Short abstract
Review of alternative functions for caspase‐1 in inflammation, including roles as an inflammatory executioner, a regulator of protein secretion, and regulator of protein cleavage.
Keywords: inflammasomes, pyroptosis, caspase‐11
Abstract
Caspase‐1 is an inflammatory caspase that is activated through formation of inflammasome complexes in response to both pathogen‐derived and endogenous mediators. The most well‐known function of active caspase‐1 is to cleave the proforms of inflammatory cytokines IL‐1β and ‐18 into their active forms in response to inflammatory stimuli in immune cells. However, recent evidence suggests that caspase‐1 has multiple functions in addition to this cytokine maturation role and that it is at the center of many cell responses to stress and inflammation. The current review focuses on roles for caspase‐1, and the closely related caspase‐11, in inflammatory forms of cell death and protein cleavage and also in protein secretion. These alternative caspase‐1 functions can influence inflammatory responses, not just in immune cells but in other cell types, such as epithelia, where inflammatory cytokine production may not be a primary cell function.
Abbreviations
- AIM
= absent in melanoma
- ASC
= apoptosis‐associated speck‐like protein containing a caspase‐recruitment domain
- BID
= BH3 interacting‐domain death agonist
- DAMP
= damage‐associated molecular pattern
- FMF
= familial Mediterranean fever
- HMGB
= high‐mobility group box protein
- I/R
= ischemia/reperfusion
- MAVS
= mitochondrial antiviral signaling protein
- NLRC
= nod‐like receptor family, CARD domain‐containing
- NLRP
= NACHT, LRR and PYD domains‐containing protein
- PAMP
= pathogen‐associated molecular patterns
- PRR
= pattern recognition receptor
- ROS
= reactive oxygen species
- RIG
= retinoic acid‐inducible gene
- TRIF
= TIR‐domain‐containing adaptor‐inducing IFN‐β
Introduction
PRRs drive innate immune responses to both pathogen‐derived mediators (PAMPs) during infection, and endogenously generated mediators (DAMPs) during cell and tissue injury or stress. Much of the influence of PRRs is exerted through the up‐regulation of expression and the release of inflammatory cytokines, such as IL‐1β, IL‐6, and TNFα from innate immune cells. Several families of PRRs have been identified, and the discovery and characterization of inflammasome formation by NLR family members leading to caspase‐1 activation was a major advance in our understanding of the formation, maturation, and release of inflammatory cytokines IL‐1β and ‐18 [1]. These findings were obviously exciting to the innate immunity field, given that IL‐1β is such an important inflammatory mediator in multiple diseases [2].
However, the role of caspase‐1 in innate immune responses is now recognized to be far larger than the cytokine maturation function originally identified, and there is now increasing evidence that activation of caspase‐1 regulates multiple cellular functions during stress, even in cell types that do not produce large amounts of inflammatory cytokine [3, 4]. Caspase‐1 is activated in many nonimmune cell types, such as keratinocytes, astrocytes, hepatocytes, and cardiomyocytes [4, 5], where IL‐1β and ‐18 are not produced at a significant level, which suggests alternative caspase‐1 functions. Recent studies have begun to implicate caspase‐1 as a regulator of cellular responses to stress through the regulation of cytoprotective responses, tissue repair, and cell death [3, 6, 7] ( Fig. 1 ). This aspect of caspase‐1 function may be especially important in epithelial cells, such as hepatocytes, neurons, and cardiomyocytes, where cell and tissue stress or damage can be the major source for DAMP release within organs [5, 8]. Caspase‐1 in these cell types may therefore be a critical driver of inflammatory responses to both sterile injury and infectious stimuli. However, this area of research remains largely understudied, and the noncytokine‐maturation role of caspase‐1 is only beginning to emerge.
Figure 1.
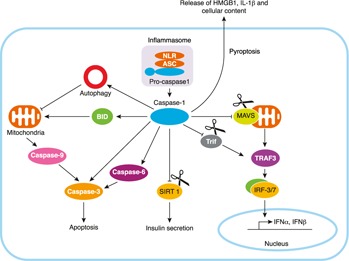
Noncytokine maturation functions of caspase‐1.
INFLAMMASOMES AND CASPASE ACTIVATION
Inflammasomes are intracellular platforms of proteins that are formed in response to wide‐ranging stimuli and result in the recruitment of procaspase‐1 leading to its subsequent cleavage into enzymatically active caspase‐1 [9]. As caspase‐1 is a proinflammatory caspase, its activity is tightly regulated by signal‐dependent autoactivation within inflammasomes. The exact nature of the regulation of inflammasome formation and how inflammasomes are stimulated is a burgeoning area of innate immune research, particularly given its relevance to the regulation of IL‐1β production. Four main inflammasomes have been identified. They involve the NLR members NLRP1, NLRP3, and NLRC4, as well as AIM2, a member of the pyrin and PYHIN family of nucleic acid receptors [9]. Activation of individual inflammasomes appears to be relatively specific and can be induced by both PAMPs and DAMPs [10]. NLRP1 and NLRC4 inflammasomes have been shown to be activated only by PAMPs such as muramyl dipeptide and flagellin, and AIM2 is specifically activated by double‐stranded DNA, which can be endogenous or pathogen‐derived. The NLRP3 inflammasome is particularly interesting, as it appears to be activated by a wide‐range of stimuli that include both pathogen and endogenous mediators, such as ROS, mitochondrial DAMPs, and ATP, as well as by crystalline structures, such as uric acid [11]. A typical inflammasome contains an NLR and the adaptor protein ASC, which allows association through CARD‐domain interactions with procaspase‐1 [12]. Procaspase‐1 then undergoes autocleavage to form the active caspase‐1 p10/p20 tetramer [12]. Active caspase‐1 is essential for the proteolytic maturation of pro‐IL1β and ‐18 into cleaved and active IL‐1β and ‐18.
