% CO2. Cells were divided and seeded in six-well plates and then transfected with 1 µg plasmid DNA per well at ~50% confluency with either the WT or mutant (p.E314K) vectors for 48 hours, after which protein lysates were collected. No DNA was used for the control transfections. Transfection reactions were conducted with the Effectene Transfection Reagent according to the manufacturer’s instructions (Qiagen, Germantown, MD). For the cycloheximide (CHX) chase assay, HEK293T cells were seeded in 12-well plates. After reaching 50% confluency, they were transfected with WT and p.E314K mutant vectors for 48 hours, and then treated for 4 hours with 25 µM CHX (a eukaryotic translation elongation blocker) (Sigma, St. Louis, MO) or vehicle in the presence of 5 µM MG-132 (an inhibitor of the 26S proteosome) (Sigma) or dimethyl sulfoxide. After 4 hours, fresh serum free media was added, and every 0.5 hours the supernatant and protein lysates were collected for Western blot analysis.
Functional assay of P450scc enzyme activity
HEK293T cells were maintained in DMEM medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin solution and incubated at 37°C with 5% CO2. Cells were divided and seeded in 12-well plates and transiently transfected at 50% confluency with WT or mutant F2 (p.E314K-F2) plasmid. After 24 hours, the culture medium was replaced with fresh serum-free medium containing 0.3, 1, 3, or 5 µM 22R-hydroxycholesterol, which is the substrate for the P450scc enzyme and is converted to pregnenolone (7, 16). Conditioned media (supernatant) was collected 24 hours later and analyzed for pregnenolone production by enzyme immunoassay (ALPCO Diagnostics, Salem, NH). Assays were performed in the linear range of the standard curve as described (7).
Western blot analysis
Proteins were extracted with RIPA Lysis Buffer supplemented with 0.5% Triton X-100 and protease and phosphatase inhibitor (CalbioChem, Burlington, MA). Pelleted cells were incubated in buffers for 10 minutes on ice and centrifuged at 13,000 rpm for 20 minutes, and supernatant was transferred and stored at −80°C. After bicinchoninic acid protein quantification, 30 μg of total protein per lane was separated by electrophoresis on 4% to 12% Bis-Tris-glycine gels (Invitrogen). After transfer to polyvinylidene difluoride membranes, they were blocked at room temperature for 1 hours in 5% nonfat milk followed by overnight incubation with primary antibody at 4°C in phosphate-buffered saline with Tween-20 (PBST) [antimouse V5 antibody (Invitrogen), anti-rabbit beta-actin (Cell Signaling Technology, Danvers, MA)]. After three washes with PBST, membranes were incubated with fluorescent conjugated anti-mouse or anti-rabbit secondary antibodies (Li-COR Biotechnology, Lincoln, NE) at 1:15,000 dilution in PBST in darkness at room temperature for 1 hour. Immunoreactivity was visualized and quantified by infrared scanning in the Odyssey system (Li-COR Biotechnology), and band intensity was quantified by using Image Studio Lite version 5.2 software (Li-COR Biotechnology).
Statistical analysis
All experiments were performed in triplicate. Statistical analyses were performed with two-tailed unpaired Student t test (SigmaPlot, San Jose, CA). Data were presented as mean (SD), unless otherwise specified. P < 0.05 was considered statistically significant.
Results
Mutational analysis
Whole exome sequencing of each proband detected two heterozygous variants in the CYP11A1 gene associated with CAH due to P450scc deficiency: one previously described frameshift mutation in exon 5 (c.835delA, p.I279Yfs*10), with an expected complete loss of enzymatic activity (7, 8, 17–19), and a missense variant of unknown significance in exon 5, changing glutamate at the position 314 to lysine (c.940G>A, p.E314K). These two variants were in trans (compound heterozygous) with biparental inheritance in both families (Fig. 1). Results were confirmed by targeted Sanger sequencing (Fulgent Genetics, Temple City, CA).
