Short abstract
Expression of dominant‐negative Trim9 in zebrafish macrophages alters their cell shape and significantly reduces their motility in vivo.
Keywords: chemotaxis, zebrafish, leukocyte, ubiquitin
Abstract
The vertebrate immune response comprises multiple molecular and cellular components that interface to provide defense against pathogens. Because of the dynamic complexity of the immune system and its interdependent innate and adaptive functionality, an understanding of the whole‐organism response to pathogen exposure remains unresolved. Zebrafish larvae provide a unique model for overcoming this obstacle, because larvae are protected against pathogens while lacking a functional adaptive immune system during the first few weeks of life. Zebrafish larvae were exposed to immune agonists for various lengths of time, and a microarray transcriptome analysis was executed. This strategy identified known immune response genes, as well as genes with unknown immune function, including the E3 ubiquitin ligase tripartite motif‐9 (Trim9). Although trim9 expression was originally described as “brain specific,” its expression has been reported in stimulated human Mϕs. In this study, we found elevated levels of trim9 transcripts in vivo in zebrafish Mϕs after immune stimulation. Trim9 has been implicated in axonal migration, and we therefore investigated the impact of Trim9 disruption on Mϕ motility and found that Mϕ chemotaxis and cellular architecture are subsequently impaired in vivo. These results demonstrate that Trim9 mediates cellular movement and migration in Mϕs as well as neurons.
Abbreviations
- BB
B‐box protein domain
- CC
coiled–coil protein domain
- COS
C‐terminal subgroup one signature protein domain
- DCC
deleted in colorectal carcinoma
- E. coli
Escherichia coli
- EGFP
enhanced GFP
- FN3
Fibronectin type‐III protein domain
- GEO
Gene Expression Omnibus
- hpe
hours post exposure
- hpf
hours post fertilization
- HSD
honest significant difference
- IRF
interferon regulatory factor
- mpeg1.1
macrophage expressed 1 tandem duplicate 1 gene
- NCBI
The National Center for Biotechnology Information (USA)
- Pam3CSK4
synthetic tripalmitoylated lipopeptide Pam3CysSerLys4
- PAMP
pathogen‐associated molecular pattern
- PolyIC
polyinosinic‐polycytidylic acid
- PRR
pattern recognition receptor
- qPCR
quantitative polymerase chain reaction
- RING
really interesting new gene
- ∆RINGTrim9
Trim9 lacking the RING domain necessary for ubiquitin ligase activity
- SPRY
SPla and RYanodine receptor protein domain
- trim‐9
tripartite motif 9 gene
- VASP
vasodilator‐stimulated phosphoprotein
Introduction
The innate immune system is capable of mounting a rapid and potent inflammatory response that is critical as a first line of defense against invading pathogens. Innate immune responses are induced when pathogens are sensed by a variety of cellular PRRs, among which the TLRs are the best characterized. TLRs bind diverse PAMPs, which trigger intracellular signaling cascades that activate NF‐κB and IRFs. These transcription factors alter the expression of a wide array of genes that mediate intra‐ and intercellular responses to enable the recruitment and activation of innate immune effector leukocytes to sites of infection or inflammation. Among leukocytes, Mϕs and neutrophils play a key role in innate immune responses through their ability to phagocytose and kill microorganisms. In addition, Mϕs act as important coordinators of the immune response through production of proinflammatory cytokines and antigen presentation to lymphocytes [1, 2]. Although innate immune responses are critical for host defense, excessive leukocyte activation and recruitment can lead to tissue damage and compromise organ function [3, 4]. Identifying innate immune response genes and defining the regulatory networks that control Mϕ and neutrophil activity will inform new therapeutic strategies for modulating inflammation. Because an effective innate immune response requires the coordination of multiple tissues and cell types, it is ideal to study this response in the context of the whole organism.
The zebrafish (Danio rerio) is a model used increasingly for studying vertebrate immune responses because of their small size, high fecundity, short generation time, and reference genome. The zebrafish immune system shares many conserved features with mammalian immune systems [5, –, 8]. By 72 h post fertilization (hpf), zebrafish express critical innate immune components, such as TLRs [9], and possess fully functional neutrophils [10, –, 12] and Mϕs [13, 14]. The ex utero development of zebrafish larvae allows easy access to the whole organism during this window of time, which is weeks before development of a functional adaptive immune response [15]. This facilitates the investigation of innate immune responses on the whole‐organism level.
Although several groups have used transcriptome analysis to investigate the immune response in larval zebrafish, these studies have focused on the response to specific bacterial or viral pathogens and/or known immune‐related genes (e.g., Ref. 16). To identify novel innate immune response genes, we defined the whole‐organism transcriptional response of larval zebrafish exposed to two different PAMPs: PolyIC, agonist of the viral PAMP sensor TLR3 [17, –, 19], and Pam3CSK4, which is an agonist of the bacterial PAMP sensor TLR2 [20, 21]. We identified a list of common larval innate immune response genes that have orthologous genes in human. One candidate innate immune response gene, trim9, was chosen for further analysis.
Trim9 is a member of the large tripartite motif protein family defined by the presence of three N‐terminal protein motifs: RING domain, B‐box domains, and a coiled‐coil region. The RING domain imparts E3 ubiquitin ligase activity [22]. Trim9 is abundantly expressed in neurons of the cerebral cortex and was originally considered to be “brain specific” [23, –, 25]. A survey of TRIM genes expression revealed that TRIM9 transcript levels increase in human primary Mϕs after stimulation with immune complex [26]. In this study, trim9 transcript levels increased in zebrafish Mϕs in vivo and in human Mϕ‐like cells in vitro after immune stimulation with TLR ligands. Further, we found a potential role for Trim9 in regulating Mϕ motility and cellular architecture in vivo in zebrafish. These results highlight the zebrafish as a comparative immunology tool and identify Trim9 as a novel intracellular mediator in Mϕs via its ubiquitin ligase activity.
MATERIALS AND METHODS
Zebrafish
Adult zebrafish were maintained in a recirculating aquarium facility (Aquatic Habitats, Apopka, FL, USA) at 28°C with a 10 h light–14 h dark cycle and fed a commercial grade zebrafish diet. Wild‐type zebrafish were obtained from EkkWill Waterlife Resources (Ruskin, FL, USA). Transgenic zebrafish lines Tg (mpeg1.1:mCherry) and Tg (mpeg1.1:EGFP) [13] were kind gifts from Graham Lieschke (Monash University, Melbourne, VIC, Australia) and David Traver (University of California at San Diego, La Jolla, CA, USA). Zebrafish embryos were obtained by natural spawning and maintained at 28°C in egg water (0.5 mg/L methylene blue and 60 mg/L aquarium salt mixture). Larvae were euthanized in 0.02% tricaine methanesulfonate (Finquel MS‐222; Argent Chemical, Redmond, WA, USA). Zebrafish husbandry and experiments involving live animals were approved by the North Carolina State University Institutional Animal Care and Use Committee.
Immune agonist exposure and RNA isolation
Before microarray analyses, zebrafish larvae (72 hpf) were exposed to 1–5 μg/ml Pam3CSK4 or 5–50 μg/ml PolyIC (both from InvivoGen, San Diego, CA, USA) for 24 and 36 h, and their phenotypes observed, and the transcriptional response of the IL1B (il1b) and the myxovirus (influenza) resistance A (mxa) genes assessed by quantitative (q)PCR. The concentrations of agonists selected for microarray analyses induced increased transcript levels of the il1b gene (5 μg/ml Pam3CSK4) or the mxa gene (10 μg/ml PolyIC), while yielding no overt phenotype or detectable microscopic damage (as assessed by a board certified veterinary pathologist).
