Short abstract
Characterization of the dynamic interactions between leukocytes and microbes within the brain of a living animal.
Keywords: phagocytes, intravital, fungus, clearance, complement
Abstract
Although neutrophils are typically the first immune cells attracted to an infection site, little is known about how neutrophils dynamically interact with invading pathogens in vivo. Here, with the use of intravital microscopy, we demonstrate that neutrophils migrate to the arrested Cryptococcus neoformans, a leading agent to cause meningoencephalitis, in the brain microvasculature. Following interactions with C. neoformans, neutrophils were seen to internalize the organism and then circulate back into the bloodstream, resulting in a direct removal of the organism from the endothelial surface before its transmigration into the brain parenchyma. C. neoformans infection led to enhanced expression of adhesion molecules macrophage 1 antigen on neutrophils and ICAM‐1 on brain endothelial cells. Depletion of neutrophils enhanced the brain fungal burden. Complement C3 was critically involved in the recognition of C. neoformans by neutrophils and subsequent clearance of the organism from the brain. Together, our finding of the direct removal of C. neoformans by neutrophils from its arrested site may represent a novel mechanism of host defense in the brain, in addition to the known, direct killing of microorganisms at the infection sites. These data are the first to characterize directly the dynamic interactions of leukocytes with a microbe in the brain of a living animal.
Abbreviations
- APC
allophycocyanin
- ATCC
American Type Culture Collection
- BBB
blood‐brain barrier
- Cn
Cryptococcus neoformans
- Cn H99‐GFP
Cryptococcus neoformans H99 expressing GFP
- CR3
complement receptor 3
- i.v.
intravenous
- IVM
intravital microscopy
- Mac‐1
macrophage 1 antigen
- MFI
mean fluorescence intensity
Introduction
Meningoencephalitis, caused by microbes, is a devastating disease associated with high mortality worldwide. To cause the illness, circulating organisms are firstly arrested in the brain vessels, followed by crossing the BBB [1]. Intravascular recognition and clearance of the arrested organisms before their BBB crossing are thought to be one of the most critical steps to prevent or ameliorate meningoencephalitis, as once the organisms reach the parenchyma, they will proliferate rapidly and cause fatal diseases [1]. However, the intravascular interactions of immune cells with the arrested organisms in the brain are largely unknown as a result of prior limitations in experimental approaches. Neutrophils, the most abundant phagocytes in the bloodstream, are typically the first immune cells to be recruited to an infection site to eliminate pathogens [2]. In the current study, with the use of IVM, we aimed to characterize how neutrophils recognized and responded to the arrested Cn, a pathogen causing human and animal meningoencephalitis, in the brain microvasculature in real time.
Cn is an encapsulated fungus with global distribution. After the aerosolized fungal cells are inhaled, they multiply in the lung, leading to infection. However if Cn disseminates from the lung into the brain via the bloodstream, this can have devastating consequences and lead to fatal meningoencephalitis, particularly in immunocompromised individuals, such as AIDS patients, organ‐transplant recipients, and cancer patients [3, 4]. There are 1 million cases of cryptococcosis and ∼600,000 related deaths worldwide annually [5].
In vitro, neutrophils are able to kill Cn via an intracellular [6, 7] or extracellular killing mechanism [8]. Both oxidative [6, 9] and nonoxidative [10] killing of Cn by neutrophils have been described in vitro. The augmenting of neutrophil defenses by administration of G‐CSF enhances anticryptococcal activity in animal models [11], as well as in AIDS patients [12, 13]. A deficiency of myeloperoxidase, an enzyme with the most abundant expression in neutrophils [14], significantly shortens the survival of Cn‐infected mice with higher brain fungal burden [15]. In addition, relative resistance to pulmonary Cn infection correlates to accumulation of neutrophils and elevated levels of the neutrophil chemokines keratinocyte‐derived chemokine/CXCL1 in the lung of mice [16]. More recently, it has been shown that type I IFN induction via polyinosinic:polycytidylic acid with carboxymethylcellulose and poly‐l‐lysine protects mice against cryptococcosis, and this protection is associated with a large, rapid, and sustained influx of neutrophils and monocytes into the lung [17]. These results suggest that neutrophils contribute to host defense in cryptococcal meningoencephalitis in vivo. However, it remains largely unknown how neutrophils dynamically interact with Cn in vivo, in particular, during the Cn dissemination into the brain.
We [18] and others [19, 20] have recently shown that disseminating Cn is arrested in the brain vessels and stays for a long period before invading the parenchyma of the brain. In this study, we revealed further that neutrophils could clear the arrested Cn through a direct removal of the fungus from the endothelium surface before its BBB invasion, with a critical involvement of complement C3 and adhesion molecules Mac‐1 and ICAM‐1.
MATERIALS AND METHODS
Ethics statement
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the NIH. The animal protocols involving mice were approved by the University of Maryland Institutional Animal Care and Use Committee under Protocol R‐12‐31.
Animals
Eight‐ to 10‐wk‐old C57BL/6J mice were purchased from the National Cancer Institute (Frederick, MD, USA). C3 knockout mice in C57BL/6J background were purchased from The Jackson Laboratory (Bar Harbor, ME, USA).
Cn
Cn H99 was obtained from the ATCC (Manassas, VA, USA; Catalog 208821). Cn H99‐GFP were obtained from Dr. Robin C. May (University of Birmingham, Edgbaston, Birmingham, UK) [21]. The organisms were grown to log phase in Sabouraud dextrose broth (Difco, Becton Dickinson, Franklin Lakes, NJ, USA) at 32°C with gentle rotation for 16 h and then washed 3 times in sterile PBS (pH 7.4) before use.