Prediction analysis
The PolyPhen2 and SIFT tools predicted the p.E314K variant to be benign or tolerated (Table 2). However, in silico analysis with the PROVEAN prediction tool suggested that this amino acid substitution was deleterious, with a PROVEAN score of −3.266 (less than −2.5 is considered deleterious). The Mutation Taster Model predicted that this variant was probably pathogenic and disease causing, and according to DUET, a protein stability predicting server, this mutation causes the protein to destabilize, with a ∆∆G of −0.171 kcal/mol.
Table 2.
In Silico Analyses of p.E314K Variant From Mutation Pathogenicity Prediction Software
| Software | Prediction |
|---|---|
| PolyPhen 2 | Benign |
| SIFT | Benign |
| PROVEAN | Deleterious |
| Mutation Taster | Disease causing |
| DUET | Destabilize |
Analysis of protein stability
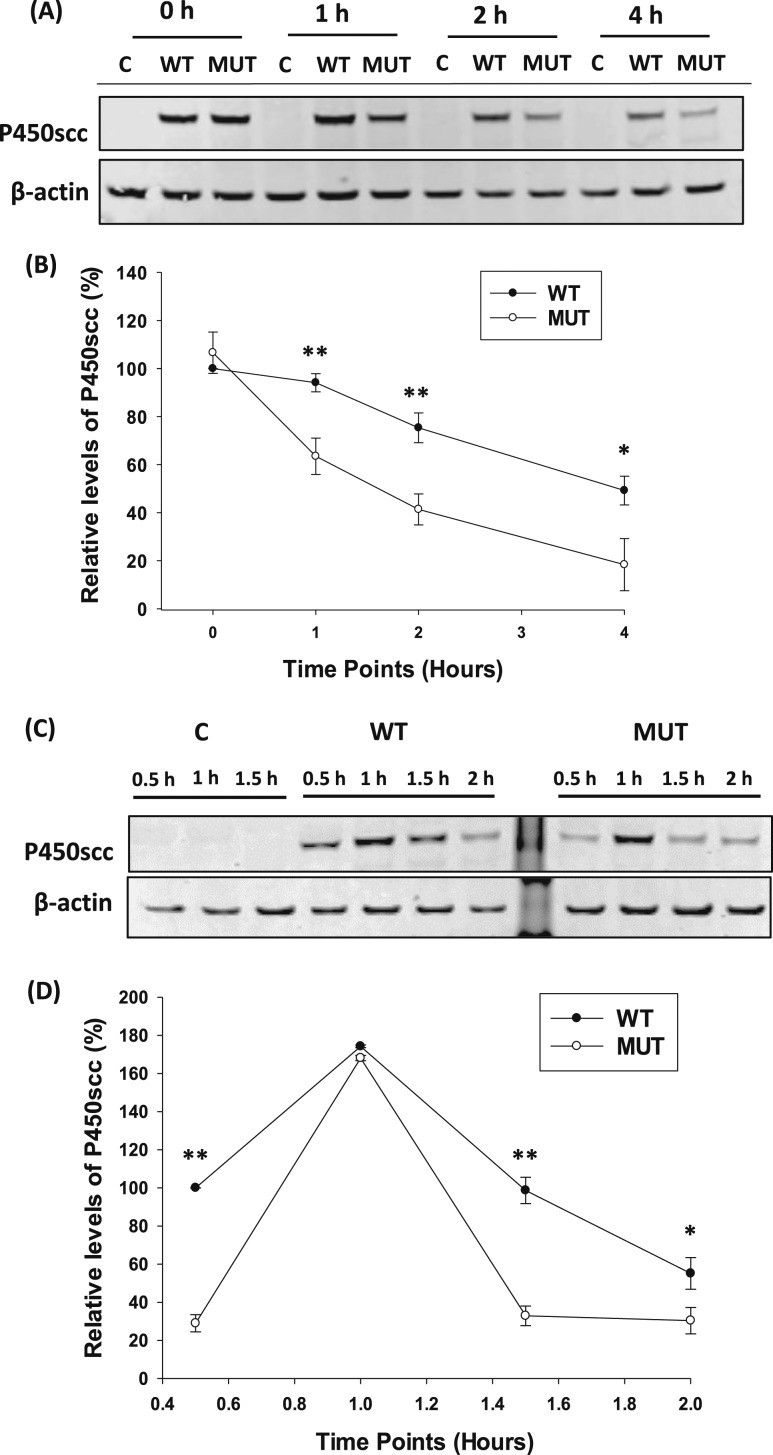
To compare the protein stability of both WT and p.E314K mutants in eukaryotic cells, an in vitro CHX chase assay was designed with the WT and p.E314K mutant vectors. Cells were treated with CHX for 4 hours, but the p.E314K mutant protein level was 30% less than the WT protein by 1 hour (P = 0.0006) and remained significantly lower until the end of the assay [Fig. 2(A), 2(B)]. MG132 treatments were done to eliminate the possibility of the p.E314K mutant undergoing proteosomal degradation. Adding MG132 did not rescue the p.E314K mutant protein (data not shown), suggesting that decreased protein was not the result of proteosomal degradation. In addition, MG132 did not significantly influence the secretion of the p.E314K mutant protein (data not shown). In the recovery period after the CHX chase assay, the levels of WT and p.E314K protein increased to similar levels within 1 hour but then differed with steady state conditions. Western blot showed a significant decrease in levels of p.E314K mutant protein compared with WT in the cells by 2 hours after removal of CHX (P = 0.01) [Fig. 2(C), 2(D)]. Based on these results, the overall p.E314K mutant protein half-life was ~50% less than WT.
