Short abstract
Review on the multiple roles of TRAF3 in B and T lymphocytes; based on Bonazinga Award lecture, annual SLB Meeting 2015.
Keywords: B cell, T cell, signal transduction
Abstract
This review summarizes the current state of knowledge regarding the roles of the signaling adapter protein tumor necrosis factor receptor (TNFR)‐associated factor 3 in regulating the functions of B and T lymphocytes. In B lymphocytes, TNFR‐associated factor 3 inhibits signaling by TNFR superfamily receptors, Toll‐like receptors, and interleukin‐6R. In contrast, signaling to B cells by the virally encoded oncogenic protein latent membrane protein 1 is promoted by TNFR‐associated factor 3. An important B cell‐specific role for TNFR‐associated factor 3 is the inhibition of homeostatic survival, directly relevant to the common occurrence of TNFR‐associated factor 3 mutations in human B cell malignancies. TNFR‐associated factor 3 was recently found to be a resident nuclear protein in B cells, where it interacts with and inhibits gene expression mediated by the cAMP response element‐binding protein transcription complex, including expression of the prosurvival protein myeloid leukemia cell differentiation protein 1. In T lymphocytes, TNFR‐associated factor 3 is required for normal signaling by the T cell antigen receptor, while inhibiting signaling by the interleukin‐2 receptor. Cytoplasmic TNFR ‐associated factor 3 restrains nuclear factor‐κB2 activation in both T and B cells. Clinical implications and future directions for the study of this context‐dependent signaling regulator are discussed.
Abbreviations
- ALCL
= anaplastic large cell lymphoma
- B‐Traf3−/−
= mice conditionally deficient in TNFR‐associated factor 3 in B lymphocytes
- CD40L/CD62L
= cluster of differentiation 40/60 ligand
- cIAP
= cellular inhibitor of apoptosis protein
- Csk
= C‐terminal Src kinase
- Foxp3
= forkhead box p3
- iNKT
= invariant NK T
- Lck
= lymphocyte‐specific protein tyrosine kinase
- LMP1
= latent membrane protein 1
- MM
= multiple myeloma
- NIK
= NF‐κB‐inducing kinase
- NLS
= nuclear localization signal
- PKC
= protein kinase C
- PTPN22
= protein tyrosine phosphatase nonreceptor type 22
- RING
= really interesting new gene
- T‐Traf3−/−
= mice conditionally deficient in TNFR‐associated factor 3 in T lymphocytes
- Tcm
= central memory T cell
- TCPTP
= T cell protein tyrosine phosphatase
- TRAF
= TNFR‐associated factor
- TRAF3−/−
= TNFR‐associated factor‐deficient
- Treg
= T regulatory cell
- WT
= wild‐type
Introduction
Over 30 yr ago, it was widely believed that activated T cells could fully activate naïve B cells in suitable proximity, exclusively via soluble lymphokines, in the absence of cognate or contact‐mediated interactions. However, a series of studies published over the following decade revealed the importance of both the ability of B cells to present cognate antigen to T cells and the requirement for contact‐mediated receptor–ligand interactions in full activation of both cell types (reviewed in ref. [1]). Within the next several years, the importance of the CD40–CD154 (CD40L) interaction in the induction of antigen‐specific B cell activation and humoral memory was discovered [2, 3]. This was followed by the understanding that multiple members of the TNFR superfamily and their ligands are expressed by both B and T cells and contribute in various ways to cellular functions and crosstalk [4, 5].
In sharp contrast to the dominant group of immunologic receptors of focus at the time the functions of CD40 were discovered, the Ig gene superfamily, CD40, and other TNFR family members do not have Src homology domains and do not appear to interact directly with cytoplasmic tyrosine kinases. Instead, protein–protein interaction studies revealed that these receptors initiate signaling primarily through interacting with a group of intracellular adapter proteins, called TRAFs [6, 7]. Seven members of the TRAF family have been described to date; they do not have a high degree of sequence homology but share a common domain structure (see Fig. 1). This features a Zn‐binding RING (except TRAF1) and Zn finger domains, coiled‐coil domains via which they homo‐ and heteromultimerize, and a TRAF‐C terminal domain (except TRAF7) that mediates many of their interactions with receptors and other signaling proteins [8, 9, 10, 11–12] ( Fig. 1 ). A large body of studies using the approach of exogenous overexpression of TRAF constructs in fibroblast or epithelial cell lines (especially 293T adenocarcinoma cells) indicated that a common feature of TRAF1, ‐2, ‐5, and ‐6 is their ability to promote the activity of NF‐κB reporter genes [13, 14, 15–16]. Exogenously overexpressed TRAFs also commonly induce activation of MAPKs, particularly JNK [17, 18–19]. However, TRAF3 is unique among TRAFs in that it does not promote NF‐κB reporter gene activation in 293 cells [20], which may underlie the relatively little interest in characterizing the functions of TRAF3 in the early years following its discovery compared with TRAF2 and ‐6.
Figure 1.
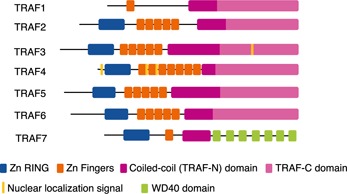
TRAF family molecules. A schematic depiction of the basic structural features of the TRAF molecules. All except TRAF1 have an N‐terminal Zn‐binding RING domain, followed by a variable number of Zn finger motifs. The coiled‐coil/TRAF‐N domain allows TRAFs to form homo‐ or heteromultimers. All except TRAF7 have a C‐terminal TRAF‐C domain used to mediate many of their protein–receptor and protein–protein interactions. TRAF7 has a unique C‐terminal structure containing multiple WD40 domains [10]. TRAF4 and recently, TRAF3 are reported to have canonical NLS sequences [11, 12].
Additionally, it was initially technically difficult to determine the biological roles played by TRAF3. A major impediment to the accurate assignment of specific physiological functions to individual TRAFs is their high degree of interaction. The canonical TRAF‐binding site present in the cytoplasmic domains of many members of the TNFR superfamily can bind TRAF2, ‐3, and ‐5 in a highly overlapping manner [21]. Thus, exogenous overexpression of one type of WT or mutant TRAF molecule can have many indirect effects, particularly as TRAFs can form heterogenous multimers with one another, complicating data interpretation. Mice with germline deficiency in TRAF2, ‐3, or ‐6 show neonatal lethality with multiple severe developmental defects [22, 23–24], so this approach also failed to reveal clearly individual TRAF functions in specific cells or tissues.
TRAF3 AS A CONTEXT‐DEPENDENT REGULATOR OF RECEPTOR FUNCTIONS
We wished to circumvent logistical barriers preventing a clear understanding of the normal functions of endogenous TRAF molecules, obstacles inherent in both exogenous overexpression and knockout mouse approaches. Lymphocytes have notoriously poor transfection efficiency, making it difficult to inhibit endogenous TRAF molecule expression sufficiently with inhibitory RNA approaches, using the relatively inefficient vectors available ∼15 yr ago. The technology for producing conditionally deficient “knockout” mouse strains was not yet available. Thus, we developed a technique for adapting the approach of gene targeting by homologous recombination, used in germ cells when making knockout mice, to the lower rate of homologous recombination in somatic cells. This strategy allowed the first creation of somatic cell lines completely and specifically deficient in individual (or multiple) TRAF molecules, without using mutagenic agents that can result in other, unknown changes [25]. Study of these cell lines revealed new information on the roles of TRAF1, ‐2, and ‐6 in signaling to B cells via various receptors [25, 26, 27–28], but the most striking and unexpected findings concerned the understudied TRAF3 molecule.
