Short abstract
Macrophages and/or dendritic cells were found to play significant roles in both antibody‐dependent and ‐independent control of B. hermsii infection, while bacterial binding of host factor H was found to be nonessential.
Abstract
Relapsing fever spirochetes, such as Borrelia hermsii, proliferate to high levels in their hosts’ bloodstream until production of IgM against borrelial surface proteins promotes bacterial clearance. The mechanisms by which B. hermsii survives in host blood, as well as the immune mediators that control this infection, remain largely unknown. It has been hypothesized that B. hermsii is naturally resistant to killing by the alternative pathway of complement activation as a result of its ability to bind factor H, a host complement regulator. However, we found that Cfh −/− mice were infected to levels identical to those seen in wild‐type mice. Moreover, only a small minority of B. hermsii in the blood of wild‐type mice had detectable levels of factor H adhered to their outer surfaces. In vitro, complement was found to play a statistically significant role in antibody‐mediated inactivation of B. hermsii, although in vivo studies indicated that complement is not essential for host control of B. hermsii. Depletion of mφ and DC from mice had significant impacts on B. hermsii infection, and depleted mice were unable to control bloodstream infections, leading to death. Infection studies using muMT indicated a significant antibody‐independent role for mφ and/or DC in host control of relapsing fever infection. Together, these findings indicate mφ and/or DC play a critical role in the production of B. hermsii‐specific IgM and for antibody‐independent control of spirochete levels.
Abbreviations
- BSK‐II
Barbour‐Stoenner‐Kelly II,
- C1qa−/−
C1qa‐deficient,
- C3−/−
C3‐deficient,
- Cfh−/−
factor H‐deficient,
- DAH
diffuse alveolar hemorrhage,
- DC
dendritic cells(s),
- HI
heat‐inactivated,
- IFA
immunofluorescence analysis, mφ, macrophage(s),
- muMT
mice deficient of B cells,
- PBS‐T
PBS‐0.05% Tween 20,
- PC
postclearance,
- TI
T cell‐independent,
- UI
uninfected wild‐type mice,
- UK
University of Kentucky,
- VMP‐1
vacuole membrane protein 1
Introduction
The causative agents of relapsing fever are a group of closely related spirochetes that include Borrelia hermsii and are transmitted by fast‐feeding soft ticks of the genus Ornithodoros or the human body louse Pediculus humanus corporis [ 1 , 2 , 3 , 4 , 5 , 6 , 7 ]. Relapsing fever is marked by alternating periods of fever and well being. During febrile episodes, relapsing fever bacteria frequently achieve densities of 103–105 bacteria/μl blood. After 2–3 days of bacteremia, the bacteria are cleared from the bloodstream, as the host produces antibodies directed against bacterial outer‐surface proteins, primarily the major outer‐surface protein (Vlp or Vsp). Allelic recombination in persisting bacteria at their vlp/vsp expression locus can lead to novel gene sequences encoding antigenically distinct Vlp/Vsp, permitting outgrowth and another wave of bacteremia with accompanying fever.
The typical mechanism for clearance of blood‐borne pathogens is through the reticuloendothelial system, where cells such as splenic mφ and liver Kupffer cells can bind bacteria directly or via bound opsonins, such as antibody or complement components [ 8 , 9 , 10 , 11 ]. Antibodies responsible for clearance of relapsing fever spirochetes are primarily of the IgM subclass [ 12 , 13 , 14 , 15 , 16 ]. These IgM molecules are produced in a TI manner by B1b cells and splenic marginal zone B cells [ 12 , 17 , 18 , 19 ]. On its own, IgM is generally considered a poor opsonin but is a potent activator of the classical complement system through binding of C1, which can subsequently promote pathogen uptake and immune activation through complement receptors on phagocytic cells.
In addition, relapsing fever Borrelia spp. must resist their hosts’ alternative pathway of complement‐mediated killing for bacterial survival within the complement‐rich environment of the blood [ 20 , 21 ]. The alternative pathway of complement is activated independently of antibodies and serves as an early defense mechanism before the production of antibodies by the humoral immune response. Activation of the complement system triggers a proteolytic cascade that leads to C3 being cleaved and deposited as C3b on surfaces of invading microorganisms, resulting in opsonization and formation of membrane‐attack complexes. Humans and other vertebrates protect their own cells from damage by the complement system by covering their surfaces with complement regulatory proteins that inactivate C3b. Factor H is the major host serum protein involved in negative regulation of the alternative pathway [ 22 ]. In vitro studies have shown that certain relapsing fever borreliae produce a surface lipoprotein, named FhbA or BhCRASP‐1, which is capable of binding factor H [ 21 , 23 , 24 ]. Binding of factor H to the outer surface of relapsing fever borreliae has been hypothesized to be essential for complement resistance [ 20 , 21 , 23 , 24 , 25 ]. Previously, there had not been any in vivo studies to address the importance of factor H directly in relapsing fever infection. In the present report, we also addressed the roles of additional immune mediators. Different complement‐deficient mouse strains were used to determine the involvement of those components, with particular interest in factor H binding for the virulence of relapsing fever borreliae. We also assessed the relative contributions of different phagocyte populations in clearing these spirochetes from the blood and whether such activities can occur independently of IgM.
MATERIALS AND METHODS
Bacteria cultivation
B. hermsii isolate DAH [ 26 ] was used for all of these studies and was obtained from Dr. Tom Schwan (Rocky Mountain Laboratories, National Institutes of Health, Hamilton, MT, USA). Upon receipt from Dr. Schwan, ∼100 μl of that culture was injected i.p. into a C57BL/6 mouse. At the first peak of bacteremia, the mouse was exsanguinated, and an aliquot of blood was inoculated into BSK‐II medium [ 27 ] containing 12% rabbit serum (Pel‐Freez Biologicals, Rogers, AR, USA) and incubated at 34°C. The outgrown bacterial culture was then divided, and aliquots were frozen for use in all experiments described herein. This method ensured that all experiments used the same genotype bacteria and that those bacteria were most likely to be infectious.
The vlp/vsp expression locus sequences of bacteria in the experimental culture were determined as follows: A 10‐μl aliquot from a 107 bacterial/ml culture was subjected directly to PCR using oligonucleotide primers corresponding to DNA sequences bordering the expression locus open‐reading frame—VMP‐1 (5′‐TAA ACT TTG AAA GTT GAG GTA TAA TGC‐3′) and VMP‐2 (5′‐TAG TAC AAA TCC CCT TGC CGC TTC‐3′) [ 7 ]. The resulting DNA was cloned into pCR2.1 (Invitrogen, Carlsbad, CA, USA) and then used to transform Escherichia coli TOP10 (Invitrogen). Twenty‐four resultant clones were chosen at random, and plasmid insert sequences were determined. Twenty‐two clones (92%) contained a sequence corresponding with vlp18, and one each (4%) contained vlp7 or vsp26.
Mice
Cfh −/− mice (lacking regulation of the alternative pathway) [ 28 , 29 ] and C1qa −/− mice (no classical pathway activity) [ 30 ] have been backcrossed onto a C57BL/6 genetic background for at least seven generations. C57BL/6 C3 −/− mice (no complement activity) [ 31 ] and C57BL/6 Igh‐6 tm1Cgn mice (muMT, lack B cells) [ 32 ] were obtained from Jackson Laboratory (Bar Harbor, ME, USA). Wild‐type C57BL/6 mice were obtained from Harlan Sprague Dawley (Indianapolis, IN, USA).
Mice were housed according to the policies of the Animal Welfare Act, and all experimental protocols used in the handling and infections of mice were approved by the University of Kentucky Institutional Animal Care and Use Committee and the UK Institutional Biosafety Committee. Mice were kept in microisolator cages and given unrestricted access to food and water.