IVM
IVM was performed on the mouse brain at various time points after i.v. injection of 20 × 106 Cn H99‐GFP [21] in 100 µl PBS by the tail vein, as described previously [18]. In brief, C57BL/6J mice were anesthetized by i.p. injection of a mixture of 10 mg/kg xylazine (MTC Pharmaceuticals, Cambridge, ON, Canada) and 200 mg/kg ketamine hydrochloride (Rogar/STB, London, ON, Canada). The body temperature of mice was kept at 36–37°C throughout the experiment. A craniotomy was performed by use of a high‐speed drill (Fine Science Tools, Foster City, CA, USA), and the durometer was gently removed to expose the underlying pial vasculature. The exposed brain was kept moist with an artificial cerebrospinal fluid throughout the experiment.
The mice were inoculated i.v. by the tail vein with Alexa Fluor 555 anti‐Ly6G (clone 1A8; BioLegend, San Diego, CA, USA) to stain neutrophils. In vivo yeast cell and immune cell interactions were observed by use of a Zeiss Axio Examiner Z1 intravital microscope with the Colibri 2 LED illumination system (Zeiss, Jena, Germany). An ORCA‐Flash4.0 complementary metal oxide silicon camera (Hamamatsu, Hamamatsu City, Japan), mounted on the microscope, was used to project the image to a monitor. All experiments were recorded for later playback and analysis.
Immunohistochemistry
Mice were euthanized and perfused 3 h after i.v. infection with 20 × 106 Cn H99, and the brain was removed for immunohistochemical staining. To avoid contamination of tissues by circulating fungi, perfusion was performed by injecting sterile saline (50 ml) into the left ventricle, as the right atrium was cut open to allow drainage during the procedure [20]. The brain tissues of infected mice were prepared for frozen sections, as described previously [18]. In brief, the tissues were removed and frozen in OCT compound. Frozen tissue blocks were cut on a cryostat microtome, and 5 μm sections were placed on coated glass slides. Tissue sections were fixed in 2% neutral‐buffered paraformaldehyde for 10 min. Sections were then incubated with 2% goat serum in PBS, followed by incubation with rabbit anti‐mouse collagen IV, mouse anti‐cryptococcal polysaccharide (E1; a gift from Dr. F. Dromer, Institut Pasteur, Molecular Mycology Unit, Paris, France), rat anti‐mouse C3b/iC3b (clone 2/11; Hycult Biotech, Plymouth Meeting, PA, USA), rat anti‐mouse Ly6G (clone 1A8; BioLegend), or rat anti‐mouse ICAM‐1 (clone YN1/1.7.4; BioLegend) at 4°C overnight. After 3 washes, sections were incubated for 30 min with Alex Fluor 647 anti‐rabbit IgG (H+L) to delineate brain microvasculature, Alexa Fluor 488 anti‐mouse IgG (H+L) to identify Cn, and Alexa Flour 555 ant‐rat IgG (H+L) to identify C3b/iC3b, neutrophils, or ICAM‐1 in the brain. The sections were rinsed and mounted with glycerol.
To stain ICAM‐1 on the brain endothelial cells following incubation with Cn in vitro, mouse brain endothelial cell line (bEnd.3; ATCC), cultured in 8‐chamber Lab‐Tek slides (Thermo Scientific, Rochester, NY, USA), was untreated or incubated with Cn H99‐GFP (2 × 106/ml) for 3 h at 37°C. The cells were stained with rat anti‐mouse ICAM‐1 (clone YN1/1.7.4; BioLegend), followed by incubation with Alexa Fluor 555 anti‐rat IgG (H+L) to identify ICAM‐1 and DAPI to label the nuclei.
Flow cytometry
Blood was taken from mice 3 h after i.v. infection with 20 × 106 Cn H99. After the erythrocyte lysis, single‐cell suspension was incubated with anti‐CD16/CD32 (clone 93; eBioscience, San Diego, CA, USA) to block nonspecific binding for 10 min. Then, the cells were stained further with APC‐Cy7 CD45 (clone 30‐F11; eBioscience), FITC anti‐Ly6G (clone 1A8; BioLegend), PE anti‐CD11a (clone M17/4; eBioscience), Alexa Fluor 647 anti‐CD11b (clone M1/70; BioLegend), APC anti‐CD11c (clone N418; eBioscience), Alexa Fluor 647 anti‐CD18 (clone M18/2; BioLegend), or isotype control antibodies. Data were acquired by use of a FACS LSR II flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed by use of FlowJo software (Tree Star, Ashland, OR, USA). The percentage of neutrophils (Ly6G+) and the MFI of CD11a, CD11b, CD11c, or CD18 were determined.
Neutrophil depletion and brain CFUs
Wild‐type mice were administrated with 200 µg anti‐Ly6G (clone 1A8; BioXcell, West Lebanon, NH, USA) or isotype control antibody in 200 µl PBS by the tail vein. The mice were i.v. infected with 5 × 104 Cn H99, 24 h after the antibody administration. In other sets of experiments, wild‐type and C3 knockout mice were directly i.v. infected with 5 × 104 Cn H99. To compare the clearance efficiency between complement and antibody opsonizations, 1 × 106 Cn H99 was preopsonized with complement or antibody by incubation for 30 min with 100 µl mouse fresh serum or 2 µg E1 mAb, respectively. C3 knockout mice were i.v. infected with 5 × 104 Cn H99, preopsonized with complement or antibody. The mice were euthanized and perfused at various time points after infection. Brain tissues were dissected and homogenized in sterile water. Appropriate dilutions of the homogenates were plated onto Sabouraud dextrose agar plates, and CFUs were enumerated after 24–48 h growth at 30°C.
