Short abstract
Novel immunoregulatory mechanism operating in the maternal‐placental interface through VIP production by trophoblast cells and the induction of Tregs involving TGFβ1.
Keywords: early pregnancy, tolerance, TGF‐β, iTreg
Abstract
Inducible regulatory T cells (Tregs) exert a timely and efficient immunosuppressive action at the critical peri‐implantation stage essential for maternal tolerance to the conceptus. Vasoactive intestinal peptide (VIP) promotes anti‐inflammatory and tolerogenic profiles through binding to VIP receptors on immune cells. We evaluated whether VIP produced by trophoblast cells induces Tregs during the early interaction of maternal leukocytes with trophoblast cells, thus contributing to maternal tolerance. We used an in vitro model of maternal leukocyte–trophoblast cell interaction represented by cocultures of fertile women's PBMCs with a human trophoblast cell line (Swan‐71) and evaluated the effect of VIP added exogenously and of the endogenous polypeptide. VIP increased the frequency of CD4+CD25+FoxP3+ cells after coculture, and these cells were able to suppress the maternal alloresponse. VIP also increased the frequency of CD4+IL10+ and CD4+TGFβ + cells, but it did not modulate IFN‐γ or IL‐17 production. Swan‐71 secreted VIP, and their coculture with maternal PBMCs significantly increased the frequency of Tregs. This effect was even more pronounced if the trophoblast cells had been pretreated with VIP. In both situations, the VIP antagonist prevented the increase in the frequency of CD4+Foxp3+ cells, reflecting a specific effect of the polypeptide after the interaction with Swan‐71 cells. Finally, the increase in CD4+CD25+FoxP3+ frequency was prevented by an anti–TGF‐β Ab and a VIP antagonist. These results suggest that VIP could have an active role in the immunoregulatory processes operating in the maternal–placental interface by contributing to the induction of Tregs through a mechanism involving TGF‐β1.
Abbreviations
- CNS1
FoxP3 enhancer conserved noncoding sequence 1
- RUNX
runt‐related class of transcription factors
- T‐bet
T‐box transcription factor
- Treg
regulatory T cell
- VIP
vasoactive intestinal peptide
Introduction
Pregnancy challenges immune cells and the immunomodulatory circuits of the mother and the developing fetus to dynamically adapt to each other in a homeostatic and tolerant environment for fetal growth. Pregnancy evolves through a predominantly proinflammatory first stage that mostly coincides with the implantation process, followed by a predominant immunosuppressant period, characterized by uterine quiescence and fetal growth, which lasts for the whole second and most of the third trimester [1, 2]. In particular, in the peri‐implantation period, a variety of cellular processes are encompassed to ensure proper trophoblast growth and invasion with intense vascular remodeling in an immunotolerant microenvironment [3, 4–5]. Several leukocyte populations and mediators contribute to tolerance induction and homeostasis maintenance. These include specialized uterine NK cells, decidual macrophages activated in an alternative profile, and tolerogenic DCs [6, 7–8], as well as various cytokines, chemokines, galectins, and other polypeptides [9, 10–11]. Consistently, a central role for Tregs has been demonstrated at the early stages of pregnancy in several animal models and in humans. They were shown to suppress alloantigen immune responses inducing maternal tolerance to the conceptus [5, 7, 8, 12]. Thus, natural Tregs that recognize self‐antigens are further expanded in the blood compartment, peaking in the second trimester [13, 14]. In contrast, newly generated inducible Tregs (iTregs) are differentiated against non–self‐antigens inherent to pregnancy such as paternal alloantigens or antigens of the trophoblast. Recently, Samstein et al. [15] have shown that iTregs are generated in placental mammals in a FoxP3 enhancer CNS1‐dependent manner and has been proposed as a mechanism that emerged to enforce maternal–fetal tolerance [16]. This intronic FoxP3 enhancer contains binding sites for Smad3 and for retinoic acid receptors that facilitate iTreg induction through TGF‐β–mediated signaling [17]. Moreover, TGF‐β can target members of the Runt‐related class of transcription factors (RUNX), such as RUNX1, which binds to the FoxP3 promoter, inducing its expression [18, 19].
In contrast, the VIP proved to have potent immunomodulatory and trophic effects through its action on VIP receptors (VPACs) on adult and embryonic tissues. Evidence of the VIP anti‐inflammatory and tolerogenic effects were provided by in vitro studies of human [20] and murine [21] cells and from studies in animal models of viral disease [22] and chronic inflammation [23, 24, 25–26]. In particular, during pregnancy, evidence from murine models and experimental designs with human leukocytes and trophoblast cells has indicated that trophoblast cells produce VIP and that it exerts immunomodulatory effects, promoting anti‐inflammatory and tolerogenic responses [27, 28, 29–30]. In experimental coculture designs with human cells, VIP modulated the immune–trophoblast cell interaction, inducing CD4+CD25+FoxP3+ cells, and reduced proinflammatory markers [31, 32]. First‐trimester human Swan‐71 trophoblast cells express VPACs, which has been shown in first‐trimester human placental trophoblasts and also in third‐trimester trophoblast cell lines [29, 30–31].
Considering that iTreg induction appears to be crucial for a successful pregnancy outcome, that their induction involves TGF‐β signaling, and that VIP exerts potent anti‐inflammatory and tolerogenic effects in various inflammatory disease models, we hypothesized that the VIP produced by trophoblast cells would induce iTregs, involving TFG‐β production and contributing to maternal tolerance. In the present study, we have shown that VIP specifically increased the frequency of maternal CD4+CD25+FoxP3+ cells after coculture with trophoblast cells, with the ability to suppress the maternal alloresponse through a mechanism involving TGF‐β1 production, and increased the frequency of CD4+IL‐10+ and CD4+TGF‐β + cells
MATERIALS AND METHODS
PBMC Samples
PBMCs were obtained from fertile volunteers, defined as women who had had 2 or more previous normal pregnancies without any miscarriages. PBMCs were isolated from heparinized peripheral blood using density gradient centrifugation on Ficoll‐Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden). The cells were extensively washed and resuspended in DMEM‐F12 (Gibco, Invitrogen, Buenos Aires, Argentina), supplemented with 10% FBS (Natocor, Cordobo, Argentina), 2 mM glutamine (Sigma‐Aldrich, St. Louis, MO, USA), and 100 μg/ml penicillin and 100 U/ml streptomycin (Invitrogen).
Cell lines
The trophoblast cell line Swan‐71 (derived by telomerase‐mediated transformation of a 7‐wk cytotrophoblast isolate described by Straszewski‐Chavez et al. [33]) was used as representative of first‐trimester trophoblast cells. The JEG‐3 (derived from human choriocarcinoma, American Type Culture Collection HTB‐36) cell line derived from a third‐trimester trophoblast was used as a control system [34, 35]. The cells were cultured in 24‐well, flat bottom, polystyrene plates (Becton Dickinson, Franklin Lakes, NJ, USA) in complete DMEM‐F12 10% FBS to 70% confluence. Swan‐71 cells were cultured in the absence or presence of VIP 10 nM (PolyPeptide Laboratories, Strasbourg, France) for 4, 8, 12, 24, and 48 h. At each time point, cells and supernatants were recovered and used for qRT‐PCR analysis and TGF‐β quantification.
Cocultures
For the cocultures, the trophoblast cell lines were cultured in 24‐well plates in complete DMEM‐F12 10% FBS at 70% of confluence (105 cells/well) in the absence or presence of maternal PBMCs (5 × 105 cells/well) with or without VIP (100 nM), anti–TGF‐β neutralizing Ab (1 μg/ml; R&D System, Minneapolis, MN, USA), recombinant TGF‐β (10 ng/ml; eBioscience, San Diego, CA, USA), and VIP antagonist (10−7 M; Peninsula‐Bachem, San Carlos, CA, USA) in several combinations. In some experiments, before culture, freshly isolated PBMCs were first labeled with 3 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR, USA), as recommended by the manufacturer.
In another set of experiments, PBMCs were cultured alone in the same experimental conditions of the cocultures to evaluate the contribution of the trophoblast cells. In pretreatment experiments performed in the presence of VIP antagonist, trophoblast cells were preincubated with VIP 10 nM for 24 h before PBMC coculture. After 48 h of culture, suspension cells were recovered and used for flow cytometry or Western blot analysis.
Flow cytometry analysis
Intracellular staining for FoxP3 detection
Flow cytometry analysis for CD4, CD25, CD127, CTLA‐4, CD39, and FoxP3 staining was performed according to the manufacturer's instructions using different combinations. In particular, for CD4, CD25, and FoxP3 staining, we used the human regulatory T cell staining kit (eBioscience). In brief, 1 × 106 cells were stained with a CD4/CD25 cocktail. After 30 min, the cells were washed with staining buffer and then incubated with the fixation/permeabilization buffer for 1 h at 4°C. After washing with permeabilization buffer, nonspecific sites were blocked by adding 2 µl (2% final) normal rat serum, in approximately 100 μl for 15 min. Next, the cells were incubated with anti‐human Ab or rat IgG2a isotype control for 30 min. Finally, the cells were washed with permeabilization buffer and analyzed. In the VIP antagonist experiments, PBMCs were stained only for CD4 (Becton Dickinson) and FoxP3 (eBioscience).
Intracellular cytokine detection
For cytokine detection in each cell population, maternal PBMCs were cultured with or without Swan‐71 cells in the presence or absence of VIP for 48 h and then incubated with StopGolgi (Becton Dickinson) for the last 4 h of culture, in accordance with the manufacturer's instructions, to promote intracellular accumulation of proteins. PBMCs were stained with a PeCy5 or APC‐conjugated anti‐CD4 Ab (eBioscience and Becton Dickinson). After fixation and permeabilization as described in the previous section, the cells were stained with PE‐conjugated anti–IL‐10, TGF‐β, IL‐17 Ab, or FITC‐conjugated IFN‐γ Ab (IL‐10 and IFN‐γ from eBioscience; IL‐17 from BioLegend, San Diego, CA, USA; and TGF‐β from IQ Products, Groningen, The Netherlands). Ten thousand events were acquired in a FACS Aria II cytometer (Becton Dickinson), and the results were analyzed using WinMDI, version 2.9, software (facs.scripps.edu/software.html). Negative control samples were incubated in parallel with an irrelevant, isotype‐matched Ab (see Figs. 1, 2, and 5). The results for positive cells are expressed as a percentage of the respective population, and the quadrant was set using irrelevant isotype‐specific Abs. In particular, for Treg analysis, positive cells were determined inside the electronically gated CD4‐positive cell population using the WinMDI, version 2.9, software.
Figure 1.
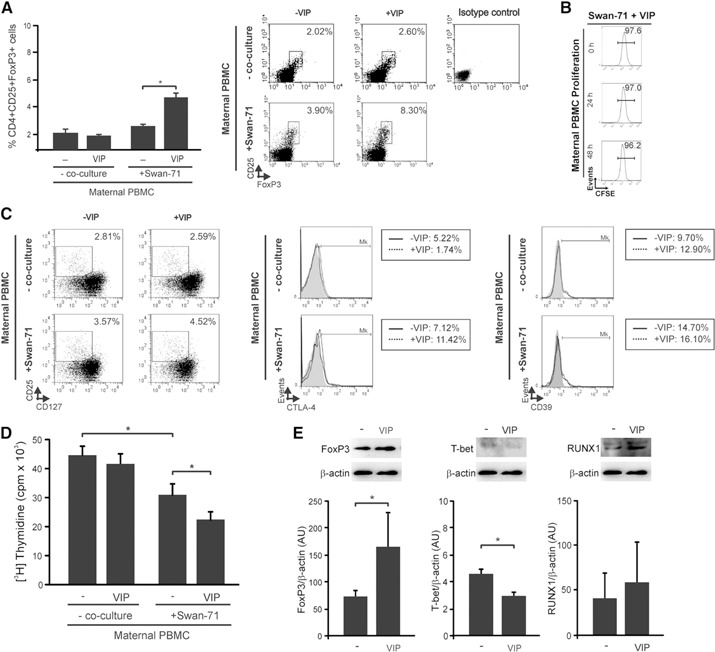
VIP increases the frequency of CD4+CD25+FoxP3 cells with suppressor ability in PBMC–Swan‐71 cell cocultures. Maternal PBMCs from fertile women were cocultured in the absence or presence of Swan‐71 at 70% of confluence with or without VIP (100 nM). After 48 h, PBMCs were recovered and (A) the frequency of CD4+CD25+FoxP3+ was evaluated by FACS analysis. Results are expressed as the mean percentage of CD4+CD25+FoxP3+ cells ± sem of at least 5 independent experiments using PBMC samples from different fertile women. *P < 0.05, Mann‐Whitney U test. (Right) Representative dot plots and the frequency of CD25+FoxP3+ cells (inside the electronically gated CD4+) after culture or not with trophoblast cells in the absence or presence of VIP. (B) In some experiments, before culture with trophoblast cells in the presence of VIP, freshly isolated PBMCs were first labeled with CFSE, and proliferation was investigated after 0, 24, and 48 h of coculture by FACS analysis. Result shown is representative of 3 similar runs. (C) After coculture, PBMCs in suspension were recovered, and the frequency of CD4+CD25+CD127−, CD4+CD25+CTLA‐4+, and CD4+CD25+CD39+ was evaluated by FACS analysis. The dot plots show the percentage of CD25+CD127− cells (inside the electronically gated CD4+ cells). The histograms show the percentage of CTLA‐4 or CD39 cells (inside the electronically gated CD4+CD25+ cells). (D) Maternal PBMCs were cultured with or without Swan‐71 cells in the absence or presence of VIP (100 nM) for 48 h. Then, PBMCs in suspension were recovered and transferred to a culture with mismatched allogeneic healthy donor PBMCs (1 × 105 cells/well). These last PBMCs had previously been treated with mitomycin C to inhibit DNA synthesis by crosslinking DNA at guanine and adenine residues to obtain a unidirectional proliferation. After 5 d, [3H]TdR was added for 18 h, and uptake was determined using a β‐scintillation counter. Results are expressed as mean cpm ± sem of at least 3 independent experiments run in triplicate. *P < 0.05, Mann Whitney U test. (E) At 48 h of coculture, PBMCs recovered were harvested and analyzed by Western blot for FoxP3, T‐bet, and RUNX1 expression. Representative immunoreactive bands and semiquantification, expressed as relative to β‐actin in A.U. ± sem are shown from 4 independent experiments. *P < 0.05, Mann Whitney U test.
Figure 2.
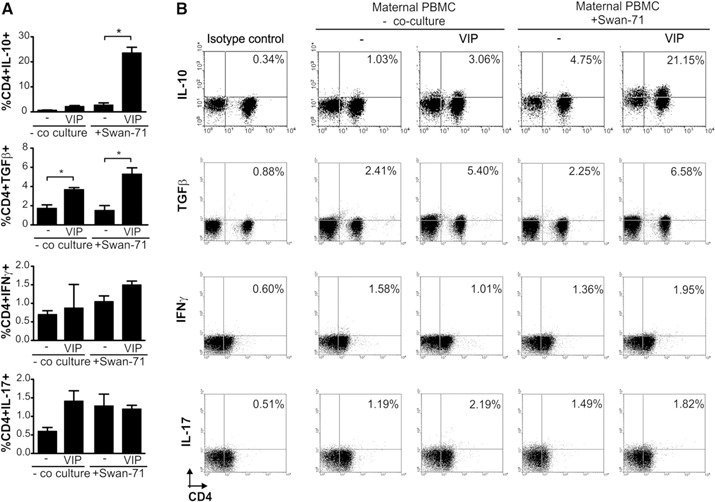
VIP induces an increase in the frequency of CD4+ cells producing anti‐inflammatory cytokines after coculture with Swan‐71 cells. (A) Maternal PBMCs obtained from fertile women were cultured with or without Swan‐71 cells at 70% confluence in the absence or presence of VIP (100 nM). After 48 h, suspension cells were recovered, and intracellular staining for IL‐10, TGF‐β, INF‐γ, and IL‐17 was performed on CD4+ cells by FACS analysis. Results are expressed as the mean percentage ± sem of at least 3 independent experiments with 3 different fertile PBMC samples. *P < 0.05, Mann‐Whitney U test. (B) Representative dot plots showing percentages corresponding to the frequency of CD4+TGF‐β +, CD4+IL‐10+, CD4+IFN‐γ +, and CD4+IL‐17+ cells ± sem from the same fertile woman in the absence or presence of VIP. Negative control samples were incubated in parallel with an irrelevant, isotype‐matched Ab and are shown for each cytokine tested.
Figure 5.
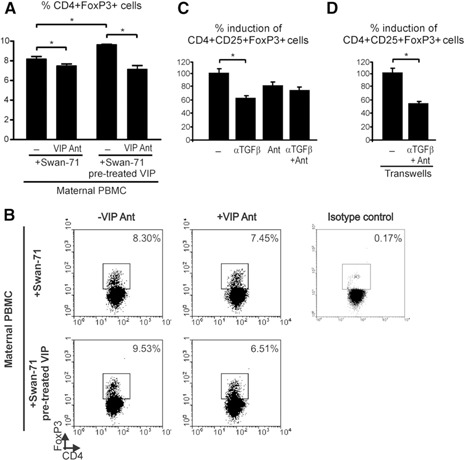
VIP produced by trophoblast cells specifically increases the frequency of Tregs during the early trophoblast–maternal interaction. The Swan‐71 cell line was pretreated or not with VIP (10 nM) for 24 h and then cocultured with maternal PBMCs in the absence or presence of VIP antagonist (Ant) (10−7 M). (A) After 48 h of culture, cells in suspension were recovered, and Treg frequency was analyzed by FACS. Results are expressed as the mean percentage of CD4+FoxP3+ cells ± sem of 3 independent PBMC preparations. *P < 0.05, Mann‐Whitney U test. (B) Representative dot plots and the frequency of CD4+FoxP3+ cells after coculture with trophoblast cells with or without VIP antagonist. Negative control also shown, corresponding to the isotype‐matched Ab. (C) Swan‐71 cells were cultured until 70% confluence, and PBMCs obtained from fertile women were added with or without a 0.4‐µm insert. In both situations, cocultures were performed in the absence or presence of anti–TGF‐β neutralizing Ab and VIP antagonist (Ant) in several combinations. After 48 h, the frequency of the CD4+CD25+FoxP3+ population was evaluated by FACS analysis. Results are expressed as the percentage of Treg induction, considering the value obtained in the presence of VIP as 100%. Results are representative of 3 independent experiments from 3 different samples from fertile women. *P < 0.05, Mann‐Whitney U test.
Western blot
Analysis of the expression of FoxP3, T‐bet, and RUNX1 was performed in PBMCs cocultured with Swan‐71 cells in the absence or presence of VIP 100 nM by Western blotting. In brief, PBMCs were recovered after 48 h of culture and washed with PBS. The cell pellet was mixed with cold lysis buffer (PBS containing 1% nonidet P‐40 and 1% SDS) with freshly added protease inhibitor cocktail (0.2 mM PMSF, 1 μg/ml leupeptin, 0.7 μg/ml pepstain, 0.1% aprotinin; Sigma‐Aldrich) and incubated for 20 min on ice, vortexing periodically. The samples were finally centrifuged at 13,000g for 15 min at 4°C, and the supernatant (cell protein lysate) was recovered and stored at −70°C until use. The protein concentration was determined using the micro‐BCA protein assay reagent kit (Pierce, Rockford, IL, USA). Equal amounts of proteins were diluted in sample buffer and separated on 12% SDS‐polyacrylamide gels. After electrophoresis, the separated proteins were transferred onto nitrocellulose membranes and probed with antibodies against RUNX1 (1:500), FoxP3 (1:500) T‐bet (1:500), or β‐actin (1:1000) (Santa Cruz Biotechnology, Dallas, TX, USA). The blots were then incubated with HRP‐conjugated anti‐rabbit IgG for RUNX1 (1:1500, Sigma‐Aldrich) or anti‐mouse IgG for FoxP3, T‐bet, and β‐actin (1:2500; BioRad, Hercules, CA, USA) and developed using and enhanced chemiluminescence detection kit (Amersham, Uppsala, Sweden) in a Bio‐Imaging Analyzer Fujifilm LAS‐1000. For each experiment, the same blot was stripped and reprobed using Restore Western Blot Stripping Buffer (Thermo Scientific, Hudson, NH, USA) to remove the primary and secondary antibodies. Immunoreactive protein bands were analyzed using ImageJ software (NIH, Bethesda, MD, USA) [36]. The results are expressed as arbitrary units (A.U.) relative to β‐actin expression.
qRT‐PCR Assay
Determination of VIP, VPAC1, and VPAC2 expression was performed in trophoblast cells, and TGF‐β1, ‐β2, and ‐β3 expression was tested in Swan‐71 cells at different time points. In brief, total RNA was isolated with TRIzol reagent (Life Technologies, Grand Island, NY, USA), followed by reverse transcription according to the manufacturer's instructions (Promega, Madison, WI, USA). For amplification of VIP, VPAC1, VPAC2, and GAPDH, 1 or 2 μl of the cDNA were used. The sample volume was increased to 20 μl with 0.25‐μM specific primers and the FastStart SYBR Green Master Mix (Roche, Indianapolis, IN, USA). For the TGF‐β1, ‐β2, and ‐β3 reaction, the KAPA SYBR FAST qPCR kit was used (KapaBiosystems, Woburn, MA, USA). Reactions were performed in the MyiQ2 Real‐Time PCR detection system (BioRad). After a predenaturation step at 95°C for 5 min, 40 cycles of a denaturation step at 95°C for 20 s, an annealing step at 56°C (VPAC2), 58°C (VPAC1), 60°C (VIP, TGF‐β1, ‐β2, and ‐β3) for 20 s, and an elongation step at 72°C for 20 s were performed. An additional extension step at 72°C for 10 min was performed. Primers were designed using the Primer‐Blast software (available at: www.ncbi.nlm.nih.gov-tools-primer-blast/). Data were analyzed using iQ5, version 2.1, optical system software (BioRad) using the comparative cycle threshold (Ct) method, with GAPDH expression as the internal control. The results are expressed as the fold change (2− ΔΔ Ct ± sem or 2− Δ Ct ± sem).
Transwell assays
Swan‐71 cells were grown in 24‐well, flat bottom, polystyrene plates in complete DMEM‐F12 10% FBS to 70% of confluence. The cells were cocultured with PBMCs obtained from fertile women (5 × 105 cells/well) with or without a 0.4‐µm cell culture insert with polycarbonate membrane (Becton Dickinson). In both situations, cocultures were performed in the absence or presence of anti–TGF‐β neutralizing Ab and VIP antagonist in several combinations. After 48 h of culture, PBMCs were recovered, stained, and analyzed by FACS for Treg frequency, as described. Data are expressed as the percentage of induction of CD4+CD25+FoxP3+.
Suppression Assays
Maternal PBMCs were obtained from fertile women and cultured with or without Swan‐71 cells at 70% of confluence in the absence or presence of VIP for 48 h. Then, PBMCs in suspension were recovered, and 1 × 105 cells/well was transferred to a culture with 1 × 105 cells/well mismatched allogeneic healthy donor PBMCs. These last PBMCs had previously been treated with mitomycin C (0.5 ng/ml; Sigma‐Aldrich) for 30 min at 37°C to inhibit DNA synthesis by crosslinking DNA at the guanine and adenine residues to obtain a unidirectional proliferation. The mixture of cells was incubated in a U‐shape microtiter plate in the presence of 10% human AB serum. After 5 d, the cells were pulsed with 1 µCi/well of methyl‐[3H]‐thymidine [3H]TdR during the last 18 h of cell culture and then harvested on glass fiber filters using a Packard Filtermate cell harvester (Packard Instruments, LaGrange, IL, USA). Incorporated radioactivity was measured in a liquid scintillation β‐counter (Packard Instruments). The tests were conducted in triplicate, and results are expressed as the mean cpm ± sem.
TGF‐β determination
Supernatants obtained from Swan‐71 cells cultured in the absence or presence of VIP (10 nM) for 12 and 24 h were quantified using TGF‐β1, ‐β2, ‐β3 Milliplex MAP Kit (TGFB‐64K‐03, Millipore, St. Charles, MO, USA). This assay is based on the Luminex xMAP technology, which allows the performance of a variety of bioassays or immunoassays on the surface of fluorescent‐coded beads known as microspheres. In brief, in a 96‐well microtiter filter plate, 25 μl of standard, quality controls or samples were mixed with 25 μl of beads and incubated overnight at 4°C. After washing twice with wash buffer, 25 μl of detection Abs were incubated for 1 h with agitation. To this mixture, 25 μl of streptavidin‐phycoerythrin was added and incubated for another 30 min at room temperature. The plate was washed twice, and the beads were resuspended in sheath fluid and read on a Luminex 200 (Luminex, Austin, TX, USA). Detection and analysis were performed using the Luminex 100 IS system (Upstate Biotechnology, Charlottesville, VA, USA). The results are expressed in pg/mL. TGF‐β3 was not detected under these conditions.
VIP determination
VIP secretion was quantified in supernatants obtained from Swan‐71 and JEG cells after 24 h of culture with VIP using the EIA Kit (Peninsula Laboratories‐Bachem, San Carlos, CA, USA). In brief, 25 μl of antiserum and 50 μl of the standard or sample were incubated in 96‐well immunoplates for 1 h at room temperature. Then, 25 μl of biotinylated tracer was added and incubated for 2 h. After 5 washings with EIA buffer, 100 μl of streptavidin‐HRP were added and incubated at room temperature for 1 h. After washing with EIA buffer, tetramethylbenzidine solution, and 2N HCl were sequentially added for color development. Absorbance was determined using the iMark Absorbance Microplate Reader (Bio‐Rad) at 650 nm and 450 nm for the blue and yellow products, respectively. The results are expressed in pg/ml.
Immunofluorescence
Trophoblast cell lines were grown over a glass slide until they reached 70% confluence. The cells were washed with PBS, fixed with cold methanol during 20 s, and then washed again with PBS. The permeabilization was performed using 1% BSA, 0.5% saponin in PBS buffer for 15 min in agitation. The cells were incubated overnight at 4°C with rabbit anti‐VIP Abs (1:50 in permeabilization buffer; Abcam, Cambridge, MA, USA) and then washed with permeabilization buffer. The secondary antibody Alexa 488‐conjugated anti‐rabbit IgG (1:80; Santa Cruz Biotechnology) was incubated for 2 h at room temperature. After washing with permeabilization buffer, DAPI staining (Cell Signaling, Danvers, MA, USA) was performed during 10 min in darkness. The cells were mounted with 20% glycerol in PBS. Photographs were acquired using a IX71 Olympus inverted fluorescence microscope (Olympus, Center Valley, PA, USA) and Micro‐Manager software [37]. A negative control was performed in the absence of anti‐VIP Abs.
Statistical analysis
The significance of the results was analyzed using Student's t test and the Mann‐Whitney U test, for nonparametric samples, using the Prism4 software (GraphPad, San Diego, CA, USA). A value of P < 0.05 was considered significant.
RESULTS
VIP increases Treg population with suppressive ability in cocultures of leukocytes with first‐trimester trophoblast cell line
On the basis that iTregs mediate fetal–maternal tolerance to non–self‐alloantigens and because VIP was shown to increase the frequency of CD4+CD25+FoxP3+ cells in an in vitro model of allogeneic response [38], we investigated the ability of VIP to modulate the frequency of maternal Tregs on interaction with first‐trimester trophoblast cells in the Swan‐71 cell line. VIP (100 nM) significantly increased the frequency of CD4+CD25+FoxP3+ cells in maternal PBMCs that were cocultured with Swan‐71 cells but not in those that were not cocultured ( Fig. 1A ). Figure 1A, right, shows representative dot plots with the frequency of CD4+CD25+FoxP3+ cells in PBMCs cocultured or not with Swan‐71 cells in the absence or presence of VIP. In addition, we quantified the proliferation by CFSE staining of maternal PBMCs after 0, 24, and 48 h of culture with trophoblast cells in the presence of VIP. Maternal lymphocytes had not entered their first cell division after 48 h (Fig. 1B). Hence, the frequency of Foxp3+ cells increased after 48 h, before any cell division could be detected by CFSE staining.
Moreover, we performed additional Treg immunostaining using different combinations of markers. We observed that VIP (100 nM) increased the frequency of CD4+CD25+FoxP3+CD127−/low, CD4+CD25+FoxP3+CTLA‐4+, and CD4+CD25+CD39+ in maternal PBMCs after coculture with Swan‐71 cells (Fig. 1C).
Because the hallmark of Tregs is their ability to suppress immune responses by inhibiting the proliferation of effector T cells [39], we evaluated whether Tregs induced in cocultures with Swan‐71 cells in the presence of VIP displayed suppressive ability. For that purpose, maternal PBMCs were cultured with or without Swan‐71 cells in the absence or presence of VIP for 48 h. Next, PBMCs in suspension were recovered and transferred to a culture with mismatched allogeneic healthy donor PBMCs. The latter PBMCs had previously been treated with mitomycin C to inhibit DNA synthesis by crosslinking DNA at guanine and adenine residues to obtain a unidirectional proliferation. After 5 d of culture, we quantified the proliferative response by thymidine incorporation. A significant decrease in proliferation was observed in the allogeneic response of those cultures performed with maternal PBMCs in the presence of trophoblast cells compared with PBMCs alone, and this effect was even more pronounced in the presence of VIP (Fig. 1D). Moreover, maternal PBMCs that had not interacted with trophoblast cells did not suppress allogeneic proliferation, regardless of whether VIP was present. Subsequently, we evaluated the expression of the transcription factors associated with a suppressive phenotype of Tregs, such as FoxP3 and RUNX1, and T‐bet as a characteristic factor of the Th1 responses. PBMCs that have interacted with trophoblast cells in the presence of VIP significantly increased FoxP3 and decreased T‐bet expression, analyzed by Western blotting (Fig. 1E). This modulation was accompanied by a trend toward an increase in RUNX1 expression, which is associated with FoxP3 at the protein level, contributing to Treg induction. Taken together, these results support that trophoblast cells have the ability to increase the frequency of functional CD4+CD25+FoxP3+ cells able to suppress the allogeneic response and that VIP can boost this effect, accompanied by an increase in FoxP3 and RUNX1 and a decrease in T‐bet protein expression.
VIP increases the frequency of CD4+ cells producing TGF‐β and IL‐10 after coculture with Swan‐71 cells
To study whether the coculture of maternal PBMCs with the first‐trimester trophoblast cell line modulated cytokine production in CD4+ T cells and the effect of VIP, we evaluated the cytokine intracellular expression by FACS in the presence or absence of VIP. VIP strongly induced CD4+TGF‐β + and CD4+IL‐10+ frequency in PBMCs cocultured with trophoblast cells ( Fig. 2 ). In contrast, no modulation was observed under these conditions in the frequency of CD4+IL‐17+ and CD4+IFN‐γ + cells. The frequency of CD4+TGF‐β + cells was also increased in cocultures in the absence of VIP, indicating the presence of additional mechanisms contributing to the induction of a tolerogenic microenvironment. Figure 2B shows representative dot plots with the frequency of CD4 cells expressing IL‐10, TGF‐β, IFN‐γ, and IL‐17 from the same fertile donor, after the interaction with Swan‐71 cells in the absence or presence of VIP.
VIP increases TGF‐β production by trophoblast cells
Several reports have indicated that the induction of FoxP3 through TGF‐β involves Smad proteins, which bind to specific sites at the CNS1 enhancer of the FoxP3 gene, inducing the expression of the transcription factor FoxP3 [15, 40]. Hence, to investigate whether the TGF‐β produced by trophoblast cells was involved in the increased frequency of Tregs observed in cocultures stimulated with VIP, we performed a kinetic analysis of TGF‐β1, ‐β2 and β3 expression in Swan‐71 cells in the absence or presence of VIP by qRT‐PCR. Swan‐71 cells increased the expression of the 3 isoforms of TGF‐β after 12 h of culture in the presence of VIP ( Fig. 3A ). In particular, VIP significantly increased TGF‐β1 expression in Swan‐71 cells at 12 h of incubation, which was mainly associated with immunomodulatory effects (Fig. 3A). Quantification of the 3 isoforms of TGF‐β in culture supernatants with Luminex determination (Luminex 200, Austin, Texas, USA) confirmed the effect. VIP significantly increased TGF‐β1 secretion in trophoblast cells after 12 h of culture (Fig. 3B). VIP did not modulate TGF‐β2 secretion, and TGF‐β3 was not detected under these conditions.
Figure 3.
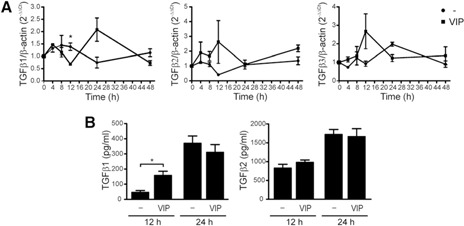
VIP induces the expression of TGF‐β in trophoblast cells. (A) A kinetic analysis of TGF‐β1, ‐β2, and ‐β3 expression on Swan‐71 cells in the absence or presence of VIP (100 nM) by qRT‐PCR. Results are expressed as the fold change relative to β‐actin (2− ΔΔ Ct ± sem) and corresponded to 4 independent experiments. *P < 0.05, Student's t test. (B) In parallel, supernatants were collected, and Luminex quantification was performed for the 3 TGF‐β isoforms after 12 and 24 h of VIP treatment. Results are expressed as the mean pg/ml ± sem of TGF‐β secretion and are representative of 3 independent assays. *P < 0.05, Student's t test. TGF‐β3 was not detected under these conditions.
VIP produced by trophoblast cells contributes to Treg induction involving TGF‐β
Because exogenously added VIP induced Tregs after interaction with Swan‐71 cells, we next investigated the ability of endogenous VIP, produced by trophoblast cells, to induce Tregs. First, we characterized the expression of VIP and its receptors, VPAC1 and VPAC2, in the first‐trimester trophoblast cell line Swan‐71. Additionally, we used the third‐trimester JEG‐3 trophoblast cell line obtained from a choriocarcinoma. The qRT‐PCR analysis revealed that although both cell lines expressed VIP and its receptors (Fig. 4A, C, and D), Swan‐71 cells secreted significantly higher levels of VIP than did the JEG‐3 cells (Fig. 4B). To confirm this result, indirect immunofluorescence was performed. Figure 4E shows microscopy photographs with higher cytoplasmic VIP staining in the Swan‐71 cells than in the JEG‐3 cells. Next, we tested the ability of endogenous VIP to induce maternal Tregs. Swan‐71 cells were cocultured with maternal PBMCs in the absence or presence of VIP antagonist, and we quantified Tregs by FACS analysis. The frequency of Tregs in the maternal PBMCs increased significantly after the interaction with Swan‐71 cells ( Fig. 5A ). This effect was even more pronounced if the trophoblast cells had been pretreated with VIP (10 nM). In both situations, VIP antagonist prevented the increase in the frequency of CD4+Foxp3+ cells, reflecting a specific VIP effect after the interaction with Swan‐71 cells. Figure 5B shows representative dot plots of PBMCs cultured with Swan‐71 cells pretreated or not with VIP in the absence or presence of VIP antagonist. Because Swan‐71 cells produce TGF‐β, we next investigated Treg induction in cocultures in the presence of an anti–TGF‐β neutralizing Ab and a VIP antagonist. CD4+CD25+FoxP3+ cell induction was significantly prevented by the anti–TGF‐β Ab (Fig. 5C), and the same trend was observed in the presence of VIP antagonist, indicating that endogenous VIP has a role in mediating this effect. No additive effect from the VIP antagonist and anti–TGF‐β Ab was found, suggesting that their actions are mediated through a common pathway. Finally, we performed transwell assays to distinguish between the contributions of soluble or membrane‐bound TGF‐β forms. Cocultures were performed in the presence of 0.4‐µm inserts in the absence or presence of an anti–TGF‐β neutralizing Ab and VIP antagonist. After 48 h of culture, the anti–TGF‐β Ab and VIP antagonist significantly prevented the increase in the frequency of Tregs, even in the presence of the transwell, suggesting that the TGF‐β soluble form is implicated in Treg modulation (Fig. 5D). Taken together, the present results support that VIP produced by Swan‐71 cells can boost Treg induction involving TGF‐β1 secretion and that exogenously added VIP can further induce this effect.
Figure 4.
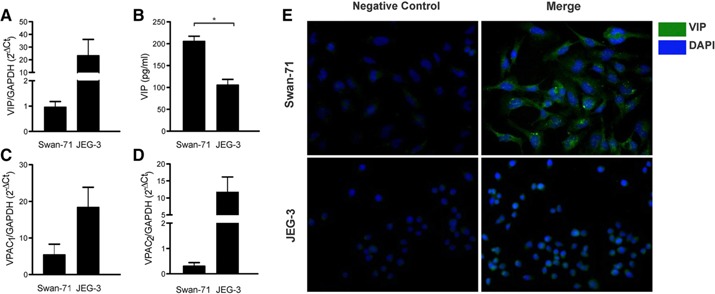
VIP/VPAC system in trophoblast cell lines. Swan‐71 and JEG‐3 cell lines were cultured at 70% confluence and recovered. (A) VIP, (C) VPAC1, and (D) VPAC2 expression was quantified by qRT‐PCR. Results are expressed as gene expression relative to GAPDH expression, 2− Δ Ct ± sem of at least 5 independent experiments. *P < 0.05, Student's t test. (B) At the same time, supernatants were collected, and VIP release was quantified by ELISA. Results are expressed as mean pg/ml ± sem of at least 3 independent experiments. *P < 0.05, Student's t test. (E) VIP production in both cell lines was evaluated by immunofluorescence staining and microscopy. Cells were permeabilized and stained with primary anti‐VIP Ab, followed by Alexa‐488 conjugated anti‐rabbit FITC Ab and DAPI for nuclei. Negative controls were performed in the absence of anti‐VIP Abs.
DISCUSSION
The initial proinflammatory response characteristic of implantation is actively modulated to a predominant anti‐inflammatory and tolerogenic profile at early gestation. Also, iTregs are essential for this immune switch, and VIP displays anti‐inflammatory and tolerogenic effects in several in vivo and in vitro models. Therefore, we analyzed the role of this neuropeptide as a physiologic local regulator of the maternal–placental interaction through the induction of Tregs with suppressor ability.
The present results provide experimental evidence that VIP produced by human first‐trimester trophoblast cells can act as a tolerance‐inducing factor, increasing the frequency of CD4+ CD25+FoxP3+ cells with increased secretion of TGF‐β. Our conclusions were based on several observations. First, VIP increased the frequency of CD4+CD25+FoxP3+ cells after maternal PBMC interaction with Swan‐71 cells. The Treg subset induced upon VIP incubation was able to suppress the maternal response against alloantigens. Moreover, VIP modulation was accompanied by an increase in FoxP3 and RUNX1 expression and a decrease in T‐bet expression in maternal PBMCs after their interaction with Swan‐71 cells. Second, VIP induced an increased frequency of CD4+ T cells that produce TGF‐β and IL‐10, amplifying the suppressive immune response. Third, first‐trimester trophoblast cells synthesize and release VIP, which specifically induces Tregs through a mechanism involving soluble TGF‐β, because VIP antagonist and anti–TGF‐β Ab prevented the effect.
Several redundant immune circuits ensure the maintenance of immune homeostasis at the early maternal–placental interface. VIP could be analyzed as an interesting example that fulfills the criteria for multiple cell targets to promote an anti‐inflammatory and tolerogenic microenvironment. For example, VIP has been proposed to favor a regulatory/suppressor macrophage phenotype through induction of IL‐10 synthesis and reduction of IL‐12, TNF‐α, and iNOS activity in human and murine macrophages through both VPAC receptor subtypes [41, 42, 43–44]. Dendritic cells are also targeted by VIP to differentiate into a tolerogenic profile, with failure to upregulate costimulatory molecules and proinflammatory cytokine expression after Toll‐like receptor signaling. In contrast, they produce significant levels of IL‐10 and induce the generation of Ag‐specific Tregs in vivo and in vitro [45, 46]. The treatment of human CD4+CD25− T cells with VIP during in vitro stimulation induces CD4+CD25+FoxP3+ cells with a potent regulatory function against allospecific effector T cells, resulting in acute graft‐vs.‐host disease protection in a mouse model of allogeneic bone marrow transplantation [38, 47].
The maternal immune system is challenged by paternal and trophoblast alloantigen stimulation and by the release of fetal antigens into the maternal circulation, a phenomenon known as microchimerism. Thus, a crucial mechanism for tolerance induction during the peri‐implantation window is the generation of Tregs specifically against non–self‐antigens through the interaction of TGF‐β with Smad3 and retinoic acid receptor binding sites in the FoxP3 promoter [48, 49–50]. We have shown that VIP increases TGF‐β1 expression and secretion in first‐trimester trophoblast cells after 12 h of culture, and the blockade of soluble TGF‐β prevented the increase in the frequency of Tregs, suggesting that VIP immunomodulatory effects on Tregs involves a TGF‐β–dependent mechanism. Also, VIP induced an increase in the expression of the transcription factors FoxP3 and RUNX1 after coculture with trophoblast cells. In particular, RUNX1 facilitates the stability of FoxP3 gene binding to CNS2 region and then promotes FoxP3 expression [18, 51].
It is important to highlight that Tregs induced in the peri‐implantation phase of pregnancy are crucial for bystander tolerance of the conceptus. Thus, the exposure to a limited repertoire of non–self‐antigens in the appropriate microenvironment generates iTregs that, once activated and fully functional, are enough to induce a tolerogenic response toward a wider repertoire of antigens expressed later on in the gestational tissues [48, 49]. Thus, the VIPs produced by first‐trimester trophoblast cells would contribute to the proper microenvironment necessary for induction of iTregs in the early stages of maternal–placental interaction. Furthermore, differences in VIP/VPAC system expression were present in the PBMCs of patients with early pregnancy complications, such as recurrent spontaneous abortion [52, 53]. In line with this, in non–obese diabetic mice with an increased embryo resorption rate at the prediabetic stage, the local expression of VIP mRNA was diminished at the resorption sites compared with that at the viable implantation sites [29, 53].
Once generated, iTregs in the regional lymphoid node must migrate into the fetal–maternal interface to exert their suppressive function. Thus, trophoblast cells contribute to the recruitment of iTregs toward the maternal–placental interface [5, 53, 54]. Nancy et al. [55] showed evidence indicating that a potential epigenetic modification of chemokine genes in decidual stromal cells could be responsible for the regulation of T cell trafficking as a mechanism of fetal survival.
Finally, Obermajer et al. [56] recently reported the conversion of Th17 cells into Tregs by which mesenchymal stem cell‐induced myeloid‐derived immunosuppressive cells mediate operational transplant tolerance. They demonstrated that retinoic acid‐related orphan receptor‐γ is a common factor in the differentiation of Tregs and Th17 cells in mice. Also, the identification of IL‐17A+ FoxP3+ cells and ex–IL‐17–producing IL‐17A−FoxP3+ T cells strongly argues for direct conversion of Th17 cells into Tregs [56]. This plasticity underlies a new mechanism of immune regulation to mediate allograft survival.
Research in the past few years has displayed a better understanding of the molecular mechanisms leading to immune tolerance and homeostasis in the maternal–fetal interface. The results we have presented suggest that VIP could have an active role in the immunoregulatory processes operating in the maternal–placental interface by contributing to the induction of functionally active iTregs.
AUTHORSHIP
C.P.L., G.M., and R.R. designed the study, supervised the experimental work, and wrote the manuscript. L.F. and E.G. performed all the experiments with Tregs and Swan‐71 cells in the cocultures. D.P. and V.H. helped with RT‐PCR data analyses and interpretation. C.P.L and R.R. supervised the whole study. All authors read and approved the final manuscript.
DISCLOSURES
The authors declare no competing financial interests.
ACKNOWLEDGMENTS
The authors thank Fulbright Commission–Argentina and Ministry of Education. They also thank Dr. G. Rabinovich for his continuous support and helpful discussion. This study was supported by grants to R.R. and C.P.L. (CONICET PIP 0602/12, UBACyT 2014‐2017, and 2012‐2015 from the University of Buenos Aires and PICT 0144/11 and PICT 1632/13 from ANPCyT).
REFERENCES
- 1. Mor, G. , Cardenas, I. (2010) The immune system in pregnancy: a unique complexity. Am. J. Reprod. Immunol. 63, 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dekel, N. , Gnainsky, Y. , Granot, I. , Mor, G. (2010) Inflammation and implantation. Am. J. Reprod. Immunol. 63, 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moser, G. , Gauster, M. , Orendi, K. , Glasner, A. , Theuerkauf, R. , Huppertz, B. (2010) Endoglandular trophoblast, an alternative route of trophoblast invasion? Analysis with novel confrontation co‐culture models. Hum. Reprod. 25, 1127–1136. [DOI] [PubMed] [Google Scholar]
- 4. Weiss, G. , Goldsmith, L.T. , Taylor, R.N. , Bellet, D. , Taylor, H.S. (2009) Inflammation in reproductive disorders. Reprod. Sci. 16, 216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guerin, L.R. , Prins, J.R. , Robertson, S.A. (2009) Regulatory T‐cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum. Reprod. Update 15, 517–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aluvihare, V.R. , Kallikourdis, M. , Betz, A.G. (2004) Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 5, 266–271. [DOI] [PubMed] [Google Scholar]
- 7. Gomez‐Lopez, N. , Guilbert, L.J. , Olson, D.M. (2010) Invasion of the leukocytes into the fetal‐maternal interface during pregnancy. J. Leukoc. Biol. 88, 625–633. [DOI] [PubMed] [Google Scholar]
- 8. Chaouat, G. , Petitbarat, M. , Dubanchet, S. , Rahmati, M. , Ledée, N. (2010) Tolerance to the foetal allograft? Am. J. Reprod. Immunol. 63, 624–636. [DOI] [PubMed] [Google Scholar]
- 9. Yoshinaga, K. (2010) Research on blastocyst implantation essential factors (BIEFs). Am. J. Reprod. Immunol. 63, 413–424. [DOI] [PubMed] [Google Scholar]
- 10. Molvarec, A. , Blois, S.M. , Stenczer, B. , Toldi, G. , Tirado‐Gonzalez, I. , Ito, M. , Shima, T. , Yoneda, S. , Vásárhelyi, B. , Rigó, J., Jr. , Saito, S. (2011) Peripheral blood galectin‐1‐expressing T and natural killer cells in normal pregnancy and preeclampsia. Clin. Immunol. 139, 48–56. [DOI] [PubMed] [Google Scholar]
- 11. Blois, S.M. , Ilarregui, J.M. , Tometten, M. , Garcia, M. , Orsal, A.S. , Cordo‐Russo, R. , Toscano, M.A. , Bianco, G.A. , Kobelt, P. , Handjiski, B. , Tirado, I. , Markert, U.R. , Klapp, B.F. , Poirier, F. , Szekeres‐Bartho, J. , Rabinovich, G.A. , Arck, P.C. (2007) A pivotal role for galectin‐1 in fetomaternal tolerance. Nat. Med. 13, 1450–1457. [DOI] [PubMed] [Google Scholar]
- 12. Saito, S. , Nakashima, A. , Shima, T. , Ito, M. (2010) Th1/Th2/Th17 and regulatory T‐cell paradigm in pregnancy. Am. J. Reprod. Immunol. 63, 601–610. [DOI] [PubMed] [Google Scholar]
- 13. Samy, E.T. , Setiady, Y.Y. , Ohno, K. , Pramoonjago, P. , Sharp, C. , Tung, K.S. K. (2006) The role of physiological self‐antigen in the acquisition and maintenance of regulatory T‐cell function. Immunol. Rev. 212, 170–184. [DOI] [PubMed] [Google Scholar]
- 14. Zenclussen, A.C. , Gerlof, K. , Zenclussen, M.L. , Sollwedel, A. , Bertoja, A.Z. , Ritter, T. , Kotsch, K. , Leber, J. , Volk, H.D. (2005) Abnormal T‐cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy‐induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am. J. Pathol. 166, 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Samstein, R.M. , Josefowicz, S.Z. , Arvey, A. , Treuting, P.M. , Rudensky, A.Y. (2012) Extrathymic generation of regulatory T cells in placental mammals mitigates maternal‐fetal conflict. Cell 150, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gobert, M. , Lafaille, J.J. (2012) Maternal‐fetal immune tolerance, block by block. Cell 150, 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng, Y. , Josefowicz, S. , Chaudhry, A. , Peng, X.P. , Forbush, K. , Rudensky, A.Y. (2010) Role of conserved non‐coding DNA elements in the Foxp3 gene in regulatory T‐cell fate. Nature 463, 808–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rudra, D. , Egawa, T. , Chong, M.M. W. , Treuting, P. , Littman, D.R. , Rudensky, A.Y. (2009) Runx‐CBFbeta complexes control expression of the transcription factor Foxp3 in regulatory T cells. Nat. Immunol. 10, 1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kitoh, A. , Ono, M. , Naoe, Y. , Ohkura, N. , Yamaguchi, T. , Yaguchi, H. , Kitabayashi, I. , Tsukada, T. , Nomura, T. , Miyachi, Y. , Taniuchi, I. , Sakaguchi, S. (2009) Indispensable role of the Runx1‐Cbfbeta transcription complex for in vivo‐suppressive function of FoxP3+ regulatory T cells. Immunity 31, 609–620. [DOI] [PubMed] [Google Scholar]
- 20. Gressens, P. , Marret, S. , Hill, J.M. , Brenneman, D.E. , Gozes, I. , Fridkin, M. , Evrard, P. (1997) Vasoactive intestinal peptide prevents excitotoxic cell death in the murine developing brain. J. Clin. Invest. 100, 390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Månsson, B. , Nilsson, B.O. , Ekström, J. (1990) Effects of repeated infusions of substance P and vasoactive intestinal peptide on the weights of salivary glands subjected to atrophying influences in rats. Br. J. Pharmacol. 101, 853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ekström, J. , Månsson, B. , Tobin, G. (1983) Vasoactive intestinal peptide evoked secretion of fluid and protein from rat salivary glands and the development of supersensitivity. Acta Physiol. Scand. 119, 169–175. [DOI] [PubMed] [Google Scholar]
- 23. Roca, V. , Larocca, L. , Calafat, M. , Aisemberg, J. , Meiss, R. , Franchi, A.M. , Leirós, C.P. (2006) Reduced nitric oxide synthase and cyclo‐oxygenase activity in the uterus of non‐obese diabetic mice. Reproduction 132, 931–938. [DOI] [PubMed] [Google Scholar]
- 24. Martinez, C. , Delgado, M. , Abad, C. , Gomariz, R.P. , Ganea, D. , Leceta, J. (1999) Regulation of VIP production and secretion by murine lymphocytes. J. Neuroimmunol. 93, 126–138. [DOI] [PubMed] [Google Scholar]
- 25. Li, J.‐M. , Southerland, L. , Hossain, M.S. , Giver, C.R. , Wang, Y. , Darlak, K. , Harris, W. , Waschek, J. , Waller, E.K. (2011) Absence of vasoactive intestinal peptide expression in hematopoietic cells enhances Th1 polarization and antiviral immunity in mice. J. Immunol. 187, 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Delgado, M. , Abad, C. , Martinez, C. , Leceta, J. , Gomariz, R.P. (2001) Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat. Med. 7, 563–568. [DOI] [PubMed] [Google Scholar]
- 27. Jovanovic, S. , Grbovic, L. , Jovanovic, A. (2000) Pregnancy does not alter the response of uterine arteries to vasoactive intestinal polypeptide. Mol. Hum. Reprod. 6, 361–368. [DOI] [PubMed] [Google Scholar]
- 28. Spong, C.Y. , Lee, S.J. , McCune, S.K. , Gibney, G. , Abebe, D.T. , Alvero, R. , Brenneman, D.E. , Hill, J.M. (1999) Maternal regulation of embryonic growth: the role of vasoactive intestinal peptide. Endocrinology 140, 917–924. [DOI] [PubMed] [Google Scholar]
- 29. Roca, V. , Calafat, M. , Larocca, L. , Ramhorst, R. , Farina, M. , Franchi, A.M. , Leirós, C.P. (2009) Potential immunomodulatory role of VIP in the implantation sites of prediabetic nonobese diabetic mice. Reproduction 138, 733–742. [DOI] [PubMed] [Google Scholar]
- 30. Fraccaroli, L. , Alfieri, J. , Larocca, L. , Calafat, M. , Roca, V. , Lombardi, E. , Ramhorst, R. , Leirós, C.P. (2009) VIP modulates the pro‐inflammatory maternal response, inducing tolerance to trophoblast cells. Br. J. Pharmacol. 156, 116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marzioni, D. , Fiore, G. , Giordano, A. , Nabissi, M. , Florio, P. , Verdenelli, F. , Petraglia, F. , Castellucci, M. (2005) Placental expression of substance P and vasoactive intestinal peptide: evidence for a local effect on hormone release. J. Clin. Endocrinol. Metab. 90, 2378–2383. [DOI] [PubMed] [Google Scholar]
- 32. Deutsch, P.J. , Sun, Y. , Kroog, G.S. (1990) Vasoactive intestinal peptide increases intracellular cAMP and gonadotropin‐alpha gene activity in JEG‐3 syncytial trophoblasts. Constraints posed by desensitization. J. Biol. Chem. 265, 10274–10281. [PubMed] [Google Scholar]
- 33. Straszewski‐Chavez, S.L. , Abrahams, V.M. , Alvero, A.B. , Aldo, P.B. , Ma, Y. , Guller, S. , Romero, R. , Mor, G. (2009) The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71. Placenta 30, 939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pattillo, R.A. , Gey, G.O. (1968) The establishment of a cell line of human hormone‐synthesizing trophoblastic cells in vitro. Cancer Res. 28, 1231–1236. [PubMed] [Google Scholar]
- 35. Kohler, P.O. , Bridson, W.E. (1971) Isolation of hormone‐producing clonal lines of human choriocarcinoma. J. Clin. Endocrinol. Metab. 32, 683–687. [DOI] [PubMed] [Google Scholar]
- 36. Schneider, C.A. , Rasband, W.S. , Eliceiri, K.W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Edelstein, A. , Amodaj, N. , Hoover, K. , Vale, R. , Stuurman, N. (2010) Computer control of microscopes using μManager. Curr. Protoc. Mol. Biol. 14.20, 14.20.1–14.20.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pozo, D. , Anderson, P. , Gonzalez‐Rey, E. (2009) Induction of alloantigen‐specific human T regulatory cells by vasoactive intestinal peptide. J. Immunol. 183, 4346–4359. [DOI] [PubMed] [Google Scholar]
- 39. Sakaguchi, S. , Sakaguchi, N. , Shimizu, J. , Yamazaki, S. , Sakihama, T. , Itoh, M. , Kuniyasu, Y. , Nomura, T. , Toda, M. , Takahashi, T. (2001) Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 182, 18–32. [DOI] [PubMed] [Google Scholar]
- 40. Chen, W. , Jin, W. , Hardegen, N. , Lei, K.‐J. , Li, L. , Marinos, N. , McGrady, G. , Wahl, S.M. (2003) Conversion of peripheral CD4+CD25‐ naive T cells to CD4+CD25+ regulatory T cells by TGF‐beta induction of transcription factor FoxP3. J. Exp. Med. 198, 1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Delgado, M. , Munoz‐Elias, E.J. , Gomariz, R.P. , Ganea, D. (1999) Vasoactive intestinal peptide and pituitary adenylate cyclase‐activating polypeptide prevent inducible nitric oxide synthase transcription in macrophages by inhibiting NF‐kappa B and IFN regulatory factor 1 activation. J. Immunol. 162, 4685–4696. [PubMed] [Google Scholar]
- 42. Gonzalez‐Rey, E. , Delgado, M. (2007) Vasoactive intestinal peptide and regulatory T‐cell induction: a new mechanism and therapeutic potential for immune homeostasis. Trends Mol. Med. 13, 241–251. [DOI] [PubMed] [Google Scholar]
- 43. Leceta, J. , Gomariz, R.P. , Martinez, C. , Carrión, M. , Arranz, A. , Juarranz, Y. (2007) Vasoactive intestinal peptide regulates Th17 function in autoimmune inflammation. Neuroimmunomodulation 14, 134–138. [DOI] [PubMed] [Google Scholar]
- 44. Gonzalez‐Rey, E. , Anderson, P. , Delgado, M. (2007) Emerging roles of vasoactive intestinal peptide: a new approach for autoimmune therapy. Ann. Rheum. Dis. 66 (Suppl 3), iii70–iii76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chorny, A. , Gonzalez‐Rey, E. , Fernandez‐Martin, A. , Ganea, D. , Delgado, M. (2006) Vasoactive intestinal peptide induces regulatory dendritic cells that prevent acute graft‐versus‐host disease while maintaining the graft‐versus‐tumor response. Blood 107, 3787–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Toscano, M.G. , Delgado, M. , Kong, W. , Martin, F. , Skarica, M. , Ganea, D. (2010) Dendritic cells transduced with lentiviral vectors expressing VIP differentiate into VIP‐secreting tolerogenic‐like DCs. Mol. Ther. 18, 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gonzalez‐Rey, E. , Ganea, D. , Delgado, M. (2010) Neuropeptides: keeping the balance between pathogen immunity and immune tolerance. Curr. Opin. Pharmacol. 10, 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Robertson, S.A. , Prins, J.R. , Sharkey, D.J. , Moldenhauer, L.M. (2013) Seminal fluid and the generation of regulatory T cells for embryo implantation. Am. J. Reprod. Immunol. 69, 315–330. [DOI] [PubMed] [Google Scholar]
- 49. Teles, A. , Thuere, C. , Wafula, P.O. , El‐Mousleh, T. , Zenclussen, M.L. , Zenclussen, A.C. (2013) Origin of Foxp3(+) cells during pregnancy. Am J Clin Exp Immunol 2, 222–233. [PMC free article] [PubMed] [Google Scholar]
- 50. Teles, A. , Zenclussen, A.C. , Schumacher, A. (2013) Regulatory T cells are baby's best friends. Am. J. Reprod. Immunol. 69, 331–339. [DOI] [PubMed] [Google Scholar]
- 51. Klunker, S. , Chong, M.M. W. , Mantel, P.‐Y. , Palomares, O. , Bassin, C. , Ziegler, M. , Rückert, B. , Meiler, F. , Akdis, M. , Littman, D.R. , Akdis, C.A. (2009) Transcription factors RUNX1 and RUNX3 in the induction and suppressive function of Foxp3+ inducible regulatory T cells. J. Exp. Med. 206, 2701–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fraccaroli, L. , Grasso, E. , Hauk, V. , Cortelezzi, M. , Calo, G. , Perez Leirós, C. , Ramhorst, R. (2012) Defects in the vasoactive intestinal peptide (VIP)/VPAC system during early stages of the placental‐maternal leucocyte interaction impair the maternal tolerogenic response. Clin. Exp. Immunol. 170, 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pérez Leirós, C. , Ramhorst, R. (2013) Tolerance induction at the early maternal‐placental interface through selective cell recruitment and targeting by immune polypeptides. Am. J. Reprod. Immunol. 69, 359–368. [DOI] [PubMed] [Google Scholar]
- 54. Ramhorst, R. , Fraccaroli, L. , Aldo, P. , Alvero, A.B. , Cardenas, I. , Leirós, C.P. , Mor, G. (2012) Modulation and recruitment of inducible regulatory T cells by first trimester trophoblast cells. Am. J. Reprod. Immunol. 67, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nancy, P. , Tagliani, E. , Tay, C.‐S. , Asp, P. , Levy, D.E. , Erlebacher, A. (2012) Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal‐fetal interface. Science 336, 1317–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Obermajer, N. , Popp, F.C. , Soeder, Y. , Haarer, J. , Geissler, E.K. , Schlitt, H.J. , Dahlke, M.H. (2014) Conversion of Th17 into IL‐17A(neg) regulatory T cells: a novel mechanism in prolonged allograft survival promoted by mesenchymal stem cell‐supported minimized immunosuppressive therapy. J. Immunol. 193, 4988–4999. [DOI] [PubMed] [Google Scholar]