Infection and quantification of B. hermsii in mice
Densities of B. hermsii in infected mouse blood were quantified as follows: Blood (5 μl) was collected from the tail vein of each mouse daily, diluted into 95 μl 0.11 M sodium citrate, pH 7.2, and counted under dark‐field microscopy using a Petroff‐Hausser counting chamber.
Efficient experimental B. hermsii infection of mice is best achieved using host‐adapted spirochetes using a technique essentially as described by Novy and Knapp [ 33 ] and others [ 34 ]. Results using this method were more reproducible than when using in vitro‐cultured spirochetes as experimental inocula. First, female 4‐ to 6‐week‐old mice (“donors”) were infected by i.p. injection with 1 × 106 B. hermsii DAH from a mid‐exponential‐phase culture. This dose was determined empirically to produce consistent, rapid infection. Times to the initial bacteremias of donor mice generally took 2–3 days longer than did mice infected with host‐adapted B. hermsii. At the peak of the first bacteremia, the donor mice were killed and exsanguinated. Bacterial concentrations in donor blood were enumerated, as described above. Experimental mice were then infected with 106 of the host‐adapted B. hermsii via i.p. or retro‐orbital inoculation. For each set of experiments, equal volumes of the same infected blood were used as inocula for all mice in that study. Use of bacteria from the first bacteremic episode ensured that extensive vlp/vsp switching did not occur and that the majority of experimental bacteria was of the same genotype.
Indirect IFA
Blood (5 μl) was collected from the tail vein of three B. hermsii‐infected wild‐type C57BL/6 mice and diluted into 45 μl 0.11 M sodium citrate, pH 7.2. Aliquots (10 μl) were then spread onto glass slides and air‐dried overnight. Specimens were fixed and permeablized by immersion in acetone for 15 min and then allowed to air‐dry. Slides were blocked overnight in PBS containing 2% BSA at 4°C. Slides were then incubated for 1 h at room temperature in goat anti‐human factor H polyclonal antiserum (Quidel, San Diego, CA, USA) or goat anti‐human plasminogen polyclonal antiserum (Novus Biologicals, Littleton, CO, USA), diluted 1:1000 in PBS‐0.2% BSA. The goat anti‐human factor H polyclonal antiserum used has been demonstrated to bind mouse factor H effectively [ 28 ]. Control slides were incubated with each of the secondary antibodies alone. Slides were washed in PBS‐0.2% BSA and incubated for 1 h at room temperature with rabbit polyclonal antiserum raised against Borrelia burgdorferi total membrane proteins, diluted 1:25,000 in PBS‐0.2% BSA [ 35 ]. This antiserum also recognizes B. hermsii cells when used at this concentration. Slides were washed and incubated simultaneously with 1:1000 dilutions, each of Alexa‐Fluor 488‐labeled donkey anti‐goat IgG and Alexa‐Fluor 594‐labeled donkey anti‐rabbit IgG (Molecular Probes, Eugene, OR, USA) for 45 min at room temperature. Slides were then washed, dried, and mounted in ProLong antifade mounting medium (Molecular Probes). Slides were analyzed at 400× magnification using a BX51 epifluorescence microscope (Olympus, Melville, NY, USA). One hundred bacteria/slide were examined for signals, indicating binding of factor H or plasminogen.
In vitro bactericidal action of antibody and complement on B. hermsii
The same culture of B. hermsii DAH described above was used for all aspects of these studies. SDS‐PAGE analyses of bacterial lysates did not detect expression of the alternative surface protein Vtp [ 26 ].
Blood was collected and serum prepared from UI or from mice infected with B. hermsii that had cleared the first wave of bacteremia (PC). Portions of the UI and PC sera were to 56°C for 30 min HI‐UI and HI‐PC, respectively, a well‐established mechanism to inhibit complement activation through the degradation of C3. Mid‐exponential‐phase (∼106 bacteria/ml)‐cultured B. hermsii were then aliquoted in 50 μl vol into separate tubes. Aliquots (50 μl) of freshly prepared UI, HI‐UI, PC, or HI‐PC mouse sera were added to bacteria. To other bacteria, 50 μl fresh culture medium was added to serve as a control. Samples were incubated at 37°C. After 2 h and 24 h, numbers of intact spirochetes and numbers of motile versus nonmotile spirochetes were determined, using dark‐field microscopy and a Petroff Hausser counting chamber. A bacterium was considered to be motile if it detectably moved in any manner. Studies were conducted in triplicate. Volumes of 20 μl (1 mm×1 mm×0.02 mm) were counted for each sample.
In vivo mφ/DC depletion
mφ and DC were depleted from mice by treatment with clodronate (dichloromethylene diphosphonate)‐encapsulated liposomes as described previously [ 36 , 37 ]. Clodronate was a gift of Roche Diagnostics GmbH (Mannheim, Germany). Briefly, mice were i.v.‐injected via the retro‐orbital plexus with 0.2 ml clodronate liposomes 2 days before B. hermsii challenge and subsequently received 0.2 ml clodronate liposomes every 3 days for the duration of the experiment. Control mice received i.v. injections of 0.2 ml PBS. mφ/DC depletion was assessed by flow cytometric analysis as described previously [ 38 ], indicated by a loss of CD11b+/Ly6G− cells from the liver and spleen compared with nondepleted mice.
In vivo neutrophil depletion
Anti‐Gr1 mAb was prepared from B cell hybridoma line RB6‐8C5 (DNAX Research Institute, Palo Alto, CA, USA) and partially purified by ammonium sulfate precipitation. The concentration of mAb was determined by SDS‐PAGE followed by staining with Coomassie brilliant blue, compared with reference concentrations of BSA standards. For depletion studies, nonspecific rat IgG (Sigma‐Aldrich, St. Louis, MO, USA) was used as the negative control. An aliquot (100 μg/mouse) of anti‐Gr1 mAb RB6‐8C5 or rat IgG control was injected i.p. 1 day prior to B. hermsii challenge and again each subsequent day [ 39 ]. Neutrophil depletion was assessed by flow cytometric analysis, indicated by a loss of CD11b+/Ly6G+ cells compared with nondepleted mice.
Analyses of B cell content
The effects of clodronate liposome treatments upon levels of B cells were assessed by flow cytometric analysis, using antibodies against B220, CD5, and CD11b to measure subsets of B cells in the spleen and peritoneum of depleted and nondepleted mice.
Tissue preparation for histology
After euthanasia, half of each mouse liver and spleen was prepared for histology. Sections were placed in cassettes and stored in 10%‐buffered formalin until being embedded in paraffin. Cross‐sections were mounted on slides, stained with H&E, and examined blindly by the UK College of Medicine histology services.
rVlp18
A clone of the B. hermsii DAH vlp18 expression locus (see above) was used as template for construction of a plasmid for production of rVlp18. The initial vlp18 clone was used as template for PCR using vlp18‐specific primers VMP‐3 (5′‐CAC CGG ACA ACA GGC AGT AGA AGC AGG G‐3′) and VMP‐4 (5′‐CTA TCT ATC TTA TTG CTG ACC TGT TGC‐3′), with the resulting amplicon cloned into pET200 TOPO (Invitrogen). The insert of one clone was sequenced completely to confirm that errors had not been introduced during cloning processes. rVlp18 protein was produced from this plasmid by transformed E. coli Rosetta 2(DE3)pLysS (Novagen, San Diego, CA, USA), which was cultivated in super broth medium (32 g/L tryptone, 20 g/L yeast extract, 5 g/L sodium chloride). Bacteria were lysed by sonication, and rVlp18 was purified using MagneHis Ni‐particles (Promega, Madison, WI, USA). Recombinant protein purity was assessed by SDS‐PAGE, followed by staining with Coomassie brilliant blue.