For microarray analyses, 72 hpf wild‐type zebrafish larvae were exposed to 10 μg/ml PolyIC, 5 μg/ml Pam3CSK4, or no agonist (negative control) by immersion for 4, 8, 12, 24, or 36 h. Exposures were executed in 24‐well plates with 10 larvae per well in a volume of 2.0 ml. Each treatment group and time point for microarray analyses included RNA pooled from 30 larvae (3 wells) and 4 biologic replicates using different clutches of embryos. RNA was isolated from euthanized larvae using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) and further purified by RNeasy MinElute Cleanup (Qiagen, Germantown, MD, USA). RNA quantity and quality was verified with a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA).
Microarrays
Microarray slides were custom designed 4 × 44 K zebrafish arrays (Agilent Technologies) as described [16] and included 43,371 probes (GEO Accession GPL7735). RNA was labeled (Cy3) and hybridized to arrays using standard methods (Cogenics, Research Triangle Park, NC, USA). Image analyses and the calculations of spot intensities were performed with Feature Extraction software, version 8.5 (Agilent Technologies).
An initial quality assessment of the raw microarray data was conducted and control spots, outliers, and spots with low average intensity were removed. To reduce the effect of inhomogeneous background hybridization across arrays, the background‐correction method normexp was applied [27]. This model‐based approach uses Agilent’s background estimates to compute positive, corrected hybridization intensities. Normexp was implemented in the Bioconductor (http://www.bioconductor.org) package limma [28]. Quantile normalization between arrays was implemented (in limma), to reduce technical variation. Finally, hybridization intensities were log2 transformed and averaged for all replicate spots on the array.
The effect of immune agonist exposure on larval transcript levels was investigated by analyzing array data with a linear model with 2 factors: 1) agonist treatment (Pam3CSK4, PolyIC, and control) and 2) time after exposure (4, 8, 12, 24, and 36 hpe). To accommodate for the dissimilarity between biologic replicates, treatment and control samples of each replicate were paired. Using the preprocessed hybridization intensities, changes in transcript levels between each treatment and control group for each time point were computed via an empirical Bayes moderated paired t test [29] using the Bioconductor package limma. Candidate genes for differential expression with a P < 0.05 were selected. To account for multiple testing, the false‐discovery rate of the extracted candidate gene list was computed from the P‐value distribution of the corresponding genes using the Bioconductor package q value [30].
To visualize expression patterns using heat maps, the log‐transformed average expression of the agonist‐treated samples were subtracted from the untreated control samples at each time point. Hierarchical cluster analyses of gene expression was used to group genes with similar expression patterns using Gene Cluster 3.0 [31], and heat maps were generated with Java Treeview [32]. Gene pathway and network analyses were conducted with Ingenuity Pathway Analysis (Qiagen).
Array data from this study have been deposited in the NCBI‐GEO database [accession no. GSE81317].
Isolation of zebrafish Mϕs
Zebrafish Mϕs were isolated from Tg (mpeg1.1:EGFP) transgenic zebrafish, in which the promoter from the mpeg1.1 (Mϕ expressed 1, tandem duplicate 1) gene drives Mϕ‐specific expression of EGFP [13]. At 120 hpf, groups of 75–100 Tg (mpeg1.1:EGFP) larvae were exposed by immersion to 10 μg/ml PolyIC, 5 μg/ml Pam3CSK4, or no agonist for 8 or 12 h. After exposure, larvae pooled by treatment group were disaggregated into single‐cell suspensions, as previously described [33] and sorted for EGFP‐positive Mϕs using a Dako Cytomation MoFlo cytometer. Mϕ identity was confirmed by Diff‐Quick staining (Siemens Medical Solutions, USA, Malvern, PA, USA). RNA was isolated from sorted cells using the RNeasy Micro kit (Qiagen).
Cell culture
Human promonocytic U‐937 cells (CRL‐1593.2; American Type Culture Collection, Manassas, VA, USA) were cultured in RPMI‐1640 medium (Corning, Manassas, VA, USA) supplemented with 10% FBS (Atlanta Biologicals, Flowery Branch, GA, USA). Differentiation was induced by 50 ng/ml PMA (Millipore‐Sigma, Billerica, MA USA) stimulation for 24 h. Differentiated cells were rested for 6 d then stimulated with 0.1 μg/ml Pam3CSK4, 10 μg/ml PolyIC, or 0.1 μg/ml ultrapure LPS from E. coli 0111:B4 (InvivoGen). At 4, 8, and 12 hpe, cells were lysed and RNA isolated using TRIzol reagent.
Quantification of trim9 transcript levels
cDNA was synthesized using Superscript III First Strand cDNA Synthesis kit (Thermo Fisher Scientific). qPCR was performed with Taqman Universal PCR Master Mix II and Taqman Gene Expression assays for zebrafish trim9 (Dr03081570_m1) and ef1α (Dr03432748_m1) and human TRIM9 (Hs00364838_m1) and ACTB (Hs01060665_g1) (Thermo Fisher Scientific). Fold changes in transcript levels were calculated using the 2−∆∆Ct method [34].
Construction of zebrafish transgenes
The mpeg1.1 promoter was amplified from an mpeg1.1:mCherry plasmid provided by Graham Lieschke [13]. Full‐length and partial [RING‐deleted (ΔRING)] trim9 sequences were amplified from a zebrafish trim9 cDNA clone (ATCC 10330554; IMAGE clone ID 6968453). Teschovirus 2A peptide and EGFP were amplified from a p3E‐2A‐EGFPpA plasmid provided by Kristen Kwan. The pDestTol2pA2 vector and pCS2FA‐transposase were obtained in the Tol2kit [35] and from Koichi Kawakami. pDestTol2pA2 was restriction digested with XhoI and ClaI (Thermo Fisher Scientific). Amplicons were cloned into the linearized pDestTol2pA2 vector using Gibson Assembly Master Mix (New England BioLabs, Ipswich, MA, USA) according to manufacturer instructions. All transgene constructs were verified by sequencing. Tol2 transposase mRNA was reverse transcribed from the pCS2FA‐transposase plasmid with the Ambion mMessage mMachine SP6 kit (Thermo Fisher Scientific).
In vivo chemotaxis assay
Double transgenic zebrafish were generated on the Tg (mpeg1.1:mCherry) background in which the mpeg1.1 promoter drives Mϕ‐specific expression of mCherry [13]. Tg (mpeg1.1:mCherry) embryos were injected with 50 pg of the Tg (mpeg1.1:trim9,EGFP), Tg (mpeg1.1:∆RINGtrim9,EGFP) or Tg (mpeg1.1:EGFP) transgene plasmid (see Fig. 3) and 50 pg of Tol2 transposase mRNA at the single cell stage to generate mosaic GFP transgenic larvae on the stable mCherry background. At 72 hpf, larvae were anesthetized in 0.016% tricaine dissolved in egg water and microinjected into the left otic vesicle with a 1.0 nl volume of PBS containing either 100 pg PolyIC or 50 pg Pam3CSK4. Injected larvae were recovered in fresh egg water at 28°C. At 2 h after injection, the larvae were reanesthetized and mounted in 1% low‐melting‐point agarose. Stacked fluorescence images of the left otic vesicle and a 250 µm segment of the tail just caudal to the urogenital opening were acquired with a laser scanning confocal microscope (AZ‐C2+; Nikon Instruments, Melville, NY, USA). Within the otic vesicle and tail, Mϕs expressing mCherry or mCherry with EGFP were counted by using NIS‐Elements AR 4.11 (Nikon). The percentage of Mϕs expressing EGFP was determined in the otic vesicle and tail and then compared, to obtain a migration index: Outlines and circularity indices for individual Mϕs in vivo in unstimulated mosaic transgenic zebrafish larvae were obtained with the NIS‐Elements AR 4.11 software.
Figure 3.
