Abstract
GH insensitivity (GHI) presents in childhood with growth failure and in its severe form is associated with extreme short stature and dysmorphic and metabolic abnormalities. In recent years, the clinical, biochemical, and genetic characteristics of GHI and other overlapping short stature syndromes have rapidly expanded. This can be attributed to advancing genetic techniques and a greater awareness of this group of disorders. We review this important spectrum of defects, which present with phenotypes at the milder end of the GHI continuum. We discuss their clinical, biochemical, and genetic characteristics. The objective of this review is to clarify the definition, identification, and investigation of this clinically relevant group of growth defects. We also review the therapeutic challenges of mild GHI.
Essential Points
The phenotypic and genotypic characterization of GH insensitivity (GHI) and other overlapping short stature syndromes have greatly expanded with increasing awareness and advancing genetic techniques
Although the severe defects classified as “classical” GHI are well characterized, there are no current reviews specifically describing the “nonclassical” mild or moderate end of the clinical spectrum
Recent evidence suggests that nonclassical GHI phenotypes are an important clinical entity, and prevalence may be higher than classical GHI
We clarify definitions and propose optimal investigation and management strategies for nonclassical GHI, and we also review the therapeutic challenges of mild GHI
Although the role of recombinant human IGF-1 replacement therapy in children with milder IGF-1 deficiency remains unclear, we present encouraging early data
Disorders of GH action have taken second place to defects of GH secretion in clinical endocrine practice. Not only do they appear to occur more rarely, but notably effective treatment has been lacking and is even now far less developed than the relatively successful and safe treatment using recombinant human GH (rhGH). In 2011, we described the continuum of disorders, which make up the group of GH insensitivity (GHI) defects (1). However, this category has continued to expand, but no current reviews have specifically described the mild or moderate end of this spectrum, in contrast to the well-documented severe defects classified as “classical” GHI. Classical GHI was first described by Laron et al. (2, 3) in 1966. This disorder, termed “Laron type dwarfism” or “Laron syndrome,” was subsequently shown to be caused by a defect in the GH receptor (GHR) gene, resulting in severe GH resistance and associated IGF-1 deficiency (4). This rare and extreme phenotype became synonymous with a diagnosis of GHI. Since the first description, however, >70 homozygous or compound heterozygous missense, nonsense, and splice site GHR mutations in >250 patients have been identified (1). Even within classical GHI patients, a significant phenotypic and biochemical variability has been observed (5).
During the last two decades, monogenic defects leading to GHI have also been uncovered in signal transducer and activator of transcription 5B (STAT5B) (6, 7), IGF1 (8), IGFALS (9), and pregnancy-associated plasma protease A2 (PAPPA2) (10). The identification of these downstream genetic abnormalities within the GH–IGF-1 axis has expanded and complicated the diagnostic spectrum of GHI, thus further supporting a continuum of GH–IGF-1 axis abnormalities associated with a wider range of phenotypes (1, 5). Depending on the genetic defect, specific clinical and dysmorphic features may include midfacial hypoplasia and frontal bossing (GHR, STAT5B) (2, 6), immune deficiency (STAT5B) (6), pubertal delay (IGFALS, STAT5B, GHR) (2, 6, 11), decreased bone mineral density (PAPPA2) (10), developmental delay, and microcephaly and intrauterine growth retardation [IGF1, IGF-1 receptor (IGF1R)] (1). More recently, it has been observed that a range of short stature disorders such as 3M syndrome, Silver-Russell syndrome (SRS), and Noonan syndrome (NS) have phenotypes that may overlap with GHI (12–15).
For many years the existence of mild or “nonclassical” GHI was poorly understood. However, recent accumulation of evidence for these nonclassical GHI phenotypes suggests that prevalence may be higher than for classical GHI and therefore should be acknowledged. In this review, we demonstrate the rapidly expanding clinical, biochemical, and genetic characteristics of GHI defects and other overlapping short stature syndromes. The key message to clinicians and scientists is that mild GHI is likely to be prevalent and should be investigated and treated.
The GH–IGF-1 Axis in Human Growth
Physiology of the GH–IGF-1 axis and linear growth
The postnatal linear growth-promoting effects of GH in humans are mediated primarily through regulating expression of IGF-1, both circulating and peripheral. The roles of circulating vs peripheral IGF-1 for GH-induced growth has evolved from the original “somatomedin hypothesis” (16, 17), in which growth was proposed to be the direct effect of GH on liver production of IGF-1 (somatomedin C), with subsequent circulating IGF-1 exerting effects on main target organs such as the cartilage and bone. Recent omic approaches (genomic, transcriptomic) support accumulating evidence that many human tissues express IGF1 mRNA (18, 19) (https://www.ncbi.nlm.nih.gov/gene/3479), although it remains unclear whether expression can be regulated by GH or whether the transcripts are translated. Rodent models in which GH injected into hypophysectomized rats resulted in direct GH regulation of Igf1 in multiple nonhepatic tissues, including the growth plate and adipose tissues (20–25), suggesting that locally produced IGF-1 has important autocrine/paracrine effects on growth. The critical role of peripheral IGF-1 has been further supported by observations that the liver-specific IgfI knockout mouse grew normally (26); however, in apparent contradiction, restoration of hepatic IgfI expression was sufficient to induce normal growth in the Igf1−/−-null mice (27). These studies, together with evidence that GH has complex anabolic effects independent of regulating IGF-1 production and that IGF-1 production can be regulated independent of GH, such as, for example, for in utero growth (28), led to several modifications of the original somatomedin hypothesis (29, 30). The central importance of GH and IGF-1 for linear growth, however, remains constant.
The GH–IGF-1 axis involves the ligands IGF-1 and IGF-2, the GH and IGF-1 receptors, six IGF-binding proteins (IGFBPs), acid-labile subunit (ALS), and multiple intracellular signaling components. Components involved in transducing GH signals include the cell surface GHR, Janus kinase 2 (JAK2), and signaling molecules such as STAT5B. The identification of genetic defects within the GH–IGF-1 axis in patients diagnosed with GHI syndrome support the key roles of these components in GH action (1). Activation of the GHR–JAK2–STAT5B pathway leads to regulation of targets IGF-1, IGFBPs, and ALS. The secreted IGF-1 is protected by complexing with IGFBPs and, in the circulation, IGF-1–IGFBP is bound in ternary complex with ALS. IGF-1 becomes bioavailable through various mechanisms with recently described human mutations in PAPPA2 (10), indicating that proteolysis of IGFBP-3 and IGFBP-5 is one such mechanism (see “PAPPA2 mutations” below). The free IGF-1 subsequently interacts with cell surface IGF-1 receptors, IGF1R, to initiate signal cascades that ultimately promote linear growth.
It is now clear that IGF-1 production regulated by GH is a postnatal event. This has recently been comprehensively reviewed (31). Molecular defects associated with the clinical conditions of GH deficiency (32) and GHI (1), both of which often result in severe IGF-1 deficiency and growth failure [postnatal height as low as −10 SD score (SDS)], affirmed that GH regulation of global IGF-1 production was crucial for normal linear growth. Moreover, the advent of multiple genetic methodologies, including next-generation genomic sequencing for evaluating molecular defects in patients who are diagnosed as GHI with short stature (14, 15, 33), has provided a better appreciation for the wide spectrum of growth impairment associated with mutations along the human GH (hGH)–IGF-1 growth axis.
The critical importance of IGF-1 for growth in humans is verified by rare homozygous IGF1 mutations, with three convincing cases reported (8, 34, 35) (see “IGF1 mutations” below). The observed intrauterine growth retardation (IUGR) and severe postnatal growth failure (height SDS below −4.9) in each case supported the importance of IGF-1 for normal growth in utero as well as postnatally. Furthermore, the microcephaly and mental retardation associated with the IGF1 mutations suggested that IGF-1 is critical for brain development, and sensorineural deafness was reported in two of the three cases (8, 34).
GHR function and postreceptor signaling
The human GHR, a homodimeric, cell-surface, transmembrane protein, is a member of the type I class of cytokine receptors, which includes the prolactin receptor, the erythropoietin receptor, and a number of IL receptors. GHR is encoded by GHR, a 297.9-kb, 10 exon–containing gene located on chromosome 5p13.1-p12. Multiple GHR transcripts are generated from the gene, with human full-length GHR mRNA widely distributed in human tissues. The two main prepeptides are the full-length GHR (GHRfl, isoform 1) and the common GHR variant that lacks exon 3 sequences. Exons 2 to 10 encode for a prepeptide of 638 amino acid residues with the first 18 amino acids, the signal peptide, proteolytically removed upon the insertion of the receptor into the plasma membrane. The mature GHR protein, 620 residues in length, is comprised of three domains: an extracellular, GH-binding domain encoded by exons 2 to 7 (246 amino acid residues), a short transmembrane domain encoded by exon 8 (24 residues), and the intracellular portion of the GHR encoded by exons 9 and exon 10 (350 residues). Exon 10 also carries 2.4 kb of the 3′ untranslated region, a region that is usually necessary for stabilizing mRNA expression and can, therefore, potentially harbor detrimental mutations (36). Posttranslational modification of the mature GHR produces a glycosylated monomer of ∼125 kDa molecular mass, and the final GHR product translocates to the cell surface as a preformed homodimer (37).
The GHR, typical of type I cytokine receptors, lacks the intrinsic kinase activity necessary to initiate signal transduction. The dimeric GHR, through very specific motifs in the intracellular domain (box 1, box 2), preferentially recruits cytosolic JAK2, with one JAK2 per monomeric GHR. Upon binding of one molecule of GH to the dimeric GHR, conformational changes in the GHR (38) lead to repositioning of the two associated JAK2 molecules, facilitating transphosphorylation (39). Activated JAK2 subsequently phosphorylates multiple tyrosines located on the intracellular domain of the GHR, with three out of the seven tyrosines serving as critical, redundant docking sites for the cytosolic STAT5B protein (40). Other pathways activated by the GH–GHR interaction include the MAPK, phosphatidylinositol 3-kinase, and STAT1 and STAT3 pathways, all of which do not appear to dock at phosphorylated tyrosines on GHR (40, 41). JAK2, however, is essential for activation of all pathways in both human GHR (40) and rodent Ghr (42). The signaling cascades culminate in gene regulation, which, in the liver, include IGF1, IGFBP3, and IGFALS (ALS). Also upregulated are negative regulators, such as the SOCS family of proteins (43), that act in a feedback loop to dampen GH–GHR signal transduction, thereby modulating IGF-1 production.
IGF-1 transport and bioavailability
IGF-1 is involved in numerous physiological processes ranging from linear growth and metabolism to cancer and obesity (44). It plays a pivotal role in fetal development and childhood and adolescent linear growth (45). In biological fluids, IGF-1s are normally bound to IGFBPs, of which there are six, that is, IGFBP-1 through IGFBP-6. Because IGFBPs have higher affinities for IGFs [equilibrium dissociation constant (Kd) ∼10−10 M] than do the cell surface IGF1Rs (Kd ∼10−8 to 10−9 M), the IGFBPs act both as carriers of IGFs, thereby prolonging the half-life of the IGFs, but also as modulators of IGF availability and activity (46, 47).
Most circulating IGF-1 is generated by the liver, regulated by insulin (in utero) and by GH (postnatal). IGF-1 circulates bound to principally IGFBP-3 and IGFBP-5, the fractions of which form ternary complexes with ALS, which is also GH-dependent and significantly prolongs the half-life of circulating IGF-1–IGFBP binary complexes (48). Mutations affecting the activity of ALS (see “IGFALS mutations” below) result in failure of formation of ternary complexes, with pharmacokinetics evidence suggesting that IGF-1 is rapidly cleared (49). Recent identification of PAPPA2 defects (see “PAPPA2 mutations” below) suggests that proteolytic cleavage of IGFBP-3 and IGFBP-5 in the ternary complexes is sufficient to release IGF-1 for biological activities (10). In addition to diminished affinities of the IGFBPs for IGFs due to proteolysis of IGFBPs, other mechanisms for increasing IGF-1 availability include potential IGFBP conformational changes induced via binding of the IGFBPs to extracellular matrices and/or to cell surface and, for rodent IGFBP-5, physical occlusion effects as regions involved in IGF and extracellular matrix interactions overlap (50). The fact that no human mutations of genes coding for any of the IGFBPs have yet been reported points to redundancy in their functions. The cell-specific actions that free IGF-1 delivers to peripheral tissues are clearly finely tuned.
GHI
Definition
GHI can be defined as impairment of all or some of the mechanisms of physiological GH action. Its etiology comprises genetic or acquired defects of the GH–IGF-1 axis, which are now numerous, as recently described (1, 44). The causes of GHI are shown in Table 1. GH actions are also complex, consisting of both direct (24) and indirect (i.e., IGF-1–independent) effects (51). Consequently, the clinical features of GHI states can vary, depending on the pathogenesis. Similarly, there may be differing degrees of GHI, again varying according to pathogenesis, with defects ranging from extreme or total absence of peripheral GHR mechanisms to milder or moderate impairment causing subtler clinical phenotypes (1, 52).
Table 1.
Classification of GH Insensitivity Disorders With Short Stature
| Defects of the GH–IGF-1 axis |
|---|
| 1. GHR defects |
| a. Extracellular mutations |
| b. Transmembrane mutations |
| c. Intracellular mutations |
| 2. GH signal transduction defects (STAT5B) |
| 3. Mutations of SHP-2 (encoded by PTPN11) |
| 4. IGF1 gene mutations or deletions |
| a. Defects causing IGF-1 deficiency |
| b. Bioinactive IGF-1 |
| 5. IGF2 gene mutations |
| 6. ALS defects (IGFALS) |
| 7. PAPPA2 mutations |
| 8. IGFIR gene mutations |
| 9. GH neutralizing antibodies in patients with GH1 gene deletion |
| Acquired disorders causing GH resistance |
| 1. Malnutrition, parenchymal liver disease, type 1 diabetes mellitus |
| 2. Chronic inflammatory and nutritional disorders (e.g., juvenile chronic arthritis, Crohn disease, celiac disease, anorexia nervosa) |
What is classical GHI?
Classical GHI [Online Mendelian Inheritance in Man (OMIM) nos. 262500 and 245590] was first described by Laron et al. (3) who reported three children with extreme growth failure from a consanguineous Jewish family of Yemenite origin and who had the phenotype of hypopituitarism with high serum GH concentrations (2). Although an abnormal GH molecule was initially suspected, this disorder, “Laron-type dwarfism” or “Laron syndrome,” was shown to be caused by a defect in the GHR, resulting in the absence of binding of 125I-GH to GHRs prepared from the patients’ liver membranes (4). Homozygous and compound heterozygous mutations of the GHR gene were subsequently demonstrated (1, 53, 54). This striking but very rare autosomal recessive phenotype occurs most commonly in populations with a high incidence of parental consanguinity (2) and has also been reported in isolated communities in Southern Ecuador (55).
The classical features are as follows: extreme postnatal growth failure, childhood and adult short stature, craniofacial disproportion with midfacial hypoplasia, small external genitalia in males, sparse and thin hair, delayed motor development, small hands and feet, delayed dentition and puberty, and hypoglycemia, which is usually asymptomatic. On clinical presentation, the most striking features are the severe growth failure, with height usually <4 SDSs below the normal population mean (2, 56), and the craniofacial disproportion, which is caused by deficient growth of the endochondral sphenoid bone. This results in a positive correlation between facial height and statural height, contrasting with the head circumference, which is only slightly decreased (57).
Biochemically, patients with classical GHI have elevated GH secretion (2). Overnight GH sampling shows that pulsatile secretion is maintained but trough GH levels, usually undetectable in normal prepubertal subjects, may remain elevated (58). Serum concentrations of IGF-1, IGFBP-3, and ALS are severely subnormal (59, 60), and the IGF-1 and IGFBP-3 responses to hGH stimulation in an IGF-1 generation test are subnormal, reflecting the severity of the GHI (61–63). Positive correlations between serum IGF-1 and IGFBP-3 and height have been reported in populations of classical GHI from Ecuador and Europe (59, 63).
Definition and evidence for nonclassical GHI
Among the endocrine scientific community, there was initially some resistance to the concept of partial or nonclassical GHI. However, the evidence for this is now apparent (1). In the large European series of subjects with unequivocal GHI recruited for rhIGF-1 therapy, a range of phenotypes and biochemical abnormalities was evident, with some subjects not conforming to the characteristics of classical GHI described above (63). Since then, as new molecular defects in the GHR and other genes have been described, patients with certain GHI but a range of moderate or mild phenotypes have been reported (44) (Table 2). The clinical description of nonclassical facial features remains subjective, and although some groups have described a “mild clinical phenotype associated with lack of GH action” (64), there is no doubt that GHI may also exist with completely normal craniofacial development and mild short stature as described recently in patients with heterozygous dominant-negative GHR mutations (see “Heterozygous dominant negative GHR mutations” below) (52).
Table 2.
Summary of Phenotypic and Biochemical Features in the Range of Genetic Defects Causing Mild GH Insensitivity
| Phenotype |
Gene Defect
|
||||||
|---|---|---|---|---|---|---|---|
| GHR Heterozygous Dominant Negative | GHR Pseudoexon | STAT5B Heterozygous Dominant Negative | IGFI | IGF2 (Heterozygous Variants) | IGFALS | PAPPA2 | |
| Severe growth failure | +/− | +/− | + | + | + | – | – |
| Midface hypoplasia | – | * | +/− | – | – | – | – |
| Other facial dysmorphism | – | – | – | + | + | – | + |
| Deafness | – | – | – | +/− | – | – | – |
| Microcephaly | – | – | – | + | – | – | +/− |
| Intellectual delay | – | – | – | + | – | – | – |
| Pubertal delay | – | – | +/− | – | – | + | – |
| Immune deficiency | – | – | + | – | – | – | – |
| Hypoglycemia | – | + | −/+ | – | n/r | – | – |
| Hyperinsulinemia | – | – | – | +/− | n/r | + | + |
| IGF-1 | ↓ | n/↓ | ↓ | n/↓ | n/↑ | ↓ | ↑ |
| IGFBP-3 | ↓ | n/↓ | ↓ | n | n/↑ | ↓ | ↑ |
| ALS | n/↓ | n/↓ | +/− | n | n/r | ↓ | ↑ |
| GH | ↑ | n/↑ | ↑ | n/↑ | n/↑ | ↑ | ↑ |
| GHBP deficiency | +/− | – | – | – | – | – | – |
The following symbols apply: +, positive; –, negative; +/−, predominantly positive; −/+, predominantly negative; *, ∼50%; ↑, increased; ↓, decreased.
Abbreviations: n, normal; n/a, not applicable; n/r, not reported.
Molecular Defects Causing Mild GHI and the Effect of Mutations on Phenotype, Linear Growth, and Metabolism
GHR mutations
Heterozygous dominant-negative GHR mutations
Reports of patients with heterozygous GHR mutations with significant short stature phenotypes have taken second place to the more frequently documented homozygous mutations. However, the molecular characterization of heterozygous dominant negative GHR mutations has now advanced to the stage where these disorders are recognized to exist at the mild or moderate end of the phenotypic and biochemical spectrum of GHI (65). Only seven patients with proven heterozygous GHR mutations and clinically relevant phenotypes have been reported. The first report by Ayling et al. (66) in 1997 described a child with short stature inherited from the maternal side of the family with mother and daughter both having heights of −3.6 SDS and carrying the same heterozygous GHR mutation exerting a dominant-negative effect on the function of the wild-type GHR. A similar case was reported the following year from Japan with inheritance by male and female siblings of a heterozygous GHR mutation (64). The heights of affected subjects in this family ranged from −2 to −3.5 SDS. None of the patients described to date had the abnormal craniofacial disproportion and facial dysmorphism of classical homozygous GHR mutation patients.
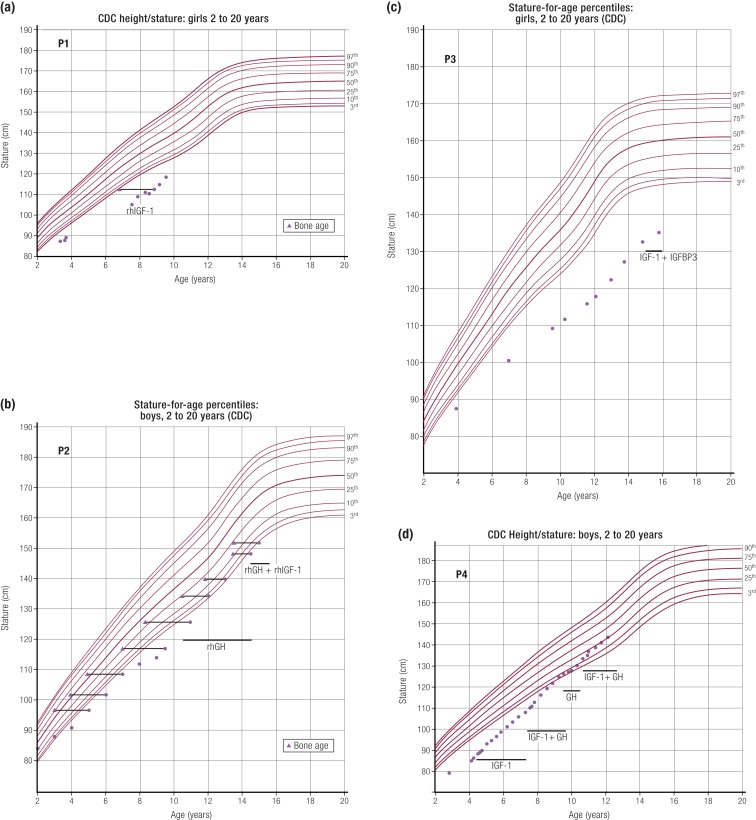
Our group recently described three novel dominant negative GHR mutations (52) with a previously reported case (67) in patients with IGF-1 deficiency and mild to moderate short stature. The range of their growth phenotypes is shown in Fig. 1. In patient 1, who has Hispanic ethnicity, height was −3.09 SDS and when evaluated at age 7.5 years, peak GH following arginine stimulation was 24.7 ng/mL and baseline IGF-1 was 24 ng/mL (normal, 39 to 198 ng/mL), increasing to 44 ng/mL after GH stimulation, indicating some GHR activity. Interestingly, IGFBP-3 was marginally subnormal at 1.2 mg/L (normal, 1.5 to 3.4 mg/L), ALS was normal, and GH-binding protein (GHBP) was slightly elevated at 1991 pmol/L (normal, 267 to 1638 pmol/L). This biochemical profile is different from that seen in a typical homozygous GHR mutation patient, where the deficiencies of IGF-1, IGFBP-3, and ALS are more severe (60, 63). The increase in IGF-1 after GH stimulation, marginally subnormal IGFBP-3, and normal ALS and GHBP are distinguishing features.
Figure 1.
Growth charts of patients carrying heterozygous GHR variants. Ranges of short stature phenotypes and responses to therapy are shown in the growth charts of four patients with dominant negative heterozygous GHR mutations (52). Patients carrying heterozygous GHR variants are (a) P1, c.964dupG; (b) P2, c.920_921ins14; (c) P3, c.945+2T>C; and (d) P4, c.899dupC (67). The time frame of rhIGF-1, rhGH, and rhIGF-1 plus IGFBP-3 treatments are indicated on the growth curves. CDC, Centers for Disease Control and Prevention. [Reproduced from Vairamani K, Merjaneh L, Casano-Sancho P, et al. Novel dominant-negative GH receptor mutations expands the spectrum of GHI and IGF-I deficiency. J Endocr Soc 2017;1:345–358.]
Patient 3 in our report shows the range of phenotypic and biochemical abnormalities in dominant-negative heterozygous GHR mutations. This Spanish girl, from a nonconsanguineous marriage, presented at age 12 years with height −4.3 SDS and normal facies. Short stature was present in three generations with the maternal grandfather’s height −4.0 SDS and mother’s height −4.3 SDS. Biochemical findings were typical of a homozygous or compound heterozygous GHR mutation, with peak GH elevated at 57.6 ng/mL, IGF-1 low at 43 ng/mL (normal, 126 to 1188 ng/mL), IGFBP-3 low at 0.87 mg/L (normal, 2.0 to 9.3 mg/L), and ALS low at 7.4 mg/L (normal, 14 to 29 mg/L). Her GHBP was significantly elevated at 35,634 pmol/L (normal, 534 to 5785 pmol/L).