NONCANONICAL INFLAMMASOME ACTIVATION
There is emerging evidence that caspase‐11, another member of the family of inflammatory caspases that is very closely related in structure to caspase‐1, mediates the noncanonical activation of NLRP3 inflammasome and subsequent caspase‐1 activation in response to intracellular LPS [13]. The first evidence was produced by Wang et al. [14] when they demonstrated an essential role for caspase‐11 in caspase‐1 activation after septic shock in vivo. More recently, Dixit and colleagues [13] demonstrated that caspase‐11 is critical for the noncanonical activation and processing of caspase‐1 and IL‐1β production in macrophages infected with Gram‐negative bacteria and certain pore‐forming toxins from organisms such as Escherichia coli, Citrobacter rodentium, and Vibrio cholera, but not after the stimulation with ATP or monosodium urate crystals. During the course of that study it was demonstrated that the available caspase‐1−/− mice actually lack both caspase‐1 and ‐11, because of a previously unrecognized inactivation mutation of caspase‐11 in B129 mice used to generate the knockouts. The proximity of caspase‐1 and ‐11 in the mouse genome prevented recombination of functional caspase‐11 in the knockout mice when backcrossed with C57BL/6 mice, which leaves them deficient in both inflammatory caspases [13]. Much of our current understanding of the role of caspase‐1 hinges on previous studies performed in what we now know are caspase‐1/caspase‐11 double‐knockout mice, so it may be necessary to re‐examine the functions of both caspase‐1 and ‐11 now that single‐mutation knockout mice are available.
CASPASE‐1, AN INFLAMMATORY EXECUTIONER
Cell death can occur in response to stress through various pathways that can be either proinflammatory (e.g., pyroptosis and necrosis) or anti‐inflammatory (e.g., apoptosis) [15]. The enzymatic activity of multiple caspases has been associated with apoptosis, but caspase‐1 has been strongly implicated in an inflammatory form of cell death termed pyroptosis [15]. The activation of the inflammasome and caspase‐1 has been associated with pyroptosis of immune cells in particular and is characterized by swelling and rapid lysis of cells with the release of the proinflammatory mediators IL‐1α and HMGB1 [16, 17–18] (Fig. 1). The release of IL‐1α and HMGB1, as well as the IL‐1β already activated in cells such as monocytes, can subsequently induce a variety of responses, including inflammatory cell chemotaxis and further release of proinflammatory cytokines from immune cells [19]. Pyroptosis as a form of caspase‐1‐dependent cell death was first described in macrophages after Salmonella infection [20, 21], but more recent evidence suggests that pyroptosis can be induced by not only PAMPs, but also DAMPs and ROS [22]. However, it is still unclear whether this caspase‐1‐dependent cell death is mediated by caspase‐1 itself or by other mediators, including caspase‐11, which has also recently been identified as crucial to initiation of pyroptosis.
Caspase‐11 is known to be involved in TLR4‐ and caspase‐1‐independent macrophage cell death, which plays a significant role in LPS‐induced lethality in vivo [23, 24]. Part of the mechanism of caspase‐11 activation to induce cell death was demonstrated in a model of Gram negative bacteria, which are able to escape into the cytosol after uptake by macrophages and trigger caspase‐11‐dependent cell death via a TRIF‐dependent pathway leading to IFN‐β release and autocrine/paracrine IFN‐β signaling to up‐regulate caspase‐11 expression [25, 26–27]. Very recently more of the mechanism behind pyroptosis has been described. Two groups have independently shown that gasdermin D, a protein associated with the regulation of epithelial apoptosis and proliferation, is essential for caspase‐11‐dependent pyroptotic cell death and IL‐1β maturation in models of endotoxic shock [28, 29] ( Table 1 ). Another study has also demonstrated that gasdermin D can be cleaved by caspase‐1 [30], and it may therefore represent a more common pyroptosis pathway. Similarly, another substrate of caspase‐11, pannexin‐1, was recently identified by Yang et al. [31] and implicated in LPS‐mediated pyroptosis. Cleavage of pannexin‐1 prevents channel formation and efflux of ATP from cells and thus prevents the subsequent influx of ATP via P2X7 ATP channels that mediate pyroptosis in response to cytosolic LPS.
Table 1.
Substrates cleaved by caspase‐1 and caspase‐11
| Protein | Substrate | Cell type | Experimental system | Downstream signaling pathway | Experiments performed using caspase‐1/11 double‐knockout mice | References |
| Cas‐1 | BID | Neuron | Neuronal I/R | Apoptosis | Yes | [35] |
| Caspase‐3 | Cardiomyocyte | Hypoxia | Apoptosis | No | [38] | |
| SIRT1 | Adipose tissue | High‐fat diet | Insulin secretion | No | [43] | |
| Glycolysis enzymes | Macrophage | Salmonella infection | Glycolysis | Yes | [41] | |
| MAVS | Neuroblast | Dengue virus infection | IFN production | No | [48] | |
| Pyrin | Epithelial cells/macrophage | Familial Mediterranean fever | NF‐κB activation | No | [42, 51] | |
| TRIF | Macrophage | Pseudomonas infection | Autophagy/IFN‐β production | Yes | [49] | |
| GATA4 | cardiomyocyte | Doxorubicin treatment | Cell death | Yes | [40] | |
| Gasdermin D | Macrophage | LPS and Nigericin | Cell death | No | [30] | |
| Cas‐11 | Gasdermin D | Epithelial cells/macrophage | Sepsis/cytosolic LPS | pyroptosis | No | [28, 29] |
| Pannexin‐1 | Macrophage | Sepsis/cytosolic LPS | P2X7‐mediated pyroptosis | No | [31] | |
| Caspase‐3 | Brain tissue | Stroke | apoptosis | No | [37] |
The role of caspase‐1 in the induction of another form of inflammatory cell death, necrosis, has also been investigated in several studies. Squires et al. [32] have shown that the impairment of plasma membrane integrity and necrosis can be triggered by caspase‐1 activation in macrophages after anthrax lethal toxin treatment. However, the cell death triggered by anthrax lethal toxin is characterized by rapid cell lysis, which is also a feature of pyroptosis. It is therefore unclear whether in this instance caspase‐1‐mediated cell death is through a distinct pathway leading to necrosis or another example of pyroptosis. Another study by Motani et al. [33] demonstrated that caspase‐1 promoted ASC‐mediated necrotic cell death in monocytes independently of the proteolytic activity of caspase‐1. This study highlights the potential of caspase‐1 to serve as a molecular determinant of the type of cell death. Activation of ASC induces necrosis in cells that express caspase‐1, whereas knockdown of caspase‐1 results in cell apoptosis, perhaps suggesting a distinct pathway of cell death triggered through caspase‐1, independent of the catalytic activity that is required for pyroptosis [34].
