Short abstract
PKCδ is essential for neutrophil, but not macrophage functions required for host defense against fungal pathogens.
Keywords: dectin‐1, Mac‐1, C. albicans, reactive oxygen species, host defense, signaling
Abstract
The C‐type lectin receptor dectin‐1 and the integrin Mac‐1 have key roles in controlling fungal infection. Here, we demonstrate that dectin‐1‐ and Mac‐1‐induced activation of protein kinase Cδ in neutrophils, independent of the Card9 adaptor, is required for reactive oxygen species production and for intracellular killing upon Candida albicans uptake. Protein kinase Cδ was also required for zymosan‐induced cytokine generation in neutrophils. In macrophages, protein kinase Cδ deficiency prevented fungi‐induced reactive oxygen species generation but had no effect on activation of TGF‐β‐activated kinase‐1, an effector of Card9, or nuclear factor κB activation, nor did it affect phagolysosomal maturation, autophagy, or intracellular C. albicans killing. In vivo, protein kinase Cδ–deficient mice were highly susceptible to C. albicans and Aspergillus fumigatus infection, which was partially rescued with adoptively transferred wild‐type neutrophils. Thus, protein kinase Cδ activation downstream of dectin‐1 and Mac‐1 has an important role in neutrophil, but not macrophage, functions required for host defense against fungal pathogens.
Abbreviations
- BM
= bone marrow
- BMDM
= bone marrow–derived macrophage
- CLR
= C‐type lectin receptor
- FcγR
= Fcγ receptor
- LAMP‐1
= lysosome‐associated membrane protein 1
- MPO
= myeloperoxidase
- PKC‐δ
= protein kinase Cδ
- ROS
= reactive oxygen species
- TAK1
= TGF‐β–activated kinase‐1
- TMB
= 3,3′,5,5′‐tetramethyl‐benzidine
- WT
= wild‐type
Introduction
The growing use of immunosuppressive therapies and AIDS has resulted in a rise in infections by opportunistic pathogens, such as Candida and Aspergillus species during the past few decades, which is associated with high mortality rates [1]. The challenge for the immune system is the ease of dimorphic transition from yeast blastoconidia to hyphae with a lack of containment of the conidia within the first 4 h postinfection, leading to extracellular germination and tissue invasion. The innate immune response, mediated primarily by neutrophils and macrophages, which generate cytotoxic ROS and nonoxidative antimicrobial components to kill internalized blastoconidia, is required to keep fungal infection under control and thus prevent systemic candidiasis. The importance of ROS in containing fungal infection is underscored by chronic granulomatous disease, a disorder arising from a genetic deficiency in NADPH oxidase components and characterized by recurrent, invasive fungal infections [2, 3]. Moreover, proteases, such as neutrophil elastase, present in the secondary granules of neutrophils, have an essential role in C. albicans clearance as assessed in mouse models [4, 5]. Cells of adaptive immunity protect against mucocutaneous infection by generating cytokines that modulate antifungal effector mechanisms [6].
In fungal infection, CLR dectin‐1, TLR, mannose receptors, and the integrin Mac‐1 have key roles in uptake of fungal components [7]. Dectin‐1 recognizes β‐glucans in the cell wall of pathogenic fungi and, in mice, is essential for host defense against fungal pathogens [8, 9]. In macrophages and dendritic cells, dectin‐1 collaborates with TLR2 to trigger NF‐κB activation and cytokine production [10]. Moreover, in murine neutrophils, dectin‐1 activation of the leukocyte integrin Mac‐1 (CD11b/C18) through Vav guanine exchange factors promotes phagocytosis of fungal particles and associated ROS generation [11], consistent with the well‐described need for activation of the integrin for productive binding and effector responses [12]. In human neutrophils, Mac‐1 has a major role in recognition and containment of hyphae [13, 14], which reduce their β‐glucan exposure during filamentous elongation [15] and gain expression and glycosylation of a Mac‐1 ligand pH‐regulated antigen 1 protein (Pra1p) [16]. Mac‐1‐mediated killing of this filamentous form may potentially occur through the generation of neutrophil extracellular traps triggered selectively by hyphae [14, 17]. The role of dectin‐1 in human neutrophils is debated [18]. Its relative contribution in fungal killing is likely determined by the strain of C. albicans, the fungal form (blastoconidia, pseudohyphae, hyphae), and thus, the extent of β‐glucan exposure and the activation status and surface expression level of Mac‐1, which changes depending on the activation state of isolated neutrophils.
Despite advances in our understanding of signaling pathways that link dectin‐1 to cytokine generation in dendritic cells and macrophages, the intracellular signaling pathway specifically connecting fungal recognition to the activation of the NADPH oxidase in innate cells remains poorly understood. Dectin‐1 signaling is initiated by phosphorylation of its cytoplasmic hemi‐ITAM by Src family kinases that result in Syk recruitment and activation, steps that parallel those found in other ITAM‐containing receptors, such as FcγR, BCR, and TCR [19]. In CLRs, this process is followed by the tyrosine phosphorylation of signaling intermediates that engage downstream pathways, including MAPK and NF‐κB needed for cytokine production [20]. A general mediator of CLR function in dendritic cells is the adaptor protein Card9, which controls canonical NF‐κB activation and cytokine production [21], and in human neutrophils, contributes to fungal killing via an ROS‐independent mechanism [22]. Work in dendritic cells suggests that PKC‐δ phosphorylation of Card9 couples CLR to Card9 activation [23]. Accordingly, a deficiency in PKC‐δ [23] or Card9 [24] significantly increases susceptibility to C. albicans infection.
Here, we provide evidence that PKC‐δ downstream of dectin‐1 and Mac‐1 promotes neutrophil ROS generation and degranulation that is required for intracellular killing of fungi, independent of Card9. PKC‐δ was also required for zymosan but was dispensable for LPS‐induced cytokine generation. Defects in these PKC‐δ dependent neutrophil functions have consequences for host defense against C. albicans or A. fumigatus infection. In contrast, PKC‐δ is dispensable for macrophage phagolysosome maturation and fungal killing. Together, our data identify a role for PKC‐δ downstream of dectin‐1 and the integrin Mac‐1 selectively in neutrophil‐mediated fungal killing through a Card9‐independent pathway.
MATERIALS AND METHODS
Mice
PKC‐δ deficient (PKC‐δ−/−) mice were F2‐generation, hybrid mice and were maintained with appropriate WT counterparts, generated from heterozygous crosses [25]. Dectin‐1 deficient (dectin‐1−/−) mice backcrossed to C57BL/6 were as described previously [26] and were provided by Dr. Yoichiro Iwakura. Card9−/− mice were generated and maintained as described previously [27]. All experiments were approved by the Harvard Medical School Animal Care and Use Committee.