Figure 2.
Comparison of P450scc stability between WT and p.E314K variant. (A) Representative Western blot analysis of P450scc expression after WT or mutant (MUT) vector transfection for 48 hours in HEK293T cells followed by 25 µM CHX treatment of the indicated time points. A mock transfection without DNA was performed as a control. (B) Quantification of P450scc levels normalized to actin and expressed as relative to the WT 0 hour time point showed impaired protein stability. (C) Representative Western immunoblot of P450scc expression after transfection with WT or MUT vectors for 48 hours, followed by CHX treatment. After 4 hours, HEK293 cells were supplemented with fresh media, and lysates were collected at the indicated time points. (D) Quantification of P450scc expression normalized to actin and expressed as relative to the WT 0.5 hour time point after the CHX chase assay showed a decrease in protein levels. Data are shown as mean ± SD from three independent experiments. *P < 0.01; **P < 0.001. Abbreviation: C, control.
In vitro analysis of P450scc enzymatic activity
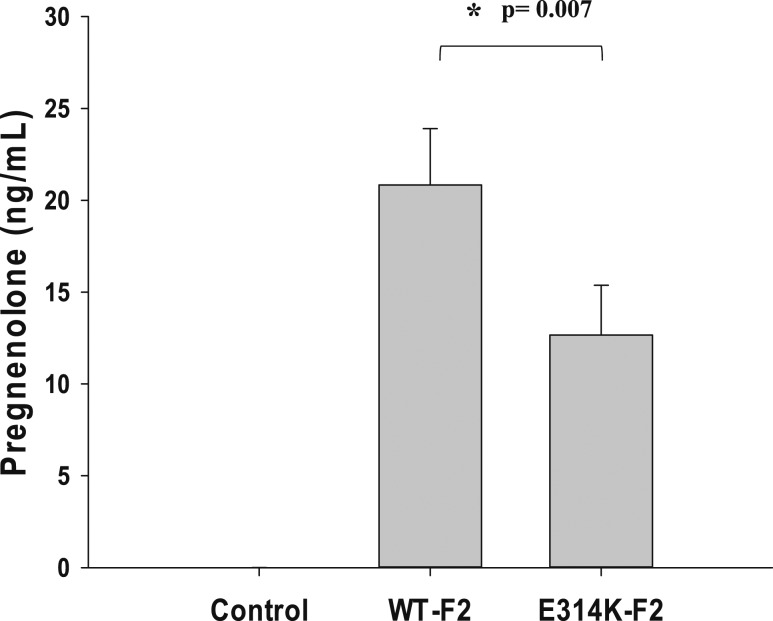
HEK293T cells expressing WT F2 plasmids produced pregnenolone 20.83 ± 3.07 ng/mL in culture medium after 5 µM 22R-hydroxycholesterol was added as a substrate, whereas cells transfected with the p.E314K mutant plasmid produced pregnenolone 12.67 ± 2.72 ng /mL (P = 0.007); control treatment lacked any detectable pregnenolone (Fig. 3). Similar results were obtained with other concentrations of substrate (data not shown).