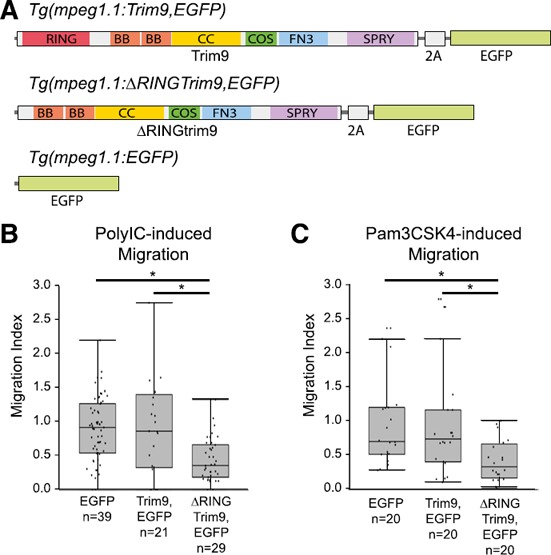
Mϕ‐specific disruption of Trim9 function results in reduced cellular chemotaxis in vivo.
(A) Three transgenes were constructed that employed the Mϕ‐specific promoter of the zebrafish mpeg1.1 gene [13]. The Tg (mpeg1.1:Trim9,EGFP) transgene expresses full‐length zebrafish Trim9 and EGFP from the same transcript but produces both proteins via a viral 2A peptide cleavage site [43]. The Tg (mpeg1.1:∆RINGTrim9,EGFP) transgene expresses both zebrafish Trim9 that lacks the RING domain and EGFP. The Tg (mpeg1.1:EGFP) transgene expresses EGFP. When these transgenes are injected into 1‐cell zebrafish embryos of the stable transgenic line Tg (mpeg1.1:mCherry) the resultant larvae are mosaic with all Mϕs expressing mCherry [13] but only a subpopulation of Mϕs expressing Tg (mpeg1.1:Trim9,EGFP), Tg (mpeg1.1:∆RINGTrim9,EGFP) or Tg (mpeg1.1:EGFP). Trim9 includes RING, B‐box (BB), coiled‐coil (CC), COS, fibronectin type‐III (FN3) and SPla and RYanodine receptor (SPRY) domains [51]. (B and C) The in vivo cellular migration index for chemotaxis toward PolyIC or Pam3CSK4 is shown for zebrafish Mϕs expressing Tg (mpeg1.1:Trim9,EGFP), Tg (mpeg1.1:∆RINGTrim9,EGFP) or Tg (mpeg1.1:EGFP) on the Tg (mpeg1.1:mCherry) genetic background as compared to Mϕs within the same individual expressing only mCherry. Each data point (dot) represents the migration index for a single larva. Raw data are provided in dataset 3. Data are presented as box‐and‐whisker plots (n = 20–39 larvae). *P < 0.05.
Time‐lapse microscopy
For time‐lapse images, 72 hpf mosaic transgenic larvae were anesthetized and injected into the otic vesicle, as described above, and mounted in 1% low‐melting‐point agarose. Fluorescent images were taken every 30 s for 2 h on a Lightsheet Z.1 microscope (Zeiss, Jena, Germany) with s‐CMOS PCO.edge cameras (PCO, AG, Kelheim, Germany) and processed with ZEN black‐imaging software (Zeiss). Cell tracking was performed with ImageJ and the MTrackJ plug‐in [36]. Migration tracks for cells visible for the full 2 h of imaging were plotted by the Ibidi Chemotaxis and Migration Tool plug‐in for Image J (https://ibidi.com/manual‐image‐analysis/171‐chemotaxis‐and‐migration‐tool.html).
Statistical analyses
For qPCR analyses, Student’s t tests were performed to compare control and treatment fold changes. Data from in vivo larval zebrafish Mϕ assays (Figs. 3–5), cell death assays and percent Mϕs (Supplemental Fig. S6) were compared by using a 1‐way ANOVA and Tukey’s HSD or Steel‐Dwass post hoc test. For all statistical tests, results were considered significant if P < 0.05, unless otherwise stated.
Figure 5.
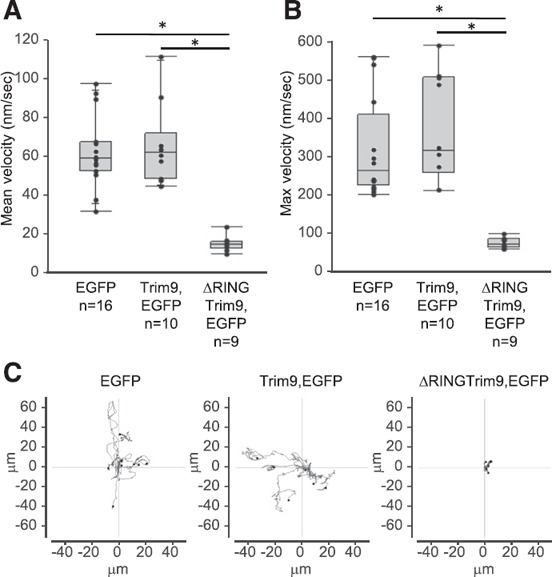
In vivo disruption of Trim9 function in Mϕs significantly disrupts Mϕ velocity.
Time‐lapse video recordings were collected documenting the in vivo movement of zebrafish Mϕs expressing Tg (mpeg1.1:EGFP), Tg (mpeg1.1:Trim9,EGFP) or Tg (mpeg1.1:∆RINGTrim9,EGFP) (see Supplemental Videos 1, 2, and 3, respectively). (A and B) The mean and maximum velocities of individual Mϕs are displayed (n = 9–16). *P < 0.05. (C) Plots of 2 h migration tracks for individual Mϕs (n = 6–8).
Online supplemental material
Supplemental materials include: microarray datasets (datasets 1 and 2); heat maps of array data (Supplemental Fig. S1); gene pathways analyses (Supplemental Fig. S2); comparisons of TRIM9 sequences (Supplemental Fig. S3); validation of Mϕ isolation (Supplemental Fig. S4); analyses of transgene expressing Mϕs in otic vesicle without stimulation (Supplemental Fig. S5); analyses of apoptotic and necrotic Mϕs (Supplemental Fig. S6); Mϕ migration index data (dataset 3); and videos of in vivo Mϕ migration (Supplemental Videos 1, 2 and 3).
RESULTS
Array results
To identify genes that are transcriptionally responsive to immune stimuli in vivo, zebrafish larvae were independently exposed to two chemically distinct immune agonists Pam3CSK4 and PolyIC. At 4, 8, 12, 24, or 36 hpe RNA was isolated from pooled larvae and subjected to microarray analyses ( Fig. 1A ). This strategy identified 574 and 1035 genes that were differentially expressed (as compared to control) at one or more time points after exposure to 5 μg/ml Pam3CSK4 or 10 μg/ml PolyIC, respectively (Fig. 1B and C; dataset 1). Hierarchal clustering of these genes reveals that more than 50% displayed altered transcript levels at 8 and 12 hpe (Fig. 1D). Smaller groups of genes were observed to have increased transcript levels at either early (4 hpe) or late (24 or 36 hpe) time points. One group of genes displayed increased transcript levels at early (4 hpe) and late (24 or 36 hpe, or both) time points (Fig. 1D). Twenty‐seven genes were observed to have increased transcript levels at multiple time points after exposure to PolyIC (as compared to Pam3CSK4 exposure, Supplemental Fig. S1A) and 4 genes were identified with increased transcript levels at 12 hpe for Pam3CSK4 (as compared to PolyIC exposure, Supplemental Fig. S1B). An interrogation of this complete gene list revealed that 8 members of the canonical CXCR4 pathway were increased significantly, representing an activation of this pathway (Supplemental Figs. S1C and S2A). The top gene network identified from this gene list is the inflammatory (NF‐κB) network, confirming that exposure of zebrafish larvae to the selected concentrations of Pam3CSK4 and PolyIC activates immune signaling pathways (Supplemental Figs. S1D and S2B).
Figure 1.