The EBV‐encoded oncogenic protein, LMP1, is a striking mimic of the CD40 receptor in B cells [29, 30–31] and can replace most CD40‐mediated functions in CD40‐deficient B cells in vivo [32, 33–34]. Similar to CD40, the cytoplasmic C‐terminal domain of LMP1 can bind TRAF1, ‐2, ‐3, ‐5, and ‐6 [20, 35, 36–37], although only CD40 and not LMP1 signaling induces the polyubiquitination and degradation of TRAF2 and ‐3 [38]. Thus, it was a natural assumption that individual TRAFs function similarly to mediate signals from CD40 and LMP1, its viral mimic. TRAF3 is the only TRAF that inhibits CD40 signals to B cells [39], as well as the synergy between CD40 and B cell antigen receptor signals [26]. However, analysis of signaling via both CD40 and LMP1 in LMP1‐transfected TRAF3−/− B cell lines found that whereas CD40 signaling is amplified in the absence of TRAF3, LMP1 signals to the same cells are markedly defective [40]. This may be related to the strongly increased avidity of LMP1 for TRAF3 compared with that of CD40 [40], which in turn, may reflect additional TRAF3‐binding contacts for LMP1 in its “noncanonical” TRAF‐binding site [41, 42]. Interestingly, a human CD40 molecule with a cytoplasmic domain polymorphism associated with a gain‐of‐function phenotype in B cells [43] displays the same increased binding of, and requirement for, TRAF3 in mediating activation signals [44]. It is intriguing that this CD40 polymorphism is associated with ethnic populations that have increased morbidity and disease activity in autoimmune diseases associated with B cell hyperactivity, such as systemic lupus erythematosus and rheumatoid arthritis [43].
TRAF3 AS A CELL TYPE‐SPECIFIC REGULATOR OF LYMPHOCYTE FUNCTIONS
The surprising discovery that TRAF3 can play different—even contrasting—roles for different receptors expressed by the same cells provided the first evidence of the highly context‐dependent nature of its function. The next question of high priority was whether TRAF3 also displays cell type‐specific roles. By this time, the powerful techniques for production of conditionally deficient mouse strains via Cre‐loxP recombination [45] had become more widely feasible and were applied to produce the first conditionally deficient, Traf3 flox/flox mice [46, 47]. These mouse strains have, in turn, been used to produce B‐Traf3 −/− and T‐Traf3 −/− and mice lacking TRAF3 in dendritic cells and macrophages [46, 47, 48, 49–50]. Roles of TRAF3 in myeloid cells have been recently reviewed [51]; here, we will focus on the multiple roles played by TRAF3 in B and T cells.
The study of B‐Traf3 −/− mice (produced by breeding Traf3 flox/flox mice with CD19‐Cre mice) revealed a critical function for TRAF3 unique to this cell type as a key inhibitor of homeostatic survival. Thus, in the absence of B cell TRAF3, mice have a marked accumulation of B lymphocytes in secondary lymphoid organs [46, 47], together with elevated serum Igs and autoantibodies, immune complex deposition in the kidney, and B cell infiltration into multiple organs [46]. The importance of TRAF3 in regulating B cell survival rather than proliferation is reflected in the markedly enhanced in vivo and in vitro survival of B cells from B‐Traf3 −/− mice in the absence of any increase in cell proliferation [46]. These mice ultimately show a propensity for development of B cell malignancies that occur later in life [52], consistent with an enhanced opportunity for additional mutagenic events provided by their abnormal B cell survival.
T‐Traf3 −/− mice (produced by breeding Traf3 flox/flox with CD4‐Cre mice) display a markedly different phenotype from their B cell counterparts. No extended T cell survival is seen in the absence of TRAF3, and numbers of total CD4+ and CD8+ T cells are similar to those of WT littermate controls, although the proportion of CD4+Foxp3+ Tregs is doubled. Sizes of secondary lymphoid organs are normal [48]. Furthermore, in contrast to B‐Traf3 −/−, T‐Traf3 −/− mice show striking defects in in vivo responses to immunization and infection [48]. The responses of splenic T cells isolated from these mice are not rescued by stimulation with agonistic anti‐CD3+ anti‐CD28 antibodies in vitro, leading to the unexpected finding that signaling via the TCR is defective in the absence of TRAF3 [48, 53].
This defect in the strength of TCR signaling results in a marked deficiency in numbers and function of iNKT in T‐Traf3 −/− mice [54], as well as in homeostatic maintenance of CD8+ Tcm cells [55].
TRAF3−/− thymic Tregs show normal Foxp3 stability. They also possess normal ability to suppress proliferation of CD4+ T cells in vitro and inhibit development of inflammatory bowel disease in vivo [56]. However, mice lacking TRAF3 only in Tregs show enhanced production of high‐affinity antibodies as a consequence of specific reduction in follicular Tregs. This, in turn, results from reduced expression of the gene encoding the molecule ICOS in TRAF3−/− Tregs [57]. A summary of TRAF3‐regulated functions in T and B lymphocytes is presented in Table 1 .
Table 1.
Regulatory functions of TRAF3 in B and T lymphocytes
| B cells | T cells |
| Inhibition of NF‐κB2 activation | Inhibition of NF‐κB2 activation |
| Inhibition of homeostatic survival | Enhancement of TCR signaling |
| Inhibition of CD40 and BAFFR signaling | Inhibition of IL‐2R signaling |
| Promotion of LMP1 signaling | Promotion of ICOS expression in Tregs |
| Inhibition of TLR signaling | |
| Inhibition of IL‐6R signaling and plasma cell differentiation | |
| Nuclear interaction with CREB complex; inhibition of CREB‐mediated transcription |
MULTIPLE MECHANISMS OF TRAF3 REGULATORY FUNCTIONS
It is now clear that TRAF3 plays distinct roles in different cell types, as well as regulating multiple functions within each cell type. How does this single signaling protein impact so many important processes in lymphocytes? Here, we will summarize the current knowledge of the molecular mechanisms by which TRAF3 regulates key pathways in B and T cells, as a membrane‐associated, intracellular, and nuclear protein.
TRAF3 as a membrane‐associated protein
TNFR superfamily receptors
TRAF3 was originally identified through its association with the cytoplasmic domain of CD40 [58]. Through this same or similar “canonical” TRAF‐binding site, TRAF3 associates with most of the TNFR superfamily members expressed by lymphocytes [6]. The specific functions of TRAF3 in regulating signaling by many of these receptors to T and/or B lymphocytes remain to be determined. The impact of TRAF3 upon CD40 signaling to B cells has been the most studied. B cell TRAF3 primarily inhibits CD40‐mediated activation events in B cells (with the exception noted above of a human CD40 gain‐of‐function variant) [44]. This includes activation of NF‐κB and the MAPKs, JNK and p38 [40], as well as CD40‐induced effector functions of antibody production and homotypic adhesion [39]. The major mechanism for this negative regulation appears to be competition with TRAF2, a positive mediator of B cell CD40 signaling, for association with their shared binding site in the CD40 cytoplasmic domain [26, 39, 59]. As discussed above, in contrast, TRAF3 is a positive regulator of these events in B cells when mediated by the viral CD40 mimic, LMP1 [60].
It was previously shown that TRAF3 may enhance CD40‐induced reactive oxygen species in the mouse B cell line WEHI 231 [61], and TRAF3 in the mouse A20 B cell line was recently reported to promote CD40‐mediated activation of the kinase AKT, a function that requires the K63 ubiquitination of TRAF3 [62]. It is not yet clear how TRAF3 enhances either of these signaling pathways; they are, to date, the only reported CD40‐mediated events enhanced rather than inhibited by TRAF3, and the findings remain to be reproduced in human B cells.
TRAF3 also indirectly inhibits signaling to B cells by the receptor for the TNF family ligand, BAFF, by down‐regulating NF‐κB activation induced by BAFFR [63]. In this case, however, the impact of TRAF3 is primarily as an intracellular protein (see below). Although BAFFR also associates with TRAF2 and ‐6, and TRAF6 is required for effective BAFFR signaling to B cells, TRAF3 does not appear to inhibit BAFFR–TRAF6 binding [64]. Signaling to B cells via CD40 or BAFFR induces the K48‐linked polyubiquitination and degradation of TRAF3 [63, 65], limiting its negative impact on their signaling. This process requires hetero‐complex formation between TRAF3 and TRAF2, with the latter acting as an E3 ubiquitin ligase, in association with cIAPs [66, 67–68]. TRAF3 has also been reported as an inhibitor of TRAF2/5‐mediated activation of NF‐κB by multiple TNFR superfamily members; however, this study examined exogenously overexpressed receptors in the epithelial cell line 293T [69]. Given the highly cell type‐dependent functions of TRAF3, it will be important to pursue this question further, studying endogenous receptors in lymphocytes.
