Short abstract
Review on the phenotypic and functional heterogeneity of MDSCs and therapeutic strategies that modulate their development, suppressive function, and differentiation in cancer‐bearing hosts.
Keywords: MDSC, MIF, immune suppression, PFKFB3, immune checkpoint inhibitors, tumor immunology
Abstract
Myeloid‐derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells that accumulate during pathologic conditions, such as cancer. Patients diagnosed with advanced metastatic cancers have an average survival of 12–24 mo, a survival time that hasn't changed significantly in the past 30 yr. Despite some encouraging improvements in response rates and overall survival in patients receiving immunotherapies, such as immune checkpoint inhibitors, most patients will ultimately progress. MDSCs contribute to immunotherapeutic resistance by actively inhibiting antitumor T cell proliferation and cytotoxic activity as well as by promoting expansion of protumorigenic T regulatory cells, thereby, dampening the host immune responses against the tumor. In addition, MDSCs promote angiogenesis, tumor invasion, and metastasis. Thus, MDSCs are potential therapeutic targets in cases of multiple cancers. This review focuses on the phenotypic and functional characteristics of MDSCs and provides an overview of the mono‐ and combinatorial–therapeutic strategies that target MDSCs with an objective of enhancing the efficacy of cancer immunotherapies.
Abbreviations
- 4‐IPP
4‐iodo‐6‐phenylpyrimidine
- 5‐FU
5‐fluorouracil
- ARG1
arginase1
- ATRA
all‐trans‐retinoic acid
- DC
dendritic cell
- dODN
decoy oligodeoxynucleotides
- HIF‐1α
hypoxia inducible factor‐1α
- HNSCC
head and neck squamous cell carcinoma
- ICI
immune checkpoint inhibitor
- IMC
immature myeloid cell
- IPI
ipilimumab
- MDS
myelodysplastic syndrome
- MDSC
myeloid‐derived suppressor cell
- MIF
migration inhibitory factor
- MMP
matrix metallopeptidase
- mRCC
metastatic renal cell carcinoma
- Nlrp3
nucleotide‐binding oligomerization domain‐like receptor family, pyrin domain‐containing 3
- NSCLC
non–small cell lung cancer
- ODN
oligodeoxynucleotides
- PFK‐1
6‐phosphofructo‐1‐kinase
- PFKFB3
6‑phosphofructo‐2‐kinase
- PMN
polymorphonuclear
- ROS
reactive oxygen species
- TAM
tumor‐associated macrophage
- VEGF
vascular endothelial growth factor
- VSSP
very small size proteoliposome
- WGP
whole‐glucan particle
Introduction
Immunotherapy‐based treatment strategies continue to transform cancer therapy and patient outcomes. The successes are based on recent scientific advances demonstrating the tolerogenic nature of cancer and the fundamental role of the tumor immune microenvironment in suppressing antitumor immunity. ICIs, such as IPI (anti–CTLA‐4; Bristol‐Myers Squibb, New York, NY, USA), pembrolizumab (anti–PD‐1; Merck & Co., Kenilworth, NJ, USA), and nivolumab (anti–PD‐1; Bristol‐Myers Squibb), have emerged as powerful therapeutic strategies for multiple types of cancers, including metastatic melanoma, lung cancer, head and neck cancer, bladder cancer, Hodgkin lymphoma, and renal cell carcinoma [1, 2–3]. These drugs “release the brakes” on the immune system and enable activation of immunity against cancer and improvements in patient survival [4, 5]. Despite encouraging objective response rates in patients receiving these immunostimulatory agents, therapeutic resistance continues to occur in most treated patients, and most patients ultimately progress [1, 2]. As such, there is an unmet clinical need to identify resistance mechanisms associated with these immunotherapies. MDSCs are potently immunosuppressive myeloid cells that accumulate in patients with advanced cancer and actively contribute to the resistance to immunotherapeutic agents [6, 7–8].
Abnormal granulocytopoiesis and accumulation of suppressor cells in the peripheral lymphoid organs because of the tumor's influence was first reported in the late 1970s [9, 10]. Recently, those suppressor cells have been the subject of extensive research and were named MDSCs in 2007 to reflect their origin and their inherent ability to suppress T cell function [11]. Although initial studies of MDSCs describe their role in cancer, recent research has highlighted the immune regulatory functions of these cells in various disease settings, including chronic inflammation, viral infections, and autoimmune diseases [12, 13–14]. MDSCs are reported to expand during pregnancy, and they participate in maintaining the maternofetal tolerance [15]. These cells are also known to expand with aging [16]. In cancer‐bearing hosts, MDSCs accumulate in peripheral blood, lymphoid tissues, and draining tumor sites [17]. MDSCs suppress T cell activation and cytotoxicity, induce the differentiation and expansion of Tregs, and inhibit NK cell activation [18]. MDSCs also participate in an array of nonimmunologic functions, such as promotion of angiogenesis and tumor metastases [19, 20].
MDSCs have been identified in patients with melanoma [21], multiple myeloma [22], hepatocarcinoma [23], NSCLC [24], renal cell carcinoma [25], and prostate cancer [26], among others. Despite evidence pointing toward the superior immune‐suppressive activity of tumor‐infiltrating MDSCs in murine tumor models [27], human studies of MDSCs have predominantly focused on peripheral blood (purified from the mononuclear fraction after Ficoll‐gradient centrifugation). The accumulation of MDSCs in the peripheral blood correlates with tumor burden, stage, and grade in multiple cancers. For example, among patients with stage IV solid tumors, those with extensive metastatic tumor burden have the highest percentage and absolute numbers of MDSCs [28].
In this review, we discuss the phenotypic and functional heterogeneity of murine and human MDSCs as well as therapeutic strategies that target MDSC development, suppressive activity, and differentiation and that aid in overcoming resistance to immunotherapies in cancer.
PHENOTYPIC HETEROGENEITY OF MDSCs
Phenotypic markers on murine MDSCs
Mouse MDSCs are characterized by expression of Gr‐1 and CD11b. Those cells are further subdivided into two major groups: CD11b+Ly6G+Ly6Clow granulocytic or PMN‐MDSC (also identified as CD11b+Gr‐1high MDSC), and CD11b+Ly6G−Ly6C+MDSC monocytic MDSC (M‐MDSCs, also identified as CD11b+Gr‐1low) [29]. Recently, murine MDSCs have been further subdivided into 5 different classes based on their relative expression of CD11b and Gr‐1 [30]. In a naive mouse, CD11b+Gr‐1+ MDSCs represent 2−4% of all nucleated splenocytes but can increase dramatically up to 50% in tumor‐bearing mice [29, 31]. These cells are a mixture of immature myeloid cells, immature granulocytes, monocyte–Mϕs, DCs, and myeloid progenitor cells [6].
Phenotypic markers on human MDSCs
In contrast to the well‐defined, murine MDSCs, human MDSCs are inadequately characterized. The phenotypic and morphologic overlap between human monocytic and granulocytic MDSCs generates confusion in delineating their roles in human disease. An international consortium of 23 laboratories immunophenotyped putative MDSC subsets in PBMCs obtained from healthy donors to define marker combinations for the identification of circulating MDSCs. Results from that study revealed very high interlaboratory variance for all MDSC subsets, especially for the granulocytic subsets, and the main variable was the gating strategy. Based on those findings, it was proposed that further efforts were required to harmonize marker combinations and gating parameters for robust enumeration of MDSC subsets [32]. A very recent report, jointly assembled by several researchers, has recommended specific gating strategies for harmonized phenotyping of human MDSCs by flow cytometry (discussed in detail in Bronte et al. [33]). Human MDSCs are defined as cells that express the common myeloid markers, such as CD14+, CD11b+, and CD33+, but are usually negative/low for HLA‐DR and lack expression of lineage‐specific Ags (Lin), such as CD3, CD56, and CD19. Human M‐MDSCs are characterized as the Lin−HLA‐DRlow/−CD11b+CD33+CD14+ phenotype. Human granulocytic or PMN‐MDSCs are defined as the CD11b+CD33+CD15+CD14− phenotype. In addition, a third subtype of CD14−HLA‐DR−CD33+CD15− cells has been identified and contains a mixture of immature progenitors; those cells have been defined as immature MDSCs, and the mouse equivalent has yet to be identified. Members of the MDSC community have now proposed to define those immature MDSCs as early stage MDSCs or eMDSCs. At this point, it appears that any characterization of MDSCs needs to include each of 3 cell populations: eMDSCs, M‐MDSCs, and PMN‐MDSCs (reviewed in Bronte et al. [33]). Current research suggests that the 3 main populations of human MDSCs are found at varied frequencies in patients with cancer; however, some of these cell populations may be exclusive to one type of cancer and may be absent in the others [34, 35].
PMN‐MDSCs and neutrophils share similarities in both phenotype and morphology. Standard Ficoll‐gradient centrifugation (at 1.077 g L−1 d) is the most widely used method for separation of neutrophils from PMN‐MDSCs. The low‐density fraction (i.e., in the mononuclear cell fraction) is enriched with PMN‐MDSCs, and the high‐density cells are the neutrophils [35]. Nevertheless, this method is not foolproof and does not distinguish between potential suppressive CD11b+CD14−CD15+ PMN‐MDSCs and nonsuppressive granulocytic cells because both cell types are sometimes present in the low‐density fraction. Consequently, an important future step in the human MDSC field is to identify cell‐surface markers that will uniquely identify these cells. Thus far, the most reliable marker for human MDSCs remains their suppressor function, which can be either direct or indirect, i.e., through the induction of Tregs.
In tumor‐bearing mice, PMN‐MDSCs suppress T cell functions and have distinct functional properties, including higher ARG1 activity, higher ROS, and lower phagocytic potential [36]. Meanwhile, in a study of acute inflammation in humans, Koenderman et al. [37] and Leliefeld et al. [38] have identified a unique subset of mature, human neutrophils (CD11chi/CD62Llo/CD11bhi/CD16hi) capable of suppressing human T cell proliferation. These circulating myeloid cells were systemically induced in response to acute inflammation caused by endotoxin challenge or by severe injury. Local release of H2O2 from the neutrophils into the immunologic synapse between the neutrophils and T cells mediated the suppression of T cell proliferation and was dependent on the expression of the integrin Mac‐1 (αMβ2) and ROS/H2O2 in the neutrophils. In addition, in patients with cancer, PMN‐MDSCs and suppressive neutrophils are isolated from the peripheral blood [39, 40]. Although the distinction between neutrophils and PMN‐MDSCs is not clear, the role of these cells in modulating the tumor‐induced immune responses is now an accepted paradigm [35, 41].
M‐MDSCs differ from the normal monocytes in healthy individuals in their ability to suppress T cell function, which is mediated by ARG1, NO, and other soluble factors (discussed below) [42]. CD14+HLA‐DR−/low M‐MDSCs not only suppress the proliferation and IFN‐γ secretion by autologous T cells but also induce CD25+Foxp3+ Tregs that are suppressive in vitro [23]. M‐MDSCs are a mixture of myeloid progenitor cells in varying stages of differentiation and can differentiate into Mϕ, DCs, or granulocytes. TAMs are mature, differentiated Mϕ that histologically resemble M‐MDSCs. In human tumors, TAMs display high expression of Mϕ‐specific markers, such as CD68 and CD163, and exhibit low expression of S100A9, and those markers can be used to discriminate between TAMs and tumor M‐MDSCs. S100 calcium‐binding protein A8 S100A8 and S100A9 belong to the family of S100 calcium‐binding proteins that have been reported to have an important role in inflammation [43]. S100A9 has recently been reported to be essential for MDSC accumulation in tumor‐bearing hosts [44]. S100A9 inhibits DC differentiation by up‐regulation of ROS and has been identified as a marker for human M‐MDSCs [45, 46].
FUNCTIONAL HETEROGENEITY OF MDSCs
Functional properties of murine MDSCs
The mechanisms underlying the suppressive activity of MDSCs are numerous, encompassing those that require direct cell–cell contact and others that are indirectly mediated by modification of the microenvironment. The functional properties of MDSCs in tumor‐bearing hosts have been extensively described in recent reviews [42, 47] and are summarized here in Fig. 1 . In mice, immune‐suppressive MDSCs: 1) produce high levels of ARG1 that deplete T cells of l‐arginine, inducing cell cycle arrest [the l‐arginine represents an important molecule central to the immune suppressive function of murine MDSCs; l‐arginine serves as a substrate for ARG1, and depletion of l‐arginine (and l‐cysteine, in some cases) causes the down‐regulation of the ζ‐chain in the TCR complex, resulting in proliferative arrest of Ag‐activated T cells] [48]; 2) stimulate production of high levels of ROS, NO, superoxide, and peroxynitrite—formed from the cooperative activities of iNOS, NADPH oxidase, and ARG1 overexpressed in MDSCs—that reduce TCR functionality [49]; 3) block migration of naive CD62L+ (l‐selectin) T cells to lymphoid organs, which ultimately inhibits the formation of effector T cells [50]; 4) release soluble factors, such as IL‐10 and TGF‐β, which stimulate Treg induction and expansion [23, 51]; and 5) increase nitrosylation of CD8 and chemokine C‐C or C‐X‐C motif ligands and receptors that affect T cell and MDSC migration, respectively [47, 52].
Figure 1.
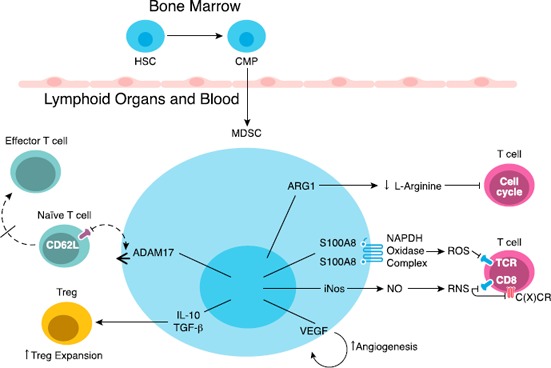
Overview of MDSC immunosuppressive mechanisms.