Reagents and antibodies
Zymosan, laminarin, PMA, luminol, and LPS were obtained from Sigma‐Aldrich (St. Louis, MO, USA). Zymosan was prepared by boiling for 10 min, followed by syringing through a 25‐gauge needle because this leads to greater β‐glucan exposure on the surface of zymosan. The following Abs were used for Western blot analysis: anti–PKC‐δ, anti–p‐PKC‐δ (Y311), anti–p‐p40phox (Thr154), anti–p‐Akt (Ser473), anti–p‐Pyk2 (Y402), anti–p‐PAK1(Thr423)/PAK2 (Thr402), anti–p‐TAK1 (Thr184/187), anti–p‐IKKα/β (Ser176/180), anti–p‐NF‐κB p65 (Ser536) (Cell Signaling Technology, Danvers, MA, USA), anti‐LAMP‐1 (Santa Cruz Biotechnologies, Dallas, TX, USA), and β‐actin (Sigma‐Aldrich).
Neutrophil and BMDM preparations
Murine neutrophils were isolated from BM as described previously [28]. In brief, BM cells were layered on Percoll (Sigma‐Aldrich) with stepwise gradients of 55%, 65%, and 75% Percoll, centrifuged at 1600 rpm (400 g relative centrifugal force) (GS‐6KR; Beckman Coulter, Indianapolis, IN, USA) for 30 min at room temperature, and the band between 65 and 75% of Percoll was collected. Cells (>95% neutrophils) were then washed and resuspended in calcium‐ and magnesium‐free PBS.
BMDM were obtained from tibias and femurs of 8–10‐wk‐old mice. The tibias and femurs were flushed, and the BM cell suspensions were passed through a 70‐mm cell strainer and cultured in plastic petri dishes for 7 d in DMEM + 10% FCS, supplemented with 100 ng/ml M‐CSF (PeproTech, Rocky Hill, NJ, USA).
Respiratory burst, phagocytosis, and iC3b–SRBC rosetting
Real‐time ROS generation was monitored by luminol‐enhanced chemiluminescence. Neutrophils or BMDM were transferred to PBS plus Ca2+/Mg2+ containing 50 μM luminol. Zymosan was added at 100 μg/ml. Chemiluminescence was measured using an AutoLumat LB‐953 tube luminometer (Berthold Technologies, Oak Ridge, TN, USA). To determine phagocytosis‐induced oxidative burst, neutrophils or BMDM were stimulated with zymosan in the presence of 1 mg/ml NBT. Cells with blue NBT deposition were scored as positive. For phagocytosis, FITC–zymosan was incubated with cells at a 10:1 ratio at 37°C for 30 min. A fluorescence microscope was used to assess the percentage of cells with ≥1 internalized FITC‐zymosan. C3‐RBC rosetting was evaluated as previously described [11]. Briefly, sheep RBCs (Lampire Biological Laboratories, Pipersville, PA, USA) were incubated with anti‐sheep RBC IgM (Cedarlane, Burlington, NC, USA), followed by incubation without or with C5‐deficient serum (Sigma‐Aldrich). Neutrophils were treated with zymosan for 15 min at 37°C. Cells were washed, C3‐RBCs were added, and the samples were spun at 800 rpm and were incubated at 37°C for 15 min. After washing, the percentage of neutrophils with ≥2 rosetted RBCs were counted.
Myeloperoxidase release assay
Neutrophils were treated with 100 μg/ml zymosan at 37°C for 30 min and centrifuged at 350 g for 5 min at 4°C to separate the supernatant from the cell pellet. Cells were lysed with 1% Triton X‐100. The substrate TMB (Thermo Fisher Scientific, Waltham, MA, USA) was added to the supernatant and the cell lysate followed by stop solution, and optical absorbance at 450 nm was measured. The MPO release ratio was calculated by dividing the enzymatic activity in the supernatant by the enzymatic activity from the supernatant and the lysate together.
In vitro C. albicans killing assay
This assay was based on a published protocol [29]; 5 × 105 C. albicans were incubated with or without 5 × 105 phagocytes in Falcon 96‐well plates in RPMI 1640 medium. Surviving C. albicans was incubated with Alamar blue (Invitrogen, Carlsbad, CA, USA) at 1:10 dilution in PBS for 12 h at 37°C, and fluorescence was measured using a SpectraMax M2 plate reader (Molecular Devices, Sunnyvale, CA, USA). Killing was calculated as 1 − [(number of C. albicans species incubated with phagocytes)/(number of C. albicans species incubated without phagocytes)].
Measurement of cytokines/chemokines
Neutrophils (5 × 105) were placed in 96‐well plates. Cell‐free supernatants were collected at indicated time points after stimulation with zymosan (10 μg/ml), LPS (100 ng/ml), or buffer control. The concentrations of TNF‐α and MIP‐2 were determined by mouse TNF‐α ELISA MAX Standard Sets (BioLegend, San Diego, CA, USA) and by a mouse CXCL2/MIP‐2 DuoSetELISA kit (R&D Systems, Minneapolis, MN, USA), respectively, according to the manufacturers’ instructions.
Mouse model of fungal infection
C. albicans (SC5314; ATCC, Manassas, VA, USA) infection was cultured, and mice were infected with blastoconidia (yeast forms), essentially as described previously [11]. After infection, mice were weighed and monitored daily. Mice were euthanized if they lost >20% of their body weight. In a separate group, the kidneys were harvested 3 d after infection. The left kidneys were photographed and homogenized for enumeration of fungal burden. The right kidneys were fixed for histologic analysis.
For tissue neutrophil accumulation, an MPO assay was performed, as previously described [30]. MPO activity in supernatants of homogenized kidney tissue was measured with TMB substrate kit (Pierce Biotechnology, Rockford, IL, USA). MPO protein quantity was calculated based on a recombinant mouse MPO standard (R&D Systems). Total protein content was measured with a BCA protein assay kit (Pierce), and MPO quantity was normalized to protein content. For A. fumigatus infection, mice were infected intranasally with 107 conidia; 48 h after inoculation, lung tissue was homogenized for enumeration of fungal burden.
Phagosome isolation
Phagosome isolation was adopted from previous protocols [31, 32]. Macrophages were incubated with 1–4 × 106 β‐1,3‐glucan beads [33] for the indicated time, and 0.5 ml of hypotonic lysis buffer (2 mM MgCl2, 6 mM β‐mercaptoethanol, 10 mM HEPES) with protease inhibitor mixture (Roche Diagnostics USA, Indianapolis, IN, USA) was added. Cells were mechanically sheared through a 1‐ml syringe with a 1.27 cm 26‐gauge needle (Thermo Fisher Scientific, Agawam, MA, USA) for 15 cycles. Hypotonic buffer was adjusted to isotonicity with 62% (w/v) sucrose solution to 40%. A discontinuous gradient was then constructed in ultracentrifugation tubes (Beckman Coulter, Brea, CA, USA) overlayering 2 ml of 62%, 40% (sample), 30%, 25%, and 10% solution. Sucrose gradients were centrifuged at 24,000 rpm (80,000 g) for 1 h at 4°C (SW‐28 rotor, L8‐M ultracentrifuge; Beckman Coulter). Following centrifugation, phagosomes were isolated at the interface of the 25%/10% sucrose layers. Phagosomes were counted using a hemacytometer and prepared for Western blot analysis.