Immunoblot analysis
B. hermsii DAH whole‐cell lysate or purified rVlp18 was separated by SDS‐PAGE, electrotransferred to nitrocellulose membranes, and then blocked overnight at 4°C with 5% nonfat dry milk in TBS‐T [20 mM Tris (pH 7.5), 150 mM NaCl, 0.05% (vol/vol) Tween 20]. Membranes were washed and then incubated for 1 h at room temperature with mouse serum samples, each diluted 1:100 in TBS‐T, using a MiniProtean II multiscreen slot‐blot apparatus (Bio‐Rad, Hercules, CA, USA). Membranes were washed and incubated with goat anti‐mouse IgM (μ‐chain) alkaline phosphatase conjugate (Sigma‐Aldrich) or goat anti‐mouse IgG (whole‐molecule) alkaline phosphatase conjugate (Sigma‐Aldrich). Immunoreactive bands were visualized using Lumi‐Phos WB (Thermo Scientific, Rockford, IL, USA).
ELISA
Serum samples were analyzed in duplicate as follows. Wells of 96‐well Maxisorp Nunc‐Immuno plates (Nalge Nunc International, Naperville, IL, USA) were coated overnight at 4°C with 10 μg/ml B. hermsii whole‐cell lysate, rVlp18, or BSA, each in 100 μl PBS. Wells were then washed in PBS‐T and blocked for 2 h at room temperature in PBS‐T containing 2% BSA. Wells were washed three times and then incubated for 1 h at room temperature with 100 μl 1:500‐diluted mouse sera. Following three additional PBS‐T washes, wells were incubated for 1 h at room temperature with goat anti‐mouse IgM HRP conjugate (Southern Biotechnology, Birmingham, AL, USA), diluted 1:8000. Wells were washed again with PBS‐T, and then 100 μl 3,3′,5,5′‐tetramethylbenzidine (Pierce, Rockford, IL, USA) was added to each well, and color was allowed to develop for 30 min at room temperature. Reactions were stopped by addition of 100 μl 2 N H2SO4 per well. The absorbance at 450 nm was then read with a VersaMax tunable microplate reader and Softmax Pro software (Molecular Devices, Sunnyvale, CA, USA).
Passive transfer of sera
Sera were collected and pooled from naïve C57BL/6 mice (uninfected sera) or from C57BL/6 mice that had been infected with B. hermsii for 6 days and had cleared the initial bacteremia (PC sera). Additional groups of C57BL/6 were depleted of mφ and DC using clodronate liposomes or received PBS for use as controls, as described above. B. hermsii were injected i.v. via the retro‐orbital plexus into all of those mice. One hour postinfection, each mouse received a 250‐μl i.p. injection of UI or PC pooled sera. Development of bacteremia was then assessed by enumeration of bacterial levels in the blood every 7 h postinfection, as described above.
RESULTS
Acquisition of host complement factor H is not essential for B. hermsii infection
Relapsing fever spirochetes such as B. hermsii must have inherent mechanisms to resist killing by their hosts’ alternative pathway of complement activation to survive and replicate in the complement‐rich environment of the blood. Although it has been proposed that this is achieved by binding the major serum regulator of the alternative pathway, factor H [ 20 , 21 , 23 , 24 , 25 ], it had not been established previously whether factor H is truly necessary for in vivo survival of relapsing fever borreliae. To that end, the ability of B. hermsii to infect Cfh −/− mice was assessed. The factor H‐binding hypothesis predicted that B. hermsii should be impaired in its ability to infect Cfh −/− mice, as the alternative pathway could not be inhibited. However, no significant differences in infection parameters were found between Cfh −/− and congenic wild‐type mice ( Fig. 1 and Supplemental Fig. 1). All six members of both mouse cohorts experienced similar initial bacteremia peaks of ∼1 × 105 B. hermsii/μl blood at approximately Day 4 postinfection. Similar rates of bacteremia, clearance, and subsequent relapses were also observed.
Figure 1.
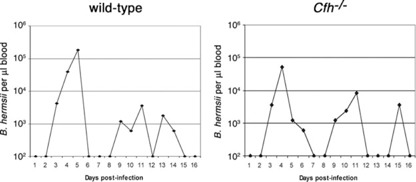
Host factor H is not essential for B. hermsii survival during mammalian infection. Representative plots of B. hermsii numbers in the blood of infected wild‐type and Cfh −/− mice over a 16‐day study, as determined by dark‐field microscopy. The limit of detection of B. hermsii in blood was 102 bacteria/μl. There were no significant differences in peak bacteremia levels for any mice (P>0.1 by Student's t‐test). See Supplemental Figure 1 for data about all experimental animals.
Although cultured B. hermsii have been demonstrated to bind factor H in vitro, the above results led us to question whether the relapsing fever spirochete also binds factor H in vivo. Blood was collected from bacteremic wild‐type mice, and B. hermsii was examined by IFA for surface‐bound factor H. Only 16% of the bloodstream spirochetes had detectable levels of factor H on their surfaces. Taken together, these results indicate that B. hermsii does not require acquisition of host factor H to resist killing by the alternative pathway of complement and apparently uses an additional and/or another complement‐avoidance mechanism(s).
A significant but nonessential role for the complement system in control of B. hermsii
IgM antibodies are critical for B. hermsii clearance, and IgM activation of the complement system can lead directly to pathogen lysis. Therefore, we examined the combined abilities of antibodies and the complement system to kill B. hermsii. For in vitro analyses, serum was collected from infected mice that had cleared initial bacteremia (PC). A portion thereof was heated to inactivate complement (HI‐PC). For use as controls, serum was also collected from UI mice and untreated or HI (UI and HI‐UI, respectively). PC sera contained high titers of IgM directed against the B. hermsii Vlp18 major outer‐surface protein, as well as a number of other borrelial proteins (data not shown). Serum‐incubated bacteria were examined for motility (indicative of retention of cellular functions) and numbers of intact spirochetes (indicative of cell lysis). Following 2 h incubation of B. hermsii in PC serum, the numbers of motile bacteria decreased significantly ( Fig. 2A ). Two‐hour incubation of bacteria in HI‐PC resulted in proportions of motile and nonmotile spirochetes that were identical to the results following incubation in UI, HI‐UI, or culture medium. No significant lysis of bacteria was observed during 2 h of incubation (Fig. 2B). Incubation of B. hermsii for 24 h in PC or HI‐PC serum resulted in significantly reduced numbers of intact bacteria (Fig. 2D). However, statistically significant differences in bacterial motility were observed with bacteria incubated for 24 h in PC versus HI‐PC serum. Of the intact spirochetes observed in those experiments, a significantly greater proportion of those in the PC serum was nonmotile, as compared with bacteria incubated in HI‐PC serum (Fig. 2C). Taken together, these results confirmed that physiologically relevant levels of B. hermsii‐directed antibodies may eventually lyse relapsing fever spirochetes in the absence of complement, yet also demonstrated that complement plus antibody led to enhanced damage to the bacteria.
Figure 2.