Under steady‐state conditions, hematopoietic stem cells (HSCs) located in the bone marrow give rise to common myeloid precursors (CMPs), which then differentiate into mature myeloid cells. During tumor progression, CMPs give rise to MDSCs, which subsequently accumulate in blood and in lymphoid organs, such as the spleen. Immunosuppressive MDSCs suppress the immune system by distinct mechanisms, including induction of Treg proliferation; production of high levels of ARG1 that depletes T cells of l‐arginine; production of high levels of ROS and nitrogen species (RNS; peroxynitrate) that lead to nitration and nitrosylation of TCR, CD8, and chemokine C(X)CRs receptors; promotion of angiogenesis; and blockade of the migration of naive CD62L+ T cells to lymphoid organs, which results in diminished expansion of effector T cells ADAM17, ADAM disintegrin, and metallopeptidase domain 17 and S100A8 and S100A9—S100 calcium‐binding proteins.
An important functional property of murine MDSCs is their inherent ability to promote tumor angiogenesis via the secretion of soluble factors, such as matrix metallopeptidase 9 and VEGF [20, 53]. MDSCs are capable of acquiring proangiogenic activity after homing into the tumor microenvironment or when exposed to tumor‐conditioned medium ex vivo [20, 54]. Additionally, a subset of MDSCs expressing CD11b, Gr‐1, CD115, and F4/80, isolated from the bone marrow and spleens of tumor‐bearing mice, can induce the development of Foxp3+ Tregs in vivo via an IFN‐γ and IL‐10–dependent mechanism [55].
In vitro, MDSCs inhibit T cell activation induced by either Ags or polyclonal stimuli through an MHC‐independent mechanism requiring cell–cell contact. Although Ag presentation to the T cells by MDSCs is not required for their in vitro suppressive activity, MDSCs can take up and cross‐present tumor‐associated Ags in the context of MHC class I in vivo [56]. The MDSC‐dependent induction of tumor Ag‐specific CD8+ T cell dysfunction leads to selective impairment of tumor‐specific immunity, indicating that MHC‐dependent responses are relevant in vivo. In this context, there are limitations when analyzing the conflicting results of MDSC‐dependent suppression of T cells. The in vitro assays evaluating the inhibitory function of MDSCs do not always accurately emulate the in vivo scenario, so in different studies, there might be variations based on both the type of stimuli and the source of T cells. For example, in vitro assays wherein the T cells are exposed to nonphysiologic numbers of MDSCs, the mechanisms governing suppression can differ from those assays where the ratio of MDSCs to T cells is the same as found in vivo (e.g., in a tumor‐bearing mouse spleen). In contrast to the in vitro assays, the ability of MDSCs to induce tolerance in tumor Ag‐specific CD8+ T cells in vivo has been proven reproducibly, although many of these studies are based on the use of Abs that deplete Gr‐1+ cells [27, 56, 57]. As such, results from murine experiments with myeloid cell–depleting Abs should be interpreted with caution because the Abs can affect cells other than MDSCs. Currently, agents specifically targeting MDSCs are not readily available; the development of such agents may result in new approaches to stimulate cancer immunity, overcome innate and/or acquired resistance to ICIs, and improve clinical outcomes.
Functional properties of human MDSCs
The molecular mechanisms governing the immune‐regulatory role of human‐tumor infiltration and circulating myeloid cells are largely unexplored. Human MDSCs in some cancers have elevated arginase activity, which is associated with a decreased CD3 ζ‐chain expression on T cells [58, 59]. In patients with head and neck, lung, and bladder cancers, MDSCs impair the migratory properties of activated T cells [52]. Depletion of l‐arginine and l‐cysteine increased NO, superoxide, peroxynitrates, and secretion of a variety of cytokines/soluble factors, such as IL‐6, IL‐10, and TGF‐β, and mediated human MDSC T cell–suppressive function [60].
REGULATORS OF MDSCs
Transcription factors and regulators that modulate murine and human MDSC suppressive activity
A large array of proinflammatory factors is involved in promoting the suppressive function of both human and murine MDSCs (reviewed in Finke et al. [60] and summarized in Fig. 2 ). TLR ligands IL‐1β or TNF‐α increase the suppressive activity of murine MDSCs by activating the NF‐κB/MyD88‐mediated pathway in those cells [61, 62–63]. MyD88‐deficient murine MDSCs lack suppressive abilities and even acquire an immunostimulatory functional activity [64]. More recently, studies have challenged the involvement of TLR‐mediated signaling in driving the suppressive function of MDSCs and, instead, suggest that TLR signaling negatively regulates the murine MDSC suppressive activity [65, 66]. IL‐1β participates in the recruitment and activation of murine MDSC in an NF‐κB–dependent manner [67, 68], and overexpression of IL‐1β by tumor cells results in more peroxynitrite‐producing murine MDSCs [69].
Figure 2.
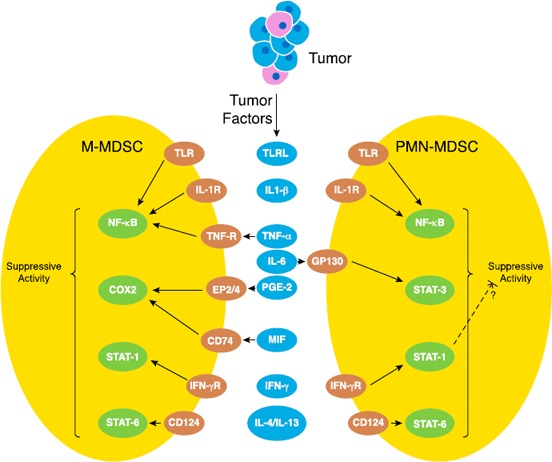
Molecular pathways involved in the maintenance of the MDSC suppressive function.
A wide variety of proinflammatory factors are involved in the induction of the suppressive function in MDSCs. Several of those pathways promote the suppressive function of both M‐MDSCs and PMN‐MDSCs (e.g., IL‐4/IL‐13–mediated STAT6 signaling and TNF‐α–induced NF‐κB activation), whereas others are more specific to maintaining the suppressive properties of either PMN‐MDSC or the M‐MDSC subsets (e.g., MIF‐cyclooxygenase 2‐PGE2 signaling in M‐MDSCs). The STAT1 pathway has been hypothesized to have contrasting roles by inducing the M‐MDSC suppressive activity but inhibiting the activity of PMN‐MDSCs.
STAT3 (activated by IL‐6), STAT1 (activated by IFN‐γ stimulation), and STAT6 [activated by CD124 (IL‐4Rα)] are among the transcription factors that regulate the suppressive activity of MDSCs [70, 71, 72–73]. STAT3 up‐regulates the expression of different components of the NADPH complex (p47, gp96, S100A9), which leads to increases in ROS production that, in turn, is associated with the superior suppressive ability of murine PMN‐MDSCs [48, 74]. In patients with HNSCC, both tumor‐infiltrating and ‐circulating human MDSC‐suppressive activities are associated with activated STAT3‐mediated events [75].
PGE2 regulates the suppressive function of murine MDSCs by up‐regulating ARG1 in MDSCs [76]. COX2/PGE2 expression correlates with the expression of ARG1 and iNOS in murine tumor‐infiltrating leukocytes [77]. Further, the COX2‐inhibitor celecoxib blocks both MDSC accumulation and function in mouse models of glioma and mesothelioma [78, 79]. PGE2 is also involved in the recruitment of MDSCs to the tumor site by inducing expression of CXCL12 [80]. Human monocytic MDSCs from patients with melanoma suppress autologous T cell proliferation via COX2/PGE2 production. In fact, PGE2 treatment alone can induce in monocytes the capability to independently suppress proliferation and IFN‐γ production in autologous T cells ex vivo [81]. As discussed above, it is very apparent that MDSC suppressive function is controlled by a network of transcription factors and regulators (Fig. 2). Many of those factors are indispensible for the pathologic activation of those cells. However, the precise functional contribution and the potential redundancy among different regulatory factors need to be elucidated. For example, based on the biologic features of M‐MDSCs and PMN‐MDSCs, the regulatory mechanisms that operate in those subpopulations are likely to be very different. However, most of the current studies are performed with total MDSCs populations and with transplantable murine tumor models. The specifics underlying those regulatory signaling events need to be elucidated, preferably, in clinically relevant settings. Such new data may lead to the development of strategies for targeted disruption of those mechanisms and thus, help to control MDSC‐mediated suppression of antitumor immunity.
TARGETING MDSCs TO OVERCOME IMMUNOTHERAPEUTIC RESISTANCE
Immune modulating agents such as ICIs have significantly improved the outcome of patients with late‐stage cancers. However, cancer‐induced accumulation of immunosuppressive cell types, including MDSCs, TAMs, and Tregs counteract that immune reaction. Resistance to anti–CTLA‐4 and anti–PD‐1 concurrent immunotherapy was recently recapitulated in an implantable murine breast‐cancer model [82]. Unresponsiveness to anti–CTLA‐4/anti–PD‐1 was found to coincide with a dramatic accumulation of circulating MDSCs and, importantly, simultaneous elimination of MDSCs by Ab depletion during anti–CTLA‐4/anti–PD‐1 treatment resulted in near‐complete eradication of established tumors [82]. In humans, higher percentages (>11%) of circulating CD14+HLA‐DRlow/− M‐MDSCs in patients with stage III/IV melanoma significantly and independently predicted patients’ risk of death [83]. Several other studies have reported similar trends of MDSC accumulation in human patients with cancer [21, 51]; it is now becoming increasingly evident that MDSCs may be centrally implicated in dictating resistance to ICIs and that therapeutic targeting of those effector cells could improve response rates and survival of patients with cancer [84, 85–86]. This section provides an overview of the mono‐ or multitherapeutic strategies that are currently being developed to target MDSCs ( Table 1 ). Also described are the novel mediators identified by our groups that participate in maintaining the suppressive function of MDSCs and the promising new directions of targeting those functional mediators as a means of reducing MDSC activity, differentiation, and immunotherapeutic resistance (Table 1).
Table 1.
Summary of preclinical and clinical inhibitors that target MDSC development, suppressive activity, and differentiation
| Inhibitors | Class of inhibitors | Mechanism of action |
|---|---|---|
| Sunitinib | Tyrosine kinase inhibitors | Inhibition of c‐kit and VEGFR functions |
| Zoledronic acid | Bisphosphonates | Posttranslational modification of MMPs; c‐Kit cleavage |
| Gemcitabine | Nucleoside analogs | Induction of MDSC apoptosis and necrosis |
| 5‐FU | Pyrimidine analogs | Induction of MDSC cytotoxicity |
| Peptibodies | Genetically fused peptide sequences | Targeting the S100 proteins on the MDSCs |
| CpG ODN | Synthetic DNA molecules | Inhibition of MDSC ARG1 and iNOS production |
| Nitroaspirin | Nonsteroidal anti‐inflammatory drugs | Inhibition of MDSC ARG1 and iNOS production |
| N‐acetyl cysteine | Cysteine derivatives | Inhibition of MDSC ARG1 and iNOS production |
| Bardoxolone methyl | Synthetic triterpenoids | Inhibition of MDSC ROS production via Stat3 inhibition and up‐regulation of antioxidant genes |
| Celecoxib | NSAID (COX2/PGE2 inhibitor) | Regulation of ARG1 expression in MDSCs |
| Sildenafil and tadalafil | Phosphodiesterase 5 inhibitors | Regulation of ARG1/iNOS expression in MDSCs |
| 4‐IPP | MIF tautomerase inhibitors | Inhibition of MDSC suppressive function and MDSC to DC differentiation |
| ATRA | Ligands of the retinoic acid receptors | MDSC to DC differentiation and Inhibition of MDSC‐mediated ROS ERK activation |
| 1α,25‐dihydroxyvitamin D3 | Vitamin D | Modulation of tumor‐derived growth factors? |
| PFK‐158 | PFKFB3 inhibitors | Inhibition of MDSC suppressive function |
STRATEGIES THAT TARGET MDSC EXPANSION
MDSCs increase in abundance under the stress of pathologic conditions, such as in inflammation and cancer. In the case of cancer, tumor‐derived growth factors cause alterations in cytokine homeostasis, and consequently, IMCs are blocked en route during differentiation from hematopoietic stem cells to mature granulocytes, Mϕs, or DCs. This cell‐fate specification blockage results in accumulation of IMCs [44]. A 2‐phase model proposed by Condamine and Gabrilovich [61] describes the development of MDSCs. The first phase includes the expansion of IMCs associated with inhibition of their terminal differentiation, and the second activation phase involves converting IMCs to MDSCs. Although the factors that govern these two processes overlap significantly, the first phase is mostly driven by tumor‐derived growth factors (e.g., GM‐CSF, G‐CSF), whereas the second phase (IMCs to MDSCs) is driven by proinflammatory cytokines produced by tumor stroma (e.g., IL‐1β, IL‐6, and TNF‐α) [87].