Statistical analysis
ANOVA with Bonferroni posttests was used when making multiple statistical comparisons on a single data set. Two‐tailed unpaired t test was used for analysis of two groups. For the analysis of nonparametrically distributed data, the 2‐tailed Mann‐Whitney U test was used. Survival data were analyzed with the log‐rank test. Results were considered statistically significant at P < 0.05.
RESULTS
PKC‐δ is downstream of dectin‐1, Mac‐1, and Vav but is not required for Mac‐1 activation in neutrophils
PKC‐δ was phosphorylated in WT neutrophils upon stimulation with zymosan, an insoluble preparation of cell wall from Saccharomyces cerevisiae, which is composed mainly of β‐glucans, mannoproteins, and chitin [34]. PKC‐δ phosphorylation required dectin‐1 because it was significantly reduced in dectin‐1−/− neutrophils, as well as in WT neutrophils incubated with a soluble β‐glucan, laminarin ( Fig. 1A ) that inhibits zymosan binding to dectin‐1. Zymosan‐induced PKC‐δ activation was also dependent on Mac‐1 (Fig. 1B). We have previously shown that dectin‐1 used Vav, a guanine‐exchange factor for Rho GTPases, to activate the integrin Mac‐1 in neutrophils [11]. We investigated whether this was PKC‐δ dependent. PKC‐δ phosphorylation required Vav‐1,3 (Fig. 1C). However, zymosan‐induced activation of Mac‐1 was not impaired in PKC‐δ−/− neutrophils, as assessed by inducible rosetting of complement‐opsonized RBCs (Fig. 1D), a bona fide readout of Mac‐1 activation [11]. Thus, PKC‐δ requires both dectin‐1 and Mac‐1 receptors as well as Vav proteins for full activation, but Mac‐1 activation per se is not PKC‐δ dependent.
Figure 1.
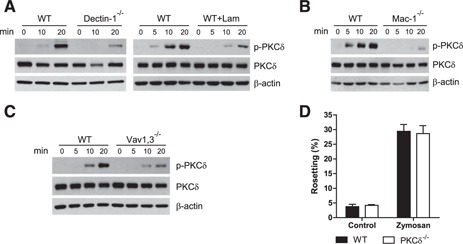
PKC‐δ is downstream of dectin‐1, Mac‐1, and Vav but is not required for Mac‐1 activation in neutrophils. (A–C) Lysates of WT, WT‐treated with laminarin, dectin‐1−/−, Mac‐1−/−, and Vav1,3−/− neutrophils stimulated with zymosan for the times listed in minutes were analyzed for PKC‐δ–Tyr311 phosphorylation. (D) Analysis of iC3b–RBC rosetting in WT and PKC‐δ−/− neutrophils after treatment with a vehicle control or zymosan. A graph of the means ± sem of 3 experiments is shown.
PKC‐δ promotes neutrophil ROS production, granule release, chemokine/cytokine generation, and C. albicans killing, and Card9 is dispensable for PKC‐δ–dependent neutrophil cytotoxic responses
PKC‐δ−/− neutrophils exhibited a significant defect in ROS production after zymosan stimulation ( Fig. 2A and B ). Because the composition of zymosan can differ with the source and with different preparations of zymosan, ROS generation in response to whole fungal cells was also evaluated using C. albicans as the model. PKC‐δ was essential for ROS generation triggered by heat‐killed (Fig. 2C) or live (Fig. 2D) C. albicans. On the other hand, Card9‐deficient neutrophils had a nonsignificant decrease in ROS production induced by live C. albicans (Fig. 2D). Notably, PMA‐induced ROS generation was also PKC‐δ dependent (Fig. 2E), suggesting a role for this kinase proximal to NADPH oxidase activation, which is consistent with the reported role of PKC‐δ in PMA‐induced phosphorylation of p40phox [35], an NADPH oxidase component needed for full ROS generation induced by various physiologic stimuli [35, 36–37]. Consistent with the reduction in zymosan‐induced ROS generation in PKC‐δ−/− neutrophils, we observed a marked decrease in zymosan‐induced phosphorylation of p40phox, whereas, on the contrary, activation of upstream signaling molecules, such as Akt, Pyk2, or MEK, was increased (Fig. 2F). PKC‐δ deficiency did not affect PAK1/2 phosphorylation (Fig. 2F), a readout of Vav/Rac activation, again suggesting that PKC‐δ is downstream of Vav proteins. Phagocytosis of zymosan particles or C. albicans yeast cells was not dependent on PKC‐δ (Fig. 2G), whereas zymosan‐induced neutrophil degranulation, as assessed by release of MPO stored in the primary granules required PKC‐δ (Fig. 2H). Moreover, we observed a striking reduction in C. albicans killing by neutrophils that were deficient in PKC‐δ (Fig. 2I), which may be attributed to the observed defect in ROS production and degranulation by these neutrophils. Notably, Card9−/− neutrophils exhibited no defect in C. albicans killing compared with WT neutrophils (Fig. 2I). Zymosan stimulation leads to a significant increase in the release of TNF‐α and MIP‐2/CXCL2 compared with untreated WT neutrophils. The release of both of these inflammatory mediators was significantly reduced in similarly treated PKC‐δ–deficient neutrophils (Fig. 2J and K, left panels), whereas both WT and PKC‐δ–deficient neutrophils produced similar amount of TNF‐α and MIP‐2/CXCL2 upon LPS treatment (Fig. 2J and K, right panels). Cell viability at the 20‐h time point was reduced by approximately 30% in both WT and PKC‐δ–null neutrophils (WT, 67.0 ± 2.1; PKC‐δ, 68.3 ± 3.1).
Figure 2.
