Short abstract
Review on how inflammation and the increase in CHI3L1 expression promote tumor progression.
Keywords: angiogenesis, inflammatory bowel, pulmonary inflammation, cytokines
Abstract
Inflammation plays a vital role at different stages of tumor progression. The development of tumors is affected by inflammatory mediators produced by the tumor and the host. YKL‐40/chitinase‐3‐like‐1 protein is often up‐regulated in inflammation‐associated diseases. With the use of chronic inflammatory disease systems, we describe the role of YKL‐40/chitinase‐3‐like‐1 protein in enhancing the inflammatory response and its implications in tumorigenesis. We also discuss how pre‐existing inflammation enhances tumor growth and metastasis. In this mini‐review, we highlight the effect of YKL‐40/chitinase‐3‐like‐1 protein‐associated inflammation in promoting tumor progression.
Abbreviations
- CBM
chitin‐binding motif
- COPD
chronic obstructive pulmonary disease
- COX‐2
cyclooxygenase‐2
- CRC
colorectal cancer
- CS
cigarette smoke
- IBD
inflammatory bowel disease
- KO
knockout
- MDSC
myeloid‐derived suppressor cells
- MMP‐9
matrix metalloproteinase 9
- PKB
protein kinase B
- shRNA
short hairpin RNA
- TAM
tumor‐associated macrophage
- TILs
tumor infiltrating lymphocytes
- VEGF
vascular endothelial growth factor
- YKL‐40/CHI3L1
chitinase‐3‐like‐1 protein
Introduction
Inflammation has been described as one of the hallmarks of cancer [1]. Approximately ¼ of all human cancers worldwide are associated with chronic inflammation or inflammatory diseases [2, 3, 4–5]. The inflammatory response is critical for tumor progression, as it influences many hallmarks of cancer, such as increased cell proliferation, evasion of cell death, increased angiogenesis, invasion, and metastasis [1, 6]. A majority of cancers are known to be linked to chronic inflammation, which can promote oncogenesis and influence tumor progression. Chronic inflammation and cancers are both characterized by increased inflammatory cellular infiltrates, inflammatory mediators, as well as angiogenic and extracellular matrix remodeling molecules [7]. One of the mediators that is up‐regulated during proinflammatory responses is YKL‐40/CHI3L1, which is expressed in the microenvironment of various solid tumors. The expression of YKL‐40/CHI3L1 is also up‐regulated during tumor growth, and the levels of this glycoprotein have been correlated with poor survival and prognosis in cancer patients [8, 9]. YKL‐40/CHI3L1, also known as breast regression protein 39, is a member of the 18 glycosyl hydrolase gene family that contains true chitinases and chitinase‐like proteins. Unlike true chitinases, chitinase‐like proteins bind to chitin but do not cleave it. YKL‐40/CHI3L1 is expressed and produced by immune cells, such as macrophages, neutrophils, and nonimmune cells, including fibroblasts, vascular smooth cells, and endothelial cells, among others. Stimulation with various cytokines, including IL‐13, IL‐6, TNF‐α, and IL‐1β, induces the production of YKL‐40/CHI3L1. In human macrophages, YKL‐40/CHI3L1 is stimulated by a key TH1 cytokine, IFN‐γ, but is suppressed by the TH2 cytokine, IL‐4 [10]. Interestingly, our studies have shown that YKL‐40/CHI3L1 down‐regulates the production of IFN‐γ in T lymphocytes [11]. As CHI3L1 is expressed by a vast array of cells, including tumor cells, it is probable that the ultimate cancer metastasis is a result of the interactions between the tumor and host tissue. These interactions could further promote inflammation, resulting in accelerated tumor growth and metastasis. Our studies that use animal models suggest that such interactions elevate CHI3L1 levels at metastatic sites and enhance metastasis, whereas CHI3L1 KO mice exhibit decreased inflammation and tumor metastasis. Although elevated serum levels of YKL‐40/CHI3L1 have been found in a broad spectrum of cancers and chronic inflammatory diseases, there is no clinical evidence to date indicating that increased YKL‐40/CHI3L1 expression in leukocytes at a target organ predisposes toward metastasis. Further studies are needed to clarify the clinical role of this molecule in promoting tumor growth and metastasis. In this review, we describe the role of YKL‐40/CHI3L1 in various inflammatory conditions and tumor progression by use of murine models of cancers and clinical correlations in humans.
TUMOR‐ASSOCIATED INFLAMMATION
Tumors are known to have local and systemic effects. Proinflammatory molecules produced by the tumor or the host immune cells in response to tumor‐derived factors can impact tumor growth, metastasis, and host survival. In the tumor microenvironment, cellular infiltration by myeloid‐derived cells, macrophages, and lymphoid cells has been reported to contribute to the inflammatory response that suppresses or promotes tumor growth. Although T cells are known for their anti‐tumor effects, infiltration of CD4+ T cells in renal and colorectal tumors was associated with poor prognosis. With the use of a mammary tumor model, we have shown that CD4+ T cell infiltration was associated with tumor growth and metastasis, as T cells from mammary tumor bearers produced MCP‐1/CCL2 and MMP‐9 [12, 13]. Lymphocytes, as immunosurveillant cells, have invasive properties and migrate from the vascular compartment to sites of inflammation by expressing MMP‐9 [14], which is up‐regulated in various cancers. We have shown increased expression of MMP‐9 in T lymphocytes from mammary tumor‐bearing mice [12]. In assessing the clinical significance of MMPs, such as MMP‐9 in melanoma patients, Lugowska et al. [15] found that serum concentrations of VEGF, MMP‐2 and ‐9, and YKL‐40/CHI3L1 were significantly higher in melanoma patients compared with the control group. YKL‐40/CHI3L1 was one of the tumor‐ and host‐derived factors that induced the expression of MMP‐9 by macrophages [16, 17].
Macrophages are the most abundant immune cell population called into the tumor site at the earlier stages of tumor progression. These macrophages orchestrate the intratumoral immune reactions that eventually lead to adverse effects. The presence of macrophages is associated with poor prognosis and survival in numerous cancer types. TAMs contribute to tumor growth and metastasis by secreting MMPs that promote extracellular matrix remodeling; angiogenic factors that increase blood vessel formation; and cytokines, chemokines, and growth factors that enhance tumor growth [18, 19]. Specifically, TAMs have been reported to produce VEGF, epidermal growth factor, basic fibroblast growth factor, platelet‐derived growth factor, IL‐6, MCP‐1, CXCL18, CXCL12, and YKL‐40/CHI3L1 [16, 20, 21, 22, 23, 24–25]. YKL‐40/CHI3L1 induces the expression of MCP‐1/CCL2 and IL‐8/CXCL2, which are chemoattractants for monocytes/macrophages and neutrophils [16]. All of these factors may serve as angiogenic molecules.
The direct angiogenic signature of YKL‐40/CHI3L1 in tumor development was demonstrated by both Mizoguchi's [25] and Shao's [26] laboratories. Implantation of breast cancer cell line MDA‐MB‐231 or colon cancer cell lines HCT‐116 or SW480, engineered to express YKL‐40/CHI3L1 in mice, showed increased blood vasculature compared with the control tumors [25, 26]. In other tumor models, such as breast and glioblastoma, tumor angiogenesis, and vessel density were suppressed when mice were implanted with YKL‐40/CHI3L1‐shRNA tumors [27, 28]. In vitro studies confirmed the angiogenic activity, as demonstrated by decreased endothelial cell migration and tube formation when human microvascular endothelial cells were exposed to YKL‐40/CHI3L1 shRNA tumor cell‐conditioned medium [27]. Angiogenesis is vital for tumor growth, and TAMs play a crucial role in controlling tumor angiogenesis. Xenografts of human colon cancer cells engineered to express YKL‐40/CHI3L1 exhibited increased macrophage infiltration, microvessel density, and tumor growth. With the use of 4T1 and DA‐3 murine mammary tumor models, Libreros et al. [11, 29] demonstrated that YKL‐40/CHI3L1 is expressed by splenic and pulmonary macrophages with a concurrent increase in tumor growth. In vivo neutralization of YKL‐40/CHI3L1 by use of anti‐YKL‐40/CHI3L1 antibodies caused decreased blood‐vessel density, production of proinflammatory mediators, and tumor growth [26, 27, 30]. More importantly, we have shown that treatment of mammary tumor‐bearing mice with chitin microparticles, the natural ligand for YKL‐40/CHI3L1, inhibited angiogenesis, production of proinflammatory mediators, YKL‐40/CHI3L1 expression, tumor growth, and metastasis [11, 29]. In line with these findings, there was decreased primary mammary tumor growth and pulmonary metastasis with an increase in survival in YKL‐40/CHI3L1 KO mice bearing mammary tumors [31]. With the use of a melanoma metastasis model, Ma et al. [32] have shown that there is decreased metastasis to the lungs in the YKL‐40/CHI3L1 KO mice. In spite of all of the evidence in the mouse models and studies correlating YKL‐40/CHI3L1 expression in patients with various cancers, to date, there is no clear‐cut evidence in published studies to indicate clinically that CHI3L1 is linked to inflammation‐associated cancer development or metastasis. This is an area that needs further investigation by use of clinical samples to demonstrate the mechanistic role of YKL‐40/CHI3L1 in patients with cancer
ALLERGIC PULMONARY INFLAMMATION AND CANCER
The incidence of allergic asthma has been on the rise, affecting 300 million people globally each day [33]. Allergic asthma is a chronic inflammatory disorder of the airways, characterized by structural changes, including fibrosis in the lamina reticularis and adventia, mucus metaplasia, myocyte hypertrophy, and angiogenesis. Airways of asthmatics are infiltrated by various leukocyte populations, including eosinophils, neutrophils, macrophages, and lymphocytes, which contribute to airway remodeling. Upon allergen exposure, these immune cells secrete a plethora of molecules that exacerbate the inflammatory response. In addition, allergic asthma is characterized by an enhanced TH2 response, with increased TH2 cytokines at the sites of inflammation [34]. A major effector molecule in TH2 pathology is IL‐13, which induces asthma‐like effects, including mucus metaplasia, airway fibrosis, and airway hyper‐responsiveness, in mice challenged with methacholine [35]. Furthermore, this interleukin has been shown to regulate the production of chitinase‐like proteins [36]. In OVA‐sensitized and challenged mice, YKL‐40/CHI3L1 expression was induced in airways presenting a significant degree of inflammation [37], which was greatly ameliorated in YKL‐40/CHI3L1 KO mice.
Until recently, the physiologic receptor of YKL‐40/CHI3L1 was not known. Ma et al. [32] demonstrated that a known decoy receptor of IL‐13, IL‐13Rα2, functions as a receptor for YKL‐40/CHI3L1. In comparing cell death of immune cells in wild‐type with YKL‐40/CHI3L1 KO mice, Lee et al. [37] showed increased cell death in CD4+ T cells, macrophages, and eosinophils in the KO mice, indicating that YKL‐40/CHI3L1 is anti‐apoptotic. In assessing the pathways that YKL‐40/CHI3L1 uses to regulate inflammatory cell apoptosis, enhanced levels of total and activated PKB/AKT were shown in wild‐type compared with YKL‐40/CHI3L1 KO mice.
To understand further the signaling pathways by which YKL‐40/CHI3L1‐IL‐13Rα2 interaction induces their effects, MAPK, ERK1/2, and Wnt/β‐catenin pathways were assessed in THP1 cells. MAPK and ERK activation and the induction of nuclear β‐catenin and c‐fos were seen in response to YKL‐40/CHI3L1, whereas there was no similar signal in YKL‐40/CHI3L1 KO mice [38]. Recent studies indicated that IL‐13Rα2 plays a role in melanoma lung metastasis. A comparison of WT and IL‐13Rα2 KO mice showed decreased metastasis to the lungs in IL‐13Rα2 KO mice [32]. He et al. [38] evaluated whether YKL‐40/CHI3L1 mediated its metastatic effects via IL‐13Rα2 and subsequent production of TGF‐β1 using a melanoma model. Comparison of wild‐type, IL‐13Rα2 null, YKL‐40/CHI3L1 transgenic, and YKL‐40/CHI3L1 transgenic/IL‐13Rα2 null mice revealed metastasis to the lungs in IL‐13Rα2 null mice compared with wild‐type. YKL‐40/CHI3L1 transgenic mice had higher metastasis compared with the YKL‐40/CHI3L1 transgenic/IL‐13Rα2 null mice.
To define further the roles of the intracellular domain of IL‐13Rα2 in signaling events, the effects of YKL‐40/CHI3L1 in cells from IL‐13Rα2 KO mice that had been transfected with full‐length IL‐13Rα2, constructs that lacked the intracellular domain of IL‐13Rα2, and controls were assessed. It was shown that MAPK and AKT activation did not require the intracellular domain of the receptor, whereas the intracellular domain did play an important role in the activation of the Wnt/β‐catenin/AP‐1 pathway [38]. Furthermore, it was found that the binding region of YKL‐40 between aa 22 and 357 was essential for binding to full‐length IL‐13Rα2 [38]. This region is important, in that it contains a CBM. On the other hand, Mizoguchi's laboratory [39] investigated the role of CBM in the regulation of IL‐8 production in colonic epithelial cells. Toward this, SW480 colonic epithelial cells were transfected with wild‐type deletion mutants of the C‐terminal CBM. It was found that the 325–339th residues of CBM in YKL‐40/CHI3L1 are critical for IL‐8 promoter activation and CXCL2/IL‐8 production [39]. These studies suggest that CBM includes regions that bind to IL‐13Rα2 and IL‐8 promoters. However, it is possible that in addition to binding IL‐13Rα2 and IL‐8 promoter regions, YKL‐40/CHI3L1 binds to other receptors and promoters, resulting in the production of other proinflammatory mediators.
During allergen‐induced pulmonary inflammation, proinflammatory leukocytes extravasate from circulation and intravasate into the lung tissue by use of mechanisms similar to tumor cell metastasis [5, 40]. Taranova et al. [41] determined whether existing pulmonary inflammation promotes secondary tumor formation. Pre‐existing inflammation augmented metastatic colonization of melanoma tumor cells by 3‐fold compared with controls [41]. The inflammatory response in this model was found to be associated with CD4+ T cells, and depletion of these CD4+ T cells decreased lung metastases. The suppression of inflammation with the corticosteroid budesonide decreased CD4+ T cell recruitment and metastasis to the lung. Another study concurred with these findings in that activated CD4+ T cells from thymic stromal lymphopoietin‐induced airway allergic inflammation resulted in increased tumor growth and lung metastasis [42]. It is well established that macrophages are one of the leukocyte subpopulations that contribute to enhanced tumor growth and metastasis. However, it is not known if altered cellular composition at a target site of metastasis, induced for inflammation, enhances tumor growth and accelerates metastasis. With the use of a combined mouse model of allergic pulmonary inflammation and breast cancer, our laboratory assessed the cellular composition of the lungs that could enhance metastasis. We reported that myeloid‐derived cells and pulmonary macrophages are increased in the lungs of allergic tumor‐bearing mice. Furthermore, it was demonstrated that these are the major cellular populations that contribute to the proinflammatory microenvironment that enhances metastasis [31]. These studies show that pre‐existing pulmonary inflammation enhances metastasis to the lung in mice‐bearing mammary tumors.
Studies by Libreros et al. [31] demonstrated that circulating levels of YKL‐40/CHI3L1 are higher during tumor progression in mice with pre‐existing allergic inflammation. In vitro treatment of macrophages with rYKL‐40/CHI3L1 increased the expression of MCP‐1/CCL2, IL‐8/CXCL2, and MMP‐9. It was shown that YKL‐40/CHI3L1 is expressed at higher levels by macrophages in the inflamed lung before tumor cell metastasis and that these levels are further increased upon tumor cell colonization in the lungs [31]. Lee et al. [43] showed that YKL‐40/CHI3L1 was induced in macrophages at sites of aeroallergen‐induced pulmonary inflammation and that this inflammation was decreased in YKL‐40/CHI3L1 KO mice. The decrease in inflammation was hypothesized to be a result of exaggerated expression of Fas, accelerated apoptosis of M2 macrophages, and CD4+ T cells in the YKL‐40/CHI3L1 KO mice [43]. With the use of YKL‐40/CHI3L1 KO mice with implanted breast tumor cells, Libreros et al. [31] demonstrated that lack of YKL‐40/CHI3L1 results in decreased inflammatory response, with decreased populations of myeloid‐derived cells/macrophages and decreased metastasis. These studies also showed that the cellular composition was altered during pulmonary inflammation in CHI3L1‐expressing hosts. The infiltrating cells in the allergic lungs of mammary tumor bearers further enhanced the levels of inflammatory mediators. All of these studies suggest that CHI3L1 plays an important role in allergic pulmonary inflammation and that this affects the rate of metastasis to the lungs.
CHRONIC PULMONARY INFLAMMATION AND CANCER
Chronic inflammation associated with COPD has been linked to the pathogenesis of lung cancer [44, 45]. Epidemiologic studies indicate that COPD is one of the most important risk factors for lung cancer among cigarette smokers and precedes lung cancer in most cases. Smokers with COPD were shown to have a 4‐ to 6‐fold increased risk in developing lung cancer [46]. Increased levels of macrophage and neutrophil infiltration are often found in the lungs of cigarette smokers. A more pronounced inflammatory response was observed in patients with COPD compared with smokers without COPD [47]. This response is characterized by epithelial injury, high cell turnover rates, and propagation of DNA errors [45]. Studies indicate that the inflammatory repair and remodeling processes promote epithelial‐mesenchymal transition and lung carcinoma through excessive release of MMPs and growth factors. The inflammatory cellular infiltrates consisting of macrophages, neutrophils, and T lymphocytes are polarized differently in COPD and lung cancer patients [48]. Whereas pulmonary T lymphocytes are polarized toward TH1 phenotype, both M1 and M2 types of macrophages were found in lungs of COPD subjects. TH1 lymphocytes secrete IFN‐γ, eliciting CXCL10 chemokine from CD8+ T cells, which then induce elastase production by macrophages. Neutrophils are also attracted to the lungs by CXCL2/IL‐8, released by macrophages in COPD patients. The inflammatory environment in the lungs of patients with COPD and lung cancer consists of macrophages and neutrophils. Reactive oxygen species released by these cells provide genotoxic stress, resulting in further DNA mutations and thereby, promoting tumor progression.
Recent studies have shown that YKL‐40/CHI3L1 plays a role in inflammatory lung diseases and that the levels of YKL‐40/CHI3L1 predict survival rate [49]. ELISA of plasma samples from patients with moderate‐to‐severe COPD showed high YKL‐40/CHI3L1 expression in severe COPD patients, and this was correlated with increased mortality [49]. In another study, it was demonstrated that YKL‐40/CHI3L1 levels were higher in smokers with COPD than those without COPD [50]. Up‐regulation of YKL‐40/CHI3L1 expression in the lungs of COPD patients is known to contribute to tissue inflammation, airway remodeling, and activation of alveolar macrophages [17]. YKL‐40/CHI3L1 induced the expression of proinflammatory mediators, such as IL‐8/CXCL2, MCP‐1/CCL2, and MMP‐9, as assessed by ELISA, in alveolar macrophages from smokers with COPD [17]. In addition to macrophages, YKL‐40/CHI3L1 is up‐regulated in airway epithelial cells and alveolar type II cells in the lungs of CS‐exposed mice compared with room air‐exposed mice [50]. There was a significant reduction in CS‐induced bronchoalveolar lavage and tissue inflammation in YKL‐40/CHI3L1 KO mice [50]. Significantly, epithelial cell apoptosis and alveolar destruction were enhanced further in the YKL‐40/CHI3L1 KO mice [50]. These findings suggest that YKL‐40/CHI3L1 is involved in preventing apoptosis in lung‐related diseases. Given that the levels of YKL‐40/CHI3L1 are increased in patients with COPD and as we and others have shown that YKL‐40/CHI3L1 plays a critical role in tumor progression, it is possible that YKL‐40/CHI3L1 contributes to COPD‐mediated tumorigenesis.
IBD AND CANCER
It is estimated that <2% of all colorectal cancers (CRCs) are inflammatory bowel disease (IBD) related. The incidence and prevalence of IBD are increasing throughout the world [51]. Furthermore, there is an increased risk of developing CRC in patients with long‐standing ulcerative colitis and Crohn's disease. The increased risk of developing CRC is associated with proinflammatory polyps and progression to advanced neoplasia, whereas a macroscopically normal colon is not associated with neoplastic risk [52, 53]. Inflammation enhances epithelial turnover in the colonic mucosa, resulting in neoplastic transformation. It was demonstrated that inflammation‐associated genes, such as COX‐2, NOS‐2, and IFN‐inducible gene 1‐8U, are up‐regulated in inflamed mucosal areas and in CRC. Studies have also shown that activation of TLR4 enhances COX‐2 expression and that increasing epidermal growth factor receptor signaling promotes CRC development [54]. Inflammation‐associated cytokines, such as IL‐6, IL‐23, and TNF‐α, also contribute to the development and growth of colitis‐associated CRC. These cytokines have been shown to enhance cell proliferation, suppress apoptosis, and thereby, promote tumor development in early stages of colitis‐associated cancer [55]. Inflammatory cells, such as phagocytic leukocytes, which produce pro‐oxidant molecules, are also increased in ulcerative colitis. Such activities pave the path for CRC induction. The activation of transcription factors NF‐κB and STAT‐3 has been linked to cell survival and production of inflammatory cytokines [55, 56]. Tran et al. [57] showed that IL‐6 and YKL‐40/CHI3L1 are required for activation of STAT‐3 in intraepithelial cells, which leads to YKL‐40/CHI3L1‐mediated NF‐κB activation. Furthermore, Chen et al. [39] demonstrated that the NF‐κB pathway is also activated by inflammation‐induced YKL‐40/CHI3L1, resulting in enhanced production of IL‐8 and TNF‐α. Other studies showed that YKL‐40/CHI3L1‐induced secretion of IL‐8/CXCL2 and MCP‐1/CCL2 in SW480 colon cancer cells was mediated through the MAPK signaling pathway [25]. The production of proinflammatory cytokines and chemokines regulated by these different pathways mediates the recruitment of macrophages and enhances angiogenesis, which could promote tumor growth.
The proinflammatory molecule YKL‐40/CHI3L1 was found to be required for the adhesion and invasion of pathogenic bacterial strains, such as Salmonella typhimurium and Escherichia coli, onto colonic epithelial cells [58]. Mizoguchi's laboratory [59] has shown that YKL‐40/CHI3L1 is induced and expressed by colonic epithelial cells and macrophages during intestinal inflammation. YKL‐40/CHI3L1 expression was assessed in ulcerative colitis patients with premalignant or malignant changes. These studies revealed increased YKL‐40/CHI3L1 expression in IBD patients with dysplasias or adenocarcinomas compared with IBD individuals without dysplasia [59]. Stimulation of SW480 cancer cells with YKL‐40/CHI3L1 resulted in the activation of the NF‐κB pathway, leading to the production of proinflammatory cytokines [60]. These cytokines attract leukocytes that are then induced by YKL‐40/CHI3L1 to produce higher levels of proinflammatory cytokines, prolonging the inflammatory response. The infection of wild‐type mice with S. typhimurium showed more severe intestinal inflammation, increased cellular infiltrates, and production of IL‐6 and IL‐8 when compared with YKL‐40/CHI3L1 KO mice infected with this organism [57]. Other chronic bacterial infections associated with cancer are Salmonella typhi infection, which promotes gallbladder cancer [61]. Although the underlying mechanism by which S. typhi infection results in gallbladder infection is poorly understood, it is possible that YKL‐40/CHI3L1 may be promoting inflammation that could contribute to tumorigenesis in the gallbladder. These studies suggest that YKL‐40/CHI3L1 plays an important role in inflammation‐associated neoplastic changes in colonic epithelial cells by promoting cell proliferation, migration, angiogenesis, and cell survival in tumor cells and macrophages [25].
SUMMARY STATEMENT
Inflammation plays a pivotal role in determining the outcome of the tumor. YKL‐40/CHI3L1 is induced during the course of inflammation and contributes to tumor progression. The delineation of the exact mechanisms by which YKL‐40/CHI3L1 affects tumor progression could yield greater insight into how inflammation affects tumors and tumor‐elicited immune responses. YKL‐40/CHI3L1 may have effects on various cells that produce cytokines, chemokines, matrix degrading enzymes, and angiogenic factors that promote tumor progression ( Fig. 1 ). YKL‐40/CHI3L1 may function as a chemoattractant, mitogenic, and antiapoptotic agent in tumor‐bearing hosts. Furthermore, the promotion of pre‐existing inflammation by YKL‐40/CHI3L1 and other mediators may differentially affect tumor outcomes. Further studies may help us to predict the correlation between YKL‐40/CHI3L1‐linked inflammation and the risk for tumor progression.
Figure 1.
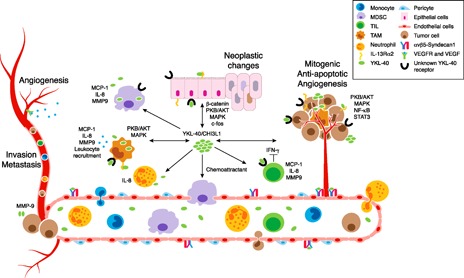
Functional activities of YKL‐40/CHI3L1 in the tumor microenvironment. YKL‐40/CHI3L1, secreted by immune and nonimmune cells in the tumor microenvironment, promotes tumor progression through its downstream effects on multiple cell types, resulting in: neoplastic changes, mitogenesis, antiapoptosis, angiogenesis, proinflammatory molecule production, immunosuppression, leukocyte recruitment, invasion, and metastasis. MDSC, Myeloid‐derived suppressor cell; TIL, tumor‐infiltrating lymphocyte.
AUTHORSHIP
S.L. designed and aided in manuscript preparation. V.I.‐C. designed and prepared the manuscript.
DISCLOSURES
The authors declare no competing financial interests.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health National Cancer Institute Grants 2‐R15‐CA135513 (to V.I.C.) and 3‐R15‐CA135513‐02A1, Research Supplement to Promote Diversity in Health‐Related Research (for S.L.), and private funding by Mr. Jack Laub (Florida Atlantic University, Boca Raton, FL, USA). The authors thank Dr. Patricia Keating for critical reading of the manuscript.
REFERENCES
- 1. Hanahan, D. , Weinberg, R.A. (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- 2. Balkwill, F. , Coussens, L.M. (2004) Cancer: an inflammatory link. Nature 431, 405–406. [DOI] [PubMed] [Google Scholar]
- 3. Perwez Hussain, S. , Harris, C.C. (2007) Inflammation and cancer: an ancient link with novel potentials. Int. J. Cancer 121, 2373–2380. [DOI] [PubMed] [Google Scholar]
- 4. Mantovani, A. , Allavena, P. , Sica, A. , Balkwill, F. (2008) Cancer‐related inflammation. Nature 454, 436–444. [DOI] [PubMed] [Google Scholar]
- 5. Coussens, L.M. , Werb, Z. (2002) Inflammation and cancer. Nature 420, 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mantovani, A. (2009) Cancer: inflaming metastasis. Nature 457, 36–37. [DOI] [PubMed] [Google Scholar]
- 7. Hanahan, D. , Weinberg, R.A. (2000) The hallmarks of cancer. Cell 100, 57–70. [DOI] [PubMed] [Google Scholar]
- 8. Johansen, J.S. , Christensen, I.J. , Riisbro, R. , Greenall, M. , Han, C. , Price, P.A. , Smith, K. , Brünner, N. , Harris, A.L. (2003) High serum YKL‐40 levels in patients with primary breast cancer is related to short recurrence free survival. Breast Cancer Res. Treat. 80, 15–21. [DOI] [PubMed] [Google Scholar]
- 9. Johansen, J.S. , Drivsholm, L. , Price, P.A. , Christensen, I.J. (2004) High serum YKL‐40 level in patients with small cell lung cancer is related to early death. Lung Cancer 46, 333–340. [DOI] [PubMed] [Google Scholar]
- 10. Kzhyshkowska, J. , Mamidi, S. , Gratchev, A. , Kremmer, E. , Schmuttermaier, C. , Krusell, L. , Haus, G. , Utikal, J. , Schledzewski, K. , Scholtze, J. , Goerdt, S. (2006) Novel stabilin‐1 interacting chitinase‐like protein (SI‐CLP) is up‐regulated in alternatively activated macrophages and secreted via lysosomal pathway. Blood 107, 3221–3228. [DOI] [PubMed] [Google Scholar]
- 11. Libreros, S. , Garcia‐Areas, R. , Shibata, Y. , Carrio, R. , Torroella‐Kouri, M. , Iragavarapu‐Charyulu, V. (2012) Induction of proinflammatory mediators by CHI3L1 is reduced by chitin treatment: decreased tumor metastasis in a breast cancer model. Int. J. Cancer 131, 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Owen, J.L. , Iragavarapu‐Charyulu, V. , Gunja‐Smith, Z. , Herbert, L.M. , Grosso, J.F. , Lopez, D.M. (2003) Up‐regulation of matrix metalloproteinase‐9 in T lymphocytes of mammary tumor bearers: role of vascular endothelial growth factor. J. Immunol. 171, 4340–4351. [DOI] [PubMed] [Google Scholar]
- 13. Owen, J.L. , Lopez, D.M. , Grosso, J.F. , Guthrie, K.M. , Herbert, L.M. , Torroella‐Kouri, M. , Iragavarapu‐Charyulu, V. (2005) The expression of CCL2 by T lymphocytes of mammary tumor bearers: role of tumor‐derived factors. Cell. Immunol. 235, 122–135. [DOI] [PubMed] [Google Scholar]
- 14. Stetler‐Stevenson, M. , Mansoor, A. , Lim, M. , Fukushima, P. , Kehrl, J. , Marti, G. , Ptaszynski, K. , Wang, J. , Stetler‐Stevenson, W.G. (1997) Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in reactive and neoplastic lymphoid cells. Blood 89, 1708–1715. [PubMed] [Google Scholar]
- 15. Lugowska, I. , Kowalska, M. , Fuksiewicz, M. , Kotowicz, B. , Mierzejewska, E. , Kosela‐Paterczyk, H. , Szamotulska, K. , and Rutkowski, P. (2015) Serum markers in early‐stage and locally advanced melanoma. Tumour Biol. DOI: 10.1007/s13277‐015‐3564–2. [DOI] [PMC free article] [PubMed]
- 16. Libreros, S. , Garcia‐Areas, R. , Iragavarapu‐Charyulu, V. (2013) CHI3L1 plays a role in cancer through enhanced production of pro‐inflammatory/pro‐tumorigenic and angiogenic factors. Immunol. Res. 57, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Létuvé, S. , Kozhich, A. , Arouche, N. , Grandsaigne, M. , Reed, J. , Dombret, M.C. , Kiener, P.A. , Aubier, M. , Coyle, A.J. , Pretolani, M. (2008) YKL‐40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J. Immunol. 181, 5167–5173. [DOI] [PubMed] [Google Scholar]
- 18. Noy, R. , Pollard, J.W. (2014) Tumor‐associated macrophages: from mechanisms to therapy. Immunity 41, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mantovani, A. , Germano, G. , Marchesi, F. , Locatelli, M. , Biswas, S.K. (2011) Cancer‐promoting tumor‐associated macrophages: new vistas and open questions. Eur. J. Immunol. 41, 2522–2525. [DOI] [PubMed] [Google Scholar]
- 20. Chong, H. , Vodovotz, Y. , Cox, G.W. , Barcellos‐Hoff, M.H. (1999) Immunocytochemical localization of latent transforming growth factor‐beta1 activation by stimulated macrophages. J. Cell. Physiol. 178, 275–283. [DOI] [PubMed] [Google Scholar]
- 21. Ganapathy, V. , Ge, R. , Grazioli, A. , Xie, W. , Banach‐Petrosky, W. , Kang, Y. , Lonning, S. , McPherson, J. , Yingling, J.M. , Biswas, S. , Mundy, G.R. , Reiss, M. (2010) Targeting the transforming growth factor‐beta pathway inhibits human basal‐like breast cancer metastasis. Mol. Cancer 9, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang, H. , Lathia, J.D. , Wu, Q. , Wang, J. , Li, Z. , Heddleston, J.M. , Eyler, C.E. , Elderbroom, J. , Gallagher, J. , Schuschu, J. , MacSwords, J. , Cao, Y. , McLendon, R.E. , Wang, X.F. , Hjelmeland, A.B. , Rich, J.N. (2009) Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth. Stem Cells 27, 2393–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dubinett, S.M. , Lee, J.M. , Sharma, S. , Mulé, J.J. (2010) Chemokines: can effector cells be redirected to the site of the tumor? Cancer J. 16, 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boimel, P.J. , Smirnova, T. , Zhou, Z.N. , Wyckoff, J. , Park, H. , Coniglio, S.J. , Qian, B.Z. , Stanley, E.R. , Cox, D. , Pollard, J.W. , Muller, W.J. , Condeelis, J. , Segall, J.E. (2012) Contribution of CXCL12 secretion to invasion of breast cancer cells. Breast Cancer Res. 14, R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kawada, M. , Seno, H. , Kanda, K. , Nakanishi, Y. , Akitake, R. , Komekado, H. , Kawada, K. , Sakai, Y. , Mizoguchi, E. , Chiba, T. (2012) Chitinase 3‐like 1 promotes macrophage recruitment and angiogenesis in colorectal cancer. Oncogene 31, 3111–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shao, R. (2013) YKL‐40 acts as an angiogenic factor to promote tumor angiogenesis. Front. Physiol. 4, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shao, R. , Hamel, K. , Petersen, L. , Cao, Q.J. , Arenas, R.B. , Bigelow, C. , Bentley, B. , Yan, W. (2009) YKL‐40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene 28, 4456–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Francescone, R. , Ngernyuang, N. , Yan, W. , Bentley, B. , Shao, R. (2014) Tumor‐derived mural‐like cells coordinate with endothelial cells: role of YKL‐40 in mural cell‐mediated angiogenesis. Oncogene 33, 2110–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Libreros, S. , Garcia‐Areas, R. , Keating, P. , Carrio, R. , Iragavarapu‐Charyulu, V.L. (2013) Exploring the role of CHI3L1 in “pre‐metastatic” lungs of mammary tumor‐bearing mice. Front. Physiol. 4, 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shao, R. , Francescone, R. , Ngernyuang, N. , Bentley, B. , Taylor, S.L. , Moral, L. , Yan, W. (2014) Anti‐YKL‐40 antibody and ionizing irradiation synergistically inhibit tumor vascularization and malignancy in glioblastoma. Carcinogenesis 35, 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Libreros, S. , Garcia‐Areas, R. , Keating, P. , Gazaniga, N. , Robinson, P. , Humbles, A. , Iragavarapu‐Charyulu, V.L. (2015) Allergen induced pulmonary inflammation enhances mammary tumor growth and metastasis: role of CHI3L1. J. Leukoc. Biol. 97, 929–940. [DOI] [PubMed] [Google Scholar]
- 32. Ma, B. , Herzog, E.L. , Lee, C.G. , Peng, X. , Lee, C.M. , Chen, X. , Rockwell, S. , Koo, J.S. , Kluger, H. , Herbst, R.S. , Sznol, M. , Elias, J.A. (2015) Role of chitinase 3‐like‐1 and semaphorin 7a in pulmonary melanoma metastasis. Cancer Res. 75, 487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. To, T. , Stanojevic, S. , Moores, G. , Gershon, A.S. , Bateman, E.D. , Cruz, A.A. , Boulet, L.P. (2012) Global asthma prevalence in adults: findings from the cross‐sectional world health survey. BMC Public Health 12, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Robinson, D.S. , Hamid, Q. , Ying, S. , Tsicopoulos, A. , Barkans, J. , Bentley, A.M. , Corrigan, C. , Durham, S.R. , Kay, A.B. (1992) Predominant TH2‐like bronchoalveolar T‐lymphocyte population in atopic asthma. N. Engl. J. Med. 326, 298–304. [DOI] [PubMed] [Google Scholar]
- 35. Zhu, Z. , Homer, R.J. , Wang, Z. , Chen, Q. , Geba, G.P. , Wang, J. , Zhang, Y. , Elias, J.A. (1999) Pulmonary expression of interleukin‐13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin. Invest. 103, 779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu, Z. , Zheng, T. , Homer, R.J. , Kim, Y.K. , Chen, N.Y. , Cohn, L. , Hamid, Q. , Elias, J.A. (2004) Acidic mammalian chitinase in asthmatic Th2 inflammation and IL‐13 pathway activation. Science 304, 1678–1682. [DOI] [PubMed] [Google Scholar]
- 37. Lee, C.G. , Hartl, D. , Lee, G.R. , Koller, B. , Matsuura, H. , Da Silva, C.A. , Sohn, M.H. , Cohn, L. , Homer, R.J. , Kozhich, A.A. , Humbles, A. , Kearley, J. , Coyle, A. , Chupp, G. , Reed, J. , Flavell, R.A. , Elias, J.A. (2009) Role of breast regression protein 39 (BRP‐39)/chitinase 3‐like‐1 in Th2 and IL‐13‐induced tissue responses and apoptosis. J. Exp. Med. 206, 1149–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. He, C.H. , Lee, C.G. , Dela Cruz, C.S. , Lee, C.M. , Zhou, Y. , Ahangari, F. , Ma, B. , Herzog, E.L. , Rosenberg, S.A. , Li, Y. , Nour, A.M. , Parikh, C.R. , Schmidt, I. , Modis, Y. , Cantley, L. , Elias, J.A. (2013) Chitinase 3‐like 1 regulates cellular and tissue responses via IL‐13 receptor α2. Cell Reports 4, 830–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen, C.C. , Llado, V. , Eurich, K. , Tran, H.T. , Mizoguchi, E. (2011) Carbohydrate‐binding motif in chitinase 3‐like 1 (CHI3L1/YKL‐40) specifically activates Akt signaling pathway in colonic epithelial cells. Clin. Immunol. 140, 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krishnamoorthy, S. , Honn, K.V. (2006) Inflammation and disease progression. Cancer Metastasis Rev. 25, 481–491. [DOI] [PubMed] [Google Scholar]
- 41. Taranova, A.G. , Maldonado III, D. , Vachon, C.M. , Jacobsen, E.A. , Abdala‐Valencia, H. , McGarry, M.P. , Ochkur, S.I. , Protheroe, C.A. , Doyle, A. , Grant, C.S. , Cook‐Mills, J. , Birnbaumer, L. , Lee, N.A. , Lee, J.J. (2008) Allergic pulmonary inflammation promotes the recruitment of circulating tumor cells to the lung. Cancer Res. 68, 8582–8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Olkhanud, P.B. , Rochman, Y. , Bodogai, M. , Malchinkhuu, E. , Wejksza, K. , Xu, M. , Gress, R.E. , Hesdorffer, C. , Leonard, W.J. , Biragyn, A. (2011) Thymic stromal lymphopoietin is a key mediator of breast cancer progression. J. Immunol. 186, 5656–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee, C.G. , Dela Cruz, C.S. , Ma, B. , Ahangari, F. , Zhou, Y. , Halaban, R. , Sznol, M. , Elias, J.A. (2012) Chitinase‐like proteins in lung injury, repair, and metastasis. Proc. Am. Thorac. Soc. 9, 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brody, J.S. , Spira, A. (2006) State of the art. Chronic obstructive pulmonary disease, inflammation, and lung cancer. Proc. Am. Thorac. Soc. 3, 535–537. [DOI] [PubMed] [Google Scholar]
- 45. Raviv, S. , Hawkins, K.A. , DeCamp, Jr., M.M. , Kalhan, R. (2011) Lung cancer in chronic obstructive pulmonary disease: enhancing surgical options and outcomes. Am. J. Respir. Crit. Care Med. 183, 1138–1146. [DOI] [PubMed] [Google Scholar]
- 46. Mannino, D.M. , Aguayo, S.M. , Petty, T.L. , Redd, S.C. (2003) Low lung function and incident lung cancer in the United States: data from the First National Health and Nutrition Examination Survey follow‐up. Arch. Intern. Med. 163, 1475–1480. [DOI] [PubMed] [Google Scholar]
- 47. Hogg, J.C. , Chu, F. , Utokaparch, S. , Woods, R. , Elliott, W.M. , Buzatu, L. , Cherniack, R.M. , Rogers, R.M. , Sciurba, F.C. , Coxson, H.O. , Paré, P.D. (2004) The nature of small‐airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 350, 2645–2653. [DOI] [PubMed] [Google Scholar]
- 48. Houghton, A.M. (2013) Mechanistic links between COPD and lung cancer. Nat. Rev. Cancer 13, 233–245. [DOI] [PubMed] [Google Scholar]
- 49. Holmgaard, D.B. , Mygind, L.H. , Titlestad, I.L. , Madsen, H. , Pedersen, S.S. , Johansen, J.S. , Pedersen, C. (2013) Plasma YKL‐40 and all‐cause mortality in patients with chronic obstructive pulmonary disease. BMC Pulm. Med. 13, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Matsuura, H. , Hartl, D. , Kang, M.J. , Dela Cruz, C.S. , Koller, B. , Chupp, G.L. , Homer, R.J. , Zhou, Y. , Cho, W.K. , Elias, J.A. , Lee, C.G. (2011) Role of breast regression protein‐39 in the pathogenesis of cigarette smoke‐induced inflammation and emphysema. Am. J. Respir. Cell Mol. Biol. 44, 777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Molodecky, N.A. , Soon, I.S. , Rabi, D.M. , Ghali, W.A. , Ferris, M. , Chernoff, G. , Benchimol, E.I. , Panaccione, R. , Ghosh, S. , Barkema, H.W. , Kaplan, G.G. (2012) Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54.e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 52. Rutter, M. , Saunders, B. , Wilkinson, K. , Rumbles, S. , Schofield, G. , Kamm, M. , Williams, C. , Price, A. , Talbot, I. , Forbes, A. (2004) Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 126, 451–459. [DOI] [PubMed] [Google Scholar]
- 53. Gupta, R.B. , Harpaz, N. , Itzkowitz, S. , Hossain, S. , Matula, S. , Kornbluth, A. , Bodian, C. , Ullman, T. (2007) Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology 133, 1099–1105, quiz 1340–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fukata, M. , Chen, A. , Vamadevan, A.S. , Cohen, J. , Breglio, K. , Krishnareddy, S. , Hsu, D. , Xu, R. , Harpaz, N. , Dannenberg, A.J. , Subbaramaiah, K. , Cooper, H.S. , Itzkowitz, S.H. , Abreu, M.T. (2007) Toll‐like receptor‐4 promotes the development of colitis‐associated colorectal tumors. Gastroenterology 133, 1869–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Grivennikov, S. , Karin, E. , Terzic, J. , Mucida, D. , Yu, G.Y. , Vallabhapurapu, S. , Scheller, J. , Rose‐John, S. , Cheroutre, H. , Eckmann, L. , Karin, M. (2009) IL‐6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis‐associated cancer. Cancer Cell 15, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sethi, G. , Sung, B. , Aggarwal, B.B. (2008) TNF: a master switch for inflammation to cancer. Front. Biosci. 13, 5094–5107. [DOI] [PubMed] [Google Scholar]
- 57. Tran, H.T. , Lee, I.A. , Low, D. , Kamba, A. , Mizoguchi, A. , Shi, H.N. , Lee, C.G. , Elias, J.A. , Mizoguchi, E. (2014) Chitinase 3‐like 1 synergistically activates IL6‐mediated STAT3 phosphorylation in intestinal epithelial cells in murine models of infectious colitis. Inflamm. Bowel Dis. 20, 835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mizoguchi, E. (2006) Chitinase 3‐like‐1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology 130, 398–411. [DOI] [PubMed] [Google Scholar]
- 59. Chen, C.C. , Pekow, J. , Llado, V. , Kanneganti, M. , Lau, C.W. , Mizoguchi, A. , Mino‐Kenudson, M. , Bissonnette, M. , Mizoguchi, E. (2011) Chitinase 3‐like‐1 expression in colonic epithelial cells as a potentially novel marker for colitis‐associated neoplasia. Am. J. Pathol. 179, 1494–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kawada, M. , Hachiya, Y. , Arihiro, A. , Mizoguchi, E. (2007) Role of mammalian chitinases in inflammatory conditions. Keio J. Med. 56, 21–27. [DOI] [PubMed] [Google Scholar]
- 61. Mager, D.L. (2006) Bacteria and cancer: cause, coincidence or cure? A review. J. Transl. Med. 4, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]