Statistics
Data were expressed as means ± sem. An ANOVA was performed to establish equal variance, and 2‐tailed Student's t test with Bonferroni correction was applied to determine statistical significance, which was defined as P < 0.05.
RESULTS AND DISCUSSION
Real‐time in vivo imaging reveals the direct removal of the arrested Cn by neutrophils from the luminal walls of the brain microvasculature
To characterize the dynamic interactions of neutrophils with Cn in the brain microvasculature, mice were i.v. infected with Cn H99‐GFP [21]. At various time points, IVM was performed on the brain as described previously [18, 22]. As a result of the fact that most Cn are arrested in the brain capillaries [18, 19–20, 23], we first determined whether and how neutrophils interacted with the arrested Cn in the capillaries, 3 h after i.v. infection. Real‐time videos were taken to image the capillary bed of the brain. As shown in Fig. 1A and Supplemental Movie 1, 2 yeast cells were arrested in the brain capillary. Six minutes later, a neutrophil moved toward the yeast cells. Thereafter, the neutrophil interacted with the yeast cells within the capillary. The yeast cells were eventually internalized by the neutrophil within minutes in the capillary. Interestingly, the 2 yeast cells appeared to stick to each other before phagocytosis and then located separately in the neutrophil after ingestion, suggesting that they were in different phagosomes of the neutrophil. After the phagocytosis, the neutrophil containing Cn crawled slowly (∼1 μm/min) and eventually circulated back into the bloodstream (Fig. 1A and Supplemental Movie 1).
Figure 1.
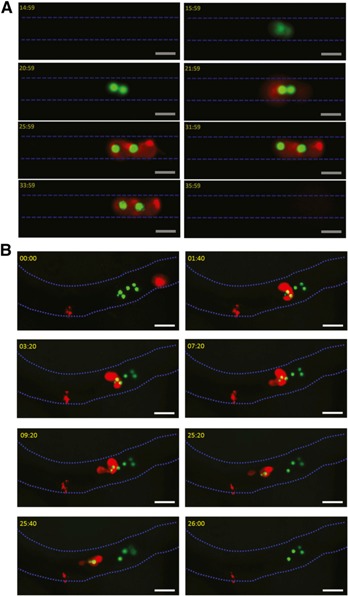
Direct removal of the arrested Cn by neutrophils from the brain microvasculature. (A) IVM was performed on the brain capillary, 3 h after i.v. infection with Cn. At 15 min after the start of image acquisition, there was no Cn in the capillary (blue dashes). At 16 min (see 15:59), 2 yeast cells sticking to each other (green) were arrested in the capillary. Six minutes later (see 21:59), a neutrophil (red) migrated toward the arrested yeast cells. At 26 min (see 25:59), the 2 yeast cells were ingested by the neutrophil and separately located within the neutrophil. The neutrophil containing Cn crawled along the vessel and eventually circulated back into the bloodstream at 36 min (see 35:59). Blood flowed from left to right. Original scale bars, 10 µm. See also Supplemental Movie 1. (B) IVM was performed on the brain postcapillary, 6 h after i.v. infection with Cn. At the start of image acquisition (00:00), 6 yeast cells (green) were present on the luminal wall of the postcapillary (blue dashes) with a neutrophil (red) behind. At 01:40, the neutrophils migrated to the yeast cells. Thereafter, the neutrophil recognized and interacted with the yeast cells, accompanied with sending out pseudopodia, resulting in phagocytosis of 2 yeast cells. At 09:20, the neutrophil containing ingested Cn started to crawl along the luminal wall. At 26:00, the crawling neutrophil carrying 2 yeast cells eventually lost its adherence and left into the bloodstream. There were still 4 yeast cells left in the vessel. Blood flowed from right to left. Original scale bars, 20 µm. See also Supplemental Movie 2.
Next, we performed IVM on the brain of mice, 6 h after i.v. infection with Cn. We found that most of the yeast cells were still located in the capillary bed. Interestingly, we could detect a small number of Cn within the postcapillaries. With the use of IVM, we have previously found that Cn does not interact with the postcapillary wall after i.v. injection of Cn during the observation period (from the beginning of i.v. injection of Cn to 60 min after the injection) [18]. Although most of the circulating Cn was arrested in capillaries within 60 min after i.v. injection, a small number of organisms could be seen passing through the brain vasculature [18]. Thus, one reasonable explanation for detecting Cn within the postcapillary wall at 6 h after infection is that infection of the brain with Cn induces endothelium to express adhesion molecules that have the ability to bind Cn that still circulates in the bloodstream. In this regard, strong evidence has been provided recently for the interactions between endothelial CD44 and Cn hyaluronic acid, which is involved in Cn transmigration into the brain parenchyma [24, 25]. In the current study, we also characterized the dynamic interactions of neutrophils, with Cn sticking to the postcapillary wall in real‐time. At the start of image acquisition, yeast cells were present in the postcapillary wall, and a neutrophil was located behind the yeast cells (Fig. 1B and Supplemental Movie 2). Approximately 2 min later, the neutrophils crawled to the yeast cells. The neutrophil recognized and interacted with the yeast cells sticking to the vessel wall, resulting in an internalization of the Cn. During the interactions of neutrophil with the Cn, we observed morphologic alterations of the neutrophil, such as sending out pseudopodia. The neutrophil containing ingested Cn started to crawl again after interactions with the Cn in the arrested site for ∼10 min. The neutrophil containing ingested Cn eventually circulated back into the bloodstream following crawling for ∼8 min, resulting in the removal of Cn from the luminal wall of the vessel. Taken together, our results demonstrated that neutrophils could clear Cn in the brain vasculature through a direct removal of the arrested Cn from the endothelial surface.