The clinical profiles of these four patients are similar to the previously described cases (64, 66). All seven reported patients had progressive growth failure, normal or high GH, and low IGF-1, that is, features of GHI, suggestive of GHR dysfunction being causal of their short stature. The identified GHR variants (52) were located in sequences encoding the intracellular domain of the GHR. The serum GHBP concentrations were high normal or even markedly elevated (patient 3), supporting in vivo expression of both normal and mutant GHR.
As well documented in the literature, patients with GHI do not show a sustained growth response to GH therapy. This is well demonstrated in patient 2 (Fig. 1) and confirms the indication in such patients for therapy with rhIGF-1 to produce clinically beneficial height gain (68). When treated at a young age with rhIGF-1, patients 1 and 4 in our report had growth acceleration (52). A combination of GH and rhIGF-1 appeared to enhance the growth response, particularly in patient 4. This combination therapy is not licensed by the Food and Drug Administration or European Medicines Agency, but the encouraging growth response complements that reported in a series of idiopathic short stature (ISS) patients with mild IGF-1 deficiency (69).
GHR pseudoexon mutations
The intronic GHR pseudoexon mutation (6Ψ) was first described in 2001 in four siblings with mild GHI from a highly consanguineous Pakistani family (70). The GHR 6Ψ mutation is a point mutation (base change A−1 to G−1) in intron 6 (c.618+792A>G) that leads to aberrant splicing and activation of a 6Ψ sequence (70). This results in an aberrant splice product of the GHR gene. This splicing process is highly variable; hence, variable quantities of normal and abnormal transcripts will be generated. Intronic mutations resulting in pseudoexon activation are rare in genetic diseases (71). The GHR 6Ψ mutation causes an inclusion of an additional 108 bases between exons 6 and 7 of the GHR gene translating to the insertion of 36 new amino acids within the extracellular domain and impaired function of the mutant GHR protein (72). In 2007, a further seven 6Ψ patients were reported (73) with more severe GHI phenotypes and heights as low as −6.0 SDS. A recent study reported the wide spectrum of clinical and biochemical features in a total of 20 6Ψ subjects, which included the 11 previously described and 9 additional patients (74) (Tables 3 and 4). This revealed marked phenotypic variability, with height SDS and IGF-1 SDS ranging between −1.7 and −5.9 and between −1.0 and −6.8, respectively, in the 20 6Ψ subjects. Additionally, only 50% patients had classical facial features of GHI (74). Phenotypic variability was also noted among affected members of the same family (74).
Table 3.
Clinical and Auxological Details of the Patients With Homozygous GHR Pseudoexon (6ψ) Mutations
| Family | Patient | Age (y) | Sex | Height SDS | BMI SDS | Birth Weight SDS | Target Height SDS | Ethnicity/Consanguinity | GHI Classical Facial Features |
|---|---|---|---|---|---|---|---|---|---|
| A | 1a | 1.3 | M | −1.7 | −4.9 | −0.2 | −2.2 | Pak/+ | No |
| 2a | 3.7 | M | −5.9 | −2.0 | 0.3 | −2.2 | Pak/+ | No | |
| 3a | 8.3 | M | −3.3 | −0.4 | NK | −1.6 | Pak/+ | No | |
| 4a | 3.8 | M | −3.6 | −0.5 | −0.1 | −1.6 | Pak/+ | No | |
| 5a | 1.2 | F | −4.4 | +1.8 | 0.7 | −2.4 | Pak/+ | No | |
| 6 | 2.5 | F | −4.4 | −0.1 | −1.8 | NK | Pak/+ | Yes | |
| B | 1a | 1.6 | F | −5.6 | −2.4 | −1.4 | −1.4 | Pak/+ | Yes |
| C | 1a | NK | M | −5.0 | NK | NK | NK | Palestine-Arab/+ | Yes |
| D | 1a | 3.3 | M | −4.9 | 0.1 | NK | NK | Pak/+ | No |
| 2a | 8.1 | M | −3.3 | −2.4 | −1.5 | NK | Pak/+ | No | |
| E | 1a | 5.4 | F | −3.5 | 0.02 | NK | NK | Pak/+ | No |
| 2a | NK | F | −4.0 | NK | NK | NK | Pak/+ | No | |
| F | 1 | 7.0 | M | −4.2 | −0.5 | −0.5 | −0.9 | Pak/+ | No |
| G | 1 | 2.6 | M | −3.8 | −2.9 | −2.9 | −1.3 | Pak/+ | Yes |
| 2 | 3.7 | F | −4.2 | −0.9 | 0.1 | 0.7 | Pak/+ | Yes | |
| H | 1 | 5.7 | M | −3.0 | −0.7 | 0.7 | −0.7 | Pak/+ | Yes |
| 2 | 1.5 | F | −4.7 | −1.2 | NK | −0.7 | Pak/+ | Yes | |
| I | 1 | 2.3 | F | −4.3 | −1.7 | −1.7 | −1.6 | Ind/− | Yes |
| J | 1 | 5.3 | F | −4.0 | 0.4 | 0.1 | −1.6 | Pak/+ | Yes |
| K | 1 | 4.3 | F | −4.1 | −0.2 | −0.3 | −0.9 | Pak/+ | Yes |
Age and height SDS are at presentation. GHI facial features include frontal bossing and midfacial hypoplasia. +, parents consanguineous; −, parents not consanguineous. [Reproduced with permission from Chatterjee S, Shapiro L, Rose SJ, et al. Phenotypic spectrum and responses to recombinant human IGF1 (rhIGF1) therapy in patients with homozygous intronic pseudoexon growth hormone receptor mutation. Eur J Endocrinol 2018;178:481–489.]
Abbreviations: Ind, Indian; NK, not known; Pak, Pakistani.
Table 4.
Biochemical Details of Patients With Homozygous GHR Pseudoexon (6ψ) Mutations
| Family | Patient | Basal GH (μg/L) | Peak GH (μg/L) | IGF1 (SDS) | IGFGT Basal/Peak (ng/mL) | IGFBP3 (SDS) |
|---|---|---|---|---|---|---|
| A | 1a | 11.0 | 10.0 | −2.5 | 23.0/24.0 | −6.0 |
| 2a | 6.0 | 14.3 | −2.5 | 21.0/26.0 | −8.9 | |
| 3a | 1.8 | 53.3 | −1.7 | 29.0/36.0 | −2.9 | |
| 4a | 17.5 | 90.0 | −2.0 | 20.0/20.0 | −3.4 | |
| 5a | 0.1 | 18.8 | −2.2 | ND | −1.72 | |
| 6 | 3.4 | 26.7 | NK | ND | ND | |
| B | 1a | 13.0 | >33.3 | −2.3 | 6.9/7.6 | −2.4 |
| C | 1a | 0.6 | NK | NK | NK | NK |
| D | 1a | 10.2 | 15.4 | −2.3 | 36.0/41.0 | −2.6 |
| 2a | 0.3 | 28.4 | −0.7 | 132.0/255.0a | −1.6 | |
| E | 1a | 2.5 | 27.0 | −1.0 | ND | −2.3 |
| 2a | 8.3 | 37.7 | −1.4 | ND | −2.3 | |
| F | 1 | 2.0 | 40.0 | −2.5 | 41.2/29.7 | −2.6 |
| G | 1 | 4.0 | >33.0 | −2.3 | 63.3/16.8 | ND |
| 2 | 16.9 | 33.3 | −2.5 | ND | ND | |
| H | 1 | 17.5 | 90.0 | −2.9 | 1.5/8.4 | −2.4 |
| 2 | 0.1 | 18.8 | −3.1 | ND | ND | |
| I | 1 | 3.4 | 26.7 | −2.1 | ND | ND |
| J | 1 | 19.3 | >40.0 | −6.8 | <25.0/<25.0 | ND |
| K | 1 | 0.6 | NK | −4.0 | <22.9/<22.9 | −2.4 |
[Reproduced with permission from Chatterjee S, Shapiro L, Rose SJ, et al. Phenotypic spectrum and responses to recombinant human IGF1 (rhIGF1) therapy in patients with homozygous intronic pseudoexon growth hormone receptor mutation. Eur J Endocrinol 2018;178:481–489.]
Abbreviations: ND, not done; NK, not known.
Positive response during IGFGT.
The characteristic facial features seen in severe GHI, namely, midfacial hypoplasia and prominent forehead, reflect the underdevelopment of the facial bones secondary to IGF1 deficiency (75, 76). It has been proposed that the degree of craniofacial changes may be more prominent in patients with more severe short stature and/or a greater degree of IGF-1 deficiency (76, 77). However, in our recent study, the presence or absence of abnormal facial features did not correlate with either the degree of short stature or the biochemical abnormalities (74).
In contrast to previous studies in patients with GHR mutations causing severe GHI deficiency (63), there was no positive correlation between height SDS and IGF-1 SDS in the 6Ψ cohort (5). The mismatch between clinical phenotype, that is, the degree of short stature and the biochemical deficiency (IGF-1 SDS) in this mutation, was striking. Many of the most severely affected patients with height SDS −5.9 to −4.0 had IGF-1 SDS values, which were in the normal range or only mildly reduced (−2.9 to −1.4) (5). The reason for this discrepancy is unclear but might be the result of additive molecular defects in other proteins downstream from the GHR, resulting in a greater degree of short stature, for example, the IGF-1 receptor or signaling molecules of the rat sarcoma viral oncogene homolog (RAS)/MAPK pathway and/or the PI3K/Akt pathway. Other genetic and/or environmental factors involved in the GHR processing, trafficking, and receptor degradation pathways may also be implicated (73). The use of different, rather than standardized/centralized IGF-1 assays, may also contribute to the observed discrepancy.
The milder phenotype could be explained by the three possible combinations of GHR dimerization, which could potentially occur in these patients. Additionally, splice mutations may not always be 100% efficient in causing aberrant splicing, and the coexistence of normal and mutant transcripts may occur. Consistent with this, the wild-type GHR transcript has been isolated in subjects with GHI caused by homozygous pseudoexon 6Ψ mutations (70). The presence of different ratios of mutant/wild-type receptor may also explain the occurrence of different phenotypes within patients with the same splice mutation (78, 79).
Other heterozygous GHR mutations
Characteristics of heterozygous family members of subjects with GHR mutations.
Heterozygous GHR mutations may have a variable effect on carriers. In extreme cases, a mutation in one allele acts in a dominant-negative fashion, leading to severe growth failure (see “Heterozygous dominant negative GHR mutations” above). Other heterozygous GHR mutation carriers, for example, relatives of severe GHI patients, exhibit a range of heights, from reduced height SDS (63, 80) to normal stature (81). A large Ecuadorian GHR deficiency cohort carrying a single GHR defect, the E180 splice mutation (c.594A>G), has allowed the extensive study of both carrier (heterozygous) and noncarrier first-degree relatives (81–84). These studies conclude that heterozygous carriers of this functionally null mutation have no reduction in circulating GHBP, IGF-1, IGF-2, IGFBP-2, or IGFBP-3 levels (83, 84). Initial studies reported no difference in height SDS between heterozygous E180 carriers and normal relatives (81, 83). However, more recently an analysis of a larger cohort of individuals suggests a modest reduction in mean height of ∼0.55 SDS in heterozygous individuals (84).
The site of the mutation within the gene and the corresponding modification at the protein level may influence the observed phenotype of heterozygous individuals (85). The genetic background of the individual may also contribute to the phenotypic diversity (85, 86).
Heterozygous GHR mutations in subjects with short stature.
The term ISS refers to short children (height >2 SDS below the corresponding mean height for age, sex, and population) with no underlying identifiable disorder or GH deficiency (87, 88). This encompasses a heterogeneous group of short children with variable GH sensitivity and IGF-1 levels (88). Hence, a subset of ISS patients has evidence of GH resistance that may play a role in their growth failure (85, 89). Consistent with this, a proportion of ISS children has a diminished response to hGH therapy (87, 90). GHBP arises from the proteolytic cleavage of the extracellular domain of the GHR. Low serum levels of GHBP characterize classical GHI (Laron syndrome), secondary to mutations located in the extracellular domain of the GHR gene, which encodes GHBP (3, 91). A proportion of children with ISS also have reduced serum GHBP concentrations. In one study, 90% of a cohort of >1500 ISS patients had GHBP concentrations below the age- and sex-adjusted mean for controls and 20% GHBP concentrations below the normal range (92). Therefore, it has been hypothesized that less deleterious GHR gene defects may cause ISS associated with features of GHI. Heterozygous GHR mutations may cause impaired GHR function, partial GHI, and a phenotype consistent with a subset of ISS patients. Consistent with this, a subset of ISS patients has higher spontaneous GH peaks than does a reference population, suggesting a diminished inhibition of GHRH (85).
Numerous studies have reported heterozygous GHR variants in ISS patient cohorts. The frequency of such variants is reported as between 5% (86, 93, 94) and 15.5% (95) of ISS patients. However, it has also been noted that GHR sequence changes are common in children with ISS and many are also identified in control subjects and normal stature family members (94, 96). These include the frequently encountered synonymous homozygous and heterozygous GHR variants: G168G and I526L (85, 93, 94, 96) and other predicted benign variants c.10T>C, c.482+9C>T, c.662−24delG, c.662−11delT, c.662−31C>T, c.662−30A>G and c.828−4delG, c.919−14delT, c.988+23delG (96) whose relative allele frequency was similar in patients and controls. Hence, many heterozygous GHR variants are not thought to play a contributory role in the etiology of short stature.
Low levels of GHBP may indicate mutations within the extracellular domain (exons 3 to 7) of the GHR. Goddard et al. (86, 97) assessed 100 ISS patients with low or undetectable GHBP levels and identified seven novel heterozygous variants and one compound heterozygous GHR variant that were not present in 100 controls. These included extracellular domain variants (mature peptide nomenclature: E44K, C122X, R161C, R211H, E224D) and an intracellular alteration (A478T). A combination of protein prediction and GH-binding assays predicted that these variants would encode a GHR with reduced function. The heterozygous R161C variant was also identified in one ISS subject in a Chilean cohort of 26 patients and one subject in another cohort of 45 patients (85, 95). In the latter cohort, the heterozygous R211H and the novel G62V variants were also identified in a further five and one ISS patients, respectively (95). Sanchez et al. (93) reported a novel nonsynonymous change (V144I) in 1 of 17 ISS subjects. Although this variant is in the extracellular domain, the GHBP level was normal. Bonioli et al. (94) reported a novel nonsynonymous heterozygous GHR variant at the same amino acid position (V144A) in 1 of 37 ISS individuals. No functional studies were performed in either study; however, it was hypothesized that this variant, close to the dimerization domain of the GHR, would result in aberrant receptor function (94). One heterozygous GHR variant (S219L) was isolated in a Japanese cohort of 86 ISS patients (33). The phenotype of these reported heterozygous GHR variant carriers was very diverse with height SDS range −5.1 to −0.2 and IGF-1 SDS −4.2 to 0.3 (85, 86, 93–95). Therefore, the spectrum of GH resistance is wider than homozygous single-gene defects. The expression of both the complete GHI phenotype in homozygous GHR patients and partial GHIS phenotype in heterozygous carriers is also highly variable.
STAT5B mutations
Homozygous STAT5B mutations
The GHR signaling pathways that are activated upon GH engagement include four of the seven STAT pathways (STAT1, 3, 5A, and 5B), all of which are expressed in multiple cell types and activated by other growth factors and cytokines (98). The identification of STAT5B mutations associated with severe growth failure, marked IGF-1 deficiency, and insensitivity to GH provided the first definitive demonstrations that the STAT5B signaling pathway is critical for GH-induced IGF-1 production and normal growth in humans (7, 99) [Table 5 (6, 100–107)]. Pathophysiologically significant mutations, both germline and somatic, have now been reported in all STAT genes (108–111) except in the STAT5A gene. However, only mutations in STAT5B are clearly linked to GHI, IGFD, and severe postnatal growth retardation. The first autosomal homozygous STAT5B mutation was reported in a 16-year-old female who not only had the growth clinical phenotype of GHR-deficient patients but also symptoms of a progressive immune deficiency that is not found in GHR-deficient patients (6, 112).
Table 5.
Phenotype of Patients Carrying Homozygous STAT5B Mutations
| STAT5B Mutation Homozygous | Sex | Age (y) | Height (SDS) | Birth | GHI | IGFD | Prolactin Elevated | Hypergamma-globulinemia | T-Cell Lymphopenia | Pulmonary Disease | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| p.Ala630Proa | F | 16.5 | −7.5 | AGA | +++ | +++ | +++ | +++ | +++ | +++ | (6) |
| c.1191insG a | F | 16.4 | −7.8 | AGA | +++ | +++ | ND | ND | ND | +++ | (100) |
| p.Arg152* | F | 15.3 | −9.9 | Unknown | ND | ND | +++ | +++ | +++ | +++ | (101) |
| p.Arg152*1 | F | 12 | −5.3 | SGA | +++ | +++ | Normal | No | No | +++ | (102) |
| c.1102insC | M | 31 | −5.9 | AGA | +++ | +++ | +++ | No | No | No | (103) |
| c.1680delG b | F | 2 | −5.8 | AGA | +++ | +++ | ND | ND | ND | + | (104) |
| c.1680delG b | F | 4 | −5.6 | AGA | +++ | +++ | ND | ND | ND | + | (104) |
| c.424_427del c | M | 6 | −5.6 | AGA | +++ | +++ | +++ | + | +++ | +++ | (105) |
| c.424_427del c | M | 2 | −3.0 | AGA | +++ | +++ | +++ | No | +++ | +++ | (105) |
| p.Phe646Ser | F | 14.8 | −5.95 | Unknown | +++ | +++ | +++ | +++ | +++ | No | (106) |
Phenotype was as described in reports. The nonsense mutation, p.Arg152*, was identified in two unrelated subjects. + to +++ indicate increasing severity of indications. [Reproduced with permission from Hwa V. STAT5B deficiency: impacts on human growth and immunity. Growth Horm IGF Res 2016;28:16–21.]
Abbreviations: AGA, appropriate for gestational age; F, female; M, male; ND, not determined; SGA, small for gestational age.
Deceased as of this review.
Siblings.
Siblings.
To date, STAT5B deficiency (MIM245590) is recognized as an autosomal recessive disorder of GHI with immunodeficiency, whereas each of the other described STAT deficiencies is associated only with distinct immunodeficiencies (108–111); growth impairments were not reported. It is noteworthy, however, that growth impairment (reported as growth less than the 5th percentile of the normal population) have been described in ∼50% of patients carrying germline gain-of-function, heterozygous STAT3 mutations (113–117), and, in the few evaluated cases, IGFD was reported (five of six patients) (114, 116, 117). Because three of the IGFD patients who were treated with GH therapy responded well to recombinant GH, growth failure has been attributed to effects secondary to the primary lymphoproliferation and autoimmunity phenotype (114, 116), which would be consistent with acquired GHI due to chronic inflammatory disorders (Table 1). Interestingly, a recent report suggests that activating STAT3 mutations can diminish GH-induced STAT5B activities and cause partial GHI (116), although the mechanism remains to be fully elucidated.
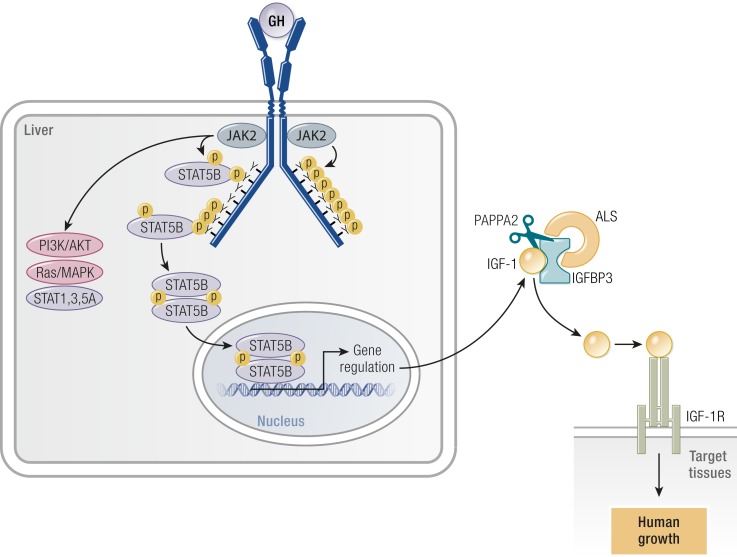
STAT5B, similar to other members of the STAT family, contain discrete functional domains, including a DNA-binding domain (DBD), an Src homology 2 (SH2) domain, which permits docking to phosphorylated tyrosine residues, and the C-terminal–transactivating domain (TAD) (Fig. 2). Following recruitment to activated receptors, the latent cytosolic STAT5B protein is phosphorylated on a single tyrosine at position 699 by kinases, including JAK2 (98), which serves to facilitate subsequent homodimerization. The activated dimeric STAT5B then mobilized into the nucleus where binding to DNA response elements regulates transcriptional activities.
Figure 2.
The role of STAT5B in the GHR intracellular signaling pathways. Following recruitment to activated receptors, the latent cytosolic STAT5B protein is phosphorylated on a single tyrosine at position 699 by kinases, including JAK2, which serves to facilitate subsequent homodimerization. The dimeric STAT5B is then mobilized into the nucleus where binding to DNA response elements regulate transcriptional activities. AKT, v-akt murine thymoma viral oncogene homolog, also known as protein kinase B; PI3K, phosphatidylinositol 3-kinase; P, phosphorylated residue; Y, tyrosine.
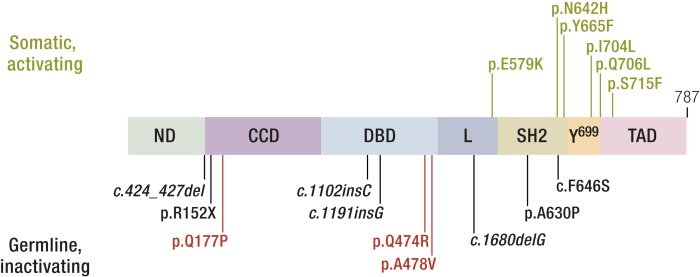
The seven homozygous-inactivating STAT5B mutations described to date (6, 100–105) are located in different protein domains (Fig. 3), with none reported in the TAD. Mutations include one nonsense mutation and four frameshifts (deletions or insertions), all of which are predicted to result in early protein termination. Only two missense mutations, p.Ala630Pro (6) and p.Phe646Ser (106), have been identified, both located within the SH2 domain, but although p.Ala630Pro was unstably expressed, loss of function for p.Phe646Ser was due, in part, to an inability to drive transcription (106, 118).
Figure 3.
Pathophysiological STAT5B mutations. Schematic of the STAT5B gene showing the discrete functional protein domains. L, linker; ND, N-terminal domain.
A summary of the growth and immune clinical phenotype of patients carrying autosomal-inactivating STAT5B mutations are shown in Table 5 and have been comprehensively reviewed (1, 107). Birth size, where documented for the STAT5B-deficient subjects, was appropriate for gestation. Postnatal growth failure was significant, consistent with the degree of IGF deficiency, and indistinguishable from those with GHI syndrome (56). Height SDS ranged from −3.0 SDS to −9.9. Bone age, when measured, was considerably delayed (101, 105). Puberty was also consistently delayed, reflecting the low levels of circulating IGF-1 and a state of chronic illness (see below). Mild facial dysmorphic features, such as a prominent forehead, depressed nasal bridge, and high-pitched voice, were noted for some of the STAT5B-deficient subjects (6, 101, 105).