Tyrosine kinase inhibitors
Proinflammatory cytokines secreted by tumors predominantly signal through the cellular tyrosine kinases. For example, IL‐6–mediated STAT3 activation stimulates expression of proliferative genes in IMCs and promotes their subsequent development to MDSCs. Therefore, compounds that block STAT3 activation are currently being investigated as potential MDSC‐targeting agents [88, 89]. Sunitinib (Pfizer, New York, NY, USA), a broad‐spectrum tyrosine kinase inhibitor, has been tested for its selective MDSC development blocking potential. Treatment with Sunitinib inhibits c‐Kit and VEGF receptor activity; these 2 receptors are involved in development of MDSCs toward an immunosuppressive phenotype [90, 91]. For patients with mRCC, sunitinib treatment has been shown to cause a 50% reduction in circulating MDSCs. In addition, sunitinib treatment resulted in diminished Treg levels with concurrent improvement in CD8+ T cell–mediated effector responses in those patients [92]. Despite these effects, sunitinib monotherapy did not enhance overall survival rates in the treated patients with mRCC [92, 93]. One plausible reason, among others, is that sunitinib does not directly enhance intratumoral T cell infiltration. In that case, combinatorial therapy involving an MDSC‐depleting agent, e.g., sunitinib, and an ICI can prove to be an effective strategy against mRCC.
Bisphosphonates
An alternative MDSC‐targeting strategy is to block the mobilization of the MDSCs formed in the bone marrow into circulation. Recently, bisphosphonates have been shown to diminish MDSC mobilization from the bone marrow. Bisphosphonates (e.g., zoledronic acid; Novartis Pharmaceuticals, Whippany, NJ, USA), currently used in the clinic to prevent loss of bone mass in patients with bone metastases, affect processes involved in the posttranslational modification of MMPs, such as MMP9 [94]. These drugs also interfere with c‐Kit cleavage [94]. Both of these processes contribute toward diminishing MDSC mobility and in vivo tumor growth [95]. The MDSC‐targeting and antitumor efficacy of these agents have not yet been widely investigated in clinical trials, and more studies are warranted.
Peptibodies
Recently, peptide‐Fc fusion proteins, named peptibodies, were developed to target the MDSCs in vivo. Peptibodies, engineered to express fusion sequences encoding mouse MDSC‐binding peptides and IgG2b Fc portion, are reported to exhibit enhanced in vivo, Ab‐dependent, cell‐mediated cytotoxicity and deplete blood, splenic, and intratumoral MDSCs [96]. Importantly, peptibody administration depleted both monocytic and PMN‐MDSCs in vivo in mouse tumor models [96]. The mode of action of these peptibodies is predicted to be via modulating the S100 proteins expressed on the MDSCs; however, more studies are needed to elucidate their exact mechanism of action.
Chemotherapeutic drugs
Anticancer drugs, such as gemcitabine and 5‐FU, are known to deplete MDSCs in vivo. Gemcitabine is a nucleoside analog that lowers MDSC levels in the spleen and, thus, can be combined with immunotherapies to enhance their antitumor activity [97, 98]. Mechanistically, gemcitabine induces MDSC cell death through apoptosis and necrosis [101]. The pyrimidine analog 5‐FU is a cytostatic drug with potent, in vivo MDSC cytotoxicity [99]. However, one disadvantage of this treatment paradigm is the 5‐FU–mediated induction of Nlrp3 inflammasome, which promotes MDSC‐derived IL‐1β secretion and angiogenesis [99, 100]. Paclitaxel, at ultralow doses, inhibits the immunosuppressive activity of tumor‐associated MDSCs in tumor‐bearing mice models by modulating p38 MAPK and S100A9 signaling [101]. Targeting MDSC‐mediated immunosuppression in the tumor microenvironment by ultralow, noncytotoxic doses of paclitaxel represents an efficient therapeutic approach to boost antitumor immune responses in tumor‐bearing hosts.
BRAF inhibitors
Selective small inhibitors of mutant BRAFV600E, such as vemurafenib (Genentech Oncology, Roche, Nutley, New Jersey) and dabrafenib (GlaxoSmithKline, Research Triangle Park, NC, USA) (BRAF inhibitors) can induce strong, although transient, reductions in the metastatic tumor burden in patients with melanoma by blocking the oncogenic signaling pathways within malignant cells [102]. The analysis of MDSC frequencies in the peripheral blood of patients with melanoma under vemurafenib therapy revealed that M‐MDSC and PMN‐MDSC percentages declined significantly over time in patients responding to the therapy [103, 104]. Thus, by neutralizing circulating, immunosuppressive MDSCs, selective BRAF inhibitors allowed Ag‐specific CD8+ T cells to efficiently target autologous tumor cells. Those observations open up the possibility of combining vemurafenib with other immunotherapeutic agents for the induction of long‐lasting tumor regression. In fact, a phase I clinical trial testing vemurafenib in combination with IPI was initiated; however, that trial had to be discontinued because of severe liver toxicity [105]. Thus, although preclinical data suggest that novel chemo‐ plus immunotherapeutic approaches can be beneficial to patients with cancer, the safety and efficacy of such unique combinatorial regimens have to be carefully assessed for immune‐related adverse events in well‐designed clinical trials.
STRATEGIES THAT TARGET MDSC TRAFFICKING
Despite the dose‐dependent, MDSC‐depleting capability of the above‐mentioned chemotherapeutic drugs, a reduction in the percentage of MDSCs does not necessarily decrease their immunosuppressive function in tumors. Newer approaches combining chemotherapeutic or immunotherapeutic drugs with agents that affect MDSC trafficking or functionality may lead to more‐robust treatment outcomes for patients with cancer.
CXCR2 antagonists
Currently, agents that inhibit MDSC trafficking to the tumor microenvironment are being developed as potential anticancer therapeutics (reviewed in Di Mitri et al. [106] and Umansky et al. [107]). Among these agents are the chemokine receptor CXCR2 antagonists that have been validated in various preclinical cancer models as potential inhibitors of MDSC recruitment [108]. Patients with prostate cancer and higher intratumoral CD33+ myeloid cells relapse after docetaxel (Sanofi‐Aventis, Bridgewater, NJ, USA) treatment, suggesting that MDSCs contribute to chemoresistance in human prostate cancer. In a murine model of prostate cancer, paracrine signaling by tumor‐infiltrating CD11b+Gr‐1+ myeloid cells triggered senescence evasion in prostate lesions of Pten‐null mice, eventually promoting tumor progression [108]. In that study, administration of an antagonist for the chemokine receptor CXCR2, in combination with the chemotherapeutic agent docetaxel, blocked tumor growth and potentiated the efficacy of chemotherapy‐induced senescence [108]. Using a prostate adenocarcinoma mouse model driven by loss of Pten and Smad4 genes, Wang et al. [109] identified CXCL5 as a cancer‐secreted chemokine that recruits CXCR2‐expressing MDSCs into the tumor microenvironment. Dual deletion of Pten and Smad4 genes in mouse prostate resulted in a rapid progression of prostate cancer and massive infiltration of PNM‐MDSCs into the tumors [109]. Mechanistically, up‐regulation of CXCL5 in the cancer cells is dependent of the Hippo‐YAP signaling pathway [109]. Thus, pharmacologic inhibition of CXCR2 is an attractive strategy to block YAP‐1‐CXCL5‐mediated MDSCs recruitment and tumor progression.
Targeting tumor‐secreted growth factors
Another novel approach to block the recruitment and function of MDSCs in tumors is to use agents that can reprogram the tumor secretome. Recently, a JAK2 inhibitor was effective in altering the tumor secretome, thereby, decreasing the levels of myeloid‐recruiting cytokines released by the tumors, which ultimately reduced the percentage of tumor‐infiltrating MDSCs and potentiated the antitumor immunity in a mouse model of prostate cancer [110]. Bindarit is an anti‐inflammatory indazolic derivative that can inhibit the synthesis of MCP‐1/CCL2. In vivo, Bindarit treatment significantly decreases the infiltration of MDSCs in murine primary breast and prostate cancer [111].
Mϕ‐CSF or CSF1 is a growth factor that promotes the migration of monocyte‐Mϕs via signaling through its receptor tyrosine kinase CSF1R (cFMS) [112, 113]. MDSCs have been shown to rely on the CSF1/CSF1R signaling axis for their recruitment into the tumors, especially in response to irradiation [114]. Radiation therapy, in a prostate cancer mouse model, augments the CSF1/CSF1R signaling, and that heightened CSF1 has a crucial role in the systemic recruitment of protumorigenic myeloid cells to the irradiated tumors [115]. Hence, the blockade of CSF1/CSF1R signaling‐mediated MDSC recruitment in combination with local irradiation and/or immunotherapy can be a durable therapeutic strategy to overcome tumor progression in various solid tumors.
STRATEGIES THAT TARGET MDSC SUPPRESSIVE ACTIVITY AND DIFFERENTIATION
Therapeutic strategies that counteract the suppressive activity of MDSCs, as well as those that promote their differentiation, have been previously reviewed [46, 116, 117–118] and are discussed below.
Oligodeoxynucleotides
CpG ODN and drugs such as nitroaspirin (NicOx, Valbonne, France) and N‐acetyl cysteine modulate ARG1 and iNOS production, which are the central mediators of MDSC suppressive activity [119, 120–121]. dODNs, which comprise STAT3‐specific DNA sequences are competitive inhibitors of STAT3 transcriptional activity [122]. STAT3 dODN linked to the TLR9 ligand cytosine guanine dinucleotide (CpG‐STAT3 dODN) is specifically internalized by TLR9‐expressing myeloid cells. Once internalized, the CpG‐STAT3 dODN conjugates quickly bind and sequester cytoplasmic STAT3, thereby inhibiting downstream gene expression in target cells [122]. Therefore, use of CpG‐STAT3 dODN can be an effective therapeutic strategy to inhibit STAT3 in MDSCs and thereby limit their T cell–immunosuppressive potential. A very recent review [123] summarizes the current preclinical data on CpG‐STAT3 inhibitors and discusses the advantages of using TLR9‐targeted delivery of oligonucleotide‐based anticancer therapeutics to specifically target myeloid cell function in cancer‐bearing hosts.
Synthetic triterpenoids
Drugs such as bardoxolone methyl inhibit MDSC‐mediated ROS production via induction of antioxidant genes as well as via inhibition of STAT3 [124, 125]. A Phase I clinical trial with pancreatic patients with cancer receiving bardoxolone methyl in combination with gemcitabine showed promising outcome with enhanced T cell responses (clinical trial RTA 402‐C‐0702).
Targeting tumor‐derived exosomes
Chalmin et al. [126] isolated exosomes from murine tumor cell lines and showed that an interaction between exosome membrane‐associated heat shock protein 72 and MDSCs modulates the suppressive activity of the MDSCs via activation of Stat3. Exosome‐mediated induction of Stat3 activation and promotion of MDSC‐suppressive function occurs in a TLR2/MyD88‐dependent manner through autocrine production of IL‐6. Targeting exosome production in vivo using dimethyl amiloride, a drug used to treat high blood pressure, blunts the suppressive activity of MDSCs and enhances the efficacy of cyclophosphamide treatment in mice bearing CT26, TS/A, or EL4 tumors [126]. In addition, MDSCs from patients treated with amiloride for metastatic colorectal carcinoma exhibit reduced T cell–suppressive activity [126].
Anti‐CD33 Abs
CD33+HLA‐DR−Lin− MDSCs have been shown to contribute significantly to the pathogenesis and disease progression of MDS. In patients with MDS, CD33+ MDSCs in the bone marrow microenvironment mediates hematopoietic suppressive function in a S100A9‐dependent manner. Recently, a fully humanized, Fc‐engineered mAb against CD33, BI 836858 (Boehringer Ingelheim, Ingelheim am Rhein, Germany), was developed and tested for its efficacy to suppress the CD33‐mediated signal transduction [127]. The results indicated that BI 836858 depleted MDSCs by Ab‐dependent, cell‐mediated cytotoxicity [127]. One of the downstream effects of the CD33 pathway activation was the increase in ROS‐induced genomic instability; BI 836858 is effective in blocking the CD33‐mediated signaling events, consequently reducing the ROS levels as well as the levels of double‐strand breaks and adducts [127]. This work provides the rationale for developing novel MDSC‐targeting therapies for patients with MDS with the ultimate goal of improving hematopoiesis in these patients.
Phosphodiesterase 5 inhibitors
Drugs such as sildenafil and tadalafil also attenuate the suppressive activity of MDSCs by regulating the expression of ARG1 and iNOS in these cells [128]. Currently, a phase II clinical trial is ongoing in which patients with HNSCC are treated with tadalafil in combination with the conventional HNSCC therapy (clinical trial NCT01697800).
COX2/PGE2 and MIF inhibitors
Celecoxib is a well‐characterized COX2/PGE2 inhibitor that regulates ARG1 expression in MDSCs and potentiates DC‐based immunotherapy [46]. More recently, our group reported that circulating MDSCs isolated from patients with late‐stage melanoma were reliant upon Mϕ MIF for suppression of Ag‐independent T cell activation, which is at least partly due to reductions in COX2/PGE2 expression in MDSCs [129]. MIF is one of the oldest cytokine activities described and has been implicated as a tumor cell– and stromal cell–derived mediator of stromal cell recruitment, polarization, and differentiation. M‐MDSCs and PMN‐MDSCs isolated from melanoma‐bearing, MIF‐deficient mice were substantially less immunosuppressive than those isolated from MIF wild‐type mice, and those reductions corresponded to reduced primary and metastatic melanoma tumor burden [130]. Importantly, 4‐IPP (a small molecule MIF tautomerase inhibitor) phenocopies MIF deficiency in vitro and in vivo and significantly attenuates MDSC immunosuppression and corresponding primary/metastatic tumor burden [130]. Additionally, MIF‐deficient, in vitro, differentiated, bone marrow‐derived MDSCs possess reduced immunosuppressive activity [129]. In an implantable mouse metastatic breast‐cancer model, tumors derived from MIF short hairpin RNA‐expressing 4T1 cells contained fewer M‐MDSCs than control tumors had [131], and reconstitution of MIF expression restored MDSC tumor infiltration and increased the metastatic potential of the tumors [131].