Figure 3.
P450scc activity of p.E314K mutant. HEK293T cells were transfected with either WT F2 or mutant F2 plasmid, and 5 µM 22R-hydroxycholesterol was added to the culture medium as a substrate. Mock transfections were performed as negative controls. Pregnenolone secreted into the culture medium was determined by enzyme immunoassay. Values are the mean ± SD from three independent transfection experiments, each performed in duplicate.
Discussion
PAI is a rare but potentially life-threatening condition. The most common cause in adults is autoimmunity, but in children a genetic etiology is most commonly found (1, 20). Autosomal recessive variants in CYP11A1 may result in partial loss of steroid hormone production, potentially affecting both adrenal and gonadal function. Thus, identifying the etiology of adrenal insufficiency presenting during childhood is essential. Here we show that a previously reported CYP11A1 nonsynonymous variant of unknown significance (c.940G>A, p.E314K) is pathogenic and is associated with a mild phenotype affecting both adrenal and gonadal function. We also show that the p.E314K variant affects P450scc stability and its half-life.
In 2001, a mutation in CYP11A1 was first reported as causing adrenal insufficiency and 46,XY sex reversal (21). Since then, <40 cases of CAH due to P450scc deficiency have been reported, with a wide range of clinical manifestations, including a milder nonclassic phenotype. The phenotype described in this nonclassic group has been diverse, although PAI was diagnosed before the age of 6 in ~90% of cases, and the remaining cases were adrenal insufficiency diagnosed later in childhood (6–9). Many patients were initially given a diagnosis of Addison disease (ie, PAI of unknown etiology) or misdiagnosed with familial glucocorticoid deficiency (22). Genitalia is variably affected; some male patients are undervirilized (ie, hypospadias or cryptorchidism), but 46,XY normal male genitalia has also been observed (8, 9). Gonadal failure may be observed with human chorionic gonadotropin stimulation testing in prepubertal children and in a subset of patients at the time of puberty (8, 9). Our patients had normal male external genitalia, and two patients had normal puberty progression with eventual peripubertal evidence of gonadal failure, whereas the other two are still age appropriately prepubertal.
Two of our male patients had peripubertal evidence of TART. TART is most commonly found in patients with CAH due to 21-hydroxylase deficiency (23), but we report TART in patients with CAH due to P450scc deficiency. Although not malignant, progressive enlargement of TART can lead to irreversible damage of the surrounding testicular tissue and azoospermia (24). TART has features of adrenal cortical tissue and responds to elevated ACTH (25). Although TART is not found in all patients with CAH and poor control, the timing and duration of ACTH elevation might contribute to its pathogenesis, suggesting that glucocorticoid replacement in patients with P450scc deficiency should be similar to the suggested therapy for 21-hydroxylase deficiency (ie, thrice daily hydrocortisone aiming to suppress nighttime ACTH secretion) (23). Moreover, because the development of TART is an additional risk factor for infertility in patients with nonclassic P450scc deficiency, early sonographic screening should be implemented.
We found that the mutant P450scc enzyme activity was less active (~60%) than WT; however, this observed decrease in enzyme activity may have been due to the reduced protein levels under steady state conditions. This finding suggests that the mutant P450scc may have the same specific activity as the WT enzyme but degrades more quickly.
Unlike in more common forms of CAH, no clear correlation has been made between the phenotype and the degree of enzymatic activity in patients with P450scc deficiency; normal development of male genitalia was reported in patients carrying a mutation corresponding to only 2.5% of residual enzyme activity (8). This finding may be due in part to different methods used across studies (9), but the influence of other genes and epigenetic factors may also play a role. The impact of other hormones on P450scc activity is largely unknown, but upregulation of CYP11A1 occurs in granulosa cells in response to the ovulatory LH surge through epigenetic changes in the CYP11A1 promoter (26).