The severe postnatal growth failure of STAT5B-deficient patients correlated with the clinical endocrine profiles (Table 5). Basal GH levels were normal, and when stimulated, GH concentrations were frequently elevated. Serum IGF-1, IGFBP-3, and ALS concentrations in all cases were abnormally low and remained low after GH treatment during an IGF-1 generation test (6, 100, 119) or GH therapy (103, 105). Some of the subjects underwent GH therapy (1 to 4 years), but the growth response was uniformly poor (6, 105). Interestingly, serum prolactin levels, when recorded, were abnormally high. Pugliese-Pires et al. (105) determined that the hyperprolactinemia state for cases 9 and 10 was not a result of macroprolactin or a pituitary tumor. It is likely that the STAT5B mutations disrupted the negative feedback loop for prolactin production, although the mechanisms involved remain to be clarified.
A distinguishing feature of STAT5B mutation patients from those carrying GHR, IGF1, IGF1R, or IGFALS mutations was symptoms of immune dysfunction. Shared symptoms in 8 of the 10 patients include severe eczema, chronic pulmonary disease manifesting as early as the first year of life (101, 102, 105), and confirmed lung fibrosis and/or lymphoid interstitial pneumonia, a condition of unknown etiology that is rare in children and often associated with autoimmune disease (120). Corticosteroid and oxygen treatments temporarily stabilize worsening pulmonary functions, but two of the patients, including the first described case of STAT5B deficiency (carrying p.Ala630Pro) (121), succumbed and died as consequences of progressive pulmonary fibrosis and respiratory failure (102). One of the patients underwent lung transplantation at age 17.5 years (105). Although this temporarily alleviated the impaired pulmonary function and oxygen requirement, the patient died aged 22 years, as did is his affected brother. Intriguingly, two of the patients lacked severe pulmonary problems, although both had symptoms of mild immune dysfunction: the patient carrying STAT5B c.1102insC was reported to have contracted hemorrhagic varicella at 16 years of age and had congenital ichthyosis and erythema, but otherwise appeared relatively healthy (103). The patient carrying STAT5B p.Phe646Ser (106) had autoimmune thyroiditis, psoriasis, and alopecia and was diagnosed with Celiac disease at age 20 years (121). The explanation for the lack of chronic pulmonary disease in these two patients remains to be elucidated.
Heterozygous dominant negative STAT5B mutations
STAT5B deficiency is established as an autosomal recessive disorder, with the STAT5B mutations causing early protein termination or affecting protein instability and/or function. Recently, the first germline dominant negative STAT5B mutations associated with short stature and mild GHIS in three unrelated families was reported (122). Whole-exome sequencing (WES) analysis confirmed that a heterozygous STAT5B variant was the top candidate in each family. The three private loss-of-function mutations were missense variants, with the two familial mutations (p.Gln474Arg and p.Ala478Val) located 4 amino acids apart within the DBD module, and the one de novo mutation located in the coiled-coiled domain (CCD; p.Gln177Pro) (Fig. 3). The two DBD mutations were unable to bind DNA, whereas p.Gln177Pro, unexpectedly, was unable to translocate into the nucleus, despite demonstrations that all three mutants were robustly tyrosine phosphorylated upon GH stimulation (122). Significantly, each mutant was capable of interacting with wild-type STAT5B, inhibiting its normal functions (122). Of note, the STAT5B p.Gln177Pro is the first reported STAT variant with a nuclear localization defect and distinct dominant negative actions. The Gln177Pro substitution in the first α-helix of the 4-α-helix bundle that comprises the CCD presumably disrupted the functional integrity of the helix bundle, which has been shown to act in concert as an unconventional nuclear localization signal (123). The affected STAT5B DBD residues, Gln474 and Ala478, are conserved among all members of the STAT family, and p.Gln474Arg and p.Ala478Val mutations are remarkably similar in functional properties to analogous, described, dominant negative STAT3 DBD mutations (124–126) associated with hyper-IgE syndrome (OMIM no. 147060) (126, 127).
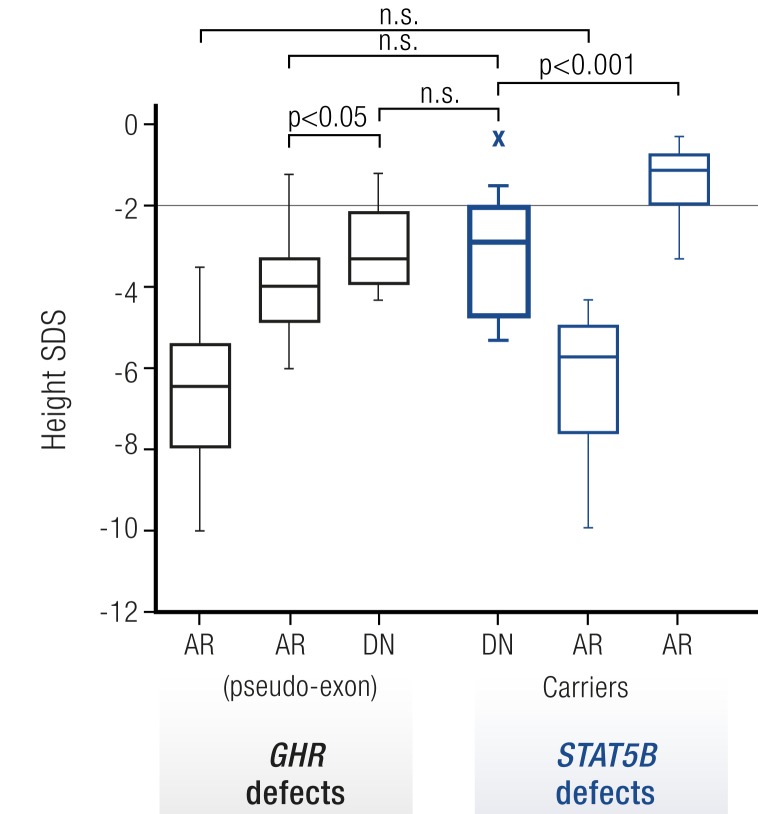
The index patients carrying the heterozygous loss-of-function STAT5B missense mutations were born appropriate for gestational age but had postnatal heights ranging from −2.9 to −5.3 SDS (Table 6). Growth impairment was associated with diminished serum IGF-1 concentrations. Intrafamilial and interfamilial variability of stature in the three families was comparable to that normally seen in autosomal recessive GHIS (1). Strikingly, the growth deficit was in the range of patients carrying dominant negative GHR mutations (52, 64, 66, 67, 128, 129) and clearly distinct from those of heterozygous relatives of autosomal recessive STAT5B-deficient patients who typically have heights within the low normal range (130) (Fig. 4).
Table 6.
Clinical Characteristics of Autosomal Dominant STAT5B-Deficient Index Patients and Comparison With Previously Reported Autosomal Recessive STAT5B Cases
|
|
Proband 1 [p.Gln177Pro] |
Proband 2 [p.Ala478Val] | Proband 3 [p.Gln474Arg] | Published AR Cases (n) |
|---|---|---|---|---|
| Sex | Male | Male | Female | Female/Male = 7/3 (10) |
| Age, y | 14.5 | 1.8 | 12.8a | 1.9–18.0 (10) |
| Birth data | ||||
| Gestational age, wk | 36 | 39 | 39 | Preterm: 6/8 (8) |
| Birth weight, g [SDS] | 2500 [−0.9] | 3460 [0.1] | 3317 [0.2] | [−2.4 to 3.0]b (6) |
| Birth length, cm [SDS] | 45 [−1.7] | nd | 48 [−0.2] | [−2.4 to 2.3]b (5) |
| Auxological features | ||||
| Weight, kg [SDS] | 28.0 [−4.5] | 9.5 [−2.3] | 22.8 [−4.7] | [−6.7 to −3.6] (4) |
| Height, cm [SDS] | 131.5 [−5.3] | 76.8 [−2.9] | 123.8 [−4.5] | [−9.9 to −4.3] (10) |
| Target height [SDS] | −0.74 | −0.83 | −1.01 | −1.98 to −0.11 (8) |
| Bone age, y | 9.6 | nd | 8.8 | Delayed: 7/7 (7) |
| Puberty | Delayed | na | Delayed | Delayed: 6/7 (7) |
| Endocrine features | ||||
| GH, basal, ng/mL | 0.4 | 3.2 | 2.0 | 0.1–17.6 (10) |
| GH, stimulated, ng/mL | 16.2 | 17.3 | 4.0 | 6.6–53.8 (7) |
| IGF1, ng/mL [SDS/reference range] | 56 [76–499] | <25 [51–303] | 208 [−1.5] | Below normal: 10/10 (10) |
| IGFBP3, mg/L [SDS/reference range] | 2.33c [−1.7] | 1.29 [0.8–3.9] | 3.80 [3.9–9.4] | Below normal: 10/10 (10) |
| IGFALS [reference range] | 418 pmol/Lc [986–1678] | nd | 13 mg/l [5.6–16.0] | Above normal: 6/6 (6) |
| Prolactin, mU/L [reference range] | 291c [86–324] | 553 [163–1039] | 621d [<383] | Above normal: 6/7 (7) |
| Immunological and pulmonary phenotype | ||||
| IgE, kU/L [reference range] | 156/340e [<200/<114] | 118 [<52] | 127 [<629] | Above normal: 4/7 (5) |
| Hemorrhagic varicella | No | No | No | 5/5 (5) |
| Chronic pulmonary disease | Recurrent infections | No | No | 8/10 (10) |
| Lung fibrosis | nd | nd | No | 6/7 (7) |
| Lymphocytic interstitial pneumonia | nd | nd | No | 7/8 (8)f |
| Eczema/skin pathology | Yes | Yes | No | 8/8 (8) |
| Otherwise disturbed immunological profiles | No | No | No | 5/7 (7) |
| Autoimmune disease | No | No | Thyroiditis; celiac disease | 6/10 (10)f |
[Reproduced with permission from Klammt J, Neumann D, Gevers EF, et al. Dominant-negative STAT5B mutations cause growth hormone insensitivity with short stature and mild immune dysregulation. Nat Commun 2018;9:2105.]
Abbreviations: na, not applicable; nd, not determined.
Patient on GH treatment of 3 mo.
Six of eight patients born appropriate for gestational age.
Determined at age 16.5 y during rhIGF treatment.
Measured at 15.3 y while on rhIGF1 for 4 mo.
Measured at two occasions.
Diagnosis confirmed or suspected.
Figure 4.
Heights of dominant negative STAT5B patients are comparable to dominant negative GHR patients. Height SDS values of GHIS patients with GHR mutations (black) were compared with height SDS values of STAT5B mutational carriers (blue). GHR defects: AR, autosomal recessive GHR mutations (n = 100); AR pseudoexon, GHR pseudoexon 6Ψ mutations (n = 21); DN, dominant negative GHR mutations (n = 16). STAT5B defects: DN, dominant negative STAT5B mutations (n = 11); AR, autosomal recessive STAT5B mutations (n = 10); AR carriers, STAT5B mutation carriers (n = 14). Box (median, 25th and 75th percentiles) and whiskers (minimum and maximum values) plots. Statistical analysis was by the Student t test. *P < 0.05, ***P < 0.001. n.s., not significant (122). [Reproduced with permission from Klammt J, Neumann D, Gevers EF, et al. Dominant-negative STAT5B mutations cause growth hormone insensitivity with short stature and mild immune dysregulation. Nat Commun 2018;9:2105.]
Additional features noted in index patients included lack of the severe immune deficiency and pathological autoimmunity associated with total STAT5B deficiency (131, 132), with immunological profiles (particularly T-lymphocytes and subsets) that were unremarkable (Table 6). Elevated IgE was detected in eight of nine subjects carrying dominant negative STAT5B mutations, and mild eczema was noted for most subjects (122). Hyperprolactinaemia usually observed with total STAT5B deficiency (107), however, was noted only in a few of the dominant negative STAT5B subjects (122).
The clinical presentation of patients carrying dominant negative STAT5B mutations and functional studies of the mutations together support residual normal STAT5B activities that manifested the milder clinical GHI syndrome with general sparing of the immune system. The broadening of the clinical spectrum of STAT5B deficiency and GHI is thus consistent with the continuum of classical to nonclassical GHI.
Note that a heterozygous loss-of-function STAT5B p.Gln206Arg (α-helix 2, CCD) was recently reported in a 33-year-old male patient with autoimmune lymphoproliferative syndrome-like features from childhood (133) who, interestingly, was of normal stature. The p.Gln206Arg mobilized into the nucleus upon IL-2 stimulation but exhibited dominant negative effects on IL-2–stimulated T-cell functions.
Other heterozygous STAT5B mutations
The frequency of dominant negative STAT5B mutations in ISS remains to be fully evaluated. Germline heterozygous STAT5B variants previously evaluated in ISS children did not prove to be functionally deficient (134, 135). Heterozygous carriers who are relatives of autosomal recessive STAT5B-deficient patients have heights within the low normal range (Fig. 4) and did not present with immunological or pulmonary complications (130). Overall, evidence supports that one copy of the wild-type STAT5B allele is likely to be sufficient for normal growth and immunity, although recurrent somatic activating heterozygous missense STAT5B mutations in the SH2 or TAD domains (Fig. 4) have been reported to be causal of lymphomas (136–138).
IGF1 mutations
Homozygous IGF1 mutations
IGF-1 is a key growth factor in intrauterine development and postnatal growth. IGF-1 circulates in a ternary complex with liver-derived IGFBP-3 and ALS. The mitogenic and metabolic effects of IGF-1 are mediated though the type 1 IGF1R, a cell surface tyrosine kinase receptor (1). Human prenatal growth is primarily regulated by nutritional supplies that influence fetal IGF-1 and IGF-2 levels (1). Targeted disruption of Igf1 or Igf2 in mice resulted in a 40% reduction in fetal growth (139). The critical importance of normal IGF-1 production has been confirmed by the severe prenatal and postnatal growth failure seen in individuals with IGF1 gene mutations.
The first human IGF1 gene defect was described by Woods et al. (8) in 1996 (1) (OMIM no. 608747). IGF1 mutations are inherited as autosomal recessive defects and since 1996, a number of other cases of homozygous IGF1 gene mutations have been described (34, 35, 140). The consistent clinical features of all of these patients are IUGR, microcephaly, retarded intellectual development, and severe postnatal growth failure. Deafness was present in all but one case (8, 34, 140). Interestingly, although there are few reported cases, there is some evidence of clinical, genetic, and biochemical heterogeneity. The patient without deafness had a relatively mild clinical phenotype (35) with cranial circumference and height SDS of −2.5 and −4.5, respectively. This compares to cranial circumference and height SDS in the more severe cases of −8.0 to −4.9 and −9.0 to −6.2, respectively (8, 34, 140).
Genetic sequencing of the mild case revealed a homozygous missense IGF1 mutation, p.R36Q, which resulted in a twofold to threefold reduction in IGF1R affinity (35). This resulted in decreased IGF1R autophosphorylation and partially, rather than severely, diminished IGF-1 activity. This case also had variable but not severely decreased IGF-1 levels (35). This is in contrast to the two most severe cases, where IGF-1 was very low or undetectable (8, 140).
Serum IGFBP-3 levels in all cases are reported as normal, consistent with the notion that its levels are controlled independently of IGF-1. In the IGF gene mutation case associated with severe IGF-1 deficiency, ALS levels (which are strongly GH-dependent) were elevated (8). In the milder case, ALS levels were normal (35). Taken together, these results support not only the independent regulation of IGF-1, IGFBP-3, and ALS by GH, but are consistent with evidence that serum ALS levels may be more reflective of hepatic responsiveness to GH than either IGFBP-3 or IGF-1 (141).
Heterozygous IGF1 mutations
Heterozygous IGF-1 mice are smaller than their wild-type littermates (142). In humans, there is also evidence to suggest that heterozygous carriers of IGF1 gene mutations exhibit a degree of prenatal and postnatal growth failure, suggesting a “dose effect.” The first patient described in 1996 had heterozygous parents with low serum IGF-1 levels and below average heights of −1.8 and −1.4 SDS, respectively (8). Walenkamp et al. (34) studied 24 family members of the homozygous inactivating IGF1 mutation index case who exhibited a severe phenotype. Nine heterozygous relatives were found to have a significantly reduced head circumference (−1.0 vs 0.5 SDS), lower mean birth weight (3048 vs 3358 g), and lower mean height (−1.0 vs −0.4 SDS) when compared with noncarrier family members (34). Consistent with these findings, serum IGF-1 levels were significantly higher and levels of bioactive IGF-1 were significantly lower in the mutation carriers compared with the noncarrier family members (34).
In 2010, van Duyvenvoorde et al. (143) reported two children and their mother and maternal grandfather with a novel heterozygous IGF1 gene mutation (143). This genetic duplication of four nucleotides of the IGF1 gene, c.243–246dupCAGC, resulted in a frameshift and premature termination codon. The two index children had severe short stature (height SDS −4.1 and −4.6) and low serum IGF-1 (−2.3 and −2.6 SDS). Interestingly, despite inheriting the same mutation, the male child had a relatively milder clinical phenotype compared with his female sibling (head circumference SDS −1.6 vs −2.4, birth weight SDS −2.9 vs −1.2, and birth length SDS −3.8 vs −1.0) (143). Neither children had deafness but the more severely affected female child also had evidence of neurodevelopmental delay. Adult carriers were significantly shorter (height SDS −3.4) compared with noncarrier family members (−1.6 SDS) and had reduced head circumferences (−1.9 vs 0.3 SDS) (143). It was hypothesized that placental dysfunction, owing to maternal IGF1 haploinsufficiency, resulted in more marked short stature in the children (143). This led to the suggestion that maternally and paternally inherited IGF1 haploinsufficiency leads to a height loss of 2 and 1 SDS, respectively (143).
Fuqua et al. (144) also reported a large kindred with short stature. The index case had severe postnatal growth failure (height SDS −4.0), low IGF-1 (−2.2 SDS), and a heterozygous IGF1 splice mutation, c.402+1G>A. This mutation is predicted to produce a frameshift resulting in IGF-1 protein truncation and segregated with the short stature in the kindred. Although the heterozygous IGF1 variant was associated with the short stature phenotype, the authors concluded that causes for the short stature were unclear (144).
In 2014, the first complete heterozygous IGF1 gene deletion was identified in a patient initially diagnosed with ISS (145). IGF-1 haploinsufficiency resulted in mild IUGR (birth weight and length −1.5 and −1.2, respectively), postnatal growth failure (height SDS −3.1 at aged 2.3 years), and low-normal serum IGF-1 (145). IGFBP-3 and ALS were normal. In contrast, next-generation sequencing of 86 unrelated Japanese ISS cases did not reveal any IGF1 mutations, suggesting that IGF1 gene defects play a limited role in the etiology of ISS (33).
The patient with a homozygous partial IGF1 gene deletion responded poorly to hGH treatment, with no change in growth rate (2.5 and 2.2 cm/y before and after treatment, respectively) (8). One heterozygous IGF1 mutation individual also failed to respond to hGH therapy with height velocity 5.0 and 4.1 cm/y before and after hGH treatment, respectively (144). Serum IGF-1 was modestly low (−2.2 SDS) and increased minimally with hGH treatment to −1.8 SDS, suggesting partial resistance to GH (144). Another two heterozygous IGF1 cases showed slightly better responses to hGH with height increases of +1.0 and +1.5 associated with an increase in serum IGF-1 levels of +3.2 and 1.3 SDS after 2 years of therapy, respectively (143).
IGF2 mutations
The roles of IGF-1 and IGF-2 in prenatal and postnatal growth in mice were extensively analyzed in a series of gene disruption studies (146–148) and both were found to contribute to intrauterine growth. Igf1−/− mice exhibited a 40% reduction in birth weight, compared with wild-type mice, and poor postnatal growth, as well as decreased viability, probably related to muscular and pulmonary hypoplasia. Homozygous Igf2−/− mice exhibited profound intrauterine growth retardation, but postnatal growth appeared relatively unimpaired and fertility and viability were preserved. As Igf2 is maternally imprinted, mice with a mutation of paternal Igf2 showed the same IUGR as mice with homozygous mutations of this gene.
A role for IGF-2 in human growth has been supported by observations in patients with SRS with molecular defects resulting in hypomethylation of the paternally derived H19 differentially methylated region (149). Similar growth restriction has been observed in patients with mutations of CDKN1C on chromosome 11p15.5 (150).
The failure for many years to identify patients with IGF2 mutations appeared to be consistent with the idea that IGF-2 was primarily a major regulator of intrauterine growth and that survival postnatally was unlikely. The first documented human IGF2 mutations were reported by Begemann et al. (151) in 2015. They reported an IGF2 variant (c.191C→A, p.Ser64Ter) in a multigenerational family in whom four members had evidence of growth restriction. Consistent with the maternal imprinting of IGF2, all inheritance was through paternal transmission. Noteworthy was that the mutation impacted both intrauterine and postnatal growth. Birth weight SDS ranged from −2.7 to −5.3, birth length SDS from −4.2 to −4.9, and latest height SDS from −1.6 to −4.0. In addition to relative microcephaly, affected individuals were noted to have triangular facies, frontal bossing, micrognathia or retrognathia, low-set ears, and clinodactyly. The protein resulting from this nonsense mutation is predicted to be ∼30% of the size of the wild-type protein (54 vs 236 amino acids) and would lack the binding sites for the IGF-1, IGF-2, and insulin receptors.
In 2017, Yamoto et al. (152) reported a de novoIGF2 mutation on the paternal allele of a patient with a Silver-Russell phenotype and ectrodactyly. Birth length SDS was −3.7 and birth weight SDS was −4.1. At 18 months of age, length SDS was −4.2. An indel IGF2 mutation was found on the paternal allele (c.110_117delinsAGGTAA, p.Leu37Glnfs*31). The most recent report, by Liu et al. (153), describes a de novo mutation of the paternal IGF2 gene in a 13-year-old Chinese boy with a birth weight SDS of −3.6, birth length SDS of −4.1, and a height SDS at age 4.5 years of −3.3. A heterozygous IGF2 variant was identified (c.101G>A, p.Gly34Asp) and located to the paternal allele.
Serum concentrations of IGF-1 were found to be normal in the cases reported by Begemann et al. (151), and IGFBP-3 concentrations were normal or slightly elevated. In the case reported by Yamoto et al. (152), serum levels of both IGF-1 and IGFBP-3 were reported as markedly elevated. These observations suggest that normal-elevated IGF-1 concentrations cannot fully compensate for the abnormal production of IGF-2. Experience with either GH or IGF-1 treatment of these cases is not available. It remains unclear whether the postnatal growth retardation reflects the persistent absence of IGF-2 action or is the consequence of abnormal programming of IGF-mediated growth in utero.
IGFALS mutations
Homozygous and compound heterozygous IGFALS mutations
Homozygous and compound heterozygous IGFALS mutations (OMIM no. 601489) are associated with GHI and severe deficiencies of circulating ALS, IGF-1, and notably IGFBP-3 (9, 154, 155). ALS is encoded by the IGFALS gene, located on chromosome 16p13.3 and spans 3.3 kb. It is a soluble glycoprotein and member of the leucine-rich repeat family, and it is expressed by hepatocytes and secreted into the bloodstream (156). GH is the main inducer of ALS synthesis and, in the circulation, ALS can be found free or bound to IGF-1 or IGF-2 and IGFBP-3 or IGFBP-5 to form a ternary complex. This prevents IGFs from leaving the circulation, thus prolonging their half-lives and decreasing their availability at a tissue level (157).
IGFALS mutations are inherited as autosomal recessive defects and have included missense and nonsense mutations, deletions, duplications, and insertions resulting in frameshift and premature stop codons and in-frame duplication mutations, leading to insertion of extra amino acid residues (1). In all homozygous cases, there was extreme deficiency of circulating ALS, with the inability to form the ternary complex (158). This results in the rapid clearance of IGF-1 and IGFBP-3, whereas local production of free IGF-1 appears to be preserved or even increased due to upregulation of GH secretion (9). Inactivation of the Igfals gene in mice results in absence of circulating ALS associated with only modest growth failure despite marked reduction of serum IGF-1 and IGFBP-3 levels (159).
The clinical presentation is characteristically of mild or moderate short stature associated with delayed puberty (154, 155). A normal pubertal growth spurt and normal adult height have been reported (154). There are no craniofacial features of classical GHI. Mutations of the IGFALS gene present a fascinating combination of genetic, biochemical, and phenotypic findings. A striking feature, seen principally with homozygous and compound heterozygous defects, is the mismatch between extreme deficiencies of circulating IGF-1, IGFBP-3, and ALS and relatively mild growth failure (1, 154, 155, 158). Insulin resistance, with hyperinsulinemia and low IGFBP-1, has also been described in these patients (158). A modest response to hGH therapy has been reported in this condition (160).
