Short abstract
Review on SAP affects neutrophil adhesion, monocyte differentiation, and macrophage polarization in the innate immune system.
Keywords: neutrophil adhesion, macrophage polarization, fibrocyte differentiation, Fcγ receptors, phagocytosis, fibrosis
Abstract
The pentraxin SAP reduces neutrophil adhesion to ECM proteins, inhibits the differentiation of monocytes into fibrocytes, attenuates profibrotic macrophages, activates the complement pathway, and promotes phagocytosis of cell debris. Together, these effects of SAP regulate key aspects of inflammation and set a threshold for immune cell activation. Here, we present a review of SAP biology with an emphasis on SAP receptor interactions and how the effect of SAP on monocytes and macrophages has been explored to develop this protein as a therapeutic for renal and lung injuries. We also discuss how there remain many unanswered questions about the role of SAP in innate immunity.
Abbreviations
- CRP
C‐reactive protein
- ECM
extracellular matrix
- NPTX1/2
neuronal pentraxin 1/2
- PTX3/4
pentraxin 3/4
- ROS
reactive oxygen species
- SAP
serum amyloid P
Introduction
The mammalian immune system is organized into two arms: innate and adaptive immunity. Innate immunity is evolutionary more ancient and constitutes the first line of defense against foreign pathogens [1]. In vertebrates, adaptive immunity complements innate immunity and provides immunological memory [2]. Pathogen recognition molecules, such as pentraxins, are at the core of innate immunity [3, 4]. Pentraxins recognize evolutionarily conserved pathogen molecules, such as C‐polysaccharide, regulate complement activation, and bind apoptotic cells to initiate and synchronize the immune response [4, –, 6, 7].
Pentraxins are a family of conserved proteins that appeared early on during the evolution of innate immunity [8] and have a 200‐aa long pentraxin domain with a conserved pentraxin signature (HxCxS/TWxS, where x=any amino acid) [9]. Pentraxins are organized into two groups: the short and the long pentraxins. The short pentraxins are identified by their pentameric structure consisting of 25 kDa monomers and include CRP and SAP (for a review on CRP, see ref. [10]). The long pentraxins have an N‐terminal domain attached to a pentraxin domain and include PTX3, PTX4, guinea pig apexin, NPTX1, NPTX2, and NPTXR (for reviews, see refs. [4, 9, 11, 12]).
The short pentraxins CRP and SAP are pattern recognition molecules secreted by the liver that interact with pathogens and cell debris to promote their removal by macrophages and neutrophils [13]. SAP binds to rough LPS, and lack of SAP causes hypersensitivity to laboratory strains of Escherichia coli [14]. In addition, CRP and SAP interact with components of the complement pathway to regulate complement activation [15, 16]. However, the regulation of the innate immune system by SAP is not limited to its effects on the complement pathway and phagocytosis. SAP binds directly to monocytes, neutrophils, and macrophages to modify their activation and alter their differentiation to modulate the immune response.
REGULATION OF NEUTROPHIL FUNCTION BY SAP
At the onset of inflammation, neutrophils are recruited to the damaged tissue, where they release ROS and promote clearance of pathogens and cell debris. This recruitment is mediated by cytokines, tissue damage, complement activation, and changes in adhesion receptors on the surface of endothelial cells [17, –, 19]. The migration and activation of neutrophils are tightly regulated by factors expressed and secreted by endothelial cells and macrophages [17]. However, factors present in plasma also affect neutrophils [17].
SAP binds to neutrophils to regulate their function
One circulating factor that regulates neutrophil accumulation in tissues is SAP [20]. SAP binds to human and murine neutrophils and decreases TNF‐α‐ and IL‐8‐induced neutrophil binding to ECM components [20, 21]. SAP also reduces TNF‐α‐induced human neutrophil adhesion to endothelial cells [22]. One possible mechanism underlying the effect of SAP on neutrophils involves SAP binding to, and thus, potentially blocking, the adhesion receptor L‐selectin on neutrophils [22]. This is supported by the observation that adding anti‐L‐selectin antibodies to human neutrophils decreases their binding to umbilical vein endothelial cells [22]. A second possible mechanism involves SAP binding to FcγRs on neutrophils [23, –, 25]. FcγRs are best known for binding IgG and in humans, include the activating receptors FcγRI, FcγRIIa, FcγRIIIa, and FcγRIIIb and the inhibitory receptor FcγRIIb [26, 27]. FcγRIIa and FcγRIIIb are expressed at high levels on human neutrophils, and activation of FcγRIIa by SAP results in the phosphorylation of the ITAM in the cytosolic region of FcγRIIa [28]. ITAM activation can then lead to conformational changes in adhesion receptors on neutrophils via inside‐out signaling [29]. Much remains to be investigated about the effects of SAP on neutrophils, and most likely, this effect involves a variety of receptors, including FcγRs and L‐selectin.