TCR complex
A major surprise of the phenotype of the T‐Traf3 −/− mouse was the finding that TRAF3 in T cells associates with, and is important for, effective signaling by the TCR complex [48]. This requirement occurs early in the TCR‐mediated signaling cascade, resulting in reduced activation of proximal TCR‐associated kinases and adapter proteins [48]. The precise nature of the interactions between TRAF3 and the TCR complex at the cell membrane is not yet defined. Initial findings indicate that T cell TRAF3 associates with Csk, which inhibits activation of Lck by phosphorylating its negative regulatory Y residue, an early event in TCR signaling [70]. Following TCR stimulation, TRAF3 association with Csk promotes relocalization of Csk from the TCR complex to the cytoplasm, thereby inhibiting Csk‐mediated, negative regulation of Lck [53].
Cytokine receptors
Although a number of TRAF3 functions are clearly distinct in B versus T lymphocytes, TRAF3 shares a common molecular mechanism for regulating cytokine receptor signaling at the cell membrane in both of these cell types, albeit involving different players in each. The intriguing 2‐fold increase in thymic Tregs of the T‐Traf3 −/− mouse prompted an extensive search for a mechanistic explanation. This cell‐intrinsic Treg increase is independent of TRAF3‐mediated regulation of NF‐κB activation, thymic selection, or signals through CD28 [56]. The increase becomes apparent in the IL‐2‐induced transition of Treg precursors to mature Tregs, prompting an examination of IL‐2 responses. Although T‐Traf3 −/− mice express unchanged amounts of the IL‐2R, they show a marked enhancement of phosphorylation of Jak1 and ‐3, as well as Stat5, in response to IL‐2 [56]. In normal T cells, TRAF3 associates with membrane Jak1, to which TRAF3 recruits TCPTP, which de‐phosphorylates Jak1 and ‐3 and Stat5. Thus, in the absence of TRAF3, enhanced IL‐2R signaling promotes Treg maturation [56].
B‐Traf3 −/− mice have an increase in the plasma cell compartment [71], consistent with the occurrence of loss‐of‐function TRAF3 mutations in human MM, a plasma cell neoplasm [72]. However, this plasma cell increase is lost when B‐Traf3 −/− mice are bred to IL‐6‐deficient mice [71]. This finding led to the discovery that similar to its T cell role in IL‐2R signaling, B cell TRAF3 normally associates with the membrane IL‐6R complex, restraining the IL‐6‐induced phosphorylation of Jak1 and Stat3 by recruitment of PTPN22. PTPN22‐deficient mice show a similar increase in the plasma cell compartment [71]. The association of TRAF3 with PTPN22 appears to play an important regulatory role in multiple immune cell types. A human PTPN22 polymorphism associated with autoimmune disease has lost TRAF3‐binding ability in myeloid cells [73]. Recent data also indicate that TRAF3 regulates PTPN22 association with the TCR complex [53].
It has been reported that TRAF3 associates with the IL‐17R and suppresses IL‐17 signaling by preventing formation of a complex of IL‐17R, TRAF6, and the adapter protein activator 1 [74]. However, this was examined using fibroblast or epithelial cell lines transfected with TRAF3 and IL‐17R constructs, so the role of endogenous TRAF3 in regulating IL‐17R signaling in immune cell types is not yet known.
In contrast to the inhibitory role played by TRAF3 in the cytokine receptor signals described above, optimal IL‐15R signaling to T cells requires TRAF3. IL‐15‐mediated Stat5 phosphorylation is significantly reduced in T‐Traf3 −/− mice [54], which share with IL‐15‐deficient mice the feature of defective iNKT cell numbers and function [75]. The mechanism by which TRAF3 promotes IL‐15 signaling in thymic iNKT cells is indirect. TCR signaling is critical for early stages of iNKT cell development [76], and this signaling is defective in T cells deficient in TRAF3 [48]. Defective TCR signaling to TRAF3−/− iNKT cell precursors leads to reduced expression of the transcription factor T‐bet at stages 2 and 3 of iNKT development, and this, in turn, results in reduced expression of the common γ‐chain cytokine receptor CD122 [54]. Defective IL‐15R signaling in T‐Traf3 −/− mice also causes a marked reduction in the CD8+ T cell subset of CD8+CD44hiCD62Lhi Tcm cells. CD8+ Tcm cells show reduced phosphorylation of the kinase ERK and of Stat5 following stimulation with IL‐15, resulting in defective homeostatic maintenance [55].
TRAF3 as an intracellular protein
NF‐κB activation
The strikingly enhanced survival of TRAF3−/− B cells correlates with constitutive activation of the noncanonical/NF‐κB2 pathway, as evidenced by nuclear localization of the NF‐κB family proteins p52 and RelB in unstimulated TRAF3−/− B cells [46, 47]. Restraint of NF‐κB2 activation in normal resting B cells is attributed primarily to constitutive degradation of cytoplasmic NIK [63]. The degradation complex is composed of TRAF3, which binds NIK, and recruits the TRAF2‐cIAP complex to serve as an E3 ubiquitin ligase, resulting in K48 polyubiquitination and proteasome‐mediated NIK degradation [77, 78]. The ability of CD40 and BAFFR to induce TRAF3 degradation in B cells by recruiting TRAF3 to their cytoplasmic domains, where it is itself degraded by the TRAF2–cIAP complex, explains how these receptors induce NF‐κB2 activation [63, 65, 79, 80].
Thus, a major paradigm was developed that the constitutive NF‐κB2 activation present in TRAF3−/− B cells fully explains their extended survival, and the primary, if not exclusive, function of B cell TRAF3 is to inhibit basal NF‐κB2 activation by inducing NIK degradation. A large number of studies have subsequently confirmed that hyperactivation of the NF‐κB2 pathway makes important contributions to the overall phenotype of TRAF3−/− B cells (recently reviewed; see ref. [81]). However, multiple discrepancies with this paradigm have also arisen, indicating that additional TRAF3‐regulated pathways are important to B cell survival and activation.
First, all TRAF3−/− immune cell types examined to date (B cells, T cells, dendritic cells, and macrophages) display constitutive NF‐κB2 activation, supporting the involvement of TRAF3 as a key factor in this pathway [46, 47–48, 82, 83]. However, only TRAF3−/− B cells display enhanced survival [46, 47–48, 82, 83]. Thus, activation of NF‐κB2 does not necessarily confer enhanced survival upon cells. Interestingly, in the human T cell line Jsl1, PMA stimulation induces production of an alternatively spliced form of TRAF3, which is associated with NF‐κB2 activation and production of the chemokine CXCL13; stimulation of normal human T cells with anti‐CD3 antibody has similar effects [84].
The relationship among TRAF3 ubiquitination, degradation, and NF‐κB2 activation has also been revealed as nonlinear in B cells. As mentioned above, loss‐of‐function human TRAF3 mutations are common in MM. One such mutant TRAF3 molecule was found to induce increased NF‐κB2 activation when introduced into TRAF3–WT B cells, but this mutant TRAF3 was not degraded in response to CD40 or BAFF stimuli [85]. Additionally, a mutant form of BAFFR that fails to stimulate NF‐κB2 activation in B cells nonetheless induces normal levels of TRAF3 degradation in these cells [85]. Taken together, these findings indicate that TRAF3 regulates B cell‐specific survival via mechanisms additional to NF‐κB2 activation.
PKCδ
Loss of TRAF3 in B cells correlates not only with increased nuclear p52/RelB but also with decreased nuclear PKCδ [46], an antiapoptotic event also seen following BAFF signaling [86]. Interestingly, PKCδ−/− B cells also show enhanced survival [86], and BAFF stimulation of TRAF3−/− B cells does not further enhance nuclear localization of PKCδ [46]. The specific molecular mechanism by which TRAF3 modulates PKCδ localization remains to be identified.