Heterozygous IGFALS mutations
Attention has recently focused on the possible effects of heterozygous IGFALS mutations on the biochemical features and clinical phenotype. In an analysis of 21 patients with homozygous or compound heterozygous IGFALS mutations and their family members who were either heterozygous carriers or homozygous wild-type normal, mean height SDS was −2.31 ± 0.87 in the homozygous patients and within individual families, and heterozygosity for IGFALS mutations resulted in ∼1.0 SDS height loss in comparison with the wild-type, whereas homozygosity or compound heterozygosity gave a further loss of 1.0 to 1.5 SD (161). A height deficit of ∼1 SDS has also been reported in heterozygous mutation carriers compared with nonmutation carriers in a Kurdish family living in the Netherlands (162) and in other reports (160, 163). The association of a short stature phenotype with heterozygous IGFALS mutations has led to the proposed terminology of partial ALS deficiency (155).
The suggestion that IGFALS mutations might influence prenatal growth, resulting in low birth weight and length (164), was recently investigated in detail in five Turkish families comprising 24 subjects with homozygous or compound heterozygous IGFALS mutations and 26 family members with heterozygous mutations (155). Postnatal growth and bone mineral density were also assessed. In summary, height, head circumference, body mass index, and birth weight SDS values were lower in the homozygous compared with the heterozygous IGFALS mutation subjects. The same was true of IGF-1, IGF-2, ALS, and IGFBP-3 concentrations. A subtle effect of heterozygous mutations on the above variables was seen. On average, height SDS was 1.3 to 1.5 lower in the subjects with homozygous mutations. Interestingly, for the first time low birth weight (below −2 SD) and head circumferences were reported in this series of homozygous mutation patients where reliable documentation was available. A potential suppressive effect of prenatal ALS deficiency on fetal IGF-1, however, remains to be confirmed.
PAPPA2 mutations
As noted above, IGF-1 is found in circulation bound in a ternary complex with an IGFBP (predominantly IGFBP-3) and ALS. The purpose of this ternary complex is to prolong the half-life of IGF-1, but it also plays a role in regulating IGF bioavailability. In the previous section, we described mutations in IGFALS that lead to low levels of IGF-1 and IGFBP-3 as a result of decreased (or absent) ternary complex formation. In 2016, the first human cases of PAPPA2 deficiency were described that lead to the opposite phenomenon, that is, increased levels of IGF-1 and IGFBP-3 due to an inability of the PAPPA2 protease to cleave IGFBP-3 (10).
PAPPA2 is a metalloproteinase member of the pappalysin family, which specifically cleaves IGFBP-3 and IGFBP-5 (165). It plays a key role in freeing IGF-1 from the ternary complex and thereby regulating the bioavailability of free IGF-1 (166). A knockout mouse model of Pappa2 demonstrated postnatal growth retardation with elevated levels of total IGF-1 and IGFBP-3 but low levels of free IGF-1 (167). In humans, PAPPA2 is expressed in a wide array of tissues with especially high levels in the placenta, and its circulating values are exponentially increased in pregnant women (168). PAPPA2 is highly homologous to the related protein PAPPA, which specifically cleaves IGFBP-4.
In the first report of human mutations in PAPPA2, Dauber et al. (10) described two families with a total of five affected children with recessive mutations of PAPPA2. The five children all presented with postnatal growth retardation with heights that were quite discordant from their midparental target height. The degree of short stature was variable, with heights ranging from −1.0 to −3.8 SDS, although some of the individuals were still growing and thus their final height SDS was yet to be determined. IGF-1 levels were markedly elevated in the patients and elevated levels of ALS, IGFBP-3 and IGFBP-5 were noted as well. Despite the markedly elevated total IGF-1 levels, serum IGF bioactivity and free IGF-1 levels were generally low with a dramatic decrease in the free IGF-1/total IGF-1 ratio representing the defect in binding protein cleavage. GH secretion was elevated in the patients, which was assumed to be due to a lack of negative feedback from the low free IGF-1 levels. One of the families had a homozygous frameshift mutation, p.D643fs25, whereas the other family had a homozygous missense mutation, p.Ala1033Val, in a highly conserved amino acid residue. In vitro studies demonstrated a complete loss of proteolytic activity of the missense mutation (10). In addition to short stature, some of the patients were noted to have moderate microcephaly, thin long bones, low bone mineral density, and insulin resistance (10, 169–171). Good (172) and moderate (169) growth responses to recombinant human IGF-1 therapy have been reported (see Recombinant “Human IGF-1 Therapy in Patients With Mild GHI Phenotypes” below).
These patients demonstrate the critical role that PAPPA2 plays in regulating IGF-1 bioavailability in humans and that defects in this system can lead to significant growth perturbations. Interestingly, common genetic variants near the PAPPA2 gene have also been associated with adult stature in large genome-wide association studies suggesting that both common and rare genetic variation influence IGF-1 bioavailability and its effect on growth (173).
Idiopathic Short Stature Patients With Apparent GHI
Definition of ISS
ISS is a term used in pediatric endocrinology to designate patients with (1) a height >2 SDS below the mean (adjusted for sex, age, and population background) and (2) a lack of evidence for any underlying nutritional, organ-system, or hormonal abnormality (174, 175). Because of this inability to identify a known cause for short stature, ISS children make up a heterogeneous mix of as yet unidentified variations of growth (174). Some ISS children may fall into the category of “extreme variations of normal.” For example, about half could be subdivided as having constitutional delay of growth and maturation (176). Another subgroup of ISS children may have normal variant familial short stature (177). However, a subset of ISS children will end up abnormally short for their genetic background. Such patients never experience a period of good catch-up growth and usually demonstrate limited response to treatment with hGH. These patients probably represent ∼1% of the general population (88, 178).
Statural growth is a complex genetically driven phenomenon with many genes influencing the adult stature achieved (179). An increasing number of genetic variants are known to be associated with variations in growth outcomes (180). For example, mutations in the GHR gene may play a role in causing ISS by impairing GHR function only to such a degree that results in a picture of short stature with minimal other phenotypic changes (97, 181). Such ISS patients have been found to demonstrate clinical and/or biochemical evidence of decreased GH action (182) (see “Other heterozygous GHR mutations” above).
Biochemical features of GHI in ISS patients
A retrospective analysis of a large multicenter registry study further analyzed 511 patients with ISS (height SD below −2 and documented GH sufficiency). The results yielded a subgroup of patients with low GHBP concentrations who also had significantly lower IGF-1 concentrations, and higher mean 12-hour GH concentrations, with all elements suggestive of some degree of GHI (89). Although the study did not show a significant correlation of GHBP values with the growth response to hGH therapy, other studies with ISS patients have demonstrated that a portion of ISS children also have a decreased response to hGH therapy (183). Such an attenuated growth response to GH treatment may be due to the presence of less severe defects of the GH–IGF-1 growth plate axis, including less severe GHR gene defects. For example, the previously described heterozygous GHR mutations may result in impaired GHR function, leading to milder GHI (86) (see “Other heterozygous GHR mutations” above).
Additional evidence that patients categorized as having ISS may be associated with GHI comes from the observation that several well-documented abnormalities of the GHR can lead to either abnormal GH or GHR binding, defective GHR dimerization, or defective GHR anchoring in the cell membrane, or result in aberrant signal GHR transduction (7, 135, 184, 185). Additional (currently unknown) mechanisms may underlie the short stature observed in certain ISS subgroups.
Genetic GHR variants in ISS
From an epidemiological perspective, a number of studies have tried to evaluate the prevalence of these genetic GHR variants in individuals with ISS. Taken together, it is likely that not >5% of patients with ISS will have (heterozygous) GHR mutations, because most appear to have normal GHR coding regions (86, 93, 97). Nevertheless, the spectrum of GHI is likely to be much larger than initially assumed based on the description of patients with extreme short stature due to homozygous single GHR gene defects. Additionally, similar to the more severely affected patients, the expression of the milder defects may show significant variability within each kindred, explaining why some patients are clinically easier to diagnose, whereas others only have mild growth deficits. Also note that some of the detected genetic variants may not necessarily be implicated in the observed growth failure, as some have been observed in normal controls or unaffected family members of ISS patients (93, 186). Therefore, additional investigations into the genetic basis of ISS are needed. A recent study tried to further assess the role of the GHR within the ISS phenotype. For this, analysis of both microsatellite polymorphisms as well as single nucleotide polymorphisms (SNPs) within the GHR was performed in two ISS cohorts and in a cohort of adult short stature (187). A significant association of a GT microsatellite polymorphism in the GHR promoter with ISS was detected. Furthermore, SNP analysis showed a consistent direction of effect for one common GHR variant in intron 2. Additional investigations in larger cohorts are needed to further identify GHI secondary to GHR gene defects as the underlying mechanism in certain ISS individuals.
“A number of other growth disorders, including 3M syndrome, SRS, and NS, have phenotypes that can overlap with GHI.”
In addition to the genetic causes of milder GHI described above (mutations of GHR, STAT5B, IGF1, IGF1R, and IGFALS), there are likely other genetic and acquired disorders that could result in reduced GH sensitivity in ISS patients. GHR synthesis may be decreased in African pygmies (188, 189). Degradation of the GHR may be increased in some ISS children, as proposed by Gil et al. (190). Abnormalities affecting the molecular mechanisms that control expression and signaling of the GHR may also potentially result in ISS. Steroid molecules, such as estrogens, may influence GHR expression and processing. Estrogens play an important role in both GH secretion in puberty, as well as the closure of the growth plates.
In a study of prepubertal and pubertal girls, compared with adults, investigators have shown that GHR mRNA production is augmented and GHR cleavage reduced, so an interplay of these hormones with the GHR may influence height outcome (191). Other growth factors, such as FGF21, may interfere with GHR function as a negative regulator of growth at the level of the growth plate through interference with the effects of GH on chondrocyte proliferation and differentiation (192). Finally, a few observations also indicate that IKKB mutations and PRKCA overexpression (with altered NF-κB action) can be associated with reduced GH sensitivity; this is most likely due to reduced activity or phosphorylation of STAT5 as a common etiology (193, 194). Although the initial patients described with these abnormalities had significant short stature (heights below −4.0 SDS), it is quite possible that other defects in signaling proteins could also modify the main growth-promoting signaling pathway, and hereby explain some case of ISS.
Genetic Analysis of GHI Patients
During the last decade, massively parallel signature sequencing (MPSS) technologies have transformed the way genetic analyses are performed in both the research setting and clinical diagnostics. We have progressed from Sanger sequencing of candidate genes, which relies on clinical phenotyping to predict the genes affected, to WES and even whole-genome sequencing (WGS), allowing the concomitant analysis of all genes in the genome without the need for prior, detailed phenotyping.
Where a classical phenotype is seen it may still be the cheapest option to sequence the genes linked to that syndrome, for example, sequencing of GHR where a classical Laron phenotype is observed. However, as we describe in this review, overlapping phenotypes and attenuated presentations can complicate the clinical picture and make MPSS a more cost-effective option for genetic diagnosis. The two main ways this has been approached are targeted gene sequencing of a panel of known/predicted growth genes (195–198) or unbiased WES (15, 179, 199, 200). There are advantages and disadvantages to each approach. The pros of panel sequencing over WES are greater read depth, better coverage of total coding regions, simplification of downstream analysis with less data, and no incidental findings (201, 202). The cons are the inability to find changes in novel genes and missing unconventional genetic changes, for example, regulatory regions, noncoding RNAs, and potential increased cost compared with WES for small numbers of patients. For gene discovery WES/WGS is the favored option, being the only way to find novel genetic etiologies, instances of nonexonic events such as pseudoexon inclusion (203), other regulatory changes, and changes in noncoding RNAs that may affect growth genes (204).
Between 30% and 48% (15, 134, 179, 197) of GHI cases are currently diagnosed genetically (Fig. 5), so where is the missing heritability? There is increasing recognition that copy number variants are prevalent in short stature (205–208). Although there are algorithms dedicated to detecting them in WES and WGS data, these are suboptimal, and hence the gold standard is still array comparative genomic hybridization or SNP arrays for their recognition (209). Another current area of research is the search for disease modifiers to explain the lack of genotype/phenotype correlation observed in the same mutation, for example, GHR pseudoexon, E180 splice, and E180X mutations (73, 74, 210). In this case, once again, MPSS can help, allowing us to elucidate all gene defects in known growth genes and pathways. Several groups have used the technique to identify disease gene modifiers in growth phenotypes in mice (211, 212) and humans (213). In dogs just six loci explain nearly half of the size determination (214); in humans many more genes are likely to be involved, and future studies investigating the contribution of heterozygous variants in known causal genes and/or oligogenic inheritance of a number of defective alleles may bear fruit.
Figure 5.
Genetic diagnoses obtained from candidate gene and WES in a selected group of 107 patients with GH insensitivity (15). [Reproduced with permission from Shapiro L, Chatterjee S, Ramadan DG, et al. Whole-exome sequencing gives additional benefits compared to candidate gene sequencing in the molecular diagnosis of children with growth hormone or IGF-1 insensitivity. Eur J Endocrinol 2017;177:485–501.]
For the future, rapidly determining the pathogenicity of variants will be the next hurdle to overcome. The bottleneck in genetic diagnosis going forward will be determination of the pathogenicity of variants discovered in MPSS. In silico prediction tools are imperfect, meaning that time-consuming functional analyses to determine causality will be needed until these algorithms improve.
Short stature syndromes that overlap with GHI
A number of other growth disorders, including 3M syndrome, SRS, and NS, have phenotypes that can overlap with GHI (12–15). NS (OMIM no. 163950) is a common autosomal dominant genetic disorder with an estimated incidence of 1 of 1000 to 1 of 2500 live births (1, 215). This syndrome is characterized by distinctive facial features, short stature, and other features, including congenital heart defects, mild-to-moderate developmental delay/learning disability, and skeletal abnormalities (216, 217). Postnatal proportionate short stature, reported in up to 80% of patients, is one of the main features of NS (217).
NS is caused by mutations in genes encoding components of the RAS/MAPK signaling pathway that plays key roles in various cellular processes, including proliferation, survival, differentiation, migration, and metabolism (1, 217, 218). The commonest mutation, present in ∼50% of cases, is in protein tyrosine phosphatase, nonreceptor type 11 (PTPN11), which codes for the cytoplasmic tyrosine phosphatase SH2 domain–containing phosphatase 2 (SHP-2) (217, 219). SHP-2 binds to and dephosphorylates signaling molecules such as STAT5b (220), which are positive regulators of the cellular response to GH. Therefore, gain-of-function mutations of PTPN11 may negatively regulate GH action and thus induce some degree of GHI at a post-GHR level (1). Consistent with this, serum IGF-1 levels were decreased in NS subjects carrying PTPN11 mutations (215, 221). PTPN11 mutation-positive subjects also show decreased growth responses to hGH therapy compared with mutation-negative subjects (222).
The mechanism of GHI in other genetic mutations associated with NS such as SOS1, RAF1, and KRAS is less well understood. It is hypothesized that aberrations in these genes hyperactivate the RAS–MAPK pathway, thus inhibiting the JAK–STAT pathway and impairing the systemic production of IGF-1, resulting in a GHI state (223). Consistent with this, inhibition of RAS–MAPK signaling in NS mice promotes an increase in IGF-1 levels in vitro and in vivo and an associated growth improvement (224). Pharmacological inhibition of the RAS–MAPK pathway also improves growth in NS mouse models (225–227).
SRS (OMIM no. 180860) is a rare but well-recognized clinically and genetically heterogeneous disorder, characterized by prenatal and postnatal growth restriction, relative macrocephaly, body asymmetry, and characteristic facial features, including a protruding forehead (228, 229). Hence, many of the clinical features overlap with classical GHI. The most common underlying mechanisms are hypomethylation of the imprinted H19/IGF2 domain of chromosome 11p15 (seen in 30% to 60% of patients) and maternal uniparental disomy for chromosome 7 (seen in ∼5% to 10% of patients) (149, 230, 231). Children with SRS and 11p15 hypomethylation have significantly higher IGF-1 and IGFBP-3 levels (even when malnourished) compared with those with maternal uniparental disomy for chromosome 7 and other children born small for gestational age, suggesting that they may display some IGF-1 resistance (232, 233). The mechanism underlying these high serum IGF-1 levels in SRS patients remains unknown (234). A previous study has shown that IGF-1 receptor defects are not present in SRS (235). IGF-2 deficiency at the cellular level, if present, may account for a change in the interaction between IGFs and insulin with their respective receptors and their binding proteins (233).
3M syndrome (OMIM no. 273750) results in prenatal and postnatal growth restriction, prominent heels, facial dysmorphism (triangular-shaped face, anteverted nares, and full fleshy lips), normal intelligence, and distinct radiological features (slender long bones and tall vertebral bodies) (236). Some clinical features of 3M syndrome such as frontal bossing, depressed nasal bridge, bitemporal hair thinning, and high-pitched voice overlap with classical GHI (237). 3M syndrome is genetically heterogeneous, and mutations in cullin 7 (CUL7) (70%), obscurin-like 1 (OBSL1) (25%), and coiled coil domain–containing 8 (CCDC8) (5%) genes have been identified (199, 238–243). Peak GH levels are normal or high and IGF-1 levels are normal or low. The growth response to hGH therapy is variable but typically poor, and therefore a degree of GH resistance is likely (237, 239).
The mechanism by which CUL7, OBSL1, and CCDC8 gene mutations cause disruption of the GH–IGF-1 axis is unclear. CUL7 is a major structural component of an E3 ligase within the ubiquitin proteasomal degradation pathway, which targets p53, cyclin D1, and the IGF-1/insulin signaling molecule, insulin receptor substrate-1 (IRS-1). Previous work (244) demonstrated that there is accumulation of the signaling molecule IRS-1 in CUL7-deficient cell lines. This may lead to an increased activation of AKT and MAPK and cellular senescence. In contrast, another study (239) did not confirm an accumulation of IRS-1 or increased AKT/MAPK activation in CUL7-deficient cell lines following GH stimulation, suggesting that GH signaling was normal. However, there was increased activation of MAPK following stimulation with IGF-1, indicating that IGF-1 signaling is disrupted therefore IGF-1 insensitivity was present (239).
OBSL1 is a putative cytoskeletal adaptor that interacts with and stabilizes CUL7 (245). Mutations in both OBSL1 and CUL7 result in an apparently identical clinical phenotype, suggesting a common molecular pathway. In support of this, CUL7 and OBSL1 coimmunoprecipitate, that is, they may physically interact in vivo and loss of OBSL1 results in downregulation of CUL7, suggesting that OBSL1 is required for the regulation of CUL7 protein levels (245). Another study showed that both GH and IGF-1 signaling are moderately diminished in OBSL1-deficient cell lines (239). This may suggest both partial GH and IGF-1 resistance in 3M syndrome patients with OBSL1 mutations.
CCDC8 is a protein of unknown molecular function (239). Co-immunoprecipitation assays revealed that CCDC8 interacts with OBSL1 but not CUL7 (246). Additionally, GH signaling was moderately reduced and early activation of IGF-1 signaling was normal in CCDC8-deficient cell lines following GH and IGF-1 stimulation, respectively, suggesting GH resistance (239). Therefore, accurate clinical and biochemical phenotyping is essential to guide genetic investigation and diagnosis in children with severe short stature and features of GHI.
Endocrine Investigation of Short Stature Patients to Identify GHI
The aim of investigating children with possible GHI is to identify an abnormality in GH action, which in combination with the clinical phenotype and possible genetic results can define the pathogenesis and facilitate therapy (Fig. 6). Clearly, the patient with mild GHI may be more difficult to distinguish from normality than the patient with extreme biochemical and clinical features. This may particularly apply to subjects with heterozygous mutations (247).
Figure 6.
Algorithm for investigation of GHI in the child with short stature.
The GH–IGF-1 axis
Investigations of the GH–IGF-1 axis consist of determination of GH secretion and exploration of the IGF system. A GH provocation test is recommended unless the child has normal auxology or a basal IGF-1 level above the mean for age (88). In a child with clinical criteria of GHD, a peak GH level of <7 to 10 ng/mL is usually used to support this diagnosis (248, 249), although criteria for this diagnosis vary from center to center. Basal IGF-1 levels should also be determined, but these may be influenced by factors such as nutrition, chronic illness, and puberty. Determination of IGFBP-3, ALS, and GHBP levels may provide additional diagnostic validation. Reliable assay performance and appropriate normative data (250, 251) are essential for the use of IGF-1 and IGFBP-3 in clinical practice.
Most patients with homozygous mutations will show detectable abnormal function of the GH–IGF-1 axis. Examples of patients with mild or moderate GHI phenotypes were four cousins with GHR pseudoexon mutations, who showed elevated pulsatile GH secretion and subnormal IGF-1 and IGFBP-3 levels (252). The moderate clinical phenotype of STAT5B deficiency was associated with IGF-1 and IGFBP-3 deficiency (106). The case with a mild defect of the IGF-1 gene had low to normal IGF-1, normal IGFBP-3, and elevated ALS (35). Patients with IGFALS mutations had deficiencies of IGF-1 and ALS and severely subnormal IGFBP-3 (155), contrasting with the children with PAPPA2 mutations who had strikingly elevated IGF-1, IGFBP-3, and ALS, again associated with a rather subtle short stature phenotype (10).
The IGF-1 generation test
The IGF-1 generation test (IGFGT) has become a popular investigation to determine GH sensitivity based on repeated injections of hGH increasing of IGF-1, IGFBP-3, and ALS concentrations and thus testing the function of the GHR. There are weaknesses of the IGFGT. First, agreed-upon protocols for the dose of hGH and normative data have not been established (253). Criteria for diagnosis of GHI in classical cases with the Laron syndrome phenotype were defined as failure to increase IGF-1 and IGFBP-3 by >15 and 400 ng/mL, respectively (254). However, as the spectrum of GHI disorders has expanded, these criteria appear to be too strict for more mildly affected subjects and even in severe GHI patients after GH increases of IGF-1 ranged from <20 to 58 ng/mL and of IGFBP-3 from 95 to 1762 ng/mL (255). Attempts to refine the IGFGT for the diagnosis of milder GHI have demonstrated that there may be an overlap with patients with ISS who produced a subnormal IGF-1 response (62), and subjects with IGF-1 deficiency who had normal GH secretion also had subnormal ability to generate IGF-1 (256). Additional sensitivity for the diagnosis of GHI was not seen with a low-dose GH protocol (257). Consequently, the IGFGT is currently not recommended for the routine investigation of short stature. Its principal value is to confirm extreme or severe GHI in cases with the classical phenotype (253).
Recombinant Human IGF-1 Therapy in Patients With Mild GHI Phenotypes
Although rhIGF-1 replacement therapy has been evaluated in children with severe IGF-1 deficiency (68), its efficacy in less severe GHI patients is less clear. No study that directly compares the efficacy of rhIGF-1 in severe and mild GHI patients has been performed. Additionally, some patients may not fulfill the currently approved Food and Drug Administration and European Medicines Agency labels for this indication (height SDS −3 or less and IGF-1 SDS −3 or less for the United States or IGF-1 below the 2.5th percentile for the European Union and normal or elevated GH). A controlled therapeutic trial of rhIGF-1 therapy in children with less severe short stature and IGF-1 deficiency (height and IGF-1 SDS −2 or less) reported an increase in first-year height velocity to 7.9 ± 1.4 cm/y compared with 5.2 ± 1.0 in an untreated control group. The response was dose-dependent and adverse events were much less frequent compared with classical GHI patients treated with rhIGF-1 (256). However, these patients had a wide range of responses (256); therefore, the optimal management of this group of patients needs to be further established.