In melanoma, circulating M‐MDSCs expressing myeloid markers are quantitatively predominant in comparison to PMN‐MDSCs [132]. In an attempt to assess whether MIF participates in human melanoma‐induced MDSC immune suppression and/or differentiation, we studied the effects of MIF inhibition in circulating M‐MDSCs, a population that is significantly expanded in the periphery in all patients with advanced melanoma [21]. Our findings indicate that small‐molecule MIF inhibitors dramatically attenuate the suppressive properties of circulating CD14+HLADRlow/− M‐MDSCs isolated from patients with late‐stage metastatic melanoma, confirming MIF as a critical mediator of MDSC‐dependent immune suppression ( Fig. 3 ) [129].
Figure 3.
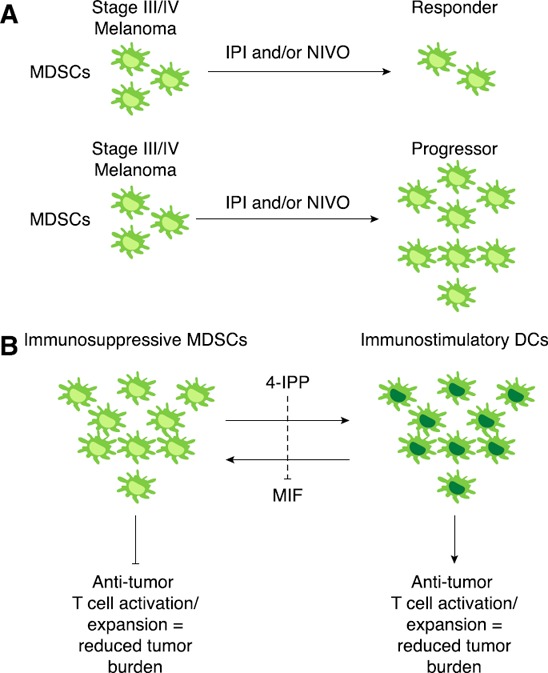
MIF inhibition induces a DC phenotype in MDSCs derived from a patient with melanoma.
(A) Aberrant accumulation of MDSCs in patients with late‐stage melanoma (stage III/IV) may represent an essential mechanism of immediate and/or acquired resistance to IPI and/or nivolumab (NIVO) immune checkpoint inhibitors. (B) Therapeutic targeting of MIF with 4‐IPP, a highly efficacious and orally bioavailable, small‐molecule MIF antagonist, attenuates MDSC immune suppression derived from patients with melanoma. Importantly, 4‐IPP induces a functional MDSC → DC‐like differentiation and thus, can attenuate the MDSC‐mediated resistance to immunotherapies.
In patients with head and neck, lung, or breast cancer, increased accumulation of MDSCs is associated with fewer DCs in their peripheral blood [133]. MDSCs isolated from patients with HLA‐A2+ cancer inhibit production of IFN‐γ by CD8+ T cells that were restimulated with tumor‐antigenic, peptide‐pulsed DCs [134]. Stimulation of MDSCs to differentiate into mature myeloid cells (e.g., DCs) without immunosuppressive properties can lead to increased antitumor immunity and negate MDSC‐mediated therapeutic resistance. Thus, a promising, clinically relevant strategy to reduce the proportion of MDSCs in tumor‐bearing hosts may be the use of agents that promote the differentiation of MDSCs into immune stimulatory DCs. Our recent studies demonstrate that inhibition of MIF with 4‐IPP during short‐term ex vivo culture of M‐MDSCs from patients with melanoma induces an MDSC → DC‐like differentiation resulting in reduced CD14 expression and increases in CD80, CD83, CD86, and CD40 expression [129]. MIF‐mediated MDSC → DC differentiation is due to reductions in COX2‐PGE2 signaling in MDSCs. Importantly, MIF‐inhibited MDSCs functionally promote Ag‐specific T cell activation suggesting that MIF inhibition in patients with late‐stage melanoma may simultaneously attenuate the immunosuppressive functionality of MDSCs and increase the numbers and immunostimulatory activities of tumor Ag‐presenting DCs (Fig. 3) [129].
We hypothesized that intrinsic resistance to ICIs (e.g., CTLA‐4 and/or PD‐1) can be overcome by targeting MIF‐dependent MDSC suppressive activity (Fig. 3). Coadministration of anti–CTLA‐4 and 4‐IPP significantly reduced tumor burden and increased survival in a model of anti–CTLA‐4–resistant B16‐F10‐bearing mice (unpublished results [135]). These initial studies using human melanoma MDSC models lend strong support to the rationale that therapeutic inhibition of MIF in MDSCs represents a clinically viable approach to enhancing antitumor T cell immunity. Our future work will address whether MIF antagonists can be used therapeutically to eliminate MDSCs in patients with cancer and whether MIF inhibition can be used to effectively induce differentiation of MDSCs into DCs with concomitant improvement in myeloid/lymphoid DC ratio, DC function, and Ag‐specific, T cell–mediated immune responses in patients with late‐stage cancer.
Retinoic acid
Ligands of the retinoic acid receptors (retinoid X receptor) are among the compounds that have been shown to stimulate differentiation of myeloid progenitors into DCs [136, 137]. In vivo, parenteral or oral administration of ATRA significantly reduces the presence of MDSCs because of the rapid stimulation of MDSC differentiation to DCs and Mϕs in vitro and in vivo [138]. A reduction in the number of Lin−HLA‐DR−CD33+ cells, accompanied by an improvement of tetanus toxoid‐specific T cell response, was observed in patients with mRCC treated with ATRA [139]. Furthermore, ATRA activated the ERK1/2 kinase pathway, leading to up‐regulation of the ROS scavenger glutathione and, thereby, reducing ROS levels in MDSCs [140]. However, ATRA has been reported to induce the development of Tregs by up‐regulating expression of Foxp3 in the CD4+ T cells [141]. Another combinatorial strategy to promote a potent antitumor response is coadministration of Treg‐depleting agents, e.g., anti–CTLA‐4 and agents that promote MDSC differentiation.
1α,25‐dihydroxyvitamin D3
Vitamin D3 has been shown to induce monocytic maturation in several leukemic cell lines [142]. Although some studies claim a direct myeloid cell differentiation potential of vitamin D3, others have suggested that the antitumor effects of vitamin D3 are indirect, i.e., via modulation of tumor‐derived growth factors [87]. In patients with NSCLC, vitamin D3 has been shown to induce differentiation of suppressive CD34+ myeloid progenitors to DC [143]. Immunohistochemistry analysis of tumors from patients with NSCLC treated with vitamin D3 for 3 wk before surgery revealed reduced infiltration of intratumoral CD34+ cells and immature DC‐SIGN+ DC and increased numbers of intratumoral DC‐LAMP+ mature DC [143].
In a follow‐up clinical study initiated with 32 newly diagnosed patients with NSCLC, tumors from the vitamin D3–treated patients showed a significant increase in the levels of intratumoral CD4+ and CD8+ T lymphocytes as well as cells expression of the activation markers CD69 [144]. In patients with HNSCC, vitamin D3 treatment was reported to differentially modulate the cytokine milieu in the plasma and tumor tissue. In patients with NSCLC, vitamin D3 treatment increased the levels of IL‐6, IL‐10, IL‐2, IFN‐γ, and TNF‐α in the tumor tissue and increased the levels of IL‐8, VEGF, IL‐1α, and IL‐1β in the plasma [145]. Additional studies are required to assess whether the differential changes to the cytokine milieu have a role in vitamin D3–induced MDSC to mature DC differentiation.
WGPs
Recent studies have been shown to induce differentiation of M‐MDSCs into cells with a DC‐like (F4/80+CD11c+) phenotype [146]. WGPs, which are microparticles of 1,3‐β‐glucan extracted from the yeast Saccharomyces cerevisiae, have been shown to activate immune cells through the stimulation of C‐type lectin receptor dectin‐1 [147, 148]. Oral treatment of lung tumor–bearing mice with WGPs significantly reduced the percentage of MDSCs and increased the percentages of Mϕ, DC, and antitumor effector CD8+ T cells in vivo, accompanied by a significant reduced tumor burden [146]. A recent report showed that particulate β‐glucan induced a cytotoxic phenotype and subsequent apoptosis in PMN‐MDSC and converted M‐MDSC to potent APCs that cross‐present Ags to prime, OVA‐specific CD8+ T cells [149]. In addition, patients with NSCLC treated with particulate β‐glucan for 2 wk had a decreased accumulation of circulating CD14− HLA‐DR−CD11b+CD33+ MDSCs in their peripheral blood as compared with the MDSC frequency that was observed pretreatment [149].
VSSPs
VSSPs are nanoparticulated adjuvants based on the combination of outer‐membrane vesicles from Neisseria meningitidis and GM3 gangliosides; these VSSPs have the capability of promoting DC maturation and enhancing CD8+ T cell effector function [150, 151–152]. Treatment of tumor‐bearing mice with VSSPs induced the accumulation of splenic MDSCs with lower immunosuppressive potential against allogeneic and Ag‐specific CTL responses [153]. VSSP treatment also induced the differentiation of MDSCs into mature APCs in tumor‐bearing mice [153]. Furthermore, when VSSP was given as an adjuvant, together with a tumor‐associated Ag (either OVA or GM3), a significant decrease in EG.7 and MCA203 tumor growth was observed, confirming the adjuvant activity of VSSP in boosting the antitumor efficacy of several vaccine candidates [153]. Based on these results, VSSPs can be used as an effective cancer vaccine adjuvant that is able to target tumor‐induced immunosuppression and stimulate a potent CD8+–mediated effector response against tumor Ags.
Inhibitors of glucose metabolism
The importance of a hypoxic tumor microenvironment in dictating the differentiation and functional properties of tumor‐infiltrating MDSCs has recently been established [154]. In particular, the transcription factor HIF‐1α was found to drive MDSC differentiation. Functionally, HIF‐1α in MDSC promotes T lymphocyte immune suppression via ARG1 and iNOS transcription [154]. An additional key transcriptional target of HIF‐1α is PFKFB3, which synthesizes fructose 2,6‐bisphosphate, a potent allosteric activator of the rate‐limiting step of glycolysis, PFK‐1 [155, 156]. We have recently found that, in murine and human melanoma MDSC‐induction models, M‐MDSCs, but not PMN‐MDSCs, express markedly increased PFKFB3, and our initial results indicate that those cells may be especially dependent on high PFKFB3 expression for their differentiation and immunosuppressive functions (unpublished observations).
In the past decade, targeting the energy metabolism pathways of immune cells has garnered interest because of its potential to uncover unique and global therapeutic targets. Our group has developed a novel PFKFB3 inhibitor, (E)‐1‐(pyridyn‐4‐yl)‐3‐(7‐(trifluoromethyl)quinolin‐2‐yl)‐prop‐2‐en‐1‐one (PFK‐158) [157, 158], which is currently being tested in a phase 1 trial at 4 institutions (clinical trial NCT02044861). In recent studies, we have found that PFKFB3 inhibition with PFK‐158 attenuates the suppressive function of circulating CD14+HLA‐DRlow/− M‐MDSCs in chemotherapy‐naive patients with stage III/IV melanoma, which is partly due to the PFK‐158–mediated modulation of the metabolic programming and ARG1 levels. These new unpublished results implicate the requirement of a novel enzyme target, PFKFB3, for the suppressive activity of MDSCs. Furthermore, therapeutic targeting of PFKFB3‐dependent metabolic pathway potentiates antitumor immune responses and can aid in diminishing resistance to other cancer immunotherapies.
CONCLUSIONS
MDSC expansion occurs via a unique myeloid cell–differentiation program induced by tumor‐derived factors. In tumor‐bearing hosts, MDSCs inhibit the innate and adaptive antitumor immune response. The functional and phenotypic discrepancies between humans and murine MDSCs reflect our incomplete understanding of this highly dynamic process of MDSC differentiation and activation in cancer. Identification of surface biomarkers and elucidation of the functional mediators unique to these two populations of MDSCs can address those issues. Although, the contribution of MDSCs to the overall impairment of antitumor T cell responses has been demonstrated, the differential effects of MDSCs on T cells may vary in relation to the metastatic disease setting and cancer type in various patients. Based on that variability, attention should be given to the selection of patients to be included in MDSC studies, particularly in terms of cancer stage and type as well as treatment history. Additionally, evaluation of MDSC frequency should be included routinely in the standard immune monitoring of patients with cancer receiving immunotherapies. Those studies should also include correlation criteria with disease course and prognosis, as well as with immune responses. Such a consistent process will allow scientists to determine whether MDSC can be used as a reliable biomarker of therapeutic response and will provide information about the definitive role of these highly heterogeneous cells in the disease outcomes of patients with cancer.
Another factor that complicates the therapeutic targeting of MDSCs is the redundancy of the regulatory mechanisms. Blockade of a specific immunosuppressive mechanism may be compensated by activation of the other existing signaling mechanisms, and the net result may be a suboptimal recovery of T cell function. We believe that combining immune‐checkpoint inhibitors or cancer vaccination with strategies that target novel, functional mediators of MDSCs that interfere with tumor‐induced immune suppression more globally will provide substantial therapeutic benefit for the treatment of cancer.
AUTHORSHIP
J.A.C., R.A.M., and K.Y. wrote the article.