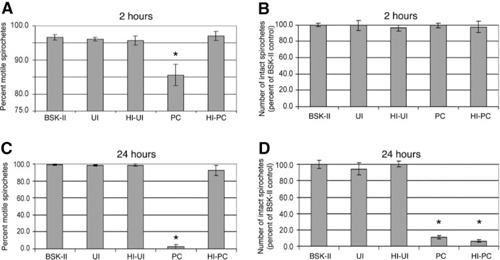
The complement system contributes significantly to antibody‐mediated killing of B. hermsii in vitro. B. hermsii were incubated in 50% freshly isolated UI, HI‐UI, PC, or HI‐PC mouse sera. BSK‐II culture medium alone served as the negative control. Samples were incubated at 37°C, and numbers of motile and intact spirochetes were counted after 2 or 24 h. There were no significant differences in IgM titers after HI of the sera samples (data not shown). (A) Motile spirochetes, as percent of intact bacteria, after 2 h incubation. (B) Number of intact spirochetes after 2 h, relative to the number of intact bacteria in the control BSK‐II wells. (C) Motile spirochetes, as percent of intact bacteria, after 24 h incubation. (D) Number of intact spirochetes after 24 h, relative to the number of intact bacteria in the control BSK‐II wells. Error bars indicate 1 sd from the mean. *, Results statistically significant from BSK‐II control (P<0.05).
Previous studies with other species of relapsing fever borreliae demonstrated that complement is not essential for control of those bacteria by infected mice [ 14 , 16 , 40 ]. To reconcile the results described above with those of earlier investigations, groups of six each wild‐type or congeneic mice defective in the classical complement pathway (C1qa −/−) or the entire complement system (C3 −/−) were infected with B. hermsii. Neither the C1qa −/− nor the C3 −/− mice showed significant differences in any aspect of disease progression when compared with wild‐type mice ( Fig. 3 and Supplemental Fig. 2). All three strains of mice experienced an initial peak bacteremia of ∼1 × 105 B. hermsii/μl at approximately Day 3 postinfection, followed by clearance and typically two relapses over the 13‐day study. Thus, although complement played a statistically significant effect on antibody‐mediated impairment of B. hermsii in vitro, the complement system is not essential for clearance of spirochetes from the bloodstream.
Figure 3.
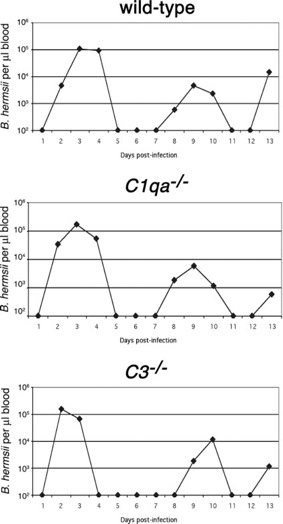
The complement system is not essential for control of B. hermsii. Representative plot of bacterial numbers in blood of B. hermsii‐infected wild‐type, C1qa −/−, and C3 −/− mice throughout 13‐day infections, as determined by dark‐field microscopy. The limit of detection of B. hermsii in blood was 102 bacteria/μl. There were no significant differences in peak bacteremia levels for any mice (P>0.1 by one‐way ANOVA). See Supplemental Figure 2 for data about all experimental animals.
mφ and/or DC are required for clearance of B. hermsii from the blood of mice
Noting that IgM bound to surfaces of bacteria can stimulate opsonization and uptake by phagocytic cells, we investigated next whether those cells play roles in controlling B. hermsii in the blood. One group of six mice was depleted of mφ and DC by i.v. administration of clodronate‐loaded liposomes, which led to 60–75% depletion of CD11b+/Ly6G− cells from the spleen and liver within 24 h of treatment, compared with nondepleted animals ( Fig. 4A ). Depletion was maintained by reinjection with liposomes every 3rd day. A second group of six mice was depleted of neutrophils by injection of anti‐Gr1 mAb [ 39 ], which led to depletion of >98% of CD11b+/Ly6G+ cells by 24 h after antibody treatment, compared with mock‐depleted animals (Fig. 4B). Neutrophil depletion was maintained by daily antibody injections. Another cohort of six mice was depleted of mφ/DC and neutrophils by simultaneous application of both of the treatments described above.
Figure 4.
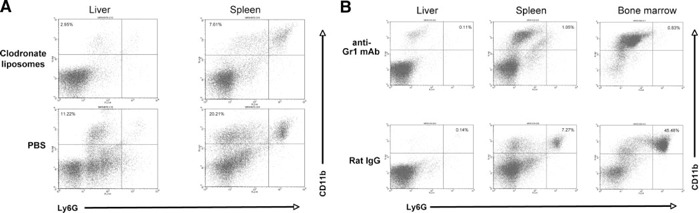
Representative flow cytometric analyses of depletion of mφ/DC and neutrophils from mice. (A) mφ/DC depletion, as demonstrated by loss of CD11b+/Ly6G− cells from the liver and spleen, compared with nondepleted mice. (B) Neutrophil depletion, demonstrated by loss of CD11b+/Ly6G+ cells from the liver, spleen, and bone marrow, as compared with nondepleted mice.
A control group of six mice was mock‐depleted and then infected with B. hermsii. All of those animals displayed typical courses of infection, with initial bacteremia peaking at an average of 2.5 × 105 spirochetes/μl blood, occurring 2 days postinfection. Detectable bacteria were cleared from the blood by Day 4 ( Fig. 5 and Supplemental Fig. 3).
Figure 5.
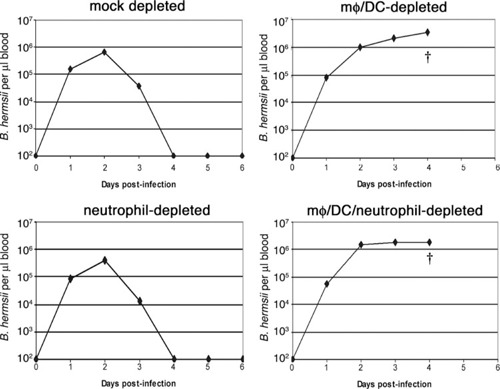
mφ and/or DC are essential for control of B. hermsii infection. Representative results of the progression of disease in mock‐, mφ/DC‐, neutrophil‐, and mφ/DC/neutrophil‐depleted mice infected with B. hermsii, as measured by enumeration of bacteria present in the blood. Bacterial numbers were quantified by dark‐field microscopy during the 6‐day study. The limit of detection of B. hermsii in blood was 102 bacteria/μl. †, Death of the mouse at the day indicated. See Supplemental Figure 3 for data about all experimental animals.
In striking contrast, mice depleted of mφ/DC were unable to clear B. hermsii from their bloodstreams (Fig. 5 and Supplemental Fig. 3). Numbers of bacteria in mφ/DC‐depleted mouse blood achieved levels approximately tenfold greater than seen in mock‐depleted mice, reaching an average of 2.5 × 106 spirochetes/μl blood by Day 4 postinfection. All infected mφ/DC‐depleted mice displayed severe morbidity including weight loss, dehydration, refusal to eat, and lethargy by Day 4 of infection and were killed for humane reasons. Such rapid onset of debilitating symptoms had not been reported from any previous study of experimental murine‐relapsing fever. Histology of the livers of mφ/DC‐depleted mice showed evidence of hemorrhaging and fibrin clotting; however, there was no extramedullary hematopoiesis or increase in megakaryocytes (data not shown). The spleens showed extensive mononuclear cell infiltration, multifocal necrosis, hemorrhage, and fibrin clotting. Splenomegaly, a typical symptom of relapsing fever [ 41 , 42 , 43 ], was not observed in any of the infected mφ/DC‐depleted mice. Conversely, histology of spleens and livers from UI mφ/DC‐depleted mice showed no apparent abnormalities. These results demonstrated that mφ and/or DC are required for the control of B. hermsii infection.