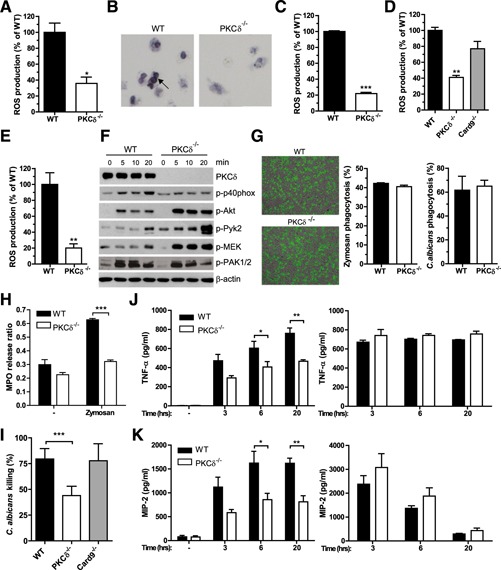
PKC‐δ, but not Card9, is required for C. albicans ROS generation and killing by neutrophils. (A) Zymosan‐induced ROS was analyzed in WT and PKC‐δ−/− neutrophils. A graph of the means ± sem of the peak value of the relative light units for samples normalized to WT cells is shown. (B) ROS production was determined by NBT reduction and representative pictures of 1 of 3 experiments are shown. (C–E) Respiratory burst of WT, PKC‐δ−/−, and Card9−/− neutrophils in response to heat‐killed C. albicans (C), live C. albicans (D), or PMA (E). A graph of the means ± sem of the peak value relative light units for samples normalized to WT cells is shown. (F) Lysates of WT and PKC‐δ−/− neutrophils stimulated with zymosan for the times in minutes shown were analyzed for phosphorylation of p40phox, Akt, Pyk2, PAK1/2, and MEK. β‐actin served as a loading control. (G) Phagocytosis of FITC‐labeled zymosan or C. albicans by WT and PKC‐δ−/− neutrophils. Representative pictures are shown. Means ± sem of results are given. (H) Release of MPO following zymosan stimulation in WT and PKC‐δ−/− neutrophils was evaluated. (I) Intracellular killing of live C. albicans by neutrophils was analyzed. TNF‐α (J) or MIP‐2 (K) levels in the supernatant of untreated cells (−) or cells treated with zymosan (left panel) or LPS (right panel) for the indicated time in hours was determined. Data represent means ± sem of 3 experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Dectin‐1–dependent PKC‐δ activation in macrophages promotes ROS generation but is dispensable for macrophage phagolysosomal maturation and fungal killing
As observed for neutrophils, zymosan treatment of macrophages resulted in PKC‐δ phosphorylation that was dectin‐1 dependent. However, in contrast to neutrophils, zymosan‐induced PKC‐δ phosphorylation was not Mac‐1 dependent ( Fig. 3A ). Thus, PKC‐δ activation is downstream of dectin‐1 but does not require Mac‐1 in macrophages, which is consistent with our previous finding that Mac‐1 is not essential for dectin‐1–mediated zymosan recognition or phagocytosis in macrophages [11]. PKC‐δ was needed for zymosan‐induced ROS production in macrophages, as assessed by both a luminol and NBT reduction assay (Fig. 3B and C). Likewise, ROS production stimulated by C. albicans was impaired in PKC‐δ−/− macrophages (data not shown). Accordingly, zymosan‐induced phosphorylation of p40phox was decreased in PKC‐δ−/− macrophages (Fig. 3D). However, phosphorylation of TAK1, IKKα/β, or p65 was normal in PKC‐δ−/− macrophages, which implies that PKC‐δ deficiency did not affect zymosan‐induced Card9 or NF‐κB activation (Fig. 3D). As in neutrophils, the uptake of zymosan particles or C. albicans was not impaired in macrophages lacking PKC‐δ (Fig. 3E and F). Phagolysosomal maturation, including recruitment of LAMP‐1 to β‐1,3‐glucan–containing phagosomes, and the progressive development of intraphagosomal, acidic environment are required for efficient fungal killing [32]. Phagosomes from WT and PKC‐δ−/− macrophages had an equivalent amount of LAMP‐1 (Fig. 3G), suggesting that PKC‐δ is not required for egress from an early to late phagolysosomal stage. Moreover, lysotracker staining, which was used to visualize phagolysosomal acidification, was equivalent in phagosomes of WT and PKC‐δ−/− macrophages containing β‐1,3‐glucan particles (Fig. 3H) or C. albicans (Fig. 3I). Finally, no difference in C. albicans killing was detected between WT and PKC‐δ−/− macrophages (Fig. 3J). Taken together, these data suggested that PKC‐δ is dispensable for macrophage phagocytosis and phagolysosome maturation and that the observed reduction in ROS and p40phox phosphorylation is not sufficient to compromise fungal killing in these cells.
Figure 3.
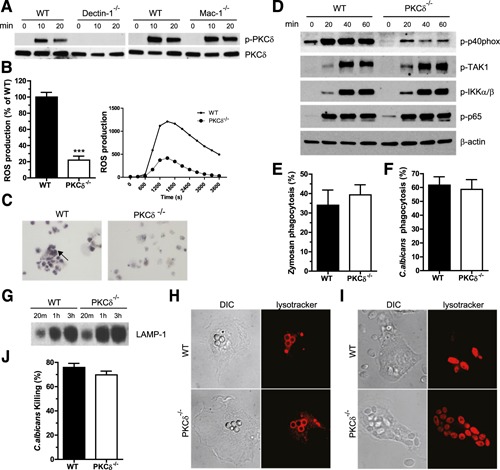
Roles of PKC‐δ in macrophages. (A) Lysates of WT, dectin‐1−/−, and Mac‐1−/− macrophages stimulated with zymosan for the times in minutes shown were analyzed for PKC‐δ–Tyr311 phosphorylation. Dectin‐1, but not Mac‐1, was required for PKC‐δ activation in macrophages. (B) Zymosan‐induced ROS was analyzed in WT and PKC‐δ−/− macrophages. A graph of the means ± sem of the peak value of the relative light units for samples normalized to WT cells and representative ROS profiles are shown. (C) ROS production was determined by NBT reduction, and representative pictures of 1 of 3 experiments are shown. (D) Lysates of WT and PKC‐δ−/− macrophages stimulated with zymosan for the times in minutes shown were analyzed for phosphorylation of p40phox, TAK1, Iκ B kinase (IKK)α/β, and p65. β‐actin served as a loading control. (E and F) Phagocytosis of FITC‐labeled zymosan (E) or C. albicans (F) by WT and PKC‐δ−/− macrophages was analyzed. Means ± sem of results are given. (G) 1 × 106 purified phagosomes from WT and PKC‐δ−/− macrophages were resolved by SDS‐PAGE and blotted for LAMP‐1. (H and I) WT and PKC‐δ−/− macrophages were incubated with β‐1,3‐glucan (H) beads (unlabeled) or heat‐killed C. albicans (I) (unlabeled) for 1 h in the presence of lysotracker (red) to visualize intracellular acidified compartments. (J) Analysis of intracellular killing of live C. albicans by macrophages. Data represent means ± sem of 3 experiments. ***P < 0.001.