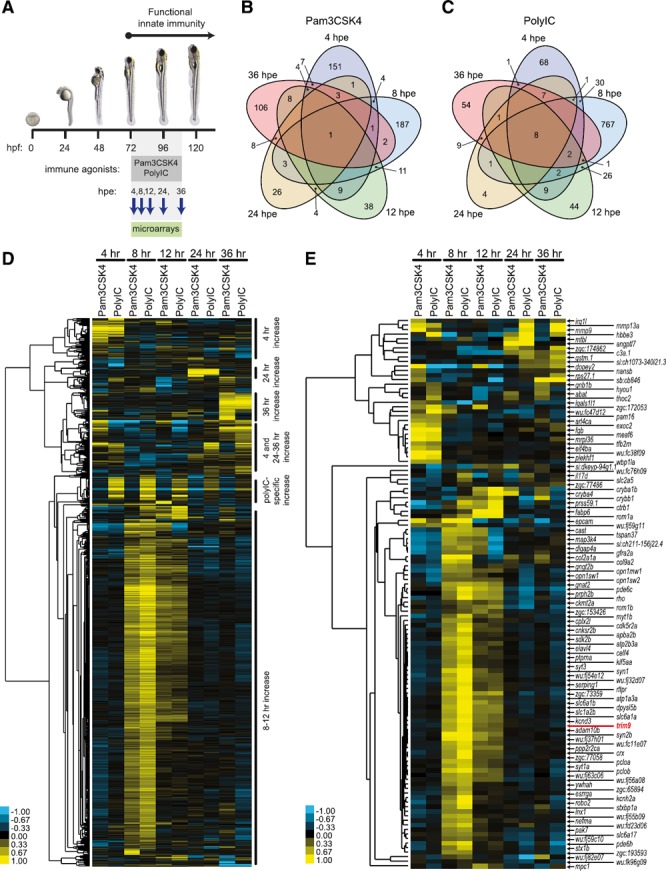
Immune agonist exposure and summary of microarray results.
(A) Zebrafish larvae (72 hpf) were exposed to 5 μg/ml Pam3CSK4, 10 μg/ml PolyIC or no agonist for 4, 8, 12, 24, or 36 h. RNA was isolated from larvae at each time point and used for microarray analyses. (B and C) Exposure to Pam3CSK4 or PolyIC led to the identification of 574 and 1035 genes, respectively, that had significantly increased or decreased transcript levels at 1 or more time points (as compared to control larvae) (dataset 1). Venn diagrams indicate the number of genes with altered transcript levels at each time point. (D) Hierarchal clustering and heat map of 1110 zebrafish genes with significantly different transcript levels (P < 0.05) after exposure to either Pam3CSK4 or PolyIC at any time point. Gene clusters with similar expression patterns are annotated on the right. (E) Hierarchal clustering and heat map of 121 zebrafish genes with significantly different transcript levels after exposure to Pam3CSK4 at any time point and significantly different transcript levels after exposure to PolyIC at any time point. The position of trim9, which displayed increased transcript levels at 8 and 12 hpe to both Pam3CSK4 and PolyIC is indicated by red text.
To identify genes that could be considered common immune response genes, we restricted the gene list to sequences that displayed significant changes in transcript levels for both immune agonists and represented defined zebrafish genes. Of the 230 zebrafish sequences that responded to both agonists (dataset 2), 121 represent defined genes including known immune response genes, (e.g., mmp9, mmp13a, c3a.1, and il17d), as well as genes with no defined immune function such as trim9 (Fig. 1E).
Trim9 transcript levels increase after immune stimulation
TRIM9 is highly conserved across vertebrate species (Supplemental Fig. S3) and plays a significant role in axonal migration [37, 38, –, 40]. Therefore, we hypothesized that Trim9 also plays a role in the motility of immune cells in zebrafish larvae. Results from qPCR confirmed the increase in trim9 transcript levels in larval zebrafish after 8 h exposure to Pam3CSK4 or PolyIC ( Fig. 2A ). Because Mϕs are one of the major innate immune effector cells active in the larval zebrafish [5, 14], we sought to determine whether trim9 transcripts are expressed in and respond to immune stimulation within these cells. EGFP‐expressing Mϕs were isolated by cell sorting (∼95% pure; Supplemental Fig. S4) from Tg (mpeg1.1:EGFP) [13] transgenic larvae, after 8 or 12 h of exposure of the whole larvae to Pam3CSK4 or PolyIC. In Mϕs, trim9 transcript levels increased significantly after exposure to PolyIC at 8 and 12 hpe and after exposure to Pam3CSK4 at 12 hpe (Fig. 2B). The relative increase in trim9 transcripts in Mϕs, which constitute a very small percentage of larval cells (Supplemental Fig. S6A), cannot account for the relative increase in trim9 on the whole animal level (compare Fig. 2A and B). It is possible that PAMP exposure increases trim9 expression in other larval immune cells (e.g., neutrophils) and/or in TLR‐expressing non‐immune cells (e.g., epithelial cells).
Figure 2.
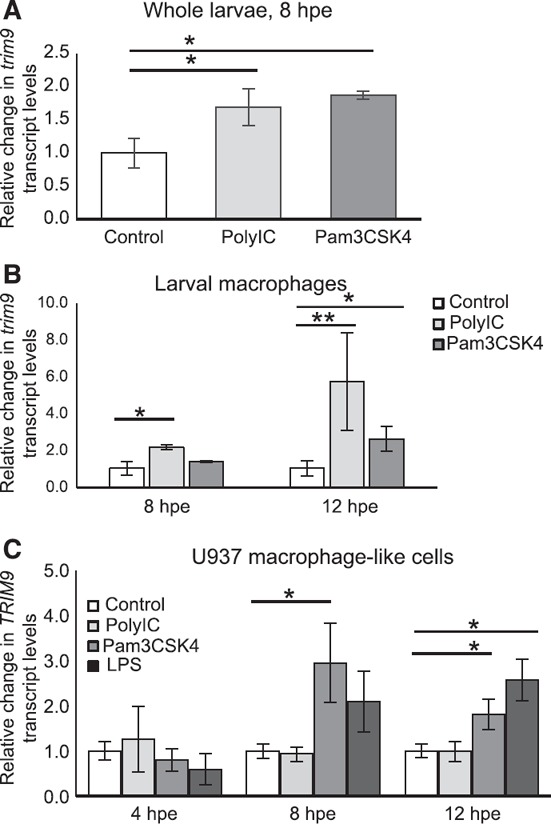
Immune agonist exposure induces increased TRIM9 transcript levels in Mϕs.
(A) Relative trim9 transcript levels were determined by qPCR from zebrafish larvae after 8 h of exposure to 5 μg/ml Pam3CSK4 or 10 μg/ml PolyIC. Larval exposure was initiated at 72 hpf. The means ± sem are shown (n = 3). *P < 0.05. (B) Relative trim9 transcript levels were determined by qPCR from larval Mϕs after 8 and 12 h exposures to 5 μg/ml Pam3CSK4 or 10 μg/ml PolyIC. Zebrafish larvae (120 hpf) of the Tg (mpeg1.1:EGFP) transgenic line were exposed to immune agonists and EGFP+ Mϕs isolated by cell sorting. The means ± sem are shown (n = 3). *P < 0.05; **P < 0.10. Relative increases for each biologic replicate are PolyIC at 8 hpe = 2.034, 2.39, and 1.99; PolyIC at 12 hpe = 10.9, 3.92, and 2.30; Pam3CSK4 at 8 hpe = 1.33, 1.48, and 1.34; Pam3CSK4 at 12 hpe = 1.52, 2.42, and 3.82. (C) The human promonocytic cell line U937 was differentiated to a Mϕ‐like phenotype and exposed to 10 μg/ml PolyIC, 0.1 μg/ml Pam3CSK4 or 0.1 μg/ml LPS for 4, 8 or 12 h. Relative transcript levels were determined by qPCR. The means ± sem are shown (n = 3). *P < 0.05.