DISCLOSURES
J.A.C. and R.A.M. are inventors on patents pertaining to PFKFB3 and MIF inhibition, respectively. K.Y. declares no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported in part by U.S. National Institutes of Health (NIH) Grant CA186661 (to R.A.M.), NIH Grant CA102285 (to R.A.M. and K.Y.), NIH Grant 1P30GM106396 (to R.A.M. and K.Y.), and NIH Grant CA149438 (to J.A.C.).
Contributor Information
Robert A. Mitchell, Email: robert.mitchell@louisville.edu
Kavitha Yaddanapudi, Email: kavitha.yaddanapudi@louisville.edu.
REFERENCES
- 1. Hodi, F. S. , O'Day, S. J. , McDermott, D. F. , Weber, R. W. , Sosman, J. A. , Haanen, J. B. , Gonzalez, R. , Robert, C. , Schadendorf, D. , Hassel, J. C. , Akerley, W. , van den Eertwegh, A. J. , Lutzky, J. , Lorigan, P. , Vaubel, J. M. , Linette, G. P. , Hogg, D. , Ottensmeier, C. H. , Lebbé, C. , Peschel, C. , Quirt, I. , Clark, J. I. , Wolchok, J. D. , Weber, J. S. , Tian, J. , Yellin, M. J. , Nichol, G. M. , Hoos, A. , Urba, W. J. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robert, C. , Long, G. V. , Brady, B. , Dutriaux, C. , Maio, M. , Mortier, L. , Hassel, J. C. , Rutkowski, P. , McNeil, C. , Kalinka‐Warzocha, E. , Savage, K. J. , Hernberg, M. M. , Lebbé, C. , Charles, J. , Mihalcioiu, C. , Chiarion‐Sileni, V. , Mauch, C. , Cognetti, F. , Arance, A. , Schmidt, H. , Schadendorf, D. , Gogas, H. , Lundgren‐Eriksson, L. , Horak, C. , Sharkey, B. , Waxman, I. M. , Atkinson, V. , Ascierto, P. A. (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330. [DOI] [PubMed] [Google Scholar]
- 3. Postow, M. A. , Chesney, J. , Pavlick, A. C. , Robert, C. , Grossmann, K. , McDermott, D. , Linette, G. P. , Meyer, N. , Giguere, J. K. , Agarwala, S. S. , Shaheen, M. , Ernstoff, M. S. , Minor, D. , Salama, A. K. , Taylor, M. , Ott, P. A. , Rollin, L. M. , Horak, C. , Gagnier, P. , Wolchok, J. D. , Hodi, F. S. (2015) Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372, 2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolchok, J. D. , Saenger, Y. (2008) The mechanism of anti‐CTLA‐4 activity and the negative regulation of T‐cell activation. Oncologist 13 (Suppl 4), 2–9. [DOI] [PubMed] [Google Scholar]
- 5. Dranoff, G. (2009) Targets of protective tumor immunity. Ann. N. Y. Acad. Sci. 1174, 74–80. [DOI] [PubMed] [Google Scholar]
- 6. Nagaraj, S. , Youn, J. I. , Gabrilovich, D. I. (2013) Reciprocal relationship between myeloid‐derived suppressor cells and T cells. J. Immunol. 191, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Solito, S. , Bronte, V. , Mandruzzato, S. (2011) Antigen specificity of immune suppression by myeloid‐derived suppressor cells. J. Leukoc. Biol. 90, 31–36. [DOI] [PubMed] [Google Scholar]
- 8. Kumar, V. , Patel, S. , Tcyganov, E. , Gabrilovich, D. I. (2016) The nature of myeloid‐derived suppressor cells in the tumor microenvironment. Trends Immunol. 37, 208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee, M. Y. , Rosse, C. (1982) Depletion of lymphocyte subpopulations in primary and secondary lymphoid organs of mice by a transplanted granulocytosis‐inducing mammary carcinoma. Cancer Res. 42, 1255–1260. [PubMed] [Google Scholar]
- 10. Tsuchiya, Y. , Igarashi, M. , Suzuki, R. , Kumagai, K. (1988) Production of colony‐stimulating factor by tumor cells and the factor‐mediated induction of suppressor cells. J. Immunol. 141, 699–708. [PubMed] [Google Scholar]
- 11. Gabrilovich, D. I. , Bronte, V. , Chen, S. H. , Colombo, M. P. , Ochoa, A. , Ostrand‐Rosenberg, S. , Schreiber, H. (2007) The terminology issue for myeloid‐derived suppressor cells. Cancer Res. 67, 425, author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goh, C. , Narayanan, S. , Hahn, Y. S. (2013) Myeloid‐derived suppressor cells: the dark knight or the joker in viral infections? Immunol. Rev. 255, 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu, B. , Bando, Y. , Xiao, S. , Yang, K. , Anderson, A. C. , Kuchroo, V. K. , Khoury, S. J. (2007) CD11b+Ly‐6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J. Immunol. 179, 5228–5237. [DOI] [PubMed] [Google Scholar]
- 14. Yin, B. , Ma, G. , Yen, C. Y. , Zhou, Z. , Wang, G. X. , Divino, C. M. , Casares, S. , Chen, S. H. , Yang, W. C. , Pan, P. Y. (2010) Myeloid‐derived suppressor cells prevent type 1 diabetes in murine models. J. Immunol. 185, 5828–5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Köstlin, N. , Kugel, H. , Spring, B. , Leiber, A. , Marmé, A. , Henes, M. , Rieber, N. , Hartl, D. , Poets, C. F. , Gille, C. (2014) Granulocytic myeloid derived suppressor cells expand in human pregnancy and modulate T‐cell responses. Eur. J. Immunol. 44, 2582–2591. [DOI] [PubMed] [Google Scholar]
- 16. Verschoor, C. P. , Johnstone, J. , Millar, J. , Dorrington, M. G. , Habibagahi, M. , Lelic, A. , Loeb, M. , Bramson, J. L. , Bowdish, D. M. (2013) Blood CD33+HLA‐DR– myeloid‐derived suppressor cells are increased with age and a history of cancer. J. Leukoc. Biol. 93, 633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Youn, J. I. , Nagaraj, S. , Collazo, M. , Gabrilovich, D. I. (2008) Subsets of myeloid‐derived suppressor cells in tumor‐bearing mice. J. Immunol. 181, 5791–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sica, A. , Bronte, V. (2007) Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Invest. 117, 1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bronte, V. , Wang, M. , Overwijk, W. W. , Surman, D. R. , Pericle, F. , Rosenberg, S. A. , Restifo, N. P. (1998) Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac‐1+/Gr‐1+ cells. J. Immunol. 161, 5313–5320. [PMC free article] [PubMed] [Google Scholar]
- 20. Yang, L. , DeBusk, L. M. , Fukuda, K. , Fingleton, B. , Green‐Jarvis, B. , Shyr, Y. , Matrisian, L. M. , Carbone, D. P. , Lin, P. C. (2004) Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor‐bearing host directly promotes tumor angiogenesis. Cancer Cell 6, 409–421. [DOI] [PubMed] [Google Scholar]
- 21. Poschke, I. , Mougiakakos, D. , Hansson, J. , Masucci, G. V. , Kiessling, R. (2010) Immature immunosuppressive CD14+HLA‐DR−/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC‐sign. Cancer Res. 70, 4335–4345. [DOI] [PubMed] [Google Scholar]
- 22. Brimnes, M. K. , Vangsted, A. J. , Knudsen, L. M. , Gimsing, P. , Gang, A. O. , Johnsen, H. E. , Svane, I. M. (2010) Increased level of both CD4+FOXP3+ regulatory T cells and CD14+HLA‐DR–/low myeloid‐derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand. J. Immunol. 72, 540–547. [DOI] [PubMed] [Google Scholar]
- 23. Hoechst, B. , Ormandy, L. A. , Ballmaier, M. , Lehner, F. , Krüger, C. , Manns, M. P. , Greten, T. F. , Korangy, F. (2008) A new population of myeloid‐derived suppressor cells in hepatocellular carcinoma patients induces CD4+CD25+Foxp3+ T cells. Gastroenterology 135, 234–243. [DOI] [PubMed] [Google Scholar]
- 24. Liu, C. Y. , Wang, Y. M. , Wang, C. L. , Feng, P. H. , Ko, H. W. , Liu, Y. H. , Wu, Y. C. , Chu, Y. , Chung, F. T. , Kuo, C. H. , Lee, K. Y. , Lin, S. M. , Lin, H. C. , Wang, C. H. , Yu, C. T. , Kuo, H. P. (2010) Population alterations of L‐arginase‐ and inducible nitric oxide synthase‐expressed CD11b+/CD14–/CD15+/CD33+ myeloid‐derived suppressor cells and CD8+ T lymphocytes in patients with advanced‐stage non–small cell lung cancer. J. Cancer Res. Clin. Oncol. 136, 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Cruijsen, H. , van der Veldt, A. A. , Vroling, L. , Oosterhoff, D. , Broxterman, H. J. , Scheper, R. J. , Giaccone, G. , Haanen, J. B. , van den Eertwegh, A. J. , Boven, E. , Hoekman, K. , de Gruijl, T. D. (2008) Sunitinib‐induced myeloid lineage redistribution in renal cell cancer patients: CD1c+ dendritic cell frequency predicts progression‐free survival. Clin. Cancer Res. 14, 5884–5892. [DOI] [PubMed] [Google Scholar]
- 26. Vuk‐Pavlović, S. , Bulur, P. A. , Lin, Y. , Qin, R. , Szumlanski, C. L. , Zhao, X. , Dietz, A. B. (2010) Immunosuppressive CD14+HLA‐DRlow/– monocytes in prostate cancer. Prostate 70, 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gallina, G. , Dolcetti, L. , Serafini, P. , De Santo, C. , Marigo, I. , Colombo, M. P. , Basso, G. , Brombacher, F. , Borrello, I. , Zanovello, P. , Bicciato, S. , Bronte, V. (2006) Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J. Clin. Invest. 116, 2777–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diaz‐Montero, C. M. , Salem, M. L. , Nishimura, M. I. , Garrett‐Mayer, E. , Cole, D. J. , Montero, A. J. (2009) Increased circulating myeloid‐derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin‐cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 58, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Youn, J. I. , Gabrilovich, D. I. (2010) The biology of myeloid‐derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur. J. Immunol. 40, 2969–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greifenberg, V. , Ribechini, E. , Rössner, S. , Lutz, M. B. (2009) Myeloid‐derived suppressor cell activation by combined LPS and IFN‐γ treatment impairs DC development. Eur. J. Immunol. 39, 2865–2876. [DOI] [PubMed] [Google Scholar]
- 31. Zhao, F. , Obermann, S. , von Wasielewski, R. , Haile, L. , Manns, M. P. , Korangy, F. , Greten, T. F. (2009) Increase in frequency of myeloid‐derived suppressor cells in mice with spontaneous pancreatic carcinoma. Immunology 128, 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mandruzzato, S. , Brandau, S. , Britten, C. M. , Bronte, V. , Damuzzo, V. , Gouttefangeas, C. , Maurer, D. , Ottensmeier, C. , van der Burg, S. H. , Welters, M. J. , Walter, S. (2016) Toward harmonized phenotyping of human myeloid‐derived suppressor cells by flow cytometry: results from an interim study. Cancer Immunol. Immunother. 65, 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bronte, V. , Brandau, S. , Chen, S. H. , Colombo, M. P. , Frey, A. B. , Greten, T. F. , Mandruzzato, S. , Murray, P. J. , Ochoa, A. , Ostrand‐Rosenberg, S. , Rodriguez, P. C. , Sica, A. , Umansky, V. , Vonderheide, R. H. , Gabrilovich, D. I. (2016) Recommendations for myeloid‐derived suppressor cell nomenclature and characterization standards. Nat. Commun. 7, 12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Solito, S. , Marigo, I. , Pinton, L. , Damuzzo, V. , Mandruzzato, S. , Bronte, V. (2014) Myeloid‐derived suppressor cell heterogeneity in human cancers. Ann. N. Y. Acad. Sci. 1319, 47–65. [DOI] [PubMed] [Google Scholar]
- 35. Dumitru, C. A. , Moses, K. , Trellakis, S. , Lang, S. , Brandau, S. (2012) Neutrophils and granulocytic myeloid‐derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol. Immunother. 61, 1155–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Youn, J. I. , Collazo, M. , Shalova, I. N. , Biswas, S. K. , Gabrilovich, D. I. (2012) Characterization of the nature of granulocytic myeloid‐derived suppressor cells in tumor‐bearing mice. J. Leukoc. Biol. 91, 167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pillay, J. , Kamp, V. M. , van Hoffen, E. , Visser, T. , Tak, T. , Lammers, J. W. , Ulfman, L. H. , Leenen, L. P. , Pickkers, P. , Koenderman, L. (2012) A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac‐1. J. Clin. Invest. 122, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leliefeld, P. H. C. , Wessels, C. M. , Leenen, L. P. H. , Koenderman, L. , Pillay, J. (2016) The role of neutrophils in immune dysfunction during severe inflammation. Crit. Care 20, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rodriguez, P. C. , Ernstoff, M. S. , Hernandez, C. , Atkins, M. , Zabaleta, J. , Sierra, R. , Ochoa, A. C. (2009) Arginase I–producing myeloid‐derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 69, 1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khaled, Y. S. , Ammori, B. J. , Elkord, E. (2014) Increased levels of granulocytic myeloid‐derived suppressor cells in peripheral blood and tumour tissue of pancreatic cancer patients. J. Immunol. Res. 2014, 879897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pillay, J. , Tak, T. , Kamp, V. M. , Koenderman, L. (2013) Immune suppression by neutrophils and granulocytic myeloid‐derived suppressor cells: similarities and differences. Cell. Mol. Life Sci. 70, 3813–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gabrilovich, D. I. , Ostrand‐Rosenberg, S. , Bronte, V. (2012) Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Foell, D. , Wittkowski, H. , Vogl, T. , Roth, J. (2007) S100 proteins expressed in phagocytes: a novel group of damage‐associated molecular pattern molecules. J. Leukoc. Biol. 81, 28–37. [DOI] [PubMed] [Google Scholar]
