Short abstract
Review on the regulatory relation between apoptosis during infection induction of Th17 responses.
Keywords: adaptive immunity, T helper 17 cells, pyroptosis, necrosis
Abstract
Microbial pathogens can initiate MOMP in host cells and as such, initiate the mitochondrial pathway of apoptosis. Innate immune recognition of cells dying in this way by infection‐induced apoptosis would involve recognition of ligands derived from the apoptotic host cell simultaneously with those derived from the infecting pathogen. The resultant signal transduction pathways engaged direct DCs to concomitantly synthesize TGF‐β and IL‐6, two cytokines that subsequently favor the differentiation of naïve CD4 T cells into Th17 cells. Citrobacter rodentium is one rodent pathogen that targets mitochondria and induces apoptosis, and blockade of apoptosis during enteric Citrobacter infection impairs the characteristic Th17 response in the intestinal LP. Here, we review these original findings. We discuss microbial infections other than Citrobacter that have been shown to induce Th17 responses, and we examine what is known about the ability of those pathogens to induce apoptosis. We also consider types of cell death other than apoptosis that can be triggered by microbial infection, and we highlight how little we know about the impact of various forms of cell death on the ensuing adaptive immune response.
Abbreviations
- ΔΨm
mitochondrial transmembrane potential
- ADPRT
ADP ribosyl transferase
- A/E
attaching and effacing
- ASC
apoptosis‐associated speck‐like protein containing a caspase recruitment domain
- BAI1
brain‐specific angiogenesis inhibitor 1
- Cag
cytotoxin‐associated gene
- CD95L
CD95 ligand
- EHEC
enterohemorrhagic strains of Escherichia coli
- EPEC
enteropathogenic Escherichia coli
- EspF
enteropathogenic Escherichia coli‐secreted protein F
- Exo
exoenzyme
- Foxp3
forkhead box p3
- GAP
GTPase‐activating protein
- GAS6
growth arrest‐specific 6
- gld
generalized lymphoproliferative disease
- HMGB1
high‐mobility group box 1 protein
- KC
keratinocyte‐derived chemokine
- LEE
locus of enterocyte effacement
- LP
lamina propria
- lpr
lymphoproliferation
- MER
c‐mer proto‐oncogene tyrosine kinase
- MFG‐E8
milk fat globule EGF8
- MMP
mitochondrial membrane permeabilization
- MOMP
mitochondrial outer membrane permeabilization
- MPT
mitochondrial permeability transition
- NLR
Nod‐like receptor
- PAI
pathogenicity island
- PS
phosphatidylserine
- Reg III
regenerating islet‐derived 3
- RIP1/3
receptor‐interacting serine‐threonine kinase 1/3
- ROR
retinoic acid‐related orphan receptor
- T3SS/T4SS
type III/IV secretion system
- TIM‐4
T cell Ig domain and mucin domain‐4
- TIR
Toll/IL‐1R homology
- Treg
T regulatory cell
- TRIF
Toll/IL‐1R homology domain‐containing adaptor‐inducing IFN‐β
- VacA
vacuolating cytotoxin A
- WT
wild type
Introduction
The immune system defends against infection by responding rapidly to microbial pathogens through a process of recognition that is based on the detection of conserved molecular structures uniquely expressed by the invading microbe. These structures are collectively known as PAMPs and are recognized by inflammatory PRRs expressed on the surface of phagocytic cells such as DCs, macrophages, and neutrophils [1]. Among the PRRs that recognize microbial pathogens, TLRs are the best characterized. TLRs are type 1 integral membrane glycoproteins, characterized by an ectodomain composed of leucine‐rich repeats responsible for recognition of PAMPs and a cytoplasmic signaling domain homologous to that of the IL‐1R, termed the TIR domain [2]. Recognition of pathogens by PRRs activates conserved host defense signaling pathways that control the expression of a variety of immune response genes, the majority of which leads to inflammatory responses [3, 4]. The TIR domain functions in the recruitment of adaptor proteins, of which, there are five: MyD88, MyD88 adaptor‐like (also known as TIR domain‐containing adaptor protein), TRIF (also known as TLR adaptor molecule 1), TIR domain‐containing adaptor‐inducing IFN‐β‐related adaptor molecule, and sterile‐α and armadillo motif‐containing protein. The ability of TLRs to recruit specific combinations of adaptors results in the activation of specific transcription factors, giving appropriate and effective responses to particular pathogens [5]. Engagement of TLRs mobilizes the activation of MAPK p38, ERK, and JNK and transcription of NF‐κB and IFN regulatory factor 1‐responsive genes, pivotal for the transcriptional initiation of a number of inflammatory, antimicrobial defense, and immune response genes [4, 5].
Importantly, PRRs also play a critical role in the recognition of dying cells in a process that is crucial for the maintenance of normal tissue turnover. Programmed cell death describes the regulated process of cell death, executed through specific intracellular signaling pathways, a process that is central to development and tissue homeostasis. Apoptotic cells express ligands, known as ″eat me″ signals, such as externalized PS [6] or ICAM‐3 [7, 8], and existing molecules that are altered by oxidation, such as oxidized low‐density lipoprotein‐like moieties [9, –, 11]. These molecules engage PRRs directly, or they can do so indirectly via specific “bridging” molecules (reviewed in refs. [12, 13]). The indirect recognition of PS can occur via bridging molecules, such as MFG‐E8, which links PS to phagocyte αvβ3 integrin [14], and GAS6 as well as Protein S, which link PS to the TYRO3, AXL, and MER receptor tyrosine kinases [15, –, 17]. Three other receptors that recognize PS include a TIM‐4 molecule on macrophages and DCs [18, 19], BAI1 [20], and stabilin‐2 [21]. Innate recognition of apoptotic cells induces the synthesis of anti‐inflammatory mediators, such as TGF‐β, PGE2, and platelet‐activating factor, by macrophages [22]. As a result, PRRs mediating the clearance of apoptotic cells are classified as noninflammatory PRRs.
APCs can discriminate between an apoptotic cell and a pathogen, according to the types of PRRs that are engaged during the activation of the innate immune response ( Fig. 1 ) [23, 24]. When pathogenic bacteria such as EPEC or Pseudomonas aeruginosa induce cell death [25, 26], the hostˈs immune system is exposed to signals from the microbial pathogen but also to signals derived from the infected dying cells [27]. Given that APCs are capable of responding to different signals by activating different PRRs, it might be expected that combinatorial signals derived from inflammatory and noninflammatory PRRs would have a large impact on host defense against microbial pathogens that induce cell death. We asked the question: what would be the resultant T cell response following simultaneous activation of inflammatory and noninflammatory PRRs in vitro and in the context of infection? Here, we discuss the evidence leading up to our hypothesis that the phagocytosis of infected apoptotic cells by DCs can induce the simultaneous production of IL‐6 and TGF‐β and lead to the polarization of Th17 cells [28]. We summarize our studies that associated the characteristic Th17 cell response, induced in response to Citrobacter rodentium infection, with the ability of Citrobacter to cause host cell apoptosis [28]. We discuss other bacterial infections known to induce Th17 and IL‐17 responses, and we consider the evidence linking these infections to the induction of host cell apoptosis. Finally, we briefly review different types of cell death and emphasize that the nature of adaptive T cell responses that might be mobilized in response to these forms of cell death is still not known.
Figure 1.
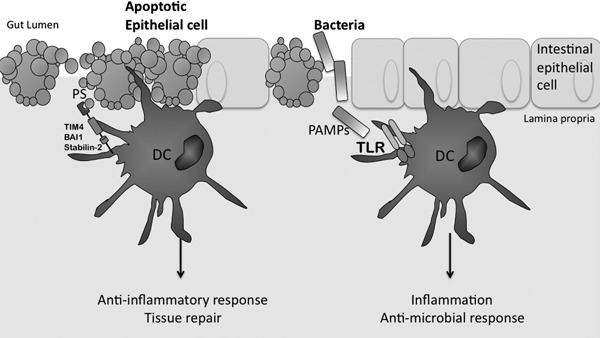
Inflammatory infection and immunosuppressive apoptosis. At steady‐state, DCs phagocytose apoptotic cells during normal tissue turnover. This figure depicts the recognition of apoptotic epithelial cells by DCs at the epithelial surface. Here, the apoptotic cell expresses the eat me signal PS, which is recognized by PRRs expressed on the cell surface of DCs, such as TIM‐4, stabilin‐2, and BAI‐1. It has been shown that the regulatory cytokine TGF‐β is induced in response to uptake of apoptotic cells, and as such, this type of phagocytosis is known as noninflammatory. In contrast, microbial pathogens, as illustrated by bacteria here, activate TLRs on the surface of DCs by presenting ligands known as PAMPs, which are associated with particular bacteria. In this case, activated DCs produce proinflammatory cytokines, such as IL‐12, IL‐6, and TNF‐α, ultimately inducing an inflammatory, antimicrobial response required to fight the infection.
THE CYTOKINES SECRETED BY ACTIVATED APCs INSTRUCT SPECIFIC ADAPTIVE IMMUNE RESPONSES
Following activation by APCs, αβ T cells differentiate in a process first described for naïve CD4 T cells on the basis of their function and the cytokines they secrete [29]. The fate of naïve Th cells is determined by three signals provided by stimulated APCs. First, the APC presents peptide on MHC molecules to the T cell through the TCR and delivers signal 1 to T cells. A second signal, referred to as ″costimulation″, is an accessory signal, required in addition to signal 1, to induce immunity [30] and is often equated to interactions among CD28, CTLA‐4, ICOS, and members of the B7 family, such as CD80 and CD86 [31]. In the absence of signal 2, Th cells become anergic, which can lead to tolerance. The third signal delivered from the APC to the T cell is through the release of proteins, primarily cytokines, which determine its differentiation into an effector cell, such as Th1 or Th2 cells [32], as well as Th17 or Treg cell populations [33].
The polarization of a T cell response strongly depends on the conditions under which the APC is stimulated, as this determines the balance of the cytokines that are produced [34]. In addition, the fate of the T cell is controlled by lineage‐specific transcription factors, and the resulting cell phenotype is characterized by a unique cytokine expression profile and effector function [35]. IL‐12 signaling through STAT4, which leads to the activation of the transcription factor T‐bet, is essential for the induction of Th1 cells [32]. IL‐4 is the critical cytokine that controls Th2 cell polarization [32]. Activation of STAT6 by IL‐4 enhances the expression of IL‐4, IL‐13, and the transcription factor GATA‐binding protein 3 [36]. TGF‐β is required for the generation of Treg cells—the natural and inducible populations [37]—both of which express the transcription factor Foxp3 [38]. IL‐6 and TGF‐β are required for murine Th17 cell differentiation from naïve CD4+ T cells [39, –, 41], and IL‐23 supports Th17 cell expansion and maintenance [41]. A role for IL‐21 induced by IL‐6 in the presence of TGF‐β has also been shown to drive Th17 cell generation [42, 43]. It was shown originally that human Th17 cell differentiation required IL‐1 plus IL‐23 or IL‐6 [44, 45]. Studies in IL‐1R1‐deficient mice demonstrated that IL‐1 was essential for promoting IL‐17 production by T cells that mediate autoimmune disease [46]. More recently, studies by Chung et al. [47] showed that IL‐1 is required for the early differentiation of murine Th17 cells and Th17 cell‐mediated autoimmunity. STAT3, the major STAT protein activated by IL‐6, is essential for Th17 cell differentiation in the mouse and human [48]. The orphan nuclear receptors RORγt (encoded by Ror‐related orphan receptor C) and to a lesser extent, RORα, are expressed specifically by mouse and human Th17 cells [49, 50], which not only play an important role in host defense against infection but also in autoimmunity [45]. Other Th cell fates have been described, including Th9 [51, 52], Th22 [53, 54], and follicular Th cells [55, –, 57, 58].
γδ T cells are a relatively rare population of T cells (2–4%) that are evolutionary conserved and found in all jawed vertebrates, but in contrast to αβ T cells, which reside primarily in secondary lymphoid organs, γδ T cells form the majority of intraepithelial lymphocytes. In the mouse, each tissue has its own specific subset of γδ T cells, each displaying a limited TCR diversity [59]. Resident γδ T cells are thought to represent a first line of defense against infection and provide certain other tissue‐specific functions, including the regulation of wound healing [60]. γδ T cells are thought to function largely like their αβ counterparts; however, there is evidence to suggest that γδ T cells do not routinely rely on APCs for antigen recognition, and they themselves have recently been shown to efficiently process and present peptide antigen to αβ T cells [61]. Along with Th17 cells, γδ T cells are an innate source of IL‐17. Studies by Sutton et al. [62] showed that αβ T cells express IL‐23R and RORγt and produce IL‐17, IL‐21, and IL‐22 in response to IL‐1β and IL‐23 without TCR engagement. Furthermore, γδ T cells activated by IL‐1β and IL‐23 promoted IL‐17 production by CD4+ T cells and increased susceptibility to experimental autoimmune encephalomyelitis, a murine model for multiple sclerosis.
APOPTOSIS: ONLY ONE OF MANY FORMS OF CELL DEATH
The term apoptosis was first used to describe a mode of cell death based on morphological features that include cell shrinkage, membrane blebbing, chromatin condensation, and formation of apoptotic bodies [63]. Apoptotic cell death is executed by members of the family of caspases, cysteinyl aspartate‐directed proteases [64]. In mammalian cells, there are two pathways that result in the activation of caspases that are involved in executing apoptosis: the extrinsic and the intrinsic pathways. The extrinsic pathway can be mediated by cell surface receptors of the TNFR family, such as TNFR1, FAS, and TRAIL‐R1 [65]. Initiator caspases, including caspase‐2, ‐8, ‐9, and ‐10, become activated, leading to the subsequent activation of downstream executioner caspases, such as caspase‐3, ‐6, and ‐7. The intrinsic pathway is triggered by various stress stimuli, such as growth factor withdrawal, UV irradiation, DNA damage, and cytotoxic drugs [66, 67]. Apoptosis occurs as a result of MMP, in particular, as a consequence of MOMP, which causes the leakage of molecules, including cytochrome c; second mitochondria‐derived activator of caspase/direct inhibitor of apoptosis‐binding protein with low isoelectric point; endonuclease G; and apoptosis‐inducing factor, among many others, which are normally confined within the space between the inner and outer mitochondrial membranes [68, 69]. Release of these molecules initiates a cascade of signaling events that results in the phenotypic characteristics associated with apoptosis, including DNA fragmentation, nuclear condensation, the formation of cytoplamic blebs and of apoptotic bodies, and exposure of eat me signals, such as PS, on the plasma membrane [66].
A form of cell death that is distinct from apoptosis is a process known as necrosis, which is caspase‐independent and characterized by cytoplasmic and organelle swelling, followed by membrane breakdown and release of cellular contents into the surrounding extracellular space [70]. Unlike apoptosis, necrosis was thought to be an unregulated process, but recent studies have shown that the kinase activities of RIP1 and RIP3 initiate a cellular pathway downstream of TNFR1 [71, –, 73] to mediate programmed necrosis, also known as necroptosis [74, 75]. Interdependent phosphorylation of RIP1 and RIP3 was linked to increased energy metabolism and production of ROS, which precede necrosis [71, 72], also likely to involve MMP; this has been proposed to occur as a result of MPT, as distinct from the MOMP, which is more typical of apoptosis [75]. MPT involves a multiprotein pore called the permeability transition pore complex, a complex that functions in exchanging small metabolites between the cytosol and the mitochondrial matrix [69].
Pyroptosis is a more recently defined form of cell death described in monocytes, macrophages, and DCs (reviewed in ref. [76]). DNA damage, as a result of pyroptosis, is observed as marked nuclear condensation without intranucleosomal fragmentation of DNA or resultant DNA laddering morphology. In contrast to apoptosis, there is no loss of MOMP, no activation of the executioner caspases 3 and 6, and no release of cytochrome c. Notably, unique to this type of cell death is the activation of the inflammatory caspase, caspase‐1, which is activated in a complex initiated by NLRs and called the inflammasome [76]. Rupture of the plasma membrane is observed as a result of caspase‐1‐mediated formation of pores, which allows efflux of ions and influx of water, resulting in osmotic lysis and release of inflammatory intracellular contents.
APOPTOSIS IN THE CONTEXT OF INFLAMMATION
Noninflammatory phagocytosis of apoptotic cells by macrophages results in the synthesis of TGF‐β [22, 77]. In contrast, the activation of APCs in response to TLR ligation induces IL‐6, a prototypical, proinflammatory cytokine [78]. Our laboratory showed that uptake of infected apoptotic cells, apoptotic cells that express TLR ligands, induced the synthesis of IL‐6 and biologically active TGF‐β, as well as IL‐23 [28]. Given the critical role that IL‐6 and TGF‐β play in the induction of Th17 cells, we hypothesized that innate recognition of an infected apoptotic cell would result in a cytokine milieu conducive for Th17 differentiation. We demonstrated that TGF‐β and IL‐6 produced by DCs in response to infected apoptotic cells promoted the differentiation of naïve CD4 T cells to the Th17 lineage ( Fig. 2 ) [28]. Th17 cell differentiation was impaired severely when TGF‐β and IL‐6 were neutralized with antibodies or when Il6 −/− or Trif −/− Myd88 /− DCs were used, indicating that these cytokines were the major drivers of Th17 cell differentiation in response to the TLR ligand carrying apoptotic cells in vitro. Furthermore, in the absence of TLR engagement, innate recognition of apoptotic cells induced the synthesis of TGF‐β, resulting in the preferential induction of Treg cells (Fig. 2). These results emphasized the activation of distinct signaling pathways in DCs in response to apoptotic cells, versus infected apoptotic cells that harbor TLR ligands and ligands derived from apoptotic cells.
Figure 2.
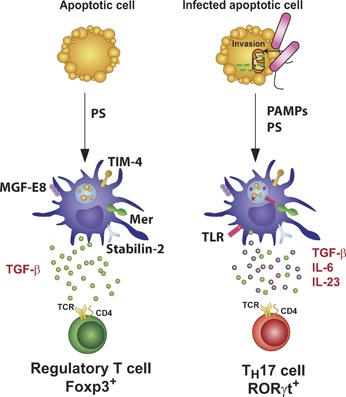
Innate immune recognition of apoptotic cells carrying TLR ligands instructs Th17 cell differentiation. Apoptotic cells express eat‐me signals, including exposure of PS at the outer leaflet of the plasma membrane, which activates PRRs such as TIM‐4, stabilin‐2, BAI‐1, MER, and the αvβ3 integrin expressed on the surface of phagocytes, such as DCs. The receptors MER or the αvβ3 integrin require interaction with bridging molecules such as GAS6 or MFG‐E8, respectively, to recognize PS and initiate phagocytosis. DCs that phagocytose apoptotic cells induce a noninflammatory response, characterized by the production of TGF‐β, resulting in the polarization of Treg cells that secrete anti‐inflammatory cytokines such as TGF‐β and IL‐10. Bacterial invasion of target cells (bacteria are shown here in pink) can induce the release of cytochrome c (green), a characteristic of apoptosis. In this case, DCs are activated in response to apoptotic cell‐derived and TLR‐derived signals. Antigens derived from the apoptotic cell and the microbial pathogen end up in the phagosome, resulting in their presentation on MHC molecules to T cells. This combination of signals induces IL‐6, TGF‐β, and IL‐23, a unique cytokine profile that triggers Th17 differentiation.
When might apoptosis occur in the context of TLR activation? Pathogenic enteric bacteria, such as EPEC, can disrupt intestinal epithelial barrier structure and function, as well as mediate apoptosis of intestinal mucosal epithelial cells [26]. Many of these bacteria possess virulence factors, such as pore‐forming toxins and adhesins. EPEC belong to a family of closely related pathogens, including EHEC, known collectively as A/E pathogens, which cause characteristic lesions when they adhere to intestinal epithelial cells, leading to effacement of brush border microvilli [26]. A 35,624‐bp genetic element known as the LEE is necessary and sufficient for the process of A/E [79, 80]. The LEE is a conserved PAI that is restricted to EPEC, EHEC, as well as the rodent pathogen C. rodentium, and encodes components of the T3SS, which mediates firm adhesion of the bacteria to the host cell and injection of various bacterial effectors directly into the host cell cytosol to alter cellular functions and survival. One of these effectors, EspF, translocates to the mitochondria to initiate MOMP and subsequent apoptosis [81, 82]. More recently, EspF has been shown to bind membrane‐ and actin‐remodeling host factors, namely SNX9 and neuronal Wiskott‐Aldrich syndrome protein, and alter their activity [83]. EPEC are known to disrupt intestinal epithelial tight junctions, a factor considered to be essential to EPEC pathogenesis [26, 83], and have been shown to induce apoptosis, as demonstrated by the exposure of PS, subsequent DNA fragmentation, and release of ATP [84, –, 86, 87, 88, 89]. C. rodentium is a commonly used rodent infection model for the study of the human infections with EPEC and EHEC.
BLOCKADE OF APOPTOSIS IMPAIRS Th17 DEVELOPMENT IN VIVO DURING A BACTERIAL INFECTION KNOWN TO TRIGGER A Th17 RESPONSE
Although it was well established that infection with C. rodentium induced Th1 immunity [90], studies by the laboratory of C. T. Weaver [39] showed that infection of mice with C. rodentium induced a robust Th17 response within the intestinal LP. The characteristic development of colitis that is associated with Citrobacter infection resolves within 2–3 weeks as a result of clearance of bacteria and development of a protective humoral and CD4 T cell‐mediated immune response [91, 92]. IL‐17A and IL‐17F are important for host defense against C. rodentium infection, such that deficiency in IL‐17A or IL‐17F resulted in splenomegaly, colonic hypertrophy, inflammation, and greater bacterial burdens in the colons of these mice compared with WT mice [93]. In addition to IL‐17A and IL‐17F, studies by the laboratory of W. Ouyang [94] showed that IL‐22 mediated protection against C. rodentium infection, such that infected Il22 −/− mice suffered 80–100% mortality in the second week postinfection. These mice exhibited mucosal hyperplasia, inflammation, colonic ulceration, and increased numbers of bacteria in the colonic crypts and within the mesenteric LNs, spleen, and liver [94]. The protective role for IL‐22 was mediated through the production of antimicrobial peptides of the Reg III family, Reg IIIβ and Reg IIIγ, as well as S100A8 and S100A9.
Having shown that innate recognition of an infected apoptotic cell by DCs induced IL‐6 and TGF‐β production and subsequent Th17 cell differentiation, we tested whether this may be the relevant physiological stimulus in vivo that induces Th17 responses. Our laboratory found that infection with C. rodentium induced Th17 immunity and that Citrobacter induction of host cell apoptosis was necessary to induce this response [28]. Previous studies by the laboratories of B.B. Finlay, M.S. Donnenberg, and C. Sasakawa [81, 82, 95, 96] had all reported that C. rodentium induced apoptosis in vitro as well as in vivo in the intestinal epithelium of infected animals. When we infected mice with the mutant strain ΔEspF, which is unable to induce host cell apoptosis, we observed a significantly reduced number of TUNEL‐positive cells in the distal colon of ΔEspF‐infected mice compared with mice infected with WT C. rodentium [28]. Furthermore, mice infected with ΔEspF, which does not induce apoptosis, had a reduction in IL‐17‐producing CD4+ T cells as compared with mice infected with WT C. rodentium. Importantly, the percentage of IFN‐γ+ CD4+ T cells was similar in the small intestinal LP of mice infected with WT or ΔEspF C. rodentium, indicating that unlike the Th17 response, the ability of ΔEspF to drive Th1 responses was not impaired [28]. Consistent with these findings, blocking apoptosis with the pan‐caspase inhibitor Q‐VD‐OPH throughout the course of Citrobacter infection, again led to a significant reduction in the number of TUNEL‐positive cells in colonic sections of treated animals compared with infected animals that were not treated with the inhibitor [28]. This correlated with a decrease in the population of IL‐17‐producing CD4 T cells in the colon of infected mice that were treated with the inhibitor. Our studies thus highlighted a novel role for host cell apoptosis during infection in determining the differentiation of Th17 cells. Whether this pathway also plays a role in triggering IL‐17 production by γδ T cells remains to be seen.
OTHER MICROBIAL PATHOGENS KNOWN TO TRIGGER IL‐17 RESPONSES AND THEIR LINK TO THE INDUCTION OF APOPTOSIS
Bacteria
Like C. rodentium, many other bacterial pathogens have been shown to elicit IL‐17 responses, including Klebsiella pneumoniae, Streprococcus pneumoniae, Borrelia species, P. aeruginosa, Helicobacter pylori [97], and Staphylococcus aureus [93]. The relationship between the ability of these pathogens to induce apoptosis and to trigger IL‐17‐associated responses is not yet clear. In many of the infections associated with these pathogens, a role for other cytokines involved in Th17 responses, such as IL‐23, IL‐22, and IL‐21, has been investigated. However, not all of these studies have shown a definitive involvement of CD4 T cells that have differentiated into the Th17 lineage, and as such, other cell types, such as γδ T cells [98, –, 100, 101, 102, 103], NKT cells [104, –, 106, 107, 108], NK cells [109, –, 111], neutrophils [112, 113], and eosinophils [114], could be contributing to the IL‐17 response being measured.
Helicobactor pylori.
H. pylori, a Gram‐negative bacterium, colonizes the proximal duodenum or distal esophagus in ∼50% of the human population [115]. Colonization is asymptomatic but has been associated with increased risk for peptic ulcers, gastritis, gastric carcinomas, and mucosa‐associated lymphomas [115]. Studies examining a link between Th17 and Helicobacter reported a consistently higher level of IL‐17 RNA transcripts in gastric mucosa and LP mononuclear cells isolated from gastric biopsies from H. pylori‐positive compared with H. pylori‐negative patients presenting with gastritis [116, 117]. Treatment of patients for H. pylori correlated with a decrease in the levels of IL‐17 protein detected within the mucosal tissue [116]. CD4 and CD8 T cells appeared to be the major source of IL‐17 in H. pylori‐positive biopsies, but notably, there was also a higher percentage of CD4 T cells that produced IFN‐γ alone in H. pylori‐positive biopsies [118]. Together with IL‐17, significantly increased levels of IL‐23 and IL‐21 were detected in biopsies of H. pylori‐positive patients compared with H. pylori‐negative patients [118, 119]. Thus, these studies collectively suggest a role for the IL‐17/IL‐23 axis in diseases associated with H. pylori colonization [120].
Has Helicobacter been reported to induce apoptosis? H. pylori secrete VacA, which has been shown to induce apoptosis of gastric epithelial cells [121, –, 123, 124, 125] ( Fig. 3 ; reviewed in ref. [126]). Addition of VacA to cells and intracellular expression of VacA cause dissipation of mitochondrial ΔΨm [125] and release of cytochrome c [122, 125], consistent with MOMP. Indeed, VacA has been reported to translocate to mitochondria, suggesting a direct role in MOMP [122]. Transient transfection of VacA resulted in activation of caspase‐3, as determined by cleavage of poly(ADP‐ribose) polymerase, and this was inhibited by cotransfection of B cell lymphoma 2 [122]. Although all H. pylori strains isolated from the stomachs of rodent model animals or humans are VacA+ and should theoretically induce apoptosis, successful induction of apoptosis in the end is determined by whether other virulence factors, such as the Helicobacter cag PAI‐encoded T4SS and the effector protein CagA, are coexpressed [115]. Therefore, studies examining IL‐17 responses in human subjects should thus take into account the CagA status of the H. pylori present in those subjects. Caruso et al. [118, 119] found no differences in the levels of IL‐21 and IL‐23 proteins among patients carrying CagA+ versus CagA– strains. On the other hand, all 51 H. pylori strains from gastric ulcer and nonulcer patients studied by Mizuno et al. [117] were CagA– and VacA+ and showed increased IL‐17 transcripts, making it tempting to speculate that the IL‐17 responses observed in those patients correlate with the lack of CagA expression and possibly an intact ability to induce apoptosis.
Figure 3.
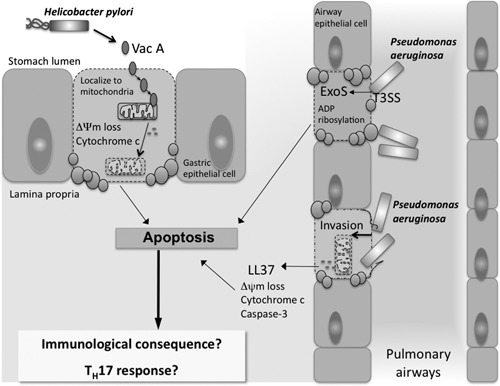
Induction of host cell apoptosis during infection with H. pylori or P. aeruginosa. H. pylori, a Gram‐negative bacterium, secretes a VacA, which causes the dissipation of ΔΨm and release of cytochrome c from gastric epithelial cells, resulting in their death by apoptosis. P. aeruginosa is another Gram‐negative bacterium, which is known to induce apoptotic cell death of lung epithelial cells. The ability P. aeruginosa to induce apoptosis relies on its expression of a T3SS, as well as several protein effectors that are injected into host cells, including ExoS, as shown in the diagram. LL‐37 is a peptide produced by epithelial cells in response to infection and inflammation and mediates important antimicrobial functions. Invasion of P. aeruginosa into epithelial cells along with the release of LL‐37 can result in the induction of epithelial cell apoptosis. Although we have established a link between C. rodentium‐induced apoptosis and Th17 immunity, little is known about the impact of H. pylori‐ or P. aeruginosa‐induced apoptosis on the IL‐17 responses reported to occur in these infections.
Klebsiella pneumoniae.
Studies have shown that IL‐23, IL‐17, and IL‐22 are critical players in the early phases of the host response to K. pneumoniae infection, even before IL‐12 and IFN‐γ responses are fully developed. Increased mRNA levels of the IL‐23 p19 subunit were detected in alveolar macrophages and DCs sooner than those for the IL‐12 p40 or p35 subunits within the BAL of mice infected with K. pneumoniae [127]. IL‐23 expression in the lung of infected mice was required for the expression of IL‐17A and IL‐17F, and IL‐12 was required for IFN‐γ. IL‐23 regulated the expression of a number of IL‐17‐dependent inflammatory mediators, including G‐CSF, IL‐6, MIP‐1α, MIP‐2, LPS‐induced CXC chemokine, and KC, within the lungs. IL‐23 was additionally important for the induction of IL‐22, which was also detectable early after infection. Deficiency in IL‐12 or IL‐23 led to reduced survival of mice during a pulmonary challenge with K. pneumoniae [127]. In a separate study, CD90 (Thy‐1)+ T cells isolated from the lungs of infected mice produced significant levels of IL‐22 [128]. Neutralization of IL‐22 in vivo led to increased mortality of mice as a result of the infection, as compared with WT or Il17a −/− mice, indicating a more critical role for IL‐22 than IL‐17 in host defense against K. pneumoniae infection.
Although there is some evidence that K. pneumoniae can induce cytotoxicity of respiratory epithelial cells [129], there has not been extensive characterization of the mode of cell death involved. Microcin E492, a 7.9‐kDa, channel‐forming bacteriocin produced by K. pneumoniae [130], induced cell shrinkage, dissipation of mitochondrial ΔΨm, release of cytochrome c, and activation of caspases 1 and 3, events that were blocked by the pan‐caspase inhibitor z‐VAD‐fmk [131]. High doses of microcin E492, which may not reflect the physiological levels of microcin E492 produced by bacteria at the infected site, induced necrosis, and a low dose was shown to induce apoptosis [131]. These data suggest that the mode of cell death induced by K. pneumoniae is more likely to be apoptosis rather than pyroptosis. However, further studies are needed to confirm whether K. pneumoniae can induce apoptosis of respiratory epithelial cells and whether this apoptosis is mediated through microcin E492 and/or dependent on the mitochondrial pathway.
Pseudomonas aeruginosa.
P. aeruginosa is a Gram‐negative, opportunistic bacterium that causes serious pneumonia by colonizing and damaging lung epithelial surfaces in immunocompromised patients and patients with cystic fibrosis [25]. P. aeruginosa is also associated with infections of skin, wounds, burns, urinary tract, and bloodstream. In searching for an association between IL‐17 and chronic neutrophilic infiltrates that are characteristic of cystic fibrosis [132], J.K. Kolls and colleagues [133] found elevated levels of IL‐23, IL‐17A, and IL‐17F in the sputum of cystic fibrosis patients colonized with P. aeruginosa. To model airway infections in cystic fibrosis patients, this group challenged mice with agarose beads containing a clinical, mucoid isolate of P. aeruginosa and found that IL‐17, KC, the metalloproteinase MMP‐9, and IL‐6 were decreased significantly in the BAL and lung homogenates of p19 −/− mice compared with WT controls [134]. As a result, significantly less airway inflammation and neutrophilic infiltrates were observed in p19 −/− mice, and the levels of IFN‐γ and TNF‐α were similar to those in WT mice. The increases in IL‐17 and IL‐23 observed in the human samples and the murine model suggested that these cytokines may be important for neutrophil recruitment and that IL‐23 could potentially be targeted in the immunotherapy of cystic fibrosis.
There is plenty of evidence pointing to the ability of P. aeruginosa to induce apoptosis (Fig. 3). P. aeruginosa possesses a T3SS that encodes several protein effectors that are injected into host cells (reviewed in ref. [25]). Of these, ExoS, ExoT, and ExoU have been linked to the induction of host cell death. ExoS and ExoT are toxins expressed by invasive strains of P. aeruginosa, and both exhibit dual GAP and ADPRT activities [25]. The GAP activity enables Pseudomonas to disrupt tight junctions within the epithelial barrier, and the ADPRT activity results in cell rounding, inhibition of DNA synthesis, vesicular traffic, and eventual cell death. The ADPRT activity of ExoT causes a form of cell death that resembles apoptosis [135]. Similarly, ExoS‐induced cell death is also associated with hallmarks of apoptosis, including membrane blebbing, cell shrinkage, caspase‐3 cleavage, DNA fragmentation, and chromatin condensation [136]. Finally, ExoU possesses PLA2 activity that causes rapid cell death mediated by the C terminus of the protein [137, –, 139, 140] and is characterized by loss of plasma membrane integrity similar to that seen in necrosis [140, 141].
A study by E. Gulbins and colleagues [142] showed a different mode of inducing cell death by P. aeruginosa, alternative to activation by T3SS proteins, and mediated by CD95/CD95L (Fas/Fas ligand) interactions. P. aeruginosa‐induced apoptosis of cultured human and murine cells was blocked by a CD95‐Fc fusion protein [142]. Similarly, fibroblasts derived from lpr mice, genetically deficient in CD95, or gld mice, genetically deficient in CD95L, were protected from P. aeruginosa‐mediated apoptosis [142]. Consistent with these results, intranasal infection with P. aeruginosa triggered apoptosis of lung epithelial cells that was absent in lpr or gld mice [142]. Although the P. aeruginosa virulence factors mediating apoptosis here were not identified, apoptosis played an important role in host defense against P. aeruginosa infection, as evidenced by increased splenic and lung bacterial burdens and increased mortality of infected lpr or gld mice.
A surprising new player in mediating apoptosis of infected epithelial cells is the human cathelicidin LL‐37, which is a cationic, amphipathic peptide produced by epithelial cells in response to infection and inflammation and mediates important antimicrobial functions (reviewed in refs. [143, 144]). D.J. Davidsonˈs group [145] showed that treatment of P. aeruginosa‐infected human bronchial epithelial cells with physiological levels of LL‐37 resulted in epithelial cell apoptosis, as determined by nuclear DNA fragmentation, cleavage of caspase‐3 and ‐9, loss of mitochondrial ΔΨm, and release of cytochrome c into the cytosol. These effects were blocked with the pan‐caspase inhibitor z‐VAD‐fmk and were not observed upon treatment of the epithelial cells with P. aeruginosa alone or LL‐37 alone, indicating an essential synergistic activity of LL‐37 and P. aeruginosa [145]. Curiously, the synergistic LL‐37/P. aeruginosa‐mediated apoptosis was lost only when a mutant of P. aeruginosa, which was incapable of invading the epithelial cells, was used. These data demonstrated that invasion of P. aeruginosa into epithelial cells, in combination with LL‐37, resulted in the induction of epithelial cell apoptosis. Whether this mode of cell death initiated by the infected epithelial cell itself can lead to Th17 responses remains to be investigated (Fig. 3).
Fungi
Candida albicans.
IL‐17A and as such, Th17 cells are considered to be protective in candidiasis and in general, against infections with fungi, findings that stemmed mainly from studies with experimental candidiasis and aspergillosis. C. albicans is a fungal pathogen that causes opportunistic infections in humans. It has been reported that Th17 cells are important for host defense against C. albicans, whereby IL‐17RA‐deficient and p19 −/− mice showed increased susceptibility to mucosal candidiasis [146] and to systemic C. albicans infection [147]. Futhermore, evidence for a requirement for a Th17 response to protect against infection in humans comes from the observations that patients with impaired Candida‐specific Th17 responses, including patients with hyper‐IgE syndrome or chronic mucocutaneous candidiasis, are highly susceptible to mucosal C. albicans infections [148]. A role for Dectin‐2 in the recognition of α‐mannans and in the induction of Th17 cell differentiation was recently shown to be essential for host defense against C. albicans [147]. The ability of C. albicans to invade and damage oral epithelial cells has been shown to be essential to establish oral infection. A recent study by Villar and Zhao [149] showed that C. albicans induce early apoptosis in oral epithelial cells, demonstrated by the activation of caspases, followed by necrotic cell death. This is in line with studies showing that systemic infection with C. albicans induced apoptosis, followed by necrosis in macrophages isolated from the peritoneal exudates of mice 30 min–2 h postinfection [150]. Whether the induction of apoptosis in response to C. albicans infection is required for the Th17 response is still unknown. Interestingly, a recent study by Martin et al. [100] showed that the number of IL‐17‐producing γδ T cells expanded rapidly following injection of mice with C. albicans hyphae, indicating that γδ T cells may also contribute to the IL‐17 response to C. albicans infection.
INNATE RECOGNITION OF OTHER FORMS OF CELL DEATH—IMMUNOLOGICAL CONSEQUENCES
Although our studies have shown that infections associated with significant apoptosis can induce Th17 immunity, the consequences of other types of cell death on the adaptive immune system are currently not well defined [151] ( Fig. 4 ). We will only briefly review what is known here. As mentioned above, apart from apoptosis, other forms of programmed cell death that have been described include necrosis and pyroptosis. Many intracellular bacterial pathogens such as Legionella, Listeria, Shigella, Salmonella, and Francisella trigger pyroptosis [152, –, 154, 155, 156, 157, 158]. Unlike apoptosis, the consequences of pyroptosis on the adaptive immune system are currently not defined [151]. In particular, whether innate immune recognition of pyroptotic cells can lead to the differentiation of Th17 cells has not been investigated. Caspase‐1 activation, which is a hallmark of pyroptosis, results in the cleavage of inflammatory cytokines such as IL‐1β and IL‐18, and their release from the pyroptotic cell. Given that pyroptosis and apoptosis are mediated by different cellular pathways and result in the release of different cytokines, the prediction would be that pyroptosis would not favor development of a Th17 response. Instead, IL‐18, in combination with IL‐12, has been reported to synergistically induce IFN‐γ production by Th1 cells [159]. Indeed, infections with Salmonella and Listeria tend to induce Th1 rather than Th17 cell responses [160, 161].
Figure 4.
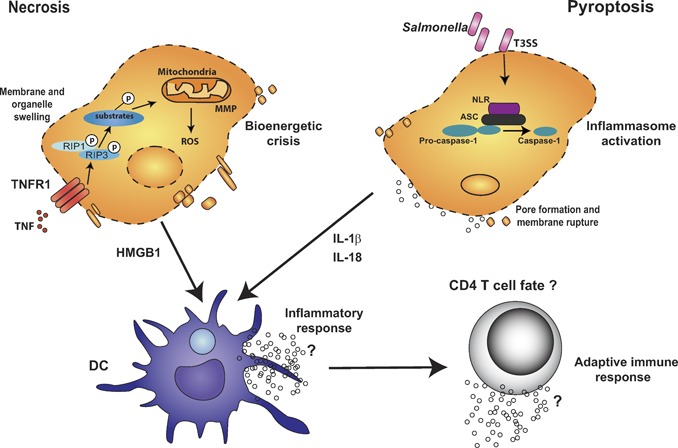
Although uptake of apoptotic cells carrying TLR ligands by phagocytes instructs Th17 differentiation, the consequence of innate recognition of other modes of cell death is yet to be defined. Phagocytosis of infected apoptotic cells by DCs induces IL‐6, TGF‐β, and IL‐23 production, a unique cytokine profile that triggers Th17 differentiation. Other types of cell death, such as necrosis and pyroptosis, also exist. Although caspase‐1 activation is typical of pyroptosis, the RIP1 and RIP3 kinases mediate death receptor‐mediated necrosis such as that downstream of TNFR1. Activation of the kinase activities of RIP1 and RIP3 targets key enzymes in bioenergetic pathways mediating glycogen breakdown, glutaminolysis, and ROS production. Caspase‐1 activation is controlled by the assembly of an inflammasome complex consisting of NLRs and the adaptor protein ASC. Activated caspase‐1 in turn mediates the processing of pro‐IL‐1β to IL‐1β. Cells undergoing pyroptosis thus release cellular contents and inflammatory cytokines such as IL‐1β, which can then activate DCs. Similarly, necrotic cells also release intracellular contents and have most typically been associated with the release of HMGB1, which can cause DC maturation. The consequence of phagocytosis of necrotic and pyroptotic cells on CD4 T cell differentiation is not yet known.
On the other hand, a study by Lin et al. [162] showed a critical role for the IL‐23‐Th17 pathway in the immune response to a live vaccine strain of Francisella tularensis, where IL‐17A but not IL‐17F or IL‐22 induced IL‐12 production by DCs and macrophages and thus, regulated the IL‐12‐Th1 pathway that is required for protective immunity against F. tularensis. In this study, IL‐17A was produced by γδ T cells as well as TCRβ+ CD4+ T cells, suggesting the development of Th17 cells. Notably, and as mentioned above, Francisella induces pyroptosis, the process of caspase‐1‐dependent programmed cell death. Activation of caspase‐1 and ASC signaling, which controls the protoelytic cleavage of caspase‐1 [163] in response to Francisella, resulted in host cell death and the release of IL‐1β and IL‐18 [164]. Futhermore, F. tularensis‐infected caspase‐1‐ and ASC‐deficient mice showed markedly increased bacterial burdens and mortality as compared with WT mice [164]. These studies demonstrate an important role for caspase‐1 and ASC in innate defense against infection by this pathogen. Given the critical role that IL‐1 plays in the induction of Th17 responses, the ability of Francisella to cause pyroptosis and as such, IL‐1β release may be important in the IL‐17‐mediated response to infection with this intracellular bacterium. However, a direct causal relationship between pyroptosis and Th17 cell differentiation in general remains to be formally demonstrated.
A number of studies have demonstrated the occurrence of necrotic cell death during infection with microbial pathogens including Shigella, HIV‐1, West Nile virus, and Coxackievirus B (reviewed in ref. [165]). Interestingly, although Shigella mediates pyroptosis in macrophages, it was found to mediate necrosis in nonepithelial cells [166]. Consistent with the notion that the presence of RIP3 permits the cells to undergo necrosis [71, –, 73], Rip3−/− mice failed to initiate vaccinia virus‐induced tissue necrosis and inflammation, which resulted in increased mortality as a result of exacerbated viral replication [71]. These results illustrate the importance of necrosis in host defense, particularly against infections with microbes that interfere with the ability of infected cells to undergo apoptosis. Necrotic cells have been shown to release proteins such as HMGB1 during primary and secondary necrosis [151], and HMGB1 can engage receptors, such as receptor for advanced glycation endproducts, TLR2, and TLR4 [167]. Given the potentially different PRRs that can be engaged by necrotic cells, the type of adaptive immune response induced in response to necrotic cell death is likely to be distinct and remains to be elucidated.
CONCLUDING REMARKS
Our studies have established a link between the differentiation of Th17 cells and apoptosis of bacterially infected cells [28]. Importantly, in this case, two signals are provided to the APC during recognition of the infected apoptotic cell. Apoptotic cell‐derived signals activate noninflammatory and likely PS‐specific PRRs on the surface of the APC, and TLR ligands provided by the invading pathogen engage TLRs and induce a proinflammatory response. Although we have shown this to occur during C. rodentium infection, this situation could conceivably occur in a number of other bacterial infections that also induce host cell apoptosis, such as H. pylori and P. aeruginosa infections. The realization that apoptosis can form an important component of the innate immune triggers which impact CD4 Th cell differentiation leads to the obvious next question as to how other forms of cell death shape the ensuing adaptive T cell response. The type of cell death induced by a pathogen is dependent on the virulence factors and mechanisms of pathogenicity unique to that pathogen. Therefore, although one pathogen induces apoptosis by targeting the intrinsic mitochondrial pathway of cell death, another might induce pyroptosis by targeting the inflammasome and caspase‐1 activation. Innate immune recognition of the particular form of cell death within the context of infection can control the induction of a specific, tailored Th cell response against the infection. Given that many pathogens have evolved mechanisms to modulate cell death, there is a clear need to better understand how cell death shapes the host immune responses to microbes.
REFERENCES
- 1. Janeway Jr., C. A. (1989) Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54, 1–13. [DOI] [PubMed] [Google Scholar]
- 2. Bowie, A. , O'Neill, L. A. (2000) The interleukin‐1 receptor/Toll‐like receptor superfamily: signal generators for pro‐inflammatory interleukins and microbial products. J. Leukoc. Biol. 67, 508–514. [DOI] [PubMed] [Google Scholar]
- 3. Medzhitov, R. , Janeway Jr., C. (2000) Innate immunity. N. Engl. J. Med. 343, 338–344. [DOI] [PubMed] [Google Scholar]
- 4. Akira, S. , Uematsu, S. , Takeuchi, O. (2006) Pathogen recognition and innate immunity. Cell 124, 783–801. [DOI] [PubMed] [Google Scholar]
- 5. O'Neill, L. A. (2006) How Toll‐like receptors signal: what we know and what we don't know. Curr. Opin. Immunol. 18, 3–9. [DOI] [PubMed] [Google Scholar]
- 6. Fadok, V. A. , Voelker, D. R. , Campbell, P. A. , Cohen, J. J. , Bratton, D. L. , Henson, P. M. (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148, 2207–2216. [PubMed] [Google Scholar]
- 7. Gregory, C. D. , Devitt, A. , Moffatt, O. (1998) Roles of ICAM‐3 and CD14 in the recognition and phagocytosis of apoptotic cells by macrophages. Biochem. Soc. Trans. 26, 644–649. [DOI] [PubMed] [Google Scholar]
- 8. Moffatt, O. D. , Devitt, A. , Bell, E. D. , Simmons, D. L. , Gregory, C. D. (1999) Macrophage recognition of ICAM‐3 on apoptotic leukocytes. J. Immunol. 162, 6800–6810. [PubMed] [Google Scholar]
- 9. Chang, M. K. , Bergmark, C. , Laurila, A. , Horkko, S. , Han, K. H. , Friedman, P. , Dennis, E. A. , Witztum, J. L. (1999) Monoclonal antibodies against oxidized low‐density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation‐specific epitopes mediate macrophage recognition. Proc. Natl. Acad. Sci. USA 96, 6353–6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oka, K. , Sawamura, T. , Kikuta, K. , Itokawa, S. , Kume, N. , Kita, T. , Masaki, T. (1998) Lectin‐like oxidized low‐density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc. Natl. Acad. Sci. USA 95, 9535–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sambrano, G. R. , Steinberg, D. (1995) Recognition of oxidatively damaged and apoptotic cells by an oxidized low density lipoprotein receptor on mouse peritoneal macrophages: role of membrane phosphatidylserine. Proc. Natl. Acad. Sci. USA 92, 1396–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ravichandran, K. S. , Lorenz, U. (2007) Engulfment of apoptotic cells: signals for a good meal. Nat. Rev. Immunol. 7, 964–974. [DOI] [PubMed] [Google Scholar]
- 13. Savill, J. , Dransfield, I. , Gregory, C. , Haslett, C. (2002) A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2, 965–975. [DOI] [PubMed] [Google Scholar]
- 14. Hanayama, R. , Tanaka, M. , Miwa, K. , Shinohara, A. , Iwamatsu, A. , Nagata, S. (2002) Identification of a factor that links apoptotic cells to phagocytes. Nature 417, 182–187. [DOI] [PubMed] [Google Scholar]
- 15. Anderson, H. A. , Maylock, C. A. , Williams, J. A. , Paweletz, C. P. , Shu, H. , Shacter, E. (2003) Serum‐derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nat. Immunol. 4, 87–91. [DOI] [PubMed] [Google Scholar]
- 16. Lemke, G. , Rothlin, C. V. (2008) Immunobiology of the TAM receptors. Nat. Rev. Immunol. 8, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagata, K. , Ohashi, K. , Nakano, T. , Arita, H. , Zong, C. , Hanafusa, H. , Mizuno, K. (1996) Identification of the product of growth arrest‐specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J. Biol. Chem. 271, 30022–30027. [DOI] [PubMed] [Google Scholar]
- 18. Kobayashi, N. , Karisola, P. , Pena‐Cruz, V. , Dorfman, D. M. , Jinushi, M. , Umetsu, S. E. , Butte, M. J. , Nagumo, H. , Chernova, I. , Zhu, B. , Sharpe, A. H. , Ito, S. , Dranoff, G. , Kaplan, G. G. , Casasnovas, J. M. , Umetsu, D. T. , Dekruyff, R. H. , Freeman, G. J. (2007) TIM‐1 and TIM‐4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity 27, 927–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miyanishi, M. , Tada, K. , Koike, M. , Uchiyama, Y. , Kitamura, T. , Nagata, S. (2007) Identification of Tim4 as a phosphatidylserine receptor. Nature 450, 435–439. [DOI] [PubMed] [Google Scholar]
- 20. Park, D. , Tosello‐Trampont, A. C. , Elliott, M. R. , Lu, M. , Haney, L. B. , Ma, Z. , Klibanov, A. L. , Mandell, J. W. , Ravichandran, K. S. (2007) BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450, 430–434. [DOI] [PubMed] [Google Scholar]
- 21. Park, S. Y. , Jung, M. Y. , Kim, H. J. , Lee, S. J. , Kim, S. Y. , Lee, B. H. , Kwon, T. H. , Park, R. W. , Kim, I. S. (2008) Rapid cell corpse clearance by stabilin‐2, a membrane phosphatidylserine receptor. Cell Death Differ. 15, 192–201. [DOI] [PubMed] [Google Scholar]
- 22. Fadok, V. A. , Bratton, D. L. , Konowal, A. , Freed, P. W. , Westcott, J. Y. , Henson, P. M. (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF‐β, PGE2, and PAF. J. Clin. Invest. 101, 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blander, J. M. , Medzhitov, R. (2004) Regulation of phagosome maturation by signals from Toll‐like receptors. Science 304, 1014–1018. [DOI] [PubMed] [Google Scholar]
- 24. Blander, J. M. , Medzhitov, R. (2006) On regulation of phagosome maturation and antigen presentation. Nat. Immunol. 7, 1029–1035. [DOI] [PubMed] [Google Scholar]
- 25. Hauser, A. R. (2009) The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat. Rev. Microbiol. 7, 654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaper, J. B. , Nataro, J. P. , Mobley, H. L. (2004) Pathogenic Escherichia coli . Nat. Rev. Microbiol. 2, 123–140. [DOI] [PubMed] [Google Scholar]
- 27. Torchinsky, M. B. , Garaude, J. , Blander, J. M. (2010) Infection and apoptosis as a combined inflammatory trigger. Curr. Opin. Immunol. 22, 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Torchinsky, M. B. , Garaude, J. , Martin, A. P. , Blander, J. M. (2009) Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature 458, 78–82. [DOI] [PubMed] [Google Scholar]
- 29. Mosmann, T. R. , Cherwinski, H. , Bond, M. W. , Giedlin, M. A. , Coffman, R. L. (1986) Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136, 2348–2357. [PubMed] [Google Scholar]
- 30. Reis e Sousa, C. (2006) Dendritic cells in a mature age. Nat. Rev. Immunol. 6, 476–483. [DOI] [PubMed] [Google Scholar]
- 31. Kroczek, R. A. , Mages, H. W. , Hutloff, A. (2004) Emerging paradigms of T‐cell co‐stimulation. Curr. Opin. Immunol. 16, 321–327. [DOI] [PubMed] [Google Scholar]
- 32. Amsen, D. , Spilianakis, C. G. , Flavell, R. A. (2009) How are T(H)1 and T(H)2 effector cells made? Curr. Opin. Immunol. 21, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Littman, D. R. , Rudensky, A. Y. (2010) Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140, 845–858. [DOI] [PubMed] [Google Scholar]
- 34. de Jong, E. C. , Smits, H. H. , Kapsenberg, M. L. (2005) Dendritic cell‐mediated T cell polarization. Springer Semin. Immunopathol. 26, 289–307. [DOI] [PubMed] [Google Scholar]
- 35. Wilson, C. B. , Rowell, E. , Sekimata, M. (2009) Epigenetic control of T‐helper‐cell differentiation. Nat. Rev. Immunol. 9, 91–105. [DOI] [PubMed] [Google Scholar]
- 36. Paul, W. E. , Zhu, J. (2010) How are T(H)2‐type immune responses initiated and amplified? Nat. Rev. Immunol. 10, 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sakaguchi, S. (2004) Naturally arising CD4+ regulatory T cells for immunologic self‐tolerance and negative control of immune responses. Annu. Rev. Immunol. 22, 531–562. [DOI] [PubMed] [Google Scholar]
- 38. Zheng, Y. , Rudensky, A. Y. (2007) Foxp3 in control of the regulatory T cell lineage. Nat. Immunol. 8, 457–462. [DOI] [PubMed] [Google Scholar]
- 39. Mangan, P. R. , Harrington, L. E. , O'Quinn, D. B. , Helms, W. S. , Bullard, D. C. , Elson, C. O. , Hatton, R. D. , Wahl, S. M. , Schoeb, T. R. , Weaver, C. T. (2006) Transforming growth factor‐β induces development of the T(H)17 lineage. Nature 441, 231–234. [DOI] [PubMed] [Google Scholar]
- 40. Bettelli, E. , Carrier, Y. , Gao, W. , Korn, T. , Strom, T. B. , Oukka, M. , Weiner, H. L. , Kuchroo, V. K. (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238. [DOI] [PubMed] [Google Scholar]
- 41. Veldhoen, M. , Hocking, R. J. , Atkins, C. J. , Locksley, R. M. , Stockinger, B. (2006) TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL‐17‐producing T cells. Immunity 24, 179–189. [DOI] [PubMed] [Google Scholar]
- 42. Korn, T. , Bettelli, E. , Gao, W. , Awasthi, A. , Jager, A. , Strom, T. B. , Oukka, M. , Kuchroo, V. K. (2007) IL‐21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 448, 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nurieva, R. , Yang, X. O. , Martinez, G. , Zhang, Y. , Panopoulos, A. D. , Ma, L. , Schluns, K. , Tian, Q. , Watowich, S. S. , Jetten, A. M. , Dong, C. (2007) Essential autocrine regulation by IL‐21 in the generation of inflammatory T cells. Nature 448, 480–483. [DOI] [PubMed] [Google Scholar]
- 44. Acosta‐Rodriguez, E. V. , Napolitani, G. , Lanzavecchia, A. , Sallusto, F. (2007) Interleukins 1β and 6 but not transforming growth factor‐β are essential for the differentiation of interleukin 17‐producing human T helper cells. Nat. Immunol. 8, 942–949. [DOI] [PubMed] [Google Scholar]
- 45. Wilson, N. J. , Boniface, K. , Chan, J. R. , McKenzie, B. S. , Blumenschein, W. M. , Mattson, J. D. , Basham, B. , Smith, K. , Chen, T. , Morel, F. , Lecron, J. C. , Kastelein, R. A. , Cua, D. J. , McClanahan, T. K. , Bowman, E. P. , de Waal Malefyt, R. (2007) Development, cytokine profile and function of human interleukin 17‐producing helper T cells. Nat. Immunol. 8, 950–957. [DOI] [PubMed] [Google Scholar]
- 46. Sutton, C. , Brereton, C. , Keogh, B. , Mills, K. H. , Lavelle, E. C. (2006) A crucial role for interleukin (IL)‐1 in the induction of IL‐17‐producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 203, 1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chung, Y. , Chang, S. H. , Martinez, G. J. , Yang, X. O. , Nurieva, R. , Kang, H. S. , Ma, L. , Watowich, S. S. , Jetten, A. M. , Tian, Q. , Dong, C. (2009) Critical regulation of early Th17 cell differentiation by interleukin‐1 signaling. Immunity 30, 576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang, X. O. , Panopoulos, A. D. , Nurieva, R. , Chang, S. H. , Wang, D. , Watowich, S. S. , Dong, C. (2007) STAT3 regulates cytokine‐mediated generation of inflammatory helper T cells. J. Biol. Chem. 282, 9358–9363. [DOI] [PubMed] [Google Scholar]
- 49. Ivanov, I. I. , McKenzie, B. S. , Zhou, L. , Tadokoro, C. E. , Lepelley, A. , Lafaille, J. J. , Cua, D. J. , Littman, D. R. (2006) The orphan nuclear receptor RORyt directs the differentiation program of proinflammatory IL‐17+ T helper cells. Cell 126, 1121–1133. [DOI] [PubMed] [Google Scholar]
- 50. Yang, X. O. , Pappu, B. P. , Nurieva, R. , Akimzhanov, A. , Kang, H. S. , Chung, Y. , Ma, L. , Shah, B. , Panopoulos, A. D. , Schluns, K. S. , Watowich, S. S. , Tian, Q. , Jetten, A. M. , Dong, C. (2008) T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR α and ROR γ. Immunity 28, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dardalhon, V. , Awasthi, A. , Kwon, H. , Galileos, G. , Gao, W. , Sobel, R. A. , Mitsdoerffer, M. , Strom, T. B. , Elyaman, W. , Ho, I. C. , Khoury, S. , Oukka, M. , Kuchroo, V. K. (2008) IL‐4 inhibits TGF‐β‐induced Foxp3+ T cells and, together with TGF‐β, generates IL‐9+ IL‐10+ Foxp3(–) effector T cells. Nat. Immunol. 9, 1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Veldhoen, M. , Uyttenhove, C. , van Snick, J. , Helmby, H. , Westendorf, A. , Buer, J. , Martin, B. , Wilhelm, C. , Stockinger, B. (2008) Transforming growth factor‐β “reprograms” the differentiation of T helper 2 cells and promotes an interleukin 9‐producing subset. Nat. Immunol. 9, 1341–1346. [DOI] [PubMed] [Google Scholar]
- 53. Duhen, T. , Geiger, R. , Jarrossay, D. , Lanzavecchia, A. , Sallusto, F. (2009) Production of interleukin 22 but not interleukin 17 by a subset of human skin‐homing memory T cells. Nat. Immunol. 10, 857–863. [DOI] [PubMed] [Google Scholar]
- 54. Trifari, S. , Kaplan, C. D. , Tran, E. H. , Crellin, N. K. , Spits, H. (2009) Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)‐17, T(H)1 and T(H)2 cells. Nat. Immunol. 10, 864–871. [DOI] [PubMed] [Google Scholar]
- 55. King, C. (2009) New insights into the differentiation and function of T follicular helper cells. Nat. Rev. Immunol. 9, 757–766. [DOI] [PubMed] [Google Scholar]
- 56. King, C. , Tangye, S. G. , Mackay, C. R. (2008) T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu. Rev. Immunol. 26, 741–766. [DOI] [PubMed] [Google Scholar]
- 57. Nurieva, R. I. , Chung, Y. , Hwang, D. , Yang, X. O. , Kang, H. S. , Ma, L. , Wang, Y. H. , Watowich, S. S. , Jetten, A. M. , Tian, Q. , Dong, C. (2008) Generation of T follicular helper cells is mediated by interleukin‐21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vogelzang, A. , McGuire, H. M. , Yu, D. , Sprent, J. , Mackay, C. R. , King, C. (2008) A fundamental role for interleukin‐21 in the generation of T follicular helper cells. Immunity 29, 127–137. [DOI] [PubMed] [Google Scholar]
- 59. Hayday, A. C. (2000) [γ][δ] Cells: a right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 18, 975–1026. [DOI] [PubMed] [Google Scholar]
- 60. Hayday, A. , Tigelaar, R. (2003) Immunoregulation in the tissues by γδ T cells. Nat. Rev. Immunol. 3, 233–242. [DOI] [PubMed] [Google Scholar]
- 61. Brandes, M. , Willimann, K. , Moser, B. (2005) Professional antigen‐presentation function by human γδ T cells. Science 309, 264–268. [DOI] [PubMed] [Google Scholar]
- 62. Sutton, C. E. , Lalor, S. J. , Sweeney, C. M. , Brereton, C. F. , Lavelle, E. C. , Mills, K. H. (2009) Interleukin‐1 and IL‐23 induce innate IL‐17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341. [DOI] [PubMed] [Google Scholar]
- 63. Kroemer, G. , El‐Deiry, W. S. , Golstein, P. , Peter, M. E. , Vaux, D. , Vandenabeele, P. , Zhivotovsky, B. , Blagosklonny, M. V. , Malorni, W. , Knight, R. A. , Piacentini, M. , Nagata, S. , Melino, G. (2005) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 12 (Suppl. 2), 1463–1467. [DOI] [PubMed] [Google Scholar]
- 64. Thornberry, N. A. , Lazebnik, Y. (1998) Caspases: enemies within. Science 281, 1312–1316. [DOI] [PubMed] [Google Scholar]
- 65. Krammer, P. H. (2000) CD95's deadly mission in the immune system. Nature 407, 789–795. [DOI] [PubMed] [Google Scholar]
- 66. Brenner, D. , Mak, T. W. (2009) Mitochondrial cell death effectors. Curr. Opin. Cell Biol. 21, 871–877. [DOI] [PubMed] [Google Scholar]
- 67. Festjens, N. , van Gurp, M. , van Loo, G. , Saelens, X. , Vandenabeele, P. (2004) Bcl‐2 family members as sentinels of cellular integrity and role of mitochondrial intermembrane space proteins in apoptotic cell death. Acta Haematol. 111, 7–27. [DOI] [PubMed] [Google Scholar]
- 68. Green, D. R. , Kroemer, G. (2004) The pathophysiology of mitochondrial cell death. Science 305, 626–629. [DOI] [PubMed] [Google Scholar]
- 69. Kroemer, G. , Galluzzi, L. , Brenner, C. (2007) Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 87, 99–163. [DOI] [PubMed] [Google Scholar]
- 70. Festjens, N. , Vanden Berghe, T. , Vandenabeele, P. (2006) Necrosis, a well‐orchestrated form of cell demise: signaling cascades, important mediators and concomitant immune response. Biochim. Biophys. Acta 1757, 1371–1387. [DOI] [PubMed] [Google Scholar]
- 71. Cho, Y. S. , Challa, S. , Moquin, D. , Genga, R. , Ray, T. D. , Guildford, M. , Chan, F. K. (2009) Phosphorylation‐driven assembly of the RIP1‐RIP3 complex regulates programmed necrosis and virus‐induced inflammation. Cell 137, 1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. He, S. , Wang, L. , Miao, L. , Wang, T. , Du, F. , Zhao, L. , Wang, X. (2009) Receptor interacting protein kinase‐3 determines cellular necrotic response to TNF‐α. Cell 137, 1100–1111. [DOI] [PubMed] [Google Scholar]
- 73. Zhang, D. W. , Shao, J. , Lin, J. , Zhang, N. , Lu, B. J. , Lin, S. C. , Dong, M. Q. , Han, J. (2009) RIP3, an energy metabolism regulator that switches TNF‐induced cell death from apoptosis to necrosis. Science 325, 332–336. [DOI] [PubMed] [Google Scholar]
- 74. Hitomi, J. , Christofferson, D. E. , Ng, A. , Yao, J. , Degterev, A. , Xavier, R. J. , Yuan, J. (2008) Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 135, 1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Galluzzi, L. , Kroemer, G. (2008) Necroptosis: a specialized pathway of programmed necrosis. Cell 135, 1161–1163. [DOI] [PubMed] [Google Scholar]
- 76. Bergsbaken, T. , Fink, S. L. , Cookson, B. T. (2009) Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Voll, R. E. , Herrmann, M. , Roth, E. A. , Stach, C. , Kalden, J. R. , Girkontaite, I. (1997) Immunosuppressive effects of apoptotic cells. Nature 390, 350–351. [DOI] [PubMed] [Google Scholar]
- 78. Iwasaki, A. , Medzhitov, R. (2004) Toll‐like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995. [DOI] [PubMed] [Google Scholar]
- 79. McDaniel, T. K. , Jarvis, K. G. , Donnenberg, M. S. , Kaper, J. B. (1995) A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92, 1664–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. McDaniel, T. K. , Kaper, J. B. (1997) A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K‐12. Mol. Microbiol. 23, 399–407. [DOI] [PubMed] [Google Scholar]
- 81. Nagai, T. , Abe, A. , Sasakawa, C. (2005) Targeting of enteropathogenic Escherichia coli EspF to host mitochondria is essential for bacterial pathogenesis: critical role of the 16th leucine residue in EspF. J. Biol. Chem. 280, 2998–3011. [DOI] [PubMed] [Google Scholar]
- 82. Nougayrède, J. P. , Donnenberg, M. S. (2004) Enteropathogenic Escherichia coli EspF is targeted to mitochondria and is required to initiate the mitochondrial death pathway. Cell. Microbiol. 6, 1097–1111. [DOI] [PubMed] [Google Scholar]
- 83. Weflen, A. W. , Alto, N. M. , Hecht, G. A. (2009) Tight junctions and enteropathogenic E. coli. Ann. N. Y. Acad. Sci. 1165, 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Abul‐Milh, M. , Wu, Y. , Lau, B. , Lingwood, C. A. , Barnett Foster, D. (2001) Induction of epithelial cell death including apoptosis by enteropathogenic Escherichia coli expressing bundle‐forming pili. Infect. Immun. 69, 7356–7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Baldwin, T. J. , Lee‐Delaunay, M. B. , Knutton, S. , Williams, P. H. (1993) Calcium‐calmodulin dependence of actin accretion and lethality in cultured HEp‐2 cells infected with enteropathogenic Escherichia coli . Infect. Immun. 61, 760–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Barnett Foster, D. , Abul‐Milh, M. , Huesca, M. , Lingwood, C. A. (2000) Enterohemorrhagic Escherichia coli induces apoptosis which augments bacterial binding and phosphatidylethanolamine exposure on the plasma membrane outer leaflet. Infect. Immun. 68, 3108–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Crane, J. K. , Majumdar, S. , Pickhardt, D. F. III (1999) Host cell death due to enteropathogenic Escherichia coli has features of apoptosis. Infect. Immun. 67, 2575–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Crane, J. K. , Olson, R. A. , Jones, H. M. , Duffey, M. E. (2002) Release of ATP during host cell killing by enteropathogenic E. coli and its role as a secretory mediator. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G74‐G86. [DOI] [PubMed] [Google Scholar]
- 89. Malish, H. R. , Freeman, N. L. , Zurawski, D. V. , Chowrashi, P. , Ayoob, J. C. , Sanger, J. W. , Sanger, J. M. (2003) Potential role of the EPEC translocated intimin receptor (Tir) in host apoptotic events. Apoptosis 8, 179–190. [DOI] [PubMed] [Google Scholar]
- 90. Higgins, L. M. , Frankel, G. , Douce, G. , Dougan, G. , MacDonald, T. T. (1999) Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect. Immun. 67, 3031–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bry, L. , Brenner, M. B. (2004) Critical role of T cell‐dependent serum antibody, but not the gut‐associated lymphoid tissue, for surviving acute mucosal infection with Citrobacter rodentium, an attaching and effacing pathogen. J. Immunol. 172, 433–441. [DOI] [PubMed] [Google Scholar]
- 92. Maaser, C. , Housley, M. P. , Iimura, M. , Smith, J. R. , Vallance, B. A. , Finlay, B. B. , Schreiber, J. R. , Varki, N. M. , Kagnoff, M. F. , Eckmann, L. (2004) Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect. Immun. 72, 3315–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ishigame, H. , Kakuta, S. , Nagai, T. , Kadoki, M. , Nambu, A. , Komiyama, Y. , Fujikado, N. , Tanahashi, Y. , Akitsu, A. , Kotaki, H. , Sudo, K. , Nakae, S. , Sasakawa, C. , Iwakura, Y. (2009) Differential roles of interleukin‐17A and ‐17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30, 108–119. [DOI] [PubMed] [Google Scholar]
- 94. Zheng, Y. , Valdez, P. A. , Danilenko, D. M. , Hu, Y. , Sa, S. M. , Gong, Q. , Abbas, A. R. , Modrusan, Z. , Ghilardi, N. , de Sauvage, F. J. , Ouyang, W. (2008) Interleukin‐22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289. [DOI] [PubMed] [Google Scholar]
- 95. Crane, J. K. , McNamara, B. P. , Donnenberg, M. S. (2001) Role of EspF in host cell death induced by enteropathogenic Escherichia coli . Cell. Microbiol. 3, 197–211. [DOI] [PubMed] [Google Scholar]
- 96. Vallance, B. A. , Deng, W. , Jacobson, K. , Finlay, B. B. (2003) Host susceptibility to the attaching and effacing bacterial pathogen Citrobacter rodentium . Infect. Immun. 71, 3443–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Curtis, M. M. , Way, S. S. (2009) Interleukin‐17 in host defense against bacterial, mycobacterial and fungal pathogens. Immunology 126, 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Girardi, M. (2006) Immunosurveillance and immunoregulation by γδ T cells. J. Invest. Dermatol. 126, 25–31. [DOI] [PubMed] [Google Scholar]
- 99. Lockhart, E. , Green, A. M. , Flynn, J. L. (2006) IL‐17 production is dominated by γδ T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 177, 4662–4669. [DOI] [PubMed] [Google Scholar]
- 100. Martin, B. , Hirota, K. , Cua, D. J. , Stockinger, B. , Veldhoen, M. (2009) Interleukin‐17‐producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity 31, 321–330. [DOI] [PubMed] [Google Scholar]
- 101. Roark, C. L. , Simonian, P. L. , Fontenot, A. P. , Born, W. K. , O'Brien, R. L. (2008) γδ T cells: an important source of IL‐17. Curr. Opin. Immunol. 20, 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shibata, K. , Yamada, H. , Hara, H. , Kishihara, K. , Yoshikai, Y. (2007) Resident Vδ1+ γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL‐17 production. J. Immunol. 178, 4466–4472. [DOI] [PubMed] [Google Scholar]
- 103. Umemura, M. , Yahagi, A. , Hamada, S. , Begum, M. D. , Watanabe, H. , Kawakami, K. , Suda, T. , Sudo, K. , Nakae, S. , Iwakura, Y. , Matsuzaki, G. (2007) IL‐17‐mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette‐Guerin infection. J. Immunol. 178, 3786–3796. [DOI] [PubMed] [Google Scholar]
- 104. Coquet, J. M. , Chakravarti, S. , Kyparissoudis, K. , McNab, F. W. , Pitt, L. A. , McKenzie, B. S. , Berzins, S. P. , Smyth, M. J. , Godfrey, D. I. (2008) Diverse cytokine production by NKT cell subsets and identification of an IL‐17‐producing CD4‐NK1.1‐ NKT cell population. Proc. Natl. Acad. Sci. USA 105, 11287–11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Michel, M. L. , Keller, A. C. , Paget, C. , Fujio, M. , Trottein, F. , Savage, P. B. , Wong, C. H. , Schneider, E. , Dy, M. , Leite‐de‐Moraes, M. C. (2007) Identification of an IL‐17‐producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J. Exp. Med. 204, 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Michel, M. L. , Mendes‐da‐Cruz, D. , Keller, A. C. , Lochner, M. , Schneider, E. , Dy, M. , Eberl, G. , Leite‐de‐Moraes, M. C. (2008) Critical role of ROR‐γt in a new thymic pathway leading to IL‐17‐producing invariant NKT cell differentiation. Proc. Natl. Acad. Sci. USA 105, 19845–19850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rachitskaya, A. V. , Hansen, A. M. , Horai, R. , Li, Z. , Villasmil, R. , Luger, D. , Nussenblatt, R. B. , Caspi, R. R. (2008) Cutting edge: NKT cells constitutively express IL‐23 receptor and RORγt and rapidly produce IL‐17 upon receptor ligation in an IL‐6‐independent fashion. J. Immunol. 180, 5167–5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Doisne, J. M. , Bartholin, L. , Yan, K. P. , Garcia, C. N. , Duarte, N. , Le Luduec, J. B. , Vincent, D. , Cyprian, F. , Horvat, B. , Martel, S. , Rimokh, R. , Losson, R. , Benlagha, K. , Marie, J. C. (2009) iNKT cell development is orchestrated by different branches of TGF‐β signaling. J. Exp. Med. 206, 1365–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cupedo, T. , Crellin, N. K. , Papazian, N. , Rombouts, E. J. , Weijer, K. , Grogan, J. L. , Fibbe, W. E. , Cornelissen, J. J. , Spits, H. (2009) Human fetal lymphoid tissue‐inducer cells are interleukin 17‐producing precursors to RORC+ CD127+ natural killer‐like cells. Nat. Immunol. 10, 66–74. [DOI] [PubMed] [Google Scholar]
- 110. Passos, S. T. , Silver, J. S. , O'Hara, A. C. , Sehy, D. , Stumhofer, J. S. , Hunter, C. A. (2010) IL‐6 promotes NK cell production of IL‐17 during toxoplasmosis. J. Immunol. 184, 1776–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Colonna, M. (2009) Interleukin‐22‐producing natural killer cells and lymphoid tissue inducer‐like cells in mucosal immunity. Immunity 31, 15–23. [DOI] [PubMed] [Google Scholar]
- 112. Li, L. , Huang, L. , Vergis, A. L. , Ye, H. , Bajwa, A. , Narayan, V. , Strieter, R. M. , Rosin, D. L. , Okusa, M. D. (2010) IL‐17 produced by neutrophils regulates IFN‐γ‐mediated neutrophil migration in mouse kidney ischemia‐reperfusion injury. J. Clin. Invest. 120, 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ferretti, S. , Bonneau, O. , Dubois, G. R. , Jones, C. E. , Trifilieff, A. (2003) IL‐17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide‐induced airway neutrophilia: IL‐15 as a possible trigger. J. Immunol. 170, 2106–2112. [DOI] [PubMed] [Google Scholar]
- 114. Molet, S. , Hamid, Q. , Davoine, F. , Nutku, E. , Taha, R. , Page, N. , Olivenstein, R. , Elias, J. , Chakir, J. (2001) IL‐17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 108, 430–438. [DOI] [PubMed] [Google Scholar]
- 115. Cover, T. L. , Blaser, M. J. (2009) Helicobacter pylori in health and disease. Gastroenterology 136, 1863–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Luzza, F. , Parrello, T. , Monteleone, G. , Sebkova, L. , Romano, M. , Zarrilli, R. , Imeneo, M. , Pallone, F. (2000) Up‐regulation of IL‐17 is associated with bioactive IL‐8 expression in Helicobacter pylori‐infected human gastric mucosa. J. Immunol. 165, 5332–5337. [DOI] [PubMed] [Google Scholar]
- 117. Mizuno, T. , Ando, T. , Nobata, K. , Tsuzuki, T. , Maeda, O. , Watanabe, O. , Minami, M. , Ina, K. , Kusugami, K. , Peek, R. M. , Goto, H. (2005) Interleukin‐17 levels in Helicobacter pylori‐infected gastric mucosa and pathologic sequelae of colonization. World J. Gastroenterol. 11, 6305–6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Caruso, R. , Fina, D. , Paoluzi, O. A. , Del Vecchio Blanco, G. , Stolfi, C. , Rizzo, A. , Caprioli, F. , Sarra, M. , Andrei, F. , Fantini, M. C. , MacDonald, T. T. , Pallone, F. , Monteleone, G. (2008) IL‐23‐mediated regulation of IL‐17 production in Helicobacter pylori‐infected gastric mucosa. Eur. J. Immunol. 38, 470–478. [DOI] [PubMed] [Google Scholar]
- 119. Caruso, R. , Fina, D. , Peluso, I. , Fantini, M. C. , Tosti, C. , Del Vecchio Blanco, G. , Paoluzi, O. A. , Caprioli, F. , Andrei, F. , Stolfi, C. , Romano, M. , Ricci, V. , MacDonald, T. T. , Pallone, F. , Monteleone, G. (2007) IL‐21 is highly produced in Helicobacter pylori‐infected gastric mucosa and promotes gelatinases synthesis. J. Immunol. 178, 5957–5965. [DOI] [PubMed] [Google Scholar]
- 120. Caruso, R. , Pallone, F. , Monteleone, G. (2007) Emerging role of IL‐23/IL‐17 axis in H pylori‐associated pathology. World J. Gastroenterol. 13, 5547–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cover, T. L. , Krishna, U. S. , Israel, D. A. , Peek Jr., R. M. (2003) Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 63, 951–957. [PubMed] [Google Scholar]
- 122. Galmiche, A. , Rassow, J. , Doye, A. , Cagnol, S. , Chambard, J. C. , Contamin, S. , de Thillot, V. , Just, I. , Ricci, V. , Solcia, E. , Van Obberghen, E. , Boquet, P. (2000) The N‐terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 19, 6361–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kuck, D. , Kolmerer, B. , Iking‐Konert, C. , Krammer, P. H. , Stremmel, W. , Rudi, J. (2001) Vacuolating cytotoxin of Helicobacter pylori induces apoptosis in the human gastric epithelial cell line AGS. Infect. Immun. 69, 5080–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Willhite, D. C. , Cover, T. L. , Blanke, S. R. (2003) Cellular vacuolation and mitochondrial cytochrome c release are independent outcomes of Helicobacter pylori vacuolating cytotoxin activity that are each dependent on membrane channel formation. J. Biol. Chem. 278, 48204–48209. [DOI] [PubMed] [Google Scholar]
- 125. Willhite, D. C. , Blanke, S. R. (2004) Helicobacter pylori vacuolating cytotoxin enters cells, localizes to the mitochondria, and induces mitochondrial membrane permeability changes correlated to toxin channel activity. Cell. Microbiol. 6, 143–154. [DOI] [PubMed] [Google Scholar]
- 126. Cover, T. L. , Blanke, S. R. (2005) Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat. Rev. Microbiol. 3, 320–332. [DOI] [PubMed] [Google Scholar]
- 127. Happel, K. I. , Dubin, P. J. , Zheng, M. , Ghilardi, N. , Lockhart, C. , Quinton, L. J. , Odden, A. R. , Shellito, J. E. , Bagby, G. J. , Nelson, S. , Kolls, J. K. (2005) Divergent roles of IL‐23 and IL‐12 in host defense against Klebsiella pneumoniae. J. Exp. Med. 202, 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Aujla, S. J. , Chan, Y. R. , Zheng, M. , Fei, M. , Askew, D. J. , Pociask, D. A. , Reinhart, T. A. , McAllister, F. , Edeal, J. , Gaus, K. , Husain, S. , Kreindler, J. L. , Dubin, P. J. , Pilewski, J. M. , Myerburg, M. M. , Mason, C. A. , Iwakura, Y. , Kolls, J. K. (2008) IL‐22 mediates mucosal host defense against Gram‐negative bacterial pneumonia. Nat. Med. 14, 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cano, V. , Moranta, D. , Llobet‐Brossa, E. , Bengoechea, J. A. , Garmendia, J. (2009) Klebsiella pneumoniae triggers a cytotoxic effect on airway epithelial cells. BMC Microbiol. 9, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lagos, R. , Tello, M. , Mercado, G. , Garcia, V. , Monasterio, O. (2009) Antibacterial and antitumorigenic properties of microcin E492, a pore‐forming bacteriocin. Curr. Pharm. Biotechnol. 10, 74–85. [DOI] [PubMed] [Google Scholar]
- 131. Hetz, C. , Bono, M. R. , Barros, L. F. , Lagos, R. (2002) Microcin E492, a channel‐forming bacteriocin from Klebsiella pneumoniae, induces apoptosis in some human cell lines. Proc. Natl. Acad. Sci. USA 99, 2696–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Chmiel, J. F. , Konstan, M. W. , Saadane, A. , Krenicky, J. E. , Lester Kirchner, H. , Berger, M. (2002) Prolonged inflammatory response to acute Pseudomonas challenge in interleukin‐10 knockout mice. Am. J. Respir. Crit. Care Med. 165, 1176–1181. [DOI] [PubMed] [Google Scholar]
- 133. McAllister, F. , Henry, A. , Kreindler, J. L. , Dubin, P. J. , Ulrich, L. , Steele, C. , Finder, J. D. , Pilewski, J. M. , Carreno, B. M. , Goldman, S. J. , Pirhonen, J. , Kolls, J. K. (2005) Role of IL‐17A, IL‐17F, and the IL‐17 receptor in regulating growth‐related oncogene‐α and granulocyte colony‐stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J. Immunol. 175, 404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Dubin, P. J. , Kolls, J. K. (2007) IL‐23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L51–L528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Shafikhani, S. H. , Morales, C. , Engel, J. (2008) The Pseudomonas aeruginosa type III secreted toxin ExoT is necessary and sufficient to induce apoptosis in epithelial cells. Cell. Microbiol. 10, 994–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kaufman, M. R. , Jia, J. , Zeng, L. , Ha, U. , Chow, M. , Jin, S. (2000) Pseudomonas aeruginosa mediated apoptosis requires the ADP‐ribosylating activity of ExoS. Microbiology 146, 2531–2541. [DOI] [PubMed] [Google Scholar]
- 137. Sato, H. , Frank, D. W. , Hillard, C. J. , Feix, J. B. , Pankhaniya, R. R. , Moriyama, K. , Finck‐Barbancon, V. , Buchaklian, A. , Lei, M. , Long, R. M. , Wiener‐Kronish, J. , Sawa, T. (2003) The mechanism of action of the Pseudomonas aeruginosa‐encoded type III cytotoxin, ExoU. EMBO J. 22, 2959–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Rabin, S. D. , Hauser, A. R. (2005) Functional regions of the Pseudomonas aeruginosa cytotoxin ExoU. Infect. Immun. 73, 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Stirling, F. R. , Cuzick, A. , Kelly, S. M. , Oxley, D. , Evans, T. J. (2006) Eukaryotic localization, activation and ubiquitinylation of a bacterial type III secreted toxin. Cell. Microbiol. 8, 1294–1309. [DOI] [PubMed] [Google Scholar]
- 140. Finck‐Barbancon, V. , Frank, D. W. (2001) Multiple domains are required for the toxic activity of Pseudomonas aeruginosa ExoU. J. Bacteriol. 183, 4330–4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Hauser, A. R. , Fleiszig, S. , Kang, P. J. , Mostov, K. , Engel, J. N. (1998) Defects in type III secretion correlate with internalization of Pseudomonas aeruginosa by epithelial cells. Infect. Immun. 66, 1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Grassmé, H. , Kirschnek, S. , Riethmueller, J. , Riehle, A. , von Kurthy, G. , Lang, F. , Weller, M. , Gulbins, E. (2000) CD95/CD95 ligand interactions on epithelial cells in host defense to Pseudomonas aeruginosa . Science 290, 527–530. [DOI] [PubMed] [Google Scholar]
- 143. Doss, M. , White, M. R. , Tecle, T. , Hartshorn, K. L. (2010) Human defensins and LL‐37 in mucosal immunity. J. Leukoc. Biol. 87, 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Tecle, T. , Tripathi, S. , Hartshorn, K. L. (2010) Review: defensins and cathelicidins in lung immunity. Innate Immun. 16, 151–159. [DOI] [PubMed] [Google Scholar]
- 145. Barlow, P. G. , Beaumont, P. E. , Cosseau, C. , Mackellar, A. , Wilkinson, T. S. , Hancock, R E. , Haslett, C. , Govan, J. R. , Simpson, A. J. , Davidson, D. J. (2010) The human cathelicidin LL‐37 preferentially promotes apoptosis of infected airway epithelium. Am. J. Respir. Cell Mol. Biol. 43, 692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Conti, H. R. , Shen, F. , Nayyar, N. , Stocum, E. , Sun, J. N. , Lindemann, M. J. , Ho, A. W. , Hai, J. H. , Yu, J. J. , Jung, J. W. , Filler, S. G. , Masso‐Welch, P. , Edgerton, M. , Gaffen, S. L. (2009) Th17 cells and IL‐17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 206, 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Saijo, S. , Ikeda, S. , Yamabe, K. , Kakuta, S. , Ishigame, H. , Akitsu, A. , Fujikado, N. , Kusaka, T. , Kubo, S. , Chung, S. H. , Komatsu, R. , Miura, N. , Adachi, Y. , Ohno, N. , Shibuya, K. , Yamamoto, N. , Kawakami, K. , Yamasaki, S. , Saito, T. , Akira, S. , Iwakura, Y. (2010) Dectin‐2 recognition of α‐mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans . Immunity 32, 681–691. [DOI] [PubMed] [Google Scholar]
- 148. Eyerich, K. , Foerster, S. , Rombold, S. , Seidl, H. P. , Behrendt, H. , Hofmann, H. , Ring, J. , Traidl‐Hoffmann, C. (2008) Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17‐associated cytokines IL‐17 and IL‐22. J. Invest. Dermatol. 128, 2640–2645. [DOI] [PubMed] [Google Scholar]
- 149. Villar, C. C. , Zhao, X. R. (2010) Candida albicans induces early apoptosis followed by secondary necrosis in oral epithelial cells. Mol. Oral Microbiol. 25, 215–225. [DOI] [PubMed] [Google Scholar]
- 150. Gasparoto, T. H. , Gaziri, L. C. , Burger, E. , de Almeida, R. S. , Felipe, I. (2004) Apoptosis of phagocytic cells induced by Candida albicans and production of IL‐10. FEMS Immunol. Med. Microbiol. 42, 219–224. [DOI] [PubMed] [Google Scholar]
- 151. Green, D. R. , Ferguson, T. , Zitvogel, L. , Kroemer, G. (2009) Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 9, 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Case, C. L. , Shin, S. , Roy, C. R. (2009) ASC and Ipaf Inflammasomes direct distinct pathways for caspase‐1 activation in response to Legionella pneumophila . Infect. Immun. 77, 1981–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Fink, S. L. , Bergsbaken, T. , Cookson, B. T. (2008) Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase‐1‐dependent pyroptosis via distinct mechanisms. Proc. Natl. Acad. Sci. USA 105, 4312–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Suzuki, T. , Franchi, L. , Toma, C. , Ashida, H. , Ogawa, M. , Yoshikawa, Y. , Mimuro, H. , Inohara, N. , Sasakawa, C. , Nunez, G. (2007) Differential regulation of caspase‐1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella‐infected macrophages. PLoS Pathog. 3, e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Rathinam, V. A. , Jiang, Z. , Waggoner, S. N. , Sharma, S. , Cole, L. E. , Waggoner, L. , Vanaja, S. K. , Monks, B. G. , Ganesan, S. , Latz, E. , Hornung, V. , Vogel, S. N. , Szomolanyi‐Tsuda, E. , Fitzgerald, K. A. (2010) The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kim, S. , Bauernfeind, F. , Ablasser, A. , Hartmann, G. , Fitzgerald, K. A. , Latz, E. , Hornung, V. (2010) Listeria monocytogenes is sensed by the NLRP3 and AIM2 inflammasome. Eur. J. Immunol. 40, 1545–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Cervantes, J. , Nagata, T. , Uchijima, M. , Shibata, K. , Koide, Y. (2008) Intracytosolic Listeria monocytogenes induces cell death through caspase‐1 activation in murine macrophages. Cell. Microbiol. 10, 41–52. [DOI] [PubMed] [Google Scholar]
- 158. Hueffer, K. , Galan, J. E. (2004) Salmonella‐induced macrophage death: multiple mechanisms, different outcomes. Cell. Microbiol. 6, 1019–1025. [DOI] [PubMed] [Google Scholar]
- 159. Nakanishi, K. , Yoshimoto, T. , Tsutsui, H. , Okamura, H. (2001) Interleukin‐18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 19, 423–474. [DOI] [PubMed] [Google Scholar]
- 160. Mittrücker, H. W. , Kaufmann, S. H. (2000) Immune response to infection with Salmonella typhimurium in mice. J. Leukoc. Biol. 67, 457–463. [DOI] [PubMed] [Google Scholar]
- 161. Neighbors, M. , Xu, X. , Barrat, F. J. , Ruuls, S. R. , Churakova, T. , Debets, R. , Bazan, J. F. , Kastelein, R. A. , Abrams, J. S. , O'Garra, A. (2001) A critical role for interleukin 18 in primary and memory effector responses to Listeria monocytogenes that extends beyond its effects on interferon γ production. J. Exp. Med. 194, 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Lin, Y. , Ritchea, S. , Logar, A. , Slight, S. , Messmer, M. , Rangel‐Moreno, J. , Guglani, L. , Alcorn, J. F. , Strawbridge, H. , Park, S. M. , Onishi, R. , Nyugen, N. , Walter, M. J. , Pociask, D. , Randall, T. D. , Gaffen, S. L. , Iwakura, Y. , Kolls, J. K. , Khader, S. A. (2009) Interleukin‐17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity 31, 799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Schroder, K. , Tschopp, J. (2010) The inflammasomes. Cell 140, 821–832. [DOI] [PubMed] [Google Scholar]
- 164. Mariathasan, S. , Weiss, D. S. , Dixit, V. M. , Monack, D. M. (2005) Innate immunity against Francisella tularensis is dependent on the ASC/caspase‐1 axis. J. Exp. Med. 202, 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Vanlangenakker, N. , Vanden Berghe, T. , Krysko, D. V. , Festjens, N. , Vandenabeele, P. (2008) Molecular mechanisms and pathophysiology of necrotic cell death. Curr. Mol. Med. 8, 207–220. [DOI] [PubMed] [Google Scholar]
- 166. Carneiro, L. A. , Travassos, L. H. , Soares, F. , Tattoli, I. , Magalhaes, J. G. , Bozza, M. T. , Plotkowski, M. C. , Sansonetti, P. J. , Molkentin, J. D. , Philpott, D. J. , Girardin, S. E. (2009) Shigella induces mitochondrial dysfunction and cell death in nonmyleoid cells. Cell Host Microbe 5, 123–136. [DOI] [PubMed] [Google Scholar]
- 167. Lotze, M. T. , Tracey, K. J. (2005) High‐mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 5, 331–342. [DOI] [PubMed] [Google Scholar]