It has been demonstrated that TRIM9 transcript levels increase in primary human Mϕs after stimulation with immune complex [26]. To determine whether a similar transcriptional response occurs after PAMP exposure, we exposed Mϕ‐like cells derived from the human U937 monocytic cell line [41] to both agonists used in the zebrafish, as well as LPS a TLR4 agonist [42]. We detected significant increases in TRIM9 transcript levels in response to Pam3CSK4 and LPS to a similar extent and in a similar time frame as seen in the larval zebrafish Mϕs (Fig. 2C). In contrast to the zebrafish, no transcriptional response was detected for PolyIC in the U937 Mϕ‐like cells. These results suggest that multiple immune stimulation pathways increase TRIM9 transcript levels in Mϕs of zebrafish and humans, indicating the potential for a conserved TRIM9 function in activated Mϕs.
Disruption of Trim9 function results in defective Mϕ chemotaxis
In neurons, TRIM9 is necessary for axon branching and attraction in response to the chemoattractant Netrin‐1/UNC‐6 [37, –, 40]. Functional TRIM9 studies have been executed in Caenorhabditis elegans and in cultured mouse cortical neurons by expression of a truncated TRIM9 that lacks the RING domain necessary for ubiquitin ligase activity (∆RINGTrim9). In these systems, ∆RINGTrim9 acts in a dominant negative manner and abolishes axon migration toward Netrin [38, 40]. During an inflammatory response, leukocytes migrate along chemoattractant gradients to reach sites of inflammation or infection. Therefore, we hypothesized that Trim9 mediates chemotaxis in Mϕs. To test this hypothesis, we designed a transgenic approach to disrupt Trim9 function in zebrafish Mϕs in vivo by using the Mϕ‐specific mpeg1.1 gene promoter [13] to drive expression of zebrafish ∆RINGTrim9 ( Fig. 3A ). A targeted transgenic strategy was used, rather than a global knockdown or knockout strategy, to avoid any adverse effects of globally disrupting Trim9 function. A teschovirus 2A peptide sequence [43] was incorporated into the transgenes to allow for coexpression of EGFP. Transgenes were introduced into Tg (mpeg1.1:mCherry) embryos to yield mosaic transgenic fish, in which all Mϕs express mCherry and a subset coexpress EGFP, indicating expression of the experimental transgene. To assess Mϕ chemotaxis in vivo, mosaic transgenic zebrafish larvae were injected into the otic vesicle at 72 hpf with either Pam3CSK4 or PolyIC, and Mϕ migration into the otic vesicle was quantified by using fluorescence microscopy. We found that Mϕs expressing ∆RINGTrim9 had a significant reduction in migration to the injected otic vesicle relative to Mϕs expressing full‐length Trim9, or EGFP alone, regardless of the inciting agonist (Fig. 3B and C and dataset 3). These results indicate that loss of Trim9 function negatively impacts Mϕ chemotaxis.
Disruption of Trim9 alters Mϕ morphology and motility
Imaging of individual transgenic Mϕs in vivo revealed a stark contrast in morphology induced by ∆RINGTrim9 expression. Migrating Mϕs extend cellular protrusions called pseudopods formed by polymerization of actin filaments that push the cell membrane forward [44]. Whereas Mϕs expressing the control (EGFP) or full‐length Trim9 transgenes were typically elongated to a stellate form because of the formation of multiple pseudopod extensions, Mϕs expressing ∆RINGTrim9 were significantly more circular with smooth cell contours ( Fig. 4A and C ). The effect of ΔRINGTrim9 on Mϕ shape appeared to be cell autonomous, in that Mϕs in larvae injected with Tg (mpeg1.1:ΔRINGTrim9,EGFP), but not expressing ΔRINGTrim9 (e.g., EGFP‐negative), were similar in shape to control Mϕs (Fig. 4B and C).
Figure 4.
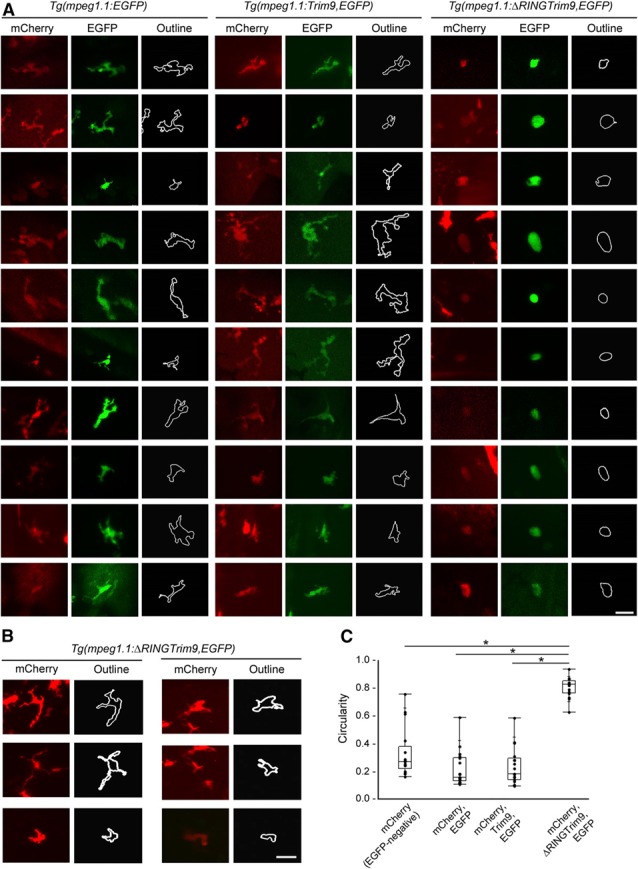
In vivo disruption of Trim9 function in Mϕs significantly alters cell shape.
(A) Zebrafish Mϕs expressing Tg (mpeg1.1:EGFP), Tg (mpeg1.1:Trim9,EGFP), or Tg (mpeg1.1:∆RINGTrim9,EGFP) on the Tg (mpeg1.1:mCherry) background were photographed to assess cell shape. Cells from each transgenic background were photographed for quantifying circularity. Scale bar, 20 μm. (B) Mϕs from Tg (mpeg1.1:mCherry) larvae injected with Tg (mpeg1.1:∆RINGTrim9,EGFP), but not expressing ∆RINGTrim9 (e.g., EGFP−) were photographed to assess cell shape. Scale bar, 20 μm. (C) Circularity scores are shown for individual cells. Mϕs that are mCherry‐positive, but EGFP‐negative were analyzed from Tg (mpeg1.1:∆RINGTrim9,EGFP) mosaic larvae including those displayed in (B). Mϕs that are mCherry‐positive and EGFP‐positive were analyzed from Tg (mpeg1.1:EGFP), Tg (mpeg1.1:Trim9,EGFP) and Tg (mpeg1.1:∆RINGTrim9,EGFP) mosaic larvae including those displayed in (A). Scale: 0–1, where 1 indicates a perfect circle. Data are presented as box‐and‐whisker plots (n = 17). *P < 0.05.
Time lapse microscopy was used to visualize the migration of transgenic Mϕs in vivo. In accordance with our migration assay data, Mϕs expressing ∆RINGTrim9 show significant reductions in velocity and travel limited distances over the 2 h imaging period compared to Mϕs expressing the control or full‐length Trim9 transgenes ( Fig. 5 ). The small migration index observed for Mϕs expressing ∆RINGTrim9 after otic vesicle PAMP injection was likely related to the presence of these Mϕs in the otic vesicle at the start of the assay and not the chemotaxis during the assay (Supplemental Fig. S5). Further, although Mϕs expressing the control or full‐length Trim9 transgenes exhibited highly dynamic pseudopod extension and retraction over the course of imaging (Supplemental Videos 1 and 2), Mϕs expressing ∆RINGTrim9 generally lacked pseudopod extensions, or occasionally exhibited short, thin cytoplasmic extensions (Supplemental Video 3). To ensure that the expression of ∆RINGTrim9 was not having a deleterious effect on the number and survival of Mϕs, we used flow cytometry to compare the number of total Mϕs and transgene‐expressing Mϕs for the 3 different transgenes. When comparing Tg (mpeg1.1:mCherry) larvae transiently expressing Tg (mpeg1.1:EGFP), Tg (mpeg1.1:Trim9,EGFP) or Tg (mpeg1.1:ΔRINGTrim9,EGFP), no significant difference in the number of total Mϕs (mCherry+) or the number of Mϕs expressing the transient transgene (mCherry+, EGFP+) were observed (Supplemental Fig. S6A and B). In addition, no significant difference was observed between the percentage of preapoptotic or necrotic Mϕs when comparing larvae expressing these transient transgenes (Supplemental Fig. S6C and D).