A study of combination rhGH plus rhIGF-1 therapy in children with short stature, low IGF-1 (below −1.0 SD) and normal GH levels assessed growth responses according to the dose of rhIGF-1 in the combination therapy. In the group receiving the highest dose of rhIGF-1 (150 μg/kg/d) combined with hGH at 45 μg/kg/d, the year 1 height velocity was 11.2 ± 2.1 compared with 9.3 ± 1.7 cm/y in a control group receiving hGH 45 at μg/kg/d alone (69). These interesting data need to be confirmed, but new studies using combination therapy are lacking due to reduced supplies of rhIGF-1.
Recently, three unrelated patients with partial GHI and three different heterozygous, dominant negative GHR variants were reported. They presented with postnatal growth failure, IGF-1 deficiency, and a relatively normal facial phenotype (52). Long-term treatment with a combination of rhGH and rhIGF-1 improved linear growth to within the normal range for one of the patients (P4, Fig. 1). Additionally, serum IGF-1 concentrations normalized with therapy (52). Similarly, improved growth rate in response to rhIGF-1 was reported in one other patient with a dominant negative GHR mutation (P1, Fig. 1), from 4 cm/y prior to therapy to annualized growth velocity of 8.1 cm/y. Of note, the family reported increased energy and muscle strength with treatment (52).
Therapeutic trials of rhIGF-1 were given to the four patients with homozygous PAPPA2 mutations who had remaining growth potential. Three of the children had a moderate improvement in growth with a height increase of 0.4 SDS after 1 year of treatment (169, 172). The fourth patient developed increased intracranial pressure, leading to discontinuation of therapy. It is not known whether patients with PAPPA2 deficiency are at increased risk of this complication with rhIGF-1 therapy. However, rhIGF-1 therapy led to the resolution of insulin resistance (169), increased bone mineral density, and improved trabecular bone structure in PAPPA2-deficient children (169, 170).
A recent study assessed the growth responses of 15 patients with GHR pseudoexon (6Ψ) mutations following rhIGF-1 therapy (74). Mean height velocity increased significantly in the first year of treatment (Fig. 7). This response was comparable to that reported in patients with other homozygous GHR defects (258) and other patients with severe IGF-1 deficiency (68, 259–261). However, in contrast to data from a large European cohort of patients treated with rhIGF1 (65), the increase in first year height velocity in the 6Ψ patients did not correlate with age of rhIGF-1 initiation or lower baseline height SDS/height velocity SDS (74). The mean cumulative change in height SDS of ∼1.4 at year 5 of treatment was also comparable to other published studies of rhIGF-1 therapy in severe GHI (259, 260).
Figure 7.
Height velocity at four different time points during treatment with rhIGF1. Box-and-whisker plots show the median, upper and lower quartiles, and range. IQR, interquartile range; n, number of patients data available/included for each time point. P values were calculated by ANOVA with Dunn-Bonferroni post hoc pairwise comparison. *P = 0.001 (74). [Reproduced with permission from Chatterjee S, Shapiro L, Rose SJ, et al. Phenotypic spectrum and responses to recombinant human IGF1 (rhIGF1) therapy in patients with homozygous intronic pseudoexon growth hormone receptor mutation. Eur J Endocrinol 2018;178:481–489.]
Consistent with other studies (68, 261), patients with GHR 6Ψ mutations did not achieve adult heights within the normal range (74). However, as previously reported in patients with severe IGF-1 deficiency (261), adult height SDS was higher than pretreatment height SDS. Given that many 6Ψ patients have a mild degree of IGF-1 deficiency, it is tempting to speculate that the response to rhIGF-1 therapy would be suboptimal. Preliminary data suggest that height outcomes are comparable to those reported in other homozygous GHR defects and severe IGF-1 deficiency.
Conclusions and Future Perspectives
The integrity of the GH–IGF-1 axis is fundamental for normal human linear growth. Defects in several of these genes are recognized to cause GHI and severe childhood growth impairment. For many years, homozygous and compound heterozygous mutations in the GHR, STAT5B, IGF1, and IGFALS genes have been synonymous with classical GHI. However, the spectrum of genetic anomalies associated with the GH–IGF-1 axis is rapidly expanding. Advancing genetic techniques and an increased awareness of this group of disorders has led to the recognition of other molecular defects that encompass milder or nonclassical GHI cases. These include heterozygous dominant negative mutations (to date reported in the GHR and STAT5B genes) and pseudoexon inclusion (reported in the GHR gene).
Genetic studies of large cohorts of short stature patients with no known diagnosis (often termed ISS) and extensive analysis of carrier and noncarrier first-degree relatives of patients with classical GHI have also allowed clinicians and researchers to determine the impact of heterozygous GH–IGF-1 axis gene mutations. As such, numerous studies have suggested a degree of height loss in individuals with specific heterozygous IGFALS, STAT5B, and GHR variants. These individuals with more modest “gene-dosage” effects suggest a possible mechanism for less severe cases of short stature with unknown etiology and features of GHI.
The relatively recent discovery of several novel gene defects, such as PAPPA2 and IGF2, have further expanded our knowledge of the GHI disorder spectrum. Ongoing work to determine new molecular causes underlying GHI phenotypes will be essential to advance the understanding of the genetic basis of GHI and short stature. This work will also be critical to discover other molecules that are integral to the correct functioning of the GH–IGF-1 axis and in turn identify new therapeutic targets.
Detailed clinical, biochemical, and genetic characterization of patients with GHI phenotypes is essential to improve our understanding of overlapping short stature syndromes and other cellular mechanisms that are necessary for normal human growth, for example, the 3M complex/RAS–MAPK pathway. Future work should also focus on the identification of new molecular mechanisms, for example, nonexonic variants and the contribution of oligogenic/polygenic inheritance, which may contribute to the development of GHI.
Acknowledgments
Financial Support: This work was supported in part by National Institutes of Health/National Institute of Child Health and Human Development Grant R01HD078592 (to V.H.).
Current Affiliation: A. Dauber’s current affiliation is the Division of Endocrinology, Children's National Medical Center, Washington, DC 20010.
Disclosure Summary: H.L.S. received research funding from Ipsen and Sandoz Pharmaceuticals. P.F.B. received research funding from Ipsen Pharmaceuticals. P.F.B. and A.D. receive medication for free from Ipsen Pharmaceuticals for a clinical study. A.D. and V.H. have a patent for the use of PAPPA2 as a growth-promoting agent. The remaining authors have nothing to disclose.
Glossary
Abbreviations
- ALS
acid-labile subunit
- CCD
coiled-coiled domain
- CCDC8
coiled coil domain–containing 8
- CUL7
cullin 7
- DBD
DNA-binding domain
- GHBP
GH-binding protein
- GHI
GH insensitivity
- GHR
GH receptor
- hGH
human GH
- IGFBP
IGF-binding protein
- IGFGT
IGF-1 generation test
- IGF1R
IGF-1 receptor
- IRS-1
insulin receptor substrate-1
- ISS
idiopathic short stature
- IUGR
intrauterine growth retardation
- JAK2
Janus kinase 2
- MPSS
massively parallel signature sequencing
- NS
Noonan syndrome
- OBSL1
obscurin-like 1
- OMIM
Online Mendelian Inheritance in Man
- PAPPA2
pregnancy-associated plasma protease A2
- PTPN11
protein tyrosine phosphatase, nonreceptor type 11
- RAS
rat sarcoma viral oncogene homolog
- rhGH
recombinant human GH
- rhIGF-1
recombinant human IGF-1
- SDS
SD score
- SH2
Src homology 2
- SHP-2
Src homology 2 domain–containing phosphatase 2
- SNP
single nucleotide polymorphism
- SRS
Silver-Russell syndrome
- STAT
signal transducer and activator of transcription
- TAD
C-terminal–transactivating domain
- WES
whole-exome sequencing
- WGS
whole-genome sequencing
References
- 1. David A, Hwa V, Metherell LA, Netchine I, Camacho-Hübner C, Clark AJ, Rosenfeld RG, Savage MO. Evidence for a continuum of genetic, phenotypic, and biochemical abnormalities in children with growth hormone insensitivity. Endocr Rev. 2011;32(4):472–497. [DOI] [PubMed] [Google Scholar]
- 2. Laron Z. Laron syndrome (primary growth hormone resistance or insensitivity): the personal experience 1958–2003. J Clin Endocrinol Metab. 2004;89(3):1031–1044. [DOI] [PubMed] [Google Scholar]
- 3. Laron Z, Pertzelan A, Mannheimer S. Genetic pituitary dwarfism with high serum concentation of growth hormone—a new inborn error of metabolism? Isr J Med Sci. 1966;2(2):152–155. [PubMed] [Google Scholar]
- 4. Eshet R, Laron Z, Pertzelan A, Arnon R, Dintzman M. Defect of human growth hormone receptors in the liver of two patients with Laron-type dwarfism. Isr J Med Sci. 1984;20(1):8–11. [PubMed] [Google Scholar]
- 5. Savage MO, Burren CP, Rosenfeld RG. The continuum of growth hormone–IGF-I axis defects causing short stature: diagnostic and therapeutic challenges. Clin Endocrinol (Oxf). 2010;72(6):721–728. [DOI] [PubMed] [Google Scholar]
- 6. Kofoed EM, Hwa V, Little B, Woods KA, Buckway CK, Tsubaki J, Pratt KL, Bezrodnik L, Jasper H, Tepper A, Heinrich JJ, Rosenfeld RG. Growth hormone insensitivity associated with a STAT5b mutation. N Engl J Med. 2003;349(12):1139–1147. [DOI] [PubMed] [Google Scholar]
- 7. Rosenfeld RG, Belgorosky A, Camacho-Hubner C, Savage MO, Wit JM, Hwa V. Defects in growth hormone receptor signaling. Trends Endocrinol Metab. 2007;18(4):134–141. [DOI] [PubMed] [Google Scholar]
- 8. Woods KA, Camacho-Hübner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med. 1996;335(18):1363–1367. [DOI] [PubMed] [Google Scholar]
- 9. Domené HM, Bengolea SV, Martínez AS, Ropelato MG, Pennisi P, Scaglia P, Heinrich JJ, Jasper HG. Deficiency of the circulating insulin-like growth factor system associated with inactivation of the acid-labile subunit gene. N Engl J Med. 2004;350(6):570–577. [DOI] [PubMed] [Google Scholar]
- 10. Dauber A, Muñoz-Calvo MT, Barrios V, Domené HM, Kloverpris S, Serra-Juhé C, Desikan V, Pozo J, Muzumdar R, Martos-Moreno GA, Hawkins F, Jasper HG, Conover CA, Frystyk J, Yakar S, Hwa V, Chowen JA, Oxvig C, Rosenfeld RG, Pérez-Jurado LA, Argente J. Mutations in pregnancy-associated plasma protein A2 cause short stature due to low IGF-I availability. EMBO Mol Med. 2016;8(4):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Domené HM, Hwa V, Argente J, Wit JM, Camacho-Hübner C, Jasper HG, Pozo J, van Duyvenvoorde HA, Yakar S, Fofanova-Gambetti OV, Rosenfeld RG; International ALS Collaborative Group . Human acid-labile subunit deficiency: clinical, endocrine and metabolic consequences [published correction appears in Horm Res. 2010;73(1):80]. Horm Res. 2009;72(3):129–141. [DOI] [PubMed] [Google Scholar]
- 12. Akawi NA, Ali BR, Hamamy H, Al-Hadidy A, Al-Gazali L. Is autosomal recessive Silver-Russel syndrome a separate entity or is it part of the 3-M syndrome spectrum? Am J Med Genet A. 2011;155(6):1236–1245. [DOI] [PubMed] [Google Scholar]
- 13. Binder G, Neuer K, Ranke MB, Wittekindt NE. PTPN11 mutations are associated with mild growth hormone resistance in individuals with Noonan syndrome. J Clin Endocrinol Metab. 2005;90(9):5377–5381. [DOI] [PubMed] [Google Scholar]
- 14. Storr HL, Dunkel L, Kowalczyk J, Savage MO, Metherell LA. Genetic characterisation of a cohort of children clinically labelled as GH or IGF1 insensitive: diagnostic value of serum IGF1 and height at presentation. Eur J Endocrinol. 2015;172(2):151–161. [DOI] [PubMed] [Google Scholar]
- 15. Shapiro L, Chatterjee S, Ramadan DG, Davies KM, Savage MO, Metherell LA, Storr HL. Whole-exome sequencing gives additional benefits compared to candidate gene sequencing in the molecular diagnosis of children with growth hormone or IGF-1 insensitivity. Eur J Endocrinol. 2017;177(6):485–501. [DOI] [PubMed] [Google Scholar]
- 16. Salmon WD Jr, Daughaday WH. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med. 1957;49(6):825–836. [PubMed] [Google Scholar]
- 17. Daughaday WH, Hall K, Raben MS, Salmon WD Jr, van den Brande JL, van Wyk JJ. Somatomedin: proposed designation for sulphation factor. Nature. 1972;235(5333):107. [DOI] [PubMed] [Google Scholar]
- 18. Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S, Danielsson A, Edlund K, Asplund A, Sjöstedt E, Lundberg E, Szigyarto CA, Skogs M, Takanen JO, Berling H, Tegel H, Mulder J, Nilsson P, Schwenk JM, Lindskog C, Danielsson F, Mardinoglu A, Sivertsson A, von Feilitzen K, Forsberg M, Zwahlen M, Olsson I, Navani S, Huss M, Nielsen J, Ponten F, Uhlén M. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13(2):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duff MO, Olson S, Wei X, Garrett SC, Osman A, Bolisetty M, Plocik A, Celniker SE, Graveley BR. Genome-wide identification of zero nucleotide recursive splicing in Drosophila. Nature. 2015;521(7552):376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lowe WL Jr, Roberts CT Jr, Lasky SR, LeRoith D. Differential expression of alternative 5′ untranslated regions in mRNAs encoding rat insulin-like growth factor I. Proc Natl Acad Sci USA. 1987;84(24):8946–8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lowe WL Jr, Lasky SR, LeRoith D, Roberts CT Jr. Distribution and regulation of rat insulin-like growth factor I messenger ribonucleic acids encoding alternative carboxyterminal E-peptides: evidence for differential processing and regulation in liver. Mol Endocrinol. 1988;2(6):528–535. [DOI] [PubMed] [Google Scholar]
- 22. Isaksson OG, Jansson JO, Gause IA. Growth hormone stimulates longitudinal bone growth directly. Science. 1982;216(4551):1237–1239. [DOI] [PubMed] [Google Scholar]
- 23. Nilsson A, Isgaard J, Lindahl A, Dahlström A, Skottner A, Isaksson OG. Regulation by growth hormone of number of chondrocytes containing IGF-I in rat growth plate. Science. 1986;233(4763):571–574. [DOI] [PubMed] [Google Scholar]
- 24. Isaksson OG, Lindahl A, Nilsson A, Isgaard J. Mechanism of the stimulatory effect of growth hormone on longitudinal bone growth. Endocr Rev. 1987;8(4):426–438. [DOI] [PubMed] [Google Scholar]
- 25. Lindahl A, Isgaard J, Isaksson OG. Growth hormone in vivo potentiates the stimulatory effect of insulin-like growth factor-1 in vitro on colony formation of epiphyseal chondrocytes isolated from hypophysectomized rats. Endocrinology. 1987;121(3):1070–1075. [DOI] [PubMed] [Google Scholar]
- 26. Yakar S, Liu J-L, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA. 1999;96(13):7324–7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu Y, Sun H, Yakar S, LeRoith D. Elevated levels of insulin-like growth factor (IGF)-I in serum rescue the severe growth retardation of IGF-I null mice. Endocrinology. 2009;150(9):4395–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saenger P, Czernichow P, Hughes I, Reiter EO. Small for gestational age: short stature and beyond. Endocr Rev. 2007;28(2):219–251. [DOI] [PubMed] [Google Scholar]
- 29. Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr Rev. 2001;22(1):53–74. [DOI] [PubMed] [Google Scholar]
- 30. Kaplan SA, Cohen P. The somatomedin hypothesis 2007: 50 years later. J Clin Endocrinol Metab. 2007;92(12):4529–4535. [DOI] [PubMed] [Google Scholar]
- 31. Ranke MB, Wit JM. Growth hormone—past, present and future. Nat Rev Endocrinol. 2018;14(5):285–300. [DOI] [PubMed] [Google Scholar]
- 32. Dattani M, Preece M. Growth hormone deficiency and related disorders: insights into causation, diagnosis, and treatment. Lancet. 2004;363(9425):1977–1987. [DOI] [PubMed] [Google Scholar]
- 33. Hattori A, Katoh-Fukui Y, Nakamura A, Matsubara K, Kamimaki T, Tanaka H, Dateki S, Adachi M, Muroya K, Yoshida S, Ida S, Mitani M, Nagasaki K, Ogata T, Suzuki E, Hata K, Nakabayashi K, Matsubara Y, Narumi S, Tanaka T, Fukami M. Next generation sequencing-based mutation screening of 86 patients with idiopathic short stature. Endocr J. 2017;64(10):947–954. [DOI] [PubMed] [Google Scholar]
- 34. Walenkamp MJ, Karperien M, Pereira AM, Hilhorst-Hofstee Y, van Doorn J, Chen JW, Mohan S, Denley A, Forbes B, van Duyvenvoorde HA, van Thiel SW, Sluimers CA, Bax JJ, de Laat JA, Breuning MB, Romijn JA, Wit JM. Homozygous and heterozygous expression of a novel insulin-like growth factor-I mutation. J Clin Endocrinol Metab. 2005;90(5):2855–2864. [DOI] [PubMed] [Google Scholar]
- 35. Netchine I, Azzi S, Houang M, Seurin D, Perin L, Ricort JM, Daubas C, Legay C, Mester J, Herich R, Godeau F, Le Bouc Y. Partial primary deficiency of insulin-like growth factor (IGF)-I activity associated with IGF1 mutation demonstrates its critical role in growth and brain development. J Clin Endocrinol Metab. 2009;94(10):3913–3921. [DOI] [PubMed] [Google Scholar]
- 36. Chatterjee S, Pal JK. Role of 5′- and 3′-untranslated regions of mRNAs in human diseases. Biol Cell. 2009;101(5):251–262. [DOI] [PubMed] [Google Scholar]
- 37. Gent J, van Kerkhof P, Roza M, Bu G, Strous GJ. Ligand-independent growth hormone receptor dimerization occurs in the endoplasmic reticulum and is required for ubiquitin system-dependent endocytosis. Proc Natl Acad Sci USA. 2002;99(15):9858–9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown RJ, Adams JJ, Pelekanos RA, Wan Y, McKinstry WJ, Palethorpe K, Seeber RM, Monks TA, Eidne KA, Parker MW, Waters MJ. Model for growth hormone receptor activation based on subunit rotation within a receptor dimer. Nat Struct Mol Biol. 2005;12(9):814–821. [DOI] [PubMed] [Google Scholar]
- 39. Brooks AJ, Dai W, O’Mara ML, Abankwa D, Chhabra Y, Pelekanos RA, Gardon O, Tunny KA, Blucher KM, Morton CJ, Parker MW, Sierecki E, Gambin Y, Gomez GA, Alexandrov K, Wilson IA, Doxastakis M, Mark AE, Waters MJ. Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science. 2014;344(6185):1249783. [DOI] [PubMed] [Google Scholar]
- 40. Derr MA, Fang P, Sinha SK, Ten S, Hwa V, Rosenfeld RG. A novel Y332C missense mutation in the intracellular domain of the human growth hormone receptor does not alter STAT5b signaling: redundancy of GHR intracellular tyrosines involved in STAT5b signaling. Horm Res Paediatr. 2011;75(3):187–199. [DOI] [PubMed] [Google Scholar]
- 41. Milward A, Metherell L, Maamra M, Barahona MJ, Wilkinson IR, Camacho-Hübner C, Savage MO, Bidlingmaier M, Clark AJL, Ross RJM, Webb SM. Growth hormone (GH) insensitivity syndrome due to a GH receptor truncated after Box1, resulting in isolated failure of STAT 5 signal transduction [published correction appears in J Clin Endocrinol Metab. 2009;94(7):2674]. J Clin Endocrinol Metab. 2004;89(3):1259–1266. [DOI] [PubMed] [Google Scholar]
- 42. Jin H, Lanning NJ, Carter-Su C. JAK2, but not Src family kinases, is required for STAT, ERK, and Akt signaling in response to growth hormone in preadipocytes and hepatoma cells. Mol Endocrinol. 2008;22(8):1825–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Flores-Morales A, Greenhalgh CJ, Norstedt G, Rico-Bautista E. Negative regulation of growth hormone receptor signaling. Mol Endocrinol. 2006;20(2):241–253. [DOI] [PubMed] [Google Scholar]
- 44. Argente J, Chowen JA, Pérez-Jurado LA, Frystyk J, Oxvig C. One level up: abnormal proteolytic regulation of IGF activity plays a role in human pathophysiology. EMBO Mol Med. 2017;9(10):1338–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yakar S, Adamo ML. Insulin-like growth factor 1 physiology: lessons from mouse models. Endocrinol Metab Clin North Am. 2012;41(2):231–247, v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16(1):3–34. [DOI] [PubMed] [Google Scholar]
- 47. Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20(6):761–787. [DOI] [PubMed] [Google Scholar]
- 48. Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab. 2000;278(6):E967–E976. [DOI] [PubMed] [Google Scholar]
