Short abstract
The in vivo effects of alternatively spliced interleukin‐4, IL‐ 4δ2, are different from those of conventional IL‐4, suggesting differential pathophysiological roles of these natural splice variants.
Keywords: cytokines, lymphocytes, lung
Abstract
IL‐4δ2 is a natural splice variant of IL‐4 that lacks the region encoded by the second exon. Numerous reports have suggested that the expression levels of IL‐4δ2 change in various diseases, especially those with pulmonary involvement, but the in vivo effects of this splice variant have never been studied. Replication‐deficient, AdV‐mediated gene delivery of mIL‐4δ2 to mouse lungs in vivo was used, and the effects compared with similar adenoviral delivery of mIL‐4 or with infection with a noncoding NULL viral construct. Overexpression of IL‐4δ2 or IL‐4 caused pulmonary infiltration by T and B lymphocytes, whereas in contrast to IL‐4, IL‐4δ2 did not induce eosinophilia or goblet cell hyperplasia. Microarray analysis of global gene expression revealed that IL‐4δ2 and IL‐4 had differential effects on gene expression. These splice variants also differentially regulated pulmonary levels of the cytokines TNF‐α, eotaxin, IL‐1α, IFN‐γ, and MCP‐1, whereas both tended to increase total lung collagen modestly. Pulmonary infiltration by lymphocytes in response to overexpression of IL‐4δ2 was attenuated but not abrogated completely by germline deficiency of IL‐4Rα or STAT6, whereas deficiency of endogenous IL‐4 had no effect. Thus, IL‐4δ2 promotes lymphocytic inflammation in vivo (although differentially from IL‐4, in part), and the effects of IL‐4δ2 are not mediated by endogenous IL‐4. Differential targeting of IL‐4δ2 and IL‐4 may therefore be considered in developing future therapeutic agents.
Abbreviations
- ADAMTS
a disintegrin‐like and metalloproteinases with thrombospondin type 1 motif
- AdV
adenovirus
- h
human
- HEK
human embryonic kidney
- m
mouse
- MMP
matrix metalloproteinase
- QPCR
quantitative PCR
Introduction
IL‐4 is a pleiotropic cytokine that acts as a central multifunctional regulator of the immune system and controls cell growth, survival, and gene expression in a multitude of diverse cells [1]. It shares sequence homology, receptor chains, and some functional effects with IL‐13 [1]. IL‐4 plays a major role in guiding the differentiation of antigen‐stimulated, naïve Th cells (CD4+ cells) toward a Th2 phenotype and is produced by activated Th2 cells, mast cells, basophils, and eosinophils. Through receptor activation, IL‐4 causes T lymphocyte proliferation, inhibition of apoptosis, Ig class‐switching in B cells, alternative activation of macrophages, and production of collagen by fibroblasts. Many diverse disease processes, including allergic disorders, asthma, parasitic infections, tuberculosis, pulmonary fibrosis, and systemic connective tissue diseases, are thought to have IL‐4 and other Th2 cytokines at the central core of their pathogenesis [2, –, 4, 5].
A naturally occurring, alternatively spliced variant of IL‐4, IL‐4δ2, was identified by us in the 1990s [6, –, 8] and has subsequently been shown to occur naturally in humans, other primates, and various other species including mice [9, –, 11]. IL‐4δ2 lacks the region encoded by the second exon of IL‐4, and the corresponding part of the protein comprised of 16 aa is absent. The precise role and function of IL‐4δ2 remain poorly understood, but previous studies in cell culture have suggested that IL‐4δ2 has no independent effects of its own and may be a naturally occurring, functional antagonist of IL‐4 and a promising therapeutic agent. Our previous study showed that in cell culture, hIL‐4δ2 did not act as a stimulator for T lymphocyte proliferation and moreover, competitively inhibited the ability of IL‐4 to act as a T lymphocyte stimulator [7]. Additionally, IL‐4δ2 inhibited IL‐4‐induced synthesis of IgE, inhibited expression of CD23 in B lymphocytes, and blocked the inhibitory action of IL‐4 on LPS‐induced COX‐2 expression and PGE‐2 secretion in monocytes [12]. The specific receptor for IL‐4δ2 is unknown, although IL‐4δ2 was found to bind specifically to human PBMCs and tumor lines known to express IL‐4R and IL‐13R, and excess unlabeled IL‐4 inhibited cellular binding of labeled IL‐4δ2, suggesting that IL‐4δ2 may bind to the same receptors as IL‐4 [7].
Although the functions of IL‐4δ2 remain poorly understood, studies have suggested that IL‐4δ2 may be involved in the underlying disease process for systemic sclerosis [8, 13], pulmonary tuberculosis [14, –, 16], asthma [17, –, 19], and sepsis [20]. Higher levels of IL‐4δ2 were associated with poorer prognosis in patients with scleroderma lung disease [8, 13]. In patients with pulmonary tuberculosis, greater absolute expression levels of IL‐4 and IL‐4δ2 in blood and lungs were associated with more extensive disease [14, –, 16]. Expression of IL‐4δ2 was increased in respiratory tract tissues of patients with asthma [17], and pulmonary fibroblasts have been reported to alter the IL‐4δ2/IL‐4 ratio in mast cells in patients with asthma [18]. A relative increase in IL‐4 and a decrease in IL‐4δ2 have been associated with better survival in patients with severe sepsis [20].
Direct functional studies of IL‐4δ2 in vivo have not been conducted previously. In this study, we used a replication‐deficient AdV system [21, –, 23] for IL‐4δ2 or IL‐4 gene delivery to mouse lung in vivo. The goal was to assess the effects of IL‐4δ2 directly and to determine whether IL‐4δ2 expression may lead to a phenotype that is different from that induced by expression of IL‐4.
MATERIALS AND METHODS
Adenoviral constructs
Adenoviral constructs encoding mIL‐4δ2 or mIL‐4 were created and validated in the same way as reported previously for constructs unrelated to the current study [21, –, 23]. Briefly, GenBank consensus sequences for these cytokines were used to artificially synthesize (GenScript, Piscataway, NJ, USA) DNA fragments corresponding to the respective cDNAs. The fragments were subcloned into a shuttle vector and transferred into a recombinant, replication‐deficient AdV vector using RAPAD® technology (ViraQuest, North Liberty, IA, USA). The constructs were designed to ensure expression of the cytokines under control of the CMV promoter. The validity of the constructs was confirmed by direct automated sequencing. The AdVs were purified by two rounds of CsCl‐gradient centrifugation, and the resulting purified virus had a concentration of ∼1 × 1012 particles/ml and an infectious titer of 4 × 1010 PFU/ml. All viruses, including control AdV NULL, which did not encode a cytokine, encoded GFP in their backbone under control of a separate promoter. Infectivity of the viruses was confirmed by GFP fluorescence of infected HEK293 cells in culture, and transcription was confirmed by RT‐QPCR with primers specific for the cytokine mRNAs. Prevalidated primers for mIL‐4δ2 and mIL‐4 were purchased from SABiosciences (Frederick, MD, USA), and primer specificity was validated further by direct sequencing of PCR products. Cell culture supernates were concentrated and electrophoretically separated, and the fraction at 10–20 kDa was excised and analyzed by liquid chromatography/mass spectrometry assays, confirming production and secretion of IL‐4δ2 or IL‐4 proteins. A battery of commercially available antibodies was screened for reactivity with mIL‐4δ2, resulting in identification of two antibodies that reacted with mIL‐4δ2 and mIL‐4 in ELISA and were used for quantification of these splice variants.
Animals and treatments
WT and germline‐deficient female C57BL/6 and Balb/c mice, ages 10–12 weeks (The Jackson Laboratory, Bar Harbor, ME, USA), were used in this study. Animals deficient in IL‐4Rα were on a BALB/c background (Stock #007746), whereas animals deficient in STAT6 were on a C57BL/6 background (Stock #005977). Most of the experiments were performed in C57Bl/6 mice, except for the studies in IL‐4Rα−/− mice, in which experimental and control groups were on the Balb/c background. The animals were treated in accordance with a research protocol that has been approved by the Institutional Animal Care and Use Committee of the University of Maryland (Baltimore, MD, USA). The AdVs were instilled intratracheally, as described previously in detail [21, –, 23].
Analyses of changes induced by IL‐4δ2
RT‐QPCR of lung homogenates was used to confirm overexpression of mRNAs for the delivered cytokines. BAL, pulmonary homogenates, histological and immunohistological analyses, and flow cytometry were all performed as described previously [21, –, 23]. Multiplex analyses (Luminex, Austin, TX, USA) of cytokine levels in BAL and lung homogenates were performed in triplicate in each sample from each animal, and the mean values and standard deviations were calculated for the triplicates. Global gene expression studies of lung homogenates were performed using Affymetrix Mouse Genome 430 2.0 short oligomer arrays, which detect ∼44,000 mouse transcripts representing over 34,000 well‐characterized mouse genes (Affymetrix, Santa Clara, CA, USA). Total RNA was isolated from 40 mg lung tissue using TRIzol (Invitrogen, Carlsbad, CA, USA); subsequent RNA quality control, cRNA synthesis and labeling, and oligonucleotide array hybridization and analysis were performed by Genome Explorations (Memphis, TN, USA). The microarray data have been uploaded to the National Center for Biotechnology Information Gene Expression Omnibus database, Accession #GSE23016.
Binding of IL‐4δ2 and IL‐4 to the cell surface
To determine whether binding of IL‐4δ2 to the surface of mouse cells was IL‐4Rα‐dependent, 1 × 107‐panned spleen cells from WT or IL‐4Rα‐deficient Balb/c mice were incubated with periodic agitation for 30 min in 1 ml cell culture supernates from HEK293 cells infected with AdV NULL, AdV‐IL‐4δ2, or AdV‐IL‐4. The concentrations of IL‐4δ2 or IL‐4 in these supernates were initially adjusted to 3 μg/ml, which was in excess of the saturating concentration of 0.5 μg/ml, established in preliminary experiments. Cells were then washed three times with 0.1% BSA in PBS, and the cell pellets were lysed in 150 μl mammalian cell lysis reagent (Sigma‐Aldrich, St. Louis, MO, USA). The concentration of IL‐4δ2 or IL‐4 bound to the cell surface was determined by sandwich ELISA using antibodies that bind both splice variants.
Separate flow cytometry experiments addressed specific binding of IL‐4δ2 to IL‐4Rα using the ability of the anti‐IL‐4Rα antibody M1 to compete with mIL‐4 for binding to IL‐4Rα [24]. Panned spleen cells from WT or IL‐4Rα‐deficient Balb/c mice were treated with 10, 100, or 300 ng/ml mIL‐4δ2 or mIL‐4 for 15 min, washed with 2% FBS in PBS, and stained with PE‐labeled M1 antibody, as described in detail in ref. [24]. Flow cytometric analyses were performed on a C6 flow cytometer (Accuri Cytometers, Ann Arbor, MI, USA).
Statistical analyses
For microarray data analysis, probe sets exhibiting significant differential expression were identified using GeneMaths XT (Applied Maths, Austin, TX, USA), based on the following criteria: 1) MAS5.0 detection P values ≤0.05 for all samples in at least one experimental group, 2) ANOVA P value ≤0.05, and 3) absolute signal log ratio ≥1.0 and independent t test P value ≤0.05 for at least one pair‐wise comparison versus the control group. Other data were analyzed using Statistica software (StatSoft, Tulsa, OK, USA). Groups were compared using Studentˈs two‐tailed t test. Differences in cytokine levels were analyzed using Mann‐Whitney U test. In all analyses, P values ≤0.05 were considered significant.
RESULTS
Replication‐deficient, AdV‐mediated gene delivery
To achieve a continuous supply of mIL‐4δ2 or mIL‐4 to the lung, adenoviral gene delivery was used. This is because rIL‐4 injected directly in mice clears rapidly [25]. The replication‐deficient AdV constructs (AdV‐IL‐4δ2, AdV‐IL‐4, and AdV NULL) caused infection in cultured HEK293 cells and in cultured human, mink, and mouse lung epithelial cells, as was evident from GFP fluorescence of the infected cells at 24–72 h after the virus had been added to the cultures, similar to our previous report (see Fig. 1 A in ref. [21]). Expression of IL‐4δ2 or IL‐4 by the infected cell culture was also confirmed by RT‐ PCR in AdV‐IL‐4δ2‐ or AdV‐IL‐4‐infected but not in AdV NULL‐infected cells. Intratracheal instillation of the adenoviral constructs in vivo resulted in pulmonary expression of GFP, as confirmed by Western blotting (Fig. 1A). RT‐PCR analyses of lung tissue homogenates using specific primers confirmed delivery of desired mRNAs in AdV‐IL‐4‐ or AdV‐IL‐4δ2‐infected mice but no detectable corresponding mRNAs in AdV NULL‐infected mice (Fig. 1B). ELISA assays of pulmonary homogenates from AdV‐IL‐4‐infected mice revealed high levels of IL‐4 and IL‐4δ2 expression in lung homogenates and BAL, whereas no IL‐4 or IL‐4δ2 protein was detected in the AdV NULL‐infected mice (Fig. 1C).
Figure 1.

AdV‐mediated gene delivery to mouse lungs, Day 14 after instillation of the indicated adenoviral viral constructs. (A) Western blotting of mouse lung homogenates for GFP (upper gel) or β‐actin. All constructs encode GFP in the adenoviral backbone and infect the lungs, as evident from GFP production in vivo. (B) Ethidium bromide gel (color‐inverted) of PCR products after amplification with primers that anneal in the first and third exons of mIL‐4. mRNA was purified from the lungs of mice (two animals/group) infected with the indicated adenoviral constructs, converted into cDNA, and amplified. Note the difference in the PCR product sizes, reflecting the presence of the second exon in IL‐4 mRNA and its absence in IL‐4δ2 mRNA. (C) ELISA of lung homogenates (dark‐shaded bars) and BAL (light‐shaded bars) for IL‐4δ2 and IL‐4, using antibodies that indiscriminately bind to either of the splice variants.
Overexpression of IL‐4δ2 induced lymphocytic infiltration of the lungs but not eosinophilia or goblet cell hyperplasia
Total and differential BAL cell counts were assessed in mice overexpressing IL‐4δ2 or IL‐4 compared with mice infected with AdV NULL. The total BAL cell counts did not differ between mice infected with AdV NULL or those infected with the encoding viruses at 7 or 14 days (P>0.05 by one‐way ANOVA). However, the relative contributions of macrophages, lymphocytes, and eosinophils changed significantly ( Fig. 2 A). Overexpression of IL‐4δ2 or IL‐4 led to significant (P<0.05 for either group compared with AdV NULL‐infected mice) recruitment of lymphocytes (Fig. 2A). Of note, only overexpression of IL‐4 caused a significant influx of eosinophils in the lungs of mice (P<0.05 compared with AdV‐IL‐4δ2‐ or AdV NULL‐infected mice). Flow cytometric assays were performed on BAL cells from IL‐4δ2‐ or IL‐4‐overexpressing mice. The BAL lymphocytes were T cells (CD4+ or CD8+) and B cells (CD19+). The results of BAL flow cytometry for hIL‐4δ2‐ or hIL‐4‐overexpressing mice are shown in Fig. 2B. There was no difference between WT C57Bl/6 and WT Balb/c mice in total cell counts, differential cell counts, or flow cytometry results (P>0.05 by Studentˈs t test) within each of the three experimental groups (infected with AdV NULL, AdV‐IL‐4, or AdV‐IL‐4δ2).
Figure 2.

Pulmonary changes caused by delivery of IL‐4δ2 or IL‐4, Day 14. (A) BAL cell counts, mean ± sd, of Giemsa‐stained cytospin slides of BAL from C57Bl/6 mice infected with Adv NULL, AdV‐IL‐4δ2, or AdV‐IL‐4. Eight to 10 animals/group were analyzed. Significant differences from corresponding subpopulations in AdV NULL‐infected mice (P<0.01, two‐tailed Studentˈs t test) are indicated with asterisks. Data pooled from two independent experiments performed by two different technicians. Other cell types (neutrophils and bronchial cells) were present in minimal amounts not exceeding 5% of total BAL cells. (B) Flow cytometry analyses of unseparated BAL cells. Lymphocytes appear within the gated population indicated on the forward (FSC)‐ versus side (SSC)‐scatter densitogram (left); they stain for CD4+, CD8+ (middle) and B220+ cells (right). The table summarizes data from two experiments, three animals/group in each experiment.
Histologically, instillation of AdV NULL caused minimal, if any, changes in the lungs of mice, but delivery of IL‐4δ2 or mIL‐4 caused profound infiltration in the perivascular and peribronchial spaces and within the alveolar septae ( Fig. 3 A). The infiltrates appeared as early as Day 3 after infection, increased on Days 7–14, and declined gradually thereafter. The infiltrates consisted predominantly of T and B cells without macrophages in IL‐4δ2‐ or IL‐4‐overexpressing mice (Fig. 3B). Goblet cell metaplasia with elevated mucus production was observed in mice overexpressing IL‐4 but not in mice overexpressing IL‐4δ2 (Fig. 3C). Goblet cells/microscopy field were counted at ×200 magnification, and the results were expressed as mean ± sd based on counts in five fields/slide, three slides/mouse, and in three mice/group. There was a significant increase (P<0.05 by one‐way ANOVA) in goblet cells in IL‐4‐expressing (25.2±9.7 cells/field) compared with IL‐4δ2‐expressing (4.3±2.4 cells/field) or AdV NULL‐infected (1.2±1.5 cells/field) mice.
Figure 3.

Histological changes caused by overexpression of IL‐4δ2 or IL‐4 in mouse lungs on Day 14 after AdV instillation (original magnification, ×200). (A) H&E staining of lung sections from mice infected with AdV NULL (left), AdV‐IL‐4δ2 (middle), or AdV‐IL‐4 (right). Perivascular and peribronchial infiltrates are indicated with arrows. (B) Immunohistochemically, the infiltrates in mice overexpressing IL‐4δ2 contained CD3+ (left) and CD4+, CD8+, CD19+, and B220+ (middle) cells but not macrophages, which appeared only sparsely throughout the lung parenchyma (arrowheads show F4/80+ cells, right). (C) Periodic acid‐Schiff staining revealed an abundance of goblet cells in mIL‐4‐overexpressing mice (arrows pointing to magenta‐colored cells in the middle; zoom‐in of the outlined area on the right) but not in mice that overexpressed IL‐4δ2 (left). Each of these stainings was performed in lung sections from at least five separate animals in each group with similar results.
Overexpression of IL‐4δ2 affects gene expression and the pulmonary cytokine milieu but does not involve endogenous IL‐4
To determine whether IL‐4δ2 or IL‐4 overexpression affects gene expression and the pulmonary cytokine milieu, mice were infected with each of the adenoviral constructs and were killed after 14 days to obtain pulmonary homogenates. Analysis of global gene expression was performed using Affymetrix oligonucleotide microarrays, whereas multiplex cytokine assays were used to test the levels of several key cytokines.
Using Affymetrix technology to profile the expression of 44,000 mouse transcripts representing over 34,000 well‐characterized mouse genes, we compared three mice in each group infected with AdV‐IL‐4δ2, AdV‐IL‐4, or AdV NULL ( Fig. 4 ). Delivery of IL‐4, but not IL‐4δ2, induced significant up‐ or down‐regulation of the expression of 283 genes represented by 312 probe set IDs (Fig. 4, right panel). There were 84 genes represented by 104 probe set IDs whose expression level was changed by IL‐4δ2 and IL‐4 (Fig. 4, upper left panel). Notable genes in the latter group included CTLA4, MHC II, C1q, and several chemokines. The expression levels of 38 genes represented by 42 probe set IDs were changed by IL‐4δ2 but not IL‐4 (Fig. 4, lower left panel), including genes for Cyr61, CD48, early growth response gene 2, Lck, lysyl oxidase, and CXCL13. Fold‐differences in the expression levels of selected genes are shown in Supplemental Table 1, and function annotation of selected groups of genes is shown in Supplemental Table 2. Thus, IL‐4δ2 induced overall smaller changes in gene expression in vivo than did IL‐4 with a subset of unique, IL‐4δ2‐specific genes.
Figure 4.
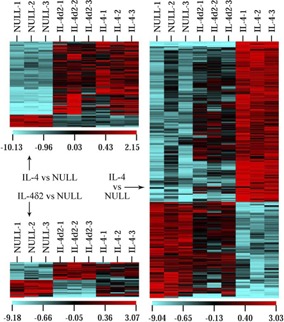
Affymetrix profiling of global gene expression in lung homogenates from mice infected with AdV NULL, AdV‐IL‐4δ2, or AdV‐IL‐4 (three mice/group, columns). Rows represent individual probe set IDs. Color denotes log‐base, twofold difference on the scales indicated below each subpanel. Only those genes whose expression changed significantly (P<0.05) compared with the AdV NULL group are shown.
Significant differences were detected in the levels of cytokine proteins shown in Table 1 ; no significant differences among the groups (P>0.05 for all pair‐wise comparisons) were found in lung homogenates for IL‐1β, IL‐12p40, IL‐12p70, IL‐13, IL‐5, IL‐6, keratinocyte‐derived chemokine (IL‐8), or TGF‐β. Of particular interest, IL‐4δ2 and IL‐4 differentially affected the pulmonary levels of IFN‐γ and MCP‐1, suggesting that IL‐4δ2 may have some Th1‐like properties (Table 1).
Table 1.
Differences in Cytokine Levels Measured by Luminex in Lung Homogenates of Mice Overexpressing IL‐4δ2 or IL‐4 on Day 14 after Infection with Indicated AdVs
| Cytokine | IL‐4δ2 median pg/ml [1st, 3rd quartile] | n | IL‐4 median pg/ml [1st, 3rd quartile] | n | NULL median pg/ml [1st, 3rd quartile] | n |
| TNF‐α | 2 [2, 3] a | 4 | 1 [1, 2] b | 8 | 0 [0, 0] | 3 |
| Eotaxin | 1310 [1250, 1330] b | 10 | 1610 [1465, 1673] a | 8 | 1050 [1010, 1060] | 3 |
| IL‐1α | 16 [12, 39] b | 10 | 47 [26, 68] a | 8 | 7 [7, 10] | 3 |
| IFN‐γ | 17 [5, 29] a | 5 | 9 [0, 11] | 3 | 5 [0, 8] | 3 |
| IL‐17 | 8 [7, 11] a | 10 | 15 [12, 17] | 8 | 17 [16, 17] | 3 |
| MCP‐1 | 94 [70, 102] b | 10 | 173 [139, 189] a | 8 | 47 [44, 48] | 3 |
Significant differences from AdV‐NULL‐infected and from animals infected with the other construct (AdV‐IL‐4δ2 or AdV‐IL‐4);
Significant differences (P < 0.05, Mann‐Whitney U test) from the AdV‐NULL‐infected animals.
Importantly, endogenous IL‐4 mRNA was not detectable in the lungs of mice overexpressing IL‐4δ2, nor was endogenous IL‐4δ2 mRNA detectable in the lungs of mice overexpressing IL‐4. Additional ELISA assays revealed no detectable IL‐4 protein in lung homogenates of mice overexpressing IL‐4δ2. To completely exclude the possibility that the effects of IL‐4δ2 are mediated by endogenous IL‐4, two similar independent experiments were performed in which WT and IL‐4 germline‐deficient mice (n=5/group) were infected with AdV‐IL‐4δ2, and the changes in differential BAL cell counts and lung histology were analyzed on Day 14. There were no significant differences (P>0.05) in total BAL cells or the content of lymphocytes, neutrophils, eosinophils, or macrophages between these groups; the histological changes were also similar, as shown for IL‐4δ2 in Fig. 2A and B. Therefore, the effects of IL‐4δ2 are not mediated by endogenous IL‐4.
Overexpression of IL‐4δ2 modestly affects total pulmonary collagen levels
Global analyses of pulmonary gene expression (Fig. 4) indicated that IL‐4δ2 and IL‐4 differentially regulated expression of ECM‐related genes. Delivery of IL‐4 caused mild elevations in mRNA level of 2.5‐, 2.2‐, and 2.5‐fold for collagens α1(I), α2(I), and α1(III), respectively. Such changes were not observed upon IL‐4δ2 delivery, although the latter led to a 2.0‐fold elevation in mRNA for lysyl oxidase, an enzyme that initiates cross‐linking of collagens and elastin. Gene delivery of IL‐4 or IL‐4δ2 caused elevation of elastin mRNA (4.9‐ and 2.4‐fold, respectively). Simultaneously, delivery of IL‐4, but not IL‐4δ2, significantly elevated levels of mRNAs for ECM‐digesting MMP12, MMP13, and MMP19 (20.2‐, 3.5‐, and 3.0‐fold, respectively), as well as mRNAs for ADAMTS2 and ADAMTS12. These observations, in combination with the observed changes in the levels of pro‐ and antifibrotic cytokines (Table 1), suggest that IL‐4 and IL‐4δ2 regulate gene expression simultaneously for deposition and turnover of ECM. To determine directly whether AdV‐mediated IL‐4 or IL‐4δ2 overexpression may increase collagen accumulation in the lungs, total lung hydroxyproline assays (a surrogate measure of collagen) and Sircol assays (a direct measure of collagen) were performed as described [21, –, 23]. In three independent experiments with five animals in each group, delivery of hIL‐4δ2 or hIL‐4 or mIL‐4δ2 or mIL‐4 tended to cause increases in total levels of pulmonary collagen compared with AdV NULL‐infected animals, but the amplitude of such increases was modest. The increase in collagen induced by IL‐4δ2 did not exceed 1.57 ± 0.24‐fold, and a similar increase induced by IL‐4 did not exceed 1.48 ± 0.27‐fold (P≥0.07 in each of the experiments and in comparisons of pooled groups). It is possible that longer expression of IL‐4δ2 could result in a more substantial accumulation of collagen in the lungs, but the adenoviral delivery model does not allow for gene delivery in excess of 3 weeks.
Effects of STAT6 and IL‐4Rα germline deficiency on the in vivo response to hIL‐4δ2
We then sought to determine whether IL‐4δ2 exerts its effects by signaling through IL‐4Rα and STAT6, which are the known key mediators of IL‐4 activities. Adenoviral delivery of mIL‐4δ2 or mIL‐4 was performed in IL‐4Rα germline‐deficient or STAT6 germline‐deficient mice compared with WT mice ( Fig. 5 ). We first validated this model by comparing eosinophil counts in BAL of STAT6 germline‐deficient, IL‐4Rα germline‐deficient, and WT mice infected with AdV‐IL‐4 (n=3 in each group, Day 14 after instillation of constructs). The WT mice overexpressing mIL‐4 had 20.1 ± 4.0% eosinophils, whereas STAT6‐ or IL‐4Rα‐deficient mice overexpressing mIL‐4 had <1% of eosinophils in their BAL. These data are consistent with previous reports [26, 27] about the role of STAT6 in mediating IL‐4‐induced eosinophilic infiltration and thus, validate the results in these knockout models below.
Figure 5.

Effects of STAT6 or IL‐4Rα deficiency in IL‐4δ2‐ or IL‐4‐expressing mice. (A) Lymphocytes in BAL, mean ± sd. Significant differences (P<0.05) are indicated with asterisks. KO, Knockout. (B) H&E staining, Day 14 after AdV instillation; original magnification, ×200. The lung sections shown were from IL‐4δ2 (left column)‐ or IL‐4 (right column)‐expressing mice, WT mice (top row), or STAT6 (middle row)‐ or IL‐4Rα (bottom row)‐deficient. Data combined from two independent experiments, four to five mice/group in each experiment. (C) ELISA for IL‐4δ2 and IL‐4 using antibodies that indiscriminately bind to either of the splice variants. Following incubation with IL‐4δ2 or IL‐4, cells were lysed and lysates tested as described in the text. (D) Flow cytometry with M1 antibody specific for IL‐4Rα. Preincubation with IL‐4, but not IL‐4δ2, attenuates binding of the antibody to the cell surface.
Overexpression of IL‐4δ2 or IL‐4 in STAT6‐deficient mice as well as overexpression of IL‐4 in IL‐4Rα‐deficient mice were manifested by a significant attenuation of lymphocytes in BAL (Fig. 5A) and in the size of pulmonary infiltrates (Fig. 5B). Deficiency of IL‐4Rα also attenuated accumulation of lymphocytes (P<0.05 by Studentˈs t test) and the size of infiltrates induced by IL‐4δ2, although the attenuating effect in this case was somewhat less profound (Fig. 5A and B). These observations suggest that IL‐4δ2 and IL‐4 share the same intracellular signaling pathway, and the effects may be dependent in part on another surface molecule in addition to IL‐4Rα. To determine whether binding of IL‐4δ2 to the surface of mouse cells was IL‐4Rα‐dependent, panned spleen cells from WT and IL‐4Rα‐deficient mice were incubated with cell culture supernates of HEK293 cells infected with AdV NULL, AdV‐IL‐4δ2, or AdV‐IL‐4, as described in Materials and Methods. Binding of IL‐4δ2 and IL‐4 by cells from IL‐4Rα−/− mice was lower than by cells from WT mice (Fig. 5C), suggesting that both splice variants indeed use this receptor chain. To assess this binding further, cells from WT mice were preincubated with 10, 100, or 300 ng/ml mIL‐4δ2 or mIL‐4 for 15 min and then stained with M1 antibody, which is known to compete with mIL‐4 for binding to IL‐4Rα [24]. Preincubation with mIL‐4, but not mIL‐4δ2, in any of the tested concentrations blocked binding of M1 to cells (Fig. 5D), suggesting that the affinity of IL‐4 for IL‐4Rα is likely higher than that of IL‐4δ2. These data are consistent with our previous report that IL‐4 readily competes with IL‐4δ2 for receptor binding in cell culture [7] but also suggest that a chain different from IL‐4Rα may be involved in IL‐4δ2 binding.
IL‐4δ2 does not potentiate the effects of IL‐4 on the lungs
We then considered the possibility that IL‐4δ2 potentiates the effects of IL‐4 on the lungs. An experiment was performed in which mice were instilled with AdV‐IL‐4δ2 + AdV NULL, AdV NULL + AdV‐IL‐4, or AdV‐IL‐4δ2 + AdV‐IL‐4 using a half‐concentration of each viral construct. The combined effects of IL‐4δ2 and IL‐4 did not exceed the sum of independent effects of each cytokine separately ( Fig. 6 ; P>0.05, one‐way ANOVA). This observation suggests that each of these splice variants acts independently on the lung and that future therapeutic targeting should consider each variant independently to avoid possible side‐effects associated with neutralization of the other splice variant.
Figure 6.

Effects of combined expression of IL‐4δ2 and IL‐4 on the lung. Mean percent ± sd differential cell counts in BAL with five to seven mice in each group. The effect of combined expression of these splice variants is lower than the sum of independent effects (P>0.05), suggesting a lack of functional synergy.
DISCUSSION
The novelty of this study is that it has addressed, for the first time, the effects of IL‐4δ2, an alternatively spliced variant of IL‐4, in vivo. The results show that in contrast to the previously observed lack of independent, short‐term effects on cultured, purified cells [7, 12], IL‐4δ2 is active in vivo, and its effects are different from those of IL‐4. Our findings provide new insight into pathophysiological mechanisms of numerous diseases, in which IL‐4δ2 appears to be involved [8, 13, –, 15, 16, 17, 18, 19, 20]. Although these previous studies explored changes in the expression levels of IL‐4δ2 in patients compared with healthy controls, they did not investigate possible effects of IL‐4δ2 on the lung. Therefore, the pathophysiological significance of these earlier findings has been unclear until now.
Expression of IL‐4δ2 in vivo caused accumulation of T and B lymphocytes (see Fig. 2) and a tendency to collagen accumulation similar to the effects of IL‐4. However, unlike IL‐4, IL‐4δ2 did not induce pulmonary eosinophilia or goblet cell hyperplasia (see Figs. 2 and 3). Also, delivery of IL‐4δ2 changed the gene‐expression pattern (see Fig. 4 and Supplemental Table 1) and the cytokine milieu (see Table 1) differentially from IL‐4. There was no detectable IL‐4 mRNA or protein in the lungs of IL‐4δ2‐expressing mice, and delivery of IL‐4δ2 to the lungs of IL‐4 germline‐deficient mice resulted in a phenotype that was indistinguishable from IL‐4δ2‐expressing WT mice. These observations suggest that the effects of IL‐4δ2 were not mediated by endogenous IL‐4. Although the increase in the levels of collagen did not reach statistical significance (P=0.07), it is important to note that this adenoviral model is short‐term, and IL‐4δ2 expression declined after 3 weeks of expression. It is possible that a more prolonged expression, similar to that observed in patients with scleroderma [8, 13], would lead to a greater accumulation of collagen in the lungs.
Deficiency of STAT6 or IL‐4Rα attenuated IL‐4δ2‐ or IL‐4‐induced lymphocytic infiltration, suggesting that these signaling molecules mediate, in part, homing of lymphocytes to the lungs in response to IL‐4δ2 or IL‐4 (see Fig. 5A and B). Consistent with previous reports [26, 27], deficiency of STAT6 completely abrogated IL‐4‐induced eosinophilic infiltration, thus validating our model. In agreement with our previous study in cell culture [7], both splice variants bound to the cell surface, partially in an IL‐4Rα‐dependent manner (see Fig. 5C), although the affinity of IL‐4 to IL‐4Rα appeared to be higher than that of IL‐4δ2 based on their differential competition with the M1 antibody for binding to the receptor chain (see Fig. 5D).
In contrast to IL‐4, IL‐4δ2 did not induce pulmonary eosinophilia or goblet cell hyperplasia, whereas IL‐4δ2 caused a proportionately higher accumulation of CD8+ cells (see Fig. 1C). The differences in eosinophil accumulation, goblet cell hyperplasia, and relative accumulation of CD8+ cells caused by IL‐4 versus IL‐4δ2 resemble the differences between patients with asthma and patients with scleroderma lung disease. Although both are considered Th2‐driven pulmonary diseases with immune inflammation [8], scleroderma lung disease is not characterized by pulmonary eosinophilia and goblet cell hyperplasia but predominantly, by elevation of CD8+ lymphocytes in the lungs [8]. Considering the previous reports about the predominance of IL‐4δ2 over IL‐4 in patients with scleroderma lung disease [8, 13], it is possible that asthma is driven predominantly by IL‐4, whereas scleroderma lung disease is driven by IL‐4δ2. Mechanistic differences in IL‐4‐ and IL‐4δ2‐induced signaling leading to the differences in cellular phenotypes need further investigation.
In contrast to observations in purified cell cultures [7, 12], IL‐4δ2 did not inhibit the effects of IL‐4 in vivo; these two splice isoforms acted on the lung in an additive manner (see Fig. 6). The reasons for the difference of IL‐4δ2 effects in cell culture and in vivo are not obvious. It is possible that prolonged exposure to IL‐4δ2 in vivo as well as cross‐talk between various cell types, which is absent in cell culture but present in vivo, contribute to such a difference. It is also possible that biological activity of IL‐4δ2 is better preserved in vivo, for example, as a result of preserved conformational stability resulting from stabilization through binding of other proteins present in vivo but not in purified IL‐4δ2 preparations.
In summary, it is demonstrated for the first time that expression of IL‐4δ2 induces lymphocytic inflammation and a tendency to collagen accumulation in vivo for the mouse model presented here; however, in contrast to IL‐4, IL‐4δ2 does not induce pulmonary eosinophilia or goblet cell hyperplasia. These two splice variants also differentially regulate gene expression and pulmonary levels of cytokines. Our observations suggest that IL‐4δ2 is an independent regulator of lymphocytic infiltration, its effects are different from and independent of the effects of IL‐4, and differential therapeutic targeting of IL‐4 and IL‐4δ2 should be considered in various diseases.
AUTHORSHIP
All authors contributed sufficiently to be included on the author list and to take responsibility for the content of this report. I.G.L. and S.P.A. contributed to the studyˈs conception. The study was designed by I.G.L. and S.P.A. with assistance from A.D.K. and J.D.H. Data were collected and analyzed by I.G.L., V.L., N.W.T., and K.H. Data interpretation was performed by I.G.L., A.D.K., J.D.H., and S.P.A. The manuscript was prepared and revised by S.P.A. with assistance from I.G.L. and N.W.T.
Supporting information
Supplementary Material Files
ACKNOWLEDGMENTS
The study was supported by the VA Merit Review Awards (I.G.L. and S.P.A.) and Arthritis Foundation Research Awards (I.G.L. and S.P.A.). We thank Mrs. Jung Choi for her excellent technical help and Dr. Paul Todd for his excellent editorial work.
REFERENCES
- 1. Nelms, K. , Keegan, A. D. , Zamorano, J. , Ryan, J. J. , Paul, W. E. (1999) The IL‐4 receptor: signaling mechanisms and biologic functions. Annu. Rev. Immunol. 17, 701–738. [DOI] [PubMed] [Google Scholar]
- 2. Corry, D. B. , Kheradmand, F. (2002) Biology and therapeutic potential of the interleukin‐4/interleukin‐13 signaling pathway in asthma. Am. J. Respir. Med. 1, 185–193. [DOI] [PubMed] [Google Scholar]
- 3. Finkelman, F. D. , Shea‐Donohue, T. , Morris, S. C. , Gildea, L. , Strait, R. , Madden, K. B. , Schopf, L. , Urban, J. F. (2004) Interleukin‐4‐ and interleukin‐13‐mediated host protection against intestinal nematode parasites. Immunol. Rev. 201, 139–155. [DOI] [PubMed] [Google Scholar]
- 4. Rook, G. A. , Hernandez‐Pando, R. , Dheda, K. , Teng Seah, G. (2004) IL‐4 in tuberculosis: implications for vaccine design. Trends Immunol. 25, 483–488. [DOI] [PubMed] [Google Scholar]
- 5. Jakubzick, C. , Kunkel, S. L. , Puri, R. K. , Hogaboam, C. M. (2004) Therapeutic targeting of IL‐4‐ and IL‐13‐responsive cells in pulmonary fibrosis. Immunol. Res. 30, 339–349. [DOI] [PubMed] [Google Scholar]
- 6. Alms, W. J. , Atamas, S. P. , Yurovsky, V. V. , White, B. (1996) Generation of a variant of human interleukin‐4 by alternative splicing. Mol. Immunol. 33, 361–370. [DOI] [PubMed] [Google Scholar]
- 7. Atamas, S. P. , Choi, J. , Yurovsky, V. V. , White, B. (1996) An alternative splice variant of human IL‐4, IL‐4 δ 2, inhibits IL‐4‐stimulated T cell proliferation. J. Immunol. 156, 435–441. [PubMed] [Google Scholar]
- 8. Atamas, S. P. , Yurovsky, V. V. , Wise, R. , Wigley, F. M. , Goter Robinson, C. J. , Henry, P. , Alms, W. J. , White, B. (1999) Production of type 2 cytokines by CD8+ lung cells is associated with greater decline in pulmonary function in patients with systemic sclerosis. Arthritis Rheum. 42, 1168–1178. [DOI] [PubMed] [Google Scholar]
- 9. Gautherot, I. , Burdin, N. , Seguin, D. , Aujame, L. , Sodoyer, R. (2002) Cloning of interleukin‐4 δ2 splice variant (IL‐4δ2) in chimpanzee and cynomolgus macaque: phylogenetic analysis of δ2 splice variant appearance, and implications for the study of IL‐4‐driven immune processes. Immunogenetics 54, 635–644. [DOI] [PubMed] [Google Scholar]
- 10. Yatsenko, O. P. , Filipenko, M. L. , Khrapov, E. A. , Voronina, E. N. , Kozlov, V. A. , Sennikov, S. V. (2004) Alternative splicing of mRNA of mouse interleukin‐4 and interleukin‐6. Cytokine 28, 190–196. [DOI] [PubMed] [Google Scholar]
- 11. Waldvogel, A. S. , Lepage, M. F. , Zakher, A. , Reichel, M. P. , Eicher, R. , Heussler, V. T. (2004) Expression of interleukin 4, interleukin 4 splice variants and interferon γ mRNA in calves experimentally infected with Fasciola hepatica . Vet. Immunol. Immunopathol. 97, 53–63. [DOI] [PubMed] [Google Scholar]
- 12. Arinobu, Y. , Atamas, S. P. , Otsuka, T. , Niiro, H. , Yamaoka, K. , Mitsuyasu, H. , Niho, Y. , Hamasaki, N. , White, B. , Izuhara, K. (1999) Antagonistic effects of an alternative splice variant of human IL‐4, IL‐4δ2, on IL‐4 activities in human monocytes and B cells. Cell. Immunol. 191, 161–167. [DOI] [PubMed] [Google Scholar]
- 13. Sakkas, L. I. , Tourtellotte, C. , Berney, S. , Myers, A. R. , Platsoucas, C. D. (1999) Increased levels of alternatively spliced interleukin 4 (IL‐4δ2) transcripts in peripheral blood mononuclear cells from patients with systemic sclerosis. Clin. Diagn. Lab. Immunol. 6, 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seah, G. T. , Scott, G. M. , Rook, G. A. (2000) Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. J. Infect. Dis. 181, 385–389. [DOI] [PubMed] [Google Scholar]
- 15. Fletcher, H. A. , Owiafe, P. , Jeffries, D. , Hill, P. , Rook, G. A. , Zumla, A. , Doherty, T. M. , Brookes, R. H.; Vacsel Study Group (2004) Increased expression of mRNA encoding interleukin (IL)‐4 and its splice variant IL‐4δ2 in cells from contacts of Mycobacterium tuberculosis, in the absence of in vitro stimulation. Immunology 112, 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dheda, K. , Chang, J. S. , Breen, R. A. , Kim, L. U. , Haddock, J. A. , Huggett, J. F. , Johnson, M. A. , Rook, G. A. , Zumla, A. (2005) In vivo and in vitro studies of a novel cytokine, interleukin 4δ2, in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 172, 501–508. [DOI] [PubMed] [Google Scholar]
- 17. Glare, E. M. , Divjak, M. , Rolland, J. M. , Walters, E. H. (1999) Asthmatic airway biopsy specimens are more likely to express the IL‐4 alternative splice variant IL‐4δ2. J. Allergy Clin. Immunol. 104, 978–982. [DOI] [PubMed] [Google Scholar]
- 18. Plante, S. , Semlali, A. , Joubert, P. , Bissonnette, E. , Laviolette, M. , Hamid, Q. , Chakir, J. (2006) Mast cells regulate procol‐lagen I (α 1) production by bronchial fibroblasts derived from subjects with asthma through IL‐4/IL‐4 δ 2 ratio. J. Allergy Clin. Immunol. 117, 1321–1327. [DOI] [PubMed] [Google Scholar]
- 19. Seah, G. T. , Gao, P. S. , Hopkin, J. M. , Rook, G. A. (2001) Interleukin‐4 and its alternatively spliced variant (IL‐4δ2) in patients with atopic asthma. Am. J. Respir. Crit. Care Med. 164, 1016–1018. [DOI] [PubMed] [Google Scholar]
- 20. Wu, H. P. , Wu, C. L. , Chen, C. K. , Chung, K. , Tseng, J. C. , Liu, Y. C. , Chuang, D. Y. (2008) The interleukin‐4 expression in patients with severe sepsis. J. Crit. Care 23, 519–524. [DOI] [PubMed] [Google Scholar]
- 21. Luzina, I. G. , Papadimitriou, J. C. , Anderson, R. , Pochetuhen, K. , Atamas, S. P. (2006) Induction of prolonged infiltration of T lymphocytes and transient T lymphocyte‐dependent collagen deposition in mouse lungs following adenoviral gene transfer of CCL18. Arthritis Rheum. 54, 2643–2655. [DOI] [PubMed] [Google Scholar]
- 22. Pochetuhen, K. , Luzina, I. G. , Lockatell, V. , Choi, J. , Todd, N. W. , Atamas, S. P. (2007) Complex regulation of pulmonary inflammation and fibrosis by CCL18. Am. J. Pathol. 171, 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luzina, I. G. , Todd, N. W. , Nacu, N. , Lockatell, V. , Choi, J. , Hummers, L. K. , Atamas, S. P. (2009) Regulation of pulmonary inflammation and fibrosis through expression of integrins αVβ3 and αVβ5 on pulmonary T lymphocytes. Arthritis Rheum. 60, 1530–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ford, A. Q. , Heller, N. M. , Stephenson, L. , Boothby, M. R. , Keegan, A. D. (2009) An atopy‐associated polymorphism in the ectodomain of the IL‐4R(α) chain (V50) regulates the persistence of STAT6 phosphorylation. J. Immunol. 183, 1607–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gea‐Sorlí, S. , Closa, D. (2009) In vitro, but not in vivo, reversibility of peritoneal macrophages activation during experimental acute pancreatitis. BMC Immunol. 10, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akimoto, T. , Numata, F. , Tamura, M. , Takata, Y. , Higashida, N. , Takashi, T. , Takeda, K. , Akira, S. (1998) Abrogation of bronchial eosinophilic inflammation and airway hyperreactivity in signal transducers and activators of transcription (STAT)6‐deficient mice. J Exp. Med. 187, 1537–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miyata, S. , Matsuyama, T. , Kodama, T. , Nishioka, Y. , Kuribayashi, K. , Takeda, K. , Akira, S. , Sugita, M. (1999) STAT6 deficiency in a mouse model of allergen‐induced airways inflammation abolishes eosinophilia but induces infiltration of CD8+ T cells. Clin. Exp. Allergy 29, 114–123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material Files