Taken together, these data suggest that Trim9 ubiquitin ligase function is necessary for proper Mϕ pseudopod formation and motility.
DISCUSSION
Gene expression studies combining zebrafish embryo infection models with transcriptome analyses have been successfully used to identify genes that are transcriptionally responsive to immune stimuli (e.g., Ref. 16) and have demonstrated the utility of the zebrafish model for identifying known immune response genes that are conserved in humans [6]. By assessing transcriptional changes in zebrafish larvae after exposure to chemically distinct immune agonists, we also have identified known immune response genes including those involved in the CXCR4 signaling pathway and in the inflammatory (NF‐κB) network.
An important goal of our study was to identify genes with an undefined role in immunity and that are well conserved in humans. To that end we chose to investigate Trim9, which is highly conserved between zebrafish and humans but at the time of our transcriptional analysis had no described immune function. Recently, dual roles for Trim9 in regulating NF‐κB and IRF3 activation have been identified. NF‐κB and IRF3 activate transcription of proinflammatory genes and IFNs, respectively, downstream of PRRs and other immune receptors. TRIM9 negatively regulates NF‐κB activation [45], but positively regulates IRF3 activation [46]. This provides additional validation of Trim9 as an innate immune mediator and of our zebrafish screen as a tool for identifying relevant immune response genes.
TRIM9 transcript levels increase in Mϕs after stimulation with agonists for TLR2 (Pam3CSK4), TLR3 (PolyIC), and TLR4 (LPS). Although these results demonstrate for the first time a TRIM9 transcriptional response to PRR stimulation in vivo and in Mϕs, the response of trim9 may depend on the immune agonist, as well as the dosing method and duration of exposure. For example, we observed increased trim9 transcript levels in zebrafish larvae that were immersed in 5 μg/ml Pam3CSK4 at 72 hpf and assessed 8 and 12 h later, whereas, trim9 was not identified as being transcriptionally responsive to Pam3CSK4 exposure in zebrafish embryos that were injected with 1.0 ng Pam3CSK4 at 27 hpf and assessed 1, 3, and 6 h later [47]. Nevertheless, the transcriptional response of TRIM9 to Pam3CSK4 was similar between zebrafish larval Mϕs and a human Mϕ‐like cell line. LPS was not tested in the zebrafish as they are highly tolerant to LPS [48]. PolyIC failed to induce a TRIM9 transcriptional response in human cells. This finding could represent a species difference in TRIM9 regulation, or reflect differences in whole organism vs. cell‐direct stimulation. A common transcription factor activated by each of these receptors is NF‐κB [17, 20, 42]. The timing of the TRIM9 transcriptional response at 8–12 h after stimulation is consistent with late‐phase NF‐κB‐activated genes [49]. This late phase is likely related to a second wave of NF‐κB activation induced by early immune response genes. This could account for the TRIM9 transcriptional response in Mϕs even when agonists were not applied directly, as in the larval zebrafish. Given that TRIM9 negatively regulates NF‐κB activation [45], upregulation of TRIM9 downstream of NF‐κB could serve as a negative feedback mechanism for regulating inflammatory signals.
Our data demonstrate that in addition to regulating innate immune signaling pathways, Trim9 mediates cellular architectural dynamics and motility in Mϕs. In invertebrate models and in mice, TRIM9 mediates axon migration toward the chemoattractant Netrin‐1/UNC‐6 [37, –, 40]. Neurons expressing ΔRINGTRIM9 fail to extend axon branches along a Netrin‐1 gradient, but otherwise respond normally to other chemoattractant signals [37, 40]. In contrast to neurons, Mϕs expressing ΔRINGTrim9 showed abnormal cell morphology with a lack of cell protrusions with or without immune stimulation. Further, they showed significant reductions in migration velocity, resulting in failure to migrate to an inflammatory site regardless of the inciting agonist. These findings suggest that Trim9 plays a broader role in cell motility in Mϕs, rather than mediating chemotaxis toward a specific chemoattractant, as in neurons. TRIM9 in neurons directly interacts with the DCC netrin‐1 receptor to mediate netrin‐1 signaling [40]. In human peripheral blood leukocytes, DCC is not detectable by immunohistochemistry or qPCR [50], suggesting that TRIM9 has an alternative means of regulation and, therefore, of function in leukocytes.
Defining the mechanism by which TRIM9 affects Mϕ motility requires further experiments; however, published reports suggest that TRIM9 modulates cytoskeletal dynamics. TRIM9 contains a C‐terminal subgroup one signature (COS) box that mediates microtubule binding [51]. To date, the role of microtubule binding in TRIM9 function remains unknown. In neurons, TRIM9 mediates axon migration through interactions with VASP [52]. VASP functions to lengthen actin polymers [53] and localizes to the tips of actin‐rich protrusions called filopodia at the leading edge of migrating axons [40]. Ubiquitination of VASP by TRIM9 limits VASP mobility to filopodia tips [52]. ΔRINGTRIM9 expressed in neurons showed prominent colocalization with VASP, an interaction that was otherwise transient, suggesting that ΔRINGTRIM9 binds and holds VASP, potentially preventing VASP function. VASP is expressed in Mϕs and mediates cytoskeletal changes required for Mϕ phagocytosis [54], making it a strong candidate for partnering with TRIM9 in regulating Mϕ motility. VASP has been shown to mediate chemoattractant‐specific chemotaxis in neutrophils [55]. Whether TRIM9 is necessary for neutrophil migration is an important question to address in future experiments. This work identified TRIM9 as a novel mediator in Mϕ function and broadened the potential role of TRIM9 in mediating motility in cells outside of the nervous system.
AUTHORSHIP
D.A.T, A.K.H., and J.A.Y. designed research. D.A.T, A.K.H., J.G., R.N.S., I.R.‐N., A.N.K., A.A.F., S.K.N., and J.M.L. performed research. D.A.T., A.K.H., D.D.J, S.H., and J.A.Y analyzed data. D.A.T. and J.A.Y. wrote the paper.
DISCLOSURES
The authors declare no conflicts of interest.
Supporting information
Supplementary Material Files
Supplementary Material Files
Supplementary Material Files
Supplementary Material Files
Supplementary Material Files
Supplementary Material Files
Supplementary Material Files
ACKNOWLEDGMENTS
This work was supported by funding from the U.S. National Institutes of Health (NIH) [R21 AI076829 (J.A.Y.), T32 OD011130 (D.A.T.), T32 GM008776 (A.K.H. and A.N.K.) and P30 ES025128], the NC State University College of Veterinary Medicine and an American Association of Immunologists Careers in Immunology Fellowship (D.A.T. and J.A.Y.). The UNC Flow Cytometry Core Facility is supported in part by NIH P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center. We thank Annemarie Meijer and Herman Spaink (University of Leiden) for the custom array design; Graham Lieschke (Monash University), and David Traver (University of California at San Diego) for transgenic lines; and Kristen Kwan (University of Utah) and Koichi Kawakami (National Institute of Genetics, Japan) for plasmids.
These authors contributed equally to this work.