- 44. Gabrilovich, D. I. , Nagaraj, S. (2009) Myeloid‐derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhao, F. , Hoechst, B. , Duffy, A. , Gamrekelashvili, J. , Fioravanti, S. , Manns, M. P. , Greten, T. F. , Korangy, F. (2012) S100A9 a new marker for monocytic human myeloid‐derived suppressor cells. Immunology 136, 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cheng, P. , Corzo, C. A. , Luetteke, N. , Yu, B. , Nagaraj, S. , Bui, M. M. , Ortiz, M. , Nacken, W. , Sorg, C. , Vogl, T. , Roth, J. , Gabrilovich, D. I. (2008) Inhibition of dendritic cell differentiation and accumulation of myeloid‐derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med. 205, 2235–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Condamine, T. , Ramachandran, I. , Youn, J. I. , Gabrilovich, D. I. (2015) Regulation of tumor metastasis by myeloid‐derived suppressor cells. Annu. Rev. Med. 66, 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rodriguez, P. C. , Quiceno, D. G. , Ochoa, A. C. (2007) l‐arginine availability regulates T‐lymphocyte cell‐cycle progression. Blood 109, 1568–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nagaraj, S. , Gupta, K. , Pisarev, V. , Kinarsky, L. , Sherman, S. , Kang, L. , Herber, D. L. , Schneck, J. , Gabrilovich, D. I. (2007) Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 13, 828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hanson, E. M. , Clements, V. K. , Sinha, P. , Ilkovitch, D. , Ostrand‐Rosenberg, S. (2009) Myeloid‐derived suppressor cells downregulate l‐selectin expression on CD4+ and CD8+ T cells. J. Immunol. 183, 937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Filipazzi, P. , Valenti, R. , Huber, V. , Pilla, L. , Canese, P. , Iero, M. , Castelli, C. , Mariani, L. , Parmiani, G. , Rivoltini, L. (2007) Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte‐macrophage colonystimulation factor‐based antitumor vaccine. J. Clin. Oncol. 25, 2546–2553. [DOI] [PubMed] [Google Scholar]
- 52. Brandau, S. , Trellakis, S. , Bruderek, K. , Schmaltz, D. , Steller, G. , Elian, M. , Suttmann, H. , Schenck, M. , Welling, J. , Zabel, P. , Lang, S. (2011) Myeloid‐derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J. Leukoc. Biol. 89, 311–317. [DOI] [PubMed] [Google Scholar]
- 53. Kusmartsev, S. , Eruslanov, E. , Kübler, H. , Tseng, T. , Sakai, Y. , Su, Z. , Kaliberov, S. , Heiser, A. , Rosser, C. , Dahm, P. , Siemann, D. , Vieweg, J. (2008) Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor‐induced immune suppression in renal cell carcinoma. J. Immunol. 181, 346–353. [DOI] [PubMed] [Google Scholar]
- 54. Finke, J. , Ko, J. , Rini, B. , Rayman, P. , Ireland, J. , Cohen, P. (2011) MDSC as a mechanism of tumor escape from sunitinib mediated antiangiogenic therapy. Int. Immunopharmacol. 11, 856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang, B. , Pan, P. Y. , Li, Q. , Sato, A. I. , Levy, D. E. , Bromberg, J. , Divino, C. M. , Chen, S. H. (2006) Gr‐1+CD115+ immature myeloid suppressor cells mediate the development of tumor‐induced T regulatory cells and T‐cell anergy in tumor‐bearing host. Cancer Res. 66, 1123–1131. [DOI] [PubMed] [Google Scholar]
- 56. Kusmartsev, S. , Nagaraj, S. , Gabrilovich, D. I. (2005) Tumor‐associated CD8+ T cell tolerance induced by bone marrow‐derived immature myeloid cells. J. Immunol. 175, 4583–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Terabe, M. , Matsui, S. , Park, J. M. , Mamura, M. , Noben‐Trauth, N. , Donaldson, D. D. , Chen, W. , Wahl, S. M. , Ledbetter, S. , Pratt, B. , Letterio, J. J. , Paul, W. E. , Berzofsky, J. A. (2003) Transforming growth factor‐β production and myeloid cells are an effector mechanism through which CD1d‐restricted T cells block cytotoxic T lymphocyte‐mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J. Exp. Med. 198, 1741–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schmielau, J. , Finn, O. J. (2001) Activated granulocytes and granulocytederived hydrogen peroxide are the underlying mechanism of suppression of T‐cell function in advanced cancer patients. Cancer Res. 61, 4756–4760. [PubMed] [Google Scholar]
- 59. Zea, A. H. , Rodriguez, P. C. , Atkins, M. B. , Hernandez, C. , Signoretti, S. , Zabaleta, J. , McDermott, D. , Quiceno, D. , Youmans, A. , O'Neill, A. , Mier, J. , Ochoa, A. C. (2005) Arginase‐producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 65, 3044–3048. [DOI] [PubMed] [Google Scholar]
- 60. Condamine, T. , Mastio, J. , Gabrilovich, D. I. (2015) Transcriptional regulation of myeloid‐derived suppressor cells. J. Leukoc. Biol. 98, 913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Condamine, T. , Gabrilovich, D. I. (2011) Molecular mechanisms regulating myeloid‐derived suppressor cell differentiation and function. Trends Immunol. 32, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Liu, Y. , Xiang, X. , Zhuang, X. , Zhang, S. , Liu, C. , Cheng, Z. , Michalek, S. , Grizzle, W. , Zhang, H. G. (2010) Contribution of MyD88 to the tumor exosome‐mediated induction of myeloid derived suppressor cells. Am. J. Pathol. 176, 2490–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Arora, M. , Poe, S. L. , Oriss, T. B. , Krishnamoorthy, N. , Yarlagadda, M. , Wenzel, S. E. , Billiar, T. R. , Ray, A. , Ray, P. (2010) TLR4/MyD88‐induced CD11b+Gr‐1 int F4/80+ non‐migratory myeloid cells suppress Th2 effector function in the lung. Mucosal Immunol. 3, 578–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hong, E. H. , Chang, S. Y. , Lee, B. R. , Kim, Y. S. , Lee, J. M. , Kang, C. Y. , Kweon, M. N. , Ko, H. J. (2013) Blockade of Myd88 signaling induces antitumor effects by skewing the immunosuppressive function of myeloid‐derived suppressor cells. Int. J. Cancer 132, 2839–2848. [DOI] [PubMed] [Google Scholar]
- 65. Yang, Y. , Zhang, R. , Xia, F. , Zou, T. , Huang, A. , Xiong, S. , Zhang, J. (2013) LPS converts Gr‐1+CD115+ myeloid‐derived suppressor cells from M2 to M1 via P38 MAPK. Exp. Cell Res. 319, 1774–1783. [DOI] [PubMed] [Google Scholar]
- 66. Wu, H. , Tao, N. , Liu, X. , Li, X. , Tang, J. , Ma, C. , Xu, X. , Shao, H. , Hou, B. , Wang, H. , Qin, Z. (2012) Polysaccharide from Lentinus edodes inhibits the immunosuppressive function of myeloid‐derived suppressor cells. PLoS One 7, e51751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bunt, S. K. , Clements, V. K. , Hanson, E. M. , Sinha, P. , Ostrand‐Rosenberg, S. (2009) Inflammation enhances myeloid‐derived suppressor cell cross‐talk by signaling through Toll‐like receptor 4. J. Leukoc. Biol. 85, 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tu, S. , Bhagat, G. , Cui, G. , Takaishi, S. , Kurt‐Jones, E. A. , Rickman, B. , Betz, K. S. , Penz‐Oesterreicher, M. , Bjorkdahl, O. , Fox, J. G. , Wang, T. C. (2008) Overexpression of interleukin‐1beta induces gastric inflammation and cancer and mobilizes myeloid‐derived suppressor cells in mice. Cancer Cell 14, 408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lu, T. , Ramakrishnan, R. , Altiok, S. , Youn, J. I. , Cheng, P. , Celis, E. , Pisarev, V. , Sherman, S. , Sporn, M. B. , Gabrilovich, D. (2011) Tumor‐infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J. Clin. Invest. 121, 4015–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Marigo, I. , Bosio, E. , Solito, S. , Mesa, C. , Fernandez, A. , Dolcetti, L. , Ugel, S. , Sonda, N. , Bicciato, S. , Falisi, E. , Calabrese, F. , Basso, G. , Zanovello, P. , Cozzi, E. , Mandruzzato, S. , Bronte, V. (2010) Tumor‐induced tolerance and immune suppression depend on the C/EBPβ transcription factor. Immunity 32, 790–802. [DOI] [PubMed] [Google Scholar]
- 71. Nefedova, Y. , Nagaraj, S. , Rosenbauer, A. , Muro‐Cacho, C. , Sebti, S. M. , Gabrilovich, D. I. (2005) Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic‐selective inhibition of the janus‐activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res. 65, 9525–9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Munera, V. , Popovic, P. J. , Bryk, J. , Pribis, J. , Caba, D. , Matta, B. M. , Zenati, M. , Ochoa, J. B. (2010) Stat 6‐dependent induction of myeloid derived suppressor cells after physical injury regulates nitric oxide response to endotoxin. Ann. Surg. 251, 120–126. [DOI] [PubMed] [Google Scholar]
- 73. Schouppe, E. , Mommer, C. , Movahedi, K. , Laoui, D. , Morias, Y. , Gysemans, C. , Luyckx, A. , De Baetselier, P. , Van Ginderachter, J. A. (2013) Tumor‐induced myeloid‐derived suppressor cell subsets exert either inhibitory or stimulatory effects on distinct CD8+ T‐cell activation events. Eur. J. Immunol. 43, 2930–2942. [DOI] [PubMed] [Google Scholar]
- 74. Corzo, C. A. , Cotter, M. J. , Cheng, P. , Cheng, F. , Kusmartsev, S. , Sotomayor, E. , Padhya, T. , McCaffrey, T. V. , McCaffrey, J. C. , Gabrilovich, D. I. (2009) Mechanism regulating reactive oxygen species in tumor‐induced myeloid‐derived suppressor cells. J. Immunol. 182, 5693–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Vasquez‐Dunddel, D. , Pan, F. , Zeng, Q. , Gorbounov, M. , Albesiano, E. , Fu, J. , Blosser, R. L. , Tam, A. J. , Bruno, T. , Zhang, H. , Pardoll, D. , Kim, Y. (2013) STAT3 regulates arginase‐I in myeloid‐derived suppressor cells from cancer patients. J. Clin. Invest. 123, 1580–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rodriguez, P. C. , Hernandez, C. P. , Quiceno, D. , Dubinett, S. M. , Zabaleta, J. , Ochoa, J. B. , Gilbert, J. , Ochoa, A. C. (2005) Arginase I in myeloid suppressor cells is induced by COX‐2 in lung carcinoma. J. Exp. Med. 202, 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Donkor, M. K. , Lahue, E. , Hoke, T. A. , Shafer, L. R. , Coskun, U. , Solheim, J. C. , Gulen, D. , Bishay, J. , Talmadge, J. E. (2009) Mammary tumor heterogeneity in the expansion of myeloid‐derived suppressor cells. Int. Immunopharmacol. 9, 937–948. [DOI] [PubMed] [Google Scholar]
- 78. Fujita, M. , Kohanbash, G. , Fellows‐Mayle, W. , Hamilton, R. L. , Komohara, Y. , Decker, S. A. , Ohlfest, J. R. , Okada, H. (2011) COX‐2 blockade suppresses gliomagenesis by inhibiting myeloid‐derived suppressor cells. Cancer Res. 71, 2664–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Veltman, J. D. , Lambers, M. E. , van Nimwegen, M. , Hendriks, R. W. , Hoogsteden, H. C. , Aerts, J. G. , Hegmans, J. P. (2010) COX‐2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid‐derived suppressor cells in mesothelioma: celecoxib influences MDSC function. BMC Cancer 10, 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Obermajer, N. , Muthuswamy, R. , Odunsi, K. , Edwards, R. P. , Kalinski, P. (2011) PGE2‐induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 71, 7463–7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mao, Y. , Poschke, I. , Wennerberg, E. , Pico de Coaña, Y. , Egyhazi Brage, S. , Schultz, I. , Hansson, J. , Masucci, G. , Lundqvist, A. , Kiessling, R. (2013) Melanoma‐educated CD14+ cells acquire a myeloid‐derived suppressor cell phenotype through COX‐2‐dependent mechanisms. Cancer Res. 73, 3877–3887. [DOI] [PubMed] [Google Scholar]