It is worth mentioning at this point that caspase‐1 and ‐11 activation in macrophages and monocytes seems to represent a death knell to these cells. However, this is not necessarily the case in long‐lived parenchymal cells such as hepatocytes. In these cells, which have important tissue‐specific functions that would be detrimental if grossly interrupted, caspase‐1 activation can be protective and does not result in pyroptosis [3]. Cell type differences in function of inflammatory mediators such as caspase‐1 will be important to take into consideration before attempting indiscriminately to block harmful inflammatory responses throughout an organism.
CASPASE‐1 AND APOPTOSIS
Even though caspase‐1 is often not associated with inflammatory cell death in nonmyeloid cells, there are some studies that suggest in some cases that the activation of caspase‐1 can be associated with apoptotic cell death independent of IL‐1β. Zhang et al. [35] have shown that caspase‐1 mediates neuronal cell death in a model of ischemia/reperfusion (I/R) through cleavage of BID, a proapoptotic protein, resulting in activation of the apoptotic caspase cascade (Fig. 1). Another study, involving serum deprivation of human neurons in vitro, induced caspase‐1 activation, which then cleaved and activated caspase‐6, an apoptotic caspase, resulting in downstream apoptosis pathway activation and cell death [36]. Caspase‐11 has also been shown to cleave procaspase‐3, an apoptosis executioner caspase, to activate apoptosis after ischemic brain injury [37]. Although cleavage of caspase‐3 has been shown in vitro and in the stroke model, more studies are needed to determine whether caspase‐11 has this effect in other immune and nonimmune cell types.
Cardiomyocytes may also be susceptible to caspase‐1‐mediated apoptosis in certain in vitro and mouse models. In models of cardiac I/R, caspase‐1 is activated in myeloid cells as well as in nonmyeloid cells in the heart, including cardiomyocytes, fibroblasts, and endothelial cells in the peri‐infarct region [38, 39]. In these studies activation of caspase‐1 via a pathway involving ATP‐mediated inflammasome activation was dependent on P2X7 receptor was associated with increased cardiomyocyte cell death after acute ischemia in mice, and this may contribute to heart failure [38]. Syed et al. [39] also demonstrated that caspase‐1 activation after hypoxia in cultured cardiomyocytes also leads to cleavage/activation of caspase‐3 and subsequent apoptosis. Another potential cleavage target for caspase‐1 in cardiomyocytes was identified in another study by Aries et al. [40] who showed that activation of caspase‐1 results in cleavage and degradation of GATA4, a transcription factor that is necessary for cardiomyocyte survival. Decreased levels of GATA4 subsequently sensitize cardiomyocytes to drug‐induced cell death, but its role in other models such as cardiac I/R has not been studied.
Our own research has focused on a role for caspase‐1 in hepatocytes in a model of mild global I/R induced by hemorrhagic shock in mice. In this cell type, activation of caspase‐1 is essential to prevent hepatocytes from undergoing apoptosis through the activation of mitochondrial autophagy. Caspase‐1 activation during shock and resuscitation increases the expression of beclin‐1, an important autophagy initiator protein, and allows the removal of damaged mitochondria to reduce excessive ROS production [22]. We are still investigating the exact mechanism involved in caspase‐1‐mediated beclin‐1 expression, but our early data suggest that caspase‐1 acts to prevent beclin‐1 cleavage, and this may involve caspase‐1‐mediated protection through cleavage of other caspases or calpains [unpublished results].
CASPASE‐1 REGULATION OF METABOLISM AND INFLAMMATION
As an aspartate‐specific cysteine protease, caspase‐1 is known to cleave IL‐1β and ‐18. In addition to this well‐established function, studies have identified multiple other caspase‐1 targets, including proteins involved in energy metabolism and inflammation [41, 42–43]. Using a proteomic approach, Shao et al. [41] identified proteins essential for mitochondrial respiration and glycolysis as potential targets of caspase‐1 in their diagonal gel screen. These included malate dehydrogenase, β subunit precursor of ATP synthase and several glycolytic enzymes that were further verified by in vitro caspase cleavage assays. Their experiments also confirmed that caspase‐1 activation after Salmonella infection leads to the cleavage of the glycolysis enzymes and reduction of the cellular glycolytic rate in macrophages, a cell type that largely depends on glycolysis for energy demand [44]. This regulation of metabolism was suggested to contribute to caspase‐1‐dependent cell death [41]. In addition, in a high‐fat diet mouse model, caspase‐1 can cleave SIRT1 [43], a histone deacetylase that promotes insulin secretion by β cells [45] and increases insulin sensitivity in peripheral tissues [46] (Fig. 1). The processing of SIRT1 by caspase‐1 after the formation of NLRP3 inflammasome results in a reduction of SIRT1 function in cells [43], providing an explanation for why mice lacking caspase‐1 or NLRP3 are protected from high‐fat diet–induced insulin resistance, metabolic dysfunction, and obesity [47]. This role for caspase‐1 in regulating metabolism may turn out to be one of the most important functions for this inflammatory caspase and highlights the links between obesity, metabolic dysfunction, and inflammation.