Signaling by innate immune receptors
Although not associating directly with innate immune receptors, TRAF3 modulates signaling pathways for a number of these receptors, many of which are intracellular. This property has been most extensively examined to date in nonimmune and myeloid cells (reviewed in refs. [87, 88]), in which innate immune receptor signaling has been most often studied. In these cells, TRAF3 promotes production of type I IFN but inhibits expression of several proinflammatory cytokines induced by innate immune receptors [83, 87, 88]. In B lymphocytes, TRAF3 inhibits cytokine production and Ig isotype switching in response to TLRs and also inhibits canonical NF‐κB activation in response to these receptors, while having no detectable impact on their activation of MAPKs [82]. Interestingly, TRAF5 also inhibits B cell TLR signaling but appears to do so primarily by inhibiting TLR‐induced JNK activation, while conversely not affecting NF‐κB activation [89]. TRAF5−/− mice show no increase in B cell survival [90].
Whereas myeloid and B cells show the greatest responsiveness to innate receptors, such as TLRs, T lymphocytes have also been reported to respond to TLR ligands, most consistently TLR2 agonists [91, 92, 93, 94–95]. Thus, it will be interesting to determine the role of T cell TRAF3 in T cell TLR signaling.
Signaling in ALCL cell lines
As TRAF3 was identified as playing an important role in normal T cell functions, its importance was examined for growth and survival of various transformed human T cell lines. Results indicate that 3 cell lines, specifically those derived from the ALCL type of T cell lymphoma, require TRAF3 for proliferation. Interestingly, this TRAF3 role is independent of the regulation of NF‐κB2, instead involving signaling by the Jak/Stat and PI3K/AKT pathways, particularly control of the latter pathway by an increase in phosphatase and tensin homolog protein [96]. It is not yet known whether TRAF3 also uses this mechanism to control proliferation in normal T cells.
TRAF3 as a nuclear protein
TRAF3 was seen by microscopy in the nucleus of human endothelial cells 15 yr ago [97], and cleavage fragments of TRAF3 were observed in the nuclei of the Jurkat human T cell line following proapoptotic signals [98]. More recently, TRAF3 was seen in the nuclei of apoptotic neurons in a rat spinal cord injury model [99]. However, nuclear functions of TRAF3 were not explored in these prior reports, and TRAF3 has been principally studied in the context of its many roles at the cell membrane and in the cytoplasm.
Upon the realization that only in B cells does TRAF3 deficiency lead to increased survival, we sought to identify TRAF3‐regulated, B cell‐specific pathways. This was pursued by performing comparative gene‐expression microarray analysis of B cells and T cells of B‐Traf3 −/− and T‐Traf3 −/− mice compared with their WT littermates. A pathway analysis of results indicated that genes regulated by the CREB transcriptional complex were preferentially up‐regulated in TRAF3−/− B cells. This prompted us to examine the localization of TRAF3 in B cells by cell fractionation and microscopy. We learned that TRAF3 is a resident nuclear protein in normal human and mouse B cells, with a canonical NLS sequence that is required for this constitutive localization [11]. TRAF3 associates with both CREB and CREB‐binding protein only in the nucleus and inhibits the transcriptional activity of a CREB reporter gene by recruiting the TRAF2–cIAP complex to the nucleus, inducing the polyubiquitination and degradation of CREB [11]. Relevant to the enhanced survival of TRAF3−/− B cells, there is increased mRNA and protein expression of the prosurvival CREB target myeloid leukemia cell differentiation protein 1 in the absence of nuclear TRAF3. Pharmacologic or small interfering RNA‐mediated CREB inhibition reduces the increased survival of TRAF3−/− B cells to that of WT B cells [11]. These results reveal a critical, new role for TRAF3, one highly relevant to the relationship between TRAF3‐mediated regulation and B cell malignancies.
The current state of knowledge regarding mechanisms by which TRAF3 regulates lymphocyte functions is illustrated in Fig. 2 .
Figure 2.
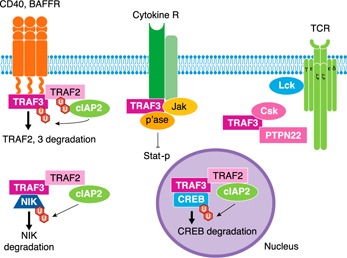
Mechanisms of TRAF3‐mediated lymphocyte regulation. Examples of the multiple ways in which TRAF3 influences lymphocyte activation events are illustrated. (Left to right) TRAF3 binds to the cytoplasmic domains of CD40 and BAFFR in B lymphocytes; in the case of CD40, TRAF3 competes with TRAF2 for this binding and inhibits TRAF2‐mediated activation events, such as canonical NF‐κB1 and JNK activation. Recruitment of TRAF2 and ‐3 to these receptors allows cIAP2 associated with TRAF2 to mediate polyubiquitination (indicated by ‘U’ in hexagons) and degradation of both TRAFs. Signaling by these receptors also impacts cytoplasmic TRAF3, which binds the kinase NIK and through recruitment of TRAF2–cIAP, mediates the ubiquitination and degradation of NIK. This limits NIK‐mediated NF‐κB activation. TRAF3 regulates cytokine Rs (IL‐6R in B cells and IL‐2R in T cells are discussed in the text) by binding to these Rs and Jak kinases and recruiting specific phosphatases (p'ase; PTPN22 to the IL‐6R, TCPTP to the IL‐2R), which dephosphorylate the Jaks, resulting in reduced downstream Stat phosphorylation (Stat‐p) and transcriptional activation. In B cells, nuclear TRAF3 binds the CREB transcriptional complex, recruiting TRAF2–cIAP to destabilize CREB, limiting CREB‐mediated transcription of prosurvival proteins. In T cells, TRAF3 associates with the TCR complex and enhances its signaling. One mechanism by which this occurs is the binding and cytoplasmic sequestration of Csk and PTPN22, allowing increased Lck activation. References are cited in the accompanying text.
FUTURE QUESTIONS AND DIRECTIONS
Many questions remain regarding roles played by TRAF3 in regulating B and T lymphocytes. Examples below represent just a few of particular interest; answering them will, no doubt, lead to more pathways to explore.
The critical function of TRAF3 in restraining B cell survival is of particular importance to the emerging role of TRAF3 as a tumor suppressor for B cell malignancies. Building upon the increasing understanding of how TRAF3 regulates survival pathways, specifically in B cells, has great potential for providing information of practical importance to cancer‐treatment decisions, as “personalized medicine” becomes a much greater factor in such choices. The targeting of TRAF3‐regulated prosurvival proteins and pathways in TRAF3−/− B cell cancers is a logical translational direction. Information on survival pathways that contribute to B cell malignancy may also have relevance, even for tumors without demonstrable TRAF3 deficiency, as CD40 and BAFFR can induce TRAF3 degradation [65, 100], whereas expression of the oncoprotein LMP1 can lead to TRAF3 sequestration away from the cytoplasm, nucleus, and other receptors [40].
How TRAF3 exerts its strong, enhancing function upon TCR signaling is a topic of current active pursuit, and TRAF3 may be worth study as a potential target in certain types of T cell lymphoma, as discussed above. Whether this role for TRAF3 is associated with the ability of its alternatively spliced form to activate NF‐κB2 remains to be determined.
The unexpected role for TRAF3 in TCR signaling has complicated the use of TRAF3−/− T cells to understand how TRAF3 regulates signaling by each of the TRAF3‐binding coregulatory receptors of the TNFR superfamily expressed by T cells. These include CD120b (TNFR2), CD27, CD30, CD134 (OX40), CD137 (4‐1BB), and glucocorticoid‐induced TNFR. Most of these receptors require effective TCR signaling for their induced expression; thus, the compromised TCR signaling of TRAF3−/− T cells precludes one from easily obtaining information on how TRAF3 serves signaling and downstream functions of each. New experimental strategies will need to be applied to pursue this important question. Additionally, activated T cells can be induced to express CD40 under some conditions [100, 102], and how TRAF3 regulates CD40 signaling to T cells versus B cells is not yet known. The new finding—that TRAF3 exerts important regulatory control of B cell functions as a resident nuclear protein—prompts the question of whether it also does so in T cells, and if so, which transcriptional complexes are impacted? The multiple context‐dependent functions of TRAF3 guarantee many interesting future questions to investigate regarding a molecule that can both surprise and fascinate.
AUTHORSHIP
G.A.B. is the sole author of this review and the corresponding author.