Neutrophils and monocytes are the main professional phagocytes in the bloodstream. Cn was also detected within monocytes circulating in capillaries of mouse brain [26], suggesting phagocytosis of Cn by monocytes in the bloodstream. In a zebrafish model, neutrophils are unable to ingest fluid‐borne bacteria, engulfing only those arrested on the walls of the infected body cavity or blood vessels in a “vacuum‐cleaner” type of behavior [27]. In stark contrast, macrophages appear able to engulf bacteria efficiently in body fluids, as well as on tissue surfaces [27]. Although we have shown that neutrophils sweep up Cn arrested in the brain vasculature, it remains unknown whether neutrophils phagocytose Cn circulating in the bloodstream. In addition, how monocytes interact with arrested and circulating Cn in the brain vasculature deserves further investigation.
Cn infection enhances the expression of adhesion molecules Mac‐1 and ICAM‐1 in vivo
Next, we sought to determine whether Cn infection enhanced the circulating neutrophils. As shown in Fig. 2A , mice showed a significantly higher percentage of neutrophils in the bloodstream 3 h after i.v. infection of Cn compared with uninfected mice. We reasoned that to interact with the arrested Cn, neutrophils must migrate toward the Cn in the brain vasculature. It is known that adhesion molecules play a critical role in leukocyte migration [2, 28]. Among various molecules, we were particularly interested in Mac‐1 (CD11b/CD18) and ICAM‐1. This is because early studies have shown that Cn can stimulate the expression of CD11b on human neutrophils in vitro [29] and up‐regulate the expression of ICAM‐1 on human brain microvascular endothelial cells in vitro [30], although these findings have never been confirmed in vivo during the infection with Cn. In addition, published work has demonstrated that interaction of Mac‐1 and ICAM‐1 is critical in arresting movement of neutrophils on HUVECs cultured in vitro [31].
Figure 2.
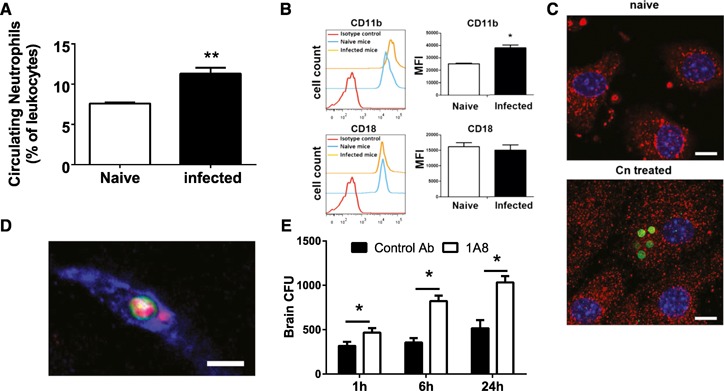
Enhanced expression of Mac‐1 and ICAM‐1 stimulated by Cn. (A) Numbers of neutrophils in the peripheral blood of mice (n = 4) were enhanced, 3 h after i.v. infection with 20 × 106 Cn (gated on CD45+ cells). (B) CD11b (upper), but not CD18 (lower), expressed on circulating neutrophils was elevated significantly in mice (n = 5), 3 h after i.v. infection with 20 × 106 Cn (gated on CD45+Ly6G+ cells). (C) ICAM‐1 staining was observed, to a greater extent, on mouse endothelial cell line (bEnd.3) treated with Cn in vitro (right) compared with untreated control (naïve; left). ICAM‐1, red; Cn, green; nuclei, blue. Original scale bars, 10 µm. (D) ICAM‐1 (red) intensity was increased around the arrested site of Cn (green) in the brain microvasculature (blue), 3 h after i.v. infection with 20 × 106 Cn. Original scale bar, 5 µm. (E) Brain CFU of mice (n = 3–4)‐treated anti‐Ly6G antibody (to deplete neutrophils) or control antibody at various time points after i.v. infection with 5 × 104 Cn. *P < 0.05; **P < 0.01. Data are presented as means ± sem. Data are representative of 2–3 independent experiments.
To this end, we purified neutrophils from naïve mice or mice 3 h after i.v. infection with Cn and evaluated their expression of CD11b/CD18 by use of flow cytometry. The expression of CD11b, but not CD18, on the neutrophils was enhanced significantly in Cn‐infected mice compared with naïve mice (Fig. 2B). Elevated expression of CD11b on neutrophils without significant enhancement of CD18 has also been reported previously [32, 33]. In addition, it has been shown that cryptococcal polysaccharides bind with CD18 on human neutrophils, which interferes the binding of the anti‐CD18 mAb to CD18 [34]. Interestingly, Cn infection did not affect the expression of CD11a and CD11c by neutrophils (data not shown). In agreement with previous findings [30], the ICAM‐1 expression was observed to a greater extent following incubation with Cn than that of the control on the mouse brain endothelial cells in vitro (Fig. 2C). In addition, enhanced staining of ICAM‐1 was detected around the arrested site of some of the Cn in the brain microvasculature by use of immunohistochemistry (Fig. 2D). These data suggested that Cn infection enhanced the expression of Mac‐1 and ICAM‐1 in vivo.