In vivo protection against fungal infection requires PKC‐δ
The role of PKC‐δ in containing fungal infection in vivo was evaluated. To assess the effect of PKC‐δ deficiency on systemic candidiasis, mice were infected with C. albicans and monitored for survival and renal fungal burden. Significant mortality ( Fig. 4A ) and an increase in renal abscesses and kidney fungal burden (Fig. 4B and C) were observed in PKC‐δ‐deficient mice compared with WT counterparts. The susceptibility to infection was observed despite normal neutrophil accumulation in the kidney of PKC‐δ–null mice compared with WT animals (Fig. 4D). The increase in susceptibility to infection could be attributed to defects in neutrophil functions in PKC‐δ–null mice because adoptive transfer of WT neutrophils resulted in a significant reduction in renal fungal burden (Fig. 4E). Consistent with our findings with C. albicans, intranasal infection with A. fumigatus, another common opportunistic fungal pathogen, led to a significant increase in fungal burden in PKC‐δ−/− vs. WT animals (Fig. 4F).
Figure 4.
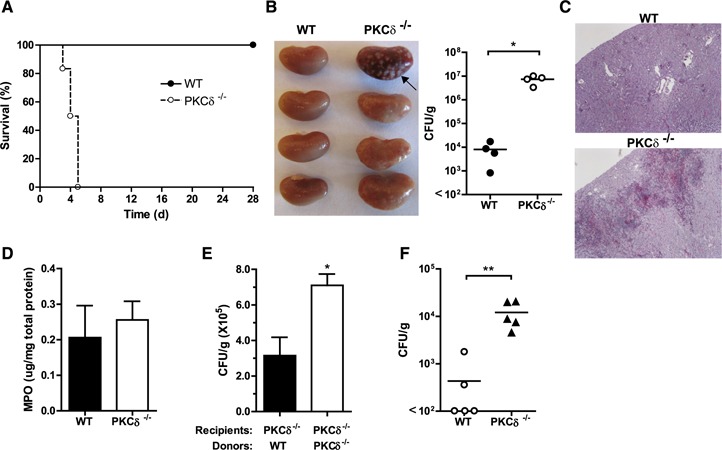
PKC‐δ is required for resistance to fungal infection. (A) PKC‐δ−/− mice and their WT cohorts (n = 8 in each) were infected intravenously with 5 × 104 C. albicans and monitored daily for survival. P = 0.0002 (log‐rank test). (B) Kidneys were harvested at d 3 after infection for gross morphology and visual inspection of abscesses (arrow) (left panel) and for quantitation of fungal burden in kidney homogenates (right panel). Each symbol represents data from an individual animal. (C) A representative photomicrograph of periodic acid–Schiff (PAS)‐stained kidney sections from wild‐type and PKC‐δ−/− mice is shown. Within kidney tissues from PKC‐δ−/− mice, large collections of neutrophils (abscess formation) surrounded by mixed inflammatory cells are present with abundant fungal forms, which are virtually absent in kidneys from wild‐type mice. (D) Kidneys harvested from mice at 16 h after infection were homogenized, and MPO activity was determined and normalized to total protein content. Means ± sem of results are given. (E) PKC‐δ−/− mice were injected with WT or PKC‐δ−/− neutrophils followed by C. albicans inoculation; 20 h later, the colony‐forming unit in kidney homogenates was determined. (F) Mice were infected intranasally with 107 A. fumigatus conidia, and 2 d later the lung was harvested. The colony‐forming unit per gram of tissue was quantitated, and data are reported as in (B). *P < 0.05; **P < 0.01.
DISCUSSION
In this study, we demonstrated that PKC‐δ, downstream of dectin‐1 and Mac‐1, promotes ROS production and granule release that is required for intracellular fungal killing by neutrophils in vitro. Our rescue experiments also suggest that PKC‐δ functions in neutrophils have a specific and important role in combating fungal infection in vivo. Thus, PKC‐δ has a central role in regulating the degradative and microbicidal properties of neutrophils required for fungal clearance.
We have previously shown that dectin‐1 and Mac‐1 in neutrophils used Vav to regulate NADPH activity and promote fungal clearance [11]. Here, we showed that PKC‐δ, downstream of Vav, was required for p40phox phosphorylation, a readout of NADPH oxidase activation. Phosphorylation of Akt, Pyk2, and MEK were not reduced and, paradoxically, tended to increase in PKC‐δ knockout neutrophils upon zymosan treatment. This observation suggests that the role of PKC‐δ is close to activation of NADPH oxidase components, consistent with this kinase's described role in p47phox phosphorylation and translocation in human monocytes in response to opsonized zymosan [38]. On the other hand, PKC‐δ may negatively regulate Akt, Pyk2, or MEK activation, which is required for other neutrophil functions. Indeed, PKC‐δ has been shown to negatively regulate PI3K activity in macrophages to limit FcγR–mediated phagocytosis [39] and, in keratinocytes, to modify apoptosis [40].
ROS in macrophages drives the autophagic factor LC3 to phagosomes to enhance fungicidal activity [41]. Despite a large reduction in ROS and p40phox phosphorylation in PKC‐δ–deficient macrophages, LC3 and subsequent fungal killing were surprisingly unaffected by the loss of PKC‐δ. One possible explanation is that macrophages use NADPH oxidase–independent mechanisms to kill fungi. In accordance with this, alveolar macrophages from WT and gp91phox −/− mice equally inhibited conidial germination in vitro, and macrophages in contact with conidia did not exhibit intracellular ROS generation in vivo [42]. In vivo, our rescue experiments showed that neutrophils and, by extension, neutrophil–mediated ROS, significantly contributed to antifungal immunity. This observation concurs with published reports showing that neutrophils, rather than macrophages, are essential in limiting pulmonary Aspergillus infection [43, 44]. Later control of fungal growth requires the NADPH oxidase in macrophages [45, 46]. Thus, neutrophil ROS generation likely has a key role in early fungal killing to contain infection, whereas macrophage‐generated superoxide and other cytotoxic mechanisms sustain antifungal immunity. Effective antifungal immunity in vivo also requires orchestration of the immune response by chemokines/cytokines released by cells of the myeloid lineage. Accordingly, we found that PKC‐δ deficiency resulted in a reduction in zymosan‐induced generation of inflammatory mediators by neutrophils. The equivalent renal accumulation of neutrophils in WT and PKC‐δ–null mice after systemic C. albicans infection suggests that PKC‐δ–dependent, neutrophil‐cytotoxic functions, rather than partial defects in generation of cytokines/chemokines that attract neutrophils, are important in controlling fungal infection.