Patients from both families carried a CYP11A1 I279Y fs*10 mutation, which has been well characterized and is reported to be pathogenic in both homozygous and compound heterozygous states (7, 18, 19, 22). This mutation leads to the deletion of an adenine nucleotide, causing an early termination/stop codon and the loss of a heme-binding domain close to the carboxyl end, thus completely ablating enzymatic activity (19). In all four of our cases, the CYP11A1 I279Y fs*10 and p.E314K variants existed as compound heterozygous and were not seen together in parents or unaffected siblings. Previously, the p.E314K variant was considered to be probably benign and functionally tolerated (22) but was reported to exist in a compound heterozygous state with other CYP11A1 variants in two patients: one patient carrying the diagnosis of familial glucocorticoid deficiency was compound heterozygous for p.E314K and a silent T330T variant at the end of exon 5 affecting splicing (22), and a 12-year-old boy with PAI and hypospadias was compound heterozygous for p.E314K and the splice site variant c.425+1G>A (27). Functional studies were not performed, and no patients have been reported to be homozygous for this variant.
Although in silico prediction tools play a valuable role in predicting that the p.E314K mutation is deleterious and would destabilize the protein integrity, these predictions are based on algorithms, and functional analyses are vital to determine the pathogenicity and correlate the effects to the clinical symptoms. The CYP11A1 p.E314K variant results in a nonconservative amino acid substitution, where glutamate, a negatively charged amino acid, is replaced by a positively charged lysine. This change in charge might affect the protein secondary structure by influencing the folding and stability. In addition, this change might affect the catalytic activity of the enzyme. However, because the mutation lies between the alpha helices, which are located away from the active site, the specific activity of the enzyme was probably not affected. The cause for protein instability is unknown.
The mechanism of progressive loss of P450scc activity in patients is not understood. Our study provides evidence of protein instability associated with a CYP11A1 variant. Previous studies of CYP11A1 variants have been restricted to studies of P450scc enzymatic activity. We show that P450scc expression levels of the p.E314K mutant were lower than in the WT in HEK293T cells. In addition, the mutant protein half-life was significantly lower than in the WT. Protein synthesis and degradation are balanced in a cell, with the degree of protein production and degradation dependent on the in vivo regulation. We found that the mutant protein turnover was higher than the WT protein turnover. The p.E314K mutant protein was not rescued with proteosome inhibitor MG-132 addition after CHX treatment, suggesting that mutant protein is not targeted for degradation in the proteasome system, a typical method of removal of any misfolded protein trapped and accumulated in the endoplasmic reticulum. Thus, altered protein stability may further contribute to the pathogenesis of this rare enzyme deficiency.
Our study shows that the CYP11A1 p.E314K variant is pathogenic in a compound heterozygous state in two unrelated and nonconsanguineous families and is a cause of PAI during childhood. Most important, patients with P450scc deficiency are also at risk for gonadal failure, irrespective of genital phenotype and the ability to attain normal pubertal progression, and male patients may develop TART. Through functional studies, we show that protein instability is associated with this CYP11A1 p.E314K variant. Children with isolated glucocorticoid deficiency should be evaluated for the rare types of CAH, including partial P450scc deficiency. Determining the underlying cause of isolated PAI is essential to providing appropriate clinical care.
Acknowledgments
We thank Dr. Niamh Cawley for his intellectual contribution to the work and Dr. Fabio Rueda Faucz for his technical support in Sanger sequencing. We also extend our gratitude to Dr. Walter L. Miller for sharing F2 plasmids for enzymatic activity studies.
Financial Support: This research was supported by the Intramural Research Program at the National Institutes of Health, Bethesda, Maryland.
Author Contributions: V.K. supervised, designed, and performed the in vitro experiments, analyzed the data, and drafted the manuscript. H.K. performed the in vitro experiments. A.T., Q.L., and C.T. participated in data collection and interpretation of results and contributed to drafting the article. Q.L. also helped in making the constructs. A.M. participated in data collection and interpretation of results. D.P.M. participated in data collection, created the study design, and supervised the project and manuscript writing. All authors approved the final version of the manuscript.