Catalytic cleavage by caspase‐1 also leads to the loss‐ or gain‐of‐function of proteins involved in inflammatory responses, such as TRIF, MAVS, and pyrin [42,48, 49]. caspase‐1 has been shown to cleave TRIF and diminish TRIF‐dependent autophagy and type I IFN production in macrophages After Pseudomonas aeruginosa infection [49]. Similarly, Yu et al. [48] demonstrated that caspase‐1 cleaves MAVS at residue D429 after Dengue virus infection, abolishing its ability to induce IFN production and therefore restricting inflammatory response after viral infection. Chae and colleagues [42, 50] suggested pyrin, a protein mutated in FMF, can be cleaved by caspase‐1 at Asp330. In contrast to the loss of function of MAVS and TRIF after their cleavage by caspase‐1, the processing of pyrin by caspase‐1 produced a 330‐residue N‐terminal fragment that enhances NF‐κB activation [42]. Moreover, they suggested that pyrin mutants associated with FMF are more susceptible to catalytic cleavage by caspase‐1 than WT pyrin, indicating a possible role for caspase‐1 in promoting inflammation through the NF‐κB pathway in the autoinflammatory disease FMF. Understanding pyrin processing may also be important for our understanding of other inflammatory diseases, not just a relatively rare genetic disease, as pyrin has been shown to form and inflammasome with ASC to activate caspase‐1 [51]. The regulation of these pathways during inflammation is therefore likely to be complex and require specific study. In addition, caspase‐1 activation has recently been shown to trigger the release of eicosanoids through calcium signaling and activation of cytosolic phospholipase A2, subsequently leading to inflammation and rapid vascular fluid loss after cytosolic delivery of flagellin [52]. This mechanism represents yet another means by which caspase‐1 rapidly initiates systemic inflammation.
CASPASE‐1 AND PROTEIN SECRETION
There is emerging evidence that associates caspase‐1 activation with unconventional secretion of proteins. Some of those include substrates of caspase‐1, such as pro‐IL‐1β and ‐18, components of the inflammasome such as caspase‐1 itself, as well as many other proteins involved in inflammation, cytoprotection, or tissue repair [6, 7, 53]. Pro‐IL‐1β and ‐18 lack the signaling peptide necessary for conventional endoplasmic reticulum–to–Golgi secretion [54, 55]. Instead, their secretion can be mediated by caspase‐1 in addition to other mechanisms, such as exosomal secretion and pathways involving lysosome exocytosis [7, 56]. In 2 separate models, one following infection by NLRC4‐activating pathogenic bacteria and the other upon the activation of NLRP3 by ATP in LPS‐primed macrophages, IL‐1β secretion was dependent on caspase‐1 activation [20, 57]. However, in both of these models IL‐1β release is accompanied by cell death, with cytokine release occurring either at the same time as, or right before, lysis of the cell. It is therefore unclear whether the caspase‐1‐mediated secretion of cytokines is an active and regulated response or a passive release upon death of the inflammatory cell; further investigation is needed to resolve this question. Caspase‐1 itself can be released into the extracellular space through this nonclassic secretion pathway from macrophages after stimulation with LPS or from UV‐irradiated keratinocytes [7]. The extracellular function of caspase‐1 is still largely unknown, but one recent study suggests that after its release from macrophages, procaspase‐1 can be further cleaved and activated by NLRP3 to subsequently induce extracellular IL‐1β maturation, thus perpetuating an inflammatory cascade during infection [6].
Caspase‐1 also regulates the secretion of other proteins involved in inflammation and tissue repair and therefore may play a role in restoring tissue homeostasis after major stress. Among proteins with caspase‐1‐regulated secretion, the most well‐studied are IL‐1α and RIG‐I [6, 53, 58]. IL‐1α is constitutively produced by activated macrophages, as well as by nonimmune cells such as keratinocytes, and it is known to trigger proinflammatory responses [79]. In vitro studies have indicated that caspase‐1 is required for IL‐1α secretion after the activation of NLRP1, NLRP3, AIM2, and NLRC4 inflammasomes [6, 7]. Although IL‐1α processing can also be mediated by calpain [60], Fettelschoss et al. [61] have shown that caspase and calpain‐mediated release are 2 separate mechanisms. Whereas the expression of cell‐surface–associated IL‐1α is dependent on calpain, the release of mature IL‐1α requires additional activation of the inflammasome and caspase‐1. Given the essential role of caspase‐1 in mediating IL‐1α secretion, it is likely that caspase‐1 serves as an important regulator of IL‐1α signaling. Lee et al. [58] have shown that caspase‐1 activation after the assembly of the NLRP3 inflammasome is able to induce IL‐1α secretion, which results in increased basal keratinocyte proliferation via the IL‐1R1 and NF‐κB pathways. However, whether this unconventional secretion pathway for IL‐1α requires the enzymatic activity of caspase‐1 remains controversial [6].
RIG‐I is a PRR that recognizes nonself (viral) dsRNA [62] and initiates an antiviral response through the activation of MAVS, which leads to the production of type 1 IFN [63]. Kim and Yoo [53] demonstrated that caspase‐1 activation mediates RIG‐I secretion, thereby reducing its intracellular level. Therefore, caspase‐1 activation has been thought to be a checkpoint to prevent overproduction of type 1 IFN through the inhibition of RIG‐I‐mediated intracellular signaling activity, thus limiting inflammation during viral and microbial infection. However, although it is known that this secretion of RIG‐I is independent of the proteolytic activity of caspase‐1, the exact mechanism of the caspase‐1‐mediated RIG‐I secretion is still not clear. In vivo studies should be performed to confirm the finding in the settings of viral infection.