Mac‐1 is also referred to as CR3. The function of Mac‐1 is described initially as the ability to bind iC3b and therefore, to mediate the phagocytosis of pathogens. On the other hand, Mac‐1 is also known to mediate neutrophil adhesion to HUVECs [35]. More recently, it has been shown that Mac‐1 is essential for intraluminal crawling of neutrophils in the liver [36], although its role has not been confirmed in the brain. Thus, in addition to the involvement of binding iC3b for Cn recognition, we suggest that Mac‐1, as an adhesion molecule, is critically involved in the intravascular encounter of neutrophils with Cn, through its biding to endothelial ICAM‐1, whose expression is also enhanced by stimulation of the arrested Cn in the brain. Thus, the targeting of these molecules might be considered as a potential therapeutic strategy for augmenting neutrophil defenses against Cn in clinical settings, as neutrophil function is relatively preserved in most patients with cryptococcosis.
We next evaluated the biologic significance of the interactions of neutrophils with Cn in the brain vasculature through depletion of circulating neutrophils. Mice were treated with anti‐Ly6G antibody (1A8) [37] or isotype control antibody and 24 h later, i.v. infected with Cn. Infected mice treated with anti‐Ly6G antibody showed significantly higher fungal burden in the brain compared with infected mice treated with control antibody (Fig. 2E), although the enhancement of fungal load was not very dramatic. Nevertheless, the statistically significant difference between infected mice treated with anti‐Ly6G antibody and control antibody demonstrated that neutrophils contribute to the clearance of Cn in the brain vasculature before its transmigration into the brain parenchyma.
Complement C3 is critically involved in the intravascular recognition of the arrested Cn by neutrophils in the brain
Next, we examined the role of C3 in the recognition and subsequent internalization of Cn by neutrophils in the brain vasculature. C3b/iC3b was detected on the surface of arrested Cn in the brain vasculature of wild‐type, but not C3 knockout, mice, 3 h after i.v. infection with Cn ( Fig. 3A ), suggesting that complement may be involved in the interactions of neutrophils with Cn under shear condition. This is in contrast to the previous observation that no binding of C3 to Cn was detected in the brain [38]. However, in the previous study, mice were euthanized 12–21 d after i.v. infection with Cn, and the observed Cn should be located in the brain parenchyma rather than vasculature of the infected mice at these time points. Thus, lack of C3 deposition on the Cn surface might be a result of limited amount of complement in the environment of brain parenchyma.
Figure 3.
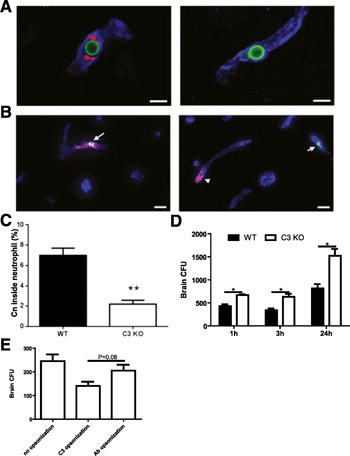
Critical role of complement C3 in the recognition and clearance of Cn by neutrophils in the brain microvasculature. (A) Complement C3b/iC3b (red) was deposited on the surface of Cn (green) arrested in the brain microvasculature (blue) in wild‐type (left) but not C3 knockout mice (right), 3 h after i.v. infection with 20 × 106 Cn. Original scale bars, 5 µm. (B) Cn (green) was seen within neutrophils (red) in the brain microvasculature (blue) of wild‐type mice (left) but hardly seen within neutrophils of C3 knockout mice (right), 3 h after i.v. infection with 20 × 106 Cn. Original scale bars, 20 µm. (C) The percentage of the yeast cells located within the neutrophils in the brain microvasculature of wild‐type (WT) and C3 knockout (KO) mice (n = 5), 3 h after i.v. infection with 20 × 106 Cn. (D) Brain CFU of wild‐type and C3 knockout mice (n = 3–4) at various time points after i.v. infection with 5 × 104 Cn. (E) Brain CFU of C3 knockout mice (n = 3), 3 h after i.v. infection, with 5 × 104 Cn preopsonized with complement or antibody or Cn without opsonization. Data are presented as means ± sem. Data are representative of 2 independent experiments. *P < 0.05; **P < 0.01.
We also observed that a considerable number of Cn were ingested by neutrophils in the brain vasculature of infected wild‐type mice. In contrast, only a limited number of Cn were internalized by neutrophils in infected C3 knockout mice (Fig. 3B). The frequency of Cn ingested by neutrophils was significantly lower in infected C3 knockout mice compared with infected wild‐type mice (Fig. 3C), suggesting that C3b/iC3b deposited on the surface of Cn was critically involved in the recognition and ingestion of Cn by neutrophils in the brain vasculature. It is worth noting that a small number of Cn were still internalized by neutrophils in C3 knockout mice. We speculate that a direct interaction of cryptococcal glucuronoxylomannan with CR3 may also mediate recognition and phagocytosis of Cn, as suggested previously [34, 39, 40].
To explore further the role of C3 in clearance of Cn in the brain vasculature, the fungal burden was determined at various time points after i.v. infection with Cn. As shown in Fig. 3D, the fungal burden in the brain of infected C3 knockout mice was significantly higher than that of infected wild‐type mice, suggesting that C3 plays a central role in clearance of Cn in the brain vasculature. In addition to C3 opsonization, it was reported that antibody opsonization was involved in Cn phagocytosis [39]. Thus, we compared the efficiency of Cn clearance in the brain vasculature between opsonization with complement and antibody. The efficiency of Cn clearance mediated by antibody in the brain vasculature was lower than that mediated by complement; however, the difference did not reach statistical significance (Fig. 3E).