DISCLOSURES
The authors declare no conflicts of interest
References
- 1. Swain, S. L. , Dutton, R. W. (1987) Consequences of the direct interaction of helper T cells with B cells presenting antigen. Immunol. Rev. 99, 263–280. [DOI] [PubMed] [Google Scholar]
- 2. Grewal, I. S. , Flavell, R. A. (1998) CD40 and CD154 in cell‐mediated immunity. Annu. Rev. Immunol. 16, 111–135. [DOI] [PubMed] [Google Scholar]
- 3. Bishop, G. A. , Hostager, B. S. (2003) The CD40‐CD154 interaction in B cell‐T cell liaisons. Cytokine Growth Factor Rev. 14, 297–309. [DOI] [PubMed] [Google Scholar]
- 4. Bishop, G. A. , Hostager, B. S. (2001) B Lymphocyte activation by contact‐mediated interactions with T lymphocytes. Curr. Opin. Immunol. 13, 278–285. [DOI] [PubMed] [Google Scholar]
- 5. Bishop, G. A. , Haxhinasto, S. A. , Stunz, L. L. , Hostager, B. S. (2003) Antigen‐specific B‐lymphocyte activation. Crit. Rev. Immunol. 23, 149–197. [DOI] [PubMed] [Google Scholar]
- 6. Arch, R. H. , Gedrich, R. W. , Thompson, C. B. (1998) Tumor necrosis factor receptor‐associated factors (TRAFs)—a family of adapter proteins that regulates life and death. Genes Dev. 12, 2821–2830. [DOI] [PubMed] [Google Scholar]
- 7. Wajant, H. , Henkler, F. , Scheurich, P. (2001) The TNF‐receptor‐associated factor family: scaffold molecules for cytokine receptors, kinases and their regulators. Cell. Signal. 13, 389–400. [DOI] [PubMed] [Google Scholar]
- 8. Grech, A. , Quinn, R. , Srinivasan, D. , Badoux, X. , Brink, R. (2000) Complete structural characterisation of the mammalian and Drosophila TRAF genes: implications for TRAF evolution and the role of RING finger splice variants. Mol. Immunol. 37, 721–734. [DOI] [PubMed] [Google Scholar]
- 9. Ha, H. , Han, D. , Choi, Y. (2009) TRAF‐mediated TNFR‐family signaling. Curr. Protoc. Immunol. Chapter 11: Unit11.9D. [DOI] [PubMed] [Google Scholar]
- 10. Zotti, T. , Vito, P. , Stilo, R. (2012) The seventh ring: exploring TRAF7 functions. J. Cell. Physiol. 227, 1280–1284. [DOI] [PubMed] [Google Scholar]
- 11. Mambetsariev, N. , Lin, W. W. , Stunz, L. L. , Hanson, B. M. , Hildebrand, J. M. , Bishop, G. A. (2016) Nuclear TRAF3 is a negative regulator of CREB in B cells. Proc. Natl. Acad. Sci. USA 113, 1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Masson, R. , Régnier, C. H. , Chenard, M. P. , Wendling, C. , Mattei, M. G. , Tomasetto, C. , Rio, M. C. (1998) Tumor necrosis factor receptor associated factor 4 (TRAF4) expression pattern during mouse development. Mech. Dev. 71, 187–191. [DOI] [PubMed] [Google Scholar]
- 13. Rothe, M. , Sarma, V. , Dixit, V. M. , Goeddel, D. V. (1995) TRAF2‐mediated activation of NF‐kP B by TNF receptor 2 and CD40. Science 269, 1424–1427. [DOI] [PubMed] [Google Scholar]
- 14. Ishida, T. , Mizushima, S. , Azuma, S. , Kobayashi, N. , Tojo, T. , Suzuki, K. , Aizawa, S. , Watanabe, T. , Mosialos, G. , Kieff, E. , Yamamoto, T. , Inoue, J. (1996) Identification of TRAF6, a novel tumor necrosis factor receptor‐associated factor protein that mediates signaling from an amino‐terminal domain of the CD40 cytoplasmic region. J. Biol. Chem. 271, 28745–28748. [DOI] [PubMed] [Google Scholar]
- 15. Ishida, T. , Tojo, T. , Aoki, T. , Kobayashi, N. , Ohishi, T. , Watanabe, T. , Yamamoto, T. , Inoue, J.‐I. (1996) TRAF5, a novel tumor necrosis factor receptor‐associated factor family protein, mediates CD40 signaling. Proc. Natl. Acad. Sci. USA 93, 9437–9442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakano, H. , Oshima, H. , Chung, W. , Williams‐Abbott, L. , Ware, C. F. , Yagita, H. , Okumura, K. (1996) TRAF5, an activator of NF‐kappaB and putative signal transducer for the lymphotoxin‐β receptor. J. Biol. Chem. 271, 14661–14664. [DOI] [PubMed] [Google Scholar]
- 17. Lee, S. Y. , Reichlin, A. , Santana, A. , Sokol, K. A. , Nussenzweig, M. C. , Choi, Y. (1997) TRAF2 is essential for JNK but not NF‐kappaB activation and regulates lymphocyte proliferation and survival. Immunity 7, 703–713. [DOI] [PubMed] [Google Scholar]
- 18. Dadgostar, H. , Cheng, G. (1998) An intact zinc ring finger is required for tumor necrosis factor receptor‐associated factor‐mediated nuclear factor‐kappaB activation but is dispensable for c‐Jun N‐terminal kinase signaling. J. Biol. Chem. 273, 24775–24780. [DOI] [PubMed] [Google Scholar]
- 19. Baud, V. , Liu, Z. G. , Bennett, B. , Suzuki, N. , Xia, Y. , Karin, M. (1999) Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino‐terminal effector domain. Genes Dev. 13, 1297–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Devergne, O. , Hatzivassiliou, E. , Izumi, K. M. , Kaye, K. M. , Kleijnen, M. F. , Kieff, E. , Mosialos, G. (1996) Association of TRAF1, TRAF2, and TRAF3 with an Epstein‐Barr virus LMP1 domain important for B‐lymphocyte transformation: role in NF‐kappaB activation. Mol. Cell. Biol. 16, 7098–7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boucher, L. M. , Marengère, L. E. , Lu, Y. , Thukral, S. , Mak, T. W. (1997) Binding sites of cytoplasmic effectors TRAF1, 2, and 3 on CD30 and other members of the TNF receptor superfamily. Biochem. Biophys. Res. Commun. 233, 592–600. [DOI] [PubMed] [Google Scholar]
- 22. Xu, Y. , Cheng, G. , Baltimore, D. (1996) Targeted disruption of TRAF3 leads to postnatal lethality and defective T‐dependent immune responses. Immunity 5, 407–415. [DOI] [PubMed] [Google Scholar]
- 23. Yeh, W. C. , Shahinian, A. , Speiser, D. , Kraunus, J. , Billia, F. , Wakeham, A. , de la Pompa, J. L. , Ferrick, D. , Hum, B. , Iscove, N. , Ohashi, P. , Rothe, M. , Goeddel, D. V. , Mak, T. W. (1997) Early lethality, functional NF‐kappaB activation, and increased sensitivity to TNF‐induced cell death in TRAF2‐deficient mice. Immunity 7, 715–725. [DOI] [PubMed] [Google Scholar]
- 24. Lomaga, M. A. , Yeh, W. C. , Sarosi, I. , Duncan, G. S. , Furlonger, C. , Ho, A. , Morony, S. , Capparelli, C. , Van, G. , Kaufman, S. , van der Heiden, A. , Itie, A. , Wakeham, A. , Khoo, W. , Sasaki, T. , Cao, Z. , Penninger, J. M. , Paige, C. J. , Lacey, D. L. , Dunstan, C. R. , Boyle, W. J. , Goeddel, D. V. , Mak, T. W. (1999) TRAF6 deficiency results in osteopetrosis and defective interleukin‐1, CD40, and LPS signaling. Genes Dev. 13, 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hostager, B. S. , Haxhinasto, S. A. , Rowland, S. L. , Bishop, G. A. (2003) Tumor necrosis factor receptor‐associated factor 2 (TRAF2)‐deficient B lymphocytes reveal novel roles for TRAF2 in CD40 signaling. J. Biol. Chem. 278, 45382–45390. [DOI] [PubMed] [Google Scholar]
- 26. Haxhinasto, S. A. , Hostager, B. S. , Bishop, G. A. (2002) Cutting edge: molecular mechanisms of synergy between CD40 and the B cell antigen receptor: role for TNF receptor‐associated factor 2 in receptor interaction. J. Immunol. 169, 1145–1149. [DOI] [PubMed] [Google Scholar]