Some other proteins that rely on caspase‐1 for secretion include those involved in regulating the dynamics of the actin cytoskeleton, including actin interacting protein 1, ARPC1, EPLIN, and actin‐binding protein coronin‐3 [7]. It has recently been shown that caspase‐1 mediates extracellular actin exposure in platelets, which plays an essential role in the release of procoagulant microparticles [64]. Activation of NLRC4 inflammasome is also associated with actin polymerization, which may serve to limit the uptake of bacteria in a cell and so may help to regulate pyroptotic cell death [65].
CASPASE‐1 AND LYSOSOMAL FUNCTION
Lysosomes are organelles that are responsible for the degradation of misfolded proteins, engulfed virus, bacteria, and dysfunctional organelles such as mitochondria [66]. Caspase‐1 has been shown to promote lysosomal degradation in macrophages after Legionella [67] and Salmonella infections [56]. NLRC4‐mediated activation of caspase‐1 restricts intracellular replication of the bacterium after Legionella infection by promoting the maturation of Legionella‐containing phagolysosomes and intracellular degradation of Legionella [67]. This effect is demonstrated to be mediated by caspase‐1‐dependent caspase‐7 activation and is independent of IL‐1β and ‐18 [68]. Caspase‐1 activation has also been shown to activate lysosome exocytosis, another step of the lysosomal degradation process, through increasing intracellular calcium levels during pyroptosis [56]. Because lysosomal degradation of mitochondria (or mitochondrial autophagy) is crucial for mitochondrial quality control under stress conditions [69], it is of great interest to investigate the relationship between caspase‐1 activation and lysosomal function after sterile injury. We have found that caspase‐1 increases the degradation of mitochondria through up‐regulation of mitochondrial autophagy in hepatocytes after sterile injury of hemorrhagic shock [3]. Therefore, it seems that, in response to danger signals, whether they are derived from pathogens or cell damage, caspase‐1 protects the host by promoting lysosomal degradation of pathogens and dysfunctional organelles.
CONCLUDING REMARKS
Caspase‐1 is expressed in multiple cell types, including in nonimmune cells, where IL‐1β and ‐18 are not produced at high levels. Instead, caspase‐1 may function to regulate protein secretion, cell death, and it may also regulate lysosomal function in cell type–specific ways. These noncytokine maturation roles of caspase‐1 may be especially important in nonimmune cells, as they may serve to restore tissue homeostasis after major stresses, including infection and sterile injury. Understanding these alternative functions of capase‐1 and its closely related inflammatory caspase, caspase‐11, will be important to any therapies designed to limit caspase‐1 activity and IL‐1β‐mediated inflammation. It may be that, to gain improved outcomes in inflammatory diseases, inhibition of pathways must be targeted to specific cell types.
AUTHORSHIP
M.J.S. initiated the review, lead discussions about the content, and contributed to writing and editing the manuscript. Q.S. contributed to discussions on the topic and content, performed the literature search, wrote the main part of the manuscript, and designed the table and figure.
DISCLOSURES
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The manuscript was supported in part by U.S. National Institutes of Health, National Institute of General Medical Sciences (NIGMS) Grant R01GM102146.
References
- 1. Takeuchi, O. , Akira, S. (2010) Pattern recognition receptors and inflammation. Cell 140, 805–820. [DOI] [PubMed] [Google Scholar]
- 2. Dinarello, C. A. (2011) Interleukin‐1 in the pathogenesis and treatment of inflammatory diseases. Blood 117, 3720–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun, Q. , Gao, W. , Loughran, P. , Shapiro, R. , Fan, J. , Billiar, T. R. , Scott, M. J. (2013) Caspase 1 activation is protective against hepatocyte cell death by up‐regulating beclin 1 protein and mitochondrial autophagy in the setting of redox stress. J. Biol. Chem. 288, 15947–15958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yazdi, A. S. , Drexler, S. K. , Tschopp, J. (2010) The role of the inflammasome in nonmyeloid cells. J. Clin. Immunol. 30, 623–627. [DOI] [PubMed] [Google Scholar]
- 5. Csak, T. , Ganz, M. , Pespisa, J. , Kodys, K. , Dolganiuc, A. , Szabo, G. (2011) Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology 54, 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gross, O. , Yazdi, A. S. , Thomas, C. J. , Masin, M. , Heinz, L. X. , Guarda, G. , Quadroni, M. , Drexler, S. K. , Tschopp, J. (2012) Inflammasome activators induce interleukin‐1α secretion via distinct pathways with differential requirement for the protease function of caspase‐1. Immunity 36, 388–400. [DOI] [PubMed] [Google Scholar]
- 7. Keller, M. , Rüegg, A. , Werner, S. , Beer, H. D. (2008) Active caspase‐1 is a regulator of unconventional protein secretion. Cell 132, 818–831. [DOI] [PubMed] [Google Scholar]
- 8. Kaczmarek, A. , Vandenabeele, P. , Krysko, D. V. (2013) Necroptosis: the release of damage‐associated molecular patterns and its physiological relevance. Immunity 38, 209–223. [DOI] [PubMed] [Google Scholar]
