Short abstract
FK506‐mediated NK cell defects are associated with impaired IL‐2 receptor signaling and selective down‐regulation of NK receptors.
Keywords: tacrolimus, IL‐2 receptor, STAT3, NK receptors
Abstract
The role of NK cells in allogeneic HCT has been increasingly appreciated, particularly in the GVL effect. Although FK506 has been used widely to prevent GVHD, its action was considered to be primarily through activated T cells. In this study, we provide direct evidence for the first time that human NK cells are immediate targets of FK506. Our in vivo data from patients undergoing peripheral blood stem cell transplantation or BMT showed a reduced number of NK cells with down‐regulated CD25 expression in their peripheral blood compartment. Likewise, FK506 caused profound inhibition of NK cell proliferation in vitro and suppressed NK cytotoxicity and cytokine secretion in response to IL‐2. These defects were accompanied by impaired cell clustering and selective down‐regulation of adhesion molecules, ICAM‐1, CD2, CD49d, and CD58. Furthermore, FK506 specifically inhibited expression of NKG2D, CD48, and DNAM1 receptors without affecting that of 2B4, NKp30, NKp44, and NKp46. As a result, natural cytotoxicity against K562 tumor targets was impaired, while leaving redirected ADCC via 2B4 intact. Finally, FK506‐treated NK cells showed impaired IL‐2R signaling and inhibition of STAT3. Collectively, these signaling impairments and selective down‐regulation of NK receptors by FK506 may underlie the proliferative and functional defects of NK cells. Thus, our data provide a new insight into the mechanism of immunosuppression by FK506, which should be considered to interpret the outcome of graft transplantation.
Abbreviations
- ABL
BCR/ABL leukemia
- APC
allophycocyanin
- ATCC
American Type Culture Collection
- BMT
bone marrow transplantation
- Cr
chromium
- CsA
cyclosporin A
- DNAM1
DNAX accessory molecule 1
- DPM
disintegrations per minute
- FasL
Fas ligand
- FKBP‐12
FK506 binding protein 12
- GVHD
graft versus host disease
- GVL
graft versus leukemia
- HCT
hematopoietic cell transplantation
- HSCT
hematopoietic stem cell transplantation
- KIR
killer Ig‐like receptor
- MTX
methroxate
- NCR
natural cytotoxicity receptor
Introduction
A major challenge in organ transplantation is to impair recipientsˈ potential to reject the donor graft while preserving immunity against opportunistic infections. FK506 (tacrolimus), initially approved for liver transplantation [1], has been an immunosuppressant of choice to control acute and chronic immune rejection processes in solid graft transplantation as well as in HCT. In particular, FK506 has been used for GVHD prophylaxis in the setting of HCT from HLA identical [2] or unrelated donors [3]. FK506 elicits its immunosuppressive action via binding to its cytosolic receptor FKBP‐12 and subsequently blocks phosphorylase activities of calcineurin, a key step for IL‐2 and other lymphokine gene expressions [4, 5]. Although main target cells for FK506 have been known to be activated T cells, recent data suggested DCs to be an additional, new target, as MHC class I‐ and class II‐restricted antigen presentation was inhibited in murine DCs by FK506 in vitro and in vivo [6, 7]. Although it needs to be verified whether this phenomenon recapitulates in human DCs, these data suggest that the mechanism underlying FK506‐mediated immune suppression may be more complicated to involve multiple target cells in vivo.
NK cells play a quintessential role in innate immunity, including viral infection, cancer surveillance, and allogeneic graft rejection processes. NK cells recognize “missing self”, including allogeneic or down‐regulated MHC class I molecules [8], and function through a series of inhibitory and activating surface receptors. Inhibitory receptors include KIRs and CD94/NKG2A, and activating receptors include NKG2D, CD16, 2B4, CD2, and NCRs, such as NKp46, NKp44, and NKp30. NK cells mount their effector function via direct cytolysis and indirectly via secreting, proinflammatory cytokines, e.g., IFN‐γ and TNF‐α. Unlike T and B cells, resting NK cells contain pre‐transcribed perforin, granzyme B, and IFN‐γ mRNAs [9, 10] and thus, react quickly against target cells.
The role of NK cells in HCT has been appreciated increasingly, as they express KIRs that recognize HLAs and consequently, could affect GVHD or GVL. The absence of cognate KIR ligands on recipient cells can result in the recognition and subsequent attack by NK cells; hence, donor‐derived NK cells have the potential to modulate GVHD and GVL [11]. Like T cells, NK cells express FKBP‐12 (unpublished results) and calcineurin [12]. Therefore, they are likely to be a target for FK506 action. However, earlier studies by Wasik et al. [13] demonstrated little or no significant inhibition of human NK cell function by FK506 in natural cytotoxicity and ADCC in a 48‐h culture in vitro. As immunosuppressive action of FK506 requires continuous maintenance of serum concentration for days or longer, the effect of FK506 on NK cells might have been underestimated in the previous studies. Therefore, this study was set up to investigate the effect of prolonged administration of FK506 on NK cells in patients following BMT or HSCT and in the parallel studies of in vitro, primary NK cell cultures obtained from healthy volunteers. Our in vivo and in vitro results unequivocally demonstrated cellular and functional defects of NK cells by FK506, which should be considered to interpret the outcome of graft transplantation and to design optimal immunosuppressive regimens.
MATERIALS AND METHODS
Clinical sample analysis
Whole blood of patients following HCT (n=11) and sex‐ and age‐matched healthy donors (n=10) was collected after obtaining informed consent. Complete blood count was performed immediately. The patients had AML or ABL and were administered with FK506 and MTX at Post‐Transplantation Days 1, 3, 6, and 11 and then only with FK506 following transplantation with unrelated, HLA‐matched bone marrow or peripheral blood stem cells (see Fig. 1 , right panel table). None of the patients had developed relapse or GVHD. Whole blood was collected 2–16 months after the transplantation. The PBMCs were stained with fluorescence‐conjugated anti‐human CD3 and anti‐human CD56 mAb, and then NK cell numbers were assessed by flow cytometry. The rest of the PBMCs were cultured in the presence of 300 u/ml human rIL‐2 (Chiron, Emeryville, CA, USA) for 1 week. The cells were stained with fluorescence‐conjugated anti‐CD3, anti‐CD56, and anti‐CD25 mAb (BD Biosciences, San Diego, CA, USA) and then analyzed by flow cytometry. All of the procedures were approved by the Institutional Review Board of Seoul National University Hospital (Korea).
Figure 1.
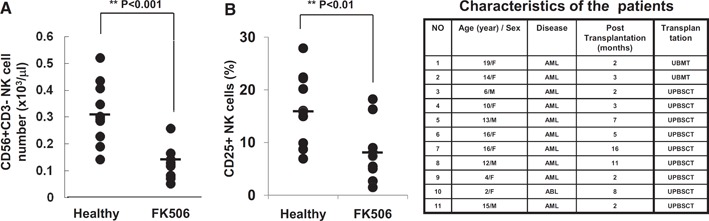
NK cell profile of patients on FK506 monotherapy following HCT. (A) NK cell numbers in PBMCs were plotted for the healthy control (n=10) and FK506‐treated group (n=11) following unrelated BMT (UBMT; right panel table) or unrelated peripheral blood stem cell transplantation (UPBSCT; right panel table). (B) PBMCs harvested from the control or FK506‐treated group were cultured with 300 u/ml human rIL‐2 in the absence of FK506 for 7 days, and the percentage of NK cells expressing CD25 was calculated. Independent sample t test was performed by SPSS.
Human primary NK cell culture
For in vitro study, human primary NK cells were isolated from PBMCs from healthy volunteers using negative selection by the RosetteSep NK cell isolation kit (Stem Cell Technology, Vancouver, BC, Canada) and Ficoll‐Paque (Amersham Pharmacia Biotech, Korea) following the manufacturersˈ instructions. The purity of CD3–CD56+ NK cells was >90%, which was measured by flow cytometry (FACSCalibur, BD Biosciences). Isolated NK cells were cultured in RPMI 1640 (Welgene, Daegu, Korea) supplemented with 10% FBS (Lonza, Walkersville, MD, USA), 100 u/ml penicillin (Lonza), 100 u/ml streptomycin (Lonza), and 300 u/ml human rIL‐2 (Chiron) with designated concentrations of FK506 (Astellas Pharma Inc., Japan) or ethanol (Sigma‐Aldrich, St. Louis, MO, USA) as a vehicle control. IL‐2 and FK506 were replenished every 3rd day in the culture. NK cells were observed and photographed by an inverted microscope (Zeiss, Göttingen, Germany). In most experiments, the assays were performed after 7 days in culture with/without FK506. All procedures were approved by Korea University Institutional Review Board (Seoul, Korea), and donors consented.
Cell culture
K562 (human caucasian chronic myelogenous leukemia cell line; ATCC, Manassas, VA, USA), U937 (human leukemic monocyte lymphoma cell line; ATCC), and CEM (human T lymphoblast cell line; ATCC) were cultured in RPMI 1640 (Welgene), supplemented with 10% FBS, 100 u/ml penicillin, and 100 u/ml streptomycin (all from Lonza), and P815 (mouse mastocytoma cell line; ATCC) was cultured in RPMI 1640 (Welgene) supplemented with 5% FBS, 100 u/ml penicillin, and 100 u/ml streptomycin (all from Lonza).
Cell proliferation assays
Live cell numbers were counted with a hemocytometer (Improved Neubauer; Hawksley Co., Lansing, UK) by trypan blue exclusion. To assess DNA duplication, 1 μCi/ml 3H‐thymidne (Amersham Pharmacia Biotech) was incorporated into NK cells overnight after 3 or 6 days incubation with/without FK506. The next day, cells were harvested onto a filter paper with a Micro96 harvester (Skatron, Norway), and DPM were counted using a β‐counter (Packard Instrument Co., Meriden, CT, USA).
Flow cytometry
Anti‐human CD2 (clone RPA‐2.10), CD3 (OKT3), CD11a (HI111), CD18 (CLB‐LFA‐1/1), CD25 (BC96), CD49d (9F10), CD56 (MEM188), CD69 (FN50), CD107a (eBioH4A3), ICAM‐1 (HA58), IFN‐γ (4S.B3), and TNF‐α (MAb11) mAb were purchased from eBioscience (San Diego, CA, USA). Anti‐human CD3 (SK7), CD48 (TU145), CD56 (B159), CD58 (1C3), CD122 (Mik‐β3), CD132 (AG184), 2B4 (2‐69), DNAM1 (DX11), NKG2D (1D11), NKp30 (p30‐15), and NKp44 (P44‐8.1) mAb were purchased from BD PharMingen (San Diego, CA, USA). Anti‐human NKp46 (195314) mAb was purchased from R&D Systems (Minneapolis, MN, USA). The mAb used were a purified or conjugated form of FITC, PE, PerCP, or APC. Isotype controls were used as negative controls. PI and Annexin V (BD PharMingen) were used according to the manufacturerˈs instructions to assess cell death. Flow cytometry was performed with a FACSCalibur (BD Biosciences) and the data were analyzed with CellQuest software (BD PharMingen). Proper live lymphocyte populations were gated by forward‐/side‐scatter.
51Cr release assays
A standard 4‐h Cr release assay was performed with minor modifications [14]. In brief, K562, U937, CEM, or P815 target cells were labeled with 51Cr (Perkin Elmer, Boston, MA, USA) at 50 μCi/5 × 105 cells and cocultured at 5 × 103 cells /well with serially diluted NK cell cultures for 4 h. For redirected ADCC, P815 cells were preincubated with anti‐human 2B4 mAb before the coculture. The γ‐scintillation of supernatant was measured by a γ‐counter (Perkin Elmer). Percent of specific lysis was calculated as follows: 100 × (experimental release–spontaneous release)/(maximum release–spontaneous release).
CD107a granule release assay and cytokine intracellular staining
CD107a assay was performed following Alter et al. [15] with minor modifications. Briefly, NK cells cultured as described above were cocultured with K562 cells at an E:T ratio of 5:1 in a round‐bottom 96‐well plate. After 1 h incubation, anti‐CD107a mAb conjugated with FITC was treated at 10 μl/well, and Golgistop (BD PharMingen) was added to the culture for the last 5 h. After incubation, samples were fixed and permeabilized using Cytofix/Cytoperm intracellular staining kits (BD PharMingen). Then, NK cells were intracellularly stained with anti‐IFN‐γ‐PE or anti‐TNF‐α‐PE Ab for an additional 30 min. After washing, cells were fixed in 1% paraformaldehyde (Sigma‐Aldrich). Flow cytometry was performed with FACSCalibur (BD Biosciences) and the data analyzed using CellQuest software (BD Biosciences).
Western blot analysis
Cells were harvested after a 14‐h incubation with IL‐2 with/without FK506 and lysed in ice‐cold protein lysis buffer (20 mM Tris, pH 7.5, 100 mM NaCl, 5 mM MgCl2, 1% Nonidet P‐40, 0.5% sodium deoxycholate) supplemented with protease and phosphatase inhibitor cocktail PhosSTOP (Roche Diagnostics, Rotkreuz, Switzerland). Cell lysates were resolved by SDS‐PAGE and transferred to nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany). Membranes were then blocked with 5% skim milk in TBST (20 mM Tris‐HCl, pH 7.4, 150 mM NaCl, 0.1% Tween‐20) overnight at 4°C and probed with anti‐mouse phospho‐STAT3 (Tyr 705; 79/86 kDa) or anti‐STAT3 (92 kDa, Cell Signaling Technology, Danvers, MA, USA). After incubation with appropriate secondary Ab coupled to HRP (Jackson ImmunoResearch Laboratories, West Grove, PA, USA), immunoreactive proteins were visualized using SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL, USA). To quantify the band intensities, densitometry was performed using LabWorks (BioImaging Systems, UVP, Cambridge, UK).
Statistics
One sample t test, ANOVA, Dunnett t test, or independent sample t test was performed with SPSS software (SPSS, Chicago, IL, USA), depending on the data. Where a P value was <0.05, the result was considered significant. *P < 0.05; **P < 0.01; ***P < 0.001.
RESULTS
Patients on FK506 following HCT show reduced NK cells with down‐regulated CD25 in PBMC
We obtained PBMC of 11 patients with malignant hematologic diseases (AML or ABL) undergoing allogeneic, unrelated BMT or HSCT ( Fig. 1 ). These patients received common regimens for GVHD prophylaxis and MTX‐FK506 regimen at Days 1, 3, 6, and 11 following transplantation and thereafter, maintained for at least 2 or more months at the serum concentration of 10 ng/ml FK506 (Fig. 1, right panel table). As the presence of MTX hinders evaluation of the direct effect of FK506 on NK cells, we analyzed patientsˈ blood samples 2 months post‐transplantation, when the only remaining regimen was FK506. Strikingly, compared with healthy donor controls (n=10), these patients showed a significantly reduced number of NK cells in their peripheral blood compartments (Fig. 1A). Furthermore, when these NK cells were stimulated with IL‐2 for 7 days and analyzed for the expression of surface‐activating receptors, we found that expression of CD25, IL‐2R‐α chain, was reduced dramatically in NK cells from FK506‐treated patients as compared with normal controls (8.5% vs. 16.5%; Fig. 1B). CD25 serves as an activation marker in NK cells, as its surface expression in the resting state is minimal and undergoes dramatic up‐regulation upon activation [16, 17]. These data reveal an effect of FK506 unidentified previously on human NK cells in patients undergoing BMT or HSCT and suggest further that the immunosuppressive action of FK506 may be mediated, not only through blocking T cell functions but also via inhibiting NK cell activation and proliferation.
FK506 impairs NK cell proliferation
To investigate the effect of FK506 on NK cells in more detail, NK cells were isolated from PBMCs of healthy donors by negative selection (CD3–CD56+ NK cell purity >90%) and cultured with 300 u/ml human rIL‐2 in the presence or absence of FK506. As shown in Fig. 2 A, no significant change in the number of FK506‐treated NK cells was observed for the first 2 days in the culture. Thereafter, NK cell numbers had decreased gradually and reached ∼55% of the control (Fig. 2A, right panel; P=0.014).
Figure 2.
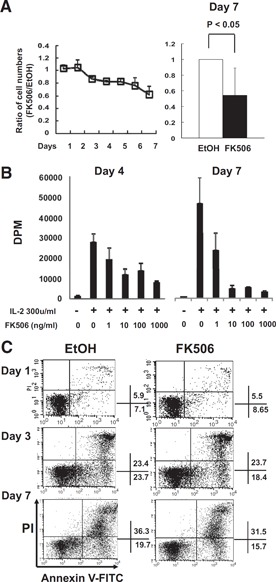
Decrease in IL‐2‐stimulated NK cell proliferation by FK506. (A) Human primary NK cells were cultured with 300 u/ml human rIL‐2 in the presence of 10 ng/ml FK506 or EtOH for 7 days. Cell numbers were counted and presented as a ratio calculated by the division of a cell number in the FK506‐treated group by that of EtOH‐treated. (Left panel) Time‐dependent changes of cell number by FK506 were shown (n=7). (Right panel) The number of NK cells treated with FK506 or EtOH at the end of culture was shown (n=7). One sample t test was performed by SPSS. (B) 3H‐Thymidine incorporation assay was performed after 4 and 7 days of IL‐2 culture with/without FK506 at designated concentrations. The data shown are from 1 particular experiment representing 3 independent experiments. Error bars show sd from triplicates. (C) PI/Annexin V assay by flow cytometry was performed with cultured NK cells as above. The percentages of PI‐AnnexinV+ early apoptotic and PI+AnnexinV+ late apoptotic/necrotic NK cells were shown. The results shown are representatives of a minimum 3 independent experiments.
To determine if the defect in NK cell number by FK506 was a result of the inhibition of cell proliferation or increased cell death, 3H‐thymidine incorporation assay and flow cytometry using Annexin V and PI were performed. As shown in Fig. 2B, DNA replication, as measured by 3H‐thymidine incorporated into NK cells in response to IL‐2, was inhibited by addition of FK506 in a dose‐dependent manner. At 1 ng/ml FK506, NK cell proliferation was inhibited by approximately 31% at Day 4 and 50% by Day 7 as compared with the vehicle control. Maximal inhibition was achieved at 10 ng/ml FK506, a clinically relevant concentration in the blood of patients following transplantation [18, 19], showing inhibition up to 57% at Day 4 and 90% at Day 7. Increasing dose of FK506 over 10 ng/ml did not increase further the level of inhibition at both days analyzed. Inhibition of DNA replication by FK506 was observed more dramatically at Day 7, compared with that of Day 4, implying that complete inhibition of DNA synthesis and blockade of cell cycle by FK506 require prolonged exposure in culture. Interestingly, the proliferation defect by FK506 seen in the 3H‐thymidine incorporation assay (Fig. 2B) was much greater than the effect on the cell numbers (Fig. 2A). This might have been a result of the fact that FK506 inhibited NK cell proliferation without affecting cell survival in culture. Consistent with this hypothesis, Annexin V/PI staining results showed no significant changes in the sign of cell death at both days following FK506 treatment (Fig. 2C). These data demonstrated that reduced NK cell numbers by FK506 in vitro appeared to be the result of the defect in cell proliferation rather than acceleration in cell death and might provide a basis underlying reduced NK cell numbers in the patients with BMT or HSCT on FK506 monotherapy in vivo (Fig. 1).
FK506 causes defective NK cell clustering accompanied by down‐regulation of cell adhesion molecules
Upon IL‐2‐induced stimulation, human primary NK cells tend to form clusters in the culture, and this clustering is highly associated with the status of NK cell activation. However, when NK cells were incubated with FK506 for 7 days, these clusters were not readily observed in culture, despite the presence of excess IL‐2 (data not shown). As cell clustering is thought to be mediated by binding of adhesion molecules on the NK cell surface with their cognate ligands on neighboring NK cells (homotypic cell‐to‐cell interaction), we analyzed the expression of a variety of adhesion molecules by flow cytometry. As shown in Fig. 3 A, IL‐2‐activated NK cells expressed significant levels of CD2 (LFA‐2), CD58 (LFA‐3; a ligand of CD2), ICAM‐1, CD11a (LFA‐1, integrin αL), CD49d (VLA‐4), and CD18 (integrin ß2). Treatment with FK506 resulted in selective down‐regulation of CD2, ICAM‐1, CD49d, and CD58 (all P<0.05), while leaving other adhesion receptors, CD11a and CD18, unaffected (Fig. 3A and B). Therefore, defective clustering in FK506‐treated NK cell culture might have been attributed to the reduced expression of adhesion receptors such as CD2, ICAM‐1, CD49d, and CD58, resulting in impaired homotypic interaction among neighboring NK cells.
Figure 3.
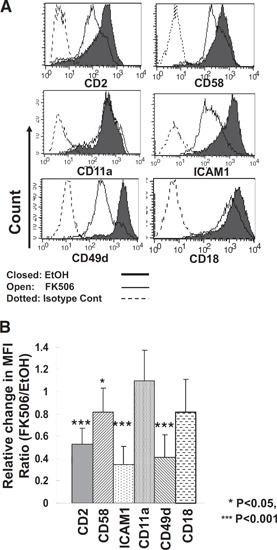
Down‐regulation of adhesion molecules by FK506. (A) Cell surface expression of adhesion molecules on CD3–CD56+ NK cells was analyzed by flow cytometry after 7 days in culture with/without 10 ng/ml FK506. Shaded lines show the surface expression profile of vehicle control, and open lines show that of FK506‐treated cells. Dotted lines represent isotype control (Cont). (B) Summary of flow cytometry data shown in A. The relative amount of expression of each cell surface molecule was calculated as a ratio of mean fluorescence intensities (MFI) of the cells cultured with FK506 to those of vehicle controls. Error bars represent sd (n=5–10). One sample t test was performed by SPSS.
FK506 selectively down‐regulates NK activating receptors
Selective down‐regulation of NK adhesion molecules by FK506 has prompted us to assess the expression of a panel of NK activating receptors in FK506‐treated NK cells. Among the receptors analyzed, the expression of NKG2D, CD48 (a ligand of 2B4), and DNAM1 was found to be down‐regulated during 7 days of culture in the presence of FK506, and the expression of 2B4, NKp30, NKp44, and NKp46 remained unchanged ( Fig. 4 A and B). The expression of NKG2D, CD48, and DNAM1 decreased significantly by 42%, 58%, and 35%, respectively (all P<0.05). Furthermore, the expression of surface activation markers, CD25 and CD69 (C‐type lectin), was also impaired in the presence of FK506 in a time‐dependent manner (Fig. 4C). Together, these data demonstrate selective down‐regulation of NK activating receptors and surface activation markers, which is likely to underlie defective proliferative responses in FK506‐treated NK cells.
Figure 4.
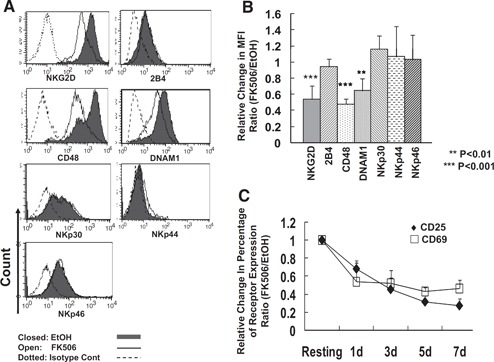
Selective down‐regulation of NK activating receptors by FK506. (A) Human primary NK cells as cultured in Fig. 2 were analyzed for the surface expression of a variety of NK receptors and activation markers; n = 5–12. (B) The relative amount of expression of each cell surface marker was calculated as a ratio of mean fluorescence intensities of the cells cultured with FK506 to those of vehicle control (n=5–12). Error bars represent sd. One sample t test was performed by SPSS. (C) The relative cell population (percent) of CD25+ and CD69+ cells out of total NK cells treated with FK506 was shown (n=3). The data shown are representatives of a minimum of 3 independent experiments.
Reduction of natural cytotoxicity and cytokine production in FK506‐treated NK cells
We next examined whether impaired expression of NK activating and adhesion receptors by FK506 could lead to functional defects of NK cells. Two effector processes, cytotoxicity and cytokine production, were monitored using IL‐2‐activated NK cells treated with or without FK506 for 7 days. As seen in Fig. 5 A, FK506‐treated NK cells showed reduced cytotoxicity against a series of allogeneic tumor targets, K562 (human caucasian chronic myelogenous leukemia cell line), CEM (human T lymphoblast cell line), and U937 (human leukemic monocyte lymphoma cell line), suggesting that the lytic pathway leading to the lysis of NK‐sensitive targets was partially impaired. Reduction in the cytotoxicity of FK506‐treated NK cells against K562 cells was correlated directly with a substantial decrease in CD107a degranulation events (Fig. 5B; P<0.001). In contrast, FK506 did not influence the ability of NK cells to induce redirected ADCC, as shown by comparable cytotoxicity between controls and FK506‐treated groups in a redirected ADCC through 2B4 (Fig. 5A). These data demonstrate that FK506‐mediated inhibition of natural cytotoxicity was likely attributed to down‐regulation of specific NK activating and/or adhesion receptors engaged in lysing allogeneic tumor targets. On the contrary, redirected ADCC through 2B4 was not affected, as FK506 did not alter surface expression of 2B4 and its downstream signaling pathways leading to degranulation.
Figure 5.
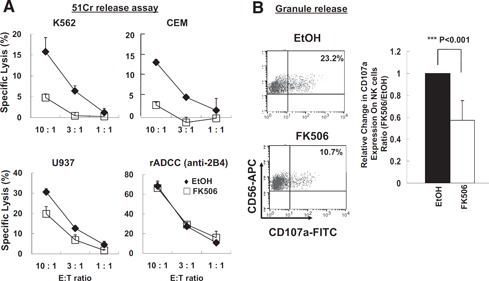
Reduced natural cytotoxicity by FK506. (A) Human NK cells cultured as in Fig. 2 were subject to 51Cr release assay against K562, CEM, and U937 targets. For redirected ADCC (rADCC), effector cells were preincubated with anti‐human 2B4 prior to exposure to FcR+P815 targets. The data are representatives from 5 independent experiments, and error bars represent sd out of triplicates. (B) NK cells upon stimulation by K562 target cells were analyzed for CD107a degranulation events. The right panel shows the relative percentages of CD56+ CD107a+ populations compared with those of vehicle control (n=8). Error bar represents sd (***P<0.001). One sample t test was performed by SPSS.
As NK cells mount dichotomous responses to cytolysis versus cytokine production, we next investigated whether FK506 affected the cytokine production of NK cells. FK506‐treated NK cells showed significantly impaired production of IFN‐γ and TNF‐α as compared with that of untreated NK cells ( Fig. 6 ). In culture with FK506, IFN‐γ (Fig. 6A)‐ and TNF‐α (Fig. 6B)‐expressing NK cell populations were decreased by approximately 72% and 67%, respectively, when compared with vehicle controls. These data demonstrate that FK506 causes functional defects in cytolysis and cytokine production in NK cells and suggest further that the down‐regulation of NK activating receptors and/or cell adhesion receptors is likely to be associated with impaired NK cell activation, e.g., reduced degranulation, subsequent natural cytotoxicity, and cytokine production.
Figure 6.
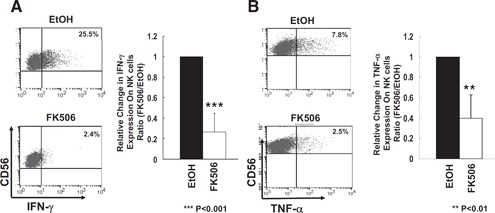
Reduced cytokine production by FK506. NK cells cultured in the presence or absence of FK506 as in Fig. 2 were mixed with K562 targets, and the production of IFN‐γ (A) and TNF‐α (B) was determined by intracellular flow cytometry. Percentages shown in the upper‐right quadrants represent (A) CD56+IFN‐γ+ or (B) CD56+TNF‐α+ NK populations (n=8 and 4, respectively). Error bars represent sd. **P < 0.01; ***P < 0.001. One sample t test was performed by SPSS.
Expression of IL‐2R subunits and activation of STAT3 were impaired by FK506
NK cells in culture responded to IL‐2 through binding to its receptor, composed of α (CD25), β (CD122), and γ (CD132) chains. As up‐regulation of the IL‐2‐α chain was strongly inhibited in FK506‐treated NK cells (Fig. 4C), we next examined whether the expression of other IL‐2R components was also affected by FK506. As shown in Fig. 7 A, expression of CD122 and CD132 was also reduced significantly following exposure to FK506 for 7 days, although not as dramatic as that of CD25 (all P<0.01). Although expression of 2B4 was not altered, that of CD25, CD122, and CD132 was down to ∼5%, 64%, and 57% of the control, respectively (Fig. 7A, right panel). We then examined if reduced IL‐2R expression by FK506 was associated with alterations in subsequent signaling events. As IL‐2 was shown previously to stimulate STAT signaling pathways, we measured the effect of FK506 on the activation of STATs. Among the STATs tested, STAT3, but not STAT1 or STAT5 (data not shown), appeared to be inhibited significantly in the presence of FK506. Phosphorylation of STAT3 was reduced by approximately 55% at 10 ng/ml and 70% at 100 ng/ml without affecting the total amount of STAT3 protein (Fig. 7B, lower panel; P<0.001). Taken together, these data suggest that reduced IL‐2R expression, leading to decreased STAT3 phosphorylation, might underlie the functional defects in the proliferation, cytotolysis, and cytokine production by FK506.
Figure 7.
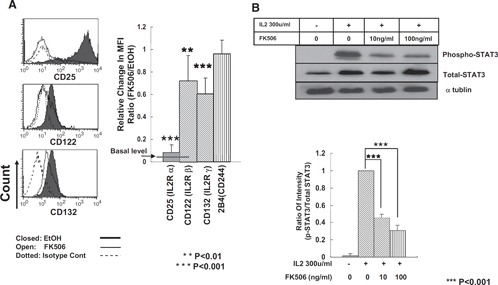
Down‐regulation of IL‐2R expression and reduced phosphorylation of STAT3 in FK506‐treated NK cells. (A) After a 7‐day culture of NK cells with 10 ng/ml FK506 in the presence of 300 u/ml human rIL‐2, the surface expression of α, β, and γ chains of IL‐2Rs was analyzed by flow cytometry. The data are representatives from 5 independent experiments. Closed histograms show vehicle control, open histograms show FK506‐treated NK cells, and dotted histograms show isotype control. The relative mean fluorescence intensities were calculated compared with those of vehicle control (n=10). **P < 0.01; ***P < 0.001. ANOVA and Dunnett t test were performed by SPSS. (B) Western blotting was performed with NK cells treated with FK506 for 14 h to measure the amount of total and phosphorylated STAT3 (p‐STAT3) protein. The relative intensities of the phosphorylated STAT3 were calculated as compared with those of total STAT3 (n=3; ***P<0.001).
DISCUSSION
FK506 is widely administered to promote engraftment and suppress GVHD following graft transplantation; however, its effect on NK cells is hitherto less elucidated. Here, we present the direct evidence for the first time that NK cells are immediate targets of FK506, which profoundly inhibited proliferation and activation of human NK cells in vitro and in vivo. Patients on FK506 following HCT showed a reduced number of NK cells with down‐regulated CD25 in their peripheral blood compartment. Similar impairment was observed and confirmed in vitro in FK506‐treated NK cells isolated from healthy volunteers. The mechanism underlying defective NK cell proliferation and their functions was, in part, attributed to the impairment of IL‐2R signaling, STAT3 activation, and expression of NK adhesion and activation receptors.
Defective proliferation of FK506‐treated NK cells was likely a result of the inability to stimulate DNA synthesis. Like CsA, FK506 is known to inhibit calcineurin [5], whose pathway is downstream of CD28 costimulation in T cells [20]. CsA and FK506 diminished IL‐2R expression on T cells [4] and inhibited antigen presentation in DCs [6, 7], thus preventing antigen‐specific T cell activation. Our data showed near‐complete inhibition of IL‐2R‐α chain (CD25) expression in NK cells by FK506; therefore, its downstream signaling events were likely to be heavily impaired. Down‐regulation of CD132, a common γ chain, by FK506 implies that not only IL‐2R but also other receptors using CD132, such as IL‐4R, ‐7R, ‐9R, ‐15R, and ‐21R, might not be optimally functional in NK cells. Those cytokines play a role in NK cell differentiation and maturation as well as effector functions; hence, not only effector functions but also differentiation processes of NK cells might have been affected by FK506. Thus, these changes might have accounted for a reduced number of NK cells in the peripheral blood of HCT patients in vivo. Along with STAT1 and STAT5, STAT3 functions as a transcription factor downstream of IL‐2R stimulation. In our experiments, FK506 selectively inhibited STAT3, leaving STAT1 and STAT5 intact, suggesting a unique role of STAT3 downstream of IL‐2R in NK cells. Upon IL‐2 stimulation, STAT3 becomes activated, phosphorylated by a Janus kinase 1 (JAK1), and then translocated to the nucleus to induce transcription of multiple genes, including Cyclin D1, TNFR, and FasL [21, 22]. Therefore, altered expression of cyclin D1, TNFR, and FasL might have contributed to cause cell cycle arrest and inhibition of the proliferation pathway in FK506‐treated NK cells.
Our data are consistent with a previous report by Wasik et al. [13], where no significant effect on human natural cytotoxicity and ADCC was observed by PBMCs incubated with FK506 for 24–48 h in culture. Our studies using purified NK cells also demonstrate no dramatic changes of NK cytotoxicity and proliferation in response to IL‐2 by FK506 within 48 h (data not shown). Thus, it appears that NK cells required a prolonged incubation time of 3 or more days with FK506 to demonstrate cytotoxic and proliferative defects. The defect in cell proliferation was accompanied by down‐regulation of IL‐2Rs and subsequent blockade of cell cycle entry, which became apparent from Day 3 following FK506 treatment. Impaired natural cytotoxicity also required a prolonged incubation time of over 3 days, allowing down‐regulation of NK activating or adhesion receptors on the cell surface. Expression of ICAM‐1, CD2, CD49d, and CD58 was down‐regulated, and that of CD18 and CD11a was not changed, suggesting that selective reduction of cell adhesion molecules appeared to be sufficient to dampen NK cell clustering. Reduced cell clustering might have potentiated the proliferation defect in FK506‐treated NK cells by preventing homotypic cell‐to‐cell costimulation events [23].
FK506 also selectively reduced the expression of NK cell activating receptors, i.e., NKG2D, CD48, and DNAM1, but not 2B4, NKp30, NKp44, and NKp46. Therefore, the capability of NK cells to kill allogeneic tumor targets primarily using NKG2D, CD48, or DNAM1 pathways would be greatly diminished, and that mediated by 2B4 or NCRs (NKp30, NKp44, NKp46) would not be affected significantly by FK506. Nevertheless, the intrinsic potential of cytotoxicity and lytic signaling components in FK506‐treated cells was intact, as shown by redirected ADCC via 2B4. Therefore, NK cells in the patients on FK506 therapy could exhibit a capacity to fully mount cytotoxicity, depending on the use of activating receptors and the nature of tumor or infected targets encountered. Interestingly, the expression of CD48, a ligand for 2B4 and CD2, was also decreased, thus the homotypic NK‐to‐NK interaction, known to occur though 2B4/CD48 [23] and CD2/CD48 (unpublished data), was likely to be impaired severely and contributed to deficient NK clustering and costimulation [24, –, 26]. These changes in the expression of NK adhesion and activation receptors seen in vitro were also confirmed in the FK506‐treated patients following allogeneic transplantation; the surface level of CD2, NKG2, and CD48 was found to be greatly diminished, and that of 2B4 remained unchanged, similar to the results obtained in vitro (Supplemental Fig. 1).
Two randomized clinical trials have shown FK506 to be superior to CsA in the prevention of acute GVHD without an increase in relapse [2, 3]. From a prospective comparison, data between FK506 with short‐term MTX and conventional CsA with MTX in adult unrelated transplants, the patients receiving FK506 showed a significantly lower occurrence of acute GVHD II–IV without altering the incidence of chronic GVHD. Side‐effects, leukemia recurrence, and overall survival were comparable in both arms. However, there was also a report demonstrating that FK506 increased the relapse rate among recipients from HLA‐matched siblings as compared with CsA [27]. Interestingly, CsA, similar to FK506, was shown to inhibit total NK cell numbers in an in vitro culture; however, it has been demonstrated to have a differential effect on NK cell subtypes, driving from the CD56+CD16+KIR+ phenotype toward CD56+CD16–KIR– phenotype [28]. We found no such transition of NK cell phenotype in FK506‐treated NK cell cultures (Supplemental Fig. 2). Unlike FK506‐treated NK cells shown here, NK cells treated with CsA showed elevated effector function, enhanced natural cytotoxicity, and more IFN‐γ as compared with control groups [28]. CsA been demonstrated to inhibit NK cell apoptosis during an encounter with tumor targets without affecting NK‐mediated cytotoxicity induced by anti‐NCRs; NKp46, NKp44, or NKp30 [29]. As FK506 treatment caused NK cells to be functionally impaired in cytolytic ability and the cytokine secretion, these cells might exhibit a reduced GVL effect and thus, contribute to the increased relapse rate in HLA‐matched siblings [27]. Although it requires further investigation, these differences might account for the differential effect of engraftment, GVHD, GVL, and the relapse rate between the FK506‐ and CsA‐treated patients. Experiments are currently in progress to compare directly the effect of CsA and FK506 on the phenotype and function of NK cells in vitro as well as in vivo via obtaining blood samples of transplanted patients. The results from these studies will provide more accurate insights into the mechanism of clinical outcomes by FK506 and CsA.
Nevertheless, it should be noted that GVHD and GVL effects in patients are complex processes involving multiple cell types such as CD4+ and CD8+ T cells, regulatory T cells, γδ T cells, NK T cells, and NK cells; thus, further detailed molecular analysis of effector cells under immunosuppressive regimens for successful engraftment awaits. Close monitoring of NK cells as well as other immune effector populations during FK506 therapy is necessary to promote successful engraftment and to reduce incidences of opportunistic infections. Collectively, our data provide a new insight into the design of immunosuppressive regimens required for individual patients by presenting the functions unidentified previously and its underlying mechanisms of FK506 in human NK cells.
AUTHORSHIP
T‐J.K. and K‐M.L. designed the research; T‐J.K. and E‐O.K. performed the experiments; H‐J.K. and H.S.A. provided the clinical samples; T‐J.K., N.K., S‐T.K., J.A.B., and K‐M.L. analyzed the results; and N.K. and K‐M.L. wrote the manuscript.
DISCLOSURE
The authors declare no conflict of interest.
Supporting information
Supplementary Material Files
Supplementary Material Files
ACKNOWLEDGMENTS
This study was supported by a grant from KICOS (K20704000007‐09A0500‐00710, K20601000002‐09E0100‐00210), the National Nuclear R&D Program (grant BAERI), the Seoul R&BD Program (10920), and the Innovative Research Institute for Cell Therapy (A062260). The authors are grateful to Dr. Juneyoung Lee (Korea University College of Medicine) for the kind advice about statistics.
REFERENCES
- 1. Bowman, L. J. , Brennan, D. C. (2008) The role of tacrolimus in renal transplantation. Expert Opin. Pharmacother. 9, 635–643. [DOI] [PubMed] [Google Scholar]
- 2. Ratanatharathorn, V. , Nash, R. A. , Przepiorka, D. , Devine, S. M. , Klein, J. L. , Weisdorf, D. , Fay, J. W. , Nademanee, A. , Antin, J. H. , Christiansen, N. P. , van der Jagt, R. , Herzig, R. H. , Litzow, M. R. , Wolff, S. N. , Longo, W. L. , Petersen, F. B. , Karanes, C. , Avalos, B. , Storb, R. , Buell, D. N. , Maher, R. M. , Fitzsimmons, W. E. , Wingard, J. R. (1998) Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft‐versus‐host disease prophylaxis after HLA‐identical sibling bone marrow transplantation. Blood 92, 2303–2314. [PubMed] [Google Scholar]
- 3. Nash, R. A. , Antin, J. H. , Karanes, C. , Fay, J. W. , Avalos, B. R. , Yeager, A. M. , Przepiorka, D. , Davies, S. , Petersen, F. B. , Bartels, P. , Buell, D. , Fitzsimmons, W. , Anasetti, C. , Storb, R. , Ratanatharathorn, V. (2000) Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft‐versus‐host disease after marrow transplantation from unrelated donors. Blood 96, 2062–2068. [PubMed] [Google Scholar]
- 4. Kino, T. , Hatanaka, H. , Miyata, S. , Inamura, N. , Nishiyama, M. , Yajima, T. , Goto, T. , Okuhara, M. , Kohsaka, M. , Aoki, H. , et al. (1987) FK‐506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK‐506 in vitro. J. Antibiot. (Tokyo) 40, 1256–1265. [DOI] [PubMed] [Google Scholar]
- 5. Liu, J. , Farmer Jr., J. D. , Lane, W. S. , Friedman, J. , Weissman, I. , Schreiber, S. L. (1991) Calcineurin is a common target of cyclophilin‐cyclo‐sporin A and FKBP‐FK506 complexes. Cell 66, 807–815. [DOI] [PubMed] [Google Scholar]
- 6. Lee, Y. R. , Yang, I. H. , Lee, Y. H. , Im, S. A. , Song, S. , Li, H. , Han, K. , Kim, K. , Eo, S. K. , Lee, C. K. (2005) Cyclosporin A and tacrolimus, but not rapamycin, inhibit MHC‐restricted antigen presentation pathways in dendritic cells. Blood 105, 3951–3955. [DOI] [PubMed] [Google Scholar]
- 7. Lee, Y. H. , Lee, Y. R. , Im, S. A. , Park, S. I. , Kim, K. H. , Gerelchuluun, T. , Song, S. , Kim, K. , Lee, C. K. (2007) Calcineurin inhibitors block MHC‐restricted antigen presentation in vivo. J. Immunol. 179, 5711–5716. [DOI] [PubMed] [Google Scholar]
- 8. Karre, K. , Ljunggren, H. G. , Piontek, G. , Kiessling, R. (1986) Selective rejection of H‐2‐deficient lymphoma variants suggests alternative immune defense strategy. Nature 319, 675–678. [DOI] [PubMed] [Google Scholar]
- 9. Fehniger, T. A. , Cai, S. F. , Cao, X. , Bredemeyer, A. J. , Presti, R. M. , French, A. R. , Ley, T. J. (2007) Acquisition of murine NK cell cytotoxicity requires the translation of a pre‐existing pool of granzyme B and perforin mRNAs. Immunity 26, 798–811. [DOI] [PubMed] [Google Scholar]
- 10. Stetson, D. B. , Mohrs, M. , Reinhardt, R. L. , Baron, J. L. , Wang, Z. E. , Gapin, L. , Kronenberg, M. , Locksley, R. M. (2003) Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 198, 1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Welniak, L. A. , Blazar, B. R. , Murphy, W. J. (2007) Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu. Rev. Immunol. 25, 139–170. [DOI] [PubMed] [Google Scholar]
- 12. Pores‐Fernando, A. T. , Gaur, S. , Doyon, M. Y. , Zweifach, A. (2009) Calcineurin‐dependent lytic granule exocytosis in NK‐92 natural killer cells. Cell. Immunol. 254, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wasik, M. , Gorski, A. , Stepien‐Sopniewska, B. , Lagodzinski, Z. (1991) Effect of FK506 versus cyclosporine on human natural and antibody‐dependent cytotoxicity reactions in vitro. Transplantation 51, 268–270. [DOI] [PubMed] [Google Scholar]
- 14. Yoon, S. H. , Yun, S. O. , Park, J. Y. , Won, H. Y. , Kim, E. K. , Sohn, H. J. , Cho, H. I. , Kim, T. G. (2009) Selective addition of CXCR3(+) CCR4(‐) CD4(+) Th1 cells enhances generation of cytotoxic T cells by dendritic cells in vitro. Exp. Mol. Med. 41, 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alter, G. , Malenfant, J. M. , Altfeld, M. (2004) CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 294, 15–22. [DOI] [PubMed] [Google Scholar]
- 16. Toomey, J. A. , Gays, F. , Foster, D. , Brooks, C. G. (2003) Cytokine requirements for the growth and development of mouse NK cells in vitro. J. Leukoc. Biol. 74, 233–242. [DOI] [PubMed] [Google Scholar]
- 17. Giavedoni, L. D. , Velasquillo, M. C. , Parodi, L. M. , Hubbard, G. B. , Hodara, V. L. (2000) Cytokine expression, natural killer cell activation, and phenotypic changes in lymphoid cells from rhesus macaques during acute infection with pathogenic simian immunodeficiency virus. J. Virol. 74, 1648–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ingels, S. C. , Koenig, J. , Scott, M. G. (1995) Stability of FK506 (tacrolimus) in whole‐blood specimens. Clin. Chem. 41, 1320–1321. [PubMed] [Google Scholar]
- 19. Wingard, J. R. , Nash, R. A. , Przepiorka, D. , Klein, J. L. , Weisdorf, D. J. , Fay, J. W. , Zhu, J. , Maher, R. M. , Fitzsimmons, W. E. , Ratanatharathorn, V. (1998) Relationship of tacrolimus (FK506) whole blood concentrations and efficacy and safety after HLA‐identical sibling bone marrow transplantation. Biol. Blood Marrow Transplant. 4, 157–163. [DOI] [PubMed] [Google Scholar]
- 20. Liao, X. C. , Fournier, S. , Killeen, N. , Weiss, A. , Allison, J. P. , Littman, D. R. (1997) Itk negatively regulates induction of T cell proliferation by CD28 costimulation. J. Exp. Med. 186, 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levy, D. E. , Lee, C. K. (2002) What does Stat3 do? J. Clin. Invest. 109, 1143–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Snyder, M. , Huang, X. Y. , Zhang, J. J. (2008) Identification of novel direct Stat3 target genes for control of growth and differentiation. J. Biol. Chem. 283, 3791–3798. [DOI] [PubMed] [Google Scholar]
- 23. Lee, K. M. , Forman, J. P. , McNerney, M. E. , Stepp, S. , Kuppireddi, S. , Guzior, D. , Latchman, Y. E. , Sayegh, M. H. , Yagita, H. , Park, C. K. , Oh, S. B. , Wulfing, C. , Schatzle, J. , Mathew, P. A. , Sharpe, A. H. , Kumar, V. (2006) Requirement of homotypic NK‐cell interactions through 2B4(CD244)/CD48 in the generation of NK effector functions. Blood 107, 3181–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barber, D. F. , Long, E. O. (2003) Coexpression of CD58 or CD48 with intercellular adhesion molecule 1 on target cells enhances adhesion of resting NK cells. J. Immunol. 170, 294–299. [DOI] [PubMed] [Google Scholar]
- 25. Latchman, Y. , Reiser, H. (1998) Enhanced murine CD4+ T cell responses induced by the CD2 ligand CD48. Eur. J. Immunol. 28, 4325–4331. [DOI] [PubMed] [Google Scholar]
- 26. Latchman, Y. , McKay, P. F. , Reiser, H. (1998) Identification of the 2B4 molecule as a counter‐receptor for CD48. J. Immunol. 161, 5809–5812. [PubMed] [Google Scholar]
- 27. Hiraoka, A. , Ohashi, Y. , Okamoto, S. , Moriyama, Y. , Nagao, T. , Kodera, Y. , Kanamaru, A. , Dohy, H. , Masaoka, T. (2001) Phase III study comparing tacrolimus (FK506) with cyclosporine for graft‐versus‐host disease prophylaxis after allogeneic bone marrow transplantation. Bone Marrow Transplant. 28, 181–185. [DOI] [PubMed] [Google Scholar]
- 28. Wang, H. , Grzywacz, B. , Sukovich, D. , McCullar, V. , Cao, Q. , Lee, A. B. , Blazar, B. R. , Cornfield, D. N. , Miller, J. S. , Verneris, M. R. (2007) The unexpected effect of cyclosporin A on CD56+CD16‐ and CD56+CD16+ natural killer cell subpopulations. Blood 110, 1530–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poggi, A. , Massaro, A. M. , Negrini, S. , Contini, P. , Zocchi, M. R. (2005) Tumor‐induced apoptosis of human IL‐2‐activated NK cells: role of natural cytotoxicity receptors. J. Immunol. 174, 2653–2660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material Files
Supplementary Material Files