Depletion of neutrophils and other Gr1‐positive cell types did not have any significant effects on B. hermsii infection (Fig. 5 and Supplemental Fig. 3). Numbers of bacteria in the bloodstream peaked at an average of 2.2 × 105 bacteria/μl after 2 days infection, and spirochetes were cleared by Day 4. Neither neutrophil‐depleted nor mock‐depleted animals exhibited any outward signs of morbidity or stress during the course of the infection. Histology performed on the livers and spleens of these two cohorts of infected mice appeared similar, with dramatic increases in extramedullary hematopoiesis and the number of megakaryocytes in both tissues. Splenomegaly was present in both infected cohorts, which was associated with large influxes of mononuclear cells. These data indicate that depletion of neutrophils did not alter the growth of bacteria in vivo or the pathology of infection, demonstrating that neither neutrophils nor other Gr‐1‐positive cell types by themselves play a significant role in the host response and clearance of B. hermsii from the bloodstream.
Infection of mice depleted of mφ/DC and neutrophils yielded results comparable with those seen with mice depleted of only mφ/DC, and bacterial loads in the blood averaged 2.1 × 106 spirochetes/μl (Fig. 5 and Supplemental Fig. 3). As with the mφ/DC‐depleted mice, the dually depleted mice all exhibited severe morbidity by Day 4 and were killed. Necropsy results were comparable with those of the mφ/DC‐depleted mice.
mφ/DC depletion impairs production of B. hermsii‐directed IgM
All infected mice from the studied described above were analyzed for serum antibodies ( Fig. 6 ). Sera from mock‐depleted mice and neutrophil‐depleted mice showed strong antibody responses to multiple borrelial proteins, primarily of the IgM isotype. As predicted from the abilities of these mice to clear the initial bacteremia, sera contained antibodies directed against the major B. hermsii outer‐surface protein, Vlp18. In contrast, mice depleted of mφ/DC or mφ/DC and neutrophils produced far lower levels of B. hermsii‐directed antibodies. ELISA indicated that mφ/DC‐ and mφ/DC/neutrophil‐depleted mice each produced approximately tenfold less IgM, which recognized all antigens of B. hermsii, and eightfold less Vlp18‐specific IgM than did mock‐ or neutrophil‐depleted animals (Fig. 6 C and D). Although there appeared to be a slight decrease in B. hermsii‐specific antibody production in the neutrophil‐depleted mice, these values were not significantly different from mock‐depleted animals. Quantification of B1 and B2 cells by flow cytometry indicated that clodronate liposome treatment did not affect either subset of B cells (Supplemental Fig. 4).
Figure 6.
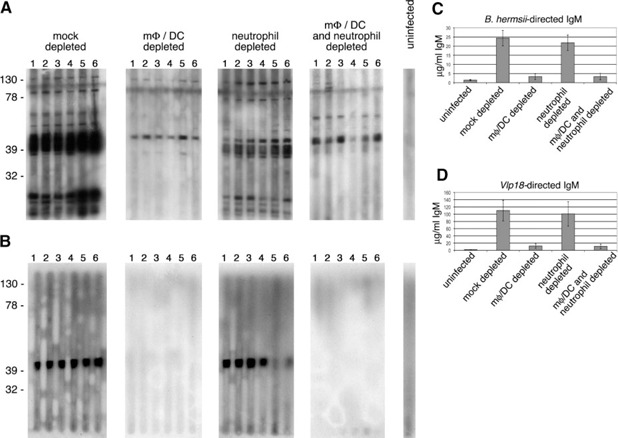
mφ/DC depletion impairs production of antibodies against B. hermsii. (A) Immunoblot analyses of B. hermsii‐directed IgM, using borrelial whole cell lysate as target. (B) Immunoblot analyses of Vlp18‐directed IgM, using rVlp18 as target. The treatment of each cohort and mouse number is indicated along the top of each column. The positions of molecular mass markers (in kDa) are indicated to the left of each row. (C and D) Results of IgM ELISA using B. hermsii whole cell lysate or rVlp18 as target.
One of the functions of mφ and DC is to present antigens to antibody‐producing cells. We therefore examined the ability of mφ/DC‐depleted mice to control B. hermsii infection if provided with antiborrelial serum by passive transfer. Mouse cohorts were depleted of mφ/DC or mock‐depleted, infected with B. hermsii, and then injected with serum from mice that had cleared initial bacteremia (PC) or from naïve mice (UI). All of the mice that received serum from mice previously UI developed bacteremia ( Fig. 7 ). Regardless of mφ/DC content, all but one mouse that received PC serum were able to control the infection.
Figure 7.
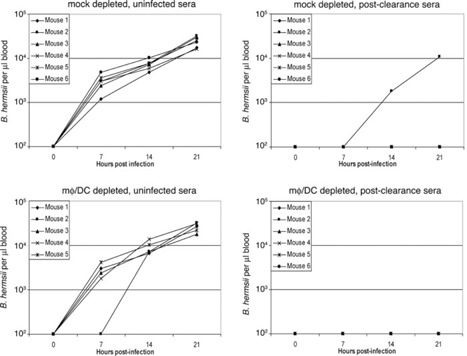
Passive transfer of B. hermsii‐directed serum is sufficient to prevent bacteremia in mΦ/DC‐depleted mice. Prior to infection, mice were depleted of mΦ/DC or mock‐depleted and then injected i.v. with B. hermsii. One hour later, mice were injected i.p. with pooled serum from UI mice or from mice that had cleared initial bacteremia (PC). Bacteria numbers in the bloodstreams of all mice were counted every 7 h. The limit of detection of B. hermsii in blood was 102 bacteria/μl.
mφ and/or DC are essential for antibody‐independent control of B. hermsii
Results of the studies described above raised the possibility that the inability of mφ/DC‐depleted mice to control B. hermsii numbers in the bloodstream was simply a result of their reduced levels of spirochete‐directed antibodies. To address that question, we used muMT mice, which lack mature B cells and are therefore unable to produce antibodies. Mock‐depleted muMT animals infected with B. hermsii developed persistent bacteremias, averaging ∼5 × 105 bacteria/μl blood ( Fig. 8 and Supplemental Fig. 5). For all of these mice, bacteria numbers in the blood decreased five‐ to tenfold after an initial peak bacteremia, indicating an antibody‐independent mechanism(s) of reducing bacteremia. Mock‐depleted mice did not exhibit any overt symptoms and behaved as would UI mice. Similar results have been reported from studies that used scid mice [ 12 , 44 ]. In contrast, infected mφ/DC‐depleted muMT mice acquired bacteremias that were approximately tenfold higher than those of the mock‐depleted animals (Fig. 8 and Supplemental Fig. 4). As with wild‐type mφ/DC‐depleted mice, the depleted muMT became debilitatingly ill by Day 4 of infection and were killed for humane reasons. These results indicate that mφ/DC perform a critical, antibody‐independent function necessary for controlling B. hermsii levels in the blood.
Figure 8.
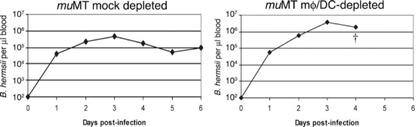
mφ/DC are essential for the control of B. hermsii, even in the absence of antibody. Representative results of bloodstream numbers of B. hermsii in mΦ/DC‐ and mock‐depleted muMT. †, Death of the mouse at the day indicated. The limit of detection of B. hermsii in blood was 102 bacteria/μl. See Supplemental Figure 4 for data about all experimental animals.
DISCUSSION
Relapsing fever Borrelia spp. replicate to high levels in their hosts’ bloodstream, achieving bacterial densities of upwards of 105 bacteria/μl. Immunocompetent hosts produce IgM antibodies targeting borrelial outer‐surface proteins, which lead to rapid clearance of spirochetes from the bloodstream. mφ and/or DC were found to be critical for production B. hermsii‐directed antibodies. In addition, our studies indicate that mφ and/or DC play a significant role(s) in antibody‐independent control of B. hermsii numbers in the bloodstream. These cell types may be involved with IL‐10‐ and/or MyD88‐dependent, antibody‐independent activity observed previously [ 44 , 45 ]. We note, however, that B. hermsii‐infected, MyD88‐deficient mice produced wild‐type levels of IgM and exhibited splenomegaly and other symptoms not observed in mφ/DC‐depleted mice [ 44 ]. Thus, we conclude that these phagocytic cells play at least two critical roles in controlling relapsing fever infection.