- 9. Latz, E. , Xiao, T. S. , Stutz, A. (2013) Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo, H. , Callaway, J. B. , Ting, J. P. (2015) Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 21, 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis, B. K. , Wen, H. , Ting, J. P. (2011) The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 29, 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schroder, K. , Tschopp, J. (2010) The inflammasomes. Cell 140, 821–832. [DOI] [PubMed] [Google Scholar]
- 13. Kayagaki, N. , Warming, S. , Lamkanfi, M. , Vande Walle, L. , Louie, S. , Dong, J. , Newton, K. , Qu, Y. , Liu, J. , Heldens, S. , Zhang, J. , Lee, W. P. , Roose‐Girma, M. , Dixit, V. M. (2011) Non‐canonical inflammasome activation targets caspase‐11. Nature 479, 117–121. [DOI] [PubMed] [Google Scholar]
- 14. Wang, S. , Miura, M. , Jung, Y. K. , Zhu, H. , Li, E. , Yuan, J. (1998) Murine caspase‐11, an ICE‐interacting protease, is essential for the activation of ICE. Cell 92, 501–509. [DOI] [PubMed] [Google Scholar]
- 15. Fink, S. L. , Cookson, B. T. (2005) Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 73, 1907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Craven, R. R. , Gao, X. , Allen, I. C. , Gris, D. , Bubeck Wardenburg, J. , McElvania‐Tekippe, E. , Ting, J. P. , Duncan, J. A. (2009) Staphylococcus aureus alpha‐hemolysin activates the NLRP3‐inflammasome in human and mouse monocytic cells. PLoS One 4, e7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lightfield, K. L. , Persson, J. , Brubaker, S. W. , Witte, C. E. , von Moltke, J. , Dunipace, E. A. , Henry, T. , Sun, Y. H. , Cado, D. , Dietrich, W. F. , Monack, D. M. , Tsolis, R. M. , Vance, R. E. (2008) Critical function for Naip5 in inflammasome activation by a conserved carboxy‐terminal domain of flagellin. Nat. Immunol. 9, 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang, Y. , Jiang, G. , Zhang, P. , Fan, J. (2015) Programmed cell death and its role in inflammation. Mil. Med. Res 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu, B. , Nakamura, T. , Inouye, K. , Li, J. , Tang, Y. , Lundbäck, P. , Valdes‐Ferrer, S. I. , Olofsson, P. S. , Kalb, T. , Roth, J. , Zou, Y. , Erlandsson‐Harris, H. , Yang, H. , Ting, J. P. , Wang, H. , Andersson, U. , Antoine, D. J. , Chavan, S. S. , Hotamisligil, G. S. , Tracey, K. J. (2012) Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488, 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fink, S. L. , Cookson, B. T. (2006) Caspase‐1‐dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol. 8, 1812–1825. [DOI] [PubMed] [Google Scholar]
- 21. Hersh, D. , Monack, D. M. , Smith, M. R. , Ghori, N. , Falkow, S. , Zychlinsky, A. (1999) The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase‐1. Proc. Natl. Acad. Sci. USA 96, 2396–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fernandes‐Alnemri, T. , Yu, J. W. , Datta, P. , Wu, J. , Alnemri, E. S. (2009) AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shi, J. , Zhao, Y. , Wang, Y. , Gao, W. , Ding, J. , Li, P. , Hu, L. , Shao, F. (2014) Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192. [DOI] [PubMed] [Google Scholar]
- 24. Kayagaki, N. , Wong, M. T. , Stowe, I. B. , Ramani, S. R. , Gonzalez, L. C. , Akashi‐Takamura, S. , Miyake, K. , Zhang, J. , Lee, W. P. , Muszyński, A. , Forsberg, L. S. , Carlson, R. W. , Dixit, V. M. (2013) Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249. [DOI] [PubMed] [Google Scholar]
- 25. Aachoui, Y. , Leaf, I. A. , Hagar, J. A. , Fontana, M. F. , Campos, C. G. , Zak, D. E. , Tan, M. H. , Cotter, P. A. , Vance, R. E. , Aderem, A. , Miao, E. A. (2013) Caspase‐11 protects against bacteria that escape the vacuole. Science 339, 975–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rathinam, V. A. , Vanaja, S. K. , Waggoner, L. , Sokolovska, A. , Becker, C. , Stuart, L. M. , Leong, J. M. , Fitzgerald, K. A. (2012) TRIF licenses caspase‐11‐dependent NLRP3 inflammasome activation by gram‐negative bacteria. Cell 150, 606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang, Q. , Stevenson, H. L. , Scott, M. J. , Ismail, N. (2015) Type I interferon contributes to noncanonical inflammasome activation, mediates immunopathology, and impairs protective immunity during fatal infection with lipopolysaccharide‐negative ehrlichiae. Am. J. Pathol. 185, 446–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kayagaki, N. , Stowe, I. B. , Lee, B. L. , O'Rourke, K. , Anderson, K. , Warming, S. , Cuellar, T. , Haley, B. , Roose‐Girma, M. , Phung, Q. T. , Liu, P. S. , Lill, J. R. , Li, H. , Wu, J. , Kummerfeld, S. , Zhang, J. , Lee, W. P. , Snipas, S. J. , Salvesen, G. S. , Morris, L. X. , Fitzgerald, L. , Zhang, Y. , Bertram, E. M. , Goodnow, C. C. , Dixit, V. M. (2015) Caspase‐11 cleaves gasdermin D for non‐canonical inflammasome signalling. Nature 526, 666–671. [DOI] [PubMed] [Google Scholar]
- 29. Shi, J. , Zhao, Y. , Wang, K. , Shi, X. , Wang, Y. , Huang, H. , Zhuang, Y. , Cai, T. , Wang, F. , Shao, F. (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665. [DOI] [PubMed] [Google Scholar]
- 30. He, W. T. , Wan, H. , Hu, L. , Chen, P. , Wang, X. , Huang, Z. , Yang, Z. H. , Zhong, C. Q. , Han, J. (2015) Gasdermin D is an executor of pyroptosis and required for interleukin‐1β secretion. Cell Res. 25, 1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang, D. , He, Y. , MunToz‐Planillo, R. , Liu, Q. , NúnTez, G. (2015) Caspase‐11 requires the pannexin‐1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity 43, 923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Squires, R. C. , Muehlbauer, S. M. , Brojatsch, J. (2007) Proteasomes control caspase‐1 activation in anthrax lethal toxin‐mediated cell killing. J. Biol. Chem. 282, 34260–34267. [DOI] [PubMed] [Google Scholar]