- 82. Kim, K. , Skora, A. D. , Li, Z. , Liu, Q. , Tam, A. J. , Blosser, R. L. , Diaz, L. A., Jr. , Papadopoulos, N. , Kinzler, K. W. , Vogelstein, B. , Zhou, S. (2014) Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid‐derived cells. Proc. Natl. Acad. Sci. USA 111, 11774–11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Weide, B. , Martens, A. , Zelba, H. , Stutz, C. , Derhovanessian, E. , Di Giacomo, A. M. , Maio, M. , Sucker, A. , Schilling, B. , Schadendorf, D. , Büttner, P. , Garbe, C. , Pawelec, G. (2014) Myeloid‐derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY‐ESO‐1‐ or melan‐A‐specific T cells. Clin. Cancer Res. 20, 1601–1609. [DOI] [PubMed] [Google Scholar]
- 84. Gebhardt, C. , Sevko, A. , Jiang, H. , Lichtenberger, R. , Reith, M. , Tarnanidis, K. , Holland‐Letz, T. , Umansky, L. , Beckhove, P. , Sucker, A. , Schadendorf, D. , Utikal, J. , Umansky, V. (2015) Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with Ipilimumab. Clin. Cancer Res. 21, 5453–5459. [DOI] [PubMed] [Google Scholar]
- 85. Sade‐Feldman, M. , Kanterman, J. , Klieger, Y. , Ish‐Shalom, E. , Olga, M. , Saragovi, A. , Shtainberg, H. , Lotem, M. , Baniyash, M. (2016) Clinical significance of circulating CD33+CD11b+HLA‐DR– myeloid cells in patients with stage IV melanoma treated with Ipilimumab. Clin. Cancer Res. 22, 5661–5672. [DOI] [PubMed] [Google Scholar]
- 86. Martens, A. , Wistuba‐Hamprecht, K. , Geukes Foppen, M. , Yuan, J. , Postow, M. A. , Wong, P. , Romano, E. , Khammari, A. , Dreno, B. , Capone, M. , Ascierto, P. A. , Di Giacomo, A. M. , Maio, M. , Schilling, B. , Sucker, A. , Schadendorf, D. , Hassel, J. C. , Eigentler, T. K. , Martus, P. , Wolchok, J. D. , Blank, C. , Pawelec, G. , Garbe, C. , Weide, B. (2016) Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with Ipilimumab. Clin. Cancer Res. 22, 2908–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Serafini, P. , Borrello, I. , Bronte, V. (2006) Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin. Cancer Biol. 16, 53–65. [DOI] [PubMed] [Google Scholar]
- 88. Ugel, S. , Delpozzo, F. , Desantis, G. , Papalini, F. , Simonato, F. , Sonda, N. , Zilio, S. , Bronte, V. (2009) Therapeutic targeting of myeloid‐derived suppressor cells. Curr. Opin. Pharmacol. 9, 470–481. [DOI] [PubMed] [Google Scholar]
- 89. Sansone, P. , Bromberg, J. (2012) Targeting the interleukin‐6/Jak/stat pathway in human malignancies. J. Clin. Oncol. 30, 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ko, J. S. , Zea, A. H. , Rini, B. I. , Ireland, J. L. , Elson, P. , Cohen, P. , Golshayan, A. , Rayman, P. A. , Wood, L. , Garcia, J. , Dreicer, R. , Bukowski, R. , Finke, J. H. (2009) Sunitinib mediates reversal of myeloid‐derived suppressor cell accumulation in renal cell carcinoma patients. Clin. Cancer Res. 15, 2148–2157. [DOI] [PubMed] [Google Scholar]
- 91. Kodera, Y. , Katanasaka, Y. , Kitamura, Y. , Tsuda, H. , Nishio, K. , Tamura, T. , Koizumi, F. (2011) Sunitinib inhibits lymphatic endothelial cell functions and lymph node metastasis in a breast cancer model through inhibition of vascular endothelial growth factor receptor 3. Breast Cancer Res. 13, R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Finke, J. H. , Rini, B. , Ireland, J. , Rayman, P. , Richmond, A. , Golshayan, A. , Wood, L. , Elson, P. , Garcia, J. , Dreicer, R. , Bukowski, R. (2008) Sunitinib reverses type‐1 immune suppression and decreases Tregulatory cells in renal cell carcinoma patients. Clin. Cancer Res. 14, 6674–6682. [DOI] [PubMed] [Google Scholar]
- 93. Amato, R. J. , Hawkins, R. E. , Kaufman, H. L. , Thompson, J. A. , Tomczak, P. , Szczylik, C. , McDonald, M. , Eastty, S. , Shingler, W. H. , de Belin, J. , Goonewardena, M. , Naylor, S. , Harrop, R. (2010) Vaccination of metastatic renal cancer patients with MVA‐5T4: a randomized, double‐blind, placebo‐controlled phase III study. Clin. Cancer Res. 16, 5539–5547. [DOI] [PubMed] [Google Scholar]
- 94. Heissig, B. , Hattori, K. , Dias, S. , Friedrich, M. , Ferris, B. , Hackett, N. R. , Crystal, R. G. , Besmer, P. , Lyden, D. , Moore, M. A. , Werb, Z. , Rafii, S. (2002) Recruitment of stem and progenitor cells from the bone marrow niche requires MMP‐9 mediated release of kit‐ligand. Cell 109, 625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Melani, C. , Sangaletti, S. , Barazzetta, F. M. , Werb, Z. , Colombo, M. P. (2007) Amino‐biphosphonate‐mediated MMP‐9 inhibition breaks the tumor‐bone marrow axis responsible for myeloid‐derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 67, 11438–11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Qin, H. , Lerman, B. , Sakamaki, I. , Wei, G. , Cha, S. C. , Rao, S. S. , Qian, J. , Hailemichael, Y. , Nurieva, R. , Dwyer, K. C. , Roth, J. , Yi, Q. , Overwijk, W. W. , Kwak, L. W. (2014) Generation of a new therapeutic peptide that depletes myeloid‐derived suppressor cells in tumor‐bearing mice. Nat. Med. 20, 676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Suzuki, E. , Kapoor, V. , Jassar, A. S. , Kaiser, L. R. , Albelda, S. M. (2005) Gemcitabine selectively eliminates splenic Gr‐1+/CD11b+ myeloid suppressor cells in tumor‐bearing animals and enhances antitumor immune activity. Clin. Cancer Res. 11, 6713–6721. [DOI] [PubMed] [Google Scholar]
- 98. Le, H. K. , Graham, L. , Cha, E. , Morales, J. K. , Manjili, M. H. , Bear, H. D. (2009) Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor‐bearing mice. Int. Immunopharmacol. 9, 900–909. [DOI] [PubMed] [Google Scholar]
- 99. Vincent, J. , Mignot, G. , Chalmin, F. , Ladoire, S. , Bruchard, M. , Chevriaux, A. , Martin, F. , Apetoh, L. , Rébé, C. , Ghiringhelli, F. (2010) 5‐Fluorouracil selectively kills tumor‐associated myeloid‐derived suppressor cells resulting in enhanced T cell‐dependent antitumor immunity. Cancer Res. 70, 3052–3061. [DOI] [PubMed] [Google Scholar]
- 100. Bruchard, M. , Mignot, G. , Derangère, V. , Chalmin, F. , Chevriaux, A. , Végran, F. , Boireau, W. , Simon, B. , Ryffel, B. , Connat, J. L. , Kanellopoulos, J. , Martin, F. , Rébé, C. , Apetoh, L. , Ghiringhelli, F. (2013) Chemotherapy‐triggered cathepsin B release in myeloid‐derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat. Med. 19, 57–64. [DOI] [PubMed] [Google Scholar]
- 101. Sevko, A. , Michels, T. , Vrohlings, M. , Umansky, L. , Beckhove, P. , Kato, M. , Shurin, G. V. , Shurin, M. R. , Umansky, V. (2013) Antitumor effect of paclitaxel is mediated by inhibition of myeloid‐derived suppressor cells and chronic inflammation in the spontaneous melanoma model. J. Immunol. 190, 2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Johnson, D. B. , Sosman, J. A. (2013) Update on the targeted therapy of melanoma. Curr. Treat. Options Oncol. 14, 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Schilling, B. , Sucker, A. , Griewank, K. , Zhao, F. , Weide, B. , Görgens, A. , Giebel, B. , Schadendorf, D. , Paschen, A. (2013) Vemurafenib reverses immunosuppression by myeloid derived suppressor cells. Int. J. Cancer 133, 1653–1663. [DOI] [PubMed] [Google Scholar]
- 104. Schilling, B. , Paschen, A. (2013) Immunological consequences of selective BRAF inhibitors in malignant melanoma: neutralization of myeloid‐derived suppressor cells. OncoImmunology 2, e25218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ribas, A. , Hodi, F. S. , Callahan, M. , Konto, C. , Wolchok, J. (2013) Hepatotoxicity with combination of vemurafenib and ipilimumab. N. Engl. J. Med. 368, 1365–1366. [DOI] [PubMed] [Google Scholar]
- 106. Di Mitri, D. , Toso, A. , Alimonti, A. (2015) Molecular pathways: targeting tumor‐infiltrating myeloid‐derived suppressor cells for cancer therapy. Clin. Cancer Res. 21, 3108–3112. [DOI] [PubMed] [Google Scholar]
- 107. Umansky, V. , Blattner, C. , Gebhardt, C. , Utikal, J. (2016) The role of myeloid‐derived suppressor cells (MDSC) in cancer progression. Vaccines (Basel) 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Di Mitri, D. , Toso, A. , Chen, J. J. , Sarti, M. , Pinton, S. , Jost, T. R. , D'Antuono, R. , Montani, E. , Garcia‐Escudero, R. , Guccini, I. , Da Silva‐Alvarez, S. , Collado, M. , Eisenberger, M. , Zhang, Z. , Catapano, C. , Grassi, F. , Alimonti, A. (2014) Tumour‐infiltrating Gr‐1+ myeloid cells antagonize senescence in cancer. Nature 515, 134–137. [DOI] [PubMed] [Google Scholar]
- 109. Wang, G. , Lu, X. , Dey, P. , Deng, P. , Wu, C. C. , Jiang, S. , Fang, Z. , Zhao, K. , Konaparthi, R. , Hua, S. , Zhang, J. , Li‐Ning‐Tapia, E. M. , Kapoor, A. , Wu, C. J. , Patel, N. B. , Guo, Z. , Ramamoorthy, V. , Tieu, T. N. , Heffernan, T. , Zhao, D. , Shang, X. , Khadka, S. , Hou, P. , Hu, B. , Jin, E. J. , Yao, W. , Pan, X. , Ding, Z. , Shi, Y. , Li, L. , Chang, Q. , Troncoso, P. , Logothetis, C. J. , McArthur, M. J. , Chin, L. , Wang, Y. A. , DePinho, R. A. (2016) Targeting YAP‐dependent MDSC infiltration impairs tumor progression. Cancer Discov. 6, 80–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Toso, A. , Revandkar, A. , Di Mitri, D. , Guccini, I. , Proietti, M. , Sarti, M. , Pinton, S. , Zhang, J. , Kalathur, M. , Civenni, G. , Jarrossay, D. , Montani, E. , Marini, C. , Garcia‐Escudero, R. , Scanziani, E. , Grassi, F. , Pandolfi, P. P. , Catapano, C. V. , Alimonti, A. (2014) Enhancing chemotherapy efficacy in Pten‐deficient prostate tumors by activating the senescence‐associated antitumor immunity. Cell Rep. 9, 75–89. [DOI] [PubMed] [Google Scholar]
- 111. Zollo, M. , Di Dato, V. , Spano, D. , De Martino, D. , Liguori, L. , Marino, N. , Vastolo, V. , Navas, L. , Garrone, B. , Mangano, G. , Biondi, G. , Guglielmotti, A. (2012) Targeting monocyte chemotactic protein‐1 synthesis with bindarit induces tumor regression in prostate and breast cancer animal models. Clin. Exp. Metastasis 29, 585–601. [DOI] [PubMed] [Google Scholar]
- 112. Hamilton, J. A. (2008) Colony‐stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 8, 533–544. [DOI] [PubMed] [Google Scholar]
- 113. Chitu, V. , Stanley, E. R. (2006) Colony‐stimulating factor‐1 in immunity and inflammation. Curr. Opin. Immunol. 18, 39–48. [DOI] [PubMed] [Google Scholar]
- 114. Priceman, S. J. , Sung, J. L. , Shaposhnik, Z. , Burton, J. B. , Torres‐Collado, A. X. , Moughon, D. L. , Johnson, M. , Lusis, A. J. , Cohen, D. A. , Iruela‐Arispe, M. L. , Wu, L. (2010) Targeting distinct tumor‐infiltrating myeloid cells by inhibiting CSF‐1 receptor: combating tumor evasion of antiangiogenic therapy. Blood 115, 1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Xu, J. , Escamilla, J. , Mok, S. , David, J. , Priceman, S. , West, B. , Bollag, G. , McBride, W. , Wu, L. (2013) CSF1R signaling blockade stanches tumor‐infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 73, 2782–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Najjar, Y. G. , Finke, J. H. (2013) Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front. Oncol. 3, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kao, J. , Ko, E. C. , Eisenstein, S. , Sikora, A. G. , Fu, S. , Chen, S. H. (2011) Targeting immune suppressing myeloid‐derived suppressor cells in oncology. Crit. Rev. Oncol. Hematol. 77, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Draghiciu, O. , Lubbers, J. , Nijman, H. W. , Daemen, T. (2015) Myeloid derived suppressor cells—an overview of combat strategies to increase immunotherapy efficacy. OncoImmunology 4, e954829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Shirota, Y. , Shirota, H. , Klinman, D. M. (2012) Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid‐derived suppressor cells. J. Immunol. 188, 1592–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. De Santo, C. , Serafini, P. , Marigo, I. , Dolcetti, L. , Bolla, M. , Del Soldato, P. , Melani, C. , Guiducci, C. , Colombo, M. P. , Iezzi, M. , Musiani, P. , Zanovello, P. , Bronte, V. (2005) Nitroaspirin corrects immune dysfunction in tumor‐bearing hosts and promotes tumor eradication by cancer vaccination. Proc. Natl. Acad. Sci. USA 102, 4185–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gao, P. , Zhang, H. , Dinavahi, R. , Li, F. , Xiang, Y. , Raman, V. , Bhujwalla, Z. M. , Felsher, D. W. , Cheng, L. , Pevsner, J. , Lee, L. A. , Semenza, G. L. , Dang, C. V. (2007) HIF‐dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell 12, 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zhang, Q. , Hossain, D. M. S. , Duttagupta, P. , Moreira, D. , Zhao, X. , Won, H. , Buettner, R. , Nechaev, S. , Majka, M. , Zhang, B. , Cai, Q. , Swiderski, P. , Kuo, Y. H. , Forman, S. , Marcucci, G. , Kortylewski, M. (2016) Serum‐resistant CpG‐STAT3 decoy for targeting survival and immune checkpoint signaling in acute myeloid leukemia. Blood 127, 1687–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kortylewski, M. , Moreira, D. (2017) Myeloid cells as a target for oligonucleotide therapeutics: turning obstacles into opportunities. Cancer Immunol. Immunother. [Epub ahead of print February 18, 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Liby, K. T. , Yore, M. M. , Sporn, M. B. (2007) Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Rev. Cancer 7, 357–369. [DOI] [PubMed] [Google Scholar]