SAP inhibits neutrophil spreading
In addition to decreasing neutrophil adhesion, SAP decreases human neutrophil spreading, a necessary step for cell polarization and migration [20, 30, –, 32, 33]. Paradoxically, SAP does not influence human neutrophil migration in response to fMLP in a Boyden chamber [20]. This inconsistency may be a result of the differences in the adhesion receptors used during neutrophil migration on matrix proteins and after stimulation with chemotactic stimuli in a Boyden chamber. On fibronectin, neutrophils use the β1 (VLA‐4 and VLA‐5) integrins to migrate, whereas in an uncoated Boyden chamber, β2 (CD11b/CD18) integrins are the key adhesion receptors [34, 35]. SAP may act as a chemoattractant of human neutrophils, although this finding has not been replicated [21]. Alternatively, it is possible that the timing of stimuli (i.e., SAP) could determine how neutrophil spreading and migration are influenced.
Indirect effects of SAP on neutrophils
Neutrophils secrete proteases, such as elastase, to degrade the ECM and facilitate tissue infiltration (for a review, see ref. [36]). SAP but not CRP binds to neutrophil elastase and inhibits its enzymatic activity [37]. This can hinder neutrophil extravasation and the secondary damage caused by the proteolytic activity of elastase in tissues [36, –, 38, 39, 40]. SAP also induces macrophages to produce the anti‐inflammatory cytokine IL‐10, which in turn, decreases TNF‐α and CXCL8 production. This then results in decreased neutrophil recruitment [23, 41, –, 43]. These observations suggest that SAP regulates many aspects of neutrophil biology to exert an anti‐inflammatory effect and set a threshold for neutrophil recruitment and activation ( Fig. 1 ). In agreement with this, we have observed that SAP injections can decrease neutrophil accumulation in a mouse model of acute respiratory distress syndrome [20].
Figure 1.
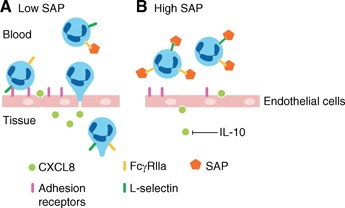
SAP inhibits neutrophil recruitment. (A) In response to chemoattractants, such as CXCL8, neutrophils begin to migrate into the tissue in a process that involves neutrophil rolling, arrest, and extravasation. All of these steps are mediated by adhesion receptors on the endothelial cells and on neutrophils. (B) In the presence of high levels of SAP, neutrophil recruitment to the tissue is reduced, as SAP‐induced IL‐10 inhibits the secretion of CXCL8. SAP also reduces neutrophil adhesion by preventing L‐selectin binding to adhesion receptors on endothelial cells. SAP may further affect neutrophil adhesion by regulating adhesion receptors on neutrophils by inside‐out signaling via FcγRs. In addition, SAP reduces neutrophil migration by inhibiting neutrophil spreading and elastase activity.
SAP INHIBITS MONOCYTE‐TO‐FIBROCYTE DIFFERENTIATION
Monocytes present within the blood are attracted to sites of injury where they differentiate into macrophages, dendritic cells, or fibrocytes [44, 45]. Fibrocytes are spindle‐shaped, fibroblast‐like cells and at least, in part, mediate tissue repair and fibrosis (for a review, see ref. [45]). Fibrocytes have been detected in human pathological conditions, including pulmonary fibrosis, keloid scars, asthma, chronic kidney disease, and nephrogenic systemic fibrosis [45, –, 47, 48, 49]. In addition to contributing to the mass of fibrotic lesions, fibrocytes promote angiogenesis, which can then promote the growth of the lesion, and secrete TGF‐β, which activates resident fibroblasts [50]. Fibrocyte differentiation is regulated by several factors, including cytokines, TLR ligands, semaphorins, and hyaluronic acid [45, 51, –, 53]. We found that when human, mouse, or rat PBMCs were cultured in serum‐free media, some of the cells became fibrocytes after 3–5 days [54]. The fibrocytes did not appear during this timeframe when serum was present [54]. We purified the fibrocyte differentiation inhibitor from human serum and identified it as SAP [54]. When PBMCs were cultured in serum that was depleted of SAP, fibrocytes appeared rapidly, indicating that SAP is the main endogenous inhibitor of fibrocyte differentiation in the blood. In agreement with this, we observed that depleting SAP from dermal wounds in pigs can facilitate fibrocyte differentiation and scar‐tissue formation [55]. We also tested whether SAP could inhibit fibrocyte differentiation and fibrosis in bleomycin‐induced lung fibrosis [56]. We found that SAP injections led to reduced numbers of fibrocytes in the lungs and reduced fibrosis in rats and mice and that delaying SAP injections until inflammation and fibrosis were already apparent could also reduce symptoms [56].
SAP inhibits fibrocyte differentiation, in part, by binding to FcγRs [57]. In support of this, we have found that cross‐linked but not monomeric IgG inhibits fibrocyte differentiation and that blocking the signal transduction pathway of the FcγRs with pharmacological inhibitors blocks the ability of SAP and cross‐linked IgG to inhibit fibrocyte differentiation [58]. In mice, deletion of the FcRγ, which is necessary for FcγRI and FcγRIIIa signaling, significantly reduces sensitivity to SAP [23, 57, 59]. However, deletions of FcγRIIb, FcγRIII, and FcγRIV do not affect sensitivity to SAP [57]. We found similar results using small interfering RNA knockdowns of human receptors [57]. However, in all cases, SAP still caused some inhibition of fibrocyte differentiation [23, 57], indicating the presence of additional SAP receptors on monocytes. These observations suggest that SAP, in part, uses FcγRI and FcRγ to inhibit fibrocyte differentiation ( Fig. 2 ).
Figure 2.
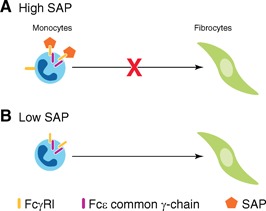
SAP inhibits fibrocyte formation. (A) When SAP is present in the tissue, as is the case in early inflammation, SAP binds to FcγRI to inhibit fibrocyte differentiation. Deletion of the FcγRI or the FcRγ significantly reduces the inhibitory effect of SAP. (B) At late stages of inflammation, when SAP levels are low, monocytes differentiate into fibroblast‐like cells called fibrocytes. Fibrocytes then secrete ECM components, such as collagen and extracellular‐modifying enzymes, to restore the architecture of the damaged tissue.
SAP REGULATES MURINE MACROPHAGE POLARIZATION
Macrophages are considered one of the most important innate effector cells (for reviews, see refs. [60, –, 62]). Macrophages can be classified into the classically activated macrophages (M1) and the alternatively activated macrophages (M2) [60, 63]. M1 macrophages are induced in response to TNF‐α, IFN‐γ, and specific TLR agonists [60, 64]. The classically activated M1 macrophages modulate host defense against intracellular pathogens, tumor cells, and tissue debris but are also responsible for tissue damage associated with their release of ROS [60, 63, 65, –, 67]. M2 is a general term for several overlapping macrophage subsets, which are induced in response to IL‐4, IL‐10, IL‐13, and SAP [23, 60, 64].The role of M2 macrophages in the immune system is highly dependent on the activating stimuli (i.e., IL‐10 vs. IL‐4) and the environmental context. The alternatively activated M2 macrophages can be classified into three main groups: immunoregulatory macrophages, profibrotic/wound‐healing macrophages, and tumor‐associated macrophages [60, 63, 68, 69]. The hallmark of immunoregulatory macrophages in humans and mice is high levels of the anti‐inflammatory cytokine IL‐10 and low levels of the proinflammatory cytokine IL‐12 [60]. Wound‐healing macrophages express high levels of IL‐10 and IL‐12, whereas tumor‐associated macrophages are identified by their secretion of a variety of angiogenic factors [60, 68].
SAP promotes immunoregulatory macrophages in mouse renal injuries
In a mouse model of systemic lupus erythematosus, macrophages in the kidneys have elevated expression of IL‐10, iNOS, and TNF‐α [64]. IL‐10 is a marker for M2 macrophages, whereas iNOS and TNF‐α are typically associated with M1 macrophages [60, 64]. When the mice were injected with SAP, the expression of the M2 markers IL‐10 and arginase 1 in the kidney macrophages was increased, whereas the levels of the M1 markers iNOS and TNF‐α decreased [64]. This change in gene expression involved the PI3K/Akt–ERK signaling pathway and indicates a shift toward an immunoregulatory phenotype in macrophages [64].
In mouse models of renal fibrosis, SAP injections decreased expression of the M1 markers Mip2a and IL‐1β, and the profibrotic M2 markers CCL17 and CCL22 on renal macrophages [23, 60]. These changes were accompanied by a significant increase in the levels of IL‐10 [23]. In IL‐10 and FcRγ knockout mice, the effects of SAP on renal fibrosis were reduced [23]. Together, these observations suggest that SAP, in two different models of renal injuries, polarizes macrophages toward an immunoregulatory phenotype.
SAP attenuates profibrotic macrophages in mouse lungs
In TGF‐β‐driven mouse models of pulmonary fibrosis, SAP alleviates fibrosis, in part, through its effect on macrophages [70, 71]. In this model of pulmonary fibrosis, SAP injections decreased M2 markers, while increasing the M1 marker CXCL10 on pulmonary macrophages [60, 70]. This is in stark contrast to the role of SAP in renal injuries, where it promotes immunoregulatory macrophages and decreases M1 macrophages. This inconsistency may be attributed to differences that exist in the milieu of kidneys and lungs. In support of this, similar to TGF‐β‐driven pulmonary fibrosis, SAP attenuated M2 macrophage activation in the spore‐induced allergic airway disease of mice [60, 72]. Furthermore, in spore‐induced allergic airway disease, SAP injections increased expression of the M1 marker IFN‐γ in lung macrophages, whereas not significantly altering levels of the immunoregulatory marker IL‐10 [72]. Together, these observations suggest that SAP has a significant role in regulating macrophage polarization, but the outcome is tissue‐dependent and at times quite different. ( Fig. 3 ).
Figure 3.
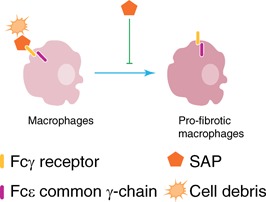
SAP inhibits profibrotic macrophages in mice. SAP attenuates profibrotic macrophages in renal and pulmonary injuries of mice in an FcRγ‐mediated manner. SAP also opsonizes cell debris to promote their removal by macrophages.
CONCLUDING REMARKS
SAP plays a significant role in the regulation of the innate immune system by binding to FcγRs. SAP decreases neutrophils adhesion, regulates macrophage activation, enhances phagocytosis of cell debris, activates the complement pathway, and inhibits fibrocyte differentiation. Together, these effects of SAP inhibit many aspects of innate immunity that contribute to inflammation and fibrosis. Depletion of SAP on dermal wounds in pigs and injections of SAP in animal models of acute respiratory distress syndrome and fibrosis, as well as in patients with pulmonary fibrosis, suggest that manipulating SAP levels may be an effective therapeutic. Despite the variety of roles that SAP plays in the innate immunity, little is known about the underlying mechanism. Furthermore, it is not clear how SAP influences the adaptive immune system. Further work will hopefully delineate how this phylogenetically ancient protein regulates leukocyte biology and affects human health.
AUTHORSHIP
N.C., D.P., and R.H.G. contributed to writing.
DISCLOSURES
D.P. and R.H.G. are inventors on patents for the use of SAP as a therapeutic for fibrosing diseases and patents for the use of SAP‐depleting materials to enhance wound healing. D.P. and R.H.G. are members of the Science Advisory Board of and have stock options from Promedior, a start‐up company that is developing SAP as a therapeutic for fibrosing diseases, and receive a share of milestone payments made by Promedior to Rice University.
ACKNOWLEDGMENTS
We thank the current and former members of the lab.
REFERENCES
- 1. Janeway C. A., Jr. , Medzhitov, R. (2002) Innate immune recognition. Annu. Rev. Immunol. 20, 197–216. [DOI] [PubMed] [Google Scholar]
- 2. Boehm, T. (2012) Evolution of vertebrate immunity. Curr. Biol. 22, R722–R732. [DOI] [PubMed] [Google Scholar]
- 3. Mantovani, A. S. , Biswas, M. Galdiero R., Sica, A. , Locati, M. (2013) Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 229, 176–185. [DOI] [PubMed] [Google Scholar]
- 4. Deban, L. , Jaillon, S. , Garlanda, C. , Bottazzi, B. , Mantovani, A. (2011) Pentraxins in innate immunity: lessons from PTX3. Cell Tissue Res. 343, 237–249. [DOI] [PubMed] [Google Scholar]
- 5. Tillett, W. S. , Francis, T. (1930) Serological reactions in pneumonia with a non‐protein somatic fraction of pneumococcus. J. Exp. Med. 52, 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Haas, C. J. , van der Tol, M. E. , Van Kessel, K. P. , Verhoef, J. , Van Strijp, J. A. (1998) A synthetic lipopolysaccharide‐binding peptide based on amino acids 27–39 of serum amyloid P component inhibits lipopoly‐saccharide‐induced responses in human blood. J. Immunol. 161, 3607–3615. [PubMed] [Google Scholar]
- 7. Litvack, M. L. , Palaniyar, N. (2010) Review: soluble innate immune pattern‐recognition proteins for clearing dying cells and cellular components: implications on exacerbating or resolving inflammation. Innate Immun. 16, 191–200. [DOI] [PubMed] [Google Scholar]
- 8. Robey, F. A. , Liu, T. Y. (1981) Limulin: a C‐reactive protein from Limulus polyphemus. J. Biol. Chem. 256, 969–975. [PubMed] [Google Scholar]
- 9. Deban, L. , Bottazzi, B. , Garlanda, C. , de la Torre, Y. M. , Mantovani, A. (2009) Pentraxins: multifunctional proteins at the interface of innate immunity and inflammation. BioFactors 35, 138–145. [DOI] [PubMed] [Google Scholar]
- 10. Du Clos, T. W. (2013) Pentraxins: structure, function, and role in inflammation. ISRN Inflamm. 2013, 379040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mantovani, A. , Valentino, S. , Gentile, S. , Inforzato, A. , Bottazzi, B. , Garlanda, C. (2013) The long pentraxin PTX3: a paradigm for humoral pattern recognition molecules. Ann. N. Y. Acad. Sci. 1285, 1–14. [DOI] [PubMed] [Google Scholar]
- 12. Martinez de la Torre, Y. , Fabbri, M. , Jaillon, S. , Bastone, A. , Nebuloni, M. , Vecchi, A. , Mantovani, A. , Garlanda, C. (2010) Evolution of the pentraxin family: the new entry PTX4. J. Immunol. 184, 5055–5064. [DOI] [PubMed] [Google Scholar]
- 13. Hutchinson, W. L. , Hohenester, E. , Pepys, M. B. (2000) Human serum amyloid P component is a single uncomplexed pentamer in whole serum. Mol. Med. 6, 482–493. [PMC free article] [PubMed] [Google Scholar]
- 14. Noursadeghi, M. , Bickerstaff, M. C. , Gallimore, J. R. , Herbert, J. , Cohen, J. , Pepys, M. B. (2000) Role of serum amyloid P component in bacterial infection: protection of the host or protection of the pathogen. Proc. Natl. Acad. Sci. USA 97, 14584–14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mold, C. , Gewurz, H. , Du Clos, T. W. (1999) Regulation of complement activation by C‐reactive protein. Immunopharmacology 42, 23–30. [DOI] [PubMed] [Google Scholar]
- 16. Ying, S. C. , Gewurz, A. T. , Jiang, H. , Gewurz, H. (1993) Human serum amyloid P component oligomers bind and activate the classical complement pathway via residues 14–26 and 76–92 of the A chain collagen‐like region of C1q. J. Immunol. 150, 169–176. [PubMed] [Google Scholar]
- 17. Kolaczkowska, E. , Kubes, P. (2013) Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175. [DOI] [PubMed] [Google Scholar]
- 18. Montecucco, F. , Steffens, S. , Burger, F. , Da, Costa, A. , Bianchi, G. , Bertolotto, M. , Mach, F. , Dallegri, F. , Ottonello, L. (2008) Tumor necrosis factor‐α (TNF‐α) induces integrin CD11b/CD18 (Mac‐1) up‐regulation and migration to the CC chemokine CCL3 (MIP‐1α) on human neutrophils through defined signalling pathways. Cell. Signal. 20, 557–568. [DOI] [PubMed] [Google Scholar]
- 19. Leonard, E. J. , Yoshimura, T. (1990) Neutrophil attractant/activation protein‐1 (NAP‐1 [interleukin‐8]). Am. J. Respir. Cell Mol. Biol. 2, 479–486. [DOI] [PubMed] [Google Scholar]
- 20. Maharjan, A. S. , Roife, D. , Brazill, D. , Gomer, R. H. (2013) Serum amyloid P inhibits granulocyte adhesion. Fibrogenesis Tissue Repair 6, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galkina, E. V. , Nazarov, P. G. , Polevschikov, A. V. , Berestovaya, L. K. , Galkin, V. E. , Bychkova, N. V. (2000) Interactions of C‐reactive protein and serum amyloid P component with interleukin‐8 and their role in regulation of neutrophil functions. Russ. J. Immunol. 5, 363–374. [PubMed] [Google Scholar]
- 22. Stibenz, D. , Grafe, M. , Debus, N. , Hasbach, M. , Bahr, I. , Graf, K. , Fleck, E. , Thanabalasingam, U. , Buhrer, C. (2006) Binding of human serum amyloid P component to L‐selectin. Eur. J. Immunol. 36, 446–456. [DOI] [PubMed] [Google Scholar]
- 23. Castaño, A. P. , Lin, S. L. , Surowy, T. , Nowlin, B. T. , Turlapati, S. A. , Patel, T. , Singh, A. , Li, S. Lupher M. L., Jr. , Duffield, J. S. (2009) Serum amyloid P inhibits fibrosis through Fc γ R‐dependent monocyte‐macro‐phage regulation in vivo. Sci. Transl. Med. 1, 5ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mold, C. , Gresham, H. D. , Du Clos, T. W. (2001) Serum amyloid P component and C‐reactive protein mediate phagocytosis through murine Fc γ Rs. J. Immunol. 166, 1200–1205. [DOI] [PubMed] [Google Scholar]
- 25. Bharadwaj, D. , Mold, C. , Markham, E. , Du Clos, T. W. (2001) Serum amyloid P component binds to Fc γ receptors and opsonizes particles for phagocytosis. J. Immunol. 166, 6735–6741. [DOI] [PubMed] [Google Scholar]
- 26. Bruhns, P. , Iannascoli, B. , England, P. , Mancardi, D. A. , Fernandez, N. , Jorieux, S. , Daeron, M. (2009) Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood 113, 3716–3725. [DOI] [PubMed] [Google Scholar]
- 27. Nimmerjahn, F. , Ravetch, J. V. (2006) Fcy receptors: old friends and new family members. Immunity 24, 19–28. [DOI] [PubMed] [Google Scholar]
- 28. Chi, M. , Tridandapani, S. , Zhong, W. , Coggeshall, K. M. , Mortensen, R. F. (2002) C‐Reactive protein induces signaling through Fc γ RIIa on HL‐60 granulocytes. J. Immunol. 168, 1413–1418. [DOI] [PubMed] [Google Scholar]
- 29. Abram, C. L. , Lowell, C. A. (2009) The ins and outs of leukocyte integrin signaling. Annu. Rev. Immunol. 27, 339–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dewitt, S. , Francis, R. J. , Hallett, M. B. (2013) Ca(2)(+) and calpain control membrane expansion during the rapid cell spreading of neutrophils. J. Cell Sci. 126, 4627–4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ley, K. , Laudanna, C. , Cybulsky, M. I. , Nourshargh, S. (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689. [DOI] [PubMed] [Google Scholar]
- 32. Sengupta, K. , Aranda‐Espinoza, H. , Smith, L. , Janmey, P. , Hammer, D. (2006) Spreading of neutrophils: from activation to migration. Biophys. J. 91, 4638–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lokuta, M. A. , Huttenlocher, A. (2005) TNF‐α promotes a stop signal that inhibits neutrophil polarization and migration via a p38 MAPK pathway. J. Leukoc. Biol. 78, 210–219. [DOI] [PubMed] [Google Scholar]
- 34. Van den Berg, J. M. , Mul, F. P. , Schippers, E. , Weening, J. J. , Roos, D. , Kuijpers, T. W. (2001) β1 Integrin activation on human neutrophils promotes β2 integrin‐mediated adhesion to fibronectin. Eur. J. Immunol. 31, 276–284. [DOI] [PubMed] [Google Scholar]
- 35. Furie, M. B. , Tancinco, M. C. , Smith, C. W. (1991) Monoclonal antibodies to leukocyte integrins CD11a/CD18 and CD11b/CD18 or intercellular adhesion molecule‐1 inhibit chemoattractant‐stimulated neutrophil transendothelial migration in vitro. Blood 78, 2089–2097. [PubMed] [Google Scholar]
- 36. Sandhaus, R. A. , Turino, G. (2013) Neutrophil elastase‐mediated lung disease. COPD 10 (Suppl. 1), 60–63. [DOI] [PubMed] [Google Scholar]
- 37. Vachino, G. , Heck, L. W. , Gelfand, J. A. , Kaplan, M. M. , Burke, J. F. , Berninger, R. W. , McAdam, K. P. (1988) Inhibition of human neutrophil and Pseudomonas elastases by the amyloid P‐component: a constituent of elastic fibers and amyloid deposits. J. Leukoc. Biol. 44, 529–534. [DOI] [PubMed] [Google Scholar]
- 38. Takai, S. , Kimura, K. , Nagaki, M. , Satake, S. , Kakimi, K. , Moriwaki, H. (2005) Blockade of neutrophil elastase attenuates severe liver injury in hepatitis B transgenic mice. J. Virol. 79, 15142–15150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Korkmaz, B. , Horwitz, M. S. , Jenne, D. E. , Gauthier, F. (2010) Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol. Rev. 62, 726–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takemasa, A. , Ishii, Y. , Fukuda, T. (2012) A neutrophil elastase inhibitor prevents bleomycin‐induced pulmonary fibrosis in mice. Eur. Respir. J. 40, 1475–1482. [DOI] [PubMed] [Google Scholar]
- 41. Wertheim, W. A. , Kunkel, S. L. , Standiford, T. J. , Burdick, M. D. , Becker, F. S. , Wilke, C. A. , Gilbert, A. R. , Strieter, R. M. (1993) Regulation of neutrophil‐derived IL‐8: the role of prostaglandin E2, dexameth‐asone, and IL‐4. J. Immunol. 151, 2166–2175. [PubMed] [Google Scholar]
- 42. Tryzmel, J. , Miskolci, V. , Castro‐Alcaraz, S. , Vancurova, I. , Davidson, D. (2003) Interleukin‐10 inhibits proinflammatory chemokine release by neutrophils of the newborn without suppression of nuclear factor‐κ B. Pediatr. Res. 54, 382–386. [DOI] [PubMed] [Google Scholar]
- 43. Shanley, T. P. , Vasi, N. , Denenberg, A. (2000) Regulation of chemokine expression by IL‐10 in lung inflammation. Cytokine 12, 1054–1064. [DOI] [PubMed] [Google Scholar]
- 44. Geissmann, F. , Manz, M. G. , Jung, S. , Sieweke, M. H. , Merad, M. , Ley, K. (2010) Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reilkoff, R. A. , Bucala, R. , Herzog, E. L. (2011) Fibrocytes: emerging effector cells in chronic inflammation. Nat. Rev. Immunol. 11, 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schmidt, M. , Sun, G. , Stacey, M. A. , Mori, L. , Mattoli, S. (2003) Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J. Immunol. 171, 380–389. [DOI] [PubMed] [Google Scholar]
- 47. Sakai, N. , Furuichi, K. , Shinozaki, Y. , Yamauchi, H. , Toyama, T. , Kitajima, S. , Okumura, T. , Kokubo, S. , Kobayashi, M. , Takasawa, K. , Takeda, S. , Yoshimura, M. , Kaneko, S. , Wada, T. (2010) Fibrocytes are involved in the pathogenesis of human chronic kidney disease. Hum. Pathol. 41, 672–678. [DOI] [PubMed] [Google Scholar]
- 48. Aiba, S. , Tagami, H. (1997) Inverse correlation between CD34 expression and proline‐4‐hydroxylase immunoreactivity on spindle cells noted in hypertrophic scars and keloids. J. Cutan. Pathol. 24, 65–69. [DOI] [PubMed] [Google Scholar]
- 49. Cowper, S. E. (2003) Nephrogenic fibrosing dermopathy: the first 6 years. Curr. Opin. Rheumatol. 15, 785–790. [DOI] [PubMed] [Google Scholar]
- 50. Wang, J. F. , Jiao, H. , Stewart, T. L. , Shankowsky, H. A. , Scott, P. G. , Tredget, E. E. (2007) Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 15, 113–121. [DOI] [PubMed] [Google Scholar]
- 51. Shao, D. D. , Suresh, R. , Vakil, V. , Gomer, R. H. , Pilling, D. (2008) Pivotal Advance: Th‐1 cytokines inhibit, and Th‐2 cytokines promote fibrocyte differentiation. J. Leukoc. Biol. 83, 1323–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maharjan, A. S. , Pilling, D. , Gomer, R. H. (2011) High and low molecular weight hyaluronic acid differentially regulate human fibrocyte differentiation. PloS One 6, e26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maharjan, A. S. , Pilling, D. , Gomer, R. H. (2010) Toll‐like receptor 2 agonists inhibit human fibrocyte differentiation. Fibrogenesis Tissue Repair 3, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pilling, D. , Buckley, C. D. , Salmon, M. , Gomer, R. H. (2003) Inhibition of fibrocyte differentiation by serum amyloid P. J. Immunol. 171, 5537–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gomer, R. H. , Pilling, D. , Kauvar, L. M. , Ellsworth, S. , Ronkainen, S. D. , Roife, D. , Davis, S. C. (2009) A serum amyloid P‐binding hydrogel speeds healing of partial thickness wounds in pigs. Wound Repair Regen. 17, 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pilling, D. , Roife, D. , Wang, M. , Ronkainen, S. D. , Crawford, J. R. , Travis, E. L. , Gomer, R. H. (2007) Reduction of bleomycin‐induced pulmonary fibrosis by serum amyloid P. J. Immunol. 179, 4035–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Crawford, J. R. , Pilling, D. , Gomer, R. H. (2012) FcγRI mediates serum amyloid P inhibition of fibrocyte differentiation. J. Leukoc. Biol. 92, 699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pilling, D. , Tucker, N. M. , Gomer, R. H. (2006) Aggregated IgG inhibits the differentiation of human fibrocytes. J. Leukoc. Biol. 79, 1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Haudek, S. B. , Trial, J. , Xia, Y. , Gupta, D. , Pilling, D. , Entman, M. L. (2008) Fc receptor engagement mediates differentiation of cardiac fibroblast precursor cells. Proc. Natl. Acad. Sci. USA 105, 10179–10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mosser, D. M. , Edwards, J. P. (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sica, A. , Mantovani, A. (2012) Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sica, A. (2010) Role of tumour‐associated macrophages in cancer‐related inflammation. Exp. Oncol. 32, 153–158. [PubMed] [Google Scholar]
- 63. Mantovani, A. , Sica, A. , Sozzani, S. , Allavena, P. , Vecchi, A. , Locati, M. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686. [DOI] [PubMed] [Google Scholar]
- 64. Zhang, W. , Xu, W. , Xiong, S. (2011) Macrophage differentiation and polarization via phosphatidylinositol 3‐kinase/Akt‐ERK signaling pathway conferred by serum amyloid P component. J. Immunol. 187, 1764–1777. [DOI] [PubMed] [Google Scholar]
- 65. Gordon, S. , Taylor, P. R. (2005) Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964. [DOI] [PubMed] [Google Scholar]
- 66. Auffray, C. , Sieweke, M. H. , Geissmann, F. (2009) Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 27, 669–692. [DOI] [PubMed] [Google Scholar]
- 67. Martinez, F. O. , Sica, A. , Mantovani, A. , Locati, M. (2008) Macrophage activation and polarization. Front. Biosci. 13, 453–461. [DOI] [PubMed] [Google Scholar]
- 68. Quatromoni, J. G. , Eruslanov, E. (2012) Tumor‐associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am. J. Translat. Res. 4, 376–389. [PMC free article] [PubMed] [Google Scholar]
- 69. Balkwill, F. R. , Mantovani, A. (2012) Cancer‐related inflammation: common themes and therapeutic opportunities. Sem. Cancer Biol. 22, 33–40. [DOI] [PubMed] [Google Scholar]
- 70. Murray, L. A. , Chen, Q. , Kramer, M. S. , Hesson, D. P. , Argentieri, R. L. , Peng, X. , Gulati, M. , Homer, R. J. , Russell, van Rooijen T. N., J. A., Elias, C. , Hogaboam, M. , Herzog, E. L. (2011) TGF‐β driven lung fibrosis is macrophage dependent and blocked by serum amyloid P. Int. J. Biochem. Cell Biol. 43, 154–162. [DOI] [PubMed] [Google Scholar]
- 71. Murray, L. A. , Rosada, R. , Moreira, A. P. , Joshi, A. , Kramer, M. S. , Hesson, D. P. , Argentieri, R. L. , Mathai, S. , Gulati, M. , Herzog, E. L. , Hogaboam, C. M. (2010) Serum amyloid P therapeutically attenuates murine bleomycin‐induced pulmonary fibrosis via its effects on macrophages. PloS One 5, e9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Moreira, A. P. , Cavassani, K. A. , Hullinger, R. , Rosada, R. S. , Fong, D. J. , Murray, L. , Hesson, D. P. , Hogaboam, C. M. (2010) Serum amyloid P attenuates M2 macrophage activation and protects against fungal spore‐induced allergic airway disease. J. Allergy Clin. Immunol. 126, 712–721. [DOI] [PubMed] [Google Scholar]


