Short abstract
RhMBL may significantly down modulate human alveolar macrophage inflammatory responses to rhMBL‐opsonized IAV, which may limit lung damage.
Keywords: innate immunity, C‐type lecin, TNF, respiratory burst, apoptosis, biological response modifier
Abstract
IAV pneumonia remains a serious global health problem, and preventative and therapeutic strategies remain limited. AM are critical effector cells in the control of influenza, impairing IAV replication, promoting IAV clearance, and promoting efferocytosis and resolution of lung inflammation. MBL, an innate immune pattern recognition molecule, present in the lungs, binds IAV, and plasma MBL deficiency is associated with increased susceptibility to IAV, although the mechanism remains incompletely understood, and the influence of MBL on the IAV‐AM interaction has not been established. In the current study, focusing on human macrophages (U937 cell line and clinically relevant human AM), data demonstrated that unopsonized IAV is readily internalized, induced release of TNF and ROS, and promoted macrophage apoptosis. In contrast, IAV, opsonized with rhMBL, reduced IAV uptake and macrophage apoptosis and dramatically reduced TNF release and ROS. Macrophage host‐defense responses were reduced further in the presence of MASPs. Taken together, these data support the concept that rhMBL may serve a protective innate host response and a critical biological response modifier function by limiting AM inflammation, oxidative injury, and AM apoptosis, which may allow effective IAV clearance while limiting collateral damage to vital organs, such as the lungs.
Abbreviations
- AM
alveolar macrophage(s)
- BLP
palmitoyl‐3‐cysteine‐serine‐lysine‐4 hydrochloride
- BSS
balanced salt solution
- H2DCFDA
2′,7′‐dichlorodihydrofluorescein diacetate
- H2O2
hydrogen peroxide
- IAV
influenza A virus
- MASP
mannose‐binding lectin‐associated serum proteases
- MBL
mannose‐binding lectin
- MOI
multiplicity of infection
- Phil82
Philippines 82/H3N2
- RCL
recombinant chimeric lectin
- RFU
relative fluorescence unit
- rhMBL
recombinant muman mannose‐binding lectin
- SPD
surfactant protein D
Introduction
Influenza A infection remains a serious and frequent life‐threatening global health problem [1], affecting three to five million persons annually and associated with 250,000–500,000 deaths worldwide [2]. In the United States, seasonal influenza affects up to 20% of the population annually, leading to 200,000 hospitalizations and >30,000 deaths [3]. Moreover, the magnitude of influenza disease on health and society is underscored during pandemics [4]. Current approaches to prophylaxis and treatment remain limited, as antiviral medications have narrow therapeutic windows for administration and are limited as a result of evolving drug resistance [5]. Furthermore, influenza vaccines are not durable, require annual administration, may not be effective in any given year, and may not be available in sufficient quantities or in a timely manner to influence outcomes. Thus, new therapeutic strategies are desperately needed to effectively manage influenza, which in turn, requires improved understanding of basic scientific mechanisms underlying effective host cell responses to influenza challenge.
AM represent critical innate immune host‐defense cells against invading lung pathogens [6], including IAV. In a murine model of influenza pneumonia, AM are critical effector cells in the initial control of influenza, impairing viral replication and promoting influenza clearance [7, 8], and resident AM promote clearance of apoptotic bodies (i.e., efferocytosis) and are critical to the resolution of inflammation [6]. AM depletion results in slowed clearance of influenza and impaired IgG and CD8+ T‐lymphocyte responses in the murine model [7, 8], and AM depletion results in high mortality in a swine model of nonlethal influenza pneumonia [9]. Studies with human macrophages reveal that IAV promotes TNF release by human monocyte‐derived macrophages [10], IAV induces proinflammatory AM genes [11], and seasonal IAV strains do not replicate significantly within AM [12]. Thus, AM play a central role in limiting morbidity and mortality during influenza A pneumonia, but influenza studies with human AM (the most abundant innate immune cells in the alveolar airspace) remain limited and incomplete.
MBL is a member of the C‐type lectin family of innate immune pattern recognition molecules [13]. MBL is an acute‐phase reactant and in part, serves as an opsonin, where carbohydrate recognition domains on multimers of trimeric MBL molecules bind distinct patterns of carbohydrates expressed on pathogen surfaces [13]. Plasma MBL deficiency is associated with increased risk of infection [14]. MBL binds [15] and neutralizes [16] IAV and reduces IAV infection of respiratory epithelial cells, and plasma deficiency increases viral lung burden in an IAV pneumonia model [17]. MBL is present in the alveolar lining fluid of humans [18], but the influence of MBL on the influenza A interaction with human AM has not been fully investigated.
The purpose of this study is to better define the host‐defense responses of human macrophages to seasonal IAV, with particular focus on clinically relevant human AM, and to determine the influence of MBL opsonization on modifying human macrophage host‐defense responses to influenza. Focusing on native and novel MBL molecules, including rhMBL and rhMBL‐ficolin chimeric molecules [19], these data showed that human lectin opsonization modestly reduced macrophage influenza A binding, internalization, and macrophage apoptosis but dramatically and significantly reduced macrophage TNF release and oxidative burst responses, especially in the presence of MASP. These data demonstrated that human MBL molecules serve an immunomodulatory role for human macrophage innate responses that may limit tissue damage and reduce pathogenesis of influenza A pneumonia while allowing for an effective host response.
MATERIALS AND METHODS
Human macrophages
Human macrophage cell lines.
As a model for studying the influence of influenza infection on human macrophage function, experiments used human promonocyte U937 cell lines [20] (American Type Culture Collection, Manassas, VA, USA). Human U937 cells were cultured in complete RPMI‐1640 medium (10% heat‐inactivated FCS, 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin). Cells in suspension were harvested during exponential growth phase, washed, and then differentiated into macrophage phenotypes using PMA (100 nM) at 37°C in 5% CO2 for 24 h, washed three times with PBS, and incubated an additional 24 h before use in experiments. Differentiation of human promonocytic U937 cells promotes adherence and acquisition of macrophage phenotype [21, 22].
Human AM.
Human AM were used to confirm critical results observed in cell lines. Prospectively recruited healthy volunteers, 18–55 years old, had no evidence of active pulmonary disease, had never smoked, and had normal spirometry. Lung immune cells were obtained by BAL using a standard technique [23]. All procedures were performed on adult volunteers after informed consent, following protocols approved by the Beth Israel Deaconess Medical Center Institutional Review Board. The cells were separated from the pooled BAL fluid, and AM were isolated by adherence for ≥72 h to plastic‐bottom, tissue‐culture plates as described previously [23]. Isolation of AM from all healthy persons yielded cells that were ≥98% viable, as determined by trypan blue dye exclusion and demonstrated >95% positive nonspecific esterase.
Influenza virus preparation
Seasonal IAV, Phil82 strain (unlabeled or FITC‐labeled), was grown in the chorioallantoic fluid of 10‐day‐old chicken eggs and purified on a discontinuous sucrose gradient, as described previously [24]. The virus was dialyzed against PBS to remove sucrose, aliquoted, and stored at −80°C until needed. The HA titer of each virus preparation was determined by titration of virus samples in PBS with thoroughly washed human type O, Rh(−) red blood cells, as described [24]. For opsonization studies, gently sonicated IAV was incubated in cold BSS++ (containing calcium and magnesium) with rhMBL (4.4 μg/mL; represents physiologic level and determined by dose‐response curve) or chimeric molecules (10 μg/mL; as used in prior experiments) [25] in final volume of 50 μl at 37°C for 30 min in a shaking incubator at 2000 rpm. Brief sonication (50% duty cycle in water‐bath sonicator) disperses IAV clumps, and IAV remains infectious as published previously [19].
Human MBL molecules
As models for human MBLs, the study used rhMBL, in addition to rhMBL‐ficolin chimeric molecules, which may have comparable activity with native rhMBL and are easier to mass produce [19, 25]. rhMBL was produced as described previously and provided by Enzon Pharmaceuticals (Bridgewater, NJ, USA) [25]. rhMBL‐ficolin chimeric lectins were produced as described previously [25]. The RCLs contained the complete MBL carbohydrate recognition domain, whereas the MBL collagenous domain was replaced with variable lengths of the ficolin collagenous domain, with RCL1 containing 126 aa, RCL2 containing 76 aa, and RCL3 containing 64 aa of the ficolin collagenous domain [25]. Each molecule contained a putative MASP‐binding domain. Experiments focused on human MBL concentrations used in prior publications [17, 19].
Mouse sera
Mouse sera from MBL null mice were used as a source for MASP. Murine MASP exhibits ∼80% homology with human MASP. MBL null mice were generated previously and fully backcrossed onto C57Black/6J [26, 27]. Sera were collected and stored at −80°C prior to the study. All animal experiments were performed under a protocol approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital (Boston, MA, USA).
Human macrophage binding and internalization of IAV
Macrophage internalization of IAV was determined by flow cytometer (Cytomics FC 500; Beckman Coulter, Brea, CA, USA), as described previously [28]. The instrument was calibrated before each measurement with standardized fluorescent particles. Opsonization of FITC‐labeled IAV at a MOI of 20:1 (AM cells) or 5:1 (U937 cells) was performed as described above, and 40 μl of each sample was then added to 500,000 macrophages in suspension and diluted to 0.5 mL. A higher IAV MOI was required for AM to improve signal:noise ratio, as a result of intrinsic AM autofluorescence. This mixture was then wrapped in aluminum foil and incubated at 37°C for 45 min in a shaking incubator at 2000 rpm and then analyzed by flow cytometry, followed by trypan blue (1% final concentration) quenching and immediate repeat flow cytometry analysis. Human macrophages were identified by the characteristic forward‐ and side‐scatter parameters, characteristic autofluoresence on unstained cells, and surface expression of HLA‐DR or CD68, distinguishing macrophages from neutrophils and lymphocytes. For select experiments, macrophage viability was measured by PI uptake [29]. Data were expressed as the percentage of cells staining positive. Samples were prepared and analyzed in duplicate, and a minimum of 5000 cells was counted for each sample.
Human macrophage cytokine release
TNF levels were measured in cultured supernatants using a specific ELISA (R&D Systems, Minneapolis, MN, USA), as described previously [30]. Adherent macrophages (24‐well plate, 5×105 cells/well) were incubated with IAV (MOI 20:1) in the presence or absence of rhMBL (4.4 μg/mL) or RCL2 or RCL3 (10 μg/mL) for 24 h [19] and cell‐free supernatants removed and stored at −80°C until assayed as described [30]. Lipid A (TLR4 ligand) from the Escherichia coli F583 (Rd mutant) (Sigma Chemical, St. Louis, MO, USA) and BLP (TLR2 ligand; Calbiochem, San Diego, CA, USA) were used as positive controls.
Human macrophage oxidative burst assay
Macrophage oxidative burst (measured as the production of H2O2) was performed by a fluorescence microplate assay, as described previously [31]. Adherent macrophages were incubated with H2DCFDA (10 uM final) and IAV (MOI 5:1) at 37°C in 5% CO2 in BSS++, in the presence and absence of rhMBL (4.4 μg/mL) and RCL2 or RCL3 (10 μg/mL), and fluorescence measurements were obtained at 20‐min intervals at an excitation wavelength of 485 nm and emission wavelength of 530 nm (CytoFluor multiplate reader series 4000; PerSeptive Biosystems, Framingham, MA, USA). Measurements were expressed as RFU. Background macrophage autofluorescence and spontaneous conversion of H2DCFDA by unstimulated macrophages were subtracted from experimental measurements at each time‐point. PMA (10 uM final; Sigma Chemical) and serum‐opsonized zymosan particles (Molecular Probes, Eugene, OR, USA) were used as positive controls.
Human macrophage apoptosis
Macrophage apoptosis was measured on cell lysates using an antigen‐capture ELISA for histone and fragmented DNA (Cell Death Detection ELISA Plus; Roche Applied Science, Indianapolis, IN, USA), according to the manufacturerˈs protocol. Adherent macrophages in 96‐well microtiter trays (4×104 cells/well) were incubated with IAV (MOI 5:1) in the presence or absence of rhMBL (4.4 μg/mL) or RCL2 or RCL3 (10 μg/mL) for 24 h. Staurosporine (PKC inhibitor; 5 uM) was used as a positive control to induce apoptosis.
Statistical analysis
All data were expressed as sem and analyzed using a nonparametric Mann‐Whitney U‐test, and a P < 0.05 was considered statistically significant. Experimental conditions were performed in duplicate or triplicate, and each experiment was performed a minimum of three times unless noted otherwise.
RESULTS
Human MBLs reduce influenza A binding and uptake by human macrophages
Internalization or ingestion of IAVs represents an important aspect of macrophage host defense against influenza, and AM are known to ingest IAV [32]. In the current study, human macrophages readily bound and internalized IAVs in vitro ( Fig. 1A ). Trypan blue quenching of extracellular FITC‐IAV identified the internalized virus ( Fig. 1B ). Human macrophage binding and internalization of influenza were MOI‐dependent ( Fig. 1C ), and the majority of macrophage‐associated IAVs was internalized ( Fig. 1C ). Internalization was dependent on endocytosis, as pretreatment of macrophages with cytochalasin b (to inhibit actin‐dependent phagocytosis) did not influence influenza A internalization by macrophages (data not shown). Human macrophage cell viability was 83–96% and was not influenced by IAV (data not shown). Opsonization of IAV with human lectins—rMBL or rMBL‐ficolin chimeric molecules—reduced cell association of influenza A with macrophages by 20.7% (P<0.0001) and 16.2% (P<0.005), respectively ( Fig. 2A ). Most of the reduction in cell‐associated virions was attributable to reduction in internalized particles, as rhMBL reduced IAV internalization by 47.9% (P<0.005), and rhMBL‐ficolin reduced IAV internalization by 41.1% (P<0.005). Macrophage viability was not influenced by opsonized IAV or by human lectins alone (data not shown).
Figure 1.
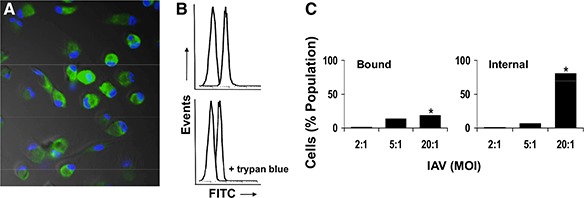
Influenza A binding and internalization by human macrophages. (A) Representative fluorescence microscopic field of adherent human AM (nuclear profile‐stained DAPI, blue) following incubation with FITC‐labeled IAV (green). Most macrophages demonstrate association of FITC‐IAV, representing bound and internalized IAV virions. Original magnification, ×200. (B and C) Quantitative analysis of IAV binding and internalization by human macrophages. (B) Flow cytometry histograms demonstrating cell‐associated FITC‐IAV (upper; representing surface‐bound and internalized virions) and following quenching of extracellular fluorescence with trypan blue (lower; representing internalized virions). For each upper and lower panel, the left histogram represents macrophage autofluoresence (in the absence of FITC‐IAV), and the right histogram represents macrophages incubated with FITC‐IAV. (C) Human AM demonstrate MOI dependence of FITC‐IAV binding and internalization. For all conditions, macrophages were incubated with FITC‐IAV for 45 min at 37°C. To normalize for biological variability, data are expressed as percent change compared with unopsonized IAV condition. *P < 0.005.
Figure 2.
Human MBLs reduce influenza A binding and uptake by human macrophages. Human U937 macrophages (A) or human AM (B) were incubated with unopsonized or MBL‐opsonized FITC‐IAV (rhMBL or MBL‐ficolin chimera RCL2), and total IAV (bound plus internalized) and internalized IAV were determined by flow cytometry. For all conditions, macrophages were incubated with FITC‐IAV for 45 min at 37°C. To normalize for biological variability, data are expressed as percent change compared with unopsonized IAV condition. *P < 0.005. Figure from representative experiments for U937 macrophage (n=10) and human AM (n=4), each with similar results.
As validation for the results with human macrophage U937 cell lines, similar patterns of IAV association and internalization were observed in clinically relevant primary human AM, where rhMBL reduced IAV internalization by 43.8% (P<0.005), and rhMBL‐ficolin reduced IAV internalization by 52.43% (P<0.005; Fig. 2B ). For the three chimeric molecules examined—RCL1, RCL2, and RCL3—all reduced internalization, and RCL2 consistently exhibited the greatest effect (data not shown) and was used predominantly throughout the remainder of the study. Thus, human MBLs reduced IAV association with human macrophages, although the reduction was modest, and the reduction in cell‐associated IAV particles was similar with rhMBL or rhMBL‐ficolin chimeric molecules.
Human MBLs reduce macrophage TNF release in response to influenza A
TNF represents an important component of an effective host response to influenza A [33]. In the current study, constitutive release of TNF‐α by unstimulated (untreated) human U937 macrophages was minimal (data not shown), whereas incubation with unopsonized IAV (MOI 20:1), lipid A (TLR4 ligand), and BLP (TLR2 ligand) all induced robust TNF‐α release that was independent of actin‐polymerization (data not shown). In marked contrast, incubation of human macrophages with IAV, opsonized with rhMBL or MBL‐ficolin chimeric molecules, resulted in a dramatic and significant reduction by 85.9% and 67.4% (P<0.005), respectively, in TNF‐α release compared with unopsonized IAV ( Fig. 3 ). A similar response pattern was observed for clinically relevant human AM, with low constitutive TNF‐α release in unstimulated (untreated) macrophages (data not shown), robust TNF‐α release to unopsonized IAV or lipid A (data not shown), and a marked reduction in macrophage TNF‐α release in response to lectin‐opsonized IAV ( Fig. 3 ). Neither rhMBL nor MBL‐ficolin chimeric molecules alone induced macrophage TNF‐α release, and neither influenced lipid A‐mediated TNF‐α release (data not shown). The reduction in TNF‐α release as a result of viral opsonization was consistently more pronounced to rhMBL than to the MBL‐ficolin chimeric molecule for human U937 macrophages and human AM. Thus, human MBL opsonization suppressed dramatically and significantly human macrophage TNF‐α release in response to IAV virions.
Figure 3.
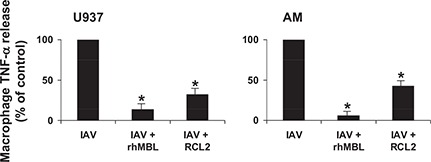
Human MBLs reduce macrophage TNF release in response to influenza A. Adherent, differentiated human U937 macrophages or human AM were incubated with unopsonized or MBL‐opsonized IAV at 37°C for 24 h, and cell‐free‐cultured supernatants were harvested and assayed for TNF‐α release by ELISA (conditions performed in triplicate). To normalize for biological variability, data were expressed as percent change from unopsonized IAV condition. *P < 0.005. Figure depicts representative experiments performed for U937 macrophages (n=4) and human AM (n=3) with similar results.
Human MBLs reduce macrophage oxidative burst response to influenza A
Phagocyte respiratory burst (or oxidative burst) response represents an important component of an effective host‐defense response to infectious challenge [34]. In the current study, spontaneous constitutive release of H2O2 ROS (as a measure of oxidative burst) by unstimulated (untreated) human U937 macrophages was low but increased gradually over 2 h (data not shown). Incubation of human macrophages with unopsonized IAV promoted an increase in H2O2 production above baseline (Table 1). However, in marked contrast, incubation of human U937 macrophages with rhMBL‐opsonized IAV significantly reduced H2O2 production to background levels, whereas incubation with rhMBL‐ficolin‐opsonized IAV dramatically reduced H2O2 production, suppressing oxidative burst response below constitutive H2O2 release. This was not influenced by cytochalasin b (data not shown). Importantly, incubating human macrophages with rhMBL or rhMBL‐ficolin chimeric molecules alone (in the absence of IAV virions) markedly reduced H2O2 release (Table 1), and rhMBL reduced zymosan‐induced H2O2 release (data not shown). A similar pattern of responses was observed using clinically relevant human AM. Thus, human MBL suppressed dramatically and significantly the human macrophage oxidative burst response, alone or following opsonization of IAV virions.
Table 1.
Human MBLs Reduce Macrophage Oxidative Burst Response to Influenza A
| Time (t; min) | t = 0 | t = 20 | t = 40 | t = 60 | t = 80 | t = 100 | t = 120 |
|---|---|---|---|---|---|---|---|
| Human macrophages (U937) | |||||||
| IAV | 212.5 | 309.0 | 409.5 | 445.0 | 457.5 | 495.0 | 610.0 |
| IAV + rhMBL | 135.5 | 130.5 | 196.5 | 185.0 | 163.5 a | 166.5 a | 173.5 a |
| IAV + RCL2 | −24.5 | −148.0 a | −228.0 a | −393.0 a | −510.0 a | −644.5 a | −768.5 a |
| rhMBL | 16.0 | −92.5 a | −76.0 a | −120.0 a | −115.0 a | −217.5 a | −182.5 a |
| RCL2 | 123.5 | 45.0 a | −7.0 a | −176.0 a | −221.0 a | −418.0 a | −526.5 a |
| Human AM | |||||||
| IAV | 69.0 | −4.0 | 22.0 | 258.0 | 452.0 | 563.0 | 866.0 |
| IAV + rhMBL | 77.0 | 48.0 | −1.0 | 22.0 | 7.0 a | −14.0 a | −6.0 a |
| IAV + RCL2 | 60.3 | −40.0 | −238.0 a | −392.7 a | −468.3 a | −605.7 a | −678.7 a |
| rhMBL | 50.0 | −42.0 | −92.0 | −108.0 a | −121.0 a | −281.0 a | −307.0 a |
| RCL2 | 11.3 | −172.7 | −417.7 a | −654.7 a | −833.0 a | −1012.7 a | −1161.3 a |
Values expressed as mean RFU. Negative RFU values represent H2O2 release below constitutive (spontaneous) macrophage release.
P < 0.005 compared with IAV condition at corresponding time‐point.
Human MBLs reduce macrophage apoptosis in response to influenza A
Macrophage apoptosis represents an important host‐defense response to infection [35]. Increased macrophage apoptosis is associated with increased susceptibility to IAV infection in MBL‐deficient mice [17]. In the current study, unopsonized IAV (MOI 5:1) induced apoptosis in human U937 macrophages above background (data not shown). In contrast, incubation with rhMBL‐opsonized IAV significantly reduced macrophage apoptosis by 20.2% (P<0.005; Fig. 4 ), whereas incubation with rhMBL‐ficolin chimeric molecules did not affect macrophage apoptosis significantly. In contrast, rhMBL and rhMBL‐ficolin‐opsonized IAV reduced apoptosis in human AM by 42.5% (P<0.005; Fig. 4 ). rhMBL or rhMBL‐ficolin molecules alone did not induce macrophage apoptosis and did not influence staurosporine‐mediated apoptosis (data not shown). Thus, human MBL significantly reduced macrophage apoptosis in response to IAV virions.
Figure 4.
Human MBLs reduce macrophage apoptosis in response to influenza A. Adherent, differentiated human U937 macrophages or human AM were incubated with unopsonized or MBL‐opsonized IAV at 37°C for 24 h, and cell‐free‐cultured supernatants were harvested and assayed by Cell Death Detection ELISA Plus (conditions performed in triplicate). To normalize for biological variability, data were expressed as percent change from unopsonized IAV condition. *P < 0.005. Figure depicts representative experiments performed for U937 macrophages (n=3) and human AM (n=3) with similar results.
MASP promotes human MBL‐mediated dampening of macrophage host‐defense responses
MASPs activate and promote MBL binding [13]. However, whether MASP influences the macrophage host‐defense responses to lectin‐opsonized IAV virions has not been established. In the current study, MASP alone did not induce macrophage TNF release, oxidative burst response, or apoptosis (data not shown) nor did macrophage pretreatment with MASP, ×24 h, influence macrophage uptake of the virus, TNF release, or oxidative burst in response to unopsonized IAV (data not shown), and MASP did not modulate lipid A‐mediated TNF release (data not shown). However, the addition of a MASP source to experimental conditions consistently augmented the effect of IAV opsonization with rhMBL or rhMBL‐ficolin chimeric molecules. MASP further, consistently reduced human macrophage uptake of IAV ( Fig. 5A ), TNF release ( Fig. 5B ), and oxidative burst responses (data not shown). Thus, in addition to effects on lectin‐mediated activation of the coagulation cascade [36, 37], MASP may influence human macrophage host‐defense responses to particles opsonized with human MBL.
Figure 5.
MASP promotes human MBL‐mediated dampening of macrophage host‐defense response. (A) Human AM were incubated with unopsonized or MBL‐opsonized IAV (MOI 20:1) in the presence or absence or MASP at 37°C for 24 h, and then IAV internalization was determined by flow cytometry. (B) Adherent human U937 macrophages were incubated with unopsonized or MBL‐opsonized IAV (MOI 5:1) in the presence or absence of MASP at 37°C for 24 h, and cell‐free‐cultured supernatants were harvested and assayed for TNF‐α release by ELISA. Experiments were performed in duplicate (n=3). To normalize for biological variability, data were expressed as percent change from unopsonized IAV condition. *P < 0.005. Figure depicts representative experiments performed for human AM (n=3) and human U937 macrophages (n=3) with similar results.
DISCUSSION
These data demonstrate that IAV induces critical human AM innate responses, and human MBL opsonization of IAV dampens these responses. Unopsonized IAV is readily bound to and internalized by human macrophages in vitro and in turn, induces a robust release of TNF and of ROS and promotes macrophage apoptosis. In contrast, IAV opsonized with human MBLs reduces IAV uptake and human macrophage apoptosis but reduces dramatically and significantly TNF and ROS. Furthermore, the human lectin‐mediated reduction in internalization, TNF release, and oxidative burst responses are augmented in the presence of MASP. Taken together, these findings demonstrate that human MBLs down‐modulate macrophage innate responses to IAV.
These data are the first to investigate the influence of MBL opsonization on the IAV interaction with human AM. Prior studies with human neutrophils demonstrate that rhMBL opsonization mildly enhances IAV binding but does not significantly promote internalization or oxidative burst responses, whereas IAV opsonization with MBL‐SPD chimeric molecules greatly enhances neutrophil binding, uptake, and respiratory burst [38]. In contrast, in the current study focusing on human macrophages, human MBL reduced IAV‐mediated binding and uptake and suppressed TNF release, oxidative burst responses, and macrophage apoptosis with comparable responses, comparing rhMBL with MBL‐ficolin chimeric molecules. The observations that rhMBL and chimeric molecules exhibit similar responses by human macrophages but different influences on human neutrophils suggest that the influence of MBL opsonization of IAV may be cell‐specific and dependent, in part, on the nature of the MBL chimeric collagenous domain [19].
MBLs represent important pattern recognition molecules in the innate immune response to IAV. MBL binds [15] and neutralizes IAV [16] and reduces infection of respiratory epithelial cells, and plasma MBL deficiency increases viral lung burden in an IAV pneumonia model [17]. Prior investigations demonstrate that human MBL and chimeric MBL‐SPD effectively inactivate IAV [38]. Furthermore, rhMBL and rhMBL‐ficolin chimeric proteins bind IAV, activate the lectin pathway, and promote IAV aggregation and inhibition of viral HA [19]. The current study extends these observations to demonstrate that rhMBL molecule opsonization can modulate the function of AM in response to IAV, in particular, by down‐modulating important host‐defense functions, including internalization and apoptosis and especially proinflammatory cytokine release and oxidative burst response. Taken together, these data suggest that the mechanism for MBL anti‐IAV function may include direct influence on the virus, such as promoting IAV aggregation, reduction of viral HA, and inhibition of virus‐mediated HA and neuraminidase activities, in addition to direct influence on immune cells, such as macrophages.
Recognizing that MBL‐deficient mice are susceptible to IAV pneumonia [17], data from the current study provide important mechanistic insights and support an immunomodulatory role of MBL opsonization to dampen human macrophage innate immune responses to IAV. The current study demonstrated robust IAV binding and uptake by human macrophages, consistent with prior investigations [32]. AM internalization with seasonal IAV is generally not productive [12] and promotes viral elimination, although this was not determined in the current study. The unexpected finding that MBL opsonization reduces IAV internalization may, in part, reflect agglutination of IAV virions [19] and limited ability to internalize large viral aggregates. The modest reduction in IAV internalization by AM may be offset by direct IAV neutralization [16], although this was not specifically investigated.
TNF‐α release and oxidative burst responses are considered important components of an effective innate immune response to IAV. However, with the recognition that TNF release does not promote IAV clearance and may contribute to lung injury and disease pathogenesis [10], the observed marked, significant reduction in macrophage TNF release to MBL‐opsonized IAV may thus be protective to the host. Furthermore, phagocyte oxidative burst responses may contribute to disease pathogenesis and lung inflammation in the murine IAV pneumonia model, while a reduced oxidative burst response may be protective [39]. Thus, in the current study, the observed marked reduction in macrophage respiratory burst response to MBL‐opsonized IAV may be protective to the host. Taken together, the reduced macrophage TNF release and oxidative burst response to IAV may represent a regulatory mechanism to allow effective clearance of IAV, while limiting collateral damage. Recognizing that complete inhibition of these critical host responses to IAV may be detrimental to the host, a balanced host response would be optimal, but the level of response remains to be established.
Plasma MBL deficiency is associated with increased macrophage apoptosis [17] and susceptibility to IAV infection [15], suggesting that macrophage apoptosis contributes to disease pathogenesis. As resident AM promote clearance of apoptotic bodies (i.e., efferocytosis) during the resolution phase of influenza infection [40] and recognizing that seasonal IAV does not replicate in AM, the observed reduction in macrophage apoptosis in the presence of MBL‐opsonized IAV may thus serve a protective function. A consequence of reduced resident macrophage apoptosis may preserve the number of resident lung macrophages available to limit viral replication and to promote resolution of the inflammatory response [40]. Collectively, these data support the concept that the MBL‐mediated down‐modulation of human macrophage TNF release, oxidative burst release, and reduced apoptosis may represent mechanisms to promote IAV clearance through efferocytosis, reduce local tissue inflammation and oxidative injury, and limit collateral damage to the lungs.
The mechanism for macrophage recognition of MBL‐opsonized IAV was not established in the current study. MBL binds distinct patterns of pathogen surface molecules through MBL carbohydrate recognition domains, and the MBL‐opsonized pathogen may be taken up by macrophage collectin receptors [41]. Although the macrophage collectin receptor has not been identified, candidate receptors include mannose receptor [42], macrophage galactose‐type lectin [43], or calreticulin [44]. Data from the current study demonstrated that rhMBL opsonization of IAV down‐modulates macrophage host‐defense responses, especially proinflammatory cytokine release and generation of ROS. The influence of rhMBL opsonization of IAV on modifying macrophage innate responses may, in part, reflect specific or distinct pathways activated by rhMBL interaction through specific macrophage collectin receptors. However, whether rhMBL binds to one or more of these candidate or other collectin receptors, perhaps in combination with other innate receptors, such as TLR2 [45], remains to be determined.
The current study demonstrated that MBL opsonization dampens critical human macrophage host‐defense responses to IAV, including binding and internalization, TNF release, oxidative burst response, and apoptosis. However, the release of other macrophage cytokines or chemokines, release of reactive nitrogen species, or other macrophage host‐defense pathways, such as autophagy, were not specifically examined. The current study focused on the IAV Phil82 strain, and whether these findings apply to other IAV isolates or other viruses was not determined. The observation that human U937 macrophages exhibited similar responses to MBL‐opsonized IAV as primary human AM supports the use of differentiated U937 cell lines as an in vitro model for human macrophage investigations with influenza. However, the influence of MBL on certain macrophage responses (such as apoptosis) may be cell‐specific. Finally, the results from the current in vitro study may not accurately reflect the influence of MBL in vivo; however, the use of clinically relevant human AM may have more direct translation to human disease. Furthermore, the findings that neither rhMBL nor rhMBL chimeric molecules promoted human macrophage inflammation are consistent with clinical trial results examining rhMBL administration to persons with MBL deficiency [46].
In conclusion, these data demonstrate that IAV opsonization by human MBLs, a component of the human alveolar‐lining material, results in down‐modulation of macrophage innate immune responses. Recognizing that plasma MBL deficiency is associated with increased morbidity and mortality in an IAV pneumonia model [17] and that this may be, in part, attributable to exuberant host responses of inflammation, ROS, and ineffective clearance, data from the current study support the concept that MBL serves as a biological response modifier of the AM host responses to IAV. MBL‐mediated down‐modulation of macrophage responses may, in turn, limit the collateral damage associated with the innate immune response to IAV challenge by reducing but not eliminating effective host responses. With the recognition that exogenous rhMBL administration facilitates IAV clearance in a MBL‐deficient mouse model of IAV pneumonia [17] and that exogenous rhMBL can be administered safely to humans with MBL deficiency [46], exogenous administration of rhMBL may represent a viable adjunctive therapeutic strategy. However, additional studies would be required to evaluate specifically the potential role for rhMBL in the treatment of IAV pneumonia.
AUTHORSHIP
B.N. is the first author who designed and performed experiments, analyzed data, and wrote the manuscript. X.Z. performed experiments and analyzed data. M.W. and K.H. designed experiments, prepared influenza virus, and analyzed data. K.T. designed experiments, prepared MBLs, and analyzed data. T.B.K. designed experiments and analyzed data. A.A. designed experiments, analyzed data, performed bronchoscopy, and wrote the manuscript. H.K. is the senior author who developed the hypothesis, designed experiments, analyzed data, and wrote the manuscript.
DISCLOSURES
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
Grant Support for this paper was from the Cystic Fibrosis Foundation (HL 063655 and AI 074503).
We are grateful to all persons who volunteered for research bronchoscopy. We thank Elizabeth Vassar‐Sternburg, Kristin Linnell, Ann Hougland, Xiomarra Guerra, Johanna Leary, Cynthia Peguero, Jose Munguia, and the entire Beth Israel Deaconess Medical Center West Procedure Center staff for excellent technical assistance with research bronchoscopy. These data were presented, in part, at the American Thoracic Society 2011 International Conference in Denver, CO, USA.
Footnotes
SEE CORRESPONDING EDITORIAL ON PAGE 702
REFERENCES
- 1. Dolin, R. (2005) Influenza—interpandemic as well as pandemic disease. N. Engl. J. Med. 353, 2535–2537. [DOI] [PubMed] [Google Scholar]
- 2. Anonymous . (2003) Influenza. World Health Organization Fact Sheet. Geneva, Switzerland, No. 211, from http://www.who.int/mediacentre/fact-sheets/2003/fs211/en/.
- 3. Thompson, W. W. , Comanor, L. , Shay, D. K. (2006) Epidemiology of seasonal influenza: use of surveillance data and statistical models to estimate the burden of disease. J. Infect. Dis. 194 (Suppl. 2), S82–S91. [DOI] [PubMed] [Google Scholar]
- 4. Belshe, R. B. (2005) The origins of pandemic influenza—lessons from the 1918 virus. N. Engl. J. Med. 353, 2209–2211. [DOI] [PubMed] [Google Scholar]
- 5. Moscona, A. (2005) Neuraminidase inhibitors for influenza. N. Engl. J. Med. 353, 1363–1373. [DOI] [PubMed] [Google Scholar]
- 6. Zhang, J. , Koziel, H. (2001) Alveolar macrophages. In Cellular Aspects of HIV Infection (Cossarizza A., Kaplan D., eds.), Wiley‐Liss, New York, NY, USA, 207–227. [Google Scholar]
- 7. Wells, M. A. , Albrecht, P. , Daniel, S. , Ennis, F. A. (1978) Host defense mechanisms against influenza virus: interaction of influenza virus with murine macrophages in vitro. Infect. Immun. 22, 758–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodgers, B. , Mims, C. A. (1981) Interaction of influenza virus with mouse macrophages. Infect. Immun. 31, 751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim, H. M. , Lee, Y. W. , Lee, K. J. , Kim, H. S. , Cho, S. W. , van Rooijen, N. , Guan, Y. , Seo, S. H. (2008) Alveolar macrophages are indispensable for controlling influenza viruses in lungs of pigs. J. Virol. 82, 4265–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cerami, A. , Beutler, B. (1988) The role of cachectin/TNF in endotoxic shock and cachexia. Immunol. Today 9, 28–31. [DOI] [PubMed] [Google Scholar]
- 11. Wang, J. , Nikrad, M. P. , Travanty, E. A. , Zhou, B. , Phang, T. , Gao, B. , Alford, T. , Ito, Y. , Nahreini, P. , Hartshorn, K. , Wentworth, D. , Dinarello, C. A. , Mason, R. J. (2012) Innate immune response of human alveolar macrophages during influenza A infection. PLoS One 7, e29879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Short, K. R. , Brooks, A. G. , Reading, P. C. , Londrigan, S. L. (2012) The fate of influenza A virus after infection of human macrophages and dendritic cells. J. Gen. Virol. 93, 2315–2325. [DOI] [PubMed] [Google Scholar]
- 13. Takahashi, K. , Ip, W. E. , Michelow, I. C. , Ezekowitz, R. A. (2006) The mannose‐binding lectin: a prototypic pattern recognition molecule. Curr. Opin. Immunol. 18, 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peterslund, N. A. , Koch, C. , Jensenius, J. C. , Thiel, S. (2001) Association between deficiency of mannose‐binding lectin and severe infections after chemotherapy. Lancet 358, 637–638. [DOI] [PubMed] [Google Scholar]
- 15. Hartshorn, K. L. , Sastry, K. , White, M. R. , Anders, E. M. , Super, M. , Ezekowitz, R. A. , Tauber, A. I. (1993) Human mannose‐binding protein functions as an opsonin for influenza A viruses. J. Clin. Invest. 91, 1414–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reading, P. C. , Morey, L. S. , Crouch, E. C. , Anders, E. M. (1997) Collectin‐mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J. Virol. 71, 8204–8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chang, W. C. , White, M. R. , Moyo, P. , McClear, S. , Thiel, S. , Hartshorn, K. L. , Takahashi, K. (2010) Lack of the pattern recognition molecule mannose‐binding lectin increases susceptibility to influenza A virus infection. BMC Immunol. 11, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hodge, S. , Hodge, G. , Jersmann, H. , Matthews, G. , Ahern, J. , Holmes, M. , Reynolds, P. N. (2008) Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 178, 139–148. [DOI] [PubMed] [Google Scholar]
- 19. Chang, W. C. , Hartshorn, K. L. , White, M. R. , Moyo, P. , Michelow, I. C. , Koziel, H. , Kinane, B. T. , Schmidt, E. V. , Fujita, T. , Takahashi, K. (2011) Recombinant chimeric lectins consisting of mannose‐binding lectin and L‐ficolin are potent inhibitors of influenza A virus compared with man‐nose‐binding lectin. Biochem. Pharmacol. 81, 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sundstrom, C. , Nilsson, K. (1976) Establishment and characterization of a human histiocytic lymphoma cell line (U‐937). Int. J. Cancer 17, 565–577. [DOI] [PubMed] [Google Scholar]
- 21. Carruba, G. , D'Agostino, P. , Miele, M. , Calabro, M. , Barbera, C. , Bella, G. D. , Milano, S. , Ferlazzo, V. , Caruso, R. , Rosa, M. L. , Cocciadiferro, L. , Campisi, I. , Castagnetta, L. , Cillari, E. (2003) Estrogen regulates cytokine production and apoptosis in PMA‐differentiated, macrophage‐like U937 cells. J. Cell. Biochem. 90, 187–196. [DOI] [PubMed] [Google Scholar]
- 22. Verhoeckx, K. C. , Bijlsma, S. , de Groene, E. M. , Witkamp, R. F. , van der Greef, J. , Rodenburg, R. J. (2004) A combination of proteomics, principal component analysis and transcriptomics is a powerful tool for the identification of biomarkers for macrophage maturation in the U937 cell line. Proteomics 4, 1014–1028. [DOI] [PubMed] [Google Scholar]
- 23. Koziel, H. , Eichbaum, Q. , Kruskal, B. A. , Pinkston, P. , Rogers, R. A. , Armstrong, M. Y. , Richards, F. F. , Rose, R. M. , Ezekowitz, R. A. (1998) Reduced binding and phagocytosis of Pneumocystis carinii by alveolar macrophages from persons infected with HIV‐1 correlates with mannose receptor downregulation. J. Clin. Invest. 102, 1332–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hartshorn, K. L. , Collamer, M. , Auerbach, M. , Myers, J. B. , Pavlotsky, N. , Tauber, A. I. (1988) Effects of influenza A virus on human neutrophil calcium metabolism. J. Immunol. 141, 1295–1301. [PubMed] [Google Scholar]
- 25. Michelow, I. C. , Dong, M. , Mungall, B. A. , Yantosca, L. M. , Lear, C. , Ji, X. , Karpel, M. , Rootes, C. L. , Brudner, M. , Houen, G. , Eisen, D. P. , Kinane, T. B. , Takahashi, K. , Stahl, G. L. , Olinger, G. G. , Spear, G. T. , Ezekowitz, R. A. , Schmidt, E. V. (2010) A novel L‐ficolin/mannose‐binding lectin chimeric molecule with enhanced activity against Ebola virus. J. Biol. Chem. 285, 24729–24739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shi, L. , Takahashi, K. , Dundee, J. , Shahroor‐Karni, S. , Thiel, S. , Jensenius, J. C. , Gad, F. , Hamblin, M. R. , Sastry, K. N. , Ezekowitz, R. A. (2004) Mannose‐binding lectin‐deficient mice are susceptible to infection with Staphylococcus aureus. J. Exp. Med. 199, 1379–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moller‐Kristensen, M. , Hamblin, M. R. , Thiel, S. , Jensenius, J. C. , Takahashi, K. (2007) Burn injury reveals altered phenotype in mannan‐binding lectin‐deficient mice. J. Invest. Dermatol. 127, 1524–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rämet, M. , Manfruelli, P. , Pearson, A. , Mathey‐Prevot, B. , Ezekowitz, R. A. (2002) Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature 416, 644–648. [DOI] [PubMed] [Google Scholar]
- 29. Koziel, H. , Li, X. , Armstrong, M. Y. , Richards, F. F. , Rose, R. M. (2000) Alveolar macrophages from human immunodeficiency virus‐infected persons demonstrate impaired oxidative burst response to Pneumocystis carinii in vitro. Am. J. Respir. Cell Mol. Biol. 23, 452–459. [DOI] [PubMed] [Google Scholar]
- 30. Tachado, S. D. , Zhang, J. , Zhu, J. , Patel, N. , Koziel, H. (2005) HIV impairs TNF‐α release in response to Toll‐like receptor 4 stimulation in human macrophages in vitro. Am. J. Respir. Cell Mol. Biol. 33, 610–621. [DOI] [PubMed] [Google Scholar]
- 31. Wan, C. P. , Myung, E. , Lau, B. H. (1993) An automated micro‐fluoro‐metric assay for monitoring oxidative burst activity of phagocytes. J. Immunol. Methods 159, 131–138. [DOI] [PubMed] [Google Scholar]
- 32. Rodgers, B. C. , Mims, C. A. (1982) Influenza virus replication in human alveolar macrophages. J. Med. Virol. 9, 177–184. [DOI] [PubMed] [Google Scholar]
- 33. Cheung, C. Y. , Poon, L. L. , Lau, A. S. , Luk, W. , Lau, Y. L. , Shortridge, K. F. , Gordon, S. , Guan, Y. , Peiris, J. S. (2002) Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 360, 1831–1837. [DOI] [PubMed] [Google Scholar]
- 34. Shepherd, V. L. (1986) The role of the respiratory burst of phagocytes in host defense. Semin. Respir. Infect. 1, 99. [PubMed] [Google Scholar]
- 35. Fairbairn, I. P. (2004) Macrophage apoptosis in host immunity to mycobacterial infections. Biochem. Soc. Trans. 32, 496–498. [DOI] [PubMed] [Google Scholar]
- 36. Krarup, A. , Wallis, R. , Presanis, J. S. , Gal, P. , Sim, R. B. (2007) Simultaneous activation of complement and coagulation by MBL‐associated serine protease 2. PLoS One 2, e623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Presanis, J. S. , Hajela, K. , Ambrus, G. , Gal, P. , Sim, R. B. (2004) Differential substrate and inhibitor profiles for human MASP‐1 and MASP‐2. Mol. Immunol. 40, 921–929. [DOI] [PubMed] [Google Scholar]
- 38. White, M. R. , Crouch, E. , Chang, D. , Sastry, K. , Guo, N. , Engelich, G. , Takahashi, K. , Ezekowitz, R. A. , Hartshorn, K. L. (2000) Enhanced antiviral and opsonic activity of a human mannose‐binding lectin and surfactant protein D chimera. J. Immunol. 165, 2108–2115. [DOI] [PubMed] [Google Scholar]
- 39. Snelgrove, R. J. , Edwards, L. , Rae, A. J. , Hussell, T. (2006) An absence of reactive oxygen species improves the resolution of lung influenza infection. Eur. J. Immunol. 36, 1364–1373. [DOI] [PubMed] [Google Scholar]
- 40. Janssen, W. J. , Barthel, L. , Muldrow, A. , Oberley‐Deegan, R. E. , Kearns, M. T. , Jakubzick, C. , Henson, P. M. (2011) Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am. J. Respir. Crit. Care Med. 184, 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ip, W. K. , Takahashi, K. , Ezekowitz, R. A. , Stuart, L. M. (2009) Mannose‐binding lectin and innate immunity. Immunol. Rev. 230, 9–21. [DOI] [PubMed] [Google Scholar]
- 42. Reading, P. C. , Miller, J. L. , Anders, E. M. (2000) Involvement of the mannose receptor in infection of macrophages by influenza virus. J. Virol. 74, 5190–5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Upham, J. P. , Pickett, D. , Irimura, T. , Anders, E. M. , Reading, P. C. (2010) Macrophage receptors for influenza A virus: role of the macrophage galactose‐type lectin and mannose receptor in viral entry. J. Virol. 84, 3730–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pagh, R. , Duus, K. , Laursen, I. , Hansen, P. R. , Mangor, J. , Thielens, N. , Arlaud, G. J. , Kongerslev, L. , Hojrup, P. , Houen, G. (2008) The chaperone and potential mannan‐binding lectin (MBL) co‐receptor calreticulin interacts with MBL through the binding site for MBL‐associated serine proteases. FEBS J. 275, 515–526. [DOI] [PubMed] [Google Scholar]
- 45. Ip, W. K. , Takahashi, K. , Moore, K. J. , Stuart, L. M. , Ezekowitz, R. A. (2008) Mannose‐binding lectin enhances Toll‐like receptors 2 and 6 signaling from the phagosome. J. Exp. Med. 205, 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Petersen, K. A. , Matthiesen, F. , Agger, T. , Kongerslev, L. , Thiel, S. , Cornelissen, K. , Axelsen, M. (2006) Phase I safety, tolerability, and pharmacokinetic study of recombinant human mannan‐binding lectin. J. Clin. Immunol. 26, 465–475. [DOI] [PubMed] [Google Scholar]