- 33. Motani, K. , Kushiyama, H. , Imamura, R. , Kinoshita, T. , Nishiuchi, T. , Suda, T. (2011) Caspase‐1 protein induces apoptosis‐associated speck‐like protein containing a caspase recruitment domain (ASC)‐mediated necrosis independently of its catalytic activity. J. Biol. Chem. 286, 33963–33972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kelk, P. , Johansson, A. , Claesson, R. , Hänström, L. , Kalfas, S. (2003) Caspase 1 involvement in human monocyte lysis induced by Actinobacillus actinomycetemcomitans leukotoxin. Infect. Immun. 71, 4448–4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang, W. H. , Wang, X. , Narayanan, M. , Zhang, Y. , Huo, C. , Reed, J. C. , Friedlander, R. M. (2003) Fundamental role of the Rip2/caspase‐1 pathway in hypoxia and ischemia‐induced neuronal cell death. Proc. Natl. Acad. Sci. USA 100, 16012–16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guo, H. , Pétrin, D. , Zhang, Y. , Bergeron, C. , Goodyer, C. G. , LeBlanc, A. C. (2006) Caspase‐1 activation of caspase‐6 in human apoptotic neurons. Cell Death Differ. 13, 285–292. [DOI] [PubMed] [Google Scholar]
- 37. Kang, S. J. , Wang, S. , Hara, H. , Peterson, E. P. , Namura, S. , Amin‐Hanjani, S. , Huang, Z. , Srinivasan, A. , Tomaselli, K. J. , Thornberry, N. A. , Moskowitz, M. A. , Yuan, J. (2000) Dual role of caspase‐11 in mediating activation of caspase‐1 and caspase‐3 under pathological conditions. J. Cell Biol. 149, 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mezzaroma, E. , Toldo, S. , Farkas, D. , Seropian, I. M. , Van Tassell, B. W. , Salloum, F. N. , Kannan, H. R. , Menna, A. C. , Voelkel, N. F. , Abbate, A. (2011) The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc. Natl. Acad. Sci. USA 108, 19725–19730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Syed, F. M. , Hahn, H. S. , Odley, A. , Guo, Y. , Vallejo, J. G. , Lynch, R. A. , Mann, D. L. , Bolli, R. , Dorn II, G. W., (2005) Proapoptotic effects of caspase‐1/interleukin‐converting enzyme dominate in myocardial ischemia. Circ. Res. 96, 1103–1109. [DOI] [PubMed] [Google Scholar]
- 40. Aries, A. , Whitcomb, J. , Shao, W. , Komati, H. , Saleh, M. , Nemer, M. (2014) Caspase‐1 cleavage of transcription factor GATA4 and regulation of cardiac cell fate. Cell Death Dis. 5, e1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shao, W. , Yeretssian, G. , Doiron, K. , Hussain, S. N. , Saleh, M. (2007) The caspase‐1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J. Biol. Chem. 282, 36321–36329. [DOI] [PubMed] [Google Scholar]
- 42. Chae, J. J. , Wood, G. , Richard, K. , Jaffe, H. , Colburn, N. T. , Masters, S. L. , Gumucio, D. L. , Shoham, N. G. , Kastner, D. L. (2008) The familial Mediterranean fever protein, pyrin, is cleaved by caspase‐1 and activates NF‐kappaB through its N‐terminal fragment. Blood 112, 1794–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chalkiadaki, A. , Guarente, L. (2012) High‐fat diet triggers inflammation‐induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 16, 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lewis, J. S. , Lee, J. A. , Underwood, J. C. , Harris, A. L. , Lewis, C. E. (1999) Macrophage responses to hypoxia: relevance to disease mechanisms. J. Leukoc. Biol. 66, 889–900. [DOI] [PubMed] [Google Scholar]
- 45. Biason‐Lauber, A. , Böni‐Schnetzler, M. , Hubbard, B. P. , Bouzakri, K. , Brunner, A. , Cavelti‐Weder, C. , Keller, C. , Meyer‐Böni, M. , Meier, D. T. , Brorsson, C. , Timper, K. , Leibowitz, G. , Patrignani, A. , Bruggmann, R. , Boily, G. , Zulewski, H. , Geier, A. , Cermak, J. M. , Elliott, P. , Ellis, J. L. , Westphal, C. , Knobel, U. , Eloranta, J. J. , Kerr‐Conte, J. , Pattou, F. , Konrad, D. , Matter, C. M. , Fontana, A. , Rogler, G. , Schlapbach, R. , Regairaz, C. , Carballido, J. M. , Glaser, B. , McBurney, M. W. , Pociot, F. , Sinclair, D. A. , Donath, M. Y. (2013) Identification of a SIRT1 mutation in a family with type 1 diabetes. Cell Metab. 17, 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun, C. , Zhang, F. , Ge, X. , Yan, T. , Chen, X. , Shi, X. , Zhai, Q. (2007) SIRT1 improves insulin sensitivity under insulin‐resistant conditions by repressing PTP1B. Cell Metab. 6, 307–319. [DOI] [PubMed] [Google Scholar]
- 47. Stienstra, R. , Joosten, L. A. , Koenen, T. , van Tits, B. , van Diepen, J. A. , van den Berg, S. A. , Rensen, P. C. , Voshol, P. J. , Fantuzzi, G. , Hijmans, A. , Kersten, S. , Müller, M. , van den Berg, W. B. , van Rooijen, N. , Wabitsch, M. , Kullberg, B. J. , van der Meer, J. W. , Kanneganti, T. , Tack, C. J. , Netea, M. G. (2010) The inflammasome‐mediated caspase‐1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 12, 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu, C. Y. , Chiang, R. L. , Chang, T. H. , Liao, C. L. , Lin, Y. L. (2010) The interferon stimulator mitochondrial antiviral signaling protein facilitates cell death by disrupting the mitochondrial membrane potential and by activating caspases. J. Virol. 