A less‐important role was found for complement. In vitro, complement played a small but statistically significant role in antibody‐mediated killing of B. hermsii. In vivo, however, the complement system was not required for control of B. hermsii infection, much as has been seen earlier for other species of relapsing fever spirochetes [ 16 , 40 ]. The present studies also suggested that binding of host factor H to B. hermsii surfaces is not required for spirochetal resistance to complement, in that wild‐type and Cfh −/− mice were equally well infected by B. hermsii, and only a small proportion of B. hermsii in the bloodstreams of wild‐type mice had detectable levels of factor H on their surfaces. The B. hermsii FhbA/BhCRASP‐1 protein, which can bind factor H in vitro, can also bind host plasminogen [ 21 ]. Relating to that function, we observed that 71% of B. hermsii found in the blood of wild‐type mice had detectable levels of plasminogen on their surfaces (data not shown), suggesting that this may be the major function of FhbA/BhCRASP‐1 in vivo. We note that relapsing fever bacteria can bind other complement regulators to their surfaces, such as C4b‐binding protein, which may contribute to resistance to complement‐mediated killing [ 20 , 46 ]. Earlier studies found that the related Lyme disease spirochete, B. burgdorferi, can also infect Cfh −/− mice efficiently [ 28 ], indicating that it, too, possesses an alternate/additional mechanism(s) to evade killing by its hosts’ alternative pathway of complement activation. These results bring into the question the general paradigm that the ability of a pathogen to bind factor H in vitro implies a significant role for that characteristic during actual infection processes. It may be wise to revisit other pathogens to elucidate the in vivo importance of their abilities to acquire factor H.
Results of these studies, in combination with data from previous work, provide a more detailed understanding of the mechanisms used by humans and other hosts to control relapsing fever infections. Depletion of marginal‐zone mφ by clod‐ronate liposomes has indicated an importance for these cells in processing and presenting certain TI antigens [ 47 ]. B. hermsii appears to have multiple components (TI‐1 and TI‐2 antigens) that contribute to induction of the TI antibody response [ 48 ]. However, it is still unclear if mφ and/or DC are required for direct antigen presentation to these cells for IgM production or whether an additional mφ/DC‐dependent mechanism is required. For example, mφ and DC may produce cytokines or other factors important for efficient production of bacteria‐specific antibodies or for survival of B cells [ 49 , 50 , 51 , 52 , 53 , 54 ]. MyD88‐deficient mice produce abundant antiborrelial IgM [ 44 ], suggesting that the mφ/DC role in antibody production is at least partially MyD88‐independent. B1b cells are triggered to produce high levels of IgM, which then eliminate B. hermsii from the bloodstream [ 12 , 19 ]. Although stimulation of B1b and marginal‐zone B cells by TLR agonists on pathogen surfaces can directly lead to their activation and production of IgM [ 48 , 55 , 56 ], our data indicate that mφ and/or DC provide additional stimulation. The exact mechanism(s) of IgM‐mediated clearance are not yet understood. In vitro, some borrelia‐specific IgM mAb are capable of lysing some strains of relapsing fever Borrelia in the absence of complement [ 13 , 15 ]. Other antiborrelial IgM mAb will agglutinate but not lyse B. hermsii directly [ 13 ]. Our results indicate that although physiologically relevant levels of serum IgM alone can be directly bactericidal to B. hermsii after prolonged exposure (24 h), significant levels of bacterial lysis did not occur within 2 h incubation with serum. Similarly, rapid inhibitory but nonlytic effects of PC serum were reported by Novy and Knapp [ 33 ] in their seminal 1906 studies of the relapsing fever organism “Spirillum obermeieri” (probably Borrelia recurrentis). Complement contributed to in vitro antibody‐mediated killing during short (2 h)‐ as well as long‐term (24 h) incubations. Similarly, the presence of serum complement has been reported to enhance bacterial killing by an anti‐B. hermsii IgM mAb [ 13 ]. IgM is a poor opsonin but a potent activator of complement that promotes C3b‐mediated bacterial uptake by phagocytic cells. However, our studies of B. hermsii mirrored those of other relapsing fever species, finding that the complement system is not essential for clearance of relapsing fever Borrelia spp [ 14 , 16 , 40 ]. These results suggest that IgM may mediate opsonization and uptake of B. hermsii directly, in the absence of complement. However, as mφ/DC depletion was not absolute, we cannot completely rule out a role for these cells in B. hermsii clearance from blood. Opsonization may be mediated through IgM directly and promote uptake of B. hermsii in the absence of complement. The complement receptors CR3 and CR4 have been reported to promote complement‐independent, antibody‐mediated phagocytosis of pathogens [ 57 ]. Additionally, an FcR, capable of binding IgM, Fcα/μ, is expressed on mφ and B cells in tissues, including liver, spleen, and kidney [ 58 ], and may play a role in B. hermsii clearance. Antiborrelial IgM might damage the outer membranes of the spirochetes, exposing a bacterial ligand(s) that could trigger phagocytosis by mφ/DC. Our current studies with B cell‐deficient mice suggest a critical, antibody‐independent role for mφ/DC control of B. hermsii in the blood, in that spirochetes infecting mφ/DC‐depleted muMT mice reached blood densities tenfold greater than did nondepleted muMT mice. In contrast, neither neutrophils nor other Gr1‐positive cells played a significant role in the clearance of B. hermsii. Polymorphonuclear leukocytes previously have been demonstrated to phagocytose B. hermsii readily in the presence of antibody in vitro [ 59 ]. However, it remains unknown whether B. hermsii are taken up inefficiently by neutrophils during infection or are able to survive within these cells by production of protective substances [ 60 ].
In conclusion, these studies demonstrate that acquisition of host factor H by B. hermsii is not necessary for bacterial resistance to the alternative pathway of complement activation and that complement plays a relatively minor role in clearing B. hermsii from the bloodstream. Significant roles were discovered for mφ/DC in production of antiborrelial antibodies and in an antibody‐independent mechanism of controlling bloodstream numbers of B. hermsii. Further studies of the host‐pathogen interactions of relapsing fever will continue to illuminate mechanisms by which humans and other hosts are overwhelmed initially by this spirochete, but later mount effective immune responses to control and eliminate bacteremia.
ACKNOWLEDGMENTS
These studies were funded by an exploratory grant to B. S. from the UK College of Medicine. We thank Subbarao Bondada, Catherine Brissette, Sarah D'Orazio, Jeff Ebersole, Beth Garvy, Alan Kaplan, Susan Straley, Sean Riley, and Ashutosh Verma for helpful comments about these studies and the manuscript.