REFERENCES
- 1. Arango Duque, G. , Descoteaux, A. (2014) Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol. 5, 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenthal, A. S. , Shevach, E. M. (1973) Function of macrophages in antigen recognition by guinea pig T lymphocytes, I. requirement for histocompatible macrophages and lymphocytes. J. Exp. Med. 138, 1194–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Casellas, F. , LÓpez‐Vivancos, J. , Vergara, M. , Malagelada, J. (1999) Impact of inflammatory bowel disease on health‐related quality of life. Dig. Dis. 17, 208–218. [DOI] [PubMed] [Google Scholar]
- 4. Cheung, A. M. , Tansey, C. M. , Tomlinson, G. , Diaz‐Granados, N. , Matté, A. , Barr, A. , Mehta, S. , Mazer, C. D. , Guest, C. B. , Stewart, T. E. , Al‐Saidi, F. , Cooper, A. B. , Cook, D. , Slutsky, A. S. , Herridge, M. S. (2006) Two‐year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 174, 538–544. [DOI] [PubMed] [Google Scholar]
- 5. Lieschke, G. J. , Oates, A. C. , Crowhurst, M. O. , Ward, A. C. , Layton, J. E. (2001) Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood 98, 3087–3096. [DOI] [PubMed] [Google Scholar]
- 6. Van der Vaart, M. , Spaink, H. P. , Meijer, A. H. (2012) Pathogen recognition and activation of the innate immune response in zebrafish. Adv. Hematol. 2012, 159807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zapata, A. , Diez, B. , Cejalvo, T. , Gutiérrez‐de Frías, C. , Cortés, A. (2006) Ontogeny of the immune system of fish. Fish Shellfish Immunol. 20, 126–136. [DOI] [PubMed] [Google Scholar]
- 8. Wittamer, V. , Bertrand, J. Y. , Gutschow, P. W. , Traver, D. (2011) Characterization of the mononuclear phagocyte system in zebrafish. Blood 117, 7126–7135. [DOI] [PubMed] [Google Scholar]
- 9. Van der Sar, A. M. , Stockhammer, O. W. , van der Laan, C. , Spaink, H. P. , Bitter, W. , Meijer, A. H. (2006) MyD88 innate immune function in a zebrafish embryo infection model. Infect. Immun. 74, 2436–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Renshaw, S. A. , Loynes, C. A. , Trushell, D. M. , Elworthy, S. , Ingham, P. W. , Whyte, M. K. (2006) A transgenic zebrafish model of neutrophilic inflammation. Blood 108, 3976–3978. [DOI] [PubMed] [Google Scholar]
- 11. Meijer, A. H. , van der Sar, A. M. , Cunha, C. , Lamers, G. E. , Laplante, M. A. , Kikuta, H. , Bitter, W. , Becker, T. S. , Spaink, H. P. (2008) Identification and real‐time imaging of a myc‐expressing neutrophil population involved in inflammation and mycobacterial granuloma formation in zebrafish. Dev. Comp. Immunol. 32, 36–49. [DOI] [PubMed] [Google Scholar]
- 12. Mathias, J. R. , Perrin, B. J. , Liu, T. X. , Kanki, J. , Look, A. T. , Huttenlocher, A. (2006) Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J. Leukoc. Biol. 80, 1281–1288. [DOI] [PubMed] [Google Scholar]
- 13. Ellett, F. , Pase, L. , Hayman, J. W. , Andrianopoulos, A. , Lieschke, G. J. (2011) mpeg1 promoter transgenes direct macrophage‐lineage expression in zebrafish. Blood 117, e49–e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herbomel, P. , Thisse, B. , Thisse, C. (1999) Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development 126, 3735–3745. [DOI] [PubMed] [Google Scholar]
- 15. Lam, S. H. , Chua, H. L. , Gong, Z. , Lam, T. J. , Sin, Y. M. (2004) Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. 28, 9–28. [DOI] [PubMed] [Google Scholar]
- 16. Stockhammer, O. W. , Zakrzewska, A. , HegedØs, Z. , Spaink, H. P. , Meijer, A. H. (2009) Transcriptome profiling and functional analyses of the zebrafish embryonic innate immune response to Salmonella infection. J. Immunol. 182, 5641–5653. [DOI] [PubMed] [Google Scholar]
- 17. Alexopoulou, L. , Holt, A. C. , Medzhitov, R. , Flavell, R. A. (2001) Recognition of double‐stranded RNA and activation of NF‐kappaB by Toll‐like receptor 3. Nature 413, 732–738. [DOI] [PubMed] [Google Scholar]
- 18. Phelan, P. E. , Mellon, M. T. , Kim, C. H. (2005) Functional characterization of full‐length TLR3, IRAK‐4, and TRAF6 in zebrafish (Danio rerio). Mol. Immunol. 42, 1057–1071. [DOI] [PubMed] [Google Scholar]
- 19. Matsuo, A. , Oshiumi, H. , Tsujita, T. , Mitani, H. , Kasai, H. , Yoshimizu, M. , Matsumoto, M. , Seya, T. (2008) Teleost TLR22 recognizes RNA duplex to induce IFN and protect cells from birnaviruses. J. Immunol. 181, 3474–3485. [DOI] [PubMed] [Google Scholar]
- 20. Aliprantis, A. O. , Yang, R. B. , Mark, M. R. , Suggett, S. , Devaux, B. , Radolf, J. D. , Klimpel, G. R. , Godowski, P. , Zychlinsky, A. (1999) Cell activation and apoptosis by bacterial lipoproteins through toll‐like receptor‐2. Science 285, 736–739. [DOI] [PubMed] [Google Scholar]
- 21. Ribeiro, C. M. , Hermsen, T. , Taverne‐thiele, A. J. , Savelkoul, H. F. , Wiegertjes, G. F. (2010) Evolution of recognition of ligands from Gram‐positive bacteria: similarities and differences in the TLR2‐mediated response between mammalian vertebrates and teleost fish. J. Immunol. 184, 2355–2368. [DOI] [PubMed] [Google Scholar]
- 22. Joazeiro, C. A. , Weissman, A. M. (2000) RING finger proteins: mediators of ubiquitin ligase activity. Cell 102, 549–552. [DOI] [PubMed] [Google Scholar]
- 23. Li, Y. , Chin, L. S. , Weigel, C. , Li, L. (2001) Spring, a novel RING finger protein that regulates synaptic vesicle exocytosis. J. Biol. Chem. 276, 40824–40833. [DOI] [PubMed] [Google Scholar]
- 24. Tanji, K. , Kamitani, T. , Mori, F. , Kakita, A. , Takahashi, H. , Wakabayashi, K. (2010) TRIM9, a novel brain‐specific E3 ubiquitin ligase, is repressed in the brain of Parkinson's disease and dementia with Lewy bodies. Neurobiol. Dis. 38, 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berti, C. , Messali, S. , Ballabio, A. , Reymond, A. , Meroni, G. (2002) TRIM9 is specifically expressed in the embryonic and adult nervous system. Mech. Dev. 113, 159–162. [DOI] [PubMed] [Google Scholar]
- 26. Carthagena, L. , Bergamaschi, A. , Luna, J. M. , David, A. , Uchil, P. D. , Margottin‐Goguet, F. , Mothes, W. , Hazan, U. , Transy, C. , Pancino, G. , Nisole, S. (2009) Human TRIM gene expression in response to interferons. PLoS One 4, e4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ritchie, M. E. , Silver, J. , Oshlack, A. , Holmes, M. , Diyagama, D. , Holloway, A. , Smyth, G. K. (2007) A comparison of background correction methods for two‐colour microarrays. Bioinformatics 23, 2700–2707. [DOI] [PubMed] [Google Scholar]
- 28. Smyth, G. K. (2005) Limma: linear Models for Microarray Data In Bioinformatics and Computational Biology Solutions Using R and Bioconductor. (Gentleman R., Carey V., Dudoit S., Irizarry R., Huber W., eds.), Springer, New York, 397–420. [Google Scholar]