Card9 appears to have various roles in leukocyte subsets. We observed that zymosan‐induced p‐TAK1 and p‐IKKα/β is not affected in PKC‐δ−/− macrophages, whereas others have shown that, in similarly treated dendritic cells, both phosphorylation events are reduced by the loss of PKC‐δ [23]. In dendritic cells, dectin‐1–triggered phosphorylation of Card9 via PKC‐δ represents a mechanism for subsequent activation of NF‐κB pathway, leading to the generation of cytokines [23, 24]. However, dectin‐1 signaling through Card9 is not required to drive NF‐κB activation and downstream TNF generation in macrophages [47]. Card9 deficiency in macrophages has been reported to lead to a partial reduction in C. albicans but not zymosan‐induced ROS production [48], whereas we show that Card9 is dispensable for C. albicans–induced ROS generation and killing in neutrophils, despite a requirement for PKC‐δ in these processes. Given these differential roles for Card9, we anticipate that the invasive candidiasis observed in patients with Card9 deficiency [49] may be the result of a primary defect in immune cell–generated chemokines that promote neutrophil recruitment and activation as reported [50], rather than an intrinsic defect in neutrophil‐mediated cytotoxic functions against conidia. On the other hand, contrary to our results, human neutrophils from patients with Card9 deficiency exhibited a defect in killing [22]. This result may reflect variations in the C. albicans strain used and, thus, different β‐glucan presentation on the cell wall and/or different in vitro assay conditions. The conidia and hyphae vary in size (with the latter preventing phagocytosis), metabolic activity, and surface molecule expression and also in the immune responses they elicit [51, 52]. In turn, immune cells control differentiation between the 2 states [53, 54–55]. Thus, prolonged incubation of neutrophils with blastoconidia in vitro in the presence of serum [22] can change the requirements for dectin‐1, Card9, and other human receptors. It is also possible that the differences could reflect a fundamental difference between human and mouse, albeit the molecular basis for this argument remains to be elucidated.
In summary, we show that PKC‐δ on neutrophils has a key role in antifungal defense. Interestingly, PKC‐δ–dependent signaling may represent one of the most ancestral defense pathways in innate immune cells because tpa‐1, a homolog of mammalian PKC‐δ in C. elegans, was found to be important for antifungal innate immunity [56].
AUTHORSHIP
X. Li, X.C., H.N., G.S., E.D., M.K.M, J.M.T., and X.S. performed the experiments and analyzed the data; X.Lin, and J.M.V. contributed to the execution of the experiments and provided editorial comments on the prepared manuscript; X. Li and T.M. conceived the experiments, analyzed and interpreted the data, wrote the article, and had access to all original data.
DISCLOSURES
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grant R01HL065095 (to T.M.); NIH National Institute of Allergy and Infectious Diseases Grants 5R01AI092084, 5R01AI097519 (to J.M.V.), and 1K08AI110655 (to M.K.M); the National Natural Science Foundation of China Grant 81302529 (to X. Li); and the Natural Science Foundation of Fujian, China Grant 2014D007 (to X. Li).
References
- 1. Chauhan, N. , Latge, J. P. , Calderone, R. (2006) Signalling and oxidant adaptation in Candida albicans and Aspergillus fumigatus. Nat. Rev. Microbiol. 4, 435–444. [DOI] [PubMed] [Google Scholar]
- 2. Brown, G. D. (2011) Innate antifungal immunity: the key role of phagocytes. Annu. Rev. Immunol. 29, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heyworth, P. G. , Cross, A. R. , Curnutte, J. T. (2003) Chronic granulomatous disease. Curr. Opin. Immunol. 15, 578–584. [DOI] [PubMed] [Google Scholar]
- 4. Reeves, E. P. , Lu, H. , Jacobs, H. L. , Messina, C. G. , Bolsover, S. , Gabella, G. , Potma, E. O. , Warley, A. , Roes, J. , Segal, A. W. (2002) Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416, 291–297. [DOI] [PubMed] [Google Scholar]
- 5. Tkalcevic, J. , Novelli, M. , Phylactides, M. , Iredale, J. P. , Segal, A. W. , Roes, J. (2000) Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity 12, 201–210. [DOI] [PubMed] [Google Scholar]
- 6. Lionakis, M. S. (2012) Genetic susceptibility to fungal infections in humans. Curr. Fungal Infect. Rep. 6, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Netea, M. G. , Brown, G. D. , Kullberg, B. J. , Gow, N. A. (2008) An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 6, 67–78. [DOI] [PubMed] [Google Scholar]
- 8. Brown, G. D. , Taylor, P. R. , Reid, D. M. , Willment, J. A. , Williams, D. L. , Martinez‐Pomares, L. , Wong, S. Y. , Gordon, S. (2002) Dectin‐1 is a major β‐glucan receptor on macrophages. J. Exp. Med. 196, 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taylor, P. R. , Tsoni, S. V. , Willment, J. A. , Dennehy, K. M. , Rosas, M. , Findon, H. , Haynes, K. , Steele, C. , Botto, M. , Gordon, S. , Brown, G. D. (2007) Dectin‐1 is required for β‐glucan recognition and control of fungal infection. Nat. Immunol. 8, 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gantner, B. N. , Simmons, R. M. , Canavera, S. J. , Akira, S. , Underhill, D. M. (2003) Collaborative induction of inflammatory responses by Dectin‐1 and Toll‐like receptor 2. J. Exp. Med. 197, 1107–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li, X. , Utomo, A. , Cullere, X. , Choi, M. M. , Milner, D. A., Jr. , Venkatesh, D. , Yun, S. H. , Mayadas, T. N. (2011) The β‐glucan receptor Dectin‐1 activates the integrin Mac‐1 in neutrophils via Vav protein signaling to promote Candida albicans clearance. Cell Host Microbe 10, 603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hynes, R. O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687. [DOI] [PubMed] [Google Scholar]
- 13. Lavigne, L. M. , Albina, J. E. , Reichner, J. S. (2006) β‐Glucan is a fungal determinant for adhesion‐dependent human neutrophil functions. J. Immunol. 177, 8667–8675. [DOI] [PubMed] [Google Scholar]