Supporting information
Supplementary Material Files
REFERENCES
- 1. Barbour, A. G. (1990) Antigenic variation of a relapsing fever Borrelia species. Annu. Rev. Microbiol. 44, 155–171. [DOI] [PubMed] [Google Scholar]
- 2. Barbour, A. G. , Dai, Q. , Restrepo, B. I. , Stoenner, H. G. , Frank, S. A. (2006) Pathogen escape from host immunity by a genome program for antigenic variation. Proc. Natl. Acad. Sci. USA 103, 18290–18295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carlisle, R. J. (1906) Two cases of relapsing fever; with notes on the occurrence of this disease throughout the world at the present day. J. Infect. Dis. 3, 233–265. [Google Scholar]
- 4. Dutton, J. E. , Todd, J. L. (1905) The nature of tick fever in the eastern part of Congo Free State. Br. Med. J. 1259–1260. [Google Scholar]
- 5. Plasterk, R. H. A. , Simon, M. I. , Barbour, A. G. (1985) Transposition of structural genes to an expression sequence on a linear plasmid causes antigenic variation in the bacterium Borrelia hermsii. Nature 318, 257–263. [DOI] [PubMed] [Google Scholar]
- 6. Stoenner, H. G. , Dodd, T. , Larsen, C. (1982) Antigenic variation in Borrelia hermsii. J. Exp. Med. 156, 1297–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dai, Q. , Restrepo, B. I. , Porcella, S. F. , Raffel, S. J. , Schwan, T. G. , Barbour, A. G. (2006) Antigenic variation by Borrelia hermsii occurs through recombination between extragenic repetitive elements on linear plasmids. Mol. Microbiol. 60, 1329–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benacerraf, B. , Sebestyen, M. M. , Schlossman, S. (1959) A quantitative study of the kinetics of blood clearance of P32‐labeled Escherichia coli and Staphylococci by the reticuloendothelial system. J. Exp. Med. 110, 27–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cousens, L. P. , Wing, E. J. (2000) Innate defenses in the liver during Listeria infection. Immunol. Rev. 174, 150–159. [DOI] [PubMed] [Google Scholar]
- 10. Altamura, M. , Caradonna, L. , Amati, L. , Pellegrino, N. M. , Urgesi, G. , Miniello, S. (2001) Splenectomy and sepsis: the role of the spleen in the immune‐mediated bacterial clearance. Immunopharmacol. Immunotoxicol. 23, 153–161. [DOI] [PubMed] [Google Scholar]
- 11. Bohnsack, J. F. , Brown, E. J. (1986) The role of the spleen in resistance to infection. Annu. Rev. Med. 37, 49–59. [DOI] [PubMed] [Google Scholar]
- 12. Alugupalli, K. R. , Gerstein, R. M. , Chen, J. , Szomolanyi‐Tsuda, E. , Woodland, R. T. , Leong, J. M. (2003) The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J. Immunol. 170, 3819–3827. [DOI] [PubMed] [Google Scholar]
- 13. Barbour, A. G. , Bundoc, V. (2001) In vitro and in vivo neutralization of the relapsing fever agent Borrelia hermsii with serotype‐specific immunoglobulin M antibodies. Infect. Immun. 69, 1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Connolly, S. E. , Benach, J. L. (2005) The versatile roles of antibodies in Borrelia infections. Nat. Rev. Microbiol. 3, 411–420. [DOI] [PubMed] [Google Scholar]
- 15. Connolly, S. E. , Thanassi, D. G. , Benach, J. L. (2004) Generation of a complement‐independent bactericidal IgM against a relapsing fever Borrelia. J. Immunol. 172, 1191–1197. [DOI] [PubMed] [Google Scholar]
- 16. Connolly, S. E. , Benach, J. L. (2001) Cutting edge: the spirochetemia of murine relapsing fever is cleared by complement‐independent bactericidal antibodies. J. Immunol. 167, 3029–3032. [DOI] [PubMed] [Google Scholar]
- 17. Alugupalli, K. R. (2008) A distinct role for B1b lymphocytes in T cell‐independent immunity. Curr. Top. Microbiol. Immunol. 319, 105–130. [DOI] [PubMed] [Google Scholar]
- 18. Newman, K. , Johnson, R. C. (1984) T‐cell‐independent elimination of Borrelia turicatae. Infect. Immun. 45, 572–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Belperron, A. A. , Dailey, C. M. , Bockenstedt, L. K (2005) Infection‐induced marginal zone B cell production of Borrelia hermsii‐specific antibody is impaired in the absence of CD1d. J. Immunol. 174, 5681–5686. [DOI] [PubMed] [Google Scholar]
- 20. Meri, T. , Cutler, S. J. , Blom, A. M. , Meri, S. , Jokiranta, T. S. (2006) Relapsing fever spirochetes Borrelia recurrentis and B. duttonii acquire complement regulators C4b‐binding protein and factor H. Infect. Immun. 74, 4157–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rossmann, E. , Kraiczy, P. , Herzberger, P. , Skerka, C. , Kirschfink, M. , Simon, M. M. , Zipfel, P. F. , Wallich, R. (2007) Dual binding specificity of a Borrelia hermsii‐associated complement regulator‐acquiring surface protein for factor H and plasminogen discloses a putative virulence factor of relapsing fever spirochetes. J. Immunol. 178, 7292–7301. [DOI] [PubMed] [Google Scholar]
- 22. Janeway, C. A. , Travers, P. , Walport, M. , Capra, J. D. (1999) Immunobiology, New York, NY, USA, Elsevier Science. [Google Scholar]
- 23. Hovis, K M. , Jones, J. P. , Sadlon, T. , Raval, G. , Gordon, D. L. , Marconi, R. T. (2006) Molecular analyses of the interaction of Borrelia hermsii FhbA with the complement regulatory proteins factor H and factor H‐like protein 1. Infect. Immun. 74, 2007–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hovis, K M. , McDowell, J. V. , Griffin, L. , Marconi, R. T. (2004) Identification and characterization of a linear‐plasmid‐encoded factor H‐binding protein (FhbA) of the relapsing fever spirochete Borrelia hermsii. J. Bacteriol. 186, 2612–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McDowell, J. V. , Tran, E. , Hamilton, D. , Wolfgang, J. , Miller, K. , Marconi, R. T. (2003) Analysis of the ability of spirochete species associated with relapsing fever, avian borreliosis, and epizootic bovine abortion to bind factor H and cleave C3b. J. Clin. Microbiol. 41, 3905–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schwan, T. G. , Hinnebusch, B. J. (1998) Bloodstream‐ versus tick‐associated variants of a relapsing fever bacterium. Science 280, 1938–1940. [DOI] [PubMed] [Google Scholar]
- 27. Barbour, A. G. (1984) Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57, 521–525. [PMC free article] [PubMed] [Google Scholar]
- 28. Woodman, M. E. , Cooley, A. E. , Miller, J. C. , Lazarus, J. J. , Tucker, K. , Bykowski, T. , Botto, M. , Hellwage, J. , Wooten, R. M. , Stevenson, B. (2007) Borrelia burgdorferi binding of host complement regulator factor H is not required for efficient mammalian infection. Infect. Immun. 75, 3131–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pickering, M. C. , Cook, H. T. , Warren, J. , Bygrave, A. E. , Moss, J. , Walport, M. J. , Botto, M. (2002) Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in factor H. Nat. Genet. 31, 424–428. [DOI] [PubMed] [Google Scholar]
- 30. Botto, M. , Dell'Agnola, C. , Bygrave, A. E. , Thompson, E. M. , Cook, H. T. , Petry, F. , Loos, M. , Pandolfi, P. P. , Walport, M. J. (1998) Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 19, 56–59. [DOI] [PubMed] [Google Scholar]