The contribution of C3 to Cn clearance is probably not limited to opsonization of the yeast cell. Deposition of C3b/iC3b will lead to activation of other components of complement cascade. Indeed, C5 knockout mice are also more susceptible to Cn infection than wild‐type mice [41]. Recently, it has been shown that C5a stimulates the enhanced expression of CD11b on neutrophils in an in vitro system, which is critical for the phagocytes to ingest Candida albicans [42]. Thus, we suggest that the enhanced expression of CD11b on neutrophils induced by Cn infection in our system may not only facilitate migration of neutrophils toward arrested Cn via interaction with ICAM‐1 expressed on brain endothelium but also plays an important role in internalization of the yeast cell by the phagocytes in the brain microvasculature.
Neutrophils, the most abundant phagocytes in the bloodstream and the key effector cells in innate immunity against microbes, are the first cells that are rapidly recruited to infection sites during an innate immune response to infection [2, 28]. Although in vitro studies have shown that neutrophils efficiently kill microbes, it still remains largely unknown how the recruited neutrophils interact with invading pathogens in vivo. Our data are the first to characterize directly the dynamic interactions of phagocytes with a microbe in the brain of a living animal. It is traditionally thought that pathogen elimination occurs at the infection sites where the pathogens are located through a direct intracellular or extracellular killing mechanism [2]. Interestingly, we found that circulating neutrophils could interact with Cn attached to the luminal wall of the vessels and carry the organism to circulate back into the bloodstream, resulting in a direct removal of the arrested Cn from the brain microvasculature. Recently, monocytes have been shown to ingest amyloid β, a protein fragment related to Alzheimer's disease, deposited on the vessel wall in the brain and carry it to leave the brain [43]. In contrast to amyloid β, Cn is an invading, infectious agent. Therefore, our findings suggest a previously undescribed mechanism for pathogen elimination in the brain, in contrast to the known, direct killing of microbes at the infection sites [2]. As a facultative intracellular pathogen, Cn can survive and grow within phagocytes [44, 45]. If phagocytes had not left the brain following ingestion of Cn, then clearance of arrested Cn would not have been efficient in the brain vasculature as a result of potential growth of Cn within the phagocytes. Indeed, depletion of neutrophils was shown to lower fungal burden in the lung, suggesting that neutrophils may ingest Cn without killing the yeast cell, leading to intracellular growth in vivo, at least in the lung [46, 47]. Therefore, carrying the ingested Cn to leave the brain vasculature might be an optimal strategy for the host to clear the fungus from the brain before its transmigration into the brain parenchyma, provided that phagocytes cannot efficiently kill the ingested fungus at the infection sites. Although the phagocytes carrying Cn back to the bloodstream may bring the organism to other organs, the risk of pathogenesis would be reduced significantly, as the brain is the target organ of Cn, and meningoencephalitis is the major and most lethal complication of cryptococcosis [3, 48]. We suggest that this novel mechanism for pathogen elimination may represent a major pathway for clearance of Cn from brain microvasculature and have a broad application to other microbes disseminating to the brain, in particular, those having the capability of growing within phagocytes.
AUTHORSHIP
M.Z. conceived of the study, performed experiments, analyzed results, and wrote the manuscript. D.S, G.L., and H.W. performed experiments. H.Z. was involved in the study design and data analysis. M.S. supervised the project, conceived of the study, analyzed results, and wrote the manuscript.
DISCLOSURES
The authors declare no conflicts of interest.
Supporting information
Supplementary data
Supplementary data
Supplementary data
ACKNOWLEDGMENTS
This work was supported by start‐up funds from the University of Maryland (to M.S.) and, in part, by U.S. National Institutes of Health National Institute of Allergy and Infectious Diseases Grant AI115086A (to M.S.). M.Z. was supported by the postdoctoral scholarship from Nanjing Medical University. The authors are grateful to Dr. Yunsheng Wang for his technical assistance with confocal microscopy and FACS analysis.