- 49. Högler W, Martin DD, Crabtree N, Nightingale P, Tomlinson J, Metherell L, Rosenfeld R, Hwa V, Rose S, Walker J, Shaw N, Barrett T, Frystyk J. IGFALS gene dosage effects on serum IGF-I and glucose metabolism, body composition, bone growth in length and width, and the pharmacokinetics of recombinant human IGF-I administration. J Clin Endocrinol Metab. 2014;99(4):E703–E712. [DOI] [PubMed] [Google Scholar]
- 50. Bramani S, Song H, Beattie J, Tonner E, Flint DJ, Allan GJ. Amino acids within the extracellular matrix (ECM) binding region (201–218) of rat insulin-like growth factor binding protein (IGFBP)-5 are important determinants in binding IGF-I. J Mol Endocrinol. 1999;23(1):117–123. [DOI] [PubMed] [Google Scholar]
- 51. Nilsson O, Marino R, De Luca F, Phillip M, Baron J. Endocrine regulation of the growth plate. Horm Res. 2005;64(4):157–165. [DOI] [PubMed] [Google Scholar]
- 52. Vairamani K, Merjaneh L, Casano-Sancho P, Sanli ME, David A, Metherell LA, Savage MO, Del Pozo JS, Backeljauw PF, Rosenfeld RG, Aisenberg J, Dauber A, Hwa V. Novel dominant-negative GH receptor mutations expands the spectrum of GHI and IGF-I deficiency. J Endocr Soc. 2017;1(4):345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Amselem S, Duquesnoy P, Attree O, Novelli G, Bousnina S, Postel-Vinay MC, Goossens M. Laron dwarfism and mutations of the growth hormone-receptor gene. N Engl J Med. 1989;321(15):989–995. [DOI] [PubMed] [Google Scholar]
- 54. Godowski PJ, Leung DW, Meacham LR, Galgani JP, Hellmiss R, Keret R, Rotwein PS, Parks JS, Laron Z, Wood WI. Characterization of the human growth hormone receptor gene and demonstration of a partial gene deletion in two patients with Laron-type dwarfism. Proc Natl Acad Sci USA. 1989;86(20):8083–8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rosenbloom AL, Guevara Aguirre J, Rosenfeld RG, Fielder PJ. The little women of Loja—growth hormone-receptor deficiency in an inbred population of southern Ecuador. N Engl J Med. 1990;323(20):1367–1374. [DOI] [PubMed] [Google Scholar]
- 56. Laron Z, Lilos P, Klinger B. Growth curves for Laron syndrome. Arch Dis Child. 1993;68(6):768–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Leonard J, Samuels M, Cotterill AM, Savage MO. Effects of recombinant insulin-like growth factor I on craniofacial morphology in growth hormone insensitivity. Acta Paediatr Suppl. 1994;83(S399):140–141. [DOI] [PubMed] [Google Scholar]
- 58. Cotterill AM, Holly JM, Taylor AM, Davies SC, Coulson VJ, Preece MA, Wass JA, Savage MO. The insulin-like growth factor (IGF)-binding proteins and IGF bioactivity in Laron-type dwarfism. J Clin Endocrinol Metab. 1992;74(1):56–63. [DOI] [PubMed] [Google Scholar]
- 59. Rosenfeld RG, Rosenbloom AL, Guevara-Aguirre J. Growth hormone (GH) insensitivity due to primary GH receptor deficiency. Endocr Rev. 1994;15(3):369–390. [DOI] [PubMed] [Google Scholar]
- 60. Burren CP, Wanek D, Mohan S, Cohen P, Guevara-Aguirre J, Rosenfeld RG. Serum levels of insulin-like growth factor binding proteins in Ecuadorean children with growth hormone insensitivity. Acta Paediatr Suppl. 1999;88(S428):185–191. [DOI] [PubMed] [Google Scholar]
- 61. Blum WF, Cotterill AM, Postel-Vinay MC, Ranke MB, Savage MO, Wilton P; Pharmacia Study Group on Insulin-like Growth Factor I Treatment in Growth Hormone Insensitivity Syndromes . Improvement of diagnostic criteria in growth hormone insensitivity syndrome: solutions and pitfalls. Acta Paediatr Suppl. 1994;83(S399):117–124. [DOI] [PubMed] [Google Scholar]
- 62. Selva KA, Buckway CK, Sexton G, Pratt KL, Tjoeng E, Guevara-Aguirre J, Rosenfeld RG. Reproducibility in patterns of IGF generation with special reference to idiopathic short stature. Horm Res. 2003;60(5):237–246. [DOI] [PubMed] [Google Scholar]
- 63. Woods KA, Dastot F, Preece MA, Clark AJ, Postel-Vinay MC, Chatelain PG, Ranke MB, Rosenfeld RG, Amselem S, Savage MO. Phenotype: genotype relationships in growth hormone insensitivity syndrome. J Clin Endocrinol Metab. 1997;82(11):3529–3535. [DOI] [PubMed] [Google Scholar]
- 64. Iida K, Takahashi Y, Kaji H, Nose O, Okimura Y, Abe H, Chihara K. Growth hormone (GH) insensitivity syndrome with high serum GH-binding protein levels caused by a heterozygous splice site mutation of the GH receptor gene producing a lack of intracellular domain. J Clin Endocrinol Metab. 1998;83(2):531–537. [DOI] [PubMed] [Google Scholar]
- 65. Bang P, Polak M, Woelfle J, Houchard A; EU IGFD Registry Study Group . Effectiveness and safety of rhIGF-1 therapy in children: the European Increlex® growth forum database experience. Horm Res Paediatr. 2015;83(5):345–357. [DOI] [PubMed] [Google Scholar]
- 66. Ayling RM, Ross R, Towner P, Von Laue S, Finidori J, Moutoussamy S, Buchanan CR, Clayton PE, Norman MR. A dominant-negative mutation of the growth hormone receptor causes familial short stature. Nat Genet. 1997;16(1):13–14. [DOI] [PubMed] [Google Scholar]
- 67. Aisenberg J, Auyeung V, Pedro HF, Sugalski R, Chartoff A, Rothenberg R, Derr MA, Hwa V, Rosenfeld RG. Atypical GH insensitivity syndrome and severe insulin-like growth factor-I deficiency resulting from compound heterozygous mutations of the GH receptor, including a novel frameshift mutation affecting the intracellular domain. Horm Res Paediatr. 2010;74(6):406–411. [DOI] [PubMed] [Google Scholar]
- 68. Chernausek SD, Backeljauw PF, Frane J, Kuntze J, Underwood LE; GH Insensitivity Syndrome Collaborative Group . Long-term treatment with recombinant insulin-like growth factor (IGF)-I in children with severe IGF-I deficiency due to growth hormone insensitivity. J Clin Endocrinol Metab. 2007;92(3):902–910. [DOI] [PubMed] [Google Scholar]
- 69. Backeljauw PF, Miller BS, Dutailly P, Houchard A, Lawson E, Hale DE, Reiner B, Sperling MA; MS316 Study Group . Recombinant human growth hormone plus recombinant human insulin-like growth factor-1 coadministration therapy in short children with low insulin-like growth factor-1 and growth hormone sufficiency: results from a randomized, multicenter, open-label, parallel-group, active treatment-controlled trial. Horm Res Paediatr. 2015;83(4):268–279. [DOI] [PubMed] [Google Scholar]
- 70. Metherell LA, Akker SA, Munroe PB, Rose SJ, Caulfield M, Savage MO, Chew SL, Clark AJ. Pseudoexon activation as a novel mechanism for disease resulting in atypical growth-hormone insensitivity. Am J Hum Genet. 2001;69(3):641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Akker SA, Misra S, Aslam S, Morgan EL, Smith PJ, Khoo B, Chew SL. Pre-spliceosomal binding of U1 small nuclear ribonucleoprotein (RNP) and heterogenous nuclear RNP E1 is associated with suppression of a growth hormone receptor pseudoexon. Mol Endocrinol. 2007;21(10):2529–2540. [DOI] [PubMed] [Google Scholar]
- 72. Maamra M, Milward A, Esfahani HZ, Abbott LP, Metherell LA, Savage MO, Clark AJ, Ross RJA. A 36 residues insertion in the dimerization domain of the growth hormone receptor results in defective trafficking rather than impaired signaling. J Endocrinol. 2006;188(2):251–261. [DOI] [PubMed] [Google Scholar]
- 73. David A, Camacho-Hübner C, Bhangoo A, Rose SJ, Miraki-Moud F, Akker SA, Butler GE, Ten S, Clayton PE, Clark AJ, Savage MO, Metherell LA. An intronic growth hormone receptor mutation causing activation of a pseudoexon is associated with a broad spectrum of growth hormone insensitivity phenotypes. J Clin Endocrinol Metab. 2007;92(2):655–659. [DOI] [PubMed] [Google Scholar]
- 74. Chatterjee S, Shapiro L, Rose SJ, Mushtaq T, Clayton PE, Ten SB, Bhangoo A, Kumbattae U, Dias R, Savage MO, Metherell LA, Storr HL. Phenotypic spectrum and responses to recombinant human IGF1 (rhIGF1) therapy in patients with homozygous intronic pseudoexon growth hormone receptor mutation. Eur J Endocrinol. 2018;178(5):481–489. [DOI] [PubMed] [Google Scholar]
- 75. Savage MO, Attie KM, David A, Metherell LA, Clark AJ, Camacho-Hübner C. Endocrine assessment, molecular characterization and treatment of growth hormone insensitivity disorders. Nat Clin Pract Endocrinol Metab. 2006;2(7):395–407. [DOI] [PubMed] [Google Scholar]
- 76. Kurtoğlu S, Hatipoglu N. Growth hormone insensitivity: diagnostic and therapeutic approaches. J Endocrinol Invest. 2016;39(1):19–28. [DOI] [PubMed] [Google Scholar]
- 77. Burren CP, Woods KA, Rose SJ, Tauber M, Price DA, Heinrich U, Gilli G, Razzaghy-Azar M, Al-Ashwal A, Crock PA, Rochiccioli P, Yordam N, Ranke MB, Chatelain PG, Preece MA, Rosenfeld RG, Savage MO. Clinical and endocrine characteristics in atypical and classical growth hormone insensitivity syndrome. Horm Res. 2001;55(3):125–130. [DOI] [PubMed] [Google Scholar]
- 78. Zhu Q, Watanabe C, Liu T, Hollenbaugh D, Blaese RM, Kanner SB, Aruffo A, Ochs HD. Wiskott-Aldrich syndrome/X-linked thrombocytopenia: WASP gene mutations, protein expression, and phenotype. Blood. 1997;90(7):2680–2689. [PubMed] [Google Scholar]
- 79. Lemahieu V, Gastier JM, Francke U. Novel mutations in the Wiskott-Aldrich syndrome protein gene and their effects on transcriptional, translational, and clinical phenotypes. Hum Mutat. 1999;14(1):54–66. [DOI] [PubMed] [Google Scholar]
- 80. Laron Z, Klinger B, Erster B, Silbergeld A. Serum GH binding protein activities identifies the heterozygous carriers for Laron type dwarfism. Acta Endocrinol (Copenh). 1989;121(4):603–608. [DOI] [PubMed] [Google Scholar]
- 81. Rosenbloom AL, Guevara-Aguirre J, Berg MA, Francke U. Stature in Ecuadorians heterozygous for growth hormone receptor gene E180 splice mutation does not differ from that of homozygous normal relatives. J Clin Endocrinol Metab. 1998;83(7):2373–2375. [DOI] [PubMed] [Google Scholar]
- 82. Fielder PJ, Guevara-Aguirre J, Rosenbloom AL, Carlsson L, Hintz RL, Rosenfeld RG. Expression of serum insulin-like growth factors, insulin-like growth factor-binding proteins, and the growth hormone-binding protein in heterozygote relatives of Ecuadorian growth hormone receptor deficient patients. J Clin Endocrinol Metab. 1992;74(4):743–750. [DOI] [PubMed] [Google Scholar]
- 83. Rosenbloom AL, Guevara-Aguirre J, Rosenfeld RG, Fielder PJ. Is there heterozygote expression of growth hormone receptor deficiency? Acta Paediatr Suppl. 1994;83(S399):125–127. [DOI] [PubMed] [Google Scholar]
- 84. Guevara-Aguirre J, Rosenbloom AL, Guevara-Aguirre M, Yariz K, Saavedra J, Baumbach L, Shuster J. Effects of heterozygosity for the E180 splice mutation causing growth hormone receptor deficiency in Ecuador on IGF-I, IGFBP-3, and stature. Growth Horm IGF Res. 2007;17(3):261–264. [DOI] [PubMed] [Google Scholar]
- 85. Sjoberg M, Salazar T, Espinosa C, Dagnino A, Avila A, Eggers M, Cassorla F, Carvallo P, Mericq MV. Study of GH sensitivity in Chilean patients with idiopathic short stature. J Clin Endocrinol Metab. 2001;86(9):4375–4381. [DOI] [PubMed] [Google Scholar]
- 86. Goddard AD, Dowd P, Chernausek S, Geffner M, Gertner J, Hintz R, Hopwood N, Kaplan S, Plotnick L, Rogol A, Rosenfield R, Saenger P, Mauras N, Hershkopf R, Angulo M, Attie K. Partial growth-hormone insensitivity: the role of growth-hormone receptor mutations in idiopathic short stature. J Pediatr. 1997;131(1 Pt 2):S51–S55. [DOI] [PubMed] [Google Scholar]
- 87. Wit JM, Clayton PE, Rogol AD, Savage MO, Saenger PH, Cohen P. Idiopathic short stature: definition, epidemiology, and diagnostic evaluation. Growth Horm IGF Res. 2008;18(2):89–110. [DOI] [PubMed] [Google Scholar]
- 88. Cohen P, Rogol AD, Deal CL, Saenger P, Reiter EO, Ross JL, Chernausek SD, Savage MO, Wit JM; 2007 ISS Consensus Workshop participants . Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008;93(11):4210–4217. [DOI] [PubMed] [Google Scholar]
- 89. Attie KM, Carlsson LM, Rundle AC, Sherman BM. Evidence for partial growth hormone insensitivity among patients with idiopathic short stature. The National Cooperative Growth Study. J Pediatr. 1995;127(2):244–250. [DOI] [PubMed] [Google Scholar]
- 90. Pedicelli S, Peschiaroli E, Violi E, Cianfarani S. Controversies in the definition and treatment of idiopathic short stature (ISS). J Clin Res Pediatr Endocrinol. 2009;1(3):105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Amselem S, Sobrier ML, Dastot F, Duquesnoy P, Duriez B, Goossens M. Molecular basis of inherited growth hormone resistance in childhood. Baillieres Clin Endocrinol Metab. 1996;10(3):353–369. [DOI] [PubMed] [Google Scholar]
- 92. Carlsson LM, Attie KM, Compton PG, Vitangcol RV, Merimee TJ. Reduced concentration of serum growth hormone-binding protein in children with idiopathic short stature. National Cooperative Growth Study. J Clin Endocrinol Metab. 1994;78(6):1325–1330. [DOI] [PubMed] [Google Scholar]
- 93. Sanchez JE, Perera E, Baumbach L, Cleveland WW. Growth hormone receptor mutations in children with idiopathic short stature. J Clin Endocrinol Metab. 1998;83(11):4079–4083. [DOI] [PubMed] [Google Scholar]
- 94. Bonioli E, Tarò M, Rosa CL, Citana A, Bertorelli R, Morcaldi G, Gastaldi R, Coviello DA. Heterozygous mutations of growth hormone receptor gene in children with idiopathic short stature. Growth Horm IGF Res. 2005;15(6):405–410. [DOI] [PubMed] [Google Scholar]
- 95. El Kholy M, Mella P, Rashad M, Buzi F, Meazza C, Zahra S, Elsedfy HH. Growth hormone/IGF-I axis and growth hormone receptor mutations in idiopathic short stature. Horm Res Paediatr. 2011;76(5):300–306. [DOI] [PubMed] [Google Scholar]
- 96. Hujeirat Y, Hess O, Shalev S, Tenenbaum-Rakover Y. Growth hormone receptor sequence changes do not play a role in determining height in children with idiopathic short stature. Horm Res. 2006;65(4):210–216. [DOI] [PubMed] [Google Scholar]
- 97. Goddard AD, Covello R, Luoh SM, Clackson T, Attie KM, Gesundheit N, Rundle AC, Wells JA, Carlsson LM; The Growth Hormone Insensitivity Study Group . Mutations of the growth hormone receptor in children with idiopathic short stature. N Engl J Med. 1995;333(17):1093–1098. [DOI] [PubMed] [Google Scholar]
- 98. Levy DE, Darnell JE Jr. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3(9):651–662. [DOI] [PubMed] [Google Scholar]
- 99. Rosenfeld RG, Hwa V. The growth hormone cascade and its role in mammalian growth. Horm Res. 2009;71(Suppl 2):36–40. [DOI] [PubMed] [Google Scholar]
- 100. Hwa V, Little B, Adiyaman P, Kofoed EM, Pratt KL, Ocal G, Berberoglu M, Rosenfeld RG. Severe growth hormone insensitivity resulting from total absence of signal transducer and activator of transcription 5b. J Clin Endocrinol Metab. 2005;90(7):4260–4266. [DOI] [PubMed] [Google Scholar]
- 101. Bernasconi A, Marino R, Ribas A, Rossi J, Ciaccio M, Oleastro M, Ornani A, Paz R, Rivarola MA, Zelazko M, Belgorosky A. Characterization of immunodeficiency in a patient with growth hormone insensitivity secondary to a novel STAT5b gene mutation. Pediatrics. 2006;118(5):e1584–e1592. [DOI] [PubMed] [Google Scholar]
- 102. Boyanovsky A, Lozano A, Testa G, Munoz L, Marino R, Bernasconi A, Belgorosky A, Miras M Growth hormone insensitivity and immunodeficiency: mutation in the STAT5B gene. 8th Joint Meeting of the Lawson Wilkins Pediatric Endocrine Society/European Society for Paediatric Endocrinology; 9-12 September 2009; New York, NY. Abstract P01-P67. [Google Scholar]
- 103. Vidarsdottir S, Walenkamp MJ, Pereira AM, Karperien M, van Doorn J, van Duyvenvoorde HA, White S, Breuning MH, Roelfsema F, Kruithof MF, van Dissel J, Janssen R, Wit JM, Romijn JA. Clinical and biochemical characteristics of a male patient with a novel homozygous STAT5b mutation. J Clin Endocrinol Metab. 2006;91(9):3482–3485. [DOI] [PubMed] [Google Scholar]
- 104. Hwa V, Camacho-Hübner C, Little BM, David A, Metherell LA, El-Khatib N, Savage MO, Rosenfeld RG. Growth hormone insensitivity and severe short stature in siblings: a novel mutation at the exon 13-intron 13 junction of the STAT5b gene. Horm Res. 2007;68(5):218–224. [DOI] [PubMed] [Google Scholar]
- 105. Pugliese-Pires PN, Tonelli CA, Dora JM, Silva PCA, Czepielewski M, Simoni G, Arnhold IJ, Jorge AAL. A novel STAT5B mutation causing GH insensitivity syndrome associated with hyperprolactinemia and immune dysfunction in two male siblings. Eur J Endocrinol. 2010;163(2):349–355. [DOI] [PubMed] [Google Scholar]
- 106. Scaglia PA, Martínez AS, Feigerlová E, Bezrodnik L, Gaillard MI, Di Giovanni D, Ballerini MG, Jasper HG, Heinrich JJ, Fang P, Domené HM, Rosenfeld RG, Hwa V. A novel missense mutation in the SH2 domain of the STAT5B gene results in a transcriptionally inactive STAT5b associated with severe IGF-I deficiency, immune dysfunction, and lack of pulmonary disease. J Clin Endocrinol Metab. 2012;97(5):E830–E839. [DOI] [PubMed] [Google Scholar]
- 107. Hwa V. STAT5B deficiency: Impacts on human growth and immunity. Growth Horm IGF Res. 2016;28:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Casanova J-L, Holland SM, Notarangelo LD. Inborn errors of human JAKs and STATs. Immunity. 2012;36(4):515–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368(2):161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hambleton S, Goodbourn S, Young DF, Dickinson P, Mohamad SM, Valappil M, McGovern N, Cant AJ, Hackett SJ, Ghazal P, Morgan NV, Randall RE. STAT2 deficiency and susceptibility to viral illness in humans. Proc Natl Acad Sci USA. 2013;110(8):3053–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Yildiz M, Li H, Bernard D, Amin NA, Ouillette P, Jones S, Saiya-Cork K, Parkin B, Jacobi K, Shedden K, Wang S, Chang AE, Kaminski MS, Malek SN. Activating STAT6 mutations in follicular lymphoma. Blood. 2015;125(4):668–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cohen AC, Nadeau KC, Tu W, Hwa V, Dionis K, Bezrodnik L, Teper A, Gaillard M, Heinrich J, Krensky AM, Rosenfeld RG, Lewis DB. Cutting edge: Decreased accumulation and regulatory function of CD4+CD25high T cells in human STAT5b deficiency. J Immunol. 2006;177(5):2770–2774. [DOI] [PubMed] [Google Scholar]
- 113. Flanagan SE, Haapaniemi E, Russell MA, Caswell R, Allen HL, De Franco E, McDonald TJ, Rajala H, Ramelius A, Barton J, Heiskanen K, Heiskanen-Kosma T, Kajosaari M, Murphy NP, Milenkovic T, Seppänen M, Lernmark Å, Mustjoki S, Otonkoski T, Kere J, Morgan NG, Ellard S, Hattersley AT. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat Genet. 2014;46(8):812–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Milner JD, Vogel TP, Forbes L, Ma CA, Stray-Pedersen A, Niemela JE, Lyons JJ, Engelhardt KR, Zhang Y, Topcagic N, Roberson ED, Matthews H, Verbsky JW, Dasu T, Vargas-Hernandez A, Varghese N, McClain KL, Karam LB, Nahmod K, Makedonas G, Mace EM, Sorte HS, Perminow G, Rao VK, O’Connell MP, Price S, Su HC, Butrick M, McElwee J, Hughes JD, Willet J, Swan D, Xu Y, Santibanez-Koref M, Slowik V, Dinwiddie DL, Ciaccio CE, Saunders CJ, Septer S, Kingsmore SF, White AJ, Cant AJ, Hambleton S, Cooper MA. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood. 2015;125(4):591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Haapaniemi EM, Kaustio M, Rajala HL, van Adrichem AJ, Kainulainen L, Glumoff V, Doffinger R, Kuusanmäki H, Heiskanen-Kosma T, Trotta L, Chiang S, Kulmala P, Eldfors S, Katainen R, Siitonen S, Karjalainen-Lindsberg ML, Kovanen PE, Otonkoski T, Porkka K, Heiskanen K, Hänninen A, Bryceson YT, Uusitalo-Seppälä R, Saarela J, Seppänen M, Mustjoki S, Kere J. Autoimmunity, hypogammaglobulinemia, lymphoproliferation, and mycobacterial disease in patients with activating mutations in STAT3. Blood. 2015;125(4):639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gutiérrez M, Scaglia P, Keselman A, Martucci L, Karabatas L, Domené S, Martin A, Pennisi P, Blanco M, Sanguineti N, Bezrodnik L, Di Giovanni D, Caldirola MS, Azcoiti ME, Gaillard MI, Denson LA, Zhang K, Husami A, Yayah Jones NH, Hwa V, Revale S, Vázquez M, Jasper H, Kumar A, Domené H. Partial growth hormone insensitivity and dysregulatory immune disease associated with de novo germline activating STAT3 mutations. Mol Cell Endocrinol. 2018;473:166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Sediva H, Dusatkova P, Kanderova V, Obermannova B, Kayserova J, Sramkova L, Zemkova D, Elblova L, Svaton M, Zachova R, Kolouskova S, Fronkova E, Sumnik Z, Sediva A, Lebl J, Pruhova S. Short stature in a boy with multiple early-onset autoimmune conditions due to a STAT3 activating mutation: could intracellular growth hormone signalling be compromised?. Horm Res Paediatr. 2017;88(2):160–166. [DOI] [PubMed] [Google Scholar]