Disclosure Summary: D.P.M. received unrelated research funds from Diurnal Limited and Millendo Therapeutics through the National Institutes of Health Cooperative Research and Development Agreement and is a commissioned officer in the US Public Health Service. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- CAH
congenital adrenal hyperplasia
- CHX
cycloheximide
- P450scc
cholesterol side-chain cleavage enzyme
- PAI
primary adrenal insufficiency
- PBST
phosphate-buffered saline with Tween-20
- PolyPhen2
Polymorphism Phenotyping version 2
- PROVEAN
Protein Variation Effect Analyzer
- SIFT
Sorting Intolerant From Tolerant
- TART
testicular adrenal rest tumor
- WT
wild type
References
- 1. El-Maouche D, Arlt W, Merke DP. Congenital adrenal hyperplasia. Lancet. 2017;390(10108):2194–2210. [DOI] [PubMed] [Google Scholar]
- 2. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bose HS, Sugawara T, Strauss JF III, Miller WL; International Congenital Lipoid Adrenal Hyperplasia Consortium . The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. N Engl J Med. 1996;335(25):1870–1878. [DOI] [PubMed] [Google Scholar]
- 4. Baker BY, Lin L, Kim CJ, Raza J, Smith CP, Miller WL, Achermann JC. Nonclassic congenital lipoid adrenal hyperplasia: a new disorder of the steroidogenic acute regulatory protein with very late presentation and normal male genitalia. J Clin Endocrinol Metab. 2006;91(12):4781–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Metherell LA, Naville D, Halaby G, Begeot M, Huebner A, Nürnberg G, Nürnberg P, Green J, Tomlinson JW, Krone NP, Lin L, Racine M, Berney DM, Achermann JC, Arlt W, Clark AJ. Nonclassic lipoid congenital adrenal hyperplasia masquerading as familial glucocorticoid deficiency. J Clin Endocrinol Metab. 2009;94(10):3865–3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rubtsov P, Karmanov M, Sverdlova P, Spirin P, Tiulpakov A. A novel homozygous mutation in CYP11A1 gene is associated with late-onset adrenal insufficiency and hypospadias in a 46,XY patient. J Clin Endocrinol Metab. 2009;94(3):936–939. [DOI] [PubMed] [Google Scholar]
- 7. Sahakitrungruang T, Tee MK, Blackett PR, Miller WL. Partial defect in the cholesterol side-chain cleavage enzyme P450scc (CYP11A1) resembling nonclassic congenital lipoid adrenal hyperplasia. J Clin Endocrinol Metab. 2011;96(3):792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tee MK, Abramsohn M, Loewenthal N, Harris M, Siwach S, Kaplinsky A, Markus B, Birk O, Sheffield VC, Parvari R, Hershkovitz E, Miller WL. Varied clinical presentations of seven patients with mutations in CYP11A1 encoding the cholesterol side-chain cleavage enzyme, P450scc [published correction appears in Endocrinol Metab. 2013;98(10):4213] J Clin Endocrinol Metab. 2013;98(2):713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parajes S, Kamrath C, Rose IT, Taylor AE, Mooij CF, Dhir V, Grötzinger J, Arlt W, Krone N. A novel entity of clinically isolated adrenal insufficiency caused by a partially inactivating mutation of the gene encoding for P450 side chain cleavage enzyme (CYP11A1). J Clin Endocrinol Metab. 2011;96(11):E1798–E1806. [DOI] [PubMed] [Google Scholar]
- 10. Brigham and Women’s Hospital/Harvard Medical School Polymorphism Phenotyping v2. Available at: http://genetics.bwh.harvard.edu/pph2. Accessed 27 June 2018.
- 11. The Genome Institute of Singapore. Sorting Intolerant From Tolerant. Available at: http://sift.bii.a-star.edu.sg/. Accessed 27 June 2018.