REFERENCES
- 1. Kim, K.S. (2008) Mechanisms of microbial traversal of the blood‐brain barrier. Nat. Rev. Microbiol. 6, 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kolaczkowska, E. , Kubes, P. (2013) Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175. [DOI] [PubMed] [Google Scholar]
- 3. Gottfredsson, M. , Perfect, J.R. (2000) Fungal meningitis. Semin. Neurol. 20, 307–322. [DOI] [PubMed] [Google Scholar]
- 4. Kwon‐Chung, K.J. , Sorrell, T.C. , Dromer, F. , Fung, E. , Levitz, S.M. (2000) Cryptococcosis: clinical and biological aspects. Med. Mycol. 38 (Suppl 1), 205–213. [PubMed] [Google Scholar]
- 5. Park, B.J. , Wannemuehler, K.A. , Marston, B.J. , Govender, N. , Pappas, P.G. , Chiller, T.M. (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23, 525–530. [DOI] [PubMed] [Google Scholar]
- 6. Diamond, R.D. , Root, R.K. , Bennett, J.E. (1972) Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J. Infect. Dis. 125, 367–376. [DOI] [PubMed] [Google Scholar]
- 7. Kozel, T.R. , Highison, B. , Stratton, C.J. (1984) Localization on encapsulated Cryptococcus neoformans of serum components opsonic for phagocytosis by macrophages and neutrophils. Infect. Immun. 43, 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qureshi, A. , Subathra, M. , Grey, A. , Schey, K. , Del Poeta, M. , Luberto, C. (2010) Role of sphingomyelin synthase in controlling the antimicrobial activity of neutrophils against Cryptococcus neoformans . PLoS One 5, e15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller, G.P. , Kohl, S. (1983) Antibody‐dependent leukocyte killing of Cryptococcus neoformans . J. Immunol. 131, 1455–1459. [PubMed] [Google Scholar]
- 10. Mambula, S.S. , Simons, E.R. , Hastey, R. , Selsted, M.E. , Levitz, S.M. (2000) Human neutrophil‐mediated nonoxidative antifungal activity against Cryptococcus neoformans . Infect. Immun. 68, 6257–6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Graybill, J.R. , Bocanegra, R. , Lambros, C. , Luther, M.F. (1997) Granulocyte colony stimulating factor therapy of experimental cryptococcal meningitis. J. Med. Vet. Mycol. 35, 243–247. [DOI] [PubMed] [Google Scholar]
- 12. Coffey, M.J. , Phare, S.M. , George, S. , Peters‐Golden, M. , Kazanjian, P.H. (1998) Granulocyte colony‐stimulating factor administration to HIV‐infected subjects augments reduced leukotriene synthesis and anticryptococcal activity in neutrophils. J. Clin. Invest. 102, 663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vecchiarelli, A. , Monari, C. , Baldelli, F. , Pietrella, D. , Retini, C. , Tascini, C. , Francisci, D. , Bistoni, F. (1995) Beneficial effect of recombinant human granulocyte colony‐stimulating factor on fungicidal activity of polymorphonuclear leukocytes from patients with AIDS. J. Infect. Dis. 171, 1448–1454. [DOI] [PubMed] [Google Scholar]
- 14. Klebanoff, S.J. (2005) Myeloperoxidase: friend and foe. J. Leukoc. Biol. 77, 598–625. [DOI] [PubMed] [Google Scholar]
- 15. Aratani, Y. , Kura, F. , Watanabe, H. , Akagawa, H. , Takano, Y. , Ishida‐Okawara, A. , Suzuki, K. , Maeda, N. , Koyama, H. (2006) Contribution of the myeloperoxidase‐dependent oxidative system to host defence against Cryptococcus neoformans . J. Med. Microbiol. 55, 1291–1299. [DOI] [PubMed] [Google Scholar]
- 16. Guillot, L. , Carroll, S.F. , Homer, R. , Qureshi, S.T. (2008) Enhanced innate immune responsiveness to pulmonary Cryptococcus neoformans infection is associated with resistance to progressive infection. Infect. Immun. 76, 4745–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sionov, E. , Mayer‐Barber, K.D. , Chang, Y.C. , Kauffman, K.D. , Eckhaus, M.A. , Salazar, A.M. , Barber, D.L. , Kwon‐Chung, K.J. (2015) Type I IFN induction via poly‐ICLC protects mice against cryptococcosis. PLoS Pathog. 11, e1005040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi, M. , Li, S.S. , Zheng, C. , Jones, G.J. , Kim, K.S. , Zhou, H. , Kubes, P. , Mody, C.H. (2010) Real‐time imaging of trapping and urease‐dependent transmigration of Cryptococcus neoformans in mouse brain. J. Clin. Invest. 120, 1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang, Y.C. , Stins, M.F. , McCaffery, M.J. , Miller, G.F. , Pare, D.R. , Dam, T. , Paul‐Satyaseela, M. , Kim, K.S. , Kwon‐Chung, K.J. (2004) Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood‐brain barrier. Infect. Immun. 72, 4985–4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Charlier, C. , Chrétien, F. , Baudrimont, M. , Mordelet, E. , Lortholary, O. , Dromer, F. (2005) Capsule structure changes associated with Cryptococcus neoformans crossing of the blood‐brain barrier. Am. J. Pathol. 166, 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Voelz, K. , Johnston, S.A. , Rutherford, J.C. , May, R.C. (2010) Automated analysis of cryptococcal macrophage parasitism using GFP‐tagged cryptococci. PLoS One 5, e15968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi, M. , Colarusso, P. , Mody, C.H. (2012) Real‐time in vivo imaging of fungal migration to the central nervous system. Cell. Microbiol. 14, 1819–1827. [DOI] [PubMed] [Google Scholar]
- 23. Olszewski, M.A. , Noverr, M.C. , Chen, G.H. , Toews, G.B. , Cox, G.M. , Perfect, J.R. , Huffnagle, G.B. (2004) Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am. J. Pathol. 164, 1761–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jong, A. , Wu, C.H. , Gonzales‐Gomez, I. , Kwon‐Chung, K.J. , Chang, Y.C. , Tseng, H.K. , Cho, W.L. , Huang, S.H. (2012) Hyaluronic acid receptor CD44 deficiency is associated with decreased Cryptococcus neoformans brain infection. J. Biol. Chem. 287, 15298–15306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jong, A. , Wu, C.H. , Shackleford, G.M. , Kwon‐Chung, K.J. , Chang, Y.C. , Chen, H.M. , Ouyang, Y. , Huang, S.H. (2008) Involvement of human CD44 during Cryptococcus neoformans infection of brain microvascular endothelial cells. Cell. Microbiol. 10, 1313–1326. [DOI] [PubMed] [Google Scholar]