- 31. Wessels, M. R. , Butko, P. , Ma, M. , Warren, H. B. , Lage, A. L. , Carroll, M. C. (1995) Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. USA 92, 11490–11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kitamura, D. , Roes, J. , Kuhn, R. , Rajewsky, K (1991) A B cell‐deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature 350, 423–426. [DOI] [PubMed] [Google Scholar]
- 33. Novy, F. G. , Knapp, R. E. (1906) Studies on Spirillum obermeieri and related organisms. J. Infect. Dis. 3, 291–393. [Google Scholar]
- 34. Garcia‐Monco, J. C. , Miller, N. S. , Backenson, P. B. , Anda, P. , Benach, J. L. (1997) A mouse model of Borrelia meningitis after intradermal injection. J. Infect. Dis. 175, 1243–1245. [DOI] [PubMed] [Google Scholar]
- 35. Miller, J. C. , von Lackum, K, Babb, K, McAlister, J. D. , Stevenson, B. (2003) Temporal analysis of Borrelia burgdorferi Erp protein expression throughout the mammal‐tick infectious cycle. Infect. Immun. 71, 6943–6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Rooijen, N. , Kors, N. , vd Ende, M. , Dijkstra, C. D. (1990) Depletion and repopulation of macrophages in spleen and liver of rat after intravenous treatment with liposome‐encapsulated dichloromethylene diphosphonate. Cell Tissue Res. 260, 215–222. [DOI] [PubMed] [Google Scholar]
- 37. Van Rooijen, N. , Sanders, A. (1994) Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174, 83–93. [DOI] [PubMed] [Google Scholar]
- 38. Qualls, J. E. , Kaplan, A. M. , van Rooijen, N. , Cohen, D. A. (2006) Suppression of experimental colitis by intestinal mononuclear phagocytes. J. Leukoc. Biol. 80, 802–815. [DOI] [PubMed] [Google Scholar]
- 39. Daley, J. M. , Thomay, A. A. , Connolly, M. D. , Reichner, J. S. , Albina, J. E. (2008) Use of Ly6G‐specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83, 64–70. [DOI] [PubMed] [Google Scholar]
- 40. Newman, K. , Johnson, R. C. (1981) In vivo evidence that an intact lytic complement pathway is not essential for successful removal of circulating Borrelia turicatae from mouse blood. Infect. Immun. 31, 465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barclay, A. J. , Coulter, J. B. (1990) Tick‐borne relapsing fever in central Tanzania. Trans. R. Soc. Trop. Med. Hyg. 84, 852–856. [DOI] [PubMed] [Google Scholar]
- 42. Fihn, S. , Larson, E. B. (1980) Tick‐borne relapsing fever in the Pacific Northwest: an underdiagnosed illness? West. J. Med. 133, 203–209. [PMC free article] [PubMed] [Google Scholar]
- 43. Gebbia, J. A. , Monco, J. C. , Degen, J. L. , Bugge, T. H. , Benach, J. L. (1999) The plasminogen activation system enhances brain and heart invasion in murine relapsing fever borreliosis. J. Clin. Invest. 103, 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bolz, D. D. , Sundsbak, R. S. , Ma, Y. , Akira, S. , Weis, J. H. , Schwan, T. G. , Weis, J. J. (2006) Dual role of MyD88 in rapid clearance of relapsing fever Borrelia spp. Infect. Immun. 74, 6750–6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Londoño, D. , Marques, A. , Hornung, R. L. , Cadavid, D. (2008) IL‐10 helps control pathogen load during high‐level bacteremia. J. Immunol. 181, 2076–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grosskinsky, S. , Schott, M. , Brenner, C. , Cutler, S. J. , Kraiczy, P. , Zipfel, P. F. , Simon, M. M. , Wallich, R. (2009) Borrelia recurrentis employs a novel multifunctional surface protein with anti‐complement, anti‐opsonic and invasive potential to escape innate immunity. PLoS ONE 4, e4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Claassen, E. , Kors, N. , Dijkstra, C. D. , van Rooijen, N. (1986) Marginal zone of the spleen and the development and localization of specific antibody‐forming cells against thymus‐dependent and thymus‐independent type‐2 antigens. Immunology 57, 399–403. [PMC free article] [PubMed] [Google Scholar]
- 48. Alugupalli, K R. , Akira, S. , Lien, E. , Leong, J. M. (2007) MyD88‐ and Bruton's tyrosine kinase‐mediated signals are essential for T cell‐independent pathogen‐specific IgM responses. J. Immunol. 178, 3740–3749. [DOI] [PubMed] [Google Scholar]
- 49. Balazs, M. , Martin, F. , Zhou, T. , Kearney, J. (2002) Blood dendritic cells interact with splenic marginal zone B cells to initiate T‐independent immune responses. Immunity 17, 341–352. [DOI] [PubMed] [Google Scholar]
- 50. Kim, H. A. , Jeon, S. H. , Seo, G. Y. , Park, J. B. , Kim, P. H. (2008) TGF‐β1 and IFN‐γ stimulate mouse macrophages to express BAFF via different signaling pathways. J. Leukoc. Biol. 83, 1431–1439. [DOI] [PubMed] [Google Scholar]
- 51. Wykes, M. , MacPherson, G. (2000) Dendritic cell‐B‐cell interaction: dendritic cells provide B cells with CD40‐independent proliferation signals and CD40‐dependent survival signals. Immunology 100, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sapoznikov, A. , Pewzner‐Jung, Y. , Kalchenko, V. , Krauthgamer, R, Shachar, I. , Jung, S. (2008) Perivascular clusters of dendritic cells provide critical survival signals to B cells in bone marrow niches. Nat. Immunol. 9, 388–395. [DOI] [PubMed] [Google Scholar]
- 53. MacLennan, I. , Vinuesa, C. (2002) Dendritic cells, BAFF, and APRIL: innate players in adaptive antibody responses. Immunity 17, 235–238. [DOI] [PubMed] [Google Scholar]
- 54. Boswell, H. S. , Ahmed, A. , Scher, I. , Singer, A. (1980) Role of accessory cells in B cell activation. II. The interaction of B cells with accessory cells results in the exclusive activation of an Lyb5+ B cell subpopulation. J. Immunol. 125, 1340–1348. [PubMed] [Google Scholar]
- 55. Rubtsov, A. V. , Swanson, C. L. , Troy, S. , Strauch, P. , Pelanda, R. , Torres, R. M. (2008) TLR agonists promote marginal zone B cell activation and facilitate T‐dependent IgM responses. J. Immunol. 180, 3882–3888. [DOI] [PubMed] [Google Scholar]
- 56. Vos, Q. , Lees, A. , Wu, Z. Q. , Snapper, C. M. , Mond, J. J. (2000) B‐cell activation by T‐cell‐independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol. Rev. 176, 154–170. [DOI] [PubMed] [Google Scholar]
- 57. Taborda, C. P. , Casadevall, A. (2002) CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement‐independent antibody‐mediated phagocytosis of Cryptococcus neoformans. Immunity 16, 791–802. [DOI] [PubMed] [Google Scholar]
- 58. Shibuya, A. , Sakamoto, N. , Shimizu, Y. , Shibuya, K, Osawa, M. , Hiroyama, T. , Eyre, H. J. , Sutherland, G. R. , Endo, Y. , Fujita, T. , Miyabayashi, T. , Sakano, S. , Tsuji, T. , Nakayama, E. , Phillips, J. H. , Lanier, L. L. , Nakauchi, H. (2000) Fc α/μ receptor mediates endocytosis of IgM‐coated microbes. Nat. Immunol. 1, 441–446. [DOI] [PubMed] [Google Scholar]
- 59. Spagnuolo, P. J. , Butler, T. , Bloch, E. H. , Santoro, C. , Tracy, J. W. , Johnson, R. C. (1982) Opsonic requirements for phagocytosis of Borrelia hermsii by human polymorphonuclear leukocytes. J. Infect. Dis. 145, 358–364. [DOI] [PubMed] [Google Scholar]
- 60. Guyard, C. , Battisti, J. M. , Raffel, S. J. , Schrumpf, M. E. , Whitney, A. R. , Krum, J. G. , Porcella, S. F. , Rosa, P. A. , DeLeo, F. R. , Schwan, T. G. (2006) Relapsing fever spirochetes produce a serine protease that provides resistance to oxidative stress and killing by neutrophils. Mol. Microbiol. 60, 710–722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material Files