- 27. Xie, P. , Hostager, B. S. , Munroe, M. E. , Moore, C. R. , Bishop, G. A. (2006) Cooperation between TNF receptor‐associated factors 1 and 2 in CD40 signaling. J. Immunol. 176, 5388–5400. [DOI] [PubMed] [Google Scholar]
- 28. Rowland, S. R. , Tremblay, M. L. , Ellison, J. M. , Stunz, L. L. , Bishop, G. A. , Hostager, B. S. (2007) A novel mechanism for TNFR‐associated factor 6‐dependent CD40 signaling. J. Immunol. 179, 4645–4653. [DOI] [PubMed] [Google Scholar]
- 29. Zimber‐Strobl, U. , Kempkes, B. , Marschall, G. , Zeidler, R. , Van Kooten, C. , Banchereau, J. , Bornkamm, G. W. , Hammerschmidt, W. (1996) Epstein‐Barr virus latent membrane protein (LMP1) is not sufficient to maintain proliferation of B cells but both it and activated CD40 can prolong their survival. EMBO J. 15, 7070–7078. [PMC free article] [PubMed] [Google Scholar]
- 30. Kilger, E. , Kieser, A. , Baumann, M. , Hammerschmidt, W. (1998) Epstein‐Barr virus‐mediated B‐cell proliferation is dependent upon latent membrane protein 1, which simulates an activated CD40 receptor. EMBO J. 17, 1700–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Busch, L. K. , Bishop, G. A. (1999) The EBV transforming protein, latent membrane protein 1, mimics and cooperates with CD40 signaling in B lymphocytes. J. Immunol. 162, 2555–2561. [PubMed] [Google Scholar]
- 32. Uchida, J. , Yasui, T. , Takaoka‐Shichijo, Y. , Muraoka, M. , Kulwichit, W. , Raab‐Traub, N. , Kikutani, H. (1999) Mimicry of CD40 signals by Epstein‐Barr virus LMP1 in B lymphocyte responses. Science 286, 300–303. [DOI] [PubMed] [Google Scholar]
- 33. Stunz, L. L. , Busch, L. K. , Munroe, M. E. , Sigmund, C. D. , Tygrett, L. T. , Waldschmidt, T. J. , Bishop, G. A. (2004) Expression of the cytoplasmic tail of LMP1 in mice induces hyperactivation of B lymphocytes and disordered lymphoid architecture. Immunity 21, 255–266. [DOI] [PubMed] [Google Scholar]
- 34. Rastelli, J. , Hömig‐Hölzel, C. , Seagal, J. , Müller, W. , Hermann, A. C. , Rajewsky, K. , Zimber‐Strobl, U. (2008) LMP1 signaling can replace CD40 signaling in B cells in vivo and has unique features of inducing class‐switch recombination to IgG1. Blood 111, 1448–1455. [DOI] [PubMed] [Google Scholar]
- 35. Miller, W. E. , Cheshire, J. L. , Raab‐Traub, N. (1998) Interaction of tumor necrosis factor receptor‐associated factor signaling proteins with the latent membrane protein 1 PXQXT motif is essential for induction of epidermal growth factor receptor expression. Mol. Cell. Biol. 18, 2835–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Devergne, O. , Cahir McFarland, E. D. , Mosialos, G. , Izumi, K. M. , Ware, C. F. , Kieff, E. (1998) Role of the TRAF binding site and NF‐kappaB activation in Epstein‐Barr virus latent membrane protein 1‐induced cell gene expression. J. Virol. 72, 7900–7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schultheiss, U. , Püschner, S. , Kremmer, E. , Mak, T. W. , Engelmann, H. , Hammerschmidt, W. , Kieser, A. (2001) TRAF6 is a critical mediator of signal transduction by the viral oncogene latent membrane protein 1. EMBO J. 20, 5678–5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown, K. D. , Hostager, B. S. , Bishop, G. A. (2001) Differential signaling and tumor necrosis factor receptor‐associated factor (TRAF) degradation mediated by CD40 and the Epstein‐Barr virus oncoprotein latent membrane protein 1 (LMP1). J. Exp. Med. 193, 943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hostager, B. S. , Bishop, G. A. (1999) Cutting edge: contrasting roles of TNF receptor‐associated factor 2 (TRAF2) and TRAF3 in CD40‐activated B lymphocyte differentiation. J. Immunol. 162, 6307–6311. [PubMed] [Google Scholar]
- 40. Xie, P. , Hostager, B. S. , Bishop, G. A. (2004) Requirement for TRAF3 in signaling by LMP1 but not CD40 in B lymphocytes. J. Exp. Med. 199, 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu, S. , Xie, P. , Welsh, K. , Li, C. , Ni, C. , Zhu, X. , Reed, J. C. , Satterthwait, A. C. , Bishop, G. A. , Ely, K. R. (2005) LMP1 protein from the Epstein‐Barr virus is a structural CD40 decoy in B lymphocytes for binding to TRAF3. J. Biol. Chem. 280, 33620–33626. [DOI] [PubMed] [Google Scholar]
- 42. Graham, J. P. , Arcipowski, K. M. , Bishop, G. A. (2010) Differential B‐lymphocyte regulation by CD40 and its viral mimic, latent membrane protein 1. Immunol. Rev. 237, 226–248. [DOI] [PubMed] [Google Scholar]
- 43. Peters, A. L. , Plenge, R. M. , Graham, R. R. , Altshuler, D. M. , Moser, K. L. , Gaffney, P. M. , Bishop, G. A. (2008) A novel polymorphism of the human CD40 receptor with enhanced function. Blood 112, 1863–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peters, A. L. , Bishop, G. A. (2010) Differential TRAF3 utilization by a variant human CD40 receptor with enhanced signaling. J. Immunol. 185, 6555–6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smith, A. J. H. , De Sousa, M. A. , Kwabi‐Addo, B. , Heppell‐Parton, A. , Impey, H. , Rabbitts, P. (1995) A site‐directed chromosomal translocation induced in embryonic stem cells by Cre‐loxP recombination. Nat. Genet. 9, 376–385. [DOI] [PubMed] [Google Scholar]
- 46. Xie, P. , Stunz, L. L. , Larison, K. D. , Yang, B. , Bishop, G. A. (2007) Tumor necrosis factor receptor‐associated factor 3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity 27, 253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gardam, S. , Sierro, F. , Basten, A. , Mackay, F. , Brink, R. (2008) TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity 28, 391–401. [DOI] [PubMed] [Google Scholar]
- 48. Xie, P. , Kraus, Z. J. , Stunz, L. L. , Liu, Y. , Bishop, G. A. (2011) TNF receptor‐associated factor 3 is required for T cell‐mediated immunity and TCR/CD28 signaling. J. Immunol. 186, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xie, P. , Poovassery, J. S. , Stunz, L. L. , Smith, S. M. , Schultz, M. L. , Carlin, L. E. , Bishop, G. A. (2011) Enhanced Toll‐like receptor (TLR) responses of TNFR‐associated factor 3 (TRAF3)‐deficient B lymphocytes. J. Leukoc. Biol. 90, 1149–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Perkins, D. J. , Polumuri, S. K. , Pennini, M. E. , Lai, W. , Xie, P. , Vogel, S. N. (2013) Reprogramming of murine macrophages through TLR2 confers viral resistance via TRAF3‐mediated, enhanced interferon production. PLoS Pathog. 9, e1003479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lalani, A. I. , Luo, C. , Han, Y. , Xie, P. (2015) TRAF3: a novel tumor suppressor gene in macrophages. Macrophage (Houst). 2, e1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moore, C. R. , Liu, Y. , Shao, C. , Covey, L. R. , Morse, H. C. , III, Xie, P. (2012) Specific deletion of TRAF3 in B lymphocytes leads to B‐lymphoma development in mice. Leukemia 26, 1122–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wallis, A. , Yi, Z. , Bishop, G. (2015) TRAF3 regulates Csk and PTPN22 to enhance T cell receptor signaling. J. Immunol. 194 (1 Suppl), 61.6. [Google Scholar]