- 14. Byrd, A. S. , O'Brien, X. M. , Johnson, C. M. , Lavigne, L. M. , Reichner, J. S. (2013) An extracellular matrix‐based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans . J. Immunol. 190, 4136–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gantner, B. N. , Simmons, R. M. , Underhill, D. M. (2005) Dectin‐1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 24, 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soloviev, D. A. , Jawhara, S. , Fonzi, W. A. (2011) Regulation of innate immune response to Candida albicans infections by αMβ2‐Pra1p interaction. Infect. Immun. 79, 1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Branzk, N. , Lubojemska, A. , Hardison, S. E. , Wang, Q. , Gutierrez, M. G. , Brown, G. D. , Papayannopoulos, V. (2014) Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 15, 1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gazendam, R. P. , van Hamme, J. L. , Tool, A. T. , van Houdt, M. , Verkuijlen, P. J. , Herbst, M. , Liese, J. G. , van de Veerdonk, F. L. , Roos, D. , van den Berg, T. K. , Kuijpers, T. W. (2014) Two independent killing mechanisms of Candida albicans by human neutrophils: evidence from innate immunity defects. Blood 124, 590–597. [DOI] [PubMed] [Google Scholar]
- 19. Bezbradica, J. S. , Medzhitov, R. (2012) Role of ITAM signaling module in signal integration. Curr. Opin. Immunol. 24, 58–66. [DOI] [PubMed] [Google Scholar]
- 20. Saijo, S. , Iwakura, Y. (2011) Dectin‐1 and Dectin‐2 in innate immunity against fungi. Int. Immunol. 23, 467–472. [DOI] [PubMed] [Google Scholar]
- 21. Roth, S. , Ruland, J. (2013) Caspase recruitment domain‐containing protein 9 signaling in innate immunity and inflammation. Trends Immunol. 34, 243–250. [DOI] [PubMed] [Google Scholar]
- 22. Drewniak, A. , Gazendam, R. P. , Tool, A. T. , van Houdt, M. , Jansen, M. H. , van Hamme, J. L. , van Leeuwen, E. M. , Roos, D. , Scalais, E. , de Beaufort, C. , Janssen, H. , van den Berg, T. K. , Kuijpers, T. W. (2013) Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood 121, 2385–2392. [DOI] [PubMed] [Google Scholar]
- 23. Strasser, D. , Neumann, K. , Bergmann, H. , Marakalala, M. J. , Guler, R. , Rojowska, A. , Hopfner, K. P. , Brombacher, F. , Urlaub, H. , Baier, G. , Brown, G. D. , Leitges, M. , Ruland, J. (2012) Syk kinase‐coupled C‐type lectin receptors engage protein kinase C‐s to elicit Card9 adaptor‐mediated innate immunity. Immunity 36, 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gross, O. , Gewies, A. , Finger, K. , Schäfer, M. , Sparwasser, T. , Peschel, C. , Förster, I. , Ruland, J. (2006) Card9 controls a non‐TLR signalling pathway for innate anti‐fungal immunity. Nature 442, 651–656. [DOI] [PubMed] [Google Scholar]
- 25. Chou, W. H. , Choi, D. S. , Zhang, H. , Mu, D. , McMahon, T. , Kharazia, V. N. , Lowell, C. A. , Ferriero, D. M. , Messing, R. O. (2004) Neutrophil protein kinase Cδ as a mediator of stroke‐reperfusion injury. J. Clin. Invest. 114, 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saijo, S. , Fujikado, N. , Furuta, T. , Chung, S. H. , Kotaki, H. , Seki, K. , Sudo, K. , Akira, S. , Adachi, Y. , Ohno, N. , Kinjo, T. , Nakamura, K. , Kawakami, K. , Iwakura, Y. (2007) Dectin‐1 is required for host defense against Pneumocystis carinii but not against Candida albicans . Nat. Immunol. 8, 39–46. [DOI] [PubMed] [Google Scholar]
- 27. Hsu, Y. M. , Zhang, Y. , You, Y. , Wang, D. , Li, H. , Duramad, O. , Qin, X. F. , Dong, C. , Lin, X. (2007) The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat. Immunol. 8, 198–205. [DOI] [PubMed] [Google Scholar]
- 28. Hirahashi, J. , Mekala, D. , Van Ziffle, J. , Xiao, L. , Saffaripour, S. , Wagner, D. D. , Shapiro, S. D. , Lowell, C. , Mayadas, T. N. (2006) Mac‐1 signaling via Src‐family and Syk kinases results in elastase‐dependent thrombohemorrhagic vasculopathy. Immunity 25, 271–283. [DOI] [PubMed] [Google Scholar]
- 29. Johnnidis, J. B. , Harris, M. H. , Wheeler, R. T. , Stehling‐Sun, S. , Lam, M. H. , Kirak, O. , Brummelkamp, T. R. , Fleming, M. D. , Camargo, F. D. (2008) Regulation of progenitor cell proliferation and granulocyte function by microRNA‐223. Nature 451, 1125–1129. [DOI] [PubMed] [Google Scholar]
- 30. Huang, W. , Na, L. , Fidel, P. L. , Schwarzenberger, P. (2004) Requirement of interleukin‐17A for systemic anti‐Candida albicans host defense in mice. J. Infect. Dis. 190, 624–631. [DOI] [PubMed] [Google Scholar]
- 31. Stuart, L. M. , Boulais, J. , Charriere, G. M. , Hennessy, E. J. , Brunet, S. , Jutras, I. , Goyette, G. , Rondeau, C. , Letarte, S. , Huang, H. , Ye, P. , Morales, F. , Kocks, C. , Bader, J. S. , Desjardins, M. , Ezekowitz, R. A. (2007) A systems biology analysis of the Drosophila phagosome. Nature 445, 95–101. [DOI] [PubMed] [Google Scholar]
- 32. Mansour, M. K. , Tam, J. M. , Khan, N. S. , Seward, M. , Davids, P. J. , Puranam, S. , Sokolovska, A. , Sykes, D. B. , Dagher, Z. , Becker, C. , Tanne, A. , Reedy, J. L. , Stuart, L. M. , Vyas, J. M. (2013) Dectin‐1 activation controls maturation of β‐1,3‐glucan‐containing phagosomes. J. Biol. Chem. 288, 16043–16054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tam, J. M. , Mansour, M. K. , Khan, N. S. , Yoder, N. C. , Vyas, J. M. (2012) Use of fungal derived polysaccharide‐conjugated particles to probe Dectin‐1 responses in innate immunity. Integr. Biol. (Camb.) 4, 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Di Carlo, F. J. , Fiore, J. V. (1958) On the composition of zymosan. Science 127, 756–757. [DOI] [PubMed] [Google Scholar]