84, 2421–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jabir, M. S. , Ritchie, N. D. , Li, D. , Bayes, H. K. , Tourlomousis, P. , Puleston, D. , Lupton, A. , Hopkins, L. , Simon, A. K. , Bryant, C. , Evans, T. J. (2014) Caspase‐1 cleavage of the TLR adaptor TRIF inhibits autophagy and β‐interferon production during Pseudomonas aeruginosa infection. Cell Host Microbe 15, 214–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chae, J. J. , Wood, G. , Masters, S. L. , Richard, K. , Park, G. , Smith, B. J. , Kastner, D. L. (2006) The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase‐1 to modulate IL‐1beta production. Proc. Natl. Acad. Sci. USA 103, 9982–9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xu, P. , Wen, Z. , Shi, X. , Li, Y. , Fan, L. , Xiang, M. , Li, A. , Scott, M. J. , Xiao, G. , Li, S. , Billiar, T. R. , Wilson, M. A. , Fan, J. (2013) Hemorrhagic shock augments Nlrp3 inflammasome activation in the lung through impaired pyrin induction. J. Immunol. 190, 5247–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Von Moltke, J. , Trinidad, N. J. , Moayeri, M. , Kintzer, A. F. , Wang, S. B. , van Rooijen, N. , Brown, C. R. , Krantz, B. A. , Leppla, S. H. , Gronert, K. , Vance, R. E. (2012) Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 490, 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim, M. J. , Yoo, J. Y. (2008) Active caspase‐1‐mediated secretion of retinoic acid inducible gene‐I. J. Immunol. 181, 7324–7331. [DOI] [PubMed] [Google Scholar]
- 54. Qu, Y. , Franchi, L. , Nunez, G. , Dubyak, G. R. (2007) Nonclassical IL‐1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J. Immunol. 179, 1913–1925. [DOI] [PubMed] [Google Scholar]
- 55. Rubartelli, A. , Cozzolino, F. , Talio, M. , Sitia, R. (1990) A novel secretory pathway for interleukin‐1 beta, a protein lacking a signal sequence. EMBO J. 9, 1503–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bergsbaken, T. , Fink, S. L. , den Hartigh, A. B. , Loomis, W. P. , Cookson, B. T. (2011) Coordinated host responses during pyroptosis: caspase‐1‐dependent lysosome exocytosis and inflammatory cytokine maturation. J. Immunol. 187, 2748–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lopez‐Castejon, G. , Brough, D. (2011) Understanding the mechanism of IL‐1β secretion. Cytokine Growth Factor Rev. 22, 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee, P. , Lee, D. J. , Chan, C. , Chen, S. W. , Ch'en, I. , Jamora, C. (2009) Dynamic expression of epidermal caspase 8 simulates a wound healing response. Nature 458, 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen, C. J. , Kono, H. , Golenbock, D. , Reed, G. , Akira, S. , Rock, K. L. (2007) Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 13, 851–856. [DOI] [PubMed] [Google Scholar]
- 60. Yazdi, A. S. , Drexler, S. K. (2013) Regulation of interleukin 1 alpha secretion by inflammasomes. Ann. Rheum. Dis. 72, ii96–ii99. [DOI] [PubMed] [Google Scholar]
- 61. Fettelschoss, A. , Kistowska, M. , LeibundGut‐Landmann, S. , Beer, H. D. , Johansen, P. , Senti, G. , Contassot, E. , Bachmann, M. F. , French, L. E. , Oxenius, A. , Kundig, T. M. (2011) Inflammasome activation and IL‐1beta target IL‐1alpha for secretion as opposed to surface expression. Proc. Natl. Acad. Sci. USA 108, 18055‐18060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Takahasi, K. , Yoneyama, M. , Nishihori, T. , Hirai, R. , Kumeta, H. , Narita, R. , Gale, M., Jr. , Inagaki, F. , Fujita, T. (2008) Nonself RNA‐sensing mechanism of RIG‐I helicase and activation of antiviral immune responses. Mol. Cell 29, 428–440. [DOI] [PubMed] [Google Scholar]
- 63. Seth, R. B. , Sun, L. , Ea, C. K. , Chen, Z. J. J. (2005) Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF‐kappaB and IRF 3. Cell 122, 669–682. [DOI] [PubMed] [Google Scholar]
- 64. Rothmeier, A. S. , Marchese, P. , Petrich, B. G. , Furlan‐Freguia, C. , Ginsberg, M. H. , Ruggeri, Z. M. , Ruf, W. (2015) Caspase‐1‐mediated pathway promotes generation of thromboinflammatory microparticles. J. Clin. Invest. 125, 1471–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Man, S. M. , Ekpenyong, A. , Tourlomousis, P. , Achouri, S. , Cammarota, E. , Hughes, K. , Rizzo, A. , Ng, G. , Wright, J. A. , Cicuta, P. , Guck, J. R. , Bryant, C. E. (2014) Actin polymerization as a key innate immune effector mechanism to control Salmonella infection. Proc. Natl. Acad. Sci. USA 111, 17588–17593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Luzio, J. P. , Pryor, P. R. , Bright, N. A. (2007) Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 8, 622–632. [DOI] [PubMed] [Google Scholar]
- 67. Amer, A. , Franchi, L. , Kanneganti, T. D. , Body‐Malapel, M. , Ozören, N. , Brady, G. , Meshinchi, S. , Jagirdar, R. , Gewirtz, A. , Akira, S. , NúnTez, G. (2006) Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J. Biol. Chem. 281, 35217–35223. [DOI] [PubMed] [Google Scholar]
- 68. Akhter, A. , Gavrilin, M. A. , Frantz, L. , Washington, S. , Ditty, C. , Limoli, D. , Day, C. , Sarkar, A. , Newland, C. , Butchar, J. , Marsh, C. B. , Wewers, M. D. , Tridandapani, S. , Kanneganti, T. D. , Amer, A. O. (2009) Caspase‐7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 5, e1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Green, D. R. , Galluzzi, L. , Kroemer, G. (2011) Mitochondria and the autophagy‐inflammation‐cell death axis in organismal aging. Science 333, 1109–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]