- 26. Chrétien, F. , Lortholary, O. , Kansau, I. , Neuville, S. , Gray, F. , Dromer, F. (2002) Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J. Infect. Dis. 186, 522–530. [DOI] [PubMed] [Google Scholar]
- 27. Colucci‐Guyon, E. , Tinevez, J.Y. , Renshaw, S.A. , Herbomel, P. (2011) Strategies of professional phagocytes in vivo: unlike macrophages, neutrophils engulf only surface‐associated microbes. J. Cell Sci. 124, 3053–3059. [DOI] [PubMed] [Google Scholar]
- 28. Hickey, M.J. , Kubes, P. (2009) Intravascular immunity: the host‐pathogen encounter in blood vessels. Nat. Rev. Immunol. 9, 364–375. [DOI] [PubMed] [Google Scholar]
- 29. Dong, Z.M. , Murphy, J.W. (1996) Cryptococcal polysaccharides induce L‐selectin shedding and tumor necrosis factor receptor loss from the surface of human neutrophils. J. Clin. Invest. 97, 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang, S.H. , Wu, C.H. , Jiang, S. , Bahner, I. , Lossinsky, A.S. , Jong, A.Y. (2011) HIV‐1 gp41 ectodomain enhances Cryptococcus neoformans binding to human brain microvascular endothelial cells via gp41 core‐induced membrane activities. Biochem. J. 438, 457–466. [DOI] [PubMed] [Google Scholar]
- 31. Diamond, M.S. , Staunton, D.E. , de Fougerolles, A.R. , Stacker, S.A. , Garcia‐Aguilar, J. , Hibbs, M.L. , Springer, T.A. (1990) ICAM‐1 (CD54): a counter‐receptor for Mac‐1 (CD11b/CD18). J. Cell Biol. 111, 3129–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barden, A. , Graham, D. , Beilin, L.J. , Ritchie, J. , Baker, R. , Walters, B.N. , Michael, C.A. (1997) Neutrophil CD11B expression and neutrophil activation in pre‐eclampsia. Clin. Sci. 92, 37–44. [DOI] [PubMed] [Google Scholar]
- 33. Orr, Y. , Taylor, J.M. , Cartland, S. , Bannon, P.G. , Geczy, C. , Kritharides, L. (2007) Conformational activation of CD11b without shedding of L‐selectin on circulating human neutrophils. J. Leukoc. Biol. 82, 1115–1125. [DOI] [PubMed] [Google Scholar]
- 34. Dong, Z.M. , Murphy, J.W. (1997) Cryptococcal polysaccharides bind to CD18 on human neutrophils. Infect. Immun. 65, 557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arnaout, M.A. , Lanier, L.L. , Faller, D.V. (1988) Relative contribution of the leukocyte molecules Mo1, LFA‐1, and p150,95 (LeuM5) in adhesion of granulocytes and monocytes to vascular endothelium is tissue‐ and stimulus‐specific. J. Cell. Physiol. 137, 305–309. [DOI] [PubMed] [Google Scholar]
- 36. Phillipson, M. , Heit, B. , Colarusso, P. , Liu, L. , Ballantyne, C.M. , Kubes, P. (2006) Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J. Exp. Med. 203, 2569–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Daley, J.M. , Thomay, A.A. , Connolly, M.D. , Reichner, J.S. , Albina, J.E. (2008) Use of Ly6G‐specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83, 64–70. [DOI] [PubMed] [Google Scholar]
- 38. Truelsen, K. , Young, T. , Kozel, T.R. (1992) In vivo complement activation and binding of C3 to encapsulated Cryptococcus neoformans . Infect. Immun. 60, 3937–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Taborda, C.P. , Casadevall, A. (2002) CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement‐independent antibody‐mediated phagocytosis of Cryptococcus neoformans . Immunity 16, 791–802. [DOI] [PubMed] [Google Scholar]
- 40. Zaragoza, O. , Taborda, C.P. , Casadevall, A. (2003) The efficacy of complement‐mediated phagocytosis of Cryptococcus neoformans is dependent on the location of C3 in the polysaccharide capsule and involves both direct and indirect C3‐mediated interactions. Eur. J. Immunol. 33, 1957–1967. [DOI] [PubMed] [Google Scholar]
- 41. Rhodes, J.C. , Wicker, L.S. , Urba, W.J. (1980) Genetic control of susceptibility to Cryptococcus neoformans in mice. Infect. Immun. 29, 494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hünniger, K. , Bieber, K. , Martin, R. , Lehnert, T. , Figge, M.T. , Löffler, J. , Guo, R.F. , Riedemann, N.C. , Kurzai, O. (2015) A second stimulus required for enhanced antifungal activity of human neutrophils in blood is provided by anaphylatoxin C5a. J. Immunol. 194, 1199–1210. [DOI] [PubMed] [Google Scholar]
- 43. Michaud, J.P. , Bellavance, M.A. , Préfontaine, P. , Rivest, S. (2013) Real‐time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta. Cell Reports 5, 646–653. [DOI] [PubMed] [Google Scholar]
- 44. Feldmesser, M. , Kress, Y. , Novikoff, P. , Casadevall, A. (2000) Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 68, 4225–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tucker, S.C. , Casadevall, A. (2002) Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc. Natl. Acad. Sci. USA 99, 3165–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mednick, A.J. , Feldmesser, M. , Rivera, J. , Casadevall, A. (2003) Neutropenia alters lung cytokine production in mice and reduces their susceptibility to pulmonary cryptococcosis. Eur. J. Immunol. 33, 1744–1753. [DOI] [PubMed] [Google Scholar]
- 47. Wozniak, K.L. , Kolls, J.K. , Wormley, F.L., Jr. (2012) Depletion of neutrophils in a protective model of pulmonary cryptococcosis results in increased IL‐17A production by γδ T cells. BMC Immunol. 13, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Casadevall, A. (2010) Cryptococci at the brain gate: break and enter or use a Trojan horse? J. Clin. Invest. 120, 1389–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data