- 35. Chessa, T. A. , Anderson, K. E. , Hu, Y. , Xu, Q. , Rausch, O. , Stephens, L. R. , Hawkins, P. T. (2010) Phosphorylation of threonine 154 in p40phox is an important physiological signal for activation of the neutrophil NADPH oxidase. Blood 116, 6027–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matute, J. D. , Arias, A. A. , Wright, N. A. , Wrobel, I. , Waterhouse, C. C. , Li, X. J. , Marchal, C. C. , Stull, N. D. , Lewis, D. B. , Steele, M. , Kellner, J. D. , Yu, W. , Meroueh, S. O. , Nauseef, W. M. , Dinauer, M. C. (2009) A new genetic subgroup of chronic granulomatous disease with autosomal recessive mutations in p40phox and selective defects in neutrophil NADPH oxidase activity. Blood 114, 3309–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ellson, C. D. , Davidson, K. , Ferguson, G. J. , O'Connor, R. , Stephens, L. R. , Hawkins, P. T. (2006) Neutrophils from p40phox‐/‐ mice exhibit severe defects in NADPH oxidase regulation and oxidant‐dependent bacterial killing. J. Exp. Med. 203, 1927–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bey, E. A. , Xu, B. , Bhattacharjee, A. , Oldfield, C. M. , Zhao, X. , Li, Q. , Subbulakshmi, V. , Feldman, G. M. , Wientjes, F. B. , Cathcart, M. K. (2004) Protein kinase Cδ is required for p47phox phosphorylation and translocation in activated human monocytes. J. Immunol. 173, 5730–5738. [DOI] [PubMed] [Google Scholar]
- 39. Hazeki, K. , Inoue, K. , Nigorikawa, K. , Hazeki, O. (2009) Negative regulation of class IA phosphoinositide 3‐kinase by protein kinase Cδ limits Fcγ receptor‐mediated phagocytosis in macrophages. J. Biochem. 145, 87–94. [DOI] [PubMed] [Google Scholar]
- 40. Li, L. , Sampat, K. , Hu, N. , Zakari, J. , Yuspa, S. H. (2006) Protein kinase C negatively regulates Akt activity and modifies UVC‐induced apoptosis in mouse keratinocytes. J. Biol. Chem. 281, 3237–3243. [DOI] [PubMed] [Google Scholar]
- 41. Tam, J. M. , Mansour, M. K. , Khan, N. S. , Seward, M. , Puranam, S. , Tanne, A. , Sokolovska, A. , Becker, C. E. , Acharya, M. , Baird, M. A. , Choi, A. M. , Davidson, M. W. , Segal, B. H. , Lacy‐Hulbert, A. , Stuart, L. M. , Xavier, R. J. , Vyas, J. M. (2014) Dectin‐1‐dependent LC3 recruitment to phagosomes enhances fungicidal activity in macrophages. J. Infect. Dis. 210, 1844–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cornish, E. J. , Hurtgen, B. J. , McInnerney, K. , Burritt, N. L. , Taylor, R. M. , Jarvis, J. N. , Wang, S. Y. , Burritt, J. B. (2008) Reduced nicotinamide adenine dinucleotide phosphate oxidase‐independent resistance to Aspergillus fumigatus in alveolar macrophages. J. Immunol. 180, 6854–6867. [DOI] [PubMed] [Google Scholar]
- 43. Mircescu, M. M. , Lipuma, L. , van Rooijen, N. , Pamer, E. G. , Hohl, T. M. (2009) Essential role for neutrophils but not alveolar macrophages at early time points following Aspergillus fumigatus infection. J. Infect. Dis. 200, 647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bonnett, C. R. , Cornish, E. J. , Harmsen, A. G. , Burritt, J. B. (2006) Early neutrophil recruitment and aggregation in the murine lung inhibit germination of Aspergillus fumigatus conidia. Infect. Immun. 74, 6528–6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grimm, M. J. , Vethanayagam, R. R. , Almyroudis, N. G. , Dennis, C. G. , Khan, A. N. , D'Auria, A. C. , Singel, K. L. , Davidson, B. A. , Knight, P. R. , Blackwell, T. S. , Hohl, T. M. , Mansour, M. K. , Vyas, J. M. , Röhm, M. , Urban, C. F. , Kelkka, T. , Holmdahl, R. , Segal, B. H. (2013) Monocyte‐ and macrophage‐targeted NADPH oxidase mediates antifungal host defense and regulation of acute inflammation in mice. J. Immunol. 190, 4175–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Philippe, B. , Ibrahim‐Granet, O. , Prévost, M. C. , Gougerot‐Pocidalo, M. A. , Sanchez Perez, M. , Van der Meeren, A. , Latgé, J. P. (2003) Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect. Immun. 71, 3034–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goodridge, H. S. , Shimada, T. , Wolf, A. J. , Hsu, Y. M. , Becker, C. A. , Lin, X. , Underhill, D. M. (2009) Differential use of CARD9 by dectin‐1 in macrophages and dendritic cells. J. Immunol. 182, 1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu, W. , Hsu, Y. M. , Bi, L. , Songyang, Z. , Lin, X. (2009) CARD9 facilitates microbe‐elicited production of reactive oxygen species by regulating the LyGDI‐Rac1 complex. Nat. Immunol. 10, 1208–1214. [DOI] [PubMed] [Google Scholar]
- 49. Glocker, E. O. , Hennigs, A. , Nabavi, M. , Schäffer, A. A. , Woellner, C. , Salzer, U. , Pfeifer, D. , Veelken, H. , Warnatz, K. , Tahami, F. , Jamal, S. , Manguiat, A. , Rezaei, N. , Amirzargar, A. A. , Plebani, A. , Hannesschläger, N. , Gross, O. , Ruland, J. , Grimbacher, B. (2009) A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 361, 1727–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jhingran, A. , Kasahara, S. , Shepardson, K. M. , Junecko, B. A. , Heung, L. J. , Kumasaka, D. K. , Knoblaugh, S. E. , Lin, X. , Kazmierczak, B. I. , Reinhart, T. A. , Cramer, R. A. , Hohl, T. M. (2015) Compartment‐specific and sequential role of MyD88 and CARD9 in chemokine induction and innate defense during respiratory fungal infection. PLoS Pathog. 11, e1004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. d'Ostiani, C. F. , Del Sero, G. , Bacci, A. , Montagnoli, C. , Spreca, A. , Mencacci, A. , Ricciardi‐Castagnoli, P. , Romani, L. (2000) Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 191, 1661–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van der Graaf, C. A. , Netea, M. G. , Verschueren, I. , van der Meer, J. W. , Kullberg, B. J. (2005) Differential cytokine production and Toll‐like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Infect. Immun. 73, 7458–7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gow, N. A. , Brown, A. J. , Odds, F. C. (2002) Fungal morphogenesis and host invasion. Curr. Opin. Microbiol. 5, 366–371. [DOI] [PubMed] [Google Scholar]
- 54. Noble, S. M. , French, S. , Kohn, L. A. , Chen, V. , Johnson, A. D. (2010) Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat. Genet. 42, 590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Biswas, S. , Van Dijck, P. , Datta, A. (2007) Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans . Microbiol. Mol. Biol. Rev. 71, 348–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ziegler, K. , Kurz, C. L. , Cypowyj, S. , Couillault, C. , Pophillat, M. , Pujol, N. , Ewbank, J. J. (2009) Antifungal innate immunity in C. elegans: PKCδ links G protein signaling and a conserved p38 MAPK cascade. Cell Host Microbe 5, 341–352. [DOI] [PubMed] [Google Scholar]