- 54. Yi, Z. , Stunz, L. L. , Bishop, G. A. (2013) TNF receptor associated factor 3 plays a key role in development and function of invariant natural killer T cells. J. Exp. Med. 210, 1079–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yi, Z. , Stunz, L. L. , Lin, W. W. , Bishop, G. A. (2014) TRAF3 regulates homeostasis of CD8+ central memory T cells. PLoS One 9, e102120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yi, Z. , Lin, W. W. , Stunz, L. L. , Bishop, G. A. (2014) The adaptor TRAF3 restrains the lineage determination of thymic regulatory T cells by modulating signaling via the receptor for IL‐2. Nat. Immunol. 15, 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chang, J.‐H. , Hu, H. , Jin, J. , Puebla‐Osorio, N. , Xiao, Y. , Gilbert, B. E. , Brink, R. , Ullrich, S. E. , Sun, S.‐C. (2014) TRAF3 regulates the effector function of regulatory T cells and humoral immune responses. J. Exp. Med. 211, 137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cheng, G. , Cleary, A. M. , Ye, Z. S. , Hong, D. I. , Lederman, S. , Baltimore, D. (1995) Involvement of CRAF1, a relative of TRAF, in CD40 signaling. Science 267, 1494–1498. [DOI] [PubMed] [Google Scholar]
- 59. He, L. , Grammer, A. C. , Wu, X. , Lipsky, P. E. (2004) TRAF3 forms heterotrimers with TRAF2 and modulates its ability to mediate NF‐kPB activation. J. Biol. Chem. 279, 55855–55865. [DOI] [PubMed] [Google Scholar]
- 60. Stunz, L. L. , Bishop, G. A. (2014) Latent membrane protein 1 and the B lymphocyte—a complex relationship. Crit. Rev. Immunol. 34, 177–198. [DOI] [PubMed] [Google Scholar]
- 61. Ha, Y. J. , Lee, J. R. (2004) Role of TNF receptor‐associated factor 3 in the CD40 signaling by production of reactive oxygen species through association with p40phox, a cytosolic subunit of nicotinamide adenine dinucleotide phosphate oxidase. J. Immunol. 172, 231–239. [DOI] [PubMed] [Google Scholar]
- 62. Fang, D.‐F. , He, K. , Wang, N. , Sang, Z. H. , Qiu, X. , Xu, G. , Jian, Z. , Liang, B. , Li, T. , Li, H.‐Y. , Li, A. L. , Zhou, T. , Gong, W.‐L. , Yang, B. , Karin, M. , Zhang, X.‐M. , Li, W.‐H. (2014) NEDD4 ubiquitinates TRAF3 to promote CD40‐mediated AKT activation. Nat. Commun. 5, 4513. [DOI] [PubMed] [Google Scholar]
- 63. Liao, G. , Zhang, M. , Harhaj, E. W. , Sun, S.‐C. (2004) Regulation of the NF‐kappaB‐inducing kinase by tumor necrosis factor receptor‐associated factor 3‐induced degradation. J. Biol. Chem. 279, 26243–26250. [DOI] [PubMed] [Google Scholar]
- 64. Hildebrand, J. M. , Luo, Z. , Manske, M. K. , Price‐Troska, T. , Ziesmer, S. C. , Lin, W. , Hostager, B. S. , Slager, S. , Witzig, T. E. , Ansell, S. M. , Cerhan, J. R. , Bishop, G. A. , Novak, A. J. (2010) A BAFF‐R mutation associated with non‐Hodgkin lymphoma alters TRAF recruitment and reveals new insights into BAFF‐R signaling. J. Exp. Med. 207, 2569–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hostager, B. S. , Catlett, I. M. , Bishop, G. A. (2000) Recruitment of CD40 and tumor necrosis factor receptor‐associated factors 2 and 3 to membrane microdomains during CD40 signaling. J. Biol. Chem. 275, 15392–15398. [DOI] [PubMed] [Google Scholar]
- 66. Brown, K. D. , Hostager, B. S. , Bishop, G. A. (2002) Regulation of TRAF2 signaling by self‐induced degradation. J. Biol. Chem. 277, 19433–19438. [DOI] [PubMed] [Google Scholar]
- 67. Moore, C. R. , Bishop, G. A. (2005) Differential regulation of CD40‐mediated TNF receptor‐associated factor degradation in B lymphocytes. J. Immunol. 175, 3780–3789. [DOI] [PubMed] [Google Scholar]
- 68. Gardam, S. , Turner, V. M. , Anderton, H. , Limaye, S. , Basten, A. , Koentgen, F. , Vaux, D. L. , Silke, J. , Brink, R. (2011) Deletion of cIAP1 and cIAP2 in murine B lymphocytes constitutively activates cell survival pathways and inactivates the germinal center response. Blood 117, 4041–4051. [DOI] [PubMed] [Google Scholar]
- 69. Hauer, J. , Püschner, S. , Ramakrishnan, P. , Simon, U. , Bongers, M. , Federle, C. , Engelmann, H. (2005) TNF receptor (TNFR)‐associated factor (TRAF) 3 serves as an inhibitor of TRAF2/5‐mediated activation of the noncanonical NF‐kappaB pathway by TRAF‐binding TNFRs. Proc. Natl. Acad. Sci. USA 102, 2874–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Brownlie, R. J. , Zamoyska, R. (2013) T Cell receptor signalling networks: branched, diversified and bounded. Nat. Rev. Immunol. 13, 257–269. [DOI] [PubMed] [Google Scholar]
- 71. Lin, W. W. , Yi, Z. , Stunz, L. L. , Maine, C. J. , Sherman, L. A. , Bishop, G. A. (2015) The adaptor protein TRAF3 inhibits interleukin‐6 receptor signaling in B cells to limit plasma cell development. Sci. Signal. 8, ra88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chng, W. J. , Keats, J. J. , Chesi, M. , Baker, A. , Bruhn, L. K. , Dispenzieri, A. , Stewart, A. K. , Carpten, J. , Fonesca, R. , Bergsagel, P. L. (2007) Prediction of response to bortezomib and dexamethasone resistance in myeloma (MM) by novel mutations in the NFKB pathway. J. Clin. Oncol. 25 (Suppl), 8007. [Google Scholar]
- 73. Wang, Y. , Shaked, I. , Stanford, S. M. , Zhou, W. , Curtsinger, J. M. , Mikulski, Z. , Shaheen, Z. R. , Cheng, G. , Sawatzke, K. , Campbell, A. M. , Auger, J. L. , Bilgic, H. , Shoyama, F. M. , Schmeling, D. O. , Balfour, H. H., Jr. , Hasegawa, K. , Chan, A. C. , Corbett, J. A. , Binstadt, B. A. , Mescher, M. F. , Ley, K. , Bottini, N. , Peterson, E. J. (2013) The autoimmunity‐associated gene PTPN22 potentiates Toll‐like receptor‐driven, type 1 interferon‐dependent immunity. Immunity 39, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhu, S. , Pan, W. , Shi, P. , Gao, H. , Zhao, F. , Song, X. , Liu, Y. , Zhao, L. , Li, X. , Qian, Y. (2010) Modulation of experimental autoimmune encephalomyelitis through TRAF3‐mediated suppression of interleukin 17 receptor signaling. J. Exp. Med. 207, 2647–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gordy, L. E. , Bezbradica, J. S. , Flyak, A. I. , Spencer, C. T. , Dunkle, A. , Sun, J. , Stanic, A. K. , Boothby, M. R. , He, Y. W. , Zhao, Z. , Van Kaer, L. , Joyce, S. (2011) IL‐15 regulates homeostasis and terminal maturation of NKT cells. J. Immunol. 187, 6335–6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Godfrey, D. I. , Berzins, S. P. (2007) Control points in NKT‐cell development. Nat. Rev. Immunol. 7, 505–518. [DOI] [PubMed] [Google Scholar]