- 125. Konopleva, M. , Zhang, W. , Shi, Y. X. , McQueen, T. , Tsao, T. , Abdelrahim, M. , Munsell, M. F. , Johansen, M. , Yu, D. , Madden, T. , Safe, S. H. , Hung, M. C. , Andreeff, M. (2006) Synthetic triterpenoid 2‐cyano‐3,12‐dioxooleana‐1,9‐dien‐28‐oic acid induces growth arrest in HER2‐overexpressing breast cancer cells. Mol. Cancer Ther. 5, 317–328. [DOI] [PubMed] [Google Scholar]
- 126. Chalmin, F. , Ladoire, S. , Mignot, G. , Vincent, J. , Bruchard, M. , Remy‐Martin, J. P. , Boireau, W. , Rouleau, A. , Simon, B. , Lanneau, D. , De Thonel, A. , Multhoff, G. , Hamman, A. , Martin, F. , Chauffert, B. , Solary, E. , Zitvogel, L. , Garrido, C. , Ryffel, B. , Borg, C. , Apetoh, L. , Rébé, C. , Ghiringhelli, F. (2010) Membrane‐associated Hsp72 from tumorderived exosomes mediates STAT3‐dependent immunosuppressive function of mouse and human myeloid‐derived suppressor cells. J. Clin. Invest. 120, 457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Eksioglu, E. A. , Chen, X. , Heider, K. H. , Rueter, B. , McGraw, K. L. , Basiorka, A. A. , Wei, M. , Burnette, A. , Cheng, P. , Lancet, J. , Komrokji, R. , Djeu, J. , List, A. , Wei, S. (2017) Novel therapeutic approach to improve hematopoiesis in low risk MDS by targeting MDSCs with the Fcengineered CD33 antibody BI 836858. Leukemia. [Epub ahead of print February 10, 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Serafini, P. , Meckel, K. , Kelso, M. , Noonan, K. , Califano, J. , Koch, W. , Dolcetti, L. , Bronte, V. , Borrello, I. (2006) Phosphodiesterase‐5 inhibition augments endogenous antitumor immunity by reducing myeloid‐derived suppressor cell function. J. Exp. Med. 203, 2691–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Yaddanapudi, K. , Rendon, B. E. , Lamont, G. , Kim, E. J. , Al Rayyan, N. , Richie, J. , Albeituni, S. , Waigel, S. , Wise, A. , Mitchell, R. A. (2016) MIF is necessary for late‐stage melanoma patient MDSC immune suppression and differentiation. Cancer Immunol. Res. 4, 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yaddanapudi, K. , Putty, K. , Rendon, B. E. , Lamont, G. J. , Faughn, J. D. , Satoskar, A. , Lasnik, A. , Eaton, J. W. , Mitchell, R. A. (2013) Control of tumor‐associated macrophage alternative activation by macrophage migration inhibitory factor. J. Immunol. 190, 2984–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Simpson, K. D. , Templeton, D. J. , Cross, J. V. (2012) Macrophage migration inhibitory factor promotes tumor growth and metastasis by inducing myeloid‐derived suppressor cells in the tumor microenvironment. J. Immunol. 189, 5533–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hillen, F. , Baeten, C. I. , van de Winkel, A. , Creytens, D. , van der Schaft, D. W. , Winnepenninckx, V. , Griffioen, A. W. (2008) Leukocyte infiltration and tumor cell plasticity are parameters of aggressiveness in primary cutaneous melanoma. Cancer Immunol. Immunother. 57, 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Almand, B. , Resser, J. R. , Lindman, B. , Nadaf, S. , Clark, J. I. , Kwon, E. D. , Carbone, D. P. , Gabrilovich, D. I. (2000) Clinical significance of defective dendritic cell differentiation in cancer. Clin. Cancer Res. 6, 1755–1766. [PubMed] [Google Scholar]
- 134. Almand, B. , Clark, J. I. , Nikitina, E. , van Beynen, J. , English, N. R. , Knight, S. C. , Carbone, D. P. , Gabrilovich, D. I. (2001) Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J. Immunol. 166, 678–689. [DOI] [PubMed] [Google Scholar]
- 135. Holmgaard, R. B. , Zamarin, D. , Munn, D. H. , Wolchok, J. D. , Allison, J. P. (2013) Indoleamine 2,3‐dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA‐4. J. Exp. Med. 210, 1389–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Gabrilovich, D. I. , Velders, M. P. , Sotomayor, E. M. , Kast, W. M. (2001) Mechanism of immune dysfunction in cancer mediated by immature Gr‐1+ myeloid cells. J. Immunol. 166, 5398–5406. [DOI] [PubMed] [Google Scholar]
- 137. Hengesbach, L. M. , Hoag, K. A. (2004) Physiological concentrations of retinoic acid favor myeloid dendritic cell development over granulocyte development in cultures of bone marrow cells from mice. J. Nutr. 134, 2653–2659. [DOI] [PubMed] [Google Scholar]
- 138. Kusmartsev, S. , Cheng, F. , Yu, B. , Nefedova, Y. , Sotomayor, E. , Lush, R. , Gabrilovich, D. (2003) All‐trans‐retinoic acid eliminates immature myeloid cells from tumor‐bearing mice and improves the effect of vaccination. Cancer Res. 63, 4441–4449. [PubMed] [Google Scholar]
- 139. Mirza, N. , Fishman, M. , Fricke, I. , Dunn, M. , Neuger, A. M. , Frost, T. J. , Lush, R. M. , Antonia, S. , Gabrilovich, D. I. (2006) All‐trans‐retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 66, 9299–9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Nefedova, Y. , Fishman, M. , Sherman, S. , Wang, X. , Beg, A. A. , Gabrilovich, D. I. (2007) Mechanism of all‐trans retinoic acid effect on tumor‐associated myeloid‐derived suppressor cells. Cancer Res. 67, 11021–11028. [DOI] [PubMed] [Google Scholar]
- 141. Ma, J. , Liu, Y. , Li, Y. , Gu, J. , Liu, J. , Tang, J. , Wang, J. , Ryffel, B. , Shen, Y. , Brand, D. , Liu, Z. , Zheng, S. G. (2014) Differential role of all‐trans retinoic acid in promoting the development of CD4+ and CD8+ regulatory T cells. J. Leukoc. Biol. 95, 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Testa, U. , Masciulli, R. , Tritarelli, E. , Pustorino, R. , Mariani, G. , Martucci, R. , Barberi, T. , Camagna, A. , Valtieri, M. , Peschle, C. (1993) Transforming growth factor‐β potentiates vitamin D3‐induced terminal monocytic differentiation of human leukemic cell lines. J. Immunol. 150, 2418–2430. [PubMed] [Google Scholar]
- 143. Kulbersh, J. S. , Day, T. A. , Gillespie, M. B. , Young, M. R. I. (2009) 1α,25‐Dihydroxyvitamin D3 to skew intratumoral levels of immune inhibitory CD34+ progenitor cells into dendritic cells. Otolaryngol. Head Neck Surg. 140, 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Walsh, J. E. , Clark, A. M. , Day, T. A. , Gillespie, M. B. , Young, M. R. I. (2010) Use of α,25‐dihydroxyvitamin D3 treatment to stimulate immune infiltration into head and neck squamous cell carcinoma. Hum. Immunol. 71, 659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Walker, D. D. , Reeves, T. D. , de Costa, A. M. , Schuyler, C. , Young, M. R. I. (2012) Immunological modulation by 1α,25‐dihydroxyvitamin D3 in patients with squamous cell carcinoma of the head and neck. Cytokine 58, 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Tian, J. , Ma, J. , Ma, K. , Guo, H. , Baidoo, S. E. , Zhang, Y. , Yan, J. , Lu, L. , Xu, H. , Wang, S. (2013) β‐Glucan enhances antitumor immune responses by regulating differentiation and function of monocytic myeloid‐derived suppressor cells. Eur. J. Immunol. 43, 1220–1230. [DOI] [PubMed] [Google Scholar]
- 147. Kerrigan, A. M. , Brown, G. D. (2010) Syk‐coupled C‐type lectin receptors that mediate cellular activation via single tyrosine based activation motifs. Immunol. Rev. 234, 335–352. [DOI] [PubMed] [Google Scholar]
- 148. Goodridge, H. S. , Reyes, C. N. , Becker, C. A. , Katsumoto, T. R. , Ma, J. , Wolf, A. J. , Bose, N. , Chan, A. S. H. , Magee, A. S. , Danielson, M. E. , Weiss, A. , Vasilakos, J. P. , Underhill, D. M. (2011) Activation of the innate immune receptor dectin‐1 upon formation of a ‘phagocytic synapse’. Nature 472, 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Albeituni, S. H. , Ding, C. , Liu, M. , Hu, X. , Luo, F. , Kloecker, G. , Bousamra II, M. , Zhang, H. G. , Yan, J. (2016) Yeast‐derived particulate β‐glucan treatment subverts the suppression of myeloid‐derived suppressor cells (MDSC) by inducing polymorphonuclear MDSC apoptosis and monocytic MDSC differentiation to APC in cancer. J. Immunol. 196, 2167–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Estevez, F. , Carr, A. , Solorzano, L. , Valiente, O. , Mesa, C. , Barroso, O. , Sierra, G. V. , Fernandez, L. E. (1999) Enhancement of the immune response to poorly immunogenic gangliosides after incorporation into very small size proteoliposomes (VSSP). Vaccine 18, 190–197. [DOI] [PubMed] [Google Scholar]
- 151. Mesa, C. , de León, J. , Rigley, K. , Fernández, L. E. (2006) Very small size proteoliposomes derived from Neisseria meningitidis: an effective adjuvant for dendritic cell activation. Vaccine 24 (Suppl 2), S2–S42, 3. [DOI] [PubMed] [Google Scholar]
- 152. Mesa, C. , De León, J. , Rigley, K. , Fernández, L. E. (2004) Very small size proteoliposomes derived from Neisseria meningitidis: an effective adjuvant for Th1 induction and dendritic cell activation. Vaccine 22, 3045–3052. [DOI] [PubMed] [Google Scholar]
- 153. Fernández, A. , Mesa, C. , Marigo, I. , Dolcetti, L. , Clavell, M. , Oliver, L. , Fernández, L. E. , Bronte, V. (2011) Inhibition of tumor‐induced myeloid‐derived suppressor cell function by a nanoparticulated adjuvant. J. Immunol. 186, 264–274. [DOI] [PubMed] [Google Scholar]
- 154. Corzo, C. A. , Condamine, T. , Lu, L. , Cotter, M. J. , Youn, J. I. , Cheng, P. , Cho, H. I. , Celis, E. , Quiceno, D. G. , Padhya, T. , McCaffrey, T. V. , McCaffrey, J. C. , Gabrilovich, D. I. (2010) HIF‐1α regulates function and differentiation of myeloid‐derived suppressor cells in the tumor microenvironment. J. Exp. Med. 207, 2439–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Telang, S. , Yalcin, A. , Clem, A. L. , Bucala, R. , Lane, A. N. , Eaton, J. W. , Chesney, J. (2006) Ras transformation requires metabolic control by 6‐phosphofructo‐2‐kinase. Oncogene 25, 7225–7234. [DOI] [PubMed] [Google Scholar]
- 156. Yalcin, A. , Telang, S. , Clem, B. , Chesney, J. (2009) Regulation of glucose metabolism by 6‐phosphofructo‐2‐kinase/fructose‐2,6‐bisphosphatases in cancer. Exp. Mol. Pathol. 86, 174–179. [DOI] [PubMed] [Google Scholar]
- 157. Clem, B. , Telang, S. , Clem, A. , Yalcin, A. , Meier, J. , Simmons, A. , Rasku, M. A. , Arumugam, S. , Dean, W. L. , Eaton, J. , Lane, A. , Trent, J. O. , Chesney, J. (2008) Small‐molecule inhibition of 6‐phosphofructo‐2‐kinase activity suppresses glycolytic flux and tumor growth. Mol. Cancer Ther. 7, 110–120. [DOI] [PubMed] [Google Scholar]
- 158. Clem, B. F. , O'Neal, J. , Tapolsky, G. , Clem, A. L. , Imbert‐Fernandez, Y. , Kerr II, D. A. , Klarer, A. C. , Redman, R. , Miller, D. M. , Trent, J. O. , Telang, S. , Chesney, J. (2013) Targeting 6‐phosphofructo‐2‐kinase (PFKFB3) as a therapeutic strategy against cancer. Mol. Cancer Ther. 12, 1461–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]