- 118. Varco-Merth B, Feigerlová E, Shinde U, Rosenfeld RG, Hwa V, Rotwein P. Severe growth deficiency is associated with STAT5b mutations that disrupt protein folding and activity. Mol Endocrinol. 2013;27(1):150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Walenkamp MJE, Vidarsdottir S, Pereira AM, Karperien M, van Doorn J, van Duyvenvoorde HA, Breuning MH, Roelfsema F, Kruithof MF, van Dissel J, Janssen R, Wit JM, Romijn JA. Growth hormone secretion and immunological function of a male patient with a homozygous STAT5b mutation. Eur J Endocrinol. 2007;156(2):155–165. [DOI] [PubMed] [Google Scholar]
- 120. Rubinstein A, Bernstein LJ, Charytan M, Krieger BZ, Ziprkowski M. Corticosteroid treatment for pulmonary lymphoid hyperplasia in children with the acquired immune deficiency syndrome. Pediatr Pulmonol. 1988;4(1):13–17. [DOI] [PubMed] [Google Scholar]
- 121. Bezrodnik L, Di Giovanni D, Caldirola MS, Azcoiti ME, Torgerson T, Gaillard MI. Long-term follow-up of STAT5B deficiency in three argentinian patients: clinical and immunological features. J Clin Immunol. 2015;35(3):264–272. [DOI] [PubMed] [Google Scholar]
- 122. Klammt J, Neumann D, Gevers EF, Andrew SF, Schwartz ID, Rockstroh D, Colombo R, Sanchez MA, Vokurkova D, Kowalczyk J, Metherell LA, Rosenfeld RG, Pfäffle R, Dattani MT, Dauber A, Hwa V. Dominant-negative STAT5B mutations cause growth hormone insensitivity with short stature and mild immune dysregulation. Nat Commun. 2018;9(1):2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Shin HY, Reich NC. Dynamic trafficking of STAT5 depends on an unconventional nuclear localization signal. J Cell Sci. 2013;126(Pt 15):3333–3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, Freeman AF, Demidowich A, Davis J, Turner ML, Anderson VL, Darnell DN, Welch PA, Kuhns DB, Frucht DM, Malech HL, Gallin JI, Kobayashi SD, Whitney AR, Voyich JM, Musser JM, Woellner C, Schäffer AA, Puck JM, Grimbacher B. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357(16):1608–1619. [DOI] [PubMed] [Google Scholar]
- 125. Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, Metin A, Karasuyama H. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448(7157):1058–1062. [DOI] [PubMed] [Google Scholar]
- 126. Renner ED, Rylaarsdam S, Anover-Sombke S, Rack AL, Reichenbach J, Carey JC, Zhu Q, Jansson AF, Barboza J, Schimke LF, Leppert MF, Getz MM, Seger RA, Hill HR, Belohradsky BH, Torgerson TR, Ochs HD. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced TH17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J Allergy Clin Immunol. 2008;122(1):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Schimke LF, Sawalle-Belohradsky J, Roesler J, Wollenberg A, Rack A, Borte M, Rieber N, Cremer R, Maass E, Dopfer R, Reichenbach J, Wahn V, Hoenig M, Jansson AF, Roesen-Wolff A, Schaub B, Seger R, Hill HR, Ochs HD, Torgerson TR, Belohradsky BH, Renner ED. Diagnostic approach to the hyper-IgE syndromes: immunologic and clinical key findings to differentiate hyper-IgE syndromes from atopic dermatitis. J Allergy Clin Immunol. 2010;126(3):611–617.e1. [DOI] [PubMed]
- 128. Iida K, Takahashi Y, Kaji H, Takahashi MO, Okimura Y, Nose O, Abe H, Chihara K. Functional characterization of truncated growth hormone (GH) receptor-(1–277) causing partial GH insensitivity syndrome with high GH-binding protein. J Clin Endocrinol Metab. 1999;84(3):1011–1016. [DOI] [PubMed] [Google Scholar]
- 129. Takagi M, Shinohara H, Nagashima Y, Hasegawa Y, Narumi S, Hasegawa T. A novel dominant negative mutation in the intracellular domain of GHR is associated with growth hormone insensitivity. Clin Endocrinol (Oxf). 2016;85(4):669–671. [DOI] [PubMed] [Google Scholar]
- 130. Scalco RC, Hwa V, Domené HM, Jasper HG, Belgorosky A, Marino R, Pereira AM, Tonelli CA, Wit JM, Rosenfeld RG, Jorge AA. STAT5B mutations in heterozygous state have negative impact on height: another clue in human stature heritability. Eur J Endocrinol. 2015;173(3):291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Jenks JA, Seki S, Kanai T, Huang J, Morgan AA, Scalco RC, Nath R, Bucayu R, Wit JM, Al-Herz W, Ramadan D, Jorge AA, Bacchetta R, Hwa V, Rosenfeld R, Nadeau KC. Differentiating the roles of STAT5B and STAT5A in human CD4+ T cells. Clin Immunol. 2013;148(2):227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Nadeau K, Hwa V, Rosenfeld RG. STAT5b deficiency: an unsuspected cause of growth failure, immunodeficiency, and severe pulmonary disease. J Pediatr. 2011;158(5):701–708. [DOI] [PubMed] [Google Scholar]
- 133. Majri SS, Fritz JM, Villarino AV, Zheng L, Kanellopoulou C, Chaigne-Delalande B, Grönholm J, Niemela JE, Afzali B, Biancalana M, Pittaluga S, Sun A, Cohen JL, Holland SM, O’Shea JJ, Uzel G, Lenardo MJ. STAT5B: a differential regulator of the life and death of CD4+ effector memory T cells. J Immunol. 2018;200(1):110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wit JM, van Duyvenvoorde HA, Scheltinga SA, de Bruin S, Hafkenscheid L, Kant SG, Ruivenkamp CA, Gijsbers AC, van Doorn J, Feigerlova E, Noordam C, Walenkamp MJ, Claahsen-van de Grinten H, Stouthart P, Bonapart IE, Pereira AM, Gosen J, Delemarre-van de Waal HA, Hwa V, Breuning MH, Domené HM, Oostdijk W, Losekoot M. Genetic analysis of short children with apparent growth hormone insensitivity. Horm Res Paediatr. 2012;77(5):320–333. [DOI] [PubMed] [Google Scholar]
- 135. Walenkamp MJ, Klammt J, Feigerlova E, Losekoot M, van Duyvenvoorde HA, Hwa V, Pfäffle R, Wit JM. Genetic analysis of GHR should contain sequencing of all coding exons and specific intron sequences, and screening for exon deletions. Horm Res Paediatr. 2013;80(6):406–412. [DOI] [PubMed] [Google Scholar]
- 136. Kiel MJ, Sahasrabuddhe AA, Rolland DC, Velusamy T, Chung F, Schaller M, Bailey NG, Betz BL, Miranda RN, Porcu P, Byrd JC, Medeiros LJ, Kunkel SL, Bahler DW, Lim MS, Elenitoba-Johnson KS. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. 2015;6(1):8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Küçük C, Jiang B, Hu X, Zhang W, Chan JK, Xiao W, Lack N, Alkan C, Williams JC, Avery KN, Kavak P, Scuto A, Sen E, Gaulard P, Staudt L, Iqbal J, Zhang W, Cornish A, Gong Q, Yang Q, Sun H, d’Amore F, Leppä S, Liu W, Fu K, de Leval L, McKeithan T, Chan WC. Activating mutations of STAT5B and STAT3 in lymphomas derived from γδ-T or NK cells. Nat Commun. 2015;6(1):6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Jiang L, Gu ZH, Yan ZX, Zhao X, Xie YY, Zhang ZG, Pan CM, Hu Y, Cai CP, Dong Y, Huang JY, Wang L, Shen Y, Meng G, Zhou JF, Hu JD, Wang JF, Liu YH, Yang LH, Zhang F, Wang JM, Wang Z, Peng ZG, Chen FY, Sun ZM, Ding H, Shi JM, Hou J, Yan JS, Shi JY, Xu L, Li Y, Lu J, Zheng Z, Xue W, Zhao WL, Chen Z, Chen SJ. Exome sequencing identifies somatic mutations of DDX3X in natural killer/T-cell lymphoma. Nat Genet. 2015;47(9):1061–1066. [DOI] [PubMed] [Google Scholar]
- 139. Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol. 2001;229(1):141–162. [DOI] [PubMed] [Google Scholar]
- 140. Bonapace G, Concolino D, Formicola S, Strisciuglio P. A novel mutation in a patient with insulin-like growth factor 1 (IGF1) deficiency. J Med Genet. 2003;40(12):913–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Camacho-Hübner C, Woods KA, Miraki-Moud F, Hindmarsh PC, Clark AJ, Hansson Y, Johnston A, Baxter RC, Savage MO. Effects of recombinant human insulin-like growth factor I (IGF-I) therapy on the growth hormone-IGF system of a patient with a partial IGF-I gene deletion. J Clin Endocrinol Metab. 1999;84(5):1611–1616. [DOI] [PubMed] [Google Scholar]
- 142. Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart TA. IGF-I is required for normal embryonic growth in mice. Genes Dev. 1993;7(12B):2609–2617. [DOI] [PubMed] [Google Scholar]
- 143. van Duyvenvoorde HA, van Setten PA, Walenkamp MJ, van Doorn J, Koenig J, Gauguin L, Oostdijk W, Ruivenkamp CA, Losekoot M, Wade JD, De Meyts P, Karperien M, Noordam C, Wit JM. Short stature associated with a novel heterozygous mutation in the insulin-like growth factor 1 gene. J Clin Endocrinol Metab. 2010;95(11):E363–E367. [DOI] [PubMed] [Google Scholar]
- 144. Fuqua JS, Derr M, Rosenfeld RG, Hwa V. Identification of a novel heterozygous IGF1 splicing mutation in a large kindred with familial short stature. Horm Res Paediatr. 2012;78(1):59–66. [DOI] [PubMed] [Google Scholar]
- 145. Batey L, Moon JE, Yu Y, Wu B, Hirschhorn JN, Shen Y, Dauber A. A novel deletion of IGF1 in a patient with idiopathic short stature provides insight Into IGF1 haploinsufficiency. J Clin Endocrinol Metab. 2014;99(1):E153–E159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. DeChiara TM, Efstratiadis A, Robertson EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345(6270):78–80. [DOI] [PubMed] [Google Scholar]
- 147. DeChiara TM, Robertson EJ, Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991;64(4):849–859. [DOI] [PubMed] [Google Scholar]
- 148. Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993;75(1):59–72. [PubMed] [Google Scholar]
- 149. Netchine I, Rossignol S, Dufourg MN, Azzi S, Rousseau A, Perin L, Houang M, Steunou V, Esteva B, Thibaud N, Demay MC, Danton F, Petriczko E, Bertrand AM, Heinrichs C, Carel JC, Loeuille GA, Pinto G, Jacquemont ML, Gicquel C, Cabrol S, Le Bouc Y. 11p15 Imprinting center region 1 loss of methylation is a common and specific cause of typical Russell-Silver syndrome: clinical scoring system and epigenetic-phenotypic correlations. J Clin Endocrinol Metab. 2007;92(8):3148–3154. [DOI] [PubMed] [Google Scholar]
- 150. Kerns SL, Guevara-Aguirre J, Andrew S, Geng J, Guevara C, Guevara-Aguirre M, Guo M, Oddoux C, Shen Y, Zurita A, Rosenfeld RG, Ostrer H, Hwa V, Dauber A. A novel variant in CDKN1C is associated with intrauterine growth restriction, short stature, and early-adulthood-onset diabetes. J Clin Endocrinol Metab. 2014;99(10):E2117–E2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Begemann M, Zirn B, Santen G, Wirthgen E, Soellner L, Büttel HM, Schweizer R, van Workum W, Binder G, Eggermann T. Paternally inherited IGF2 mutation and growth restriction. N Engl J Med. 2015;373(4):349–356. [DOI] [PubMed] [Google Scholar]
- 152. Yamoto K, Saitsu H, Nakagawa N, Nakajima H, Hasegawa T, Fujisawa Y, Kagami M, Fukami M, Ogata T. De novo IGF2 mutation on the paternal allele in a patient with Silver-Russell syndrome and ectrodactyly. Hum Mutat. 2017;38(8):953–958. [DOI] [PubMed] [Google Scholar]
- 153. Liu D, Wang Y, Yang XA, Liu D. De novo mutation of paternal IGF2 gene causing Silver-Russell syndrome in a sporadic patient. Front Genet. 2017;8:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Domené HM, Martínez AS, Frystyk J, Bengolea SV, Ropelato MG, Scaglia PA, Chen JW, Heuck C, Wolthers OD, Heinrich JJ, Jasper HG. Normal growth spurt and final height despite low levels of all forms of circulating insulin-like growth factor-I in a patient with acid-labile subunit deficiency. Horm Res. 2007;67(5):243–249. [DOI] [PubMed] [Google Scholar]
- 155. Işık E, Haliloglu B, van Doorn J, Demirbilek H, Scheltinga SA, Losekoot M, Wit JM. Clinical and biochemical characteristics and bone mineral density of homozygous, compound heterozygous and heterozygous carriers of three novel IGFALS mutations. Eur J Endocrinol. 2017;176(6):657–667. [DOI] [PubMed] [Google Scholar]
- 156. Leong SR, Baxter RC, Camerato T, Dai J, Wood WI. Structure and functional expression of the acid-labile subunit of the insulin-like growth factor-binding protein complex. Mol Endocrinol. 1992;6(6):870–876. [DOI] [PubMed] [Google Scholar]
- 157. Boisclair YR, Rhoads RP, Ueki I, Wang J, Ooi GT. The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: an important but forgotten component of the circulating IGF system. J Endocrinol. 2001;170(1):63–70. [DOI] [PubMed] [Google Scholar]
- 158. Heath KE, Argente J, Barrios V, Pozo J, Díaz-González F, Martos-Moreno GA, Caimari M, Gracia R, Campos-Barros A. Primary acid-labile subunit deficiency due to recessive IGFALS mutations results in postnatal growth deficit associated with low circulating insulin growth factor (IGF)-I, IGF binding protein-3 levels, and hyperinsulinemia. J Clin Endocrinol Metab. 2008;93(5):1616–1624. [DOI] [PubMed] [Google Scholar]
- 159. Ueki I, Ooi GT, Tremblay ML, Hurst KR, Bach LA, Boisclair YR. Inactivation of the acid labile subunit gene in mice results in mild retardation of postnatal growth despite profound disruptions in the circulating insulin-like growth factor system. Proc Natl Acad Sci USA. 2000;97(12):6868–6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Grandone A, Miraglia del Giudice E, Cirillo G, Abbondanza C, Cioffi M, Romano T, Micillo F, Marzuillo P, Perrone L. Clinical features of a new acid-labile subunit (IGFALS) heterozygous mutation: anthropometric and biochemical characterization and response to growth hormone administration. Horm Res Paediatr. 2014;81(1):67–72. [DOI] [PubMed] [Google Scholar]
- 161. Fofanova-Gambetti OV, Hwa V, Wit JM, Domene HM, Argente J, Bang P, Högler W, Kirsch S, Pihoker C, Chiu HK, Cohen L, Jacobsen C, Jasper HG, Haeusler G, Campos-Barros A, Gallego-Gómez E, Gracia-Bouthelier R, van Duyvenvoorde HA, Pozo J, Rosenfeld RG. Impact of heterozygosity for acid-labile subunit (IGFALS) gene mutations on stature: results from the international acid-labile subunit consortium. J Clin Endocrinol Metab. 2010;95(9):4184–4191. [DOI] [PubMed] [Google Scholar]
- 162. van Duyvenvoorde HA, Kempers MJ, Twickler TB, van Doorn J, Gerver WJ, Noordam C, Losekoot M, Karperien M, Wit JM, Hermus AR. Homozygous and heterozygous expression of a novel mutation of the acid-labile subunit. Eur J Endocrinol. 2008;159(2):113–120. [DOI] [PubMed] [Google Scholar]
- 163. Domené HM, Scaglia PA, Martínez AS, Keselman AC, Karabatas LM, Pipman VR, Bengolea SV, Guida MC, Ropelato MG, Ballerini MG, Lescano EM, Blanco MA, Heinrich JJ, Rey RA, Jasper HG. Heterozygous IGFALS gene variants in idiopathic short stature and normal children: impact on height and the IGF system. Horm Res Paediatr. 2013;80(6):413–423. [DOI] [PubMed] [Google Scholar]
- 164. Storr HL, Prasad R, Temple IK, Metherell LA, Savage MO, Walker JM. Heterogeneity of the growth phenotype and birth size in acid-labile subunit (ALS) deficiency. J Endocrinol Invest. 2015;38(4):407–412. [DOI] [PubMed] [Google Scholar]
- 165. Overgaard MT, Haaning J, Boldt HB, Olsen IM, Laursen LS, Christiansen M, Gleich GJ, Sottrup-Jensen L, Conover CA, Oxvig C. Expression of recombinant human pregnancy-associated plasma protein-A and identification of the proform of eosinophil major basic protein as its physiological inhibitor. J Biol Chem. 2000;275(40):31128–31133. [DOI] [PubMed] [Google Scholar]
- 166. Oxvig C. The role of PAPP-A in the IGF system: location, location, location. J Cell Commun Signal. 2015;9(2):177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Conover CA, Boldt HB, Bale LK, Clifton KB, Grell JA, Mader JR, Mason EJ, Powell DR. Pregnancy-associated plasma protein-A2 (PAPP-A2): tissue expression and biological consequences of gene knockout in mice. Endocrinology. 2011;152(7):2837–2844. [DOI] [PubMed] [Google Scholar]
- 168. Wang J, Qiu Q, Haider M, Bell M, Gruslin A, Christians JK. Expression of pregnancy-associated plasma protein A2 during pregnancy in human and mouse. J Endocrinol. 2009;202(3):337–345. [DOI] [PubMed] [Google Scholar]
- 169. Cabrera-Salcedo C, Mizuno T, Tyzinski L, Andrew M, Vinks AA, Frystyk J, Wasserman H, Gordon CM, Hwa V, Backeljauw P, Dauber A. Pharmacokinetics of IGF-1 in PAPP-A2-deficient patients, growth response, and effects on glucose and bone density. J Clin Endocrinol Metab. 2017;102(12):4568–4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Hawkins-Carranza FG, Muñoz-Calvo MT, Martos-Moreno GA, Allo-Miguel G, Del Río L, Pozo J, Chowen JA, Pérez-Jurado LA, Argente J. rhIGF-1 treatment increases bone mineral density and trabecular bone structure in children with PAPP-A2 deficiency. Horm Res Paediatr. 2018;89(3):200–204. [DOI] [PubMed] [Google Scholar]
- 171. Argente J, Perez-Jurado LA. Letter to the Editor: History and clinical implications of PAPP-A2 in human growth: when reflecting on idiopathic short stature leads to a specific and new diagnosis: understanding the concept of “low IGF-I availability.” Growth Horm IGF Res. 2018;40:17–19. [DOI] [PubMed] [Google Scholar]
- 172. Muñoz-Calvo MT, Barrios V, Pozo J, Chowen JA, Martos-Moreno GA, Hawkins F, Dauber A, Domené HM, Yakar S, Rosenfeld RG, Pérez-Jurado LA, Oxvig C, Frystyk J, Argente J. treatment with recombinant human insulin-like growth factor-1 improves growth in patients with PAPP-A2 Deficiency. J Clin Endocrinol Metab. 2016;101(11):3879–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Lango Allen H, Estrada K, Lettre G, Berndt SI, Weedon MN, Rivadeneira F, Willer CJ, Jackson AU, Vedantam S, Raychaudhuri S, Ferreira T, Wood AR, Weyant RJ, Segrè AV, Speliotes EK, Wheeler E, Soranzo N, Park JH, Yang J, Gudbjartsson D, Heard-Costa NL, Randall JC, Qi L, Vernon Smith A, Mägi R, Pastinen T, Liang L, Heid IM, Luan J, Thorleifsson G, Winkler TW, Goddard ME, Sin Lo K, Palmer C, Workalemahu T, Aulchenko YS, Johansson A, Zillikens MC, Feitosa MF, Esko T, Johnson T, Ketkar S, Kraft P, Mangino M, Prokopenko I, Absher D, Albrecht E, Ernst F, Glazer NL, Hayward C, Hottenga JJ, Jacobs KB, Knowles JW, Kutalik Z, Monda KL, Polasek O, Preuss M, Rayner NW, Robertson NR, Steinthorsdottir V, Tyrer JP, Voight BF, Wiklund F, Xu J, Zhao JH, Nyholt DR, Pellikka N, Perola M, Perry JR, Surakka I, Tammesoo ML, Altmaier EL, Amin N, Aspelund T, Bhangale T, Boucher G, Chasman DI, Chen C, Coin L, Cooper MN, Dixon AL, Gibson Q, Grundberg E, Hao K, Juhani Junttila M, Kaplan LM, Kettunen J, Konig IR, Kwan T, Lawrence RW, Levinson DF, Lorentzon M, McKnight B, Morris AP, Müller M, Suh Ngwa J, Purcell S, Rafelt S, Salem RM, Salvi E, Sanna S, Shi J, Sovio U, Thompson JR, Turchin MC, Vandenput L, Verlaan DJ, Vitart V, White CC, Ziegler A, Almgren P, Balmforth AJ, Campbell H, Citterio L, De Grandi A, Dominiczak A, Duan J, Elliott P, Elosua R, Eriksson JG, Freimer NB, Geus EJ, Glorioso N, Haiqing S, Hartikainen AL, Havulinna AS, Hicks AA, Hui J, Igl W, Illig T, Jula A, Kajantie E, Kilpeläinen TO, Koiranen M, Kolcic I, Koskinen S, Kovacs P, Laitinen J, Liu J, Lokki ML, Marusic A, Maschio A, Meitinger T, Mulas A, Paré G, Parker AN, Peden JF, Petersmann A, Pichler I, Pietilainen KH, Pouta A, Ridderstrale M, Rotter JI, Sambrook JG, Sanders AR, Schmidt CO, Sinisalo J, Smit JH, Stringham HM, Bragi Walters G, Widen E, Wild SH, Willemsen G, Zagato L, Zgaga L, Zitting P, Alavere H, Farrall M, McArdle WL, Nelis M, Peters MJ, Ripatti S, van Meurs JB, Aben KK, Ardlie KG, Beckmann JS, Beilby JP, Bergman RN, Bergmann S, Collins FS, Cusi D, den Heijer M, Eiriksdottir G, Gejman PV, Hall AS, Hamsten A, Huikuri HV, Iribarren C, Kähönen M, Kaprio J, Kathiresan S, Kiemeney L, Kocher T, Launer LJ, Lehtimäki T, Melander O, Mosley TH Jr, Musk AW, Nieminen MS, O’Donnell CJ, Ohlsson C, Oostra B, Palmer LJ, Raitakari O, Ridker PM, Rioux JD, Rissanen A, Rivolta C, Schunkert H, Shuldiner AR, Siscovick DS, Stumvoll M, Tonjes A, Tuomilehto J, van Ommen GJ, Viikari J, Heath AC, Martin NG, Montgomery GW, Province MA, Kayser M, Arnold AM, Atwood LD, Boerwinkle E, Chanock SJ, Deloukas P, Gieger C, Grönberg H, Hall P, Hattersley AT, Hengstenberg C, Hoffman W, Lathrop GM, Salomaa V, Schreiber S, Uda M, Waterworth D, Wright AF, Assimes TL, Barroso I, Hofman A, Mohlke KL, Boomsma DI, Caulfield MJ, Cupples LA, Erdmann J, Fox CS, Gudnason V, Gyllensten U, Harris TB, Hayes RB, Jarvelin MR, Mooser V, Munroe PB, Ouwehand WH, Penninx BW, Pramstaller PP, Quertermous T, Rudan I, Samani NJ, Spector TD, Volzke H, Watkins H, Wilson JF, Groop LC, Haritunians T, Hu FB, Kaplan RC, Metspalu A, North KE, Schlessinger D, Wareham NJ, Hunter DJ, O’Connell JR, Strachan DP, Wichmann HE, Borecki IB, van Duijn CM, Schadt EE, Thorsteinsdottir U, Peltonen L, Uitterlinden AG, Visscher PM, Chatterjee N, Loos RJ, Boehnke M, McCarthy MI, Ingelsson E, Lindgren CM, Abecasis GR, Stefansson K, Frayling TM, Hirschhorn JN. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467(7317):832–838.