- 77. Vallabhapurapu, S. , Matsuzawa, A. , Zhang, W. , Tseng, P.‐H. , Keats, J. J. , Wang, H. , Vignali, D. A. A. , Bergsagel, P. L. , Karin, M. (2008) Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK‐dependent alternative NF‐kappaB signaling. Nat. Immunol. 9, 1364–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zarnegar, B. J. , Wang, Y. , Mahoney, D. J. , Dempsey, P. W. , Cheung, H. H. , He, J. , Shiba, T. , Yang, X. , Yeh, W.‐C. , Mak, T. W. , Korneluk, R. G. , Cheng, G. (2008) Noncanonical NF‐kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat. Immunol. 9, 1371–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Coope, H. J. , Atkinson, P. G. P. , Huhse, B. , Belich, M. , Janzen, J. , Holman, M. J. , Klaus, G. G. B. , Johnston, L. H. , Ley, S. C. (2002) CD40 regulates the processing of NF‐kappaB2 p100 to p52. EMBO J. 21, 5375–5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kayagaki, N. , Yan, M. , Seshasayee, D. , Wang, H. , Lee, W. , French, D. M. , Grewal, I. S. , Cochran, A. G. , Gordon, N. C. , Yin, J. , Starovasnik, M. A. , Dixit, V. M. (2002) BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF‐kappaB2. Immunity 17, 515–524. [DOI] [PubMed] [Google Scholar]
- 81. Lin, W. W. , Hostager, B. S. , Bishop, G. A. (2015) TRAF3, ubiquitination, and B‐lymphocyte regulation. Immunol. Rev. 266, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xie, P. , Brown, L. , Stunz, L. , Bishop, G. (2008) TRAF3 inhibits signaling by Toll‐like receptors in B lymphocytes. FASEB J. 22, 1066.5. [Google Scholar]
- 83. Lalani, A. I. , Moore, C. R. , Luo, C. , Kreider, B. Z. , Liu, Y. , Morse, H. C. , III, Xie, P. (2015) Myeloid cell TRAF3 regulates immune responses and inhibits inflammation and tumor development in mice. J. Immunol. 194, 334–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Michel, M. , Wilhelmi, I. , Schultz, A.‐S. , Preussner, M. , Heyd, F. (2014) Activation‐induced tumor necrosis factor receptor‐associated factor 3 (Traf3) alternative splicing controls the noncanonical nuclear factor kPB pathway and chemokine expression in human T cells. J. Biol. Chem. 289, 13651–13660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lin, W. W. , Hildebrand, J. M. , Bishop, G. A. (2013) A complex relationship between TRAF3 and non‐canonical NF‐kPB2 activation in B lymphocytes. Front. Immunol. 4, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mecklenbräuker, I. , Kalled, S. L. , Leitges, M. , Mackay, F. , Tarakhovsky, A. (2004) Regulation of B‐cell survival by BAFF‐dependent PKCdelta‐mediated nuclear signalling. Nature 431, 456–461. [DOI] [PubMed] [Google Scholar]
- 87. Hildebrand, J. M. , Yi, Z. , Buchta, C. M. , Poovassery, J. S. , Stunz, L. L. , Bishop, G. A. (2011) Roles of tumor necrosis factor receptor associated factor 3 (TRAF3) and TRAF5 in immune cell functions. Immunol. Rev. 244, 55–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Häcker, H. , Tseng, P.‐H. , Karin, M. (2011) Expanding TRAF function: TRAF3 as a tri‐faced immune regulator. Nat. Rev. Immunol. 11, 457–468. [DOI] [PubMed] [Google Scholar]
- 89. Buchta, C. M. , Bishop, G. A. (2014) TRAF5 negatively regulates TLR signaling in B lymphocytes. J. Immunol. 192, 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nakano, H. , Sakon, S. , Koseki, H. , Takemori, T. , Tada, K. , Matsumoto, M. , Munechika, E. , Sakai, T. , Shirasawa, T. , Akiba, H. , Kobata, T. , Santee, S. M. , Ware, C. F. , Rennert, P. D. , Taniguchi, M. , Yagita, H. , Okumura, K. (1999) Targeted disruption of Traf5 gene causes defects in CD40‐ and CD27‐mediated lymphocyte activation. Proc. Natl. Acad. Sci. USA 96, 9803–9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. MacLeod, H. , Wetzler, L. M. (2007) T Cell activation by TLRs: a role for TLRs in the adaptive immune response. Sci. STKE 2007, pe48. [DOI] [PubMed] [Google Scholar]
- 92. McCarron, M. , Reen, D. J. (2009) Activated human neonatal CD8+ T cells are subject to immunomodulation by direct TLR2 or TLR5 stimulation. J. Immunol. 182, 55–62. [DOI] [PubMed] [Google Scholar]
- 93. Mercier, B. C. , Cottalorda, A. , Coupet, C.‐A. , Marvel, J. , Bonnefoy‐Bérard, N. (2009) TLR2 engagement on CD8 T cells enables generation of functional memory cells in response to a suboptimal TCR signal. J. Immunol. 182, 1860–1867. [DOI] [PubMed] [Google Scholar]
- 94. Nyirenda, M. H. , Sanvito, L. , Darlington, P. J. , O'Brien, K. , Zhang, G. X. , Constantinescu, C. S. , Bar‐Or, A. , Gran, B. (2011) TLR2 stimulation drives human naive and effector regulatory T cells into a Th17‐like phenotype with reduced suppressive function. J. Immunol. 187, 2278–2290. [DOI] [PubMed] [Google Scholar]
- 95. Nyirenda, M. H. , Morandi, E. , Vinkemeier, U. , Constantin‐Teodosiu, D. , Drinkwater, S. , Mee, M. , King, L. , Podda, G. , Zhang, G.‐X. , Ghaemmaghami, A. , Constantinescu, C. S. , Bar‐Or, A. , Gran, B. (2015) TLR2 stimulation regulates the balance between regulatory T cell and Th17 function: a novel mechanism of reduced regulatory T cell function in multiple sclerosis. J. Immunol. 194, 5761–5774. [DOI] [PubMed] [Google Scholar]
- 96. Muro, I. , Fang, G. , Gardella, K. A. , Mahajan, I. M. , Wright, C. W. (2014) The TRAF3 adaptor protein drives proliferation of anaplastic large cell lymphoma cells by regulating multiple signaling pathways. Cell Cycle 13, 1918–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Urbich, C. , Mallat, Z. , Tedgui, A. , Clauss, M. , Zeiher, A. M. , Dimmeler, S. (2001) Upregulation of TRAF‐3 by shear stress blocks CD40‐mediated endothelial activation. J. Clin. Invest. 108, 1451–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lee, Z. H. , Lee, S. E. , Kwack, K. , Yeo, W. , Lee, T. H. , Bae, S. S. , Suh, P. G. , Kim, H. H. (2001) Caspase‐mediated cleavage of TRAF3 in FasL‐stimulated Jurkat‐T cells. J. Leukoc. Biol. 69, 490–496. [PubMed] [Google Scholar]
- 99. Wu, Y. , Zheng, M. , Wang, S. , Song, C. , Wang, C. , Xiao, Y. , Xu, L. , Xu, X. (2014) Spatiotemporal pattern of TRAF3 expression after rat spinal cord injury. J. Mol. Histol. 45, 541–553. [DOI] [PubMed] [Google Scholar]
- 100. Xu, L.‐G. , Shu, H.‐B. (2002) TNFR‐associated factor‐3 is associated with BAFF‐R and negatively regulates BAFF‐R‐mediated NF‐kappa B activation and IL‐10 production. J. Immunol. 169, 6883–6889. [DOI] [PubMed] [Google Scholar]
- 101. Munroe, M. E. (2009) Functional roles for T cell CD40 in infection and autoimmune disease: the role of CD40 in lymphocyte homeostasis. Semin. Immunol. 21, 283–288. [DOI] [PubMed] [Google Scholar]
- 102. Yi, Z. , Stunz, L. L. , Bishop, G. A. (2014) CD40‐mediated maintenance of immune homeostasis in the adipose tissue microenvironment. Diabetes 63, 2751–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]