- 29. Smyth, G.K. (2004) Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol., 3, Article3. [DOI] [PubMed] [Google Scholar]
- 30. Storey, J. D. (2002) A direct approach to false discovery rates. J. R. Stat. Soc. Series B 63, 479–498. [Google Scholar]
- 31. de Hoon, M. J. , Imoto, S. , Nolan, J. , Miyano, S. (2004) Open source clustering software. Bioinformatics 20, 1453–1454. [DOI] [PubMed] [Google Scholar]
- 32. Saldanha, A. J. (2004) Java Treeview: extensible visualization of microarray data. Bioinformatics 20, 3246–3248. [DOI] [PubMed] [Google Scholar]
- 33. Manoli, M. , Driever, W. (2012) Fluorescence‐activated cell sorting (FACS) of fluorescently tagged cells from zebrafish larvae for RNA isolation. Cold Spring Harb. Protoc. 2012, pdb.prot069633. [DOI] [PubMed]
- 34. Livak, K. J. , Schmittgen, T. D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- 35. Kwan, K. M. , Fujimoto, E. , Grabher, C. , Mangum, B. D. , Hardy, M. E. , Campbell, D. S. , Parant, J. M. , Yost, H. J. , Kanki, J. P. , Chien, C. B. (2007) The Tol2kit: a multisite gateway‐based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088–3099. [DOI] [PubMed] [Google Scholar]
- 36. Meijering, E. , Dzyubachyk, O. , Smal, I. (2012) Methods for cell and particle tracking. Methods Enzymol. 504, 183–200. [DOI] [PubMed] [Google Scholar]
- 37. Hao, J. C. , Adler, C. E. , Mebane, L. , Gertler, F. B. , Bargmann, C. I. , Tessier‐Lavigne, M. (2010) The tripartite motif protein MADD‐2 functions with the receptor UNC‐40 (DCC) in Netrin‐mediated axon attraction and branching. Dev. Cell 18, 950–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alexander, M. , Selman, G. , Seetharaman, A. , Chan, K. K. , D'Souza, S. A. , Byrne, A. B. , Roy, P. J. (2010) MADD‐2, a homolog of the Opitz syndrome protein MID1, regulates guidance to the midline through UNC‐40 in Caenorhabditis elegans. Dev. Cell 18, 961–972. [DOI] [PubMed] [Google Scholar]
- 39. Song, S. , Ge, Q. , Wang, J. , Chen, H. , Tang, S. , Bi, J. , Li, X. , Xie, Q. , Huang, X. (2011) TRIM‐9 functions in the UNC‐6/UNC‐40 pathway to regulate ventral guidance. J. Genet. Genomics 38, 1–11. [DOI] [PubMed] [Google Scholar]
- 40. Winkle, C. C. , McClain, L. M. , Valtschanoff, J. G. , Park, C. S. , Maglione, C. , Gupton, S. L. (2014) A novel Netrin‐1‐sensitive mechanism promotes local SNARE‐mediated exocytosis during axon branching. J. Cell Biol. 205, 217–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baek, Y. S. , Haas, S. , Hackstein, H. , Bein, G. , Hernandez‐Santana, M. , Lehrach, H. , Sauer, S. , Seitz, H. (2009) Identification of novel transcriptional regulators involved in macrophage differentiation and activation in U937 cells. BMC Immunol. 10, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chow, J. C. , Young, D. W. , Golenbock, D. T. , Christ, W. J. , Gusovsky, F. (1999) Toll‐like receptor‐4 mediates lipopolysaccharide‐induced signal transduction. J. Biol. Chem. 274, 10689–10692. [DOI] [PubMed] [Google Scholar]
- 43. Kim, J. H. , Lee, S. R. , Li, L. H. , Park, H. J. , Park, J. H. , Lee, K. Y. , Kim, M. K. , Shin, B. A. , Choi, S. Y. (2011) High cleavage efficiency of a 2A peptide derived from porcine teschovirus‐1 in human cell lines, zebrafish and mice. PLoS One 6, e18556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hartwig, J. , Stossel, T. 1985. Macrophage movements In Mononuclear phagocytes: Characteristics, Physiology and Function. (van Furth R., ed.), Martinus Nijhoff Publishers, Leiden, 329–335. [Google Scholar]
- 45. Shi, M. , Cho, H. , Inn, K. S. , Yang, A. , Zhao, Z. , Liang, Q. , Versteeg, G. A. , Amini‐Bavil‐Olyaee, S. , Wong, L. Y. , Zlokovic, B. V. , Park, H. S. , García‐Sastre, A. , Jung, J. U. (2014) Negative regulation of NF‐κB activity by brain‐specific TRIpartite Motif protein 9. Nat. Commun. 5, 4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qin, Y. , Liu, Q. , Tian, S. , Xie, W. , Cui, J. , Wang, R. F. (2016) TRIM9 short isoform preferentially promotes DNA and RNA virus‐induced production of type I interferon by recruiting GSK3ß to TBK1. Cell Res. 26, 613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang, S. , Marín‐Juez, R. , Meijer, A. H. , Spaink, H. P. (2015) Common and specific downstream signaling targets controlled by Tlr2 and Tlr5 innate immune signaling in zebrafish. BMC Genomics 16, 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Novoa, B. , Bowman, T. V. , Zon, L. , Figueras, A. (2009) LPS response and tolerance in the zebrafish (Danio rerio). Fish Shellfish Immunol. 26, 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Han, S. J. , Ko, H. M. , Choi, J. H. , Seo, K. H. , Lee, H. S. , Choi, E. K. , Choi, I. W. , Lee, H. K. , Im, S. Y. (2002) Molecular mechanisms for lipopolysaccharide‐induced biphasic activation of nuclear factor‐kappa B (NF‐kappa B). J. Biol. Chem. 277, 44715–44721. [DOI] [PubMed] [Google Scholar]
- 50. Ly, N. P. , Komatsuzaki, K. , Fraser, I. P. , Tseng, A. A. , Prodhan, P. , Moore, K. J. , Kinane, T. B. (2005) Netrin‐1 inhibits leukocyte migration in vitro and in vivo. Proc. Natl. Acad. Sci. USA 102, 14729–14734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Short, K. M. , Cox, T. C. (2006) Subclassification of the RBCC/TRIM superfamily reveals a novel motif necessary for microtubule binding. J. Biol. Chem. 281, 8970–8980. [DOI] [PubMed] [Google Scholar]
- 52. Menon, S. , Boyer, N. P. , Winkle, C. C. , McClain, L. M. , Hanlin, C. C. , Pandey, D. , Rothenfußer, S. , Taylor, A. M. , Gupton, S. L. (2015) The E3 ubiquitin ligase TRIM9 is a filopodia off switch required for netrin‐dependent axon guidance. Dev. Cell 35, 698–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hansen, S. D. , Mullins, R. D. (2010) VASP is a processive actin polymerase that requires monomeric actin for barbed end association. J. Cell Biol. 191, 571–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Coppolino, M. G. , Krause, M. , Hagendorff, P. , Monner, D. A. , Trimble, W. , Grinstein, S. , Wehland, J. , Sechi, A. S. (2001) Evidence for a molecular complex consisting of Fyb/SLAP, SLP‐76, Nck, VASP and WASP that links the actin cytoskeleton to Fcgamma receptor signalling during phagocytosis. J. Cell Sci. 114, 4307–4318. [DOI] [PubMed] [Google Scholar]
- 55. Neel, N. F. , Barzik, M. , Raman, D. , Sobolik‐Delmaire, T. , Sai, J. , Ham, A. J. , Mernaugh, R. L. , Gertler, F. B. , Richmond, A. (2009) VASP is a CXCR2‐interacting protein that regulates CXCR2‐mediated polarization and chemotaxis. J. Cell Sci. 122, 1882–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material Files
Supplementary Material Files
Supplementary Material Files
Supplementary Material Files
Supplementary Material Files
Supplementary Material Files
Supplementary Material Files