- 174. Murray PG, Clayton PE, Chernausek SD. A genetic approach to evaluation of short stature of undetermined cause. Lancet Diabetes Endocrinol. 2018;6(7):564–574. [DOI] [PubMed] [Google Scholar]
- 175. Grimberg A, DiVall SA, Polychronakos C, Allen DB, Cohen LE, Quintos JB, Rossi WC, Feudtner C, Murad MH; Drug and Therapeutics Committee and Ethics Committee of the Pediatric Endocrine Society . Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm Res Paediatr. 2016;86(6):361–397. [DOI] [PubMed] [Google Scholar]
- 176. Wit JM. Definition and subcategorization of idiopathic short stature: between consensus and controversy. Horm Res Paediatr. 2011;76(Suppl 3):3–6. [DOI] [PubMed] [Google Scholar]
- 177. Rosenbloom AL. Idiopathic short stature: conundrums of definition and treatment. Int J Pediatr Endocrinol. 2009;2009(1):470378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Wit JM, Reiter EO, Ross JL, Saenger PH, Savage MO, Rogol AD, Cohen P. Idiopathic short stature: management and growth hormone treatment. Growth Horm IGF Res. 2008;18(2):111–135. [DOI] [PubMed] [Google Scholar]
- 179. Guo MH, Shen Y, Walvoord EC, Miller TC, Moon JE, Hirschhorn JN, Dauber A. Whole exome sequencing to identify genetic causes of short stature. Horm Res Paediatr. 2014;82(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. de Bruin C, Dauber A. Genomic insights into growth and its disorders: an update. Curr Opin Endocrinol Diabetes Obes. 2016;23(1):51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Savage MO, Burren CP, Blair JC, Woods KA, Metherell L, Clark AJ, Camacho-Hübner C. Growth hormone insensitivity: pathophysiology, diagnosis, clinical variation and future perspectives. Horm Res. 2001;55(Suppl 2):32–35. [DOI] [PubMed] [Google Scholar]
- 182. Hintz RL, Attie KM, Baptista J, Roche A; Genentech Collaborative Group . Effect of growth hormone treatment on adult height of children with idiopathic short stature. N Engl J Med. 1999;340(7):502–507. [DOI] [PubMed] [Google Scholar]
- 183. Cohen P, Rogol AD, Howard CP, Bright GM, Kappelgaard AM, Rosenfeld RG; American Norditropin Study Group . Insulin growth factor-based dosing of growth hormone therapy in children: a randomized, controlled study. J Clin Endocrinol Metab. 2007;92(7):2480–2486. [DOI] [PubMed] [Google Scholar]
- 184. Derr MA, Aisenberg J, Fang P, Tenenbaum-Rakover Y, Rosenfeld RG, Hwa V. The growth hormone receptor (GHR) c.899dupC mutation functions as a dominant negative: insights into the pathophysiology of intracellular GHR defects. J Clin Endocrinol Metab. 2011;96(11):E1896–E1904. [DOI] [PubMed] [Google Scholar]
- 185. Fang P, Riedl S, Amselem S, Pratt KL, Little BM, Haeusler G, Hwa V, Frisch H, Rosenfeld RG. Primary growth hormone (GH) insensitivity and insulin-like growth factor deficiency caused by novel compound heterozygous mutations of the GH receptor gene: genetic and functional studies of simple and compound heterozygous states. J Clin Endocrinol Metab. 2007;92(6):2223–2231. [DOI] [PubMed] [Google Scholar]
- 186. Iida K, Takahashi Y, Kaji H, Onodera N, Takahashi MO, Okimura Y, Abe H, Chihara K. The C422F mutation of the growth hormone receptor gene is not responsible for short stature. J Clin Endocrinol Metab. 1999;84(11):4214–4219. [DOI] [PubMed] [Google Scholar]
- 187. Dias C, Giordano M, Frechette R, Bellone S, Polychronakos C, Legault L, Deal CL, Goodyer CG. Genetic variations at the human growth hormone receptor (GHR) gene locus are associated with idiopathic short stature. J Cell Mol Med. 2017;21(11):2985–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Bozzola M, Travaglino P, Marziliano N, Meazza C, Pagani S, Grasso M, Tauber M, Diegoli M, Pilotto A, Disabella E, Tarantino P, Brega A, Arbustini E. The shortness of Pygmies is associated with severe under-expression of the growth hormone receptor. Mol Genet Metab. 2009;98(3):310–313. [DOI] [PubMed] [Google Scholar]
- 189. Meazza C, Pagani S, Bozzola M. The pygmy short stature enigma. Pediatr Endocrinol Rev. 2011;8(4):394–399. [PubMed] [Google Scholar]
- 190. Gil AP, Kostopoulou E, Karageorgou I, Kamzelas K, Spiliotis BE. Increased growth hormone receptor (GHR) degradation due to over-expression of cytokine inducible SH2 domain-containing protein (CIS) as a cause of GH transduction defect (GHTD). J Pediatr Endocrinol Metab. 2012;25(9–10):897–908. [DOI] [PubMed] [Google Scholar]
- 191. Pagani S, Meazza C, Gertosio C, Bozzola E, Bozzola M. Growth hormone receptor gene expression in puberty. Horm Metab Res. 2015;47(8):581–584. [DOI] [PubMed] [Google Scholar]
- 192. Wu S, Levenson A, Kharitonenkov A, De Luca F. Fibroblast growth factor 21 (FGF21) inhibits chondrocyte function and growth hormone action directly at the growth plate. J Biol Chem. 2012;287(31):26060–26067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Wu S, Walenkamp MJ, Lankester A, Bidlingmaier M, Wit JM, De Luca F. Growth hormone and insulin-like growth factor I insensitivity of fibroblasts isolated from a patient with an IκBα mutation. J Clin Endocrinol Metab. 2010;95(3):1220–1228. [DOI] [PubMed] [Google Scholar]
- 194. Mul D, Wu S, de Paus RA, Oostdijk W, Lankester AC, Duyvenvoorde HA, Ruivenkamp CA, Losekoot M, Tol MJ, De Luca F, van de Vosse E, Wit JM. A mosaic de novo duplication of 17q21–25 is associated with GH insensitivity, disturbed in vitro CD28-mediated signaling, and decreased STAT5B, PI3K, and NF-κB activation. Eur J Endocrinol. 2012;166(4):743–752. [DOI] [PubMed] [Google Scholar]
- 195. Wang SR, Carmichael H, Andrew SF, Miller TC, Moon JE, Derr MA, Hwa V, Hirschhorn JN, Dauber A. Large-scale pooled next-generation sequencing of 1077 genes to identify genetic causes of short stature. J Clin Endocrinol Metab. 2013;98(8):E1428–E1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Clayton P, Bonnemaire M, Dutailly P, Maisonobe P, Naudin L, Pham E, Zhang Z, Grupe A, Thiagalingam A, Denèfle P; EPIGROW Study Group . Characterizing short stature by insulin-like growth factor axis status and genetic associations: results from the prospective, cross-sectional, epidemiogenetic EPIGROW study [published corrections appear in J Clin Endocrinol Metab. 2013;98(10):4213, J Clin Endocrinol Metab. 2014;99(6):2315, and J Clin Endocrinol Metab. 2014;99(7):2619]. J Clin Endocrinol Metab. 2013;98(6):E1122–E1130. [DOI] [PubMed] [Google Scholar]
- 197. Grosse G, Hilger A, Ludwig M, Reutter H, Lorenzen F, Even G, Holterhus PM, Woelfle J; German GHI Study Group . Targeted resequencing of putative growth-related genes using whole exome sequencing in patients with severe primary IGF-I deficiency. Horm Res Paediatr. 2017;88(6):408–417. [DOI] [PubMed] [Google Scholar]
- 198. Meyer R, Soellner L, Begemann M, Dicks S, Fekete G, Rahner N, Zerres K, Elbracht M, Eggermann T.. Targeted next generation sequencing approach in patients referred for Silver-Russell syndrome testing increases the mutation detection rate and provides decisive information for clinical management. J Pediatr. 2017;187:206–212.e1. [DOI] [PubMed]
- 199. Dauber A, Stoler J, Hechter E, Safer J, Hirschhorn JN. Whole exome sequencing reveals a novel mutation in CUL7 in a patient with an undiagnosed growth disorder. J Pediatr. 2013;162:202–204.e1. [DOI] [PMC free article] [PubMed]
- 200. Dauber A. New genetic tools in the diagnosis of growth defects. Growth Horm IGF Res. 2018;38:24–28. [DOI] [PubMed] [Google Scholar]
- 201. Seaver LH, Irons M; American College of Medical Genetics (ACMG) Professional Practice and Guidelines Committee . ACMG practice guideline: genetic evaluation of short stature. Genet Med. 2009;11(6):465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Dhir A, Buratti E. Alternative splicing: role of pseudoexons in human disease and potential therapeutic strategies. FEBS J. 2010;277(4):841–855. [DOI] [PubMed] [Google Scholar]
- 204. Schierding W, Antony J, Cutfield WS, Horsfield JA, O’Sullivan JM. Intergenic GWAS SNPs are key components of the spatial and regulatory network for human growth. Hum Mol Genet. 2016;25(15):3372–3382. [DOI] [PubMed] [Google Scholar]
- 205. Zahnleiter D, Uebe S, Ekici AB, Hoyer J, Wiesener A, Wieczorek D, Kunstmann E, Reis A, Doerr HG, Rauch A, Thiel CT. Rare copy number variants are a common cause of short stature. PLoS Genet. 2013;9(3):e1003365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. van Duyvenvoorde HA, Lui JC, Kant SG, Oostdijk W, Gijsbers AC, Hoffer MJ, Karperien M, Walenkamp MJ, Noordam C, Voorhoeve PG, Mericq V, Pereira AM, Claahsen-van de Grinten HL, van Gool SA, Breuning MH, Losekoot M, Baron J, Ruivenkamp CA, Wit JM. Copy number variants in patients with short stature. Eur J Hum Genet. 2014;22(5):602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Dauber A, Yu Y, Turchin MC, Chiang CW, Meng YA, Demerath EW, Patel SR, Rich SS, Rotter JI, Schreiner PJ, Wilson JG, Shen Y, Wu BL, Hirschhorn JN. Genome-wide association of copy-number variation reveals an association between short stature and the presence of low-frequency genomic deletions. Am J Hum Genet. 2011;89(6):751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Canton AP, Costa SS, Rodrigues TC, Bertola DR, Malaquias AC, Correa FA, Arnhold IJ, Rosenberg C, Jorge AA. Genome-wide screening of copy number variants in children born small for gestational age reveals several candidate genes involved in growth pathways. Eur J Endocrinol. 2014;171(2):253–262. [DOI] [PubMed] [Google Scholar]
- 209. Yau C, Holmes CC. CNV discovery using SNP genotyping arrays. Cytogenet Genome Res. 2008;123(1–4):307–312. [DOI] [PubMed] [Google Scholar]
- 210. Fang P, Girgis R, Little BM, Pratt KL, Guevara-Aguirre J, Hwa V, Rosenfeld RG. Growth hormone (GH) insensitivity and insulin-like growth factor-I deficiency in Inuit subjects and an Ecuadorian cohort: functional studies of two codon 180 GH receptor gene mutations. J Clin Endocrinol Metab. 2008;93(3):1030–1037. [DOI] [PubMed] [Google Scholar]
- 211. Hernández-Porras I, Jiménez-Catalán B, Schuhmacher AJ, Guerra C. The impact of the genetic background in the Noonan syndrome phenotype induced by K-RasV14I. Rare Dis. 2015;3(1):e1045169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Egeblad M, Shen HC, Behonick DJ, Wilmes L, Eichten A, Korets LV, Kheradmand F, Werb Z, Coussens LM. Type I collagen is a genetic modifier of matrix metalloproteinase 2 in murine skeletal development. Dev Dyn. 2007;236(6):1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Montalbano A, Juergensen L, Roeth R, Weiss B, Fukami M, Fricke-Otto S, Binder G, Ogata T, Decker E, Nuernberg G, Hassel D, Rappold GA. Retinoic acid catabolizing enzyme CYP26C1 is a genetic modifier in SHOX deficiency. EMBO Mol Med. 2016;8(12):1455–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Rimbault M, Beale HC, Schoenebeck JJ, Hoopes BC, Allen JJ, Kilroy-Glynn P, Wayne RK, Sutter NB, Ostrander EA. Derived variants at six genes explain nearly half of size reduction in dog breeds. Genome Res. 2013;23(12):1985–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Yart A, Edouard T. Noonan syndrome: an update on growth and development. Curr Opin Endocrinol Diabetes Obes. 2018;25(1):67–73. [DOI] [PubMed] [Google Scholar]
- 216. Roberts AE, Allanson JE, Tartaglia M, Gelb BD. Noonan syndrome. Lancet. 2013;381(9863):333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Tartaglia M, Gelb BD, Zenker M. Noonan syndrome and clinically related disorders. Best Pract Res Clin Endocrinol Metab. 2011;25(1):161–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Tidyman WE, Rauen KA. Expansion of the RASopathies. Curr Genet Med Rep. 2016;4(3):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Kim SO, Jiang J, Yi W, Feng GS, Frank SJ. Involvement of the Src homology 2-containing tyrosine phosphatase SHP-2 in growth hormone signaling. J Biol Chem. 1998;273(4):2344–2354. [DOI] [PubMed] [Google Scholar]
- 220. Stofega MR, Herrington J, Billestrup N, Carter-Su C. Mutation of the SHP-2 binding site in growth hormone (GH) receptor prolongs GH-promoted tyrosyl phosphorylation of GH receptor, JAK2, and STAT5B. Mol Endocrinol. 2000;14(9):1338–1350. [DOI] [PubMed] [Google Scholar]
- 221. Limal JM, Parfait B, Cabrol S, Bonnet D, Leheup B, Lyonnet S, Vidaud M, Le Bouc Y. Noonan syndrome: relationships between genotype, growth, and growth factors. J Clin Endocrinol Metab. 2006;91(1):300–306. [DOI] [PubMed] [Google Scholar]
- 222. Binder G. Response to growth hormone in short children with Noonan syndrome: correlation to genotype. Horm Res. 2009;72(Suppl 2):52–56. [DOI] [PubMed] [Google Scholar]
- 223. Padidela R, Camacho-Hübner C, Attie KM, Savage MO. Abnormal growth in Noonan syndrome: genetic and endocrine features and optimal treatment. Horm Res. 2008;70(3):129–136. [DOI] [PubMed] [Google Scholar]
- 224. De Rocca Serra-Nédélec A, Edouard T, Tréguer K, Tajan M, Araki T, Dance M, Mus M, Montagner A, Tauber M, Salles JP, Valet P, Neel BG, Raynal P, Yart A. Noonan syndrome-causing SHP2 mutants inhibit insulin-like growth factor 1 release via growth hormone-induced ERK hyperactivation, which contributes to short stature. Proc Natl Acad Sci USA. 2012;109(11):4257–4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Nakamura T, Gulick J, Pratt R, Robbins J. Noonan syndrome is associated with enhanced pERK activity, the repression of which can prevent craniofacial malformations. Proc Natl Acad Sci USA. 2009;106(36):15436–15441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Wu X, Simpson J, Hong JH, Kim KH, Thavarajah NK, Backx PH, Neel BG, Araki T. MEK-ERK pathway modulation ameliorates disease phenotypes in a mouse model of Noonan syndrome associated with the Raf1L613V mutation. J Clin Invest. 2011;121(3):1009–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Chen PC, Wakimoto H, Conner D, Araki T, Yuan T, Roberts A, Seidman C, Bronson R, Neel B, Seidman JG, Kucherlapati R. Activation of multiple signaling pathways causes developmental defects in mice with a Noonan syndrome–associated Sos1 mutation. J Clin Invest. 2010;120(12):4353–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Wakeling EL, Brioude F, Lokulo-Sodipe O, O’Connell SM, Salem J, Bliek J, Canton AP, Chrzanowska KH, Davies JH, Dias RP, Dubern B, Elbracht M, Giabicani E, Grimberg A, Grønskov K, Hokken-Koelega AC, Jorge AA, Kagami M, Linglart A, Maghnie M, Mohnike K, Monk D, Moore GE, Murray PG, Ogata T, Petit IO, Russo S, Said E, Toumba M, Tümer Z, Binder G, Eggermann T, Harbison MD, Temple IK, Mackay DJ, Netchine I. Diagnosis and management of Silver-Russell syndrome: first international consensus statement. Nat Rev Endocrinol. 2017;13(2):105–124. [DOI] [PubMed] [Google Scholar]
- 229. Ishida M. New developments in Silver-Russell syndrome and implications for clinical practice. Epigenomics. 2016;8(4):563–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Gicquel C, Rossignol S, Cabrol S, Houang M, Steunou V, Barbu V, Danton F, Thibaud N, Le Merrer M, Burglen L, Bertrand AM, Netchine I, Le Bouc Y. Epimutation of the telomeric imprinting center region on chromosome 11p15 in Silver-Russell syndrome. Nat Genet. 2005;37(9):1003–1007. [DOI] [PubMed] [Google Scholar]
- 231. Schönherr N, Meyer E, Eggermann K, Ranke MB, Wollmann HA, Eggermann T. (Epi)mutations in 11p15 significantly contribute to Silver-Russell syndrome: but are they generally involved in growth retardation? Eur J Med Genet. 2006;49(5):414–418. [DOI] [PubMed] [Google Scholar]
- 232. Iliev DI, Kannenberg K, Weber K, Binder G. IGF-I sensitivity in Silver-Russell syndrome with IGF2/H19 hypomethylation. Growth Horm IGF Res. 2014;24(5):187–191. [DOI] [PubMed] [Google Scholar]
- 233. Binder G, Seidel AK, Martin DD, Schweizer R, Schwarze CP, Wollmann HA, Eggermann T, Ranke MB. The endocrine phenotype in Silver-Russell syndrome is defined by the underlying epigenetic alteration. J Clin Endocrinol Metab. 2008;93(4):1402–1407. [DOI] [PubMed] [Google Scholar]
- 234. Giabicani E, Netchine I, Brioude F. New clinical and molecular insights into Silver-Russell syndrome. Curr Opin Pediatr. 2016;28(4):529–535. [DOI] [PubMed] [Google Scholar]
- 235. Binder G, Mavridou K, Wollmann HA, Eggermann T, Ranke MB. Screening for insulin-like growth factor-I receptor mutations in patients with Silver-Russell syndrome. J Pediatr Endocrinol Metab. 2002;15(8):1167–1171. [DOI] [PubMed] [Google Scholar]
- 236. Miller JD, McKusick VA, Malvaux P, Temtamy S, Salinas C. The 3-M syndrome: a heritable low birthweight dwarfism. Birth Defects Orig Artic Ser. 1975;11(5):39–47. [PubMed] [Google Scholar]
- 237. Clayton PE, Hanson D, Magee L, Murray PG, Saunders E, Abu-Amero SN, Moore GE, Black GC. Exploring the spectrum of 3-M syndrome, a primordial short stature disorder of disrupted ubiquitination. Clin Endocrinol (Oxf). 2012;77(3):335–342. [DOI] [PubMed] [Google Scholar]
- 238. Hanson D, Murray PG, Black GC, Clayton PE. The genetics of 3-M syndrome: unravelling a potential new regulatory growth pathway. Horm Res Paediatr. 2011;76(6):369–378. [DOI] [PubMed] [Google Scholar]
- 239. Hanson D, Murray PG, Coulson T, Sud A, Omokanye A, Stratta E, Sakhinia F, Bonshek C, Wilson LC, Wakeling E, Temtamy SA, Aglan M, Rosser EM, Mansour S, Carcavilla A, Nampoothiri S, Khan WI, Banerjee I, Chandler KE, Black GC, Clayton PE. Mutations in CUL7, OBSL1 and CCDC8 in 3-M syndrome lead to disordered growth factor signalling. J Mol Endocrinol. 2012;49(3):267–275. [DOI] [PubMed] [Google Scholar]
- 240. Demir K, Altıncık A, Böber E. Severe short stature due to 3-M syndrome with a novel OBSL1 gene mutation. J Pediatr Endocrinol Metab. 2013;26(1–2):147–150. [DOI] [PubMed] [Google Scholar]
- 241. Lugli L, Bertucci E, Mazza V, Elmakky A, Ferrari F, Neuhaus C, Percesepe A. Pre- and post-natal growth in two sisters with 3-M syndrome. Eur J Med Genet. 2016;59(4):232–236. [DOI] [PubMed] [Google Scholar]
- 242. Liao L, Gan HW, Hwa V, Dattani M, Dauber A. Two siblings with a mutation in CCDC8 presenting with mild short stature: a case of 3-M syndrome. Horm Res Paediatr. 2017;88(5):364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Keskin M, Muratoğlu Şahin N, Kurnaz E, Bayramoğlu E, Savaş Erdeve Ş, Aycan Z, Çetinkaya S. A rare cause of short stature: 3M syndrome in a patient with novel mutation in OBSL1 gene. J Clin Res Pediatr Endocrinol. 2017;9(1):91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Xu X, Sarikas A, Dias-Santagata DC, Dolios G, Lafontant PJ, Tsai SC, Zhu W, Nakajima H, Nakajima HO, Field LJ, Wang R, Pan ZQ. The CUL7 E3 ubiquitin ligase targets insulin receptor substrate 1 for ubiquitin-dependent degradation. Mol Cell. 2008;30(4):403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Hanson D, Murray PG, Sud A, Temtamy SA, Aglan M, Superti-Furga A, Holder SE, Urquhart J, Hilton E, Manson FD, Scambler P, Black GC, Clayton PE. The primordial growth disorder 3-M syndrome connects ubiquitination to the cytoskeletal adaptor OBSL1. Am J Hum Genet. 2009;84(6):801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Hanson D, Murray PG, O’Sullivan J, Urquhart J, Daly S, Bhaskar SS, Biesecker LG, Skae M, Smith C, Cole T, Kirk J, Chandler K, Kingston H, Donnai D, Clayton PE, Black GC. Exome sequencing identifies CCDC8 mutations in 3-M syndrome, suggesting that CCDC8 contributes in a pathway with CUL7 and OBSL1 to control human growth. Am J Hum Genet. 2011;89(1):148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Wit JM, Oostdijk W, Losekoot M, van Duyvenvoorde HA, Ruivenkamp CA, Kant SG. Mechanisms in endocrinology: novel genetic causes of short stature. Eur J Endocrinol. 2016;174(4):R145–R173. [DOI] [PubMed] [Google Scholar]
- 248. Growth Hormone Research Society; GH Research Society . Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. J Clin Endocrinol Metab. 2000;85(11):3990–3993. [DOI] [PubMed] [Google Scholar]
- 249. Wagner IV, Paetzold C, Gausche R, Vogel M, Koerner A, Thiery J, Arsene CG, Henrion A, Guettler B, Keller E, Kiess W, Pfaeffle R, Kratzsch J. Clinical evidence-based cutoff limits for GH stimulation tests in children with a backup of results with reference to mass spectrometry. Eur J Endocrinol. 2014;171(3):389–397. [DOI] [PubMed] [Google Scholar]
- 250. Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jørgensen K, Müller J, Hall K, Skakkebaek NE. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol Metab. 1994;78(3):744–752. [DOI] [PubMed] [Google Scholar]
- 251. Juul A, Dalgaard P, Blum WF, Bang P, Hall K, Michaelsen KF, Müller J, Skakkebaek NE. Serum levels of insulin-like growth factor (IGF)-binding protein-3 (IGFBP-3) in healthy infants, children, and adolescents: the relation to IGF-I, IGF-II, IGFBP-1, IGFBP-2, age, sex, body mass index, and pubertal maturation. J Clin Endocrinol Metab. 1995;80(8):2534–2542. [DOI] [PubMed] [Google Scholar]
- 252. Bjarnason R, Banerjee K, Rose SJ, Rosberg S, Metherell L, Clark AJ, Albertsson-Wikland K, Savage MO. Spontaneous growth hormone secretory characteristics in children with partial growth hormone insensitivity. Clin Endocrinol (Oxf). 2002;57(3):357–361. [DOI] [PubMed] [Google Scholar]
- 253. Coutant R, Dörr HG, Gleeson H, Argente J. Diagnosis of endocrine disease: limitations of the IGF1 generation test in children with short stature. Eur J Endocrinol. 2012;166(3):351–357. [DOI] [PubMed] [Google Scholar]
- 254. Blum WF, Ranke MB, Savage MO, Hall K; The Kabi Pharmacia Study Group on Insulin-like Growth Factor I Treatment in Growth Hormone Insensitivity Syndromes . Insulin-like growth factors and their binding proteins in patients with growth hormone receptor deficiency: suggestions for new diagnostic criteria. Acta Paediatr Suppl. 1992;383:125–126. [PubMed] [Google Scholar]
- 255. Rosenfeld RG. The IGF system: new developments relevant to pediatric practice. Endocr Dev. 2005;9:1–10. [DOI] [PubMed] [Google Scholar]
- 256. Midyett LK, Rogol AD, Van Meter QL, Frane J, Bright GM; MS301 Study Group . Recombinant insulin-like growth factor (IGF)-I treatment in short children with low IGF-I levels: first-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2010;95(2):611–619. [DOI] [PubMed] [Google Scholar]
- 257. Buckway CK, Guevara-Aguirre J, Pratt KL, Burren CP, Rosenfeld RG. The IGF-I generation test revisited: a marker of GH sensitivity. J Clin Endocrinol Metab. 2001;86(11):5176–5183. [DOI] [PubMed] [Google Scholar]
- 258. Klinger B, Laron Z. Three year IGF-I treatment of children with Laron syndrome. J Pediatr Endocrinol Metab. 1995;8(3):149–158. [DOI] [PubMed] [Google Scholar]
- 259. Backeljauw PF, Underwood LE. Therapy for 6.5–7.5 years with recombinant insulin-like growth factor I in children with growth hormone insensitivity syndrome: a clinical research center study. J Clin Endocrinol Metab. 2001;86(4):1504–1510. [DOI] [PubMed] [Google Scholar]
- 260. Ranke MB, Savage MO, Chatelain PG, Preece MA, Rosenfeld RG, Blum WF, Wilton P. Insulin-like growth factor I improves height in growth hormone insensitivity: two years’ results. Horm Res. 1995;44(6):253–264. [DOI] [PubMed] [Google Scholar]
- 261. Backeljauw PF, Kuntze J, Frane J, Calikoglu AS, Chernausek SD. Adult and near-adult height in patients with severe insulin-like growth factor-I deficiency after long-term therapy with recombinant human insulin-like growth factor-I. Horm Res Paediatr. 2013;80(1):47–56. [DOI] [PubMed] [Google Scholar]