Abstract
Objective: To investigate the hyaluronic acid (HA) modified, doxorubicin (DOX) and gallic acid (GA) co-delivered lipid-polymeric hybrid nano-system for leukemia therapy.
Methods: We produced a kind of lipid-polymer hybrid nanoparticle (LPHN) with a core-shell structure in which DOX and GA were co-loaded. In vitro and in vivo leukemia therapeutic effects of the HA modified, DOX and GA co-delivered LPHNs (HA-DOX/GA-LPHNs) were evaluated in DOX resistant human HL-60 promyelocytic leukemia cells (HL-60/ADR cells), DOX resistant human K562 chronic myeloid leukemia cells (K562/ADR cells), and HL-60/ADR cells bearing mouse model.
Results: The sizes and zeta potentials of HA modified LPHNs were about 160 nm and −40 mV. HA-DOX/GA-LPHNs showed the most prominent cytotoxicity and the best synergistic effect was obtained when DOX/GA ratio was 2/1. In vivo studies revealed that HA-DOX/GA-LPHNs inhibited tumor growth from 956 mm3 to 213 mm3, with an inhibition rate of 77.7%.
Conclusion: In summary, the study showed that HA-DOX/GA-LPHNs can be applied as a promising leukemia therapy system.
Keywords: acute myeloid leukemia, doxorubicin, gallic acid, multidrug resistance, lipid-polymer hybrid nanoparticles
Introduction
Acute myeloid leukemia (AML) is the most common form of acute leukemia among adults.1 It causes the maximum number of deaths among leukemia patients every year. It is one of the most refractory diseases due to its uncontrolled proliferation characteristics.2 The most common symptom of leukemia is pancytopenia or leukemic infiltration.3 As a myeloproliogenic disease, it is associated with increased white blood cells, anemia, splenomegaly, weight loss, and drowsiness.4 Therefore, timely treatment should be given to destroy these cancer cells so that the bone marrow can produce normal cells.5
Doxorubicin (DOX) is a first-line cancer chemotherapy drug for the treatment of leukemia, lung, breast, cervical, ovarian, prostate, and bladder cancers.6 However, the efficacy of DOX is often affected by multidrug resistance (MDR) mechanisms involving P-glycoprotein.7 In recent years, a variety of anticancer drugs combined with DOX have been widely developed for chemotherapy to reduce MDR and the side effects caused by drug dose reduction.8–10 Gallic acid (GA) is a polyhydroxyphenol compound which can be found in green tea, grapes, strawberries, bananas, and many other natural products.11 It can be used as a free radical scavenger and as an inducer of differentiation and apoptosis in leukemia, lung, and colon cancer cell lines. GA and its derivatives can induce Ca2+ dependent apoptosis in leukemia cells, thus exhibiting anti-leukemic ability in human HL-60 and K562 leukemia cells.12–14 In addition, when GA was combined with other chemotherapeutic drugs, better efficiency may have been achieved compared to a single drug.15 However, since different drugs have different physical and chemical properties, how to obtain significant antitumor effects while reducing the toxicity of normal tissues, is still a challenge.16 Therefore, there is an urgent need to develop new treatment strategies for these hard-to-treat cases of leukemia, in addition to reducing the side effects on all leukemia patients.17
In order to overcome the inherent defects of drugs, including short half-life and undesired distribution in normal tissues and organs, nanocarriers have been developed for drug delivery.18 For instance, nanocarriers have been developed to reduce the side effects of DOX and increase therapeutic effect in leukemia.19 Receptor-mediated internalization may introduce the binding of ligands to receptors, which may help with the release of drugs from the nanocarriers into the cell.20 Therefore, receptor-mediated uptake of anticancer drugs can be used as an effective drug delivery system for leukemia treatment.21 CD44 has been reported to be overexpressed in many leukemia cells such as human AML cells, T-cell prolymphocytic leukemia cells, and B-cell chronic lymphocytic leukemia cells.22 Hyaluronic acid (HA) is a natural polysaccharide which has a strong affinity for CD44 receptors.23 Thus, HA was conjugated with polyethylene glycol-distearoyl phosphoethanolamine (PEG-DSPE) to form HA-PEG-DSPE and was used as targeted ligands for the decoration of nanoparticles.
In the present research, we produced lipid-polymer hybrid nanoparticles (LPHNs) with a core-shell structure in which DOX and GA were co-loaded. By combining the bionic properties of lipids with the structural advantages of polymer cores, an outstanding delivery system was obtained.24 In vitro and in vivo leukemia therapeutic effects of the HA modified, DOX and GA co-delivered LPHNs (HA-DOX/GA-LPHNs) were evaluated in DOX resistant human HL-60 promyelocytic leukemia cells (HL-60/ADR cells), DOX resistant human K562 chronic myeloid leukemia cells (K562/ADR cells), and HL-60/ADR cells bearing mouse model.
Materials and methods
Chemicals and reagents
HA (molecular weight: 9.5 kDa) was obtained from Shandong Freda
Biopharm Co., Ltd. (Ji’nan, People's Republic of China). NH2-PEG3400-DSPE was purchased from Ponsure Biotechnology, Shanghai, People's Republic of China. Poly-ε-caprolactone (PCL, molecular weight: 14 kDa), lecithin (99% phosphatidylcholine), dicyclohexylcarbodiimide (DCC), MTT, FBS, and RPMI 1640 were supplied by Sigma-Aldrich Co. (St Louis, MO, USA).
Cells and animals
Human HL-60 promyelocytic leukemia cells (HL-60 cells) and K562 chronic myeloid leukemia cells (K562 cells) were provided by American Type Culture Collection (Manassas, VA, USA). HL-60/ADR cells and K562/ADR cells were established with the continuous increase of DOX concentration and selected in the growth medium.25 FBS (10%,v/v) was added to RPMI 1640 at 37°C to form the cell culture medium. HL-60 and K562 cells were cultured in the medium with the presence of penicillin (1%, v/v, 100 U/mL), streptomycin (100 mg/ml), and L-glutamine (2 mM), in a 5% CO2 humidified atmosphere. HL-60/ADR and K562/ADR cells were maintained in DOX (0.2 μM) containing medium to keep the drug resistance phenotype.
BALB/c nude mice (18–22 g) were purchased from Key Biological Technology Co., Ltd. (Shanghai, People's Republic of China). The chronic myeloid leukemia mouse model was created by subcutaneous injected of HL-60/ADR cells (107 cells in 200 μL of PBS) into the right flank of mice (HL-60 cells and K562 chronic myeloid leukemia cells K562 cells). Animal experiments were performed under the guidance of the National Institutes of Health for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978) and was approved by the ethics committee of Hebei Province Hospital of Chinese Medicine and Taizhou Hospital of Zhejiang Province.
Synthesis of HA-PEG-DSPE
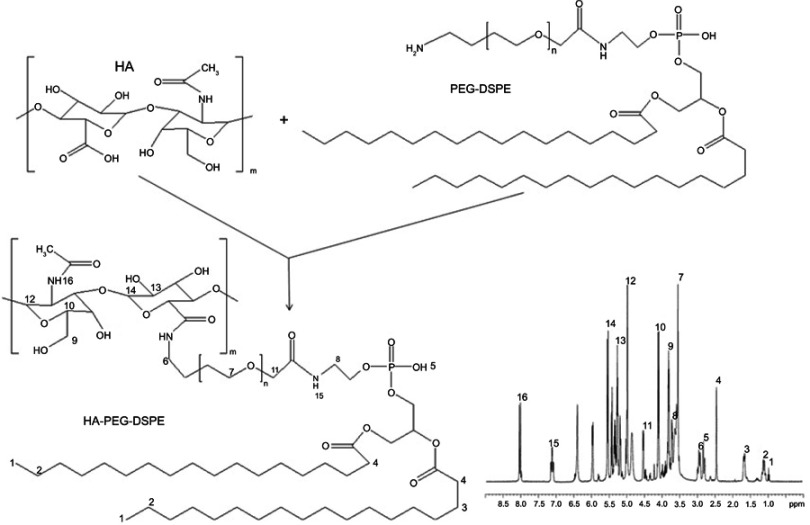
HA-PEG-DSPE (Figure 1) was prepared by the conjugation of the HA (carboxyl group) with the NH2-PEG3400-DSPE (amine group).26 HA was firstly dissolved in DMSO, then DCC (1.1 equivalents) was added and stirred in an ice bath. NH2-PEG3400-DSPE (1 equivalent, dissolved in DMSO) was and added into HA solution drop by drop and stirred for 12 hours at 20°C–25°C. HA-PEG-DSPE was lyophilized and characterized by 1H-NMR analysis.
Figure 1.
Synthesis of HA-PEG-DSPE and 1H-NMR analysis. Abbreviations: HA, hyaluronic acid; PEG-DSPE, polyethylene glycol-distearoyl phosphoethanolamine.
Preparation of HA-DOX/GA-LPHNs
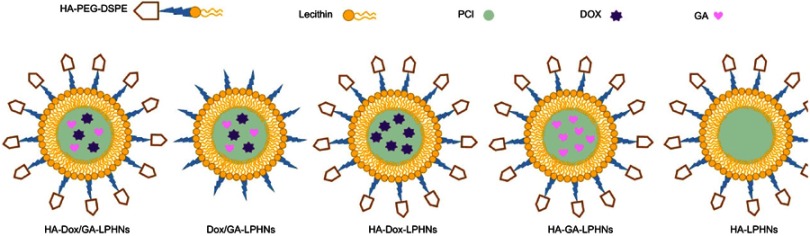
HA-DOX/GA-LPHNs (Figure 2) were self-assembled with PCL, lecithin, and HA-PEG-DSPE by one-step nanoprecipitation.27 Briefly, lecithin (100 mg) and HA-PEG-DSPE (50 mg) were added in acetone (20 mL) and heated (65°C) to form a liquid lipid phase. DOX (20 mg), GA (10 mg), and PCL polymer (100 mg) were dissolved in acetone (20 mL) and then added dropwise into the 65°C lipid phase which was stirred at 300 rpm. The acetone and free molecules were removed by washing the HA-DOX/GA-LPHNs solution three times using a centrifugal filter with a molecular weight cut-off (MWCO) of 10 kDa.
Figure 2.
Scheme graphs of HA-DOX/GA-LPHNs, DOX/GA-LPHNs, HA-DOX-LPHNs, HA-GA-LPHNs, and HA-LPHNs. Abbreviations: HA, hyaluronic acid; DOX, doxorubicin; GA, gallic acid; LPHNs, lipid-polymer hybrid nanoparticles.
Non-HA modified, DOX and GA co-delivered LPHNs (DOX/GA-LPHNs) were assembled in the same way using PEG-DSPE instead of HA-PEG-DSPE. Single drug loaded LPHNs were prepared by the same method using single DOX (40 mg) or single GA (20 mg) dissolved along with PCL polymer, named HA-DOX-LPHNs and HA-GA-LPHNs, respectively. To prepare drug-free blank LPHNs, no drug was added during preparation, named HA-LPHNs (Figure 2).
Particle size, zeta potential, and drug loading (DL) capacity
HA-DOX/GA-LPHNs, DOX/GA-LPHNs, HA-DOX-LPHNs, HA-GA-LPHNs, and HA-LPHNs were diluted in phosphate buffer (pH 7.4), and their sizes, polydispersity index (PDI), and zeta potentials were analyzed in triplicate by dynamic light scattering (Zeta sizer Nano ZS, Malvern Instruments, Malvern, UK) at room temperature.28 To evaluate the DL capacity, HA-DOX/GA-LPHNs, DOX/GA-LPHNs, HA-DOX-LPHNs, and HA-GA-LPHNs were dissolved in 1 mL of methanol under vigorous vortexing. The solutions were centrifuged (20,000 rpm, 20 minutes) and the amount of DOX was analyzed by UV spectrophotometry (Shimadzu, Japan) at 478 nm.29 The amount of GA was measured by high liquid performance chromatography (HPLC):30 A 20 mL aliquot of the supernatant obtained after centrifugation was injected into a Luna C18 15 cm ×3.0 mm HPLC column (Phenomenex, Torrance, CA, USA). Gradient elution was conducted at a flow rate of 1.5 mL/minute using methanol and 0.5% phosphoric acid at ratios of 70:30. The absorbance at 270 nm was detected. The capacity of DL and drug entrapment efficiency (EE) were calculated as follows:
Storage stability
The LPHNs were stored at 2°C–8°C. The stability of LPHNs was evaluated for 4months.31 At predetermined time points, the particle size and PDI of HA-DOX/GA-LPHNs, DOX/GA-LPHNs, HA-DOX-LPHNs, HA-GA-LPHNs, and HA-LPHNs were tested by the methods in the previoussection.
In vitro drug release
The DOX and GA released from LPHNs were investigated by dialysis method.32 HA-DOX/GA-LPHNs, DOX/GA-LPHNs, HA-DOX-LPHNs, and HA-GA-LPHNs were dispersed into PBS (pH 7.4). Then, the LPHN suspensions were sealed in dialysis bags (MWCO: 5 kDa) with 30 mL PBS (pH 7.4) in the outside as dialysis medium. At predetermined time points, samples (0.5 mL) were taken out from the dialysis bags and fresh PBS was (0.5 mL) added into the bags. The release of DOX and GA were calculated by the method described in “DL capacity” section.
In vitro cytotoxicity
In vitro cytotoxicity of LPHNs was evaluated on HL-60/ADR and K562/ADR cells using the MTT assay.33 Briefly, HL-60/ADR or K562/ADR cells (200 µL) were seeded into 96-well plates at a density of 5×104 cells/mL and incubated for 24 hours. Various concentrations of HA-DOX/GA-LPHNs, DOX/GA-LPHNs, HA-DOX-LPHNs, HA-GA-LPHNs, HA-LPHNs, and free DOX and GA mixed solution (free DOX/GA) were added and incubated for 72 hours. After removal of the medium, MTT solution (20 µL, 5 mg/mL in PBS) was added to each well for 4 hours. DMSO (200 µL) was then added and absorbance was read at 570 nm.
Synergistic effects analysis
To investigate the synergistic effects of the DOX and GA co-delivered HA-DOX/GA-LPHNs on HL-60/ADR cells, half-maximal inhibitory concentration (IC50) was calculated and combination index (CI) analysis was done by Chou and Talalay’s method.34 CI values for DOX and GA combinations were d according to the following equation: CI = (D)DOX/(Dx)DOX + (D)GA/(Dx)GA were used to calculate the CI values of DOX and GA in HA-DOX/GA-LPHNs. (D)DOX and (D)GA: concentrations of DOX and GA in the combination system at the ICx value; (Dx)DOX and (Dx)GA: ICx value of DOX alone and GA alone. CIx <1 and >1 represent synergism and antagonism, respectively. In this study, x=50.
In vivo biodistribution
HA-DOX/GA-LPHNs, DOX/GA-LPHNs, HA-DOX-LPHNs, HA-GA-LPHNs, HA-LPHNs, free DOX/GA (each contained 10 mg/kg of DOX and 5 mg/kg of GA), and 0.9% normal saline were intravenously injected in different groups (eight mice in each group) of AML bearing mice.35 Each group of mice was sacrificed. At 1 hour and 48 hours after intravenous injection, mice were killed by cervical vertebra dislocation and tumor tissue and other organs were taken out and homogenized in saline (tissue/water =1:5, w/v) and the amount of DOX and GA were determined using the technique described in “DL capacity” section.
In vivo antitumor efficacy
HA-DOX/GA-LPHNs, DOX/GA-LPHNs, HA-DOX-LPHNs, HA-GA-LPHNs, HA-LPHNs, free DOX/GA (each contained 10 mg/kg of DOX and 5 mg/kg of GA), and 0.9% normal saline were intravenously injected through tail vein in different groups (eight mice in each group) of AML bearing mice every 3 days.36 At 21 days after administration, the mice were sacrificed. Tumors were taken out and the weight and volume were measured. Tumor volume (TV) and tumor inhibition rate (TIR) were calculated according to the following equations: TV = the long axis × (the short axis)2/2; TIR (%) = (tumor weight of the control group - tumor weight of the treated group)/tumor weight of the control group ×100. Body weight changes of the mice were monitored every 3 days to evaluate systemic toxicity of the LPHNs.
Statistical analysis
Means ± SD was used to present the data. An unpaired Student's t-test between two groups was applied to analyze the data with SPSS 20.0 software. The level of significance in all statistical analyses was set at a probability of P<0.05.
Results
Characterization of HA-PEG-DSPE
Figure 1 showed the 1H-NMR of HA-PEG-DSPE and the peaks were marked in the chemical structure. The peaks of 1, 2, 3, 4, and 5 were the protons of DSPE; the peaks of 9, 12, 13, 14, and 16 belonged to the structure of HA; peak 7 belonged to PEG; the peaks of 6, 8, 11, and 15 were the protons in or next to the amide linkage.
Particle size, zeta potential, and DL capacity
Table 1 showed the sizes of HA modified LPHNs were about 160 nm, which means DL did not increase the size of the particles. However, non-HA decorated LPHNs had a smaller size of 130 nm. Zeta potential of HA-DOX/GA-LPHNs and DOX/GA-LPHNs were −41.3 and −30.5 mV, respectively. EEs of drug loaded LPHNs erre around 85%, with different DLs ranging from 5.1%−13.5%. Figure 3 illustrated no obvious change in size or PDI of LPHNs during 4 months of study, which could be evidence of the good stability of the system.
Table 1.
Characterization of lipid-polymer hybrid nanoparticles (LPHNs) (mean ± SD, n=3)
| Characteristics | Particle size (nm) | PDI | Zeta potential (mV) | DL (%) | EE (%) | ||
|---|---|---|---|---|---|---|---|
| DOX | GA | DOX | GA | ||||
| HA-DOX/GA-LPHNs | 165.7±4.6 | 0.167±0.021 | −41.3±2.8 | 10.2±0.9 | 5.3±0.5 | 88.9±3.7 | 85.6±3.5 |
| DOX/GA-LPHNs | 131.2±3.5 | 0.129±0.011 | −30.5±2.3 | 13.5±1.1 | 6.4±0.4 | 86.9±3.4 | 84.7±3.9 |
| HA-DOX-LPHNs | 162.6±4.1 | 0.151±0.019 | −40.5±2.9 | 10.3±0.8 | N/A | 87.4±3.3 | N/A |
| HA-GA-LPHNs | 164.1±4.3 | 0.133±0.017 | −39.7±2.6 | N/A | 5.1±0.3 | N/A | 86.1±3.1 |
| HA-LPHNs | 161.9±3.9 | 0.141±0.012 | −38.9±3.1 | N/A | N/A | N/A | N/A |
Figure 3.

Storage stability of evaluated by the mean particle size (A) and polydispersity index (B) at various time points up to 120 days (mean ± SD, n=3).
In vitro drug release
Figure 4 showed the DOX (Figure 4A) and GA (Figure 4B) release profiles of HA-DOX/GA-LPHNs, DOX/GA-LPHNs, HA-DOX-LPHNs, and HA-GA-LPHNs. Firstly, all the drug loaded LPHNs exhibited sustained release patterns. Secondly, all the formulas had over 80% of drug release during the time of study. Most importantly, HA modified LPHNs revealed slower release than the non-modified DOX/GA-LPHNs, this may have been caused by the HA coating on the LPHNs' surface which delayed the drug release.
Figure 4.

DOX (A) and GA (B) release profiles of HA-DOX/GA-LPHNs, DOX/GA-LPHNs, HA-DOX-LPHNs, and HA-GA-LPHNs (mean ± SD, n=3). Abbreviations: HA, hyaluronic acid; DOX, doxorubicin; GA, gallic acid; LPHNs, lipid-polymer hybrid nanoparticles.
In vitro cytotoxicity
HA-LPHNs showed no significant cytotoxicity effects on HL-60/ADR and K562/ADR cells (Figure 5). When loaded with drugs, HA-DOX/GA-LPHNs, DOX/GA-LPHNs, HA-DOX-LPHNs, and HA-GA-LPHNs exhibited significant cytotoxicity. Although free DOX/GA exhibited obvious toxicity and the efficiency was enhanced over time, cell inhibition effect of drugs co-delivered LPHNs groups was more remarkable (P<0.05). HA-DOX/GA-LPHNs showed the most prominent cytotoxicity compared to DOX/GA-LPHNs, HA-DOX-LPHNs, and HA-GA-LPHNs groups (P<0.05).
Figure 5.

In vitro cytotoxicity of LPHNs and free drugs in HL-60/ADR (A) and K562/ADR (B) cells (mean ± SD, n=8). Abbreviation: LPHNs, lipid-polymer hybrid nanoparticles.
Synergistic effects
DOX/GA ratio in the LPHNs was selected by CI50 values to get the best synergistic effect, and were calculated according to the IC50 values of DOX and GA in HA-DOX/GA-LPHNs (Table 2). Figure 6 showed that the best synergistic effect was obtained when DOX/GA ratio was 2/1. Back to the preparation of HA-DOX/GA-LPHNs, 20 mg of DOX and 10 mg of GA were determined.
Table 2.
IC50 values of doxorubicin (DOX) and gallic acid (GA) when different ratios were used in hyaluronic acid-DOX/GA-lipid-polymer hybrid nanoparticles (mean ± SD, n=3)
| DOX/GA ration (w/w) | IC50 of DOX (μM) | IC50 of GA (μM) |
|---|---|---|
| 10:1 | 9.73±0.82 | 0.97±0.12 |
| 5:1 | 2.95±0.33 | 0.59±0.09 |
| 2:1 | 0.46±0.06 | 0.23±0.05 |
| 1:1 | 1.03±0.17 | 1.03±0.19 |
| 1:2 | 1.79±0.22 | 3.58±0.49 |
| 1:5 | 2.18±0.37 | 10.92±0.86 |
Figure 6.
CI50 values when different DOX/GA ratios were applied (mean ± SD, n=8).
Abbreviations: DOX, doxorubicin; GA, gallic acid.
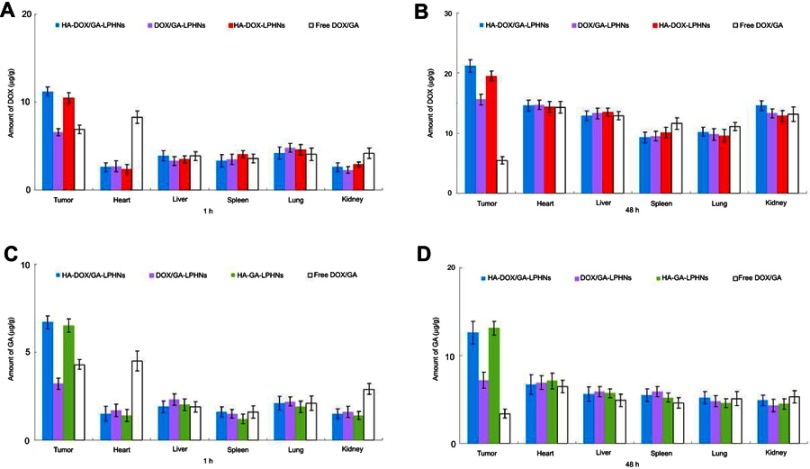
In vivo biodistribution
In vivo distributions of drugs in tumor and other organs are presented in Figure 7. At 1 hour, distributions of DOX and GA in free DOX/GA were higher in the heart and kidney than loaded in LPHNs (P<0.05). When tested at both 1 hour and 48 hours, HA modified LPHNs exhibited higher distribution in tumor compared to free DOX/GA and non-HA modified LPHNs (P<0.05). No significant differences in drug distribution were found in the other organs.
Figure 7.
In vivo distributions of drugs in tissues and tumors: DOX distribution at 1 hour (A) and 48 hours (B); GA distribution at 1 hour (C) and 48 hours (D) (mean ± SD, n=8). Abbreviations: DOX, doxorubicin; GA, gallic acid.
In vivo antitumor efficacy
In vivo antitumor efficacy of LPHNs was investigated in an AML bearing mouse model (Figure 8A). Firstly, drug loaded LPHNs groups remarkably inhibited the tumor growth in contrast with the free DOX/GA group (P<0.05). Secondly, HA-DOX/GA-LPHNs showed remarkably higher inhibition rates than single drug loaded HA-DOX-LPHNs and HA-GA-LPHNs (P<0.05). Finally, the tumors of mice treated with HA-DOX/GA-LPHNs were significantly smaller than non-HA modified DOX/GA-LPHNs (P<0.05). Body weight results revealed that an obvious decrease in weight was found in the control, free DOX/GA, and HA-LPHNs groups (Figure 8B). However, no significant body weight changes were found in the drug loaded LPHNs group. The TIRs of all the treated groups were summarized in Table 3.
Figure 8.

In vivo antitumor efficacy of LPHNs investigated on AML bearing mouse model (A) and body weight changes (B) (mean ± SD, n=8). Abbreviations: LPHNs, lipid-polymer hybrid nanoparticles; AML, acute myeloid leukemia.
Table 3.
The tumor inhibition rates of the treated groups (mean ± SD, n=3)
| Samples | Tumor inhibition rate (%) |
|---|---|
| HA-DOX/GA-LPHNs | 77.7±3.2 |
| DOX/GA-LPHNs | 61.1±2.1 |
| HA-DOX-LPHNs | 51.4±2.6 |
| HA-GA-LPHNs | 34.0±1.8 |
| Free DOX/GA | 27.3±1.5 |
Discussion
In this study, PCL was applied as polymeric material to encapsulate poorly water soluble drugs and lecithin was used to construct the lipid shell at the surface of LPHNs. HA was covalently conjugated to PEG-DSPE as modified lipid to form the shell of the LPHNs. The LPHNs were self-assembled through a single-step nanoprecipitation method. The sizes of HA modified LPHNs (160 nm) were larger than non-HA decorated LPHNs (130 nm), in the meantime, zeta potentials of HA modified LPHNs were more negative (−41.3 mV) than DOX/GA-LPHNs (−30.5 mV). These results mean that the existence of HA would increase the overall size of the LPHNs and bring more negative charge to the systems.
The stability of any nanoparticle system needs to be evaluated and optimized, as disruption of the nanoparticles in the drug delivery system may affect its therapeutic potential.37 The phospholipids that constitute the shell of the LPHNs may act as surfactants to stabilize the nanoparticles. No obvious changes in size or PDI of LPHNs were found during 4 months of study, which could be evidence of the good stability of the system.
A significant advantage of the nano drug delivery system is the sustained or controlled release, which greatly enhances the bioavailability of the drug and reduces the side effects of the drug on healthy human tissues.38 The drugs were released from LPHNs in sustained behaviors. The DOX and GA were mainly located in the polymeric core and the shell needed to be firstly destabilized and then the core may be corroded to slowly release the drugs.39 HA modified LPHNs revealed slower release than the non-modified DOX/GA-LPHNs, this may have been caused by the HA coating on the LPHNs' surface which delayed the drug release.
Blank HA-LPHNs showed no significant cytotoxicity effects on HL-60/ADR and K562/ADR cells, which means the carriers themselves did not bring about toxicity. HA-DOX/GA-LPHNs showed more prominent cytotoxicity than HA-DOX-LPHNs and HA-GA-LPHNs, this may be proof of the synergism of DOX and GA. The efficacy of DOX can be influenced by MDR mechanisms and in this study, GA was applied to combine with DOX to reduce MDR and the side effects caused by drug dose reduction. The assessment of drug-drug interactions is important in all areas of medicine, especially in combination with cancer chemotherapy.40 The term “combination index“ (CI) was introduced by Chou and Talalay in 1983 to quantify the synergistic or antagonistic effects of the two drugs.41 Firstly, we summarized the IC50 values of DOX and GA when different DOX/GA ratios were applied in HA-DOX/GA-LPHNs preparation. Since these two drugs were designed at different ratios and had different efficiency in the formulations, the IC50 values of DOX and GA were different for different formulations. Then, the best synergistic effect was calculated and the best DOX/GA ratio was 2/1. This section demonstrated the synergistic efficiency and DOX/GA ratio of the two drug systems.
In vivo tissue distribution behavior of LPHNs was investigated in AML bearing mice. LPHNs were more widely distributed in tumor tissues and less widely distributed in the heart and kidney, thus, they can reduce side effects during cancer treatment.42 Instead, the drug solution samples were mainly distributed in the heart and kidneys, which may cause systemic toxicity. It was reported that CD44 mediates the uptake and degradation of hyaluronan by rodent alveolar macrophages and transformed fibroblasts. The accumulation of LPHNs in the lung is not high, indicating that the other CD44 receptor expressed cells may not affect the ability of LPHNs.43 The accumulation of LPHNs in tumor tissues was higher than that in other normal tissues. The drug concentration of LPHNs in tumor tissues remained high 48 hours after injection, indicating that LPHNs has a sustained release effect. The long cycling effect is due to the presence of PEG chains on the particle surface, which provides the stealth characteristic.44 Compared with non-HA modified DOX/GA-LPHNs, HA-DOX/GA-LPHNs accumulated more in tumors, indicating that HA’s targeting ability increased the accumulation of LPHNs at tumor sites.
In vivo antitumor studies illustrated that HA-DOX/GA-LPHNs showed better tumor growth inhibition than DOX/GA-LPHNs in an AML nude mouse xenograft, which may be due to the increased release time and targeted effect of HA modification.45,46 In addition, LPHNs significantly inhibited tumor growth and had no significant adverse effects (weight loss) compared with free drugs. The finding may provide evidence that LPHNs is considered a safe system for drug delivery in leukemia treatment. The in vivo TIR results were consistent with the in vitro synergistic effects of the dual drug delivery system. This is because the LPHNs system has high structural integrity, stability, and slow release performance. The value of the co-delivering nano-system constructed in this study compared to the currently approved targeted drugs for AML/CML may be the smaller amount of DOX, higher affinity for cancer cells, and lower toxicity to the body. The HA-modified DOX/GA co-loaded LPHNs could be efficient in AML therapy, with the least systemic toxic side effects.
Conclusion
In summary, this study showed that HA-DOX/GA-LPHNs were successfully developed with good anti-AML effects. Further research is needed before the preparation can be used in patients with cancer.
Acknowledgment
The work was supported by the Taizhou Science and Technology Bureau (No. 162yw01): Study on the Jak-stat pathway mediated HLX regulation of AML cell carcinogenesis and mechanism.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.O’Donnell MR, Tallman MS, Abboud CN, et al. Acute myeloid leukemia, version 3.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(7):926–957. doi: 10.6004/jnccn.2017.0116 [DOI] [PubMed] [Google Scholar]
- 2.Deng R, Shen N, Yang Y, et al. Targeting epigenetic pathway with gold nanoparticles for acute myeloid leukemia therapy. Biomaterials. 2018;167:80–90. doi: 10.1016/j.biomaterials.2018.03.013 [DOI] [PubMed] [Google Scholar]
- 3.Mendes AN, Filgueiras LA, Siqueira MRP, et al. Encapsulation of Piper cabralanum (Piperaceae) nonpolar extract in poly(methyl methacrylate) by miniemulsion and evaluation of increase in the effectiveness of antileukemic activity in K562 cells. Int J Nanomedicine. 2017;12:8363–8373. doi: 10.2147/IJN.S134756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faderl S, Kantarjian HM, Talpaz M. Chronic myelogenous leukemia: update on biology and treatment. Oncology (Williston Park). 1999;13(2):169–80; discussion 181, 184. [PubMed] [Google Scholar]
- 5.Tang R, Cohen S, Perrot JY, et al. P-gp activity is a critical resistance factor against AVE9633 and DM4 cytotoxicity in leukaemia cell lines, but not a major mechanism of chemoresistance in cells from acute myeloid leukaemia patients. BMC Cancer. 2009;9:199. doi: 10.1186/1471-2407-9-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang P, Li J, Ghazwani M, et al. Effective co-delivery of doxorubicin and dasatinib using a PEG-Fmoc nanocarriers for combination cancer chemotherapy. Biomaterials. 2015;67:104–114. doi: 10.1016/j.biomaterials.2015.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Misra R, Sahoo SK. Coformulation of doxorubicin and curcumin in poly(D,L-lactide-co-glycolide) nanoparticles suppresses the development of multidrug resistance in K562 cells. Mol Pharm. 2011;8(3):852–866. doi: 10.1021/mp100455h [DOI] [PubMed] [Google Scholar]
- 8.He Y, Su Z, Xue L, Xu H, Zhang C. Co-delivery of erlotinib and doxorubicin by pH-sensitive charge conversion nanocarrier for synergistic therapy. J Control Release. 2016;229:80–92. doi: 10.1016/j.jconrel.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Yang C, Wang W, et al. Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer. Sci Rep. 2016;6:21225. doi: 10.1038/srep21225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Tang Z, Lin J, et al. Synergistic antitumor effects of doxorubicin-loaded carboxymethyl cellulose nanoparticle in combination with endostar for effective treatment of non-small-cell lung cancer. Adv Healthc Mater. 2014;3(11):1877–1888. doi: 10.1002/adhm.201400108 [DOI] [PubMed] [Google Scholar]
- 11.Singh A, Fatima K, Srivastava A, et al. Anticancer activity of gallic acid template-based benzylidene indanone derivative as microtubule destabilizer. Chem Biol Drug Des. 2016;88(5):625–634. doi: 10.1111/cbdd.12805 [DOI] [PubMed] [Google Scholar]
- 12.Isuzugawa K, Inoue M, Ogihara Y. Ca2+-Dependent caspase activation by gallic acid derivatives. Biol Pharm Bull. 2001;24(7):844–847. [DOI] [PubMed] [Google Scholar]
- 13.Madlener S, Illmer C, Horvath Z, et al. Gallic acid inhibits ribonucleotide reductase and cyclooxygenases in human HL-60 promyelocytic leukemia cells. Cancer Lett. 2007;245(1–2):156–162. doi: 10.1016/j.canlet.2006.01.001 [DOI] [PubMed] [Google Scholar]
- 14.Chandramohan Reddy T, Bharat Reddy D, Aparna A, et al. Anti-leukemic effects of gallic acid on human leukemia K562 cells: downregulation of COX-2, inhibition of BCR/ABL kinase and NF-κB inactivation. Toxicol In Vitro. 2012;26(3):396–405. doi: 10.1016/j.tiv.2011.12.018 [DOI] [PubMed] [Google Scholar]
- 15.Gu R, Zhang M, Meng H, Xu D, Xie Y. Gallic acid targets acute myeloid leukemia via Akt/mTOR-dependent mitochondrial respiration inhibition. Biomed Pharmacother. 2018;105:491–497. doi: 10.1016/j.biopha.2018.05.158 [DOI] [PubMed] [Google Scholar]
- 16.Mayer LD, Harasym TO, Tardi PG, et al. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5(7):1854–1863. doi: 10.1158/1535-7163.MCT-06-0118 [DOI] [PubMed] [Google Scholar]
- 17.Basha R, Sabnis N, Heym K, Bowman WP, Lacko AG. Targeted nanoparticles for pediatric leukemia therapy. Front Oncol. 2014;4:101. doi: 10.3389/fonc.2014.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Termsarasab U, Yoon IS, Park JH, Moon HT, Cho HJ, Kim DD. Polyethylene glycol-modified arachidyl chitosan-based nanoparticles for prolonged blood circulation of doxorubicin. Int J Pharm. 2014;464(1–2):127–134. doi: 10.1016/j.ijpharm.2014.01.015 [DOI] [PubMed] [Google Scholar]
- 19.Ma P, Dong X, Swadley CL, et al. Development of idarubicin and doxorubicin solid lipid nanoparticles to overcome Pgp-mediated multiple drug resistance in leukemia. J Biomed Nanotechnol. 2009;5(2):151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387 [DOI] [PubMed] [Google Scholar]
- 21.Mooberry LK, Nair M, Paranjape S, McConathy WJ, Lacko AG. Receptor mediated uptake of paclitaxel from a synthetic high density lipoprotein nanocarrier. J Drug Target. 2010;18(1):53–58. doi: 10.3109/10611860903156419 [DOI] [PubMed] [Google Scholar]
- 22.Qiu J, Cheng R, Zhang J, et al. Glutathione-sensitive hyaluronic acid-mercaptopurine prodrug linked via carbonyl vinyl sulfide: a robust and CD44-targeted nanomedicine for leukemia. Biomacromolecules. 2017;18(10):3207–3214. doi: 10.1021/acs.biomac.7b00846 [DOI] [PubMed] [Google Scholar]
- 23.Zhong Y, Goltsche K, Cheng L, et al. Hyaluronic acid-shelled acid-activatable paclitaxel prodrug micelles effectively target and treat CD44-overexpressing human breast tumor xenografts in vivo. Biomaterials. 2016;84:250–261. doi: 10.1016/j.biomaterials.2016.01.049 [DOI] [PubMed] [Google Scholar]
- 24.Mandal B, Bhattacharjee H, Mittal N, et al. Core-shell-type lipid-polymer hybrid nanoparticles as a drug delivery platform. Nanomedicine. 2013;9(4):474–491. doi: 10.1016/j.nano.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 25.Zhu B, Zhang H, Yu L. Novel transferrin modified and doxorubicin loaded Pluronic 85/lipid-polymeric nanoparticles for the treatment of leukemia: in vitroand in vivo therapeutic effect evaluation. Biomed Pharmacother. 2017;86:547–554. doi: 10.1016/j.biopha.2016.11.121 [DOI] [PubMed] [Google Scholar]