- 12. J. Craig Venter Institute Protein Variation Effect Analyzer. Available at: http://provean.jcvi.org/index.php. Accessed 27 June 2018.
- 13. Berlin Institute of Health. Mutation Taster. Available at: http://www.mutationtaster.org/. Accessed 27 June 2018.
- 14.DUET protein predicting Server: University of Cambridge. Available at: http://biosig.unimelb.edu.au/duet/. Accessed 27 June 2018.
- 15. Harikrishna JA, Black SM, Szklarz GD, Miller WL. Construction and function of fusion enzymes of the human cytochrome P450scc system. DNA Cell Biol. 1993;12(5):371–379. [DOI] [PubMed] [Google Scholar]
- 16. Toaff ME, Schleyer H, Strauss JF III. Metabolism of 25-hydroxycholesterol by rat luteal mitochondria and dispersed cells. Endocrinology. 1982;111(6):1785–1790. [DOI] [PubMed] [Google Scholar]
- 17. al Kandari H, Katsumata N, Alexander S, Rasoul MA. Homozygous mutation of P450 side-chain cleavage enzyme gene (CYP11A1) in 46, XY patient with adrenal insufficiency, complete sex reversal, and agenesis of corpus callosum. J Clin Endocrinol Metab. 2006;91(8):2821–2826. [DOI] [PubMed] [Google Scholar]
- 18. Kim CJ, Lin L, Huang N, Quigley CA, AvRuskin TW, Achermann JC, Miller WL. Severe combined adrenal and gonadal deficiency caused by novel mutations in the cholesterol side chain cleavage enzyme, P450scc. J Clin Endocrinol Metab. 2008;93(3):696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hiort O, Holterhus PM, Werner R, Marschke C, Hoppe U, Partsch CJ, Riepe FG, Achermann JC, Struve D. Homozygous disruption of P450 side-chain cleavage (CYP11A1) is associated with prematurity, complete 46,XY sex reversal, and severe adrenal failure. J Clin Endocrinol Metab. 2005;90(1):538–541. [DOI] [PubMed] [Google Scholar]
- 20. Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, Husebye ES, Merke DP, Murad MH, Stratakis CA, Torpy DJ. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tajima T, Fujieda K, Kouda N, Nakae J, Miller WL. Heterozygous mutation in the cholesterol side chain cleavage enzyme (p450scc) gene in a patient with 46,XY sex reversal and adrenal insufficiency. J Clin Endocrinol Metab. 2001;86(8):3820–3825. [DOI] [PubMed] [Google Scholar]
- 22. Chan LF, Campbell DC, Novoselova TV, Clark AJ, Metherell LA. Whole-exome sequencing in the differential diagnosis of primary adrenal insufficiency in children. Front Endocrinol (Lausanne). 2015;6:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC; Endocrine Society . Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(9):4133–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Claahsen-van der Grinten HL, Otten BJ, Hermus AR, Sweep FC, Hulsbergen-van de Kaa CA. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia can cause severe testicular damage. Fertil Steril. 2008;89(3):597–601. [DOI] [PubMed] [Google Scholar]
- 25. Claahsen-van der Grinten HL, Otten BJ, Sweep FC, Span PN, Ross HA, Meuleman EJ, Hermus AR. Testicular tumors in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency show functional features of adrenocortical tissue. J Clin Endocrinol Metab. 2007;92(9):3674–3680. [DOI] [PubMed] [Google Scholar]
- 26. Okada M, Lee L, Maekawa R, Sato S, Kajimura T, Shinagawa M, Tamura I, Taketani T, Asada H, Tamura H, Sugino N. Epigenetic changes of the Cyp11a1 promoter region in granulosa cells undergoing luteinization during ovulation in female rats. Endocrinology. 2016;157(9):3344–3354. [DOI] [PubMed] [Google Scholar]
- 27. Lara-Velazquez M, Perdomo-Pantoja A, Blackburn PR, Gass JM, Caulfield TR, Atwal PS. A novel splice site variant in CYP11A1 in trans with the p.E314K variant in a male patient with congenital adrenal insufficiency. Mol Genet Genomic Med. 2017;5(6):781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]