Short abstract
Lipogenesis and myeloid differentiation can be differentially regulated by galectin‐12.
Keywords: acute promyelocytic leukemia, lipid droplets, PPARγ, acute myeloid leukemia, myeloid differentiation
Abstract
As a member of the galectin family of animal lectins, galectin‐12 is preferentially expressed in adipocytes and leukocytes. In adipocytes, galectin‐12 is associated with lipid droplets and regulates lipid metabolism and energy balance, whereas its role in leukocytes is not clear. Analysis of galectin‐12 expression in a public data set of acute myeloid leukemia (AML) samples revealed that it is selectively overexpressed in the M3 subtype, which is also known as acute promyelocytic leukemia (APL). To investigate the role of galectin‐12 in APL cells, we manipulated its expression in the APL cell line, NB4, and measured resultant effects on all‐trans‐retinoic acid (ATRA)–induced granulocytic differentiation. With a doxycycline‐inducible gene knockdown system, we found that suppression of galectin‐12 promoted ATRA‐induced neutrophil differentiation but inhibited lipid droplet formation. Our results indicate that overexpression of galectin‐12 contributes to a differentiation block in APL cells, and suppression of galectin‐12 facilitates granulocytic differentiation. Furthermore, these data suggest that lipogenesis and other aspects of myeloid differentiation can be differentially regulated. Taken together, these findings suggest that galectin‐12 may be a target for treatment of the ATRA‐resistant subset of APL.
Abbreviations
- 15dPGJ2
= 15‐deoxy‐Δ12,14‐prostaglandin J2
- Akt
= protein kinase B
- AML
= acute myeloid leukemia
- APL
= acute promyelocytic leukemia
- ATRA
= all‐trans‐retinoic acid
- C/EBPα
= CCAAT/enhancer‐binding protein‐α
- C/EBPβ
= CCAAT/enhancer‐binding protein‐β
- CRD
= carbohydrate‐recognition domain
- NBT
= nitro blue tetrazolium
- PGZ
= pioglitazone
- PML‐RARα
= promyelocytic leukemia‐retinoic acid receptor α
- PPARγ
= peroxisome proliferator‐activated receptor‐γ
- ROS
= reactive oxygen species
Introduction
Galectins are an ancient family of animal lectins with conserved CRDs that have affinities for β‐galactosides [1]. There are 3 subfamilies of galectins, namely, prototype (galectin‐1, ‐2, ‐5, ‐7, ‐10, ‐11, ‐13, and ‐14) with 1 CRD, chimera type (only galectin‐3) with 1 CRD and a proline/glycine‐rich domain, and tandem repeat type (galectin‐4, ‐6, ‐8, ‐9, and ‐12) with 2 CRDs connected by a short linker [2, 3]. Galectin‐12 is a 2‐CRD galectin with its N‐terminal CRD homologous to those of other galectins, whereas its C‐terminal CRD exhibits considerable divergence from the consensus sequences [4, 5]. Full‐length galectin‐12 exhibits lactose‐binding activity, which is most likely contributed by its N‐terminal CRD [4].
Galectin‐12 mRNA is weakly expressed or undetectable in many tissues and cell types. In Jurkat cells, galectin‐12 was up‐regulated when cells were synchronized in G1 phase or at the G1/S boundary of the cell cycle. This expression was associated with cell‐cycle arrest in the G1 phase, which indicated that galectin‐12 is a negative regulator of cell growth [4]. In addition, galectin‐12 has been shown to be associated with cell differentiation [6, 7]. For example, it was demonstrated that galectin‐12 is predominately produced in adipocytes. In mouse preadipocyte cell line 3T3‐L1, galectin‐12 is up‐regulated in response to adipogenic hormone stimulation and induces cell cycle arrest, which in turn makes the cells competent to undergo differentiation [6]. Galectin‐12 expression is also associated with cell‐cycle arrest and terminal differentiation in sebaceous gland cells [7].
In adipocytes, galectin‐12 mainly localizes on lipid droplets [8]. Galectin‐12 is an important regulator of preadipocyte differentiation, as down‐regulation of galectin‐12 expression severely reduced adipogenic signaling as well as expression of the major adipogenic transcription factors, C/EBPα, C/EBPβ, and PPARγ [6]. Furthermore, ablation of the galectin‐12 gene in mice resulted in elevated cAMP levels, enhanced protein kinase A signaling, and an increased rate of lipolysis [8]. Thus, galectin‐12 may function as a scaffold protein for the compartmentalization of protein kinase A signaling. Galectin‐12–null mice exhibit reduced adipose tissue mass, increased mitochondrial respiration, and improved insulin sensitivity and glucose tolerance [8].
Galectin‐12 is also expressed in human peripheral blood leukocytes, especially myeloid cells [4]. Through analysis of galectin‐12 expression in a public data set of AML samples, we found that galectin‐12 was highly expressed in only the FAB subtype M3, also known as APL. APL cells are characterized by a chromosomal translocation that involves chromosomes 15 and 17 and generates PML‐RARα fusion protein [9]. ATRA is a ligand for RAR and can induce terminal differentiation toward the granulocytic pathway [10]. Moreover, ATRA is the most effective differentiating agent for APL [11]. When combined with chemotherapy, ATRA leads to complete remission in 72–90% of patients with APL with the oncogenic transcriptional repressor PML‐RARα [12, 13]; however, ∼10–30% of these patients experience relapse and develop resistance to ATRA [14]. Therefore, we decided to test the hypothesis that galectin‐12 overexpression in APL contributes to differentiation block of APL cells.
The human APL cell line NB4 can be induced to differentiate via either the neutrophilic or monocytic pathways by different stimuli [10]. For example, ATRA can induce NB4 cells to differentiate into neutrophil‐like cells, whereas PMA and 1α,25‐dihydroxyvitamin D3 can induce these cells to differentiate into monocyte‐like cells. Differentiation toward the neutrophil lineage can be assessed by cell proliferation arrest, morphologic changes, and expression of cell‐surface markers. Respiratory burst to generate reactive oxygen species (ROS) via NADPH oxidase subunits (p47phox and p67phox) is one of the well‐characterized markers for myeloid differentiation.
A prominent phenomenon during myeloid differentiation into neutrophils is accumulation of lipid droplets or lipid bodies [15], which are lipid‐rich organelles found in a variety of cells. Although the number of lipid droplets in resting human leukocytes is rather low, it increases a great deal when inflammation occurs [16]. Lipid droplets are not merely lipid storage organelles, but they also contain a variety of enzymes and cytokines and are mobilized during leukocyte activation, which leads to secretion of inflammatory factors during inflammation [17]. Thus, lipid droplets are a key marker for leukocyte activation and are potential targets for anti‐inflammatory therapies [18, 19].
As galectin‐12 has roles in adipogenic signaling as well as in adipocyte differentiation and lipogenesis, here, we explored the function of galectin‐12 in myeloid differentiation and lipid droplet accumulation to better understand the myeloid differentiation mechanism. Specifically, we used human promyelocytic leukemia cell line NB4 induced by ATRA to neutrophil differentiation to assess the roles of galectin‐12 in the differentiation and lipogenesis processes.
MATERIALS AND METHODS
Cell culture and antibodies
The human NB4 promyelocytic leukemia cell line was cultured in RPMI 1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Thermo Fisher Scientific) and was maintained at 37°C in an atmosphere of 5% CO2.
Rabbit antibodies against galectin‐12 were purchased from GeneTex (San Antonio, TX, USA); mouse anti‐PPARγ and rabbit anti‐C/EBPα and ‐C/EBPβ were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA); rabbit anti–p‐CREB, anti‐CREB, anti–p‐ERK, and mouse anti‐ERK were purchased from Cell Signaling Technology (Beverly, MA, USA); rabbit anti–β‐tublin was purchased from AnaSpec (San Jose, CA, USA); rabbit anti‐p47phox was purchased from Assay BioTech (San Francisco, CA, USA); and anti–CD11b‐FITC (clone M1/70) was purchased from eBioscience (San Diego, CA, USA). All antibodies were used at a 1:1000 dilution for immunoblotting.
Doxycycline‐inducible knockdown system construction
Stable conditional galectin‐12 knockdown NB4 cell lines were generated as previously described [20], with LacZ gene knockdown as a control. In brief, cells were transduced with pMA2641 retrovirus in the presence of 8 μg/ml polybrene for 24 h. Successfully transduced cells were then selected in fresh medium that contained 10 μg/ml blasticidin S for 5 d. Subsequently, cells were transduced with pMA2867 lentiviruses that targeted LacZ or galectin‐12 genes. Successfully transduced cells were then selected in fresh medium that contained 1 μg/ml puromycin for 5 d. To induce gene knockdown, cells were treated with 1 μg/ml doxycycline (Calbiochem, San Diego, CA, USA) for 3 d, and knockdown efficiency was determined by RT‐PCR and immunoblot analyses.
Induction of neutrophil differentiation of NB4 cells
For induction of differentiation, 2 × 105/ml cells were seeded in 6‐well plates and grown in RPMI 1640 medium in the presence or absence of 1 μM ATRA (Acros Organics, Geel, Belgium) together with 1 μg/ml doxycycline for 72 h.
Quantitation of ROS production by NBT reduction assay
Cells (1 × 106) were resuspended in 500 μl of medium with 1 mg/ml NBT (Affymetrix, Santa Clara, CA, USA) and 0.6 μg/ml PMA (Sigma‐Aldrich, St. Louis, MO, USA), and were incubated for 1 h at 37°C in the dark. Cells were then rinsed twice with PBS, once with methanol, and air dried. Formazan product was dissolved into 240 μl of 2 M KCl and 280 μl DMSO for 10 min at room temperature with gentle shaking. Solution was then transferred to a 96‐well plate and the absorbance was measured at 620 nm.
Morphology
Cells (2.5 × 104) were cytospun onto glass slides and air dried. Cells were then subjected to Wright's stain (Amresco, Solon, OH, USA) for 30 s at room temperature. Slides were immediately placed in distilled water for 10 min, rinsed 1 more time, and air dried. Cell morphology was studied by light microscopy.
CD11b cell‐surface expression
CD11b is a marker of granulocyte differentiation. Cells (1 × 106) were harvested after 72 h ATRA treatment, washed, resuspended in 100 μl washing buffer (PBS that contained 1% FBS and 0.1% NaN3) that contained 1 μL anti‐human CD11b‐FITC, then incubated for 30 min in the dark. Cells were then washed twice and resuspended in 1 ml washing buffer. FITC (green) fluorescence was measured by flow cytometry, and median fluorescence intensity was analyzed by FlowJo V.10.1 software (FlowJo, Ashland, OR, USA).
Quantitative RT‐PCR
Total RNA was extracted from NB4 cells with RiboZol Reagent (Amresco). First‐strand cDNA was synthesized with 50 U/μl MultiScribe reverse transcriptase (Applied Biosystems, Foster City, CA, USA). RT reaction was amplified in EvaGreen solution (Biotium, Hayward, CA, USA) by real‐time PCR with 5 U/μl AmpliTaq Gold DNA polymerase (Applied Biosystems). Specific oligonucleotide primers used herein were as follows: 5′‐agcctctggcttctctctgcaatgg‐3′ and 5′‐gtagatcctcgttccaggagccctgc‐3′ for human galectin‐12; and 5′‐ctgcactgccaagactgaatggctggatgg‐3′ and 5′‐ggacgctctcctgagctacagaaggaatgg‐3′ for human cyclophilin A. PCR was performed at 95°C for 10 min, followed by 35 cycles of 95°C for 15 s, 55°C for 30 s, and 72°C for 30 s, and a final extension step at 72°C for 5 min. Products were verified by electrophoresis on 3% agarose gel and visualized with ethidium bromide staining.
ROS production
After ATRA and/or doxycycline treatment for 72 h, NB4 cells from different groups were treated with 30 ng/ml PMA for 5 min, rinsed with PBS, stained with 1 μM dihydroethidium (Molecular Probes, Eugene, OR, USA) in 0.5% BSA for 30 min at 37°C, and washed with PBS. Cells were subjected to flow cytometry, and median fluorescence intensity was determined using FlowJo V.10.1 software.
Oil Red O staining
Oil Red O staining is a common method to evaluate lipid droplets. Cells were cytospun onto glass slides, fixed in 4% paraformaldehyde for 1 h at room temperature, rinsed with distilled water, then with 100% propylene glycol, and stained with 0.5% Oil Red O (Sigma‐Aldrich) dissolved in propylene glycol for 15 min at room temperature. Slides were treated with 85% propylene glycol for 5 min, washed with distilled water twice, and air dried. Lipid droplets were then observed under light microscopy.
Immunoblot analysis
After 72 h treatment with or without ATRA together with doxycycline, cells were washed and whole‐cell extracts were prepared with a lysis buffer that contained 0.2% Nonidet P‐40, 1 mM PMSF, 1.5 μg/ml protease inhibitor cocktail (Roche, Indianapolis, IN, USA), and 0.2 mM Na3VO4. Protein concentrations were determined by the bicinchoninic acid (Thermo Fisher Scientific) method, and equal amounts of protein were run on 10% denaturing SDS‐PAGE gels and transferred onto polyvinylidene fluoride membranes. Proteins were immunoblotted with indicated antibodies, and bands were developed by using Luminata Crescendo Western HRP substrate (Millipore, Bedford, MA, USA).
Extraction of cell membrane fractions
Cells were treated with 30 ng/ml PMA for 5 min at 37°C, rinsed with ice‐cold PBS, cell pellets were resuspended in ice‐cold lysis buffer (10 mM HEPES, 100 mM KCl, 3 mM NaCl, 3.5 mM MgCl2, 1 mM ATP, 0.1% Triton X‐100, 1 mM PMSF, 100 U/ml aprotinin, and pH 7.3), and incubated on ice for 30 min. After 30 strokes in a Potter homogenizer, cells were centrifuged at 500 g for 15 min to remove nuclei and unbroken cells. Supernatants were collected and centrifuged at 16,000 g for 30 min, and pellets were then collected (plasma membranes fractions).
Statistical analysis
Data are expressed as means ± sd. Differences were evaluated by 1‐way ANOVA analyses, and P < 0.05 was considered significant.
RESULTS
Galectin‐12 is highly expressed in APL but not in other subtypes of AML
To explore the involvement of galectin‐12 in myeloid leukemia, we analyzed galectin‐12 expression in a public data set of 526 AML samples of various FAB subtypes [21]. We found that galectin‐12 gene was highly expressed in FAB subtype M3 (APL), but not in other subtypes ( Fig. 1 ). APL is characterized by abnormal accumulation of immature granulocytes called promyelocytes because of a differentiation block at the promyelocytic stage of myeloid differentiation. Overexpression of galectin‐12 in APL relative to other AML subtypes suggests that this protein may contribute to block of myeloid differentiation at the promyelocytic stage.
Figure 1.
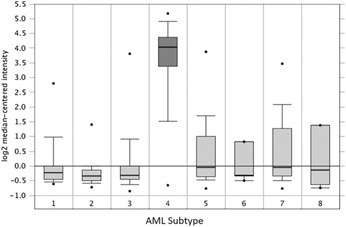
Expression of galectin‐12 in AML subtypes. Box plot of galectin‐12 expression in 526 AML samples of various FAB subtypes in the Wouters leukemia data set [21] were accessed and analyzed by using the Oncomine platform [41]. Gene expression in the data set was measured by using the Human Genome U133 Plus 2.0 Array [21]. Data show that galectin‐12 gene is specifically overexpressed in the M3 subtype (APL). FAB subtypes are 1 = M0; 2 = M1; 3 = M2; 4 = M3; 5 = M4; 6 = M4 with eosinophilia; 7 = M5; and 8 = M6.
Inducible knockdown of galectin‐12 in NB4 cells
To evaluate the role of galectin‐12 in myeloid differentiation, we constructed a doxycycline‐inducible conditional knockdown system with APL cell line NB4 [20]. With this system, we generated LacZ gene knockdown (control) and galectin‐12 gene knockdown cells. These cells were cultured in the presence of 1 μg/ml doxycycline for 72 h. Subsequently, galectin‐12 mRNA and protein levels were measured to determine knockdown efficiency induced by doxycycline. As shown in Fig. 2A , without doxycycline treatment, galectin‐12 mRNA levels in the 2 groups did not differ significantly. After doxycycline treatment, galectin‐12 mRNA levels in the LacZ gene knockdown control group did not significantly change, which suggests that doxycycline itself had little effect on galectin‐12 gene expression. In contrast, after doxycycline treatment, galectin‐12 mRNA was not detectable in the galectin‐12 gene knockdown group, which indicated that doxycycline induced efficient galectin‐12 gene knockdown. Results in Fig. 2B show changes in galectin‐12 protein levels, with and without doxycycline treatment, of the different groups.
Figure 2.
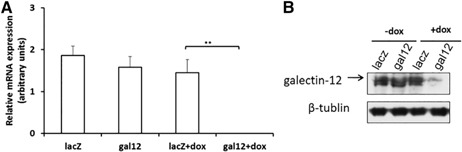
Knockdown efficiency of galectin‐12. (A) NB4 cells from different groups were treated with or without 1 μg/ml doxycycline (dox) for 72 h as described in MATERIALS AND METHODS, then galectin‐12 mRNA expression levels were measured by quantitative RT‐PCR. Results were normalized to the housekeeping gene cyclophilin A. (B) Galectin‐12 protein levels were measured by immunoblot assay.
Galectin‐12 knockdown enhances cell growth arrest during neutrophil differentiation
ATRA can induce APL cells to differentiate via the neutrophil pathway [10]. Growth arrest is one of the markers of ATRA‐induced granulocytic differentiation. Here, we treated NB4 cells with or without ATRA for 72 h and examined the differentiated ratio. Results shown in Fig. 3A suggest that cell growth was inhibited by ATRA, where the number of cells was decreased from an average of 3.5 × 106 to <3 × 106. After ATRA treatment, the cell number in the galectin‐12 knockdown group was significantly lower than that of the control LacZ knockdown group, whereas there was little difference between groups without ATRA treatment, which indicates that galectin‐12 affects cell differentiation induced by ATRA.
Figure 3.
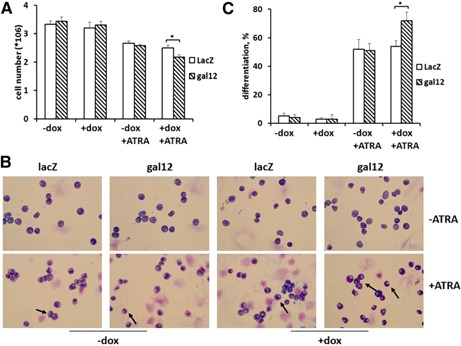
Effect of galectin‐12 knockdown on NB4 cell morphology. (A) Cells with potential LacZ or galectin‐12 gene knockdown were treated with or without 1 μg/ml doxycycline (dox) for 72 h, and with the presence or absence of 1 μM ATRA. Cells were then collected and counted under light microscopy. Data are presented as means ± sd of at least 5 independent experiments. (B) Wright's stain of NB4 cells treated with or without doxycycline and/or ATRA for 72 h. (C) Percentage of neutrophil‐like cells in each group from 5 independent counts of at least 100 cells each. Original magnification, ×400. *P < 0.05.
Galectin‐12 knockdown enhances neutrophil differentiation
To assess whether galectin‐12 knockdown enhances cell differentiation, a series of experiments was performed. First, cell morphology was assessed by Wright's stain, as the formation of horseshoe‐shaped nuclei is a characteristic feature of neutrophils (indicated by the arrows in Fig. 3B). As shown in Fig. 3C, there was little neutrophil differentiation without ATRA treatment. After ATRA treatment, ∼50% of cells underwent differentiation in the control group, and galectin‐12 knockdown dramatically increased the percentage of differentiated cells to >70%. Increased neutrophil differentiation in galectin‐12 knockdown cells was further evaluated by measuring cell‐surface expression of CD11b ( Fig. 4A ) and NBT reduction (Fig. 4B). Taken together, these results indicate that cell differentiation is increased in the absence of galecin‐12. Thus, galectin‐12 inhibits myeloid cell differentiation into neutrophils.
Figure 4.
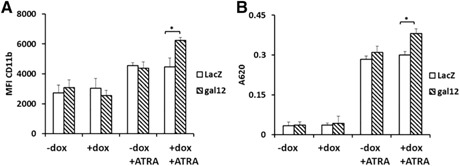
Effect of galectin‐12 knockdown on NB4 cell differentiation. (A) Expression of differentiation maker CD11b under different treatment conditions. Cells were treated with or without doxycycline (dox) in the presence or absence ATRA for 72 h, then incubated with anti–CD11b‐FITC. At least 1 × 104 cells were analyzed in each group by flow cytometry. Histograms represent median fluorescence intensity (MFI) of CD11b expression in each treatment group from 3 independent experiments. (B) Induction of NBT‐reducing activity of NB4 cells under different treatment conditions. After the same treatments as described for Fig. 4A, cells were coincubated with NBT and PMA as described in MATERIALS AND METHODS. Formazan was then dissolved in 2 M KCl and DMSO, and absorbance at 620 nm was measured. *P < 0.05.
Enhanced neutrophil differentiation by galectin‐12 knockdown is associated with modulation of ROS levels
As shown in Fig. 4B, NBT‐reducing activity increases during granulocytic differentiation by ATRA, which induces oxidative responses [22], hence respiratory burst activity is one of the most prominent features of myeloid differentiation [23]. In the current study, we used dihydroethidium to monitor cellular ROS production [24]. As shown in Fig. 5A , ROS production increased significantly after ATRA‐induced NB4 differentiation. In the galectin‐12 knockdown group, ROS production was much higher than that of the control group after induction of differentiation, which suggests that galectin‐12 knockdown also promotes functional differentiation of granulocytes. To gain insight into this mechanism, we examined the location and expression of NADPH oxidase subunit P47phox, which is one of the key indicators of oxidase activation [25]. In NB4 cells, ATRA is known to induce expression of all components of NADPH oxidase, including gp91, p67, p47, p40, p22, and Rac 2 [26, 27]. Upon activation by PMA, P47phox as well as other cytosolic components translocate to the plasma membrane and become associated with membrane components to form the NADPH oxidase complex [28]. As shown in Fig. 5B, before ATRA treatment, there was little P47phox expression on the membrane. ATRA treatment caused a 3‐fold increase in cell membrane–associated P47phox levels in the control group (LacZ knockdown with doxycycline). Galectin‐12 knockdown further increased membrane‐associated P47phox in ATRA‐treated cells. Cytosolic P47phox levels were similar across all groups. Results indicate that galectin‐12 knockdown increases P47phox translocation to the plasma membrane and promotes ROS generation.
Figure 5.
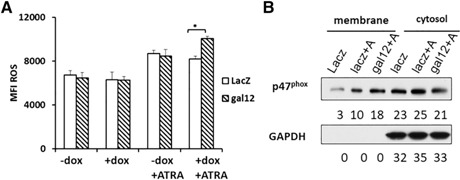
Modulation of ROS production by galectin‐12 knockdown. (A) After 72 h treatment with doxycycline (dox) and/or ATRA, cells were stained with 1 μM dihydroethidium and ROS production was evaluated by flow cytometry. Median fluorescence intensity (MFI) of ROS production with or without ATRA treatment from 3 independent experiments. (B) Relative expression of p47phox on the plasma membrane and in the cytosol. After 72 h of treatment, cells were separated into membrane and cytosolic fractions, then immunoblot assays were performed using indicated antibodies. Band intensities relative to the total in each group are indicated in numbers (%) below the immunoblot, which were quantified with ImageJ software. The loading amount of each membrane fraction was 3 times higher than that of the corresponding cytosolic fraction. *P < 0.05.
Galectin‐12 is indispensable for lipid droplet accumulation via regulation of PPARγ expression
Lipid droplets are lipid‐rich organelles that exist in a variety of cell types, including leukocytes. Whereas resting leukocytes contain few lipid droplets, the number of lipid droplets greatly increases upon cell activation. This is observed in diverse inflammatory responses, as lipid droplets contain many different inflammatory factors, enzymes, and cytokines in addition to lipids [18, 19]. Galectin‐12, a key molecule for adipogenesis, is indispensable for lipid droplet formation in adipocytes by regulating expression of C/EBPα, C/EBPβ, and PPARγ [6]. Thus, we explored the role of galectin‐12 in accumulation of lipid droplets and its role during NB4 cell differentiation via the neutrophil pathway. As shown in Fig. 6 , more lipid droplets accumulated after cells were treated with ATRA in the control group; however, lipid droplet accumulation was significantly lower in the galectin‐12 knockdown group. These results indicate that, similar to the function of galectin‐12 in adipocytes, galectin‐12 exerts a lipogenic effect in neutrophils; therefore, galectin‐12 is of pivotal importance for lipid droplet accumulation in cell types other than adipocytes.
Figure 6.
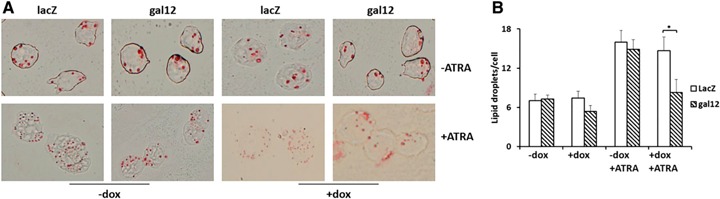
Effect of galectin‐12 knockdown on lipid droplet accumulation. (A) Cells were stained after 72 h of treatment with or without doxycycline (dox) and/or ATRA with Oil Red O to measure lipid droplet accumulation. (B) Average number of lipid droplets per cell from each treatment group from 5 independent counts of at least 100 cells each. Original magnification, ×400. *P < 0.05.
PPARγ is a member of the nuclear hormone receptor superfamily of ligand‐dependent transcription factors and has a central role in adipogenesis [29, 30]. It was previously reported that PPARγ is a master regulator of lipogenesis in NB4 cell differentiation induced by ATRA [15], as PPARγ antagonist bisphenol A diglycidyl ether can inhibit lipid droplet formation [15] and PPARγ ligands PGZ and 15dPGJ2 can stimulate lipid droplet formation in differentiated neutrophils by ATRA induction [31]. We therefore tested whether galectin‐12 modulates lipogenesis via regulation of PPARγ expression. As shown in Fig. 7 , after cell differentiation, PPARγ expression was lower in galectin‐12 knockdown cells compared with control cells. We also evaluated levels of p‐CREB, C/EBPα, and C/EBPβ and found them also to be lower in galectin‐12 knockdown cells compared with those in controls. C/EBPs are transcription factors of the basic leucine zipper class and have critical roles in adipogenesis [32, 33]. During adipogenesis, C/EBPβ expression is induced first, which can activate expression of C/EBPα and PPARγ to set off the process of lipogenesis [33]. Activation of ERK contributes to CREB‐directed transcription, and thus, we also measured PI3K and p‐Akt, as PI3K/Akt signaling is also critical for adipogenesis [34]. However, no changes were observed (data not shown), which suggests that PI3K/Akt signaling may not be affected by galectin‐12 in neutrophils.
Figure 7.
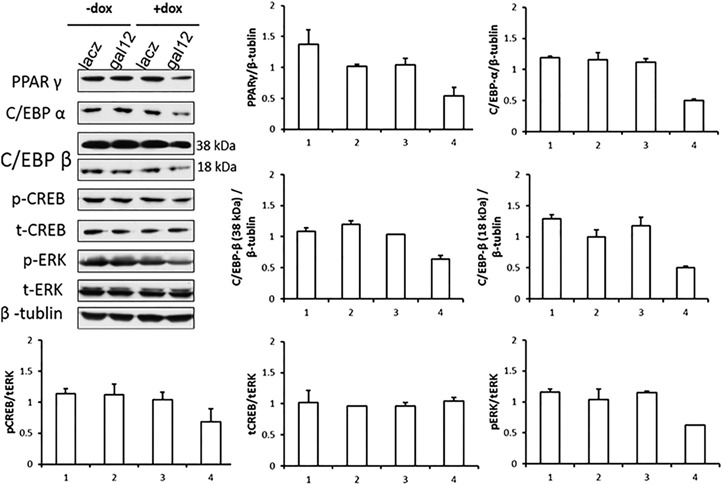
Effects of galectin‐12 knockdown on lipogenic signaling pathways in neutrophils. Cells in different groups were treated with or without doxycycline (dox) in the presence of ATRA, then washed with PBS and lysed. Lysates were then subjected to immunoblot analysis. Band intensities relative to the total in each group were quantified by ImageJ software and expressed as percentages. In the bar graphs, ratios of PPARγ, C/EBPα, and C/EBPβ isoforms (38 and 18 kDa) to β tubulin, and the ratios of p‐CREB, total CREB (t‐CREB) and p‐ERK to total ERK (t‐ERK) are presented. Numbers on the x axis represent lane assignments in the immunoblot. Each experiment was performed at least 3 times.
DISCUSSION
In the current study, we investigated the role of galectin‐12 in myeloid differentiation by using the human promyelocytic leukemia cell line NB4 as a model. We found that galectin‐12 knockdown promoted many aspects of functional and morphologic differentiation induced by ATRA, but dampened lipid droplet accumulation. Thus, galectin‐12 differentially regulates lipogenesis and other aspects of neutrophil differentiation. We also found that the inhibitory effect on differentiation was associated with p47phox translocation and ROS production, whereas it was not related to the cell cycle, as galectin‐12 knockdown cells exhibited cell‐cycle distribution similar to that of control cells, with or without ATRA treatment (data not shown).
Formation of lipid droplets, or lipogenesis, is an important part of neutrophil differentiation that has attracted increasing attention. PPARγ is a crucial transcription factor that regulates lipogenesis. There is evidence that PPARγ ligand, 2‐cyano‐3,12‐dioxooleana‐1,9‐dien‐28‐oic acid, in combination with ATRA, can induce ATRA‐sensitive and ‐resistant APL cells to differentiate into granulocytes more strongly than ATRA treatment alone [35]. PPARγ ligands PGZ and 15dPGJ2 have effects similar to those of 2‐cyano‐3,12‐dioxooleana‐1,9‐dien‐28‐oic acid on differentiation when combined with ATRA [31]. Specifically, PGZ and 15dPGJ2, in combination with ATRA, promote increased lipid droplet formation, which indicates that there is a close association between myeloid differentiation and lipogenesis [31]. Another group reported that bisphenol A diglycidyl ether, a PPARγ antagonist, inhibited lipid droplet formation stimulated by ATRA and/or G‐CSF in NB4 cells without affecting other aspects of granulocyte differentiation. The authors concluded that PPARγ mainly participated in lipogenesis, but not in other processes of NB4 cell differentiation [15]. This is consistent with our results herein that galectin‐12 knockdown promoted differentiation but inhibited lipogenesis, which indicates that galectin‐12 inhibits cell differentiation into neutrophils but is indispensable for lipid droplet formation. These results show that cell differentiation and lipogenesis can be dissociated during neutrophil differentiation. Furthermore, the galectin‐12 knockdown group exhibited decreased PPARγ expression, which suggests that the effect of this protein on lipogenesis is mediated by PPARγ.
Lipid droplets are unique cytoplasmic organelles that exist in a broad variety of cells, including adipocytes and leukocytes. There is increasing evidence that lipid droplets play important roles in leukocyte functions. In these immune cells, lipid droplets act in lipid storage to regulate lipid metabolism and are also involved in inflammatory and innate immune responses. Moreover, accumulation of these organelles is a common feature in immunopathological conditions, as lipid droplets are the platforms for inflammatory mediators, cytokines, and enzymes, etc.; therefore, lipid droplets are also called inflammatory organelles, as they are critical in different inflammatory responses and infectious diseases [16, 17, 18–19, 36]. There is also evidence that bacterial cell wall components, such as Escherichia coli LPS and Mycobacterium bovis bacillus Calmette‐Guérin, can induce lipid droplet accumulation in a time‐ and dose‐dependent manner [37, 38]. Furthermore, it has been reported that TLRs, the major pathogen‐associated molecular pattern recognition receptors which recognize a variety of pathogens, are involved in lipid droplet formation induced by infectious agents. Thus, LPS could not induce lipid droplet formation in animals with TLR4 mutations or when the TLR4 signaling pathway was inactivated [37, 38]. TLR2 has also been implicated in lipid droplet formation induced by bacillus Calmette‐Guérin [38, 39]. Therefore, galectin‐12 may have an important role in immune responses by regulating lipid droplet formation.
Taken together, our results indicate that overexpression of galectin‐12 contributes to a differentiation block in APL cells, and down‐regulation of galectin‐12 promotes terminal differentiation into neutrophils. In addition, galectin‐12 is required for lipogenic signaling via modulation of PPARγ expression. Therefore, regulation of myeloid differentiation and lipogenesis by galectin‐12 represents an attractive target for therapeutic interventions aimed at immune responses and for such diseases as myeloid leukemia. Although the vast majority of APL cases (98%) have the t[15,17](q22;q21) translocation that results in the PML‐RAR fusion and that respond to ATRA, some variants of APL with different genotypes have been described that are resistant to ATRA [40]. The status of galectin‐12 in those variants, and whether their response to ATRA can be sensitized by suppressing galectin‐12 expression, awaits further research.
AUTHORSHIP
H.X. acquired, analyzed, and interpreted the data and drafted and revised the manuscript; R.‐Y.Y. conceived and designed the study and revised the article; G.T. and F.‐T.L. conceived the study and revised the manuscript. All authors reviewed the results and approved the final version of the manuscript.
DISCLOSURES
The authors declare no competing financial interests.
ACKNOWLEDGMENTS
This work was supported by the Chinese Scholarship Council (CSC) and U.S. National Institutes of Health Grant R01‐AR56343 (to F.‐T.L.).
Footnotes
SEE CORRESPONDING EDITORIAL ON PAGE 640
References
- 1. Barondes, S. H. , Castronovo, V. , Cooper, D. N. , Cummings, R. D. , Drickamer, K. , Feizi, T. , Gitt, M. A. , Hirabayashi, J. , Hughes, C. , Kasai, K. , Leffler, H. , Liu, F.‐T. , Lotan, R. , Mercurio, A. M. , Monslgny, M. , Pillai, S. , Porier, F. , Raz, A. , Rigby, P. J. , Rini, J. M. , Wang, J. L. (1994) Galectins: a family of animal β‐galactoside‐binding lectins. Cell 76, 597–598. [DOI] [PubMed] [Google Scholar]
- 2. Yang, R.‐Y. , Rabinovich, G. A. , Liu, F.‐T. (2008) Galectins: structure, function and therapeutic potential. Expert Rev. Mol. Med. 10, e17. [DOI] [PubMed] [Google Scholar]
- 3. Liu, F.‐T. , Rabinovich, G. A. (2005) Galectins as modulators of tumour progression. Nat. Rev. Cancer 5, 29–41. [DOI] [PubMed] [Google Scholar]
- 4. Yang, R.‐Y. , Hsu, D. K. , Yu, L. , Ni, J. , Liu, F.‐T. (2001) Cell cycle regulation by galectin‐12, a new member of the galectin superfamily. J. Biol. Chem. 276, 20252–20260. [DOI] [PubMed] [Google Scholar]
- 5. Hotta, K. , Funahashi, T. , Matsukawa, Y. , Takahashi, M. , Nishizawa, H. , Kishida, K. , Matsuda, M. , Kuriyama, H. , Kihara, S. , Nakamura, T. , Tochino, Y. , Bodkin, N. L. , Hansen, B. C. , Matsuzawa, Y. (2001) Galectin‐12, an adipose‐expressed galectin‐like molecule possessing apoptosis‐inducing activity. J. Biol. Chem. 276, 34089–34097. [DOI] [PubMed] [Google Scholar]
- 6. Yang, R.‐Y. , Hsu, D. K. , Yu, L. , Chen, H.‐Y. , Liu, F.‐T. (2004) Galectin‐12 is required for adipogenic signaling and adipocyte differentiation. J. Biol. Chem. 279, 29761–29766. [DOI] [PubMed] [Google Scholar]
- 7. Harrison, W. J. , Bull, J. J. , Seltmann, H. , Zouboulis, C. C. , Philpott, M. P. (2007) Expression of lipogenic factors galectin‐12, resistin, SREBP‐1, and SCD in human sebaceous glands and cultured sebocytes. J. Invest. Dermatol. 127, 1309–1317. [DOI] [PubMed] [Google Scholar]
- 8. Yang, R.‐Y. , Yu, L. , Graham, J. L. , Hsu, D. K. , Lloyd, K. C. , Havel, P. J. , Liu, F.‐T. (2011) Ablation of a galectin preferentially expressed in adipocytes increases lipolysis, reduces adiposity, and improves insulin sensitivity in mice. Proc. Natl. Acad. Sci. USA 108, 18696–18701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dos Santos, G. A. , Kats, L. , Pandolfi, P. P. (2013) Synergy against PML‐RARa: targeting transcription, proteolysis, differentiation, and self‐renewal in acute promyelocytic leukemia. J. Exp. Med. 210, 2793–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khanna‐Gupta, A. , Kolibaba, K. , Zibello, T. A. , Berliner, N. (1994) NB4 cells show bilineage potential and an aberrant pattern of neutrophil secondary granule protein gene expression. Blood 84, 294–302. [PubMed] [Google Scholar]
- 11. Nichol, J. N. , Garnier, N. , Miller, W. H., Jr. (2014) Triple A therapy: the molecular underpinnings of the unique sensitivity of leukemic promyelocytes to anthracyclines, all‐trans‐retinoic acid and arsenic trioxide. Best Pract. Res. Clin. Haematol. 27, 19–31. [DOI] [PubMed] [Google Scholar]
- 12. Huang, M. E. , Ye, Y. C. , Chen, S. R. , Chai, J.‐R. , Lu, J.‐X. , Zhoa, L. , Gu, L.‐J. , Wang, Z.‐Y. (1988) Use of all‐trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood 72, 567–572. [PubMed] [Google Scholar]
- 13. Mandelli, F. , Diverio, D. , Avvisati, G. , Luciano, A. , Barbui, T. , Bernasconi, C. , Broccia, G. , Cerri, R. , Falda, M. , Fioritoni, G. , Leoni, F. , Liso, V. , Petti, M. C. , Rodeghiero, F. , Saglio, G. , Vegna, M. L. , Visani, G. , Jehn, U. , Willemze, R. , Muus, P. , Pelicci, P. G. , Biondi, A. , Lo Coco, F. (1997) Molecular remission in PML/RAR α‐positive acute promyelocytic leukemia by combined all‐trans retinoic acid and idarubicin (AIDA) therapy. Gruppo Italiano‐Malattie Ematologiche Maligne dell'Adulto and Associazione Italiana di Ematologia ed Oncologia Pediatrica Cooperative Groups. Blood 90, 1014–1021. [PubMed] [Google Scholar]
- 14. Lehmann‐Che, J. , Bally, C. , de Thé, H. (2014) Resistance to therapy in acute promyelocytic leukemia. N. Engl. J. Med. 371, 1170–1172. [DOI] [PubMed] [Google Scholar]
- 15. Inazawa, Y. , Nakatsu, M. , Yasugi, E. , Saeki, K. , Yuo, A. (2003) Lipid droplet formation in human myeloid NB4 cells stimulated by all trans retinoic acid and granulocyte colony‐stimulating factor: possible involvement of peroxisome proliferator‐activated receptor gamma. Cell Struct. Funct. 28, 487–493. [DOI] [PubMed] [Google Scholar]
- 16. Saka, H. A. , Valdivia, R. (2012) Emerging roles for lipid droplets in immunity and host‐pathogen interactions. Annu. Rev. Cell Dev. Biol. 28, 411–437. [DOI] [PubMed] [Google Scholar]
- 17. Bozza, P. T. , MagalhaTes, K. G. , Weller, P. F. (2009) Leukocyte lipid bodies ‐ biogenesis and functions in inflammation. Biochim. Biophys. Acta 1791, 540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walther, T. C. , Farese, R. V., Jr. (2012) Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 81, 687–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Viola, J. P. , Cruz, A. L. , Werneck, M. B. , Bozza, P. T. (2013) Formation and function of lipid droplets in inflammation and cancer In Trends in Stem Cell Proliferation and Cancer Research (Resende R. R., Ulrich H., eds.), Springer Science + Business Media, New York, 139–165 [Google Scholar]
- 20. Alexeyev, M. F. , Fayzulin, R. , Shokolenko, I. N. , Pastukh, V. (2010) A retro‐lentiviral system for doxycycline‐inducible gene expression and gene knockdown in cells with limited proliferative capacity. Mol. Biol. Rep. 37, 1987–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wouters, B. J. , Löwenberg, B. , Erpelinck‐Verschueren, C. A. , van Putten, W. L. , Valk, P. J. , Delwel, R. (2009) Double CEBPA mutations, but not single CEBPA mutations, define a subgroup of acute myeloid leukemia with a distinctive gene expression profile that is uniquely associated with a favorable outcome. Blood 113, 3088–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sham, R. L. , Phatak, P. D. , Belanger, K. A. , Packman, C. H. (1995) Functional properties of HL60 cells matured with all‐trans‐retinoic acid and DMSO: differences in response to interleukin‐8 and fMLP. Leuk. Res. 19, 1–6. [DOI] [PubMed] [Google Scholar]
- 23. Borregaard, N. (2014) What doesn't kill you makes you stronger: the anti‐inflammatory effect of neutrophil respiratory burst. Immunity 40, 1–2. [DOI] [PubMed] [Google Scholar]
- 24. Owusu‐Ansah, E. , Yavari, A. , Mandal, S. , Banerjee, U. (2008) Distinct mitochondrial retrograde signals control the G1‐S cell cycle checkpoint. Nat. Genet. 40, 356–361. [DOI] [PubMed] [Google Scholar]
- 25. Makni‐Maalej, K. , Boussetta, T. , Hurtado‐Nedelec, M. , Belambri, S. A. , Gougerot‐Pocidalo, M.‐A. , El‐Benna, J. (2012) The TLR7/8 agonist CL097 primes N‐formyl‐methionyl‐leucyl‐phenylalanine‐stimulated NADPH oxidase activation in human neutrophils: critical role of p47phox phosphorylation and the proline isomerase Pin1. J. Immunol. 189, 4657–4665. [DOI] [PubMed] [Google Scholar]
- 26. N'Diaye, E. N. , Vaissiere, C. , Gonzalez‐Christen, J. , Grégoire, C. , Le Cabec, V. , Maridonneau‐Parini, I. (1997) Expression of NADPH oxidase is induced by all‐trans retinoic acid but not by phorbol myristate acetate and 1,25‐dihydroxyvitamin D3 in the human promyelocytic cell line NB4. Leukemia 11, 2131–2136. [DOI] [PubMed] [Google Scholar]
- 27. Wang, J. , Li, L. , Cang, H. , Shi, G. , Yi, J. (2008) NADPH oxidase‐derived reactive oxygen species are responsible for the high susceptibility to arsenic cytotoxicity in acute promyelocytic leukemia cells. Leuk. Res. 32, 429–436. [DOI] [PubMed] [Google Scholar]
- 28. Brandes, R. P. , Weissmann, N. , Schröder, K. (2014) Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic. Biol. Med. 76, 208–226. [DOI] [PubMed] [Google Scholar]
- 29. Schulman, I. G. , Shao, G. , Heyman, R. A. (1998) Transactivation by retinoid X receptor‐peroxisome proliferator‐activated receptor γ (PPARgamma) heterodimers: intermolecular synergy requires only the PPARgamma hormone‐dependent activation function. Mol. Cell. Biol. 18, 3483–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamaguchi, Y. , Cavallero, S. , Patterson, M. , Shen, H. , Xu, J. , Kumar, S. R. , Sucov, H. M. (2015) Adipogenesis and epicardial adipose tissue: a novel fate of the epicardium induced by mesenchymal transformation and PPARγ activation. Proc. Natl. Acad. Sci. USA 112, 2070–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yasugi, E. , Horiuchi, A. , Uemura, I. , Okuma, E. , Nakatsu, M. , Saeki, K. , Kamisaka, Y. , Kagechika, H. , Yasuda, K. , Yuo, A. (2006) Peroxisome proliferator‐activated receptor γ ligands stimulate myeloid differentiation and lipogenensis in human leukemia NB4 cells. Dev. Growth Differ. 48, 177–188. [DOI] [PubMed] [Google Scholar]
- 32. Lekstrom‐Himes, J. , Xanthopoulos, K. G. (1999) CCAAT/enhancer binding protein epsilon is critical for effective neutrophil‐mediated response to inflammatory challenge. Blood 93, 3096–3105. [PubMed] [Google Scholar]
- 33. Cristancho, A. G. , Lazar, M. A. (2011) Forming functional fat: a growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 12, 722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Franke, T. F. (2008) PI3K/Akt: getting it right matters. Oncogene 27, 6473–6488. [DOI] [PubMed] [Google Scholar]
- 35. Tabe, Y. , Konopleva, M. , Kondo, Y. , Contractor, R. , Tsao, T. , Konoplev, S. , Shi, Y. , Ling, X. , Watt, J. C. , Tsutsumi‐Ishii, Y. , Ohsaka, A. , Nagaoka, I. , Issa, J. P. , Kogan, S. C. , Andreeff, M. (2007) PPARgamma‐active triterpenoid CDDO enhances ATRA‐induced differentiation in APL. Cancer Biol. Ther. 6, 1967–1977. [DOI] [PubMed] [Google Scholar]
- 36. Reue, K. (2011) A thematic review series: lipid droplet storage and metabolism: from yeast to man. J. Lipid Res. 52, 1865–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pacheco, P. , Bozza, F. A. , Gomes, R. N. , Bozza, M. , Weller, P. F. , Castro‐Faria‐Neto, H. C. , Bozza, P. T. (2002) Lipopolysaccharide‐induced leukocyte lipid body formation in vivo: innate immunity elicited intracellular Loci involved in eicosanoid metabolism. J. Immunol. 169, 6498–6506. [DOI] [PubMed] [Google Scholar]
- 38. D'Avila, H. , Melo, R. C. , Parreira, G. G. , Werneck‐Barroso, E. , Castro‐Faria‐Neto, H. C. , Bozza, P. T. (2006) Mycobacterium bovis bacillus Calmette‐Guérin induces TLR2‐mediated formation of lipid bodies: intracellular domains for eicosanoid synthesis in vivo. J. Immunol. 176, 3087–3097. [DOI] [PubMed] [Google Scholar]
- 39. D'Ávila, H. , Almeida, P. E. , Roque, N. R. , Castro‐Faria‐Neto, H. C. , Bozza, P. T. (2007) Toll‐like receptor‐2‐mediated C‐C chemokine receptor 3 and eotaxin‐driven eosinophil influx induced by Mycobacterium bovis BCG pleurisy. Infect. Immun. 75, 1507–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Redner, R. L. (2002) Variations on a theme: the alternate translocations in APL. Leukemia 16, 1927–1932. [DOI] [PubMed] [Google Scholar]
- 41. Rhodes, D. R. , Yu, J. , Shanker, K. , Deshpande, N. , Varambally, R. , Ghosh, D. , Barrette, T. , Pandey, A. , Chinnaiyan, A. M. (2004) ONCOMINE: a cancer microarray database and integrated data‐mining platform. Neoplasia 6, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