- 26.Yang F, Li A, Liu H, Zhang H. Gastric cancer combination therapy: synthesis of a hyaluronic acid and cisplatin containing lipid prodrug coloaded with sorafenib in a nanoparticulate system to exhibit enhanced anticancer efficacy and reduced toxicity. Drug Des Devel Ther. 2018;12:3321–3333. doi: 10.2147/DDDT.S176879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Chan JM, Gu FX, et al. Self-assembled lipid–polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano. 2008;2(8):1696–1702. doi: 10.1021/nn800275r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bi D, Zhao L, Yu R, et al. Surface modification of doxorubicin-loaded nanoparticles based on polydopamine with pH-sensitive property for tumor targeting therapy. Drug Deliv. 2018;25(1):564–575. doi: 10.1080/10717544.2018.1440447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guhagarkar SA, Majee SB, Samad A, Devarajan PV. Evaluation of pullulan-functionalized doxorubicin nanoparticles for asialoglycoprotein receptor-mediated uptake in Hep G2 cell line. Cancer Nanotechnol. 2011;2(1–6):49–55. doi: 10.1007/s12645-011-0012-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daduang J, Palasap A, Daduang S, Boonsiri P, Suwannalert P, Limpaiboon T. Gallic acid enhancement of gold nanoparticle anticancer activity in cervical cancer cells. Asian Pac J Cancer Prev. 2015;16(1):169–174. [DOI] [PubMed] [Google Scholar]
- 31.Tan S, Wang G. Lung cancer targeted therapy: folate and transferrin dual targeted, glutathione responsive nanocarriers for the delivery of cisplatin. Biomed Pharmacother. 2018;102:55–63. doi: 10.1016/j.biopha.2018.03.046 [DOI] [PubMed] [Google Scholar]
- 32.Song YF, Liu DZ, Cheng Y, et al. Charge reversible and mitochondria/nucleus dual target lipid hybrid nanoparticles to enhance antitumor activity of doxorubicin. Mol Pharm. 2018;15(3):1296–1308. doi: 10.1021/acs.molpharmaceut.7b01109 [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Zhang P, Zhu T. Ovarian carcinoma biological nanotherapy: comparison of the advantages and drawbacks of lipid, polymeric, and hybrid nanoparticles for cisplatin delivery. Biomed Pharmacother. 2019;109:475–483. doi: 10.1016/j.biopha.2018.10.158 [DOI] [PubMed] [Google Scholar]
- 34.Zhang R, Ru Y, Gao Y, Li J, Mao S. Layer-by-layer nanoparticles co-loading gemcitabine and platinum (IV) prodrugs for synergistic combination therapy of lung cancer. Drug Des Devel Ther. 2017;11:2631–2642. doi: 10.2147/DDDT.S143047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang G, Wang Z, Li C, et al. RGD peptide-modified, paclitaxel prodrug-based, dual-drugs loaded, and redox-sensitive lipid-polymer nanoparticles for the enhanced lung cancer therapy. Biomed Pharmacother. 2018;106:275–284. doi: 10.1016/j.biopha.2018.06.137 [DOI] [PubMed] [Google Scholar]
- 36.Li S, Wang L, Li N, Liu Y, Su H. Combination lung cancer chemotherapy: design of a pH-sensitive transferrin-PEG-Hz-lipid conjugate for the co-delivery of docetaxel and baicalin. Biomed Pharmacother. 2017;95:548–555. doi: 10.1016/j.biopha.2017.08.090 [DOI] [PubMed] [Google Scholar]
- 37.He P, Zhu X. Phospholipid-assisted synthesis of size-controlled gold nanoparticles. Mat Res Bul. 2007;42:1310–1315. doi: 10.1016/j.materresbull.2006.10.014 [DOI] [Google Scholar]
- 38.Ma D, Lin QM, Zhang LM, Liang YY, Xue W. A star-shaped porphyrin-arginine functionalized poly(L-lysine) copolymer for photo-enhanced drug and gene co-delivery. Biomaterials. 2014;35:4357–4367. doi: 10.1016/j.biomaterials.2014.01.070 [DOI] [PubMed] [Google Scholar]
- 39.Balakrishnan P, Song CK, Jahn A, Cho HJ. Ceramide and N,N,N-trimethylphytosphingosine-iodide (TMP-I)-based lipid nanoparticles for cancer therapy. Pharm Res. 2016;33(1):206–216. doi: 10.1007/s11095-015-1780-5 [DOI] [PubMed] [Google Scholar]
- 40.Gao Z, Li Z, Yan J, Wang P. Irinotecan and 5-fluorouracil-co-loaded, hyaluronic acid-modified layer-by-layer nanoparticles for targeted gastric carcinoma therapy. Drug Des Devel Ther. 2017;11:2595–2604. doi: 10.2147/DDDT.S140797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chou TC, Talalay P. Analysis of combined drug effects: a new look at a very old problem. Trends Pharmacol Sci. 1983;4:450–454. doi: 10.1016/0165-6147(83)90490-X [DOI] [Google Scholar]
- 42.Duan W, Liu Y. Targeted and synergistic therapy for hepatocellular carcinoma: monosaccharide modified lipid nanoparticles for the co-delivery of doxorubicin and sorafenib. Drug Des Devel Ther. 2018;12:2149–2161. doi: 10.2147/DDDT.S166402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Culty M, O’Mara TE, Underhill CB, Yeager H Jr, Swartz RP. Hyaluronan receptor (CD44) expression and function in human peripheral blood monocytes and alveolar macrophages. J Leukoc Biol. 1994;56(5):605–611. doi: 10.1002/jlb.56.5.605 [DOI] [PubMed] [Google Scholar]
- 44.Li M, Fei X, Shi F, et al. Homoharringtonine delivered by high proportion PEG of long- circulating liposomes inhibits RPMI8226 multiple myeloma cells in vitro and in vivo. Am J Transl Res. 2016;8(3):1355–1368. [PMC free article] [PubMed] [Google Scholar]
- 45.Li W, Yi X, Liu X, Zhang Z, Fu Y, Gong T. Hyaluronic acid ion-pairing nanoparticles for targeted tumor therapy. J Control Release. 2016;225:170–182. doi: 10.1016/j.jconrel.2016.01.049 [DOI] [PubMed] [Google Scholar]
- 46.Kim CE, Lim SK, Kim JS. In vivo antitumor effect of cromolyn in PEGylated liposomes for pancreatic cancer. J Control Release. 2012;157(2):190–195. doi: 10.1016/j.jconrel.2011.09.066 [DOI] [PubMed] [Google Scholar